Abstract
Complement receptor type 3 (CR3) was initially described as an opsonic receptor. Subsequently, CR3-mediated lectin-sugar recognition mechanisms have been shown to play a major role in the nonopsonic phagocytosis of several pathogens, among them Mycobacterium tuberculosis. Little is known about the binding and signal transduction mechanisms operating during nonopsonic ingestion through CR3 of different microorganisms. In the present study, we used CHO cells stably transfected with CR3 to show that CR3 was able to mediate internalization of zymosan and pathogenic mycobacteria (Mycobacterium kansasii and Mycobacterium avium) but not that of nonpathogenic species (Mycobacterium smegmatis and Mycobacterium phlei). A combination of mannan and β-glucan inhibited the phagocytosis of zymosan but had no effect on M. kansasii ingestion. Among six monoclonal antibodies (MAbs) directed against the CD11b subunit of CR3 that decreased zymosan ingestion, only three inhibited M. kansasii phagocytosis. In particular, MAbs known to block the CR3 lectin site affected only internalization of zymosan. Using U937 macrophages, we observed that zymosan ingestion through CR3 induced superoxide production measured by cytochrome c reduction and by translocation of the NADPH oxidase cytosolic component p47phox to the phagosomal membrane, whereas phagocytosis of viable or heat-killed M. kansasii did not. Furthermore, lack of superoxide anion production during phagocytosis of M. kansasii was not due to inhibition of NADPH oxidase per se or superoxide anion scavenging. Together, our results indicate that (i) nonopsonic phagocytosis of zymosan and M. kansasii by CR3 implicates different molecular mechanisms involving multiple and distinct epitopes of CD11b and (ii) CR3 may transduce different cellular responses depending on the sites mediating nonopsonic phagocytosis.
Phagocytes play a crucial role in host defense through their ability to recognize, ingest, and destroy invading microorganisms. Phagocyte-specific membrane receptors bind to their corresponding ligands on a microbe's surface and induce the internalization of microorganisms by phagocytosis. Concomitantly, signal transduction pathways are initiated, which may lead to activation of the respiratory burst enzyme NADPH oxidase, fusion of lysosomal granules with phagosomes, and eventually the killing of microbes.
Some microorganisms are able to survive within phagocytes, depending on the selective use of particular phagocytic receptors which mediate phagocytosis without inducing bactericidal functions (1, 23, 57). For example, phagosomes containing Mycobacterium tuberculosis, the etiological agent of tuberculosis, fuse with lysosomes when the bacteria are serum opsonized prior to macrophage infection, thus recruiting opsonic receptors for phagocytosis, but do not fuse under nonopsonic conditions (1). Similarly, NADPH oxidase activity is stimulated only when mycobacteria enter human macrophages under opsonic conditions (2). The choice of host cell receptor and the mechanisms of binding (opsonic versus nonopsonic) may thus influence the fate of intracellular pathogens. There is therefore considerable interest in identifying the receptors responsible for specific recognition of pathogens and the cellular consequences of such recognition. Among phagocytic receptors, complement receptor type 3 (CR3) is of particular interest, since it is the target of diverse groups of intracellular parasites (6, 16, 22, 35, 38, 47, 61), such as M. tuberculosis (8, 9, 21, 52, 53; L. S. Schlesinger, A. Frist, T. Kaufmann, R. R. Ingalls, R. Li, D. T. Galenbock, and M. A. Arnaout, Keystone Conference, abstr. 223, 1999).
CR3 (also termed Mac1) is a member of the β2 family of integrins expressed on the plasma membranes of mammalian phagocytes and natural killer cells (see references 17, 42, and 49 for a review). It is a heterodimeric type I transmembrane glycoprotein, consisting of a CD11b α chain noncovalently associated with the CD18 β subunit (17, 42, 49). It was first described as an adhesion molecule involved in phagocyte diapedesis through interaction with ICAM-1 expressed on endothelial cells or with the extracellular matrix (17) and as an opsonic receptor that recognizes complement fragment iC3b deposited on microorganisms (42, 49). More recent data indicate that CR3 also serves in the nonopsonic recognition of microbes by interacting directly with a wide spectrum of molecules on their surfaces (9, 20, 38, 44, 58, 62).
Distinct functional binding domains have been predicted or identified in the extracellular portion of the CD11b subunit of CR3 by immunologic, mutagenic, and biochemical approaches (3, 7, 12, 14, 19, 26, 31, 51, 55, 56, 58, 59, 65). The first binding domain of the CD11b subunit, called the I or A domain, is essential for iC3b binding but also supports ICAM-1, fibrinogen, and factor X recognition (14). However, the binding sites are not identical for all of these ligands, since ICAM-1 and iC3b interact with overlapping but distinct sites within the I domain of CD11b (14). The existence of a second binding domain responsible for nonopsonic binding to CR3 was demonstrated by using anti-CR3 monoclonal antibodies (MAbs) or synthetic peptides that blocked the binding of iC3b but not that of nonopsonic ligands and vice versa (12, 40, 63). This second domain, which presents lectin activity, has been identified and located C terminal to the I domain (55). The lectin domain binds to soluble β-glucan and mediates phagocytosis of particles containing β-glucan, such as zymosan (10, 40). For this reason, it has been suggested that CR3 corresponds to the phagocyte β-glucan receptor (10, 41, 55). More recently, it has been reported that CR3 has a broader sugar specificity than originally appreciated, since it also interacted with mannose, N-acetyl-d-glucosamine (NADG), and glucose (55). This dual specificity for mannose- and glucose-containing polysaccharides has suggested that CR3 has either two lectin sites or a unique site which recognizes different types of sugars (55, 65).
Zymosan, isolated from Saccharomyces cerevisiae, is mainly composed of mannan and β(1–3)-glucan (15), whereas mycobacteria present a large variety of complex sugars on their surfaces (11). Both particles are ingested nonopsonically by macrophages via CR3 (8, 9, 21, 40, 52, 53; Schlesinger et al.) and mannose receptor (2, 45, 50). Phagocytosis of zymosan through its glucan component has been shown to trigger the production of superoxide anions (O2−) (2, 10, 24), whereas the ingestion of mycobacteria did not (2). Because CR3 seems to possess distinct nonopsonic lectin sites, and because zymosan and mycobacteria display different sugar compositions, we asked whether the different behaviors of these two particles could be related to a specific mode of interaction with CR3. To address this question and to further characterize the nonopsonic binding site(s) of CR3, we measured the effects of polysaccharides and blocking antibodies on the internalization of zymosan and mycobacteria by CR3-transfected Chinese hamster ovary (CHO) cells. In addition, using differentiated U937 cells, which express CR3 but not the mannose receptor, we compared O2− production in response to nonopsonic phagocytosis of zymosan and mycobacteria.
MATERIALS AND METHODS
Media and reagents.
