Abstract
Background Dystonia is characterized by sustained or intermittent muscle contractions, leading to abnormal posturing and twisting movements. In pediatric patients, dystonia often negatively influences quality of life. Pharmacological treatment for dystonia is often inadequate and causes adverse effects. Deep brain stimulation (DBS) appears to be a valid therapeutic option for pharmacoresistant dystonia in children.
Methods To illustrate the current clinical practice, we hereby describe two pediatric cases of monogenetic movement disorders presenting with dystonia and treated with DBS. We provide a literature review of similar previously described cases and on different clinical aspects of DBS in pediatric dystonia.
Results The first patient, a 6-year-old girl with severe dystonia, chorea, and myoclonus due to an ADCY5 gene mutation, received DBS in an elective setting. The second patient, an 8-year-old boy with GNAO1 -related dystonia and chorea, underwent emergency DBS due to a pharmacoresistant status dystonicus. A significant amelioration of motor symptoms (65% on the Burke-Fahn-Marsden Dystonia Rating Scale) was observed postoperatively in the first patient and her personal therapeutic goals were achieved. DBS was previously reported in five patients with ADCY5 -related movement disorders, of which three showed objective improvement. Emergency DBS in our second patient resulted in the successful termination of his GNAO1 -related status dystonicus, this being the eighth case reported in the literature.
Conclusion DBS can be effective in monogenetic pediatric dystonia and should be considered early in the disease course. To better evaluate the effects of DBS on patients' functioning, patient-centered therapeutic goals should be discussed in a multidisciplinary approach.
Keywords: deep brain stimulation, pediatric dystonia, status dystonicus, GNAO1 gene , ADCY5 gene , monogenetic movement disorders
Introduction
Dystonia is defined as an involuntary motor pattern with sustained or intermittent twisting movements and abnormal postures, 1 and may present isolated or in combination with other movement disorders. 2 In extreme cases, dystonic episodes can develop into severe, life-threatening generalized dystonia, known as “status dystonicus.” This may lead to progressive respiratory failure, metabolic aberrations, and exhaustion, with a mortality of 10%. 3 Pediatric dystonia can negatively impact quality of life by interfering with participation in daily life activities (DLA), affecting children's mood and executive functioning. 4
When conservative management and oral medication in generalized dystonia fail due to disappointing effect or common adverse effects, 5 invasive treatment options such as deep brain stimulation (DBS) or intrathecal baclofen (ITB) may be considered. ITB may be a good option in patients with a combination of dystonia and spasticity and in children with cerebral palsy (CP), in whom the effect of DBS is often less satisfactory. 6
DBS is being increasingly used in the treatment of medically refractory pediatric dystonia, especially in the inherited forms. 7 Currently, there are only sparse studies on DBS in children, and consensus regarding DBS indications and treatment recommendations in pediatric dystonia is still lacking.
DBS is a neuromodulation technique applied through stereotactic neurosurgery, where high-frequent stimulation modulates the activity of specific deep brain areas by means of implanted electrodes. These are mostly placed in the globus pallidus internus (GPi) for dystonia treatment. 7 Pathologically, low frequencies (4–12 Hz), measured by GPi local field potentials, are increased in dystonia and are associated with dystonia severity. 8 These low frequencies are thought to lead to the disturbed functioning of motor circuits. 8 By targeting the GPi, DBS may suppress this pathological activity. GPi-DBS has been shown to have successful effects in pediatric dystonia 9 and it is nowadays considered a good treatment option for severe drug-resistant dystonia, with a quicker response in dystonia with mobile components. 10
DBS in children is implanted under general anesthesia, guided by fusion of a detailed three-dimensional-rendering of a preoperative magnetic resonance imaging (MRI) scan (for target determination) to an intraoperative computed tomographic-scan (to obtain implantation coordinates). 11 The electrodes are placed in the GPi, fixed to the skull and connected to subcutaneous extension cables, which are tunneled behind the ear and subsequently through the neck. The electrodes are then connected to the implantable pulse generator (IPG). The IPG is placed subcutaneously on the abdominal or chest wall, depending on patient size and proximity to other devices (i.e., baclofen pump, tracheostomy, gastrostomy). 12 The procedure is usually performed in a single session.
