Abstract
Background
Although smoking is classified as a risk factor for severe COVID-19 outcomes, there is a scarcity of studies on prevalence of smoking during the COVID-19 pandemic. Thus, this study aims to analyze the trends of prevalence of smoking in adolescents over the COVID-19 pandemic period.
Methods
The present study used data from middle to high school adolescents between 2005 and 2021 who participated in the Korea Youth Risk Behavior Web-based Survey (KYRBS). We evaluated the smoking prevalence (ever or daily) by year groups and estimated the slope in smoking prevalence before and during the pandemic.
Results
A total of 1,137,823 adolescents participated in the study [mean age, 15.04 years [95% confidence interval (CI) 15.03–15.06]; and male, 52.4% (95% CI 51.7–53.1)]. The prevalence of ever smokers was 27.7% (95% CI 27.3–28.1) between 2005 and 2008 but decreased to 9.8% (95% CI 9.3–10.3) in 2021. A consistent trend was found in daily smokers, as the estimates decreased from 5.4% (95% CI 5.2–5.6) between 2005 and 2008 to 2.3% (95% CI 2.1–2.5) in 2021. However, the downward slope in the overall prevalence of ever smokers and daily smokers became less pronounced in the COVID-19 pandemic period than in the pre-pandemic period. In the subgroup with substance use, the decreasing slope in daily smokers was significantly more pronounced during the pandemic than during the pre-pandemic period.
Conclusions
The proportion of ever smokers and daily smokers showed a less pronounced decreasing trend during the pandemic. The findings of our study provide an overall understanding of the pandemic’s impact on smoking prevalence in adolescents.
Supplementary file2 (MP4 64897 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s12519-022-00673-8.
Keywords: Adolescent, COVID-19, Daily smokers, Ever smokers, Pandemic, Smoking
Introduction
The global pandemic of the novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has affected daily lives over the past three years [1–3]. With the prolonged pandemic, many studies have been carried out to discover risk factors for severe COVID-19 outcomes, with smoking being identified as an important risk factor [4,5]. Smoking can accelerate the entry of angiotensin-converting enzyme-2 (ACE-2) receptor-mediated virus by stimulating the ACE-2 receptor in COVID-19, leading to worse outcomes [6]. However, despite the increased risk due to smoking, there are limited data on smoking prevalence during the pandemic, especially during 2021. Previous studies were mainly based on data from 2020, the early pandemic period, and could not evaluate the overall change throughout the pandemic [4,7]. Moreover, existing studies on smoking among adolescents during the COVID-19 period have important limitations. The studies were generally performed in the short term, did not include the mid-pandemic period, or included a small sample size; thus, further robust research is needed [8–11]. As warnings of the next COVID-19 resurgence advent, more public health studies will be needed.
Therefore, through this study, we will examine adolescents’ smoking status and make recommendations for policy and practice. This study aimed to analyze a nationwide survey based on more than one million adolescents from 2005 to 2021 to estimate how smoking prevalence has changed over the pandemic period in this/the latter population. The proportion of students with experience of smoking and students who smoked every day was compared by year group to analyze their trends. We also evaluated whether the changes in smoking rates showed different patterns in the groups divided by correlative factors.
Methods
Study population and data sources
This study included data provided by the Korea Youth Risk Behavior Web-based Survey (KYRBS) from 2005 to 2021 [12]. The KYRBS is a national representative dataset carried out by the South Korean Ministry of Education for government use. A two-step stratification, sample clustering, and weights based on school and class were used when selecting the study population. It is based on national representative sampling and approximates the total adolescent population of South Korea [12–14]. The serial survey enrolled middle to high school students aged 12–18 years, and their response rate was > 95%. Participants voluntarily submitted the survey at their respective schools. The protocol used in the study was approved by the Korea Disease Control and Prevention Agency (KDCA) and Sejong University (SJU-HR-E-2020-003). Informed written consent was obtained from all participants at enrollment.
Endpoints
The smoking status was based on whether the participant had ever smoked and included vaping as a form of smoking. The frequency of smoking was defined by how many days participants smoked in the past 30 days. The number of days was categorized into seven groups (0 days, 1–2 days, 3–5 days, 6–9 days, 10–19 days, 20–29 days, every day).
The participants were asked if they had ever smoked in their life and how often they smoked during the past 30 days. Ever smokers were defined as lifetime smokers, and daily smokers were defined as those who had smoked every day for the previous 30 days. The 17-year trend in the prevalence of lifetime smoking status (ever smokers) and everyday smoking status within the past 30 days (daily smokers) to assess whether the COVID-19 pandemic has altered these trends were the primary outcomes of interest. Furthermore, the trend changes in both prevalence during COVID-19 were evaluated based on five subgroups: sex, grade, residence area, self-reported depressive symptoms (sadness or despair), and self-reported substance use. The subgroups were set to analyze whether the smoking prevalence appears differently depending on correlative factors.
Covariate definitions
Residential areas were classified into two groups, urban and rural areas [15]. Urban areas included Seoul, Gyeonggi, Busan, Incheon, Daegu, Daejeon, Gwangju, Ulsan, and Sejong. Rural areas included the rest of the residential areas, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gangwon, Gyeongbuk, Gyeongnam, and Jeju. Three self-reported datasets, including parents’ educational levels, economic status, and school performance, were also analyzed by group. Parents’ educational levels were divided into three groups (high school or lower, college or higher, and unknown) regarding the higher educational level between parents [12,13]. Economic status and school performance were divided into five groups (high, middle-high, middle, middle-low, and low) according to the participants’ data. Sadness or despair was defined as having had these feelings during the past year. Substance use was based on whether the participant had experienced inhalants such as butane gas and bond, stimulants, heroines, amphetamines, drugs such as cannabis, and large amounts of tranquilizer doses. Smoking was excluded from the definition of substance use.
