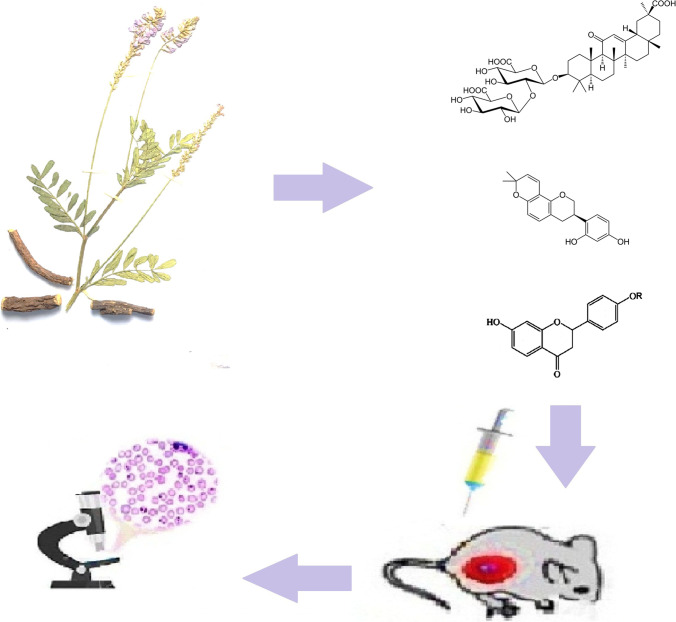
Abstract
Glycyrrhiza glabra L., Fabaceae, or licorice has shown potential therapeutic effects on fever, gastric ulcers, hepatic disorders, and malaria. This study aimed to assess the antimalarial activity of different fractions of root extract from twelve ecotypes from Iran. In this regard, mice were then randomly divided into 8 groups of 5 mice. Four hours after mice were infected by Plasmodium berghei, they received methanolic plant extract by intraperitoneal injection. The treatment was continued for 4 consecutive days (every 24 h), then on the fifth and seventh days, blood samples were taken from the tails of the mice and the parasitic percentages were calculated by microscopy technique. In comparison to control, every analyzed ecotype has a remarkable parasite inhibitory effect, whereas the source of the root also has a drastic difference in its antimalarial effects. The highest percentage of inhibition on days 5 and 7 was subjected to the extract of Semirom ecotype with suppression of 86.37 and 83%, respectively. On the other hand, 13.21 and 9.19% parasite growth inhibition was shown in the extracts of Shahrbabak and Haji Abad, respectively. The significant difference between these 12 ecotypes was shown with Mann–Whitney U pairwise comparison to variable parasitemia day 5 and parasitemia day 7 (p < 0.001).
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s43450-022-00353-8.
Keywords: Iranian native ecotypes, Malaria, Root extract, Antimalarial activity, Methanolic plant extract
Introduction
One of the parasitic diseases around the globe is malaria that, in 2019, left 419,000 deaths and 229 million infected cases. This result showed a 60–75% decrease from 2000 to 2019 interval in Africa and the Southeast Asian region. But in 2020, the world faced another major disease, COVID-19, and its similarity to malaria left patients of this neglected parasitic infections unnoticed and as a consequence, the death rate will rise in these high-alert regions (Teboh et al. 2021). Another major issue is drug resistance which has been elevated in the malaria parasite Plasmodium falciparum. This situation highlights the significance for the discovery of novel anti-plasmodial therapeutics (Ramazani et al. 2017, 2018; Bao et al. 2020; Bilia et al. 2020).
Antimalarial drugs nowadays fall into three major categories: (1) aryl aminoalcohols, (2) antifolates, and (3) artemisinin derivatives (White 2004). Quinine, a basic amine of the first category, and artemisinin are natural products from plant sources (Weinreb 2001; Kong and Tan 2015; Triemer et al. 2018; Yang et al. 2019), so drug discovery in this field could lead to additional or alternative therapeutic agents for the treatment of malaria. Traditional healers have used herbal medicine for treating infectious diseases like malaria for centuries in Africa and Asia. One of these herbal plants is Glycyrrhiza glabra L., Fabaceae (licorice), which is traditionally accepted for common cold, fever, and infections and has effects on liver enzymes and lipid profile (Ramazani et al. 2018). The main antimalarial compounds in licorice are glycyrrhizin (Cheema et al. 2014) and glycyrrhetinic acid (Kalani et al. 2013). Its use in Iranian folk herbal medicine is well documented (Ramazani et al. 2018). In recent years, total root extracts of this plant have been investigated in vitro and in vivo for their antimalarial, anti-protozoal (Gavarić et al. 2015), anti-viral (Fiore et al. 2008), and anti-microbial (Gupta et al. 2008) activities.