In this article, we present two pediatric cases with monogenetic, drug-resistant movement disorders with prominent dystonia due to mutations in the ADCY5 and GNAO1 genes, who underwent DBS in an elective and an emergency setting, respectively. Additionally, we will review the current literature with regard to the indications, timing, clinical evaluation, follow-up, and possible complications of DBS in pediatric dystonia patients, and we will provide an overview of previously described pediatric cases of ADCY5- and GNAO1 -related movement disorders treated with DBS.
Case Presentation (1)
Video 1 Patient 1 (ADCY5 gene mutation) pre-DBS treatment.
Video 2 Patient 1 (ADCY5 gene mutation) post-DBS treatment.
A 6-year-old girl with ADCY5 -related chorea, severe dystonia, and myoclonus was referred by her pediatric neurologist to the Pediatric Movement Disorders' Clinic of the Amsterdam University Medical Center (Amsterdam UMC) for the evaluation of DBS. She was born full term after an uncomplicated pregnancy. At 14 months, she progressively developed dysphagia, axial hypotonia, and chorea. Family history was negative for neurological disorders. Brain MRI showed no abnormalities. At 2 years, she was diagnosed, through whole exome sequencing (WES), with a de novo, pathogenic mutation in the ADCY5 gene (NM_183357.3:c.1252 C > T; p.Arg428Trp). At presentation, she was cognitively unaffected and was able to speak a few words with severe dysarthric speech. She could walk short distances with support of one hand but was mainly using a walking aid. She could only eat minced and moist foods (International Dysphagia Diet Standardization Initiative, IDDSI 5) and drink with a straw (IDDSI 0 and 1), due to the risk of choking. Neurological examination showed a severe, generalized movement disorder with spontaneous myoclonus of the limbs, chorea, and a variable degree of orofacial, axial, and truncal dystonia (see Video 1 ). The most invalidating symptoms were recurrent, painful episodes with dystonia and chorea, occurring with variable frequency at night and refractory to medication, which consisted of gabapentin, clonazepam, and caffeine. Trihexyphenidyl was tried with insufficient effect. The Burke-Fahn-Marsden Dystonia Rating scale (BFMDRS) score was 96 (out of 120 points) on the motor scale. A multidisciplinary evaluation (including pediatric neurologists, movement disorder specialists, neurosurgeons, pediatric physiatrist, speech therapist, occupational therapist, and psychologist) determined the indication for elective GPi-DBS, with the main goal of reducing the recurrent, painful refractory attacks with dystonia and chorea. GPi-DBS was performed under general anesthesia. Two electrodes with segmented contacts (Boston Scientific, Marlborough, US) were implanted bilaterally in the GPi and a rechargeable neurostimulator was placed on the abdominal wall. The electrodes were activated 2 days after the operation, with the following stimulation parameters: bilateral monopolar case (+), contact 2,3,4 (−) (omnidirectional stimulation, ring mode of the lower segmented contacts), pulse width 60 μs, 130 Hz, 1,5 mA. A positive effect on her speech intelligibility was noticed a couple of hours after activation of the DBS electrodes. During subsequent visits, the stimulation parameters were changed to right case (+), contact 2,3,4 (−), pulse width 60 μs, 130 Hz, 2 mA; left case (+), contact 2,3,4 (−), pulse width 60 μs, 130 Hz, 2,1 mA. Two months after the operation, she showed sporadic dystonic episodes and experienced less pain. She was also more energetic and had gained axial stability (she was able to ride her horse without any support for the first time). She was still treated with caffeine and gabapentin and she used clonazepam once per week instead of daily. Six months after the operation, she showed a BFMDRS score of 34/120 on the motor scale (65% improvement), and a significant reduction in pain. She was able to walk with the support of one hand. Her speech-intelligibility, assessed by a speech therapist, improved from 2–3 to 5–6 on the 7 points Likert scale (where 1= no intelligible speech and 7= always intelligible for familiar communication partners). Additionally, her speech had improved from severe-to-mild dysarthria and the communication with her familiar conversational partners became clearer (function level 3 and participation level 2 on the Nijmegen Dysarthria scale, respectively). She had more energy and went to school 5 days a week instead of four. At her latest follow-up, 11th months after DBS implantation, her BFMDRS motor score was unchanged (34/120). However, she showed strikingly improved fine motor skills (she was able to write better and color within the given lines). For the first time, she was able to walk without support for a couple of meters (see Video 2 ). Her speech intelligibility improved further, she was now able to communicate her needs clearly and at a faster pace. No clear improvement was noticed in her myoclonus and axial hypotonia. She tolerated the increase in stimulation parameters, which are now set at: bilateral case (+), contact 2,3,4 (−), pulse width 60 μs, 130 Hz, 3.0 to 3.1 mA (right and left, respectively).