Statistical analyses
The data source was the KYRBS conducted between 2005 and 2021. The KYRBS is conducted every October and thus can significantly represent the early- and mid-COVID-19 pandemic periods. The trend of change in the ratio of ever smokers and daily smokers was analyzed based on the stratification by sex, grade, residence area, sadness or despair, and substance use. To obtain stable estimates for prevalence, the pre-COVID-19 period was arranged into four consecutive year groups (2005–2008, 2009–2012, 2013–2016, and 2017–2019), and the COVID-19 pandemic period was arranged into two groups (2020 and 2021). A weighted complex sampling analysis was presented, followed by binary and linear logistic regression models. The results of the analyses are displayed as weighted odds ratios (ORs) with 95% confidence intervals (CIs) or weighted β-coefficients with 95% CIs [16,17]. The smoking prevalence in KYRBS cycles was analyzed as a continuous variable set from 2005 to 2008, 2009 to 2012, 2013 to 2016, 2017 to 2019, 2020 (early COVID-19 pandemic), and 2021 (mid-COVID-19 pandemic) in linear regression and as a categorical variable set by last pre-pandemic (2017 to 2019) versus the COVID-19 pandemic (2020 and 2021) in binary logistic regression. During the progress of the analysis, those with missing values were excluded. All analyses were carried out in SAS (version 9.4; SAS Institute Inc., Cary, NC, USA) and SPSS (version 26.0; IBM Corp., Armonk, NY, USA). Statistical significance was defined as a two-sided P value less than 0.05.
Patient and public involvement
None of the patients were directly involved in designing the research questions or executing the research. They were not asked for advice on the process of interpreting or writing the results. We will develop a publicly available website to inform the relevant patient community.
Results
A total of 1,137,823 adolescents were included in the KYRBS from 2005 to 2021. Among the participants, 51.5% (n = 586,132) were male, and the weighted estimate was 52.4% (95% CI 51.7–53.1). The weighted estimate of the mean age in adolescents was 15.04 years (95% CI 15.03–15.06), including 672,799 (weighted %, 57.8%) middle school students and 465,024 (weighted %, 42.2%) high school students (Table 1).
Table 1.
Baseline characteristics of the adolescent participants in the KYRBS, 2005–2021 (n = 1,137,823)
| Characteristics | Weighted sample, n (%) or weighted % (95% CI) | Crude sample, n (%) or median (IQR) |
|---|---|---|
| Number | 1,137,823 (100.0) | 1,137,823 |
| Age, y | 15.04 (15.03–15.06) | 15.00 (14.00–16.00) |
| Grade | ||
| 7th–9th grade (middle school) | 57.8 (57.5–58.2) | 672,799 (59.1) |
| 10th–12th grade (high school) | 42.2 (41.8–42.5) | 465,024 (40.9) |
| Sex, male | 52.4 (51.7–53.1) | 586,132 (51.5) |
| Region of residence | ||
| Rural | 54.1 (53.8–54.5) | 611,281 (53.7) |
| Urban | 45.9 (45.5–46.2) | 526,542 (46.3) |
| Smoking | 21.3 (21.1–21.5) | 240,142 (21.1) |
| The highest educational level of parents | ||
| High school or lower | 33.9 (33.7–34.2) | 399,719 (35.1) |
| College or higher | 50.8 (50.5–51.1) | 553,153 (48.6) |
| Unknown | 15.2 (15.1–15.4) | 184,951 (16.3) |
| Economic level | ||
| High | 8.2 (8.1–8.2) | 90,618 (8.0) |
| Middle-high | 26.9 (26.7–27.0) | 297,553 (26.2) |
| Middle | 46.6 (46.4–46.7) | 534,538 (47.0) |
| Middle-low | 14.4 (14.3–14.6) | 168,333 (14.8) |
| Low | 4.0 (3.9–4.0) | 46,781 (4.1) |
| School performance | ||
| High | 12.2 (12.1–12.3) | 138,952 (12.2) |
| Middle-high | 25.3 (25.2–25.4) | 286,555 (25.2) |
| Middle | 28.4 (28.3–28.5) | 323,593 (28.4) |
| Middle-low | 23.5 (23.4–23.6) | 267,096 (23.5) |
| Low | 10.6 (10.5–10.7) | 121,627 (10.7) |
| Smoking frequency | ||
| 0 d/mon | 90.3 (90.2–90.5) | 1,030,117 (90.5) |
| 1–2 d/mon | 1.9 (1.8–1.9) | 21,411 (1.9) |
| 3–5 d/mon | 0.8 (0.8–0.8) | 8979 (0.8) |
| 6–9 d/mon | 0.7 (0.6–0.7) | 7326 (0.6) |
| 10–19 d/mon | 0.8 (0.8–0.9) | 9374 (0.8) |
| 20–29 d/mon | 0.9 (0.9–0.9) | 10,078 (0.9) |
| Every day | 4.6 (4.5–4.7) | 50,538 (4.4) |
| Sadness or despair | 31.6 (31.5–31.8) | 357,900 (31.5) |
| Substance use | 1.2 (1.1–1.2) | 12,754 (1.1) |
CI confidence interval, IQR interquartile range, KYRBS, Korea Youth Risk Behavior Web-Based Survey
The trend changes and proportion of ever smokers and daily smokers from 2005 to 2021 are shown in Tables 2 and 3. Most prevalence in the following years steadily decreased during every predefined period (Fig. 1a, b). However, the downward slope in overall ever smokers and daily smokers became less pronounced during the pandemic; the slope value was −0.312 (95% CI −0.323 to −0.300) before the pandemic and −0.123 (95% CI −0.141 to −0.105) during the pandemic for ever smokers (βdiff, 0.189; 95% CI 0.168–0.210). For daily smokers, the rate was −0.208 (95% CI −0.230 to −0.187) in the pre-pandemic years and −0.094 (95% CI −0.124 to −0.065) during the pandemic (βdiff, 0.114; 95% CI 0.077–0.151).
Table 2.