Statti et al. (2004) examined the effect of licorice root and several of its active ingredients in various biological activities in Italy and concluded that glycyrrhizinic acid was found in different amounts as harvested from 9 regions. Also, the effect of the region on the amount of glycyrrhizic acid (1) was significant, which was due to different environmental conditions such as sunlight, altitude, and latitude. In another study, Chinese researchers determined the amount of glycyrrhizic acid in licorice roots from 14 regions in China and found that licorice plants in different regions had different glycyrrhizic acid levels ranging from 5.75 to 11.4% (Hui-yan et al. 2002). To study the phenotypic characteristics and the amount of active substances in the Abadan and Khorramshahr regions, in December 2009, licorice roots from 4 regions was collected. Phenotypic traits and percentage of glycyrrhizic acid in licorice roots were evaluated. The amount of glycyrrhizic acid in the fresh weight of the root was between 0.85 and 2.74%. Morphological traits also exhibited high diversity. Montoro and colleagues (Montoro et al. 2011) examined the profiles of licorice root metabolites from various sources. Glycyrrhizic acid levels were reported in different samples from Italy, China, Turkey, and Iran at 51, 53, 33, and 32 mg/g dry, respectively. Also, a wide range of phenols and flavonoids were identified in these samples, the quantitative and qualitative values of which showed high diversity.
This study is an ongoing investigation of the antimalarial activity of G. glabra (Ramazani et al. 2018) where the anti-Plasmodium effects of twelve ecotypes native to Iran, namely Bardsir, Kazerun, Rabat, Kashmar, Bajgah, Semirom, Haji Abad, Yasuj, Saqqez, Eqlid, Darab, and Shahrbabak, were analyzed to establish different growth inhibitory effects on parasites and the concentrations of their active ingredients such as glycyrrhizic acid (1), glabridin (2), liquiritin (3), and liquiritigenin (4).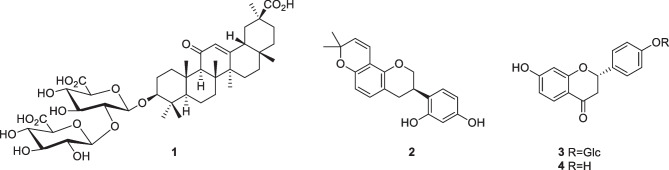
Materials and Methods
Twelve ecotypes of Glycyrrhiza glabra L., Fabaceae, were selected for this study as their anti-inflammatory and antioxidant effects in traditional medicine are well documented. According to the resources and facilities available in the herbarium of Zanjan University of Medical Sciences (ZUMS), information was gathered in relation to the collecting sites of these specimens in native areas of this plant, as well as growing season and plant morphology. Finally, the specimens were collected, identified by Prof. Ali Sonboli, and voucher specimens were deposited at the ZUMS herbarium (Table S1).
For extraction, powder plant material (1 g) from each ecotype was transferred to a test tube; then, 20 ml of 80% MeOH was added to each sample (16 ml of MeOH and 4 ml of H2O) and sonicated for 30 min (2 ×). The time between the two sonications was 20 min, and the test tube was gently shaken during this time. Centrifugation was then performed for 10 min at 2800 to 4032 × g, and the supernatant was poured into a plate and evaporated under the hood for 24 h. The dried extracts were collected as a powder from the bottom of the plate and poured into microtubes. The total extract was stored at − 20 °C until further use. At this stage, the total extract is ready for biological activity. The yield of the extractive process was 9 ± 1%.
For the quantification of the active ingredients of the extracts of G. glabra, the HPLC system (Knauer, Germany) that consisted of two pumps (model-Wellchron K1001) and a PDA detector (model 2800 K) was applied. Reversed-phase chromatographic analysis was carried out using a Luna C-18 column (250 × 4.6 mm, particle size 5 μm, Phenomenex Inc.). Samples were evaluated at an absorption and emission wavelength of 250 nm. Analytical conditions were as follows: solvent system, CH3CN-H2O (1:1) with a flow rate of 1 ml/min; temperature, 25 °C; sample injection, 20 μl. For drawing the calibration curve and registering the quantitative measurement, different concentrations were prepared (10 to 125 ppm) and injected into column C-18 (Esmaeili et al. 2019).