Case Presentation (2)
An 8-year-old boy with pharmacoresistant GNAO1- related dystonia and chorea was referred by his pediatric neurologist to the movement disorders outpatient clinic of the Amsterdam UMC for the evaluation of DBS. He was born at full term after an uneventful pregnancy and an uncomplicated birth. Family history was negative for neurological disorders. He had a developmental delay, axial hypotonia, and gait instability in his first years of life. Brain MRI showed no abnormalities. Through WES, a de novo , pathogenic mutation in the GNAO1 gene (NM_020988.3:c.626G > A; p.Arg209His) was found. From the age of 6 years, he developed a movement disorder mainly characterized by dystonia. With his right hand, he operated his electric wheelchair joystick and played computer games. He was able to properly understand speech and to adequately react through eye movements and blinking, also using gestures and a speech-generating device for communication. His swallowing function was good, he could independently drink and eat finely chopped foods. The neurological examination showed axial hypotonia and orofacial- and generalized dystonia, mostly affecting the left side of his body. His mouth was mainly open. His left hand was closed, his left arm in extension and endorotation, and his left leg in extension. He often experienced episodic deterioration of the movement disorder, with severe bilateral choreatic movements of increasing frequency, disturbing sleep. These episodes were mainly triggered by heat (i.e., fever, hot weather) or infections. Several medications were tried without effect, such as gabapentin, clonazepam, and low-dose trihexyphenidyl (which seemed to worsen his movement disorder). Because of pharmacological resistance, DBS was discussed with parents. An elective preoperative admission was planned. In the meantime, however, the patient presented to our emergency department with a severe exacerbation of his movement disorder, with a combination of continuous, severe generalized chorea, dystonia, and ballism, without a clear trigger. Sleep was disturbed, body temperature was 36 °C. Creatine kinase was 302 U/L (normal range: 20–30 U/L). In the acute setting, clonazepam and gabapentin were increased, and tetrabenazine and haloperidol were started without sufficient effect. Due to progression to status dystonicus, the patient was admitted to the pediatric intensive care unit, where he was intubated and treated with intravenous midazolam, propofol, fentanyl, haloperidol, clonazepam, and clonidine. During this period, dystonia was only absent when he was deeply sedated. Under these circumstances, an emergency DBS surgery was scheduled on the 10th day of admission. Due to his severe clinical condition, it was not possible to assess his preoperative BFMDRS score.