Mannan, β-glucan from barley [a mixture of β(1,4)glucan and β(1,3)glucan], NADG, laminarin [β(1,3)glucan], glycogen, superoxide dismutase, ferricytochrome c, and fluorescein isothiocyanate (FITC) were from Sigma Chemical Co. (St. Louis, Mo.). α-Glucan, purified from M. tuberculosis as previously described (30), was kindly provided by M. Daffé (Toulouse, France). 9-cis retinoic acid (RA) was from ICN (Orsay, France), and 1,25-dihydroxy vitamin D3 (VD3) was kindly provided by U. Fischer and P. Weber (Hoffmann-La Roche, Basel, Switzerland). RPMI 1640, alpha-modified Eagle medium (α-MEM), l-glutamine, and antibiotics were purchased from Gibco (Cergy Pontoise, France).
Monoclonal anti-CR3 and other antibodies.
A panel of mouse MAbs that had previously been reported to bind and functionally block distinct epitopes of CD11b extracellular domains were used: 2LPM {immunoglobulin G1(κ) [IgG1(κ)]; Dako, Glostrup, Denmark}, LM2/1 (IgG1), and OKM1 (IgG2b), kindly provided by M. R. W. Ehlers (Cape Town Medical School, South Africa) (8), and CBRM 1/20 (IgG1), CBRM 1/23 (IgG2a), and CBRM 1/32 (IgG1), generously provided by T. A. Springer (Harvard Medical School, Boston, Mass.) (14). The regions of CD11b reactive with the MAbs are illustrated below (see Fig. 3).
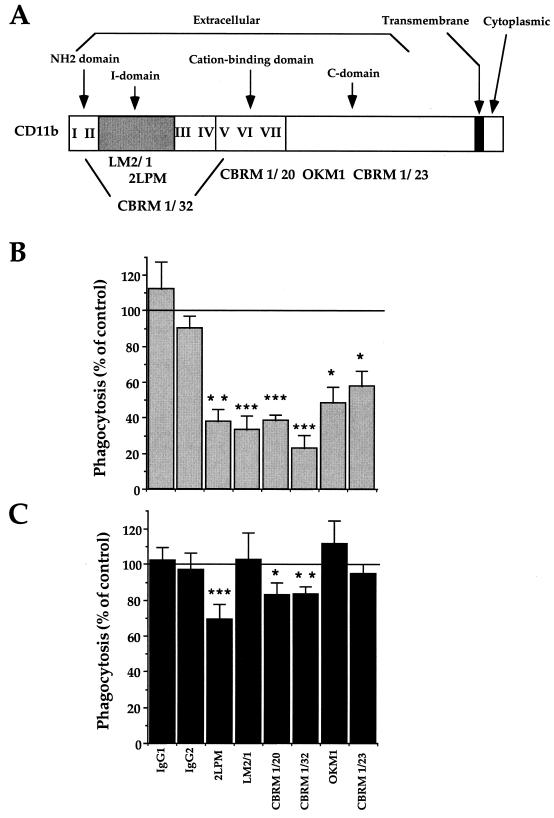
FIG. 3.
Inhibition of CR3-mediated phagocytosis of zymosan and M. kansasii by anti-CR3 MAbs. (A) Schematic map of the regions of the human CD11b α chain recognized by the different MAbs used in this study (3, 8, 31). (B and C) CR3-transfected CHO cells were preincubated with the indicated nonrelevant IgG1 or IgG2 or with anti-CD11b MAbs for 30 min at 37°C before incubation with zymosan (B) or M. kansasii (C). The data are expressed as the percentage of phagocytosis compared to control values (100%, no antibody). The values are means + SEM of three to six separate experiments performed in duplicate. Statistical differences were measured for cells pretreated with MAbs compared to untreated cells. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
Nonrelevant IgG1 and IgG2 were purchased from Dako and Pharmingen. Anti-mycobacterium rabbit antibodies (camelia) were obtained by immunizing rabbits with heat-killed mycobacteria (Mycobacterium smegmatis, Mycobacterium phlei, Mycobacterium avium, and Mycobacterium kansasii). FITC and tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-rabbit and anti-mouse antibodies were purchased from Sigma.
CR3-transfected and WT CHO cells.
CR3-transfected CHO-Mac1 cells, obtained from T. A. Springer, are CHO cells stably expressing wild-type (WT) human CR3 (14). A subclone of CHO-Mac1 cells which expresses CD11b/CD18 at a high rate (8) was used in the present study. WT and CR3-transfected CHO cells were cultured in α-MEM supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine, and, for CR3-transfected cells, 0.1 μM methotrexate (8). The cells were subcultured every 3 to 4 days with phosphate-buffered saline (PBS) containing 5 mM EDTA.
U937 cell culture and differentiation.
U937, a human monoblast cell line, was cultured in RPMI 1640 supplemented with 10% heat inactivated fetal bovine serum, l-glutamine, and antibiotics in a 5% CO2 humidified atmosphere. Differentiation into macrophages was induced with a combination of RA (0.1 μM) and VD3 (0.1 μM) for 3 days (33). On day 2, fresh medium containing the differentiation agents was added (32). The extent of differentiation was assessed by monitoring growth arrest, adhesion, and morphological changes (May-Grünwald-Giemsa staining) (27), expression of the differentiation marker CD11b was monitored by flow cytometry analysis (see below), and mannose receptor was monitored by Western blotting (2).
NADPH oxidase activity assessment.
The capacity to generate superoxide anions was assessed by the superoxide dismutase-inhibitable ferricytochrome c reduction method (29). Translocation of the p47phox cytosolic subunit of NADPH oxidase was analyzed by immunofluorescence microscopy (13). Briefly, 1 h after infection with either zymosan or M. kansasii, both stained by FITC, U937 cells were fixed and permeabilized in methanol. The cells were incubated in PBS supplemented with 3% bovine serum albumin (BSA) for 30 min at room temperature and then incubated with rabbit anti-p47phox antibodies (1:1,000) for 1 h at room temperature, washed in PBS, and revealed by TRITC-conjugated goat anti-rabbit antibodies. Anti-p47phox antibodies were generously provided by W. B. Nauseef (Veterans Administration Medical Center, University of Iowa) (13). The specificity of the staining was assessed by omission of the primary antibody. Slides were viewed using a Zeiss confocal microscope. In each case, at least 20 cells per slide from each of the three experiments performed were viewed.
Analysis of CR3 expression by flow cytometry.
CR3-transfected CHO and U937 cells were washed twice with PBS supplemented with 0.5% BSA, pH 7.4 (PBS-BSA), at 4°C, and incubated for 30 min on ice with or without the primary antibody (2LPM, diluted 1/50). The cells were washed three times in PBS-BSA and incubated with FITC-conjugated goat anti-mouse IgG for 30 min at 4°C. After being washed, the cells were fixed with 3.7% paraformaldehyde for 45 min on ice, washed again, and resuspended in PBS. The adherent cells were gently scraped off and resuspended in PBS. The cells were then analyzed on a FACScan (Becton Dickinson, San Jose, Calif.) as previously described (27).
Bacterial culture and FITC staining.
M. kansasii (ATCC 124478), M. smegmatis (ATCC 607), and M. phlei (ATCC 11758) were grown at 37°C as surface pellicles in Sauton broth medium; M. avium (IP 140310013) was cultured in suspension in Middlebrook 7H9 medium (Difco, Bonneuil sur Marne, France). Mycobacteria were prepared as previously described (34). For FITC staining, 109 bacteria were added to 1 ml of 0.01% FITC in 0.2 M Na2CO3–NaHCO3 buffer, pH 10.2, for 10 min and washed with PBS, pH 7.4 (34).