In vivo antimalarial activity in albino mice was performed as follows: NMRI male mice (20–25 g) were purchased from Pasteur Institute, Tehran, Iran. Animals were kept under standard laboratory conditions. First, Plasmodium berghei (Zanjan University of Medical Sciences, Iran) was removed from liquid nitrogen and after thawing at room temperature, it was injected intraperitoneally into two mice (Rashidzadeh et al. 2021, 2022). During this period, sampling and smear were prepared from the tail of two mice and the amount of parasitism was evaluated. The number of extracts and required doses were calculated and the rats were anesthetized with ether. Blood samples were taken from their hearts with a 2-ml syringe. For every 50 µl of blood, 10 ml of salt phosphate buffer was needed to dilute, and some heparin was poured into the blood to prevent coagulation. Then, 5 parasite-infected blood mice were injected.
After a few days, by increasing parasites in the mice, blood sampling was performed and suspension of 107 infected red blood cells in 200 µl was prepared. Male albino mice weighing approximately 20–25 g were then injected intraperitoneally with an insulin syringe. Mice were randomly divided into 8 groups of 5 individuals. Four hours after mice were infected, they receive 0.2 ml of each ecotype extract (200 mg/kg) by intraperitoneal injection (day zero). The treatment was continued for 4 consecutive days (every 24 h) and on the 5th and 7th days, blood was taken from the tails of mice. Afterwards, a drop of blood was placed at a distance of 2 cm from the edge of a clean glass slide and a second slide was angled at 45°. Blood was drawn and then pulled up to create a candle flame. After that, a thin blood smear was prepared on the slide and the sample was coded with a pencil. The blood smears were fixed with methanol on slides. Then, the slides were stained with Giemsa. A drop of emulsifying oil was poured on the slide and the number of infected red blood cells per 1000 infected red blood cells ratio was obtained by magnifying at × 100 (parasitic percentage).
In this study, two control groups were used. A positive control group was treated with 5 mg/kg chloroquine and a negative control group was injected with 0.2 ml of phosphate saline as a placebo. The rate of the inhibition of parasite growth in mice was calculated by comparing the treatment group with the positive and negative control groups. The study of the parasite in the body of mice was continued until the death of the last mice in the negative control group. Then, the mortality rate was calculated.
Data (means ± SD for at least three replications) were analyzed using a two-way analysis of variance (ANOVA). Mann–Whitney U test was run pairwise for the significance of differences between every two ecotypes.
Results and Discussion
In Iran, the origin of licorice was between 23 to 38° latitude and 45 to 58° longitude. The results of this study showed that the habitats of licorice are found at the altitude of 897 to 2338 m with an average annual rainfall of 31 to 865 mm, an average annual temperature of 11.68 to 24.35 °C, the number of frost days in the year zero up to 133 days, relative humidity 32 to 62% and humid, and Mediterranean, arid, and semi-arid climates (Table S2).