Electrodes with segmented contacts (Boston Scientific, inc.) were implanted bilaterally within the GPi ( Fig. 1 ) and a neurostimulator was placed on the right lower abdominal wall. The electrodes were activated 1 day after the operation, with the following stimulation parameters: bilateral monopolar case (+), contact 2,3,4 (−), pulse width 60 μs, 130 Hz, 2 mA. On the third postoperative day, the parents were asked to fill in subjective scores hourly and daily, in order to evaluate the severity of dystonia ( Fig. 2 ). During the first postoperative days, sedation was still necessary due to the presence of severe dyskinesia of the arms and a combination of chorea and dystonia. Sedation was phased out 8 days after the surgery. The final DBS parameters were: left lead double monopolar case (+), contact 2,3,4 and 5,6,7 (−), right lead monopolar case (+), contact 2,3,4 (−), pulse width 90 μs, 130 Hz, 3 mA. Emergency DBS terminated his status dystonicus, with improvements in his voluntary motor pattern and a gradual decrease in his dystonia. He was discharged 45 days postsurgery, with the following medications: phenobarbital, gabapentin, haloperidol, lorazepam, and tetrabenazine. At 6 months follow-up, his hand function had improved, especially of the left hand. There was still axial hypotonia and dystonia had increased to a small extent, compared to before the status dystonicus. He had not experienced any exacerbations of his movement disorder and his medication was slowly being reduced (i.e., phenobarbital, tetrabenazine, and haloperidol were phased out and stopped, and lorazepam dosage was diminished). He started to go to school again. The 6 months postoperative BFMDRS score was 49/120 on the motor scale. In the meantime, there was a subacute deterioration of his condition, with increasing left hemidystonia. Nine months after the operation, reposition of the left lead took place due to lead dislocation. Hereafter, the DBS settings were adjusted to left lead double monopolar case (+), contact 1,2,3,4 (−), pulse width 90 μs, 130 Hz, 3 mA. He is currently still currently recovering from the surgery and a coronavirus disease 2019 infection; however, his dystonia has substantially improved, compared to before the lead repositioning.
Fig. 1.
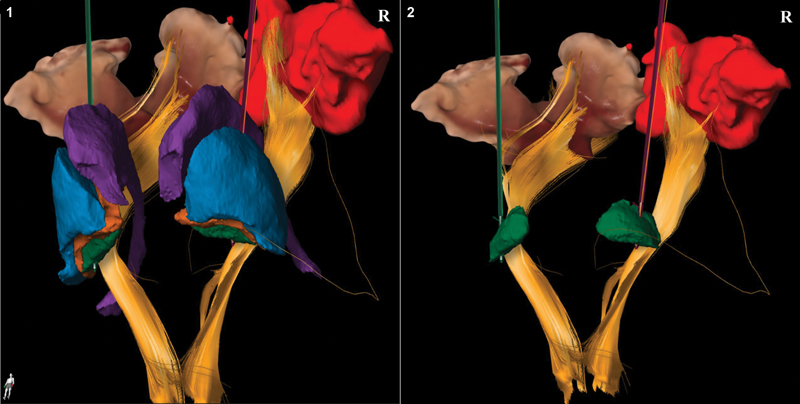
Three-dimensional representation of intraoperative placement of deep brain stimulation (DBS) leads in patient 2. Different colors are used to represent the anatomical structures adjacent to the globus pallidus interna (GPi) for DBS lead placement. The lead in green indicated the left lead, whereas the lead in purple depicts the right lead. Left panel: anterior view of the basal ganglia and corticospinal tracts. The following structures are depicted: GPi (green), globus pallidus externa (orange), putamen/ lentiform nucleus (blue), caudate nucleus (purple), corticospinal tracts (yellow). Right panel: location of the DBS leads in the GPi (green) with adjacent structures removed.
Fig. 2.