Opsonization of zymosan and mycobacteria.
Zymosan or mycobacteria were incubated in pooled normal or heat-inactivated human serum for 20 min at 37°C, washed twice in PBS, pH 7.4, and resuspended in PBS containing 1 mM CaCl2 and 0.5 mM MgCl2 (28). The number of particles or bacteria after opsonization was estimated by quantification in a Thoma chamber.
Infection of CHO and U937 cells and phagocytosis assay by immunofluorescence microscopy.
WT and CR3-transfected CHO cells (5 × 104/ml) or U937 cells (2 × 105/ml) were seeded on 12-mm-diameter glass coverslips in 24-well plates. The CHO cells were grown overnight at 37°C, and the U937 cells were differentiated for 3 days on coverslips as described above. To remove all traces of seric proteins, the cells were washed twice in α-MEM or RPMI medium supplemented with l-glutamine and were preincubated at 37°C for 30 min. All further incubations were performed in the absence of serum. Zymosan particles or FITC-labeled mycobacteria were then added at a multiplicity of infection of 50:1. When indicated, zymosan and the mycobacteria were opsonized in human serum or the mycobacteria were heat killed (30 min at 80°C) prior to infection. The cells and particles were left in contact overnight for CR3-transfected CHO cells or for 1 h for U937 cells. The cells were then extensively washed with α-MEM or RPMI medium and fixed. For zymosan, the cells were fixed and permeabilized with methanol for 6 min at −20°C, rinsed in PBS containing 0.1% Tween 20, and unspecifically stained with FITC-conjugated antibodies (28). After this treatment, the cells were uniformly fluorescent and extracellular zymosan was readily distinguished from internalized particles, which appeared as yellowish grains within a dark phagosome bordered by diffuse green staining. For mycobacteria, the cells were fixed for 45 min at room temperature with 3.7% paraformaldehyde in PBS containing 15 mM sucrose, and aldehyde groups were neutralized with 50 mM NH4Cl. The cells were washed in PBS, and extracellular mycobacteria were stained with rabbit anti-mycobacterium antibodies revealed by TRITC-conjugated anti-rabbit antibodies. Since the cells were not permeabilized, anti-mycobacterium antibodies did not reach intracellular bacteria, which thus appeared as green fluorescent particles (bacteria are prestained with FITC prior to infection experiments). Extracellular mycobacteria were stained with antibodies and were therefore fluorescent in red and green. For each set of conditions, duplicate experiments were performed, and at least 100 cells per slide were counted by fluorescence microscopy to determine the percentage of cells that had ingested at least one particle or bacterium.
Inhibition of phagocytosis of zymosan and mycobacteria.
To block the phagocytosis of zymosan particles or mycobacteria, the transfected CR3-CHO or U937 cells were preincubated for 30 min at 37°C with the indicated MAbs or polysaccharides diluted in serum-free α-MEM or RPMI 1640 prior to the addition of zymosan or M. kansasii. We verified that the preincubation time and temperature were optimal to obtain an efficient blockade of CR3. Different dilutions of MAbs and concentrations of polysaccharides were tested for inhibition of zymosan or M. kansasii internalization by CR3-CHO cells, and the most efficient conditions were as follows: 100 μg of laminarine/ml, 1 mg of β-glucan/ml (from barley), 500 μg of α-glucan/ml, 1 mg of NADG/ml, 1 mg of glycogen/ml, 1 mg of mannan/ml, 1/50 dilution (2 μg/ml) of 2LPM, 1/5 dilution of LM2/1 hybridoma supernatant, 1/100 dilution of CBRM antibodies, 1/100 dilution of OKM1, and 5 μg of IgG1 and IgG2 controls/ml. The effects of MAbs and polysaccharides on cell viability and morphology were assessed by trypan blue exclusion and light microscopy.
Statistics.
Data are presented as the mean ± standard error of the mean (SEM) of the indicated number of experiments performed in duplicate. The significance of differences was determined by the paired or unpaired Student t test. Unless otherwise stated, data are not significantly different from control values.
RESULTS
Opsonic and nonopsonic phagocytosis of zymosan and mycobacteria by CR3-transfected and WT CHO cells.
To study whether zymosan and mycobacteria interacted with distinct lectin sites on CR3, CR3-transfected CHO cells were used to avoid interaction with other macrophage receptors. As previously observed (8, 14), expression of CR3 on transfected CHO cells was homogenous, since a single cell population was detected by immunofluorescence flow cytometry (Fig. 1A).
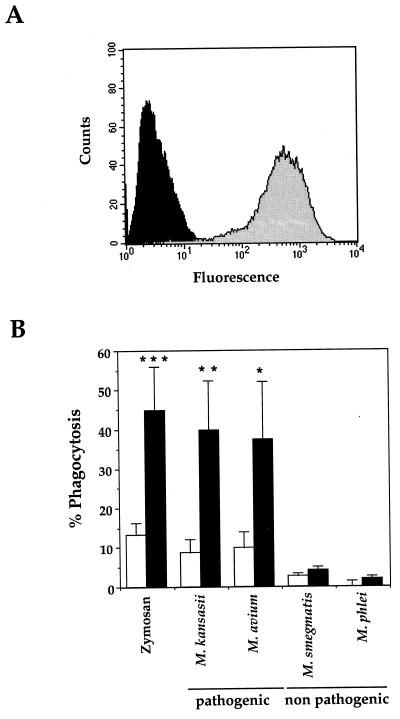
FIG. 1.
Characterization of CR3-transfected CHO cells. (A) Surface expression of CR3 was analyzed by flow cytometry. Cells were stained with anti-CD11b MAbs (2LPM) and FITC-coupled rabbit anti-mouse secondary antibodies prior to fixation with paraformaldehyde (shaded histogram); in the control, the primary antibody was omitted (solid histogram). The histograms show the fluorescence (expressed in arbitrary units) measured on the complete cell population. (B) Phagocytosis of zymosan and the indicated species of pathogenic or nonpathogenic mycobacteria by WT (open bars) and CR3-expressing (solid bars) CHO cells was measured by fluorescence microscopy. At least 100 cells per slide were counted under fluorescence microscopy. The percentage of phagocytic cells (% Phagocytosis) having ingested at least one particle or bacterium (number of phagocytic cells/number of total cells × 100) is expressed as the means + SEM of 3 to 29 separate experiments performed in duplicate. Statistical differences were measured for CR3-transfected versus WT CHO cells. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
The capacity of CR3-transfected CHO cells to ingest particles under nonopsonic conditions was tested with either zymosan or mycobacteria. CR3-transfected cells ingested zymosan and the pathogenic species M. kansasii and M. avium but did not internalize nonpathogenic mycobacterial species such as M. smegmatis or M. phlei (Fig. 1B). When CR3-transfected CHO cells were incubated in the presence of zymosan opsonized in fresh human serum, (74.6 ± 11.0)% of the cells ingested particles (mean ± SEM of 11 experiments performed in duplicate). These experiments indicated that the phagocytic capacities of CR3 were fully conserved when expressed in CHO cells, since it was able to internalize both complement-coated and nonopsonized particles. Furthermore, we confirmed that under nonopsonic conditions, CR3 is able to distinguish between nonpathogenic and pathogenic mycobacteria (8).