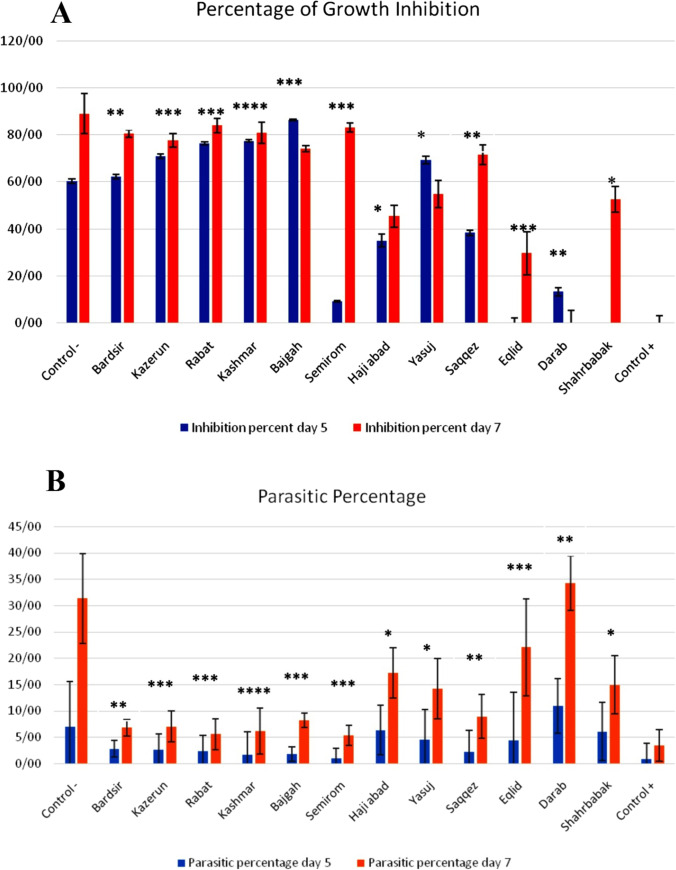
Glycyrrhizic acid (1), glabridin (2), liquiritin (3), and liquiritigenin (4) were detected in different amounts. Analysis of variance (ANOVA) showed that there was a significant difference between the populations for glycyrrhizic acid, glabridin, liqueurite, and liquiritigenin at the level of 1% probability (ESM Table 1). In this test, 12 ecotypes with the same concentrations and positive and negative control groups in 2 days (5th and 7th days after infection of mice with Plasmodium berghei) were compared (ESM Table 1). First, the percentage of parasitism of each group was calculated and the average of parasitism with standard deviation was obtained. Then, the growth percentage and the percentage of the inhibition of parasitic growth of each population and their p-value compared to the negative control group on the 5th and 7th days were calculated (Fig. 1A, B). Figure 2 illustrates the spread of the infection on mice after treatment with the Saqqez population on the fifth day, and live parasites (ring). This ecotype displayed a significant growth inhibition percentage in terms of its antimalarial properties.
Fig. 1.
Comparison chart of the percentage of parasitic growth inhibition (A) and parasitic growth (B) on the 5th and 7th days. *p < 0.05; **p < 0.01; ***correlation is significant at the p < 0.001; ****p < 0.0001 (2-tailed)
Fig. 2.
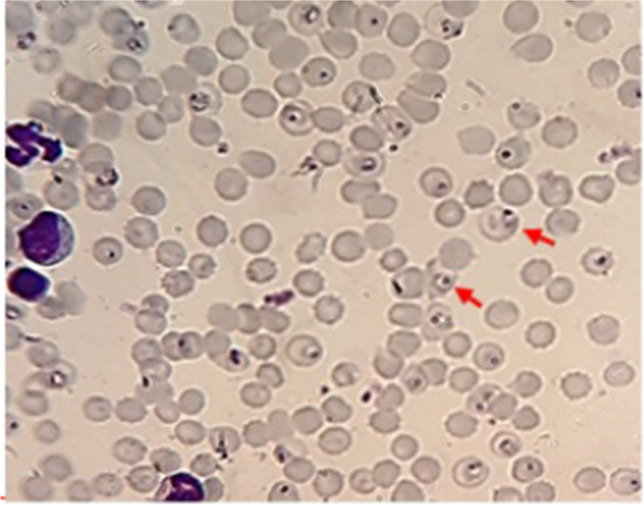
Microscopic illustration of the spread of the infection on mice after treatment with the Saqqez population on the 5th day of the treatment. Red arrows indicate live parasite (ring) × 100
To evaluate the anti-Plasmodium activity of active extracts in mice, after 4 consecutive days of intraperitoneal injection of the extract into mice infected with Plasmodium berghei, on the 5th and 7th day, blood samples were taken from the tails of mice and the parasite was examined under a light microscope. Dead and live cells were examined and the percentage of parasitism in each group was calculated, as well as the percentage of growth inhibition of each extract was obtained. In this test, chloroquine was used as a positive control and PBS as a negative control. As summarized in Table S1, total extracts of all 12 licorice ecotypes reduced parasite growth relative to controls. The highest percentage of inhibition on the fifth and seventh days was related to the total extract of Semirom ecotypes and licorice, respectively, which prevented the growth of parasites in mice with 86.37 and 83%, respectively. The lowest percentage of inhibition on the 5th and 7th days was related to the total extract of Shahrbabak and Haji Abad, which prevented the growth of the parasite in the body of mice by 13.21 and 9.19%, respectively. It is worth mentioning that G. glabra antimalarial activity is not centered on one or two components and the correlation between them and suppression of parasite percentage on day 5 and day 7 was not found (Table S2). Antimalarial activity of the ecotypes of this plant varies based on the type and amount of active ingredients. On the other hand, factors such as soil type and compounds determine the amount of active substance production, and climatic conditions can somehow produce secondary metabolites in the plant.