Weighted estimation of prevalence and trend in adolescent ever smokers in South Korea, 2005–2021
| Characteristics | 2005–2008 | 2009–2012 | 2013–2016 | 2017–2019 | 2020 (Early pandemic) | 2021 (Mid pandemic) | The trend before the pandemic, β (95% CI)a | The trend during the pandemic, β (95% CI)a | Trend difference, βdiff (95% CI) | OR (95% CI)b |
|---|---|---|---|---|---|---|---|---|---|---|
| Smoking, weighted % (95% CI) | ||||||||||
| Overall | 27.7 (27.3–28.1) | 25.9 (25.5–26.3) | 18.3 (17.9–18.8) | 13.6 (13.2–14.0) | 10.2 (9.7–10.7) | 9.8 (9.3–10.3) | −0.312 (−0.323 to −0.300) | − 0.123 (− 0.141 to − 0.105) | 0.189 (0.168 to 0.210) | 0.704 (0.669 to 0.741) |
| Sex | ||||||||||
| Male | 32.8 (32.3–33.3) | 33.3 (32.8–33.8) | 26.3 (25.9–26.8) | 19.5 (19.0–19.9) | 13.8 (13.1–14.5) | 13.1 (12.4–13.7) | −0.237 (−0.249 to −0.225) | −0.155 (−0.172 to −0.137) | 0.082 (0.061 to 0.103) | 0.642 (0.610 to 0.676) |
| Female | 21.9 (21.5–22.4) | 17.6 (17.2–18.1) | 9.6 (9.3–9.9) | 7.3 (7.0–7.6) | 6.3 (5.8–6.7) | 6.4 (6.0–6.8) | −0.461 (−0.476 to −0.446) | −0.054 (−0.078 to −0.029) | 0.407 (0.378 to 0.436) | 0.853 (0.798 to 0.912) |
| Grade | ||||||||||
| 7th–9th grade (middle school) | 22.4 (22.0–22.8) | 21.2 (20.9–21.6) | 13.0 (12.7–13.3) | 8.7 (8.4–9.0) | 5.7 (5.4–6.1) | 5.1 (4.8–5.5) | −0.369 (−0.382 to −0.357) | −0.174 (−0.194 to −0.155) | 0.195 (0.172 to 0.218) | 0.605 (0.570 to 0.642) |
| 10th–12th grade (high school) | 36.2 (35.5–36.9) | 32.1 (31.4–32.9) | 25.5 (24.8–26.2) | 19.6 (19.0–20.3) | 15.5 (14.6–16.5) | 15.5 (14.6–16.4) | −0.297 (−0.315 to −0.280) | −0.100 (−0.124 to −0.076) | 0.197 (0.167 to 0.227) | 0.750 (0.701 to 0.802) |
| Region of residence | ||||||||||
| Rural | 28.6 (28.0–29.3) | 26.6 (26.0–27.3) | 18.9 (18.4–19.5) | 14.2 (13.7–14.7) | 10.9 (10.2–11.7) | 10.2 (9.5–10.9) | −0.319 (−0.336 to −0.302) | −0.122 (−0.146 to −0.098) | 0.197 (0.168 to 0.226) | 0.714 (0.667 to 0.764) |
| Urban | 26.7 (26.2–27.2) | 25.1 (24.5–25.6) | 17.6 (17.0–18.2) | 12.9 (12.3–13.5) | 9.1 (8.4–9.9) | 9.3 (8.7–10.1) | −0.306 (−0.322 to −0.290) | −0.124 (−0.152 to −0.097) | 0.182 (0.150 to 0.214) | 0.687 (0.635 to 0.744) |
| Sadness or despair | ||||||||||
| Without symptoms | 23.7 (23.3–24.1) | 22.1 (21.7–22.5) | 16.0 (15.6–16.4) | 11.8 (11.4–12.2) | 8.3 (7.8–8.9) | 8.0 (7.5–8.4) | −0.298 (−0.312 to −0.285) | −0.141 (−0.162 to −0.121) | 0.157 (0.132 to 0.182) | 0.665 (0.626 to 0.706) |
| With symptoms | 34.2 (33.7–34.8) | 33.1 (32.5–33.6) | 24.8 (24.2–25.4) | 18.7 (18.2–19.3) | 15.6 (14.8–16.5) | 14.9 (14.1–15.8) | −0.265 (−0.278 to −0.251) | −0.090 (−0.112 to −0.068) | 0.175 (0.149 to 0.201) | 0.782 (0.736 to 0.830) |
| Substance use | ||||||||||
| No use | 27.1 (26.7–27.5) | 25.4 (25.0–25.8) | 18.1 (17.7–18.5) | 13.3 (13.0–13.7) | 10.1 (9.5–10.6) | 9.7 (9.2–10.2) | −0.308 (−0.320 to −0.296) | −0.118 (−0.136 to −0.100) | 0.190 (0.168 to 0.212) | 0.713 (0.677 to 0.751) |
| With substance use | 62.0 (60.2–63.8) | 60.2 (58.4–62.1) | 58.2 (55.6–60.8) | 45.1 (42.5–47.6) | 25.5 (21.0–30.7) | 24.9 (20.1–30.4) | −0.200 (−0.242 to −0.157) | −0.271 (−0.336 to −0.207) | − 0.071 (− 0.148 to 0.006) | 0.411 (0.332 to 0.509) |
| The highest educational level of parents | ||||||||||
| High school or lower | 31.7 (31.2–32.2) | 30.0 (29.4–30.5) | 23.1 (22.5–23.7) | 18.0 (17.4–18.7) | 13.4 (12.4–14.4) | 13.0 (12.1–14.0) | −0.214 (−0.227 to −0.202) | −0.111 (−0.133 to −0.089) | 0.103 (0.078 to 0.128) | 0.694 (0.643 to 0.748) |
| College or higher | 23.5 (23.0–24.0) | 22.4 (22.0–22.9) | 15.9 (15.5–16.4) | 12.3 (11.8–12.7) | 8.4 (7.9–8.9) | 8.0 (7.5–8.5) | −0.285 (−0.300 to −0.271) | −0.151 (−0.171 to −0.130) | 0.134 (0.109 to 0.159) | 0.638 (0.599 to 0.679) |
| Economic level | ||||||||||
| High to middle high | 23.6 (23.2–24.1) | 22.5 (22.0–23.0) | 15.7 (15.3–16.1) | 12.6 (12.2–13.1) | 9.4 (8.8–10.1) | 9.0 (8.4–9.6) | −0.304 (−0.320 to −0.288) | −0.124 (−0.147 to −0.101) | 0.180 (0.139 to 0.221) | 0.702 (0.657 to 0.750) |
| Middle to low | 30.2 (29.7–30.7) | 27.6 (27.1–28.1) | 20.0 (19.6–20.5) | 14.6 (14.2–15.0) | 10.7 (10.1–11.4) | 10.5 (10.0–11.0) | −0.306 (−0.318 to −0.294) | −0.126 (−0.145 to −0.107) | 0.180 (0.158 to 0.202) | 0.694 (0.657 to 0.734) |
The numbers in bold indicate a significant difference (P < 0.05)
CI confidence interval, KYRBS, Korea Youth Risk Behavior Web-based Survey, OR, odds ratio
aWeighted β was considered using the linear regression technique, and this method included the KYRBS cycle (2005–2008, 2009–2012, 2013–2016, 2017–2019, 2020, and 2021) as a continuous variable
bWeighted OR was considered using the logistic regression technique, and this method included the KYRBS cycle (2017–2019 versus 2020–2021 [COVID-19 pandemic]) as a categorical variable
Table 3.