Differential inhibitory effects of polysaccharides on phagocytosis of zymosan and M. kansasii by CR3-transfected CHO cells.
The above-mentioned results showed that zymosan and pathogenic mycobacteria can both be ingested nonopsonically by CR3. Because CR3 can also bind to a variety of polysaccharides, we then investigated whether distinct sugars could compete for zymosan or M. kansasii recognition.
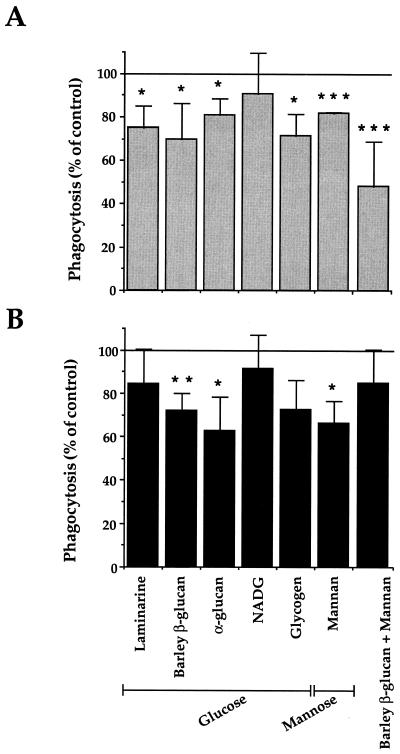
At the concentrations used, all the polysaccharides tested except NADG inhibited, but to a low degree, phagocytosis of zymosan and M. kansasii (Fig. 2). No further inhibition was obtained when larger amounts of sugar were used (not shown). Even α-glucan, which accounts for 70% of the polysaccharides in the capsule of M. kansasii (30), only partially inhibited the ingestion of mycobacteria. With a combination of β-glucan and mannan, the inhibitory effects of individual sugars were additive on zymosan phagocytosis but, surprisingly, not on M. kansasii phagocytosis. Although difficult to interpret, this result suggests that zymosan and M. kansasii may not use the same lectin site of CR3. However, given the small inhibitory effects of individual sugars, another approach was required to support this hypothesis.
FIG. 2.
Inhibition by polysaccharides of CR3-mediated zymosan and M. kansasii phagocytosis. CR3-transfected CHO cells were preincubated as indicated with glucose- or mannose-containing polysaccharides or β-glucan plus mannan before incubation with either zymosan (A) or M. kansasii (B). The data are expressed as the percentage of phagocytosis reported compared to control values (100%, no polysaccharide). The values are means + SEM of three to four separate experiments performed in duplicate. Statistical differences were measured for cells pretreated with sugars compared to untreated cells. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
Differential inhibitory effects of anti-CR3 MAbs on zymosan and M. kansasii phagocytosis by CR3-transfected CHO cells.
For this purpose, we used a panel of well-characterized MAbs directed against different epitopes of the CD11b extracellular domain of CR3. A schematic representation of the regions recognized by these different MAbs is shown in Fig. 3A.
A drastic difference was observed between the inhibition profiles of zymosan and M. kansasii internalization by the antibodies (Fig. 3B and C). Each MAb strongly inhibited phagocytosis of zymosan, whereas that of M. kansasii was reduced by 30% with 2LPM MAbs and to a lesser extent, but significantly, by CBRM 1/20 or CBRM 1/32 MAbs.
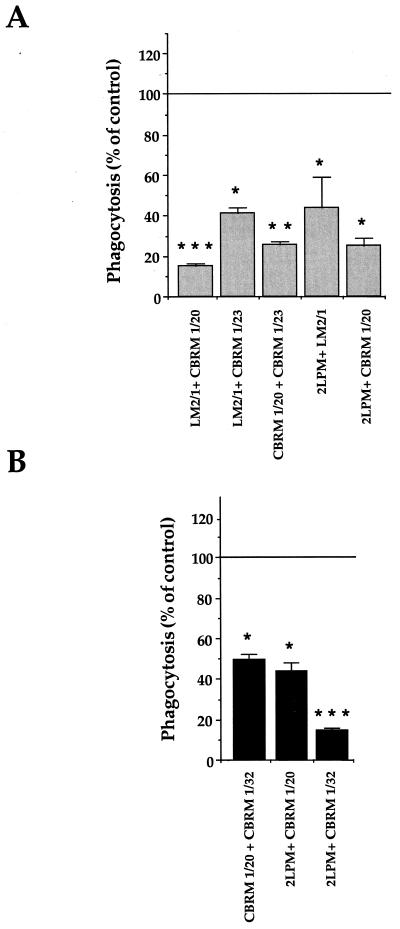
We then investigated whether combinations of the antibodies showing an inhibitory effect when used alone could exert additive effects on the inhibition of particle phagocytosis. Inhibition of zymosan phagocytosis was indeed potentiated by combining LM2/1 and CBRM 1/20 MAbs (P < 0.05 compared to LM2/1 alone, and P < 0.005 compared to CBRM 1/20 alone) or CBRM 1/20 and CBRM 1/23 MAbs (P < 0.01 compared to each MAb alone) (Fig. 4A). Inhibition of M. kansasii phagocytosis was also clearly potentiated by using the three possible pairs of MAbs which had only a minimal effect when used individually (P < 0.01 for all three pairs compared to each MAb alone) (Fig. 4B).
FIG. 4.
Inhibition of the phagocytosis of zymosan and M. kansasii by CR3-transfected CHO cells by anti-CR3 MAb combinations. As described in the legend to Fig. 3, the cells were preincubated with the indicated combinations of MAbs before incubation with zymosan (A) or M. kansasii (B). The values are the means + SEM of three separate experiments performed in duplicate. Statistical differences were measured for cells pretreated with MAbs compared to untreated cells. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
Together with the sugar inhibition experiments, these results showed that distinct molecular determinants of CR3 are involved in phagocytosis of zymosan and M. kansasii.
Differential induction of NADPH oxidase activity by zymosan and M. kansasii following their phagocytosis via CR3 in differentiated U937 cells.
It has been shown that nonopsonic phagocytosis of zymosan by macrophages elicited O2− production (2, 10, 24) whereas that of M. kansasii did not (2). We thus wondered whether such different effects could be related to the above-mentioned results showing distinct types of phagocytosis of these two particles by CR3. This was investigated using U937 cells, because upon differentiation into macrophages, these cells express CR3 and the O2−-generating enzyme NADPH oxidase but do not express the mannose receptor (Table 1), which is also involved in the nonopsonic phagocytosis of zymosan and mycobacteria (2, 45, 50).
TABLE 1.