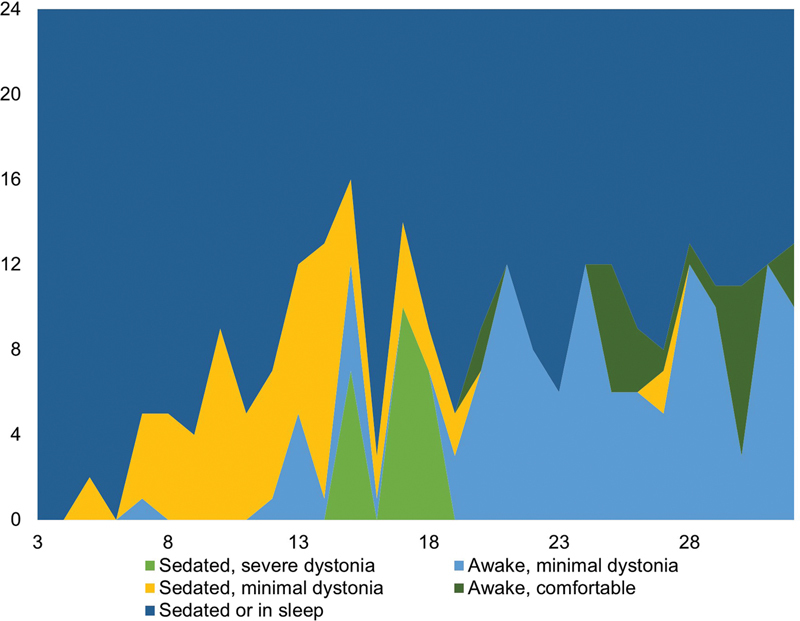
Postoperative dystonia severity scores in patient 2 (with status dystonicus due to a GNAO1 gene mutation). The x-axis represents the days after the operation; on the y-axis are the 24 hours of a day. Nota bene : postoperative dystonia severity scores were recorded from the third postoperative day.
Discussion
In this article, we described two pediatric cases of drug-resistant, severe, generalized, monogenetic dystonia treated with bilateral GPi-DBS, one of whom was operated electively (patient 1, with an ADCY5 gene mutation) and the other in the emergency setting, during a status dystonicus (patient 2, with a GNAO1 gene mutation). Both showed a positive response to DBS at 6 months follow-up. At 1 year follow-up, the first patient has achieved more than her preoperatively set goals and has so far not experienced complications associated with DBS. The second patient was reoperated 9 months after DBS surgery due to left lead dislocation.
Despite a lack of consensus, the main pediatric indications for DBS include dystonia, Tourette's syndrome, and juvenile parkinsonism. 13 Resistance to pharmacological therapy is becoming an increasingly common indication for DBS in childhood dystonia. 13 In a large meta-analysis, Elkaim et al 14 found that 66% of children with dystonia (i.e., onset before 21 years of age, with various etiologies) showed a clinically significant (>20%) improvement in the BFMDRS motor score after DBS (median follow-up of 12 months). 14
There is no general agreement on the optimal timing of DBS placement in children, as the effects of age in general, age at dystonia onset, and disease duration at time of surgery on treatment response are still object of debate. 14 A meta-analysis including 321 children found that older age at dystonia onset, rather than age at surgery or disease duration at time of surgery, was associated with a better DBS outcome. 14 Other studies found that a shorter interval between the onset of dystonia and DBS placement (i.e., shorter disease duration) is linked to a more favorable treatment response and longer-lasting benefits. 15 16 17 The influence of disease duration and age at dystonia onset on DBS outcome may be mediated by aberrant synaptic plasticity, likely present in dystonia patients, leading to maladaptive brain restructuring. 15 16 It is hypothesized that DBS modulation of these abnormal brain reorganization processes may be more difficult in patients with longer disease duration. 17 These implications could therefore justify early consideration of DBS in medically refractory pediatric dystonia.