Weighted estimation of prevalence and trend in adolescent daily smokers in South Korea, 2005–2021
| Trends in daily smokers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 2005–2008 | 2009–2012 | 2013–2016 | 2017–2019 | 2020 (Early pandemic) | 2021 (Mid pandemic) | The trend before the pandemic, β (95% CI)a | The trend during the pandemic, β (95% CI)a | Trend difference, βdiff (95% CI) | OR (95% CI)b |
| Smoking, weighted % (95% CI) | ||||||||||
| Overall | 5.4 (5.2 to 5.6) | 5.9 (5.7–6.2) | 4.0 (3.9–4.2) | 3.0 (2.8–3.2) | 2.3 (2.1–2.5) | 2.3 (2.1–2.5) | –0.208 (–0.230 to –0.187) | –0.094 (–0.124 to –0.065) | 0.114 (0.077 to 0.151) | 0.759 (0.696 to 0.828) |
| Sex | ||||||||||
| Male | 7.5 (7.2–7.8) | 8.8 (8.5–9.1) | 6.4 (6.2–6.7) | 4.6 (4.4–4.9) | 3.3 (3.0–3.7) | 3.2 (3.0–3.5) | –0.166 (–0.186 to –0.146) | –0.123 (–0.152 to –0.093) | 0.043 (0.007 to 0.079) | 0.700 (0.640 to 0.765) |
| Female | 3.0 (2.8–3.2) | 2.8 (2.6–2.9) | 1.5 (1.3–1.6) | 1.2 (1.1–1.4) | 1.2 (1.0–1.4) | 1.3 (1.1–1.5) | –0.343 (–0.376 to –0.309) | –0.005(–0.046 to –0.056) | 0.338 (0.304 to 0.372) | 0.994 (0.869 to 1.137) |
| Grade | ||||||||||
| 7th–9th grade (middle school) | 2.4 (2.3–2.5) | 2.9 (2.7–3.0) | 1.6 (1.5–1.7) | 1.1 (1.0–1.2) | 0.8 (0.7–0.9) | 0.7 (0.6–0.8) | –0.254 (–0.279 to –0.228) | –0.141 (–0.186 to –0.096) | 0.113 (0.061 to 0.165) | 0.661 (0.577 to 0.757) |
| 10th–12th grade (high school) | 10.2 (9.7–10.7) | 10.1 (9.7–10.5) | 7.3 (7.0–7.7) | 5.3 (5.0–5.6) | 4.2 (3.7–4.6) | 4.2 (3.8–4.6) | –0.241 (–0.266 to –0.216) | –0.086 (–0.119 to –0.052) | 0.155 (0.113 to 0.197) | 0.778 (0.705 to 0.858) |
| Region of residence | ||||||||||
| Rural | 5.6 (5.2–5.9) | 6.2 (5.8–6.5) | 4.1 (3.9–4.4) | 3.2 (2.9–3.4) | 2.4 (2.1–2.8) | 2.5 (2.2–2.8) | –0.215 (–0.245 to –0.185) | –0.085 (–0.123 to –0.047) | 0.130 (0.082 to 0.178) | 0.777 (0.695 to 0.868) |
| Urban | 5.2 (4.9–5.5) | 5.7 (5.4–6.0) | 3.9 (3.7–4.2) | 2.8 (2.6–3.1) | 2.1 (1.8–2.5) | 2.0 (1.7–2.3) | –0.203 (–0.233 to –0.174) | –0.110 (–0.157 to –0.064) | 0.093 (0.038 to 0.148) | 0.731 (0.635 to 0.840) |
| Sadness or despair | ||||||||||
| Without symptoms | 4.1 (3.9–4.3) | 4.6 (4.4–4.8) | 3.3 (3.1–3.4) | 2.4 (2.3–2.6) | 1.7 (1.5–1.9) | 1.7 (1.5–1.9) | –0.193 (–0.217 to –0.168) | –0.117 (–0.152 to –0.082) | 0.076 (0.033 to 0.119) | 0.706 (0.636 to 0.784) |
| With symptoms | 7.5 (7.1–7.8) | 8.5 (8.2–8.9) | 6.2 (5.9–6.5) | 4.6 (4.3–4.9) | 4.1 (3.6–4.5) | 3.8 (3.5–4.3) | –0.150 (–0.173 to –0.126) | –0.062 (–0.099 to –0.026) | 0.088 (0.045 to 0.131) | 0.847 (0.763 to 0.940) |
| Substance use | ||||||||||
| No use | 5.2 (4.9–5.4) | 5.8 (5.5–6.0) | 3.9 (3.8–4.1) | 2.9 (2.7–3.1) | 2.3 (2.0–2.5) | 2.3 (2.1–2.5) | –0.203 (–0.225 to –0.182) | –0.087 (–0.117 to –0.057) | 0.116 (0.079 to 0.153) | 0.776 (0.711 to 0.847) |
| With substance use | 19.0 (17.5–20.7) | 19.5 (18.0–21.1) | 21.1 (19.0–23.4) | 15.4 (13.6–17.3) | 8.0 (5.6–11.4) | 7.7 (5.2–11.2) | –0.042 (–0.097 to –0.012) | –0.216 (–0.298 to –0.135) | –0.174 (–0.266 to –0.082) | 0.471 (0.343 to 0.646) |