U937 cells acquire a macrophagelike phenotype upon differentiationa
| Parameter | Value
|
|
|---|---|---|
| Undifferentiated | Differentiated | |
| Morphology | Monoblast | Macrophage |
| Adhesion | No | Yes |
| Proliferation index | 3.3 ± 0.1 | 1.31 ± 0.25 |
| Bactericidal activity in response to OZ | ||
| Phagocytosis (%) | 12 ± 3 | 68 ± 12 |
| O2− production (nmol of O2−/60 min/106 cells) | 3 ± 10 | 35 ± 2 |
| Receptor expression | ||
| Mannose receptor | No | No |
| CR3 (arbitrary units) | 4.9 ± 0.7 | 84.5 ± 30.7 |
RA-VD3-differentiated and undifferentiated U937 cells were analyzed in parallel for morphology (May Grünwald-Giemsa staining), adhesion to a plastic surface (observed by light microscopy), proliferation index (multiplication factor after 3 days of culture in the presence [differentiated] or the absence [undifferentiated] of RA plus VD3), acquisition of bactericidal functions (phagocytosis of opsonized zymosan particles [OZ] and O2− production induced by OZ), mannose receptor expression (Western blot analysis), and CR3 expression (flow cytometric analysis of CD11b expression; mean fluorescence is expressed in arbitrary units). The data are expressed as means ± SEM of at least four independent experiments.
Based on different criteria, we first established that upon differentiation with a combination of RA and VD3, these cells indeed acquired a macrophage phenotype (Table 1). In particular, we observed that expression of CR3 at the surfaces of differentiated U937 cells was greatly enhanced compared to that on undifferentiated cells (Table 1).
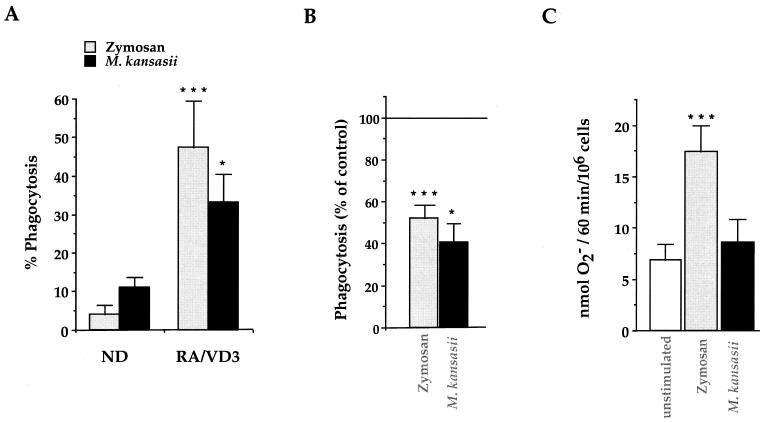
Phagocytosis of M. kansasii or zymosan was then measured in both undifferentiated and differentiated U937 cells (Fig. 5A). In differentiated cells, this process largely occurred through CR3, since it correlated with the increase in CR3 expression and was blocked by the MAb 2LPM (Fig. 5B).
FIG. 5.
Bactericidal responses of RA-VD3-differentiated U937 cells in response to zymosan and M. kansasii. (A) Phagocytosis of zymosan and M. kansasii was measured in undifferentiated (ND) and differentiated (RA/VD3) U937 cells after 1 h of incubation. The data are expressed as the percentage of phagocytosis, and the values are the means + SEM of three to six separate experiments performed in duplicate. Statistical differences were measured for differentiated cells compared to undifferentiated cells. (B) RA-VD3-differentiated U937 cells were preincubated with 2LPM MAbs prior to incubation with zymosan or M. kansasii to assess the involvement of CR3 in particle internalization. The cells were then processed for measurement of phagocytosis. The data are expressed as the percentage of phagocytosis reported compared to control values, and the values are the means + SEM of three to six separate experiments performed in duplicate. Statistical differences were calculated for cells pretreated with MAbs compared to untreated cells. (C) RA-VD3-differentiated U937 cells were incubated for 1 h either alone (open bar) or in the presence of zymosan or M. kansasii and processed for measurement of O2− production. The data are expressed in nanomoles of O2− produced by 106 cells in 1 h, and the values are the means + SEM of three separate experiments performed in duplicate. Statistical differences were measured for cells incubated with zymosan or M. kansasii compared to unstimulated cells. ∗, P < 0.05; ∗∗∗, P < 0.005.
Activation of NADPH oxidase following the ingestion of either zymosan or M. kansasii was next measured in differentiated U937 cells using either the cytochrome c reduction assay to measure the extracellular production of O2− (superoxide anions) or immunofluorescence microscopy to detect p47phox translocation to phagosomes. p47phox is a cytosolic component of NADPH oxidase which translocates to membranes to participate in the functional assembly of the enzyme (13). Zymosan was able to significantly activate the production of O2− and the translocation of p47phox, whereas M. kansasii was not (Fig. 5C and 6). Several controls were performed to ensure that the absence of O2− production did not result from an active effect of mycobacteria (Table 2). Production of O2− was not modified when viable M. kansasii were coincubated with stimulating agents, such as phorbol myristate acetate (PMA), opsonized zymosan, or zymosan, excluding a free-radical-scavenging effect of these bacteria or a direct inhibitory effect on the enzyme per se. Furthermore, heat-killed M. kansasii gave results similar to those with live mycobacteria. Finally, when IgG and complement-opsonized mycobacteria were internalized through receptors known to trigger O2− generation, the O2− production was indeed stimulated further, indicating that M. kansasii was unable to inhibit NADPH oxidase.
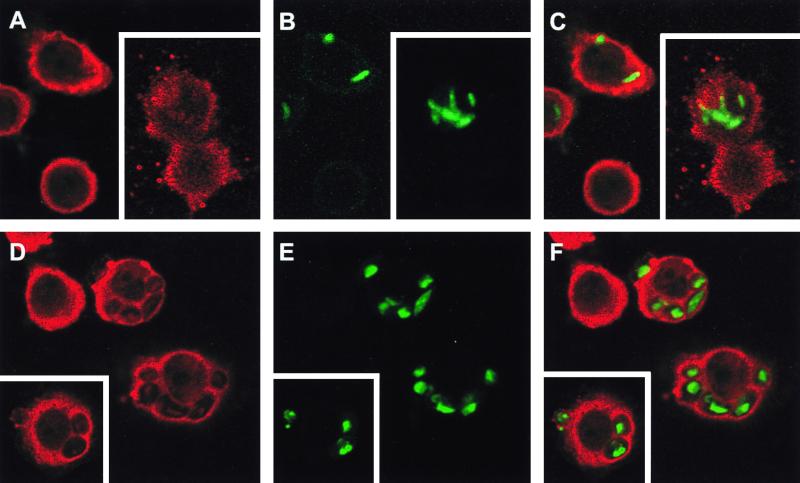
FIG. 6.
Analysis of NADPH oxidase assembly at the phagosomal membrane by confocal microscopy. Phagocytosis of FITC-labeled M. kansasii (A to C) or FITC-labeled zymosan (D to F) was not synchronized in order to obtain phagosomes at different maturation stages. Following phagocytosis, differentiated U937 cells were fixed, permeabilized, and stained with anti-p47phox antibodies revealed by TRITC-conjugated anti-rabbit antibodies. The stained cells are representative of three independent experiments, and phagosomes visualized by confocal microscopy are shown. (A and D) p47phox staining alone; (B and E) FITC-labeled particles alone; (C and F) superimposition of both types of staining. Insets, representative of a second experiment.
TABLE 2.