Furthermore, the underlying etiology of dystonia plays a role in predicting the outcome of pediatric DBS. Hale et al 16 identified 19 studies reporting a total of 76 children who underwent DBS for dystonia at the age of 13.8 ± 3.9 (mean ± standard deviation) years and a follow-up of 2.8 ± 2.8 years. The vast majority (86%) of these patients had inherited dystonia, of whom 56% had DYT1. 18 They found that patients with inherited dystonia showed more improvement compared to patients with acquired causes of dystonia (respectively, 56 vs. 21% improvement on the BFMDRS motor scores). Inherited dystonia, presenting without structural nervous system pathology, and idiopathic dystonia were identified as positive predictors for better treatment response in the meta-analysis of Elkaim et al as well. 14 CP and other forms of acquired dystonia showed more heterogeneous and overall poorer outcomes in comparison to monogenetic or idiopathic forms of dystonia, possibly due to pre-existing structural damage to the basal ganglia and thalamus. 7 14 This may be explained by the fact that, due to aberrant neuroplasticity and malfunctioning neuronal cortico-basal-ganglia and thalamocortical networks, 19 the therapeutic effect of DBS in acquired dystonia may be limited to local modulation of pathological basal ganglia activity, thus not inducing long-term changes in the abnormal motor cortical network. 7 19
In their recent review, Tisch and Kumar (2021) describe variability in gene-specific response to DBS treatment. 10 The most successful outcomes are seen in DYT- TOR1A (DYT1), myoclonus dystonia due to SCGE mutations (DYT11), and X-linked dystonia Parkinsonism (Lubag disease, DYT3). Poorer DBS outcomes are seen in dystonia with laryngeal and oromandibular involvement (i.e., DYT- THAP1 , also known as DYT6). 10 Studies on other forms of monogenetic dystonia present variable results in relatively small patient cohorts and are therefore difficult to compare with relatively more common forms of inherited dystonia. 14
In this report, we described the case of a girl with an ADCY5 -related movement disorder who experienced great benefit from DBS treatment. The role of the ADCY5 gene in movement disorders has recently been discovered. 18 Despite the broad phenotypic variability, the hyperkinetic movement disorders related to specific mutations of this gene mainly have an early onset and are associated with axial hypotonia, dystonia, chorea, nocturnal paroxysmal dyskinesia, and movement-related pain. Cognition is mostly intact. 18 The disease course is characterized by frequent, episodic exacerbations with a waxing-waning pattern and little to no progression. The exacerbations mostly occur at nighttime, when falling asleep or upon awakening, or during intercurrent illnesses. 18 While caffeine and clonazepam have been reported to improve sleep disorders in some patients, 20 21 other symptomatic treatment options have been reported with variable effect. 21
In the current literature, only five patients with ADCY5 -related dystonia (four children and a young adult) are reported to have undergone bilateral GPi-DBS (see Table 1 ). We are aware of adult patients with ADCY5 -related movement disorders described in literature, but in this review, we focused on pediatric cases. Mean age of disease presentation was 14 months and mean age at DBS treatment was 10 years. The effect of DBS was variable, with follow-up ranging from 7 months to 8 years. Subjective general improvements were described in all five patients, with reduction in nocturnal episodic dyskinesia reported in two. Objective amelioration of dystonia, quantified using pre- and postoperative BFMDRS motor scores, was reported in three patients with a variable degree of improvement (4–38%, see Table 1 ). In our patient, postoperative BFMDRS motor score showed strong improvement (65%) at 6 months follow-up, adding to the current literature on the efficacy of DBS treatment in ADCY5 patients. Interestingly, the most important goal for our patient (namely, decreasing the frequent nocturnal exacerbations) was met, but this was not reflected in the BFMDRS score. Choosing the right assessment tools to evaluate DBS treatment outcomes remains an important issue in this context, as we will discuss further below.
Table 1. Literature overview of DBS treatment in pediatric ADCY5 patients .
| Year | 1st author | Mutation | Age presentation (mo) | Movement disorder | Indication of DBS | Age of DBS (y) | Outcome | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| 2016 | Dy | c.2080 _2088del, p.K694 _M696 | 5 | Choreoathetosis/ ballism | Elective | 3 | Midline movements and purposeful vocalization | 10 mo |
| c.1252C > T (p.R418W) | 12 | Sleep myoclonus/dystonia/choreoathetosis | Elective | 8 | BFMDRS-m 45 > 38 (improvement: 16%), decreased frequency and duration of myoclonic storms | 7 mo | ||
| 2016 | Meijer | p.R418 W | 48 | Dystonia/myoclonus | Elective | 12 | BFMDRS-m 50 > 31, BFMDRS-d 22 > 15.5 (improvement: 38%, 30%) | 3.9 y |
| 2020 | de Almeida Marcelino | c1252C > T; p.Arg418Trp | 6 | Dystonia/myoclonus/choreoathetosis | Elective | 20 | BFMDRS-m 56 = 56, AIMS 20 > 18, GCI 50%/ 90%, improvement of nocturnal exacerbations | 8 y |
| 4 | Myoclonus/dystonia/choreoathetosis | Elective | 13 | BFMDRS-m 49 > 47.5 (improvement: 4%), AIMS 22 > 20, GCI 50% | 8 y |
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BFMDRS, Burke-Fahn-Marsden dystonia rating scale; -d, disability subscore; DBS, deep brain stimulation; GCI, Global Clinical Impression; GPi, globus pallidus internus; -m, motor subscore; mo, months; y, years.