| The highest educational level of parents | ||||||||||
| High school or lower | 6.7 (6.4–7.0) | 7.6 (7.3–7.9) | 5.7 (5.4–7.0) | 4.3 (4.0–4.6) | 3.1 (2.6–3.6) | 3.0 (2.6–3.5) | –0.110 (–0.133 to –0.087) | –0.106 (–0.143 to –0.069) | 0.004 (– 0.040 to 0.048) | 0.698 (0.611 to 0.797) |
| College or higher | 4.0 (3.8–4.3) | 4.7 (4.4–4.9) | 3.2 (3.1–3.4) | 2.4 (2.3–2.6) | 1.6 (1.4–1.9) | 1.6 (1.4–1.8) | –0.188 (–0.215 to –0.162) | –0.142 (–0.179 to –0.105) | 0.046 (0.000 to 0.092) | 0.647 (0.574 to 0.729) |
| Economic level | ||||||||||
| High to middle high | 4.2 (4.0–4.4) | 5.1 (4.8–5.4) | 3.4 (3.2–3.6) | 3.0 (2.8–3.2) | 2.3 (2.0–2.6) | 2.1 (1.9–2.4) | –0.152 (–0.182 to –0.123) | –0.112 (–0.150 to –0.074) | 0.040 (–0.008 to 0.088) | 0.727 (0.648 to 0.816) |
| Middle to low | 6.3 (6.0–6.5) | 6.5 (6.2–6.7) | 4.5 (4.3–4.8) | 3.2 (3.0–3.4) | 2.4 (2.2–2.7) | 2.5 (2.3–2.7) | –0.218 (–0.240 to –0.197) | –0.093 (–0.125 to –0.061) | 0.125 (0.086 to 0.164) | 0.756 (0.687 to 0.831) |
The numbers in bold indicate a significant difference (P < 0.05)
CI confidence interval, KYRBS Korea Youth Risk Behavior Web-Based Survey, OR odds ratio
aWeighted β was considered using the linear regression technique, and this method included the KYRBS cycle (2005–2008, 2009–2012, 2013–2016, 2017–2019, 2020, and 2021) as a continuous variable
bWeighted OR was considered using the logistic regression technique, and this method included the KYRBS cycle (2017–2019 versus 2020–2021 [COVID-19 pandemic]) as a categorical variable
Fig. 1.
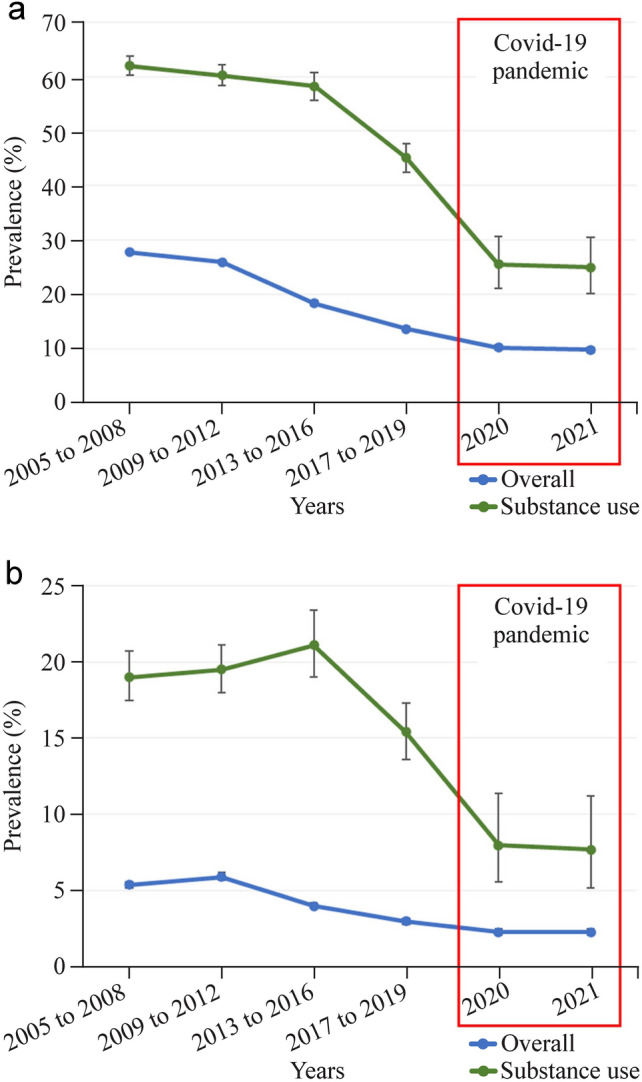
a The 17-year trend in adolescent ever smokers from 2005 to 2021; b the 17-year trend in adolescent daily smokers from 2005 to 2021
The national weighted prevalence of ever smokers was 27.7% (95% CI 27.3–28.1) from 2005 to 2008, but this decreased to 10.2% (95% CI 9.7–10.7) in 2020 and 9.8% (95% CI 9.3–10.3) in 2021. The slope of years 2005–2021 trend in overall prevalence of ever smokers was consistent in subgroups by sex (male: βdiff, 0.082; 95% CI 0.061–0.103; female: βdiff, 0.407; 95% CI 0.378–0.436), grade (7–9th grade: βdiff, 0.195; 95% CI 0.172–0.218; 10–12th grade: βdiff, 0.197; 95% CI 0.167–0.227), residence area (rural: βdiff, 0.197; 95% CI 0.168–0.226; urban: βdiff, 0.182; 95% CI 0.150–0.214), depressive symptoms (sadness or despair) (without symptoms: βdiff, 0.157; 95% CI 0.132–0.182; and with symptoms: βdiff, 0.175; 95% CI 0.149–0.201), highest educational level of parents (high school or lower: βdiff, 0.103; 95% CI 0.078–0.128; and college or higher: βdiff, 0.134; 95% CI 0.109–0.159), and economic level (high to middle high: βdiff, 0.180; 95% CI 0.139–0.221; and middle to low: βdiff, 0.180; 95% CI 0.158–0.202). The subgroups by substance use showed a steady decreasing trend (no use: βdiff, 0.190; 95% CI 0.168–0.212; with substance use: βdiff, −0.071; 95% CI −0.148–0.006).