Absence of O2− production upon ingestion of M. kansasii by U937 macrophages does not result from an active effect of mycobacteria
| Stimulus | O2− production (nmol of O2−/60 min/106 cells) |
|---|---|
| None | 7.54 ± 1.68 |
| PMAa | |
| Without M. kansasii | 36.37 ± 1.32 |
| With M. kansasii | 29.83 ± 1.53 |
| OZa | |
| Without M. kansasii | 29.64 ± 0.97 |
| With M. kansasii | 32.07 ± 0.58 |
| Za | |
| Without M. kansasii | 17.10 ± 2.01 |
| With M. kansasii | 19.04 ± 1.82 |
| Viable M. kansasiib | 9.85 ± 3.51 |
| Heat-killed M. kansasiib | 10.25 ± 0.97 |
| IgG- and complement-opsonized M. kansasiib | 21.19 ± 3.85c |
RA-VD3-differentiated U937 cells were either not stimulated or stimulated for 1 h with PMA (100 ng/ml), opsonized zymosan (OZ), or zymosan (Z) in the presence or in the absence of M. kansasii, and O2− production was measured.
Differentiated U937 cells were incubated for 1 h with viable or heat-killed M. kansasii, or M. kansasii opsonized in human serum.
P < 0.005 compared to unstimulated cells or cells stimulated with viable or heat-killed mycobacteria. The values are the means ± SEM of three separate experiments performed in duplicate.
DISCUSSION
In the present study we showed that distinct regions of CR3 are involved in the nonopsonic phagocytosis of mycobacteria and zymosan and transduce different intracellular signals, since zymosan phagocytosis triggers production of O2− whereas M. kansasii phagocytosis does not.
Only pathogenic mycobacterial species such as M. kansasii and M. avium were efficiently internalized under nonopsonic conditions by CR3-transfected CHO cells, whereas the nonpathogenic species M. smegmatis and M. phlei were not. Under similar conditions, M. tuberculosis, but not M. smegmatis, efficiently binds to CR3 in CHO-transfected cells (8). This discriminative property of CR3 also occurred in U937 cells, where upregulation of CR3 induced by differentiation correlated with a parallel increase in phagocytosis of M. kansasii (Table 2 and Fig. 5A) but not M. phlei (unpublished results). This suggests that CR3 may constitute a safe portal of entry for pathogenic mycobacteria, underlying its role in mycobacterial-mediated infectious diseases.
The double specificity of CR3 for mannose- and glucose-containing polysaccharides (55) has suggested that CR3 has either two lectin sites or a unique site with degenerated specificity (55, 65). To determine whether zymosan and M. kansasii are recognized by CR3 through distinct sugars, competition experiments were performed with single mono- or polysaccharides. The clear inhibition of zymosan ingestion obtained by combining β-glucan and mannan suggested that internalization of this particle might involve two subsites of CR3 specific for glucose and mannose moieties, respectively. In contrast, this sugar combination did not produce a significant inhibitory effect on M. kansasii phagocytosis, in line with the previous observation that laminarin (a polymer of β-glucan) and mannan minimally inhibited the adherence of nonopsonized M. tuberculosis to CR3-transfected CHO cells (Schlesinger et al.). It is possible that ingestion of mycobacteria by CR3 either involves different molecules or more complex saccharides than ingestion of zymosan or is highly sensitive to the sugar environment on the bacteria. Indeed, antigen 85C, present on the surfaces of mycobacteria, has been shown to promote the binding of microbeads coated with mycobacterial products to CR3 (20). In the same way, the affinity of the mannose receptor is significantly increased when mannose moieties are contained in complex mycobacterial glycoconjugates (48, 54). Also, lectins, such as type 1-fimbriae from Escherichia coli, could bind to glycosylated proteins at the surfaces of host cells (18), but none has been specifically described in pathogenic mycobacteria. In any case, the different inhibitory actions of sugars on nonopsonic phagocytosis of zymosan and M. kansasii by CR3 suggested that the respective underlying mechanisms are quite different.
The differential blocking effects of a panel of MAbs on the CD11b extracellular domain further support the above conclusion. Indeed, LM2/1 and OKM1 MAbs, which have both been reported to block the lectin site of CR3 (8, 14, 55), inhibited ingestion of zymosan but not M. kansasii. This shows that the β-glucan site of CR3 is probably not involved in M. kansasii ingestion but confirms its major role in the recognition of zymosan. Furthermore, almost all of the other MAbs used, with their cognate epitopes scattered throughout the extracellular domain of CD11b (Fig. 3A), were able to individually block zymosan ingestion. Only combinations of pairs of three of these, which recognized epitopes restricted to the N-terminal part of CD11b, efficiently blocked M. kansasii phagocytosis.
The patterns of antibody inhibition of M. tuberculosis (8) and M. kansasii (this study) differ to some extent. This could be explained by differences between the strains used, because even in a single strain, differences have been reported: of two substrains of H37Rv, one binds to CR3 and the other does not (9), and, depending on the physiological state of M. avium, it can or cannot be ingested through CR3 (5).
We also report that an antibody, 2LPM, directed against the recognition site of iC3b can efficiently inhibit the nonopsonic phagocytosis of both zymosan and M. kansasii. It is noteworthy, however, that binding of iC3b impairs subsequent binding of nonopsonized yeast and β-glucan particles (41), and 2LPM MAbs have been shown to reduce nonopsonic binding of M. tuberculosis to CR3 (8). These observations suggest that once the iC3b binding site is occupied either by its natural ligand or by antibodies, this affects the functionality of or sterically blocks the β-glucan binding site, as previously proposed (40, 55). Whatever the mode of action of the antibodies used (i.e., steric hindrance or conformational modifications), their differential effects on the phagocytosis of zymosan versus that of M. kansasii again highlights the fact that these two types of particles do not interact in similar ways with CR3 under nonopsonic conditions.
Although we do not exclude the possibility that other receptors could contribute to nonopsonic phagocytosis, in differentiated U937 cells which do not express the mannose receptor, ingestion of zymosan and M. kansasii largely occurred through CR3. Indeed, phagocytosis correlated with the increase in CR3 expression and was inhibited by the MAb 2LPM. As in other phagocytes, CR3 expressed in U937 cells may interact with accessory molecules, such as CD87 or CD14 (see reference 43 for a review). In these cells, it has been demonstrated (43) that such interactions may generate conformational changes of the I domain recognized by 2LPM MAbs. Such accessory receptors may be different in CHO cells, explaining the smaller inhibitory effect of 2LPM MAbs on M. kansasii phagocytosis compared to that observed in U937 cells.
Finally, using U937 cells, we also showed that CR3-mediated phagocytosis of zymosan induced O2− production, whereas ingestion of M. kansasii did not. The observation that CR3 can initiate distinct cell responses depending on the binding site recognized by particles correlates with previous data obtained with neutrophils. In these cells, which also express CR3 but not the mannose receptor, unopsonized zymosan stimulates O2− production whereas it fails to do so when particles are iC3b coated (60, 64). We demonstrated that M. kansasii and zymosan use the same receptor, but their coincubation (Table 2) did not affect the production of O2− triggered by zymosan, probably because the number of receptors at the cell surface is not limiting. The fact that phagocytosis of M. kansasii did not modify O2− production induced by different stimulating agents demonstrates that the bacteria did not directly inhibit NADPH oxidase activity per se or scavenged O2−. These data suggest that phagocytosis via CR3 may occur through several cellular pathways, the one leading to nonopsonic internalization of M. kansasii being either not coupled to NADPH oxidase activation or specifically inhibited by a bacterial component(s) before O2− production is initiated. In this respect, it is noteworthy that the mannose receptor, which is thought to be an important receptor for nonopsonic phagocytosis of mycobacteria, also appears not to be coupled to bactericidal responses (2).