GPi-DBS was performed in all patients. “Age presentation” is expressed in months, referring to the first symptom presentation of the movement disorder(s). “Age DBS” refers to the age in years at DBS treatment. References can be found in Supplementary File I >.
The second case we described was a boy with GNAO1 -related dystonia and an emergency DBS indication due to status dystonicus. GNAO1 -related disorders include a heterogenous group of neurological phenotypes, ranging from epilepsy to developmental delay, hypotonia and movement disorders. 22 The phenomenology of movement disorders in GNAO1 gene mutations mainly includes chorea, orofacial dyskinesia, and dystonia. In patients with a movement disorder, phenotype frequent exacerbations are common, often leading to status dystonicus, requiring intensive care unit admissions. 23 So far, 18 pediatric cases of GNAO1 -related movement disorders treated with GPi-DBS have been described in detail in the literature (see Table 2 ). Their mean age of dystonia presentation was 33 months, with a mean age of 9 years at DBS. DBS outcomes were mainly positive, with follow-up ranging from 4 months to 16 years. We are aware of two other pediatric GNAO1- related dystonia cases, reported by Koy et al 24 and by Malaquias et al, 25 who were under evaluation for GPi-DBS at the time of writing. Furthermore, Axeen et al 26 have reported ten patients with GNAO1 -related movement disorders who underwent DBS. However, due to the unreported final decision in the first two patients, and the lack of further information (i.e., demographics, gene mutations, DBS indication, and outcomes) in the other patients, we did not include these twelve patients in our overview.
Table 2. Literature overview of DBS treatment in pediatric GNAO1 patients .
| Year | 1st author | Mutation | Age presentation (mo) | Movement disorder | Indication of DBS | Age of DBS (y) | Outcome | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| 2016 | Kulkarni | c.626G > A(p.Arg209His) | 34 | Choreoathetosis | Elective | 5 | Motor improvement | – |
| c.626G > A(p.Arg209His) | 24 | Choreoathetosis | Elective | 7 | BFMDRS-m 66 >34 (improvement: 48%) |
10 mo | ||
| 2016 | Yilmaz | c.698A > C(p.Q233P) | 13 | Chorea | Elective | 5 | BFMDRS-m 89 >9 (improvement: 90%) |
4 mo |
| 2017 | Danti | c.737A > G(p.Glu246Gly) | 6 | Dystonia/spasticity | Emergency (status dystonicus) | 7 | Complete remission | 16 y |
| 2018 | Waak 23 | c.709G > A(p.Glu237Lys) | 3 | Tremor/chorea/spasticity | Emergency | 11 | Episodic exacerbations | 26 mo |
| c.736G > A(p.Glu246Lys) | 4 | Chorea/dystonia/spasticity | Emergency | 6 | Return to baseline | 28 mo | ||
| c.625C > T(p.Arg209Cys) | 12 | Parkinsonism/dystonia | Emergency | 10 | Improved functioning | 16 mo | ||
| 2018 | Koy 24 | c.723 + 1G > T | 36 | Dystonia/chorea/dyskinesia | Elective | 9 | BFMDRS-m 77 > 67, BFMDRS-d 29 > 18 (improvement: 13%, 38%) |
– |
| c.625C > T(p.Arg209Cys) | – | Dystonia/chorea | Elective | 14 | BFMDRS-m 114 > 85, BFMDRS-d 30 > 27 (improvement: 25%, 10%) |
10 y | ||