As the result indicates, in comparison to the control, every 12 ecotypes have remarkable parasite inhibition effects, but the source of the roots has a drastic result on its antimalarial effects. The highest percentage of inhibition on days 5 and 7 were subjected to the extract of Semirom ecotypes and negative control (licorice), respectively, which prevented the growth of parasites in mice with 86.37 and 83%, respectively. On the other hand, 13.21 and 9.19% parasite growth inhibition was shown in the extracts of Shahrbabak and Haji Abad, respectively. Results using the Mann–Whitney U pairwise comparison to variable parasitemia day 5 and parasitemia day 7 with p < 0.001 showed a significant difference between these 12 ecotypes.
Total extracts of Bardsir, Kazerun, Rabat, Kashmar, Bajgah, Semirom, Haji Abad, Yasuj, Saqqez, Eqlid, Darab, and Shahrbabak ecotypes of licorice with the daily concentration of 200 mg/kg body weight on the fifth day of sampling can efficiently prevent the growth of Plasmodium berghei parasite in albino mice in comparison with control. According to the comparison of growth percentages on the fifth and seventh days of sampling, it can be said that over time, all of the above root extracts except Bajgah, Yasuj, and Darab ecotypes can effectively prevent parasite growth in mice. Since, just 4 components of the root extract, namely glycyrrhizic acid (1), glabridin (2), liquiritin (3), and liquiritigenin (4), were evaluated, the percentage of growth inhibition cannot be directly compared with the content of these compounds. Therefore, they cannot determine the antimalarial effect of the plant alone and the presence of other compounds, such as flavonoids, could result in synergistic and antagonistic effects that may also affect the observed antimalarial activity.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
AR and MT conceived the study and revised the final manuscript. FM, TSH, and SMA wrote the manuscript. HR prepared figures and tables and wrote the manuscript. GE, MS, AK, and MA helped with the performance of experiments. The final version of the manuscript was read and approved by all authors.
Funding
Financial support of this project (grant number: A-12–349-40&41) was provided by the deputy of Zanjan University of Medical Sciences.
Declarations
Ethical Disclosures
The authors declare that the experiments with animals were performed in compliance with international rules on the care and use of laboratory animals. The study was approved by the local Ethics Committee for the use of animals in pharmacological and toxicological testing of Zanjan University of Medical Sciences (ethical code: IR.ZUMS.REC.1399.162).
Footnotes
Hamid Rashidzadeh, Fereshteh Sadat Mosavi, Tahereh Shafiee, and Seyed Masih Adyani contributed equally.
References
- Bao Y, Li Z, Chen SH, Gao LZ, Liu ZL, Cheng L, Peng Y, Tong XL, Dai FY (2020) Artemisinin is highly soluble in polyethylene glycol 4000 and such solution has multiple biological effects. Acta Biochim Pol 67:203–211. 10.18388/abp.2020_5190 [DOI] [PubMed]
- Bilia AR, Bergonzi MC, Boulos JC, Efferth T. Nanocarriers to enhance solubility, bioavailability, and efficacy of artemisinin. World J Tradit Chin Med. 2020;6:26. doi: 10.4103/wjtcm.wjtcm_2_20. [DOI] [Google Scholar]
- Cheema HS, Prakash O, Pal A, Khan F, Bawankule DU, Darokar MP. Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum. Parasitol Int. 2014;63:349–358. doi: 10.1016/j.parint.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Esmaeili H, Karami A, Hadian J, Saharkhiz MJ, Ebrahimi SN. Variation in the phytochemical contents and antioxidant activity of Glycyrrhiza glabra populations collected in Iran. Ind Crops Prod. 2019;137:248–259. doi: 10.1016/j.indcrop.2019.05.034. [DOI] [Google Scholar]