Although phagocytosis is more effective in the presence of serum (21, 45, 46), nonopsonic internalization of mycobacteria by phagocytes has clearly been demonstrated (2, 8, 21, 34, 45, 46, 52). The question of nonopsonic versus opsonic binding of mycobacteria to phagocytes is important, because seric opsonins may be limiting in the alveolar space where the tuberculosis primary infection takes place (8, 39). We showed here that CR3, which is expressed in lung alveolar macrophages, selectively internalized pathogenic mycobacteria under nonopsonic conditions, and this did not elicit production of O2−. We therefore suggest that CR3 might constitute a safe portal of entry for mycobacteria. In addition to CR3, mycobacteria have been shown to enter nonopsonically into macrophages through several receptors (2, 25, 36, 37, 66), but their relative participation in in vivo infections is difficult to establish. In CD18-deficient mice, a comparable level of tissue infection by M. avium has been reported, suggesting to us that when a phagocytic receptor is lacking, mycobacteria use another one (4). It is therefore difficult to conclude from these experiments whether CR3 is involved in primary mycobacterial infection in human lungs. To determine the receptor hierarchy in nonopsonic phagocytosis of mycobacteria, experiments on both human macrophages and cells transfected with one or combinations of phagocytic receptors should be performed. This would shed light on invasion strategies of pathogens and help to determine whether the mode of binding and the route of entry play roles in the subsequent bactericidal responses and the fate of mycobacteria.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministère de l'Education Nationale de la Recherche et de la Technologie, Programme de Microbiologie, from Sidaction, from E. C. grant no. QLK2CT 1999–01093 TB vaccine cluster, and from the Région Midi-Pyrénées (grant no. 97002346).
We gratefully acknowledge T. A. Springer, M. R. W. Ehlers, and W. B. Nauseef for the generous gifts of anti-CD11b MAbs, CR3-transfected CHO cells, and anti-p47phox antibodies. We thank M. Daffé for α-glucan and, together with L. Emorine and C. Astarie-Dequecker, for discussion and critical evaluation of the manuscript.
REFERENCES
- 1.Armstrong J A, Hart P D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astarie-Dequeker C, N'Diaye E N, Le Cabec V, Rittig M G, Prandy J, Maridonneau-Parini I. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect Immun. 1999;67:469–477. doi: 10.1128/iai.67.2.469-477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balsam L B, Liang T W, Parkos C A. Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J Immunol. 1998;160:5058–5065. [PubMed] [Google Scholar]
- 4.Bermudez L E, Goodman J, Petrofsky M. Role of complement receptors in uptake of Mycobacterium avium by macrophages in vivo: evidence from studies using CD18-deficient mice. Infect Immun. 1999;67:4912–4916. doi: 10.1128/iai.67.9.4912-4916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E, Parker A, Goodman J R. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect Immun. 1997;65:1916–1925. doi: 10.1128/iai.65.5.1916-1925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez L E, Young L S, Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991;59:1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper A, Rosen H, Blackwell J M. Monoclonal antibodies that recognize distinct epitopes of the macrophage type three complement receptor differ in their ability to inhibit binding of Leishmania promastigotes harvested at different phases of their growth cycle. Immunology. 1988;65:511–514. [PMC free article] [PubMed] [Google Scholar]
- 8.Cywes C, Godenir N L, Hoppe H C, Scholle R R, Steyn L M, Kirsch R E, Ehlers M R W. Nonopsonic binding of Mycobacterium tuberculosis to human complement receptor type 3 expressed in Chinese hamster ovary cells. Infect Immun. 1996;64:5373–5383. doi: 10.1128/iai.64.12.5373-5383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cywes C, Hoppe H C, Daffé M, Ehlers M R W. Nonopsonic binding of Mycobacterium tuberculosis to complement receptor type 3 is mediated by capsular polysaccharides and is strain dependent. Infect Immun. 1997;65:4258–4266. doi: 10.1128/iai.65.10.4258-4266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czop J K, Austen K F. A β-glucan inhibitable receptor on human monocytes: its identity with the phagocytic receptor for particulate activators of the alternative complement pathway. J Immunol. 1985;134:2588–2593. [PubMed] [Google Scholar]
- 11.Daffé M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microbiol Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 12.Dana N, Styrt B, Griffin J D, Todd R F, Klemper M S, Arnaout M A. Two functional domains in the phagocyte membrane glycoprotein Mo1 identified with monoclonal antibodies. J Immunol. 1986;137:3259–3263. [PubMed] [Google Scholar]
- 13.DeLeo F R, Allen L A H, Apicella M, Nauseef W M. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 14.Diamond M S, Garcia-Aguilar J, Bickfotd J K, Corbi A L, Springer T A. The I-domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Carlo F J, Fiore J V. On the composition of zymosan. Science. 1957;127:756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- 16.Drevets D A, Campbell P A. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991;59:2645–2652. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahmberg C G. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997;9:643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- 18.Gbarah A, Gahmberg C G, Ofek I, Jacobi U, Sharon N. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect Immun. 1991;59:4524–4530. doi: 10.1128/iai.59.12.4524-4530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham I L, Brown E J. Extracellular calcium results in a conformational change in Mac1 (CD11b/CD18) on neutrophils. Differentiation of adhesion and phagocytosis functions of Mac1. J Immunol. 1991;146:685–691. [PubMed] [Google Scholar]
- 20.Hetland G, Wiker H G. Antigen 85C on Mycobacterium bovis, BCG and M. tuberculosis promotes monocyte-CR3-mediated uptake of microbeads coated with mycobacterial products. Immunology. 1994;82:445–449. [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch C S, Ellner J J, Russell D G, Rich E A. Complement receptor-mediated uptake and tumor necrosis factor-α-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–753. [PubMed] [Google Scholar]
- 22.Hondalus M K, Diamond M S, Rosenthal L A, Springer T A, Mosser D M. The intracellular bacterium Rhodococcus equi requires Mac-1 to bind to mammalian cells. Infect Immun. 1993;61:2919–2929. doi: 10.1128/iai.61.7.2919-2929.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joiner K A, Fuhrman S A, Miettinen H M, Kasper L H, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuole in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 24.Kadish J L, Choi C C, Czop J K. Phagocytosis of unopsonized zymosan particles by trypsin-sensitive and β-glucan-inhibitable receptors on bone marrow-derived murine macrophages. Immunol Res. 1986;5:129–138. doi: 10.1007/BF02917587. [DOI] [PubMed] [Google Scholar]
- 25.Khanna K V, Choi C S, Gekker G, Peterson P K, Molitor T W. Differential infection of porcine alveolar macrophage subpopulations by nonopsonized Mycobacterium bovis involves CD14 receptors. J Leukoc Biol. 1996;60:214–220. doi: 10.1002/jlb.60.2.214. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn S E, Nardin A, Klebba P E, Taylor R P. Escherichia coli bound to the primate erythrocyte complement receptor via bispecific monoclonal antibodies are transferred to and phagocytosed by human monocytes in an in vitro model. J Immunol. 1998;160:5088–5097. [PubMed] [Google Scholar]