The national weighted prevalence of daily smokers was 5.4% (95% CI 5.2–5.6) between 2005 and 2008, but this decreased to 2.3% (95% CI 2.1–2.5) in 2020 and 2021 (Table 3). The slope of the 17-year trend in subgroups showed a similar trend with that of the overall prevalence of daily smokers, disregarding sex (male: βdiff, 0.043; 95% CI 0.007–0.079; female: βdiff, 0.338; 95% CI 0.304–0.372), grade (7–9th grade: βdiff, 0.113; 95% CI 0.061–0.165; 10–12th grade: βdiff, 0.155; 95% CI 0.113–0.197), residence area (rural: βdiff, 0.130; 95% CI 0.082–0.178; urban: βdiff, 0.093; 95% CI 0.038–0.148), and depressive symptoms (i.e., sadness or despair) (without symptoms: βdiff, 0.076; 95% CI 0.033–0.119; and with symptoms: βdiff, 0.088; 95% CI 0.045–0.131). Some subgroups defined by parents’ highest educational level (high school or lower: βdiff, 0.004; 95% CI −0.040–0.048; and college or higher: βdiff, 0.048; 95% CI 0.000–0.092) and economic status (high to middle high: βdiff, 0.040; 95% CI −0.008–0.092; and middle to low: βdiff, 0.125; 95% CI 0.086–0.164) did not show a significant difference before and during the pandemic (Supplementary Figs. 1–15). However, the subgroup of adolescents with substance use experienced a more significant decreasing trend (no use: βdiff, 0.116; 95% CI 0.079–0.153; with substance use: βdiff, −0.174; 95% CI −0.266 to −0.082).
Discussion
This study analyzed the 17-year trend in the prevalence of ever smokers and daily smokers based on nationally representative data of adolescents in South Korea from 2005 to 2021. The overall estimated prevalence of ever smokers and daily smokers showed a continuously decreasing trend; however, the overall fall in the prevalence slowed down during the pandemic (2020–2021). A consistent trend was found in subgroups based on sex, grade, residence area, and depressive symptoms. Meanwhile, a different pattern was observed in the direction of the subset with substance use. The prevalence of daily smokers with substance use during the pandemic showed a more pronounced decreasing slope than expected based on pre-pandemic trends. Thus, by conducting a trend analysis over the long term, we evaluated how the pandemic influenced adolescents’ smoking status. Our results provide the foundations to help policymakers and physicians establish interventions to reduce smoking levels among adolescents.
Comparison with previous studies
Previous studies have found inconsistent results in relation to smoking prevalence in adolescents during the COVID-19 pandemic. Studies of adolescent smoking conducted in Norway (n = 227,258) [18] and Sweden (n = 1818) [9] stated no significant change in smoking prevalence during the pandemic. The study from Sweden concluded that tobacco use increased during the pandemic only for high-risk groups such as those with narcotic use. A similar result was found in a study from the Netherlands (n = 287) [10], which showed that the smoking rate increased during the pandemic. In contrast, studies conducted in the United States (n = 14,541) [8] and Central Catalonia (n = 303) [11] concluded that smoking prevalence decreased during the COVID-19 pandemic. Limitations of these studies include small and heterogeneous samples, data that are not nationally representative due to convenience sampling, compound bias, recall bias, and selection bias, which likely contribute to the inconsistency of the results [8–11].
Our results contradict those of a study from Norway [18], which found that there was no significant change in smoking prevalence pre- and post-COVID. The discrepancy may be because although the two studies were conducted in the same year, the period was different. The survey in Norway was conducted from January to March, whereas our study was conducted in October. As Norway’s data from 2020 include the pre-pandemic period and the data from 2021 are also difficult to classify as the mid-pandemic period, these data may not be reliable as evidence to confirm the change in smoking prevalence of the mid-pandemic period. Moreover, the population of the Korean survey is more significant than that of Norway, even considering that there were fewer survey samples due to the pandemic in 2020 and 2021. Racial and cultural differences should also be considered.
Possible explanations
The decrease in adolescents’ smoking status may be owing to the government’s policy efforts to reduce the smoking rate among adolescents. The Korean Ministry of Health and Welfare has been promoting the “school smoking prevention education project” to prevent youth smoking since 1999 and has been operating the “No Smoking Leading School” as part of its core project [19]. In addition, raising cigarette prices and restricting smoking in indoor workspaces and public places such as schools likely contributed to the decrease in smoking prevalence [20]. Other than governmental efforts, policies in relation to television advertisements, posters, and other media messages to oppose cigarette advertising also likely had a positive impact on the decrease in smoking prevalence among adolescents [21]. Similar patterns found in other developed countries support these hypotheses [22].
However, during the pandemic, the decrease in the prevalence of smoking eased. This may be due to the increase in depressive symptoms among adolescents. The long duration of quarantine leaving adolescents only active at home and the loss of interaction with peers potentially isolate adolescents, increasing the risk of depression [23]. Statistics showed that the rate of adolescents who experienced sadness or despair increased during the mid-pandemic period [24,25]. A likely increase in mental health complications may have resulted in adolescents using cigarettes to cope with the stress [26]. The COVID-19 pandemic also caused an inefficiency in education that likely affected smoking status. Youth smoking prevention education, which was previously implemented in schools, was not sufficiently conducted during the pandemic [27]. In addition, wearing a mask during the pandemic may have also increased adolescents’ access to cigarettes [27] owing to the mandatory wearing of masks making it challenging to check the age of buyers [28]. Interestingly, the decreasing rate was more significant in the subgroup of adolescents with substance use. This may be caused by the reinforcement of regulations on substance use by adolescents. The widespread use of substances by adolescents decreased over 17 years. As regulations on substances were tightened, adolescents who have tried substances may have also reduced cigarette smoking.
Policy implication
As previous studies did not analyze the long-term trend of adolescents’ smoking status, only positive interpretations were made claiming that the smoking rate decreased during the pandemic [11]. However, our results imply that the decrease in smoking rates during COVID-19 was less than expected based on pre-COVID trends, thus requiring further efforts to reduce the smoking prevalence. In recent years, smoking cessation and prevention policies have not been a priority due to COVID-19. However, policy involvement is needed, as smoking in adolescence can lead to lifelong heavy smoking [29]. Moreover, adolescent smoking is harder to mediate than adult smoking, as there is no initial treatment for adolescent smokers. This study can be used to check the current status of adolescent smoking, and thus help to preemptively prevent it. Possible policy efforts to prevent the aforementioned factors may include regulating the ease of purchase of cigarettes by adolescents, strengthening smoking prevention education, and regulating tobacco advertisements. In this regard, the World Health Organization (WHO) suggests MPOWER policies to cost-effectively reduce smoking. MPOWER measures include monitoring tobacco use, protecting people from tobacco use, offering to quit, warning of the dangers, enforcement of bans on advertising, and raising taxes on tobacco [30]. Policymakers should be cognizant of the fact that the decline in smoking prevalence has not been sufficient during the COVID-19 pandemic and that further efforts should be made to reduce the number of adolescents smoking.
Strength and limitations
This is the first large-scale, long-term serial, nationally representative study of adolescent smoking, including the mid-pandemic period (2020–2021). However, the findings must be interpreted in light of the study limitations. First, the data we used in this study were based on an anonymized, self-reported web-based survey conducted at respective schools. Thus, the information of students absent at the date could not be obtained. The missing data of absent students may lead to bias in these data since the group of absent students may have different characteristics than those in school. Second, we cannot rule out the possibility of other unmeasured confounding variables [31]. Third, there were cases in which the types of responses to the questionnaire changed over the years. We have conducted data mining of the responses for the relevant year, but some errors might have occurred during this process. Fourth, the results were derived from a survey conducted only on Korean adolescents. Accordingly, the racial and cultural diversity of the study population is low; thus, future studies in other countries are necessary. Fifth, although we included vaping as a form of smoking in our study, we could not collect the data on vaping separately. Finally, there was no objective measurement of exposure to products of tobacco, e.g., cotinine.
In conclusion, this study confirmed that the prevalence of smoking in adolescents decreased less during the pandemic compared to the pre-pandemic period by conducting a long-term trend analysis for ever smokers and daily smokers. A different tendency was found in the subgroup with substance use, as the rate of ever smokers decreased as before and more significantly for daily smokers. The present results provide a comprehensive picture of the past and current smoking prevalence trends of South Korean adolescents during the COVID-19 pandemic. The findings suggest that a political solution is required to maintain the decrease in smoking rates in adolescents at a similar level to the pre-pandemic period.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
DKY: data curation, formal analysis, conceptualization, writing–original draft, writing–review and editing, supervision. RK: formal analysis, writing–review and editing. SWL: supervision, writing–review and editing. All the other authors: writing–review and editing. All authors approved the final version before submission.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C0233) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF2021R1I1A2059735). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
Data are available on reasonable request. Study protocol, statistical code: available from DKY (email: yonkkang@gmail.com). Data set: available from the Korean Centers for Disease Control and Prevention Agency (KCDA) through a data use agreement.
Declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of the article.
Ethical approval
The KYRBS data were anonymous and the study protocol was approved by the Korean Centers for Disease Control and Prevention Agency (KCDA) and Institutional Review Board of Sejong University (SJU-HR-E-2020-003). Informed written consent was obtained from all participants at enrollment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seung Won Lee, Email: lsw2920@gmail.com.
Dong Keon Yon, Email: yonkkang@gmail.com.
Sunyoung Kim, Email: ggutsun@khu.ac.kr.
References
- 1.Eisenhut M, Shin JI. COVID-19 vaccines and coronavirus 19 variants including alpha, delta, and omicron: present status and future directions. Life Cycle. 2022;2:e4. doi: 10.54724/lc.2022.e4. [DOI] [Google Scholar]
- 2.Smith L, Shin JI, Koyanagi A. Vaccine strategy against COVID-19 with a focus on the omicron and stealth omicron variants: life cycle committee recommendations. Life Cycle. 2022;2:e5. doi: 10.54724/lc.2022.e5. [DOI] [Google Scholar]
- 3.Choi YJ, Acharya KP. How serious is the omicron variant? transmissibility, genomics, and responses to COVID-19 vaccines, and ‘stealth’ omicron variants. Life Cycle. 2022;2:e7. doi: 10.54724/lc.2022.e7. [DOI] [Google Scholar]
- 4.Almazeedi S, Al-Youha S, Jamal MH, Al-Haddad M, Al-Muhaini A, Al-Ghimlas F, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garnett C, Oldham M, Shahab L, Tattan-Birch H, Cox S. Characterising smoking and smoking cessation attempts by risk of alcohol dependence: a representative, cross-sectional study of adults in England between 2014–2021. Lancet Reg Health Eur. 2022;18:100418. doi: 10.1016/j.lanepe.2022.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson SE, Garnett C, Shahab L, Oldham M, Brown J. Association of the COVID-19 lockdown with smoking, drinking and attempts to quit in England: an analysis of 2019–20 data. Addiction. 2021;116:1233–1244. doi: 10.1111/add.15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentzke AS, Wang TW, Jamal A, Park-Lee E, Ren C, Cullen KA, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881–1888. doi: 10.15585/mmwr.mm6950a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapetanovic S, Ander B, Gurdal S, Sorbring E. Adolescent smoking, alcohol use, inebriation, and use of narcotics during the Covid-19 pandemic. BMC Psychol. 2022;10:44. doi: 10.1186/s40359-022-00756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Deelen TRD, Van den Putte B, Kunst AE, Kuipers MAG. Dutch youth’s smoking behaviour during a partial Covid-19 lockdown. J Public Health Res. 2022;11:jphr.2021.2106. doi: 10.4081/jphr.2021.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogés J, Bosque-Prous M, Colom J, Folch C, Barón-Garcia T, González-Casals H, et al. Consumption of alcohol, cannabis, and tobacco in a cohort of adolescents before and during COVID-19 confinement. Int J Environ Res Public Health. 2021;18:7849. doi: 10.3390/ijerph18157849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MJ, Lee KH, Lee JS, Kim N, Song JY, Shin YH, et al. Trends in body mass index changes among Korean adolescents between 2005–2020, including the COVID-19 pandemic period: a national representative survey of one million adolescents. Eur Rev Med Pharmacol Sci. 2022;26:4082–4091. doi: 10.26355/eurrev_202206_28978. [DOI] [PubMed] [Google Scholar]
- 13.Noh H, An J, Kim MJ, Sheen YH, Yoon J, Welsh B, et al. Sleep problems increase school accidents related to allergic diseases. Pediatr Allergy Immunol. 2020;31:98–103. doi: 10.1111/pai.13132. [DOI] [PubMed] [Google Scholar]
- 14.Lee KH, Yon DK, Suh DI. Prevalence of allergic diseases among Korean adolescents during the COVID-19 pandemic: comparison with pre-COVID-19 11-year trends. Eur Rev Med Pharmacol Sci. 2022;26:2556–2568. doi: 10.26355/eurrev_202204_28492. [DOI] [PubMed] [Google Scholar]
- 15.Woo A, Lee SW, Koh HY, Kim MA, Han MY, Yon DK. Incidence of cancer after asthma development: 2 independent population-based cohort studies. J Allergy Clin Immunol. 2021;147:135–143. doi: 10.1016/j.jaci.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Lee SW. Methods for testing statistical differences between groups in medical research: statistical standard and guideline of Life cycle committee. Life Cycle. 2022;2:e1. doi: 10.54724/lc.2022.e1. [DOI] [Google Scholar]
- 17.Lee SW. Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life cycle committee. Life Cycle. 2022;2:e3. doi: 10.54724/lc.2022.e3. [DOI] [Google Scholar]
- 18.von Soest T, Kozák M, Rodríguez-Cano R, Fluit DH, Cortés-García L, Ulset VS, et al. Adolescents’ psychosocial well-being one year after the outbreak of the COVID-19 pandemic in Norway. Nat Hum Behav. 2022;6:217–228. doi: 10.1038/s41562-021-01255-w. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Jang M, Yoo S, JeKarl J, Chung JY, Cho SI. School-based tobacco control and smoking in adolescents: evidence from multilevel analyses. Int J Environ Res Public Health. 2020;17:3422. doi: 10.3390/ijerph17103422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Hasselt M, Kruger J, Han B, Caraballo RS, Penne MA, Loomis B, et al. The relation between tobacco taxes and youth and young adult smoking: what happened following the 2009 US federal tax increase on cigarettes? Addict Behav. 2015;45:104–109. doi: 10.1016/j.addbeh.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoklosa M, Pogorzelczyk K, Balwicki Ł. Cigarette price increases, advertising ban, and pictorial warnings as determinants of youth smoking initiation in Poland. Nicotine Tob Res. 2022;24:820–825. doi: 10.1093/ntr/ntab262. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins SS, Bach N, Baum CF. Impact of tobacco control policies on adolescent smoking. J Adolesc Health. 2016;58:679–685. doi: 10.1016/j.jadohealth.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guessoum SB, Lachal J, Radjack R, Carretier E, Minassian S, Benoit L, et al. Adolescent psychiatric disorders during the COVID-19 pandemic and lockdown. Psychiatry Res. 2020;291:113264. doi: 10.1016/j.psychres.2020.113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones EAK, Mitra AK, Bhuiyan AR. Impact of COVID-19 on mental health in adolescents: a systematic review. Int J Environ Res Public Health. 2021;18:2470. doi: 10.3390/ijerph18052470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SW, Yang JM, Moon SY, Kim N, Ahn YM, Kim JM, et al. Association between mental illness and COVID-19 in South Korea: a post-hoc analysis. Lancet Psychiatry. 2021;8:271–272. doi: 10.1016/S2215-0366(21)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2017;19:3–13. doi: 10.1093/ntr/ntw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaiha SM, Cheng J, Halpern-Felsher B. Association between youth smoking, electronic cigarette use, and COVID-19. J Adolesc Health. 2020;67:519–523. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haischer MH, Beilfuss R, Hart MR, Opielinski L, Wrucke D, Zirgaitis G, et al. Who is wearing a mask? Gender-, age-, and location-related differences during the COVID-19 pandemic. PLoS ONE. 2020;15:e0240785. doi: 10.1371/journal.pone.0240785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwon SH, Jeong S. Factors influencing adolescent lifetime smoking and current smoking in South Korea: using data from the 10th (2014) Korea youth risk behavior web-based survey. J Korean Acad Nurs. 2016;46:552–561. doi: 10.4040/jkan.2016.46.4.552. [DOI] [PubMed] [Google Scholar]
- 30.Lancet T. Tobacco control: far from the finish line. Lancet. 2021;398:1939. doi: 10.1016/S0140-6736(21)02650-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee SW, Lee J, Moon SY, Jin HY, Yang JM, Ogino S, et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med. 2021;56:901. doi: 10.1136/bjsports-2021-104203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. Study protocol, statistical code: available from DKY (email: yonkkang@gmail.com). Data set: available from the Korean Centers for Disease Control and Prevention Agency (KCDA) through a data use agreement.