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Bielenberg AD, J Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavarić N, Kovač J, Kretschmer N, Kladar N, Možina SS, Bucar F, Bauer R, Božin B (2015) Natural products as antibacterial agents-antibacterial potential and safety of post-distillation and waste material from Thymus vulgaris L., Lamiaceae. In: Bobbarala V (Ed) Concepts, compounds and the alternatives of antibacterials. London: IntechOpen, pp 123–151. 10.5772/60869
- Gupta VK, Fatima A, Faridi U, Negi AS, Shanker K, Kumar JK, Rahuja N, Luqman S, Sisodia BS, Saikia D, Daroka MP. Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol. 2008;116:377–380. doi: 10.1016/j.jep.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Hui-yan G, Li-dong G, Jing-hua Y. Measurement and comparison of glycyrrhizic acid contents in root of licorice (Glycyrrhiza uralensis Fisch.) from different cultivating areas. J For Res. 2002;13:141–143. doi: 10.1007/BF02857240. [DOI] [Google Scholar]
- Kalani K, Agarwal J, Alam S, Khan F, Pal A, Srivastava SK. In silico and in vivo anti-malarial studies of 18β glycyrrhetinic acid from Glycyrrhiza glabra. PLoS ONE. 2013;8:e74761. doi: 10.1371/journal.pone.0074761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LY, Tan RX. Artemisinin, a miracle of traditional Chinese medicine. Nat Prod. 2015;32:1617–1621. doi: 10.1039/c5np00133a. [DOI] [PubMed] [Google Scholar]
- Montoro P, Maldini M, Russo M, Postorino S, Piacente S, Pizza C. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J Pharm Biomed Anal. 2011;54:535–544. doi: 10.1016/j.jpba.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Ramazani A, Khosravani B, Taran J. Evaluation of novel α-(acyloxy)-α-(quinolin-4-yl) acetamides as antiplasmodial agents. Iran J Pharm Res. 2017;16:924. [PMC free article] [PubMed] [Google Scholar]
- Ramazani A, Tavakolizadeh M, Ramazani S, Kheiri-Manjili H, Eskandari M (2018) Antiplasmodial property of Glycyrrhiza glabra traditionally used for malaria in Iran: promising activity with high selectivity index for malaria. J Arthropod Borne Dis 12:135. 10.18502/jad.v12i2.39 [PMC free article] [PubMed]
- Rashidzadeh H, Tabatabaei Rezaei SJ, Adyani SM, Abazari M, Rahamooz Haghighi S, Abdollahi H, Ramazani A. Recent advances in targeting malaria with nanotechnology-based drug carriers. Pharm Dev Technol. 2021;26:807–823. doi: 10.1080/10837450.2021.1948568. [DOI] [PubMed] [Google Scholar]
- Rashidzadeh H, Rezaei SJ, Danafar H, Ramazani A. Multifunctional pH-responsive nanogel for malaria and cancer treatment: hitting two targets with one arrow. J Drug Deliv Sci Technol. 2022;76:103740. doi: 10.1016/j.jddst.2022.103740. [DOI] [Google Scholar]
- Statti GA, Tundis R, Sacchetti G, Muzzoli M, Bianchi A, Menichini F. Variability in the content of active constituents and biological activity of Glycyrrhiza glabra. Fitoterapia. 2004;75:371–374. doi: 10.1016/j.fitote.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Teboh E, Miranda I, Gideon AN. COVID-19 in malaria-endemic regions: potential consequences for malaria intervention coverage, morbidity, and mortality. Lancet Infect Dis. 2021;21:5–6. doi: 10.1016/S1473-3099(20)30763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triemer S, Gilmore K, Vu GT, Seeberger PH, Seidel-Morgenstern A. Literally green chemical synthesis of artemisinin from plant extracts. Angew Chem Int Ed Engl. 2018;57:5525–5528. doi: 10.1002/anie.201801424. [DOI] [PubMed] [Google Scholar]
- Weinreb SM. Synthetic lessons from quinine. Nature. 2001;411:429–431. doi: 10.1038/35078178. [DOI] [PubMed] [Google Scholar]
- White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GZ, Zhu JK, Yin XD, Yan YF, Wang YL, Shang XF, Liu YQ, Zhao ZM, Peng JW, Liu H. Design, synthesis, and antifungal evaluation of novel quinoline derivatives inspired from natural quinine alkaloids. J Agric Food Chem. 2019;67:11340–11353. doi: 10.1021/acs.jafc.9b04224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.