- 27.Le Cabec V, Calafat J, Borregaard N. Sorting of the specific granule protein, NGAL, during granulocytic maturation of HL-60 cells. Blood. 1997;89:2113–2121. [PubMed] [Google Scholar]
- 28.Le Cabec V, Maridonneau-Parini I. Annexin 3 is associated with cytoplasmic granules in neutrophils and monocytes and translocates to the plasma membrane in activated cells. Biochem J. 1994;303:481–487. doi: 10.1042/bj3030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Cabec V, Maridonneau-Parini I. Complete and reversible inhibition of NADPH oxidase in human neutrophils by phenylarsine oxide at a step distal to membrane translocation of the enzyme subunits. J Biol Chem. 1995;270:2067–2073. doi: 10.1074/jbc.270.5.2067. [DOI] [PubMed] [Google Scholar]
- 30.Lemassu A, Ortalo-Magne A, Bardou F, Silve G, Lanéelle M A, Daffé M. Extracellular and surface-exposed polysaccharides of non-tuberculous mycobacteria. Microbiology. 1996;142:1513–1520. doi: 10.1099/13500872-142-6-1513. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Oxvig C, Springer T A. The structure of the β-propeller domain and C-terminal region of the integrin αM subunit. Dependence on β subunit association and prediction of domains. J Biol Chem. 1998;273:15138–15147. doi: 10.1074/jbc.273.24.15138. [DOI] [PubMed] [Google Scholar]
- 32.Maridonneau-Parini I, Yang C Z, Bornens M, Goud B. Increase in the expression of a family of small guanosine triphosphate-binding proteins, rab proteins, during induced phagocyte differentiation. J Clin Investig. 1991;87:901–907. doi: 10.1172/JCI115096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima H, Kizaki M, Ueno H, Muto A, Takayama N, Matsushita H, Sonoda A, Ikeda Y. All-trans and 9-cis retinoic acid enhance 1,25-dihydroxyvitamin D3-induced monocytic differentiation of U937 cells. Leukemia Res. 1996;20:665–676. doi: 10.1016/0145-2126(96)00020-3. [DOI] [PubMed] [Google Scholar]
- 34.N'Diaye E N, Darzacq X, Astarie-Dequeker C, Daffé M, Calafat J, Maridonneau-Parini I. Fusion of azurophil granules with phagosomes and activation of the tyrosine kinase Hck are specifically inhibited during phagocytosis of mycobacteria by human neutrophils. J Immunol. 1998;161:4983–4991. [PubMed] [Google Scholar]
- 35.Payne N R, Horwitz M A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987;166:1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson P K, Gekker G, Hu S, Sheng W S, Anderson W R, Ulevitch R J, Tobias P S, Gustafson K V, Molitor T W, Chao C C. CD14 receptor-mediated uptake of nonopsonized Mycobacterium tuberculosis by human microglia. Infect Immun. 1995;63:1598–1602. doi: 10.1128/iai.63.4.1598-1602.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao S P, Ogata K, Catanzaro A. Mycobacterium avium-M. intracellulare binds to the integrin receptor αvβ3 on human monocyte-derived macrophages. Infect Immun. 1993;61:663–670. doi: 10.1128/iai.61.2.663-670.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds H Y, Newball H N. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974;84:559–573. [PubMed] [Google Scholar]
- 40.Ross G D, Cain J A, Lachmann P J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin and functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985;134:3307–3314. [PubMed] [Google Scholar]
- 41.Ross G D, Cain J A, Myones B L, Newman S L, Lachman P J. Specificity of membrane complement receptor type three (CR3) for β-glucans. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- 42.Ross G D, Vetvicka V. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993;92:181–184. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross G D, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and β-glucan in cancer. Immunopharmacology. 1999;42:61–74. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 44.Russell D G, Wright S D. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988;168:279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 46.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 47.Schlesinger L S, Horwitz M A. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J Clin Investig. 1990;85:1304–1314. doi: 10.1172/JCI114568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlesinger L S, Hull S R, Kaufman T M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 49.Sengelov H. Complement receptors in neutrophils. Crit Rev Immunol. 1995;15:107–131. [PubMed] [Google Scholar]
- 50.Speert D P, Silverstein S C. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: maturation and inhibition by mannan. J Leukoc Biol. 1985;38:655–658. doi: 10.1002/jlb.38.5.655. [DOI] [PubMed] [Google Scholar]
- 51.Steadman R, Petersen M M, Topley N, Williams D, Matthews N, Spur B, Williams J D. Differential augmentation by recombinant human tumor necrosis factor-α of neutrophil responses to particulate zymosan and glucan. J Immunol. 1990;144:2712–2718. [PubMed] [Google Scholar]
- 52.Stokes R W, Haidl I D, Jefferies W A, Speert D P. Mycobacteria-macrophage interactions: macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1993;151:7067–7076. [PubMed] [Google Scholar]
- 53.Stokes R W, Thorson L M, Speert D P. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160:5514–5521. [PubMed] [Google Scholar]
- 54.Taylor M E, Drickamer K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J Biol Chem. 1993;268:399–404. [PubMed] [Google Scholar]
- 55.Thornton B P, Vetvicka V, Pitman M, Goldman R C, Ross G D. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 56.Ugarova T P, Solovjov D A, Zhang L, Loukinov D I, Yee V C, Medved L V, Plow E F. Identification of a novel recognition sequence for integrin αMβ2 within the γ-chain of fibrinogen. J Biol Chem. 1998;273:22519–22527. doi: 10.1074/jbc.273.35.22519. [DOI] [PubMed] [Google Scholar]
- 57.Valentin-Weigand P, Benkel P, Rohde M, Chhatwal G S. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996;64:2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Strijp J A G, Russel D G, Tuomanen E, Brown E J, Wright S D. Ligand specificity of purified complement receptor type three (CD11b/CD18, αMβ2, Mac1) J Immunol. 1993;151:3324–3336. [PubMed] [Google Scholar]
- 59.Vetvicka V, Thornton B P, Ross G D. Soluble β-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Investig. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams J D, Topley N, Alobaidi H M, Harber M J. Activation of human polymorphonuclear leukocytes by particulate zymosan is related to both its major carbohydrate components: glucan and mannan. Immunology. 1986;58:117–124. [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson S D, Pearson R D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988;56:363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright S D, Jong T C. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright S D, Levin S M, Jong M T C, Chad Z, Kabbash L G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989;169:175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright S D, Silverstein S C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu X, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross G D. The β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–2290. [PubMed] [Google Scholar]
- 66.Zimmerli S, Edwards S, Ernst J. Selective receptor blockage during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Respir Cell Mol Biol. 1996;15:760–770. doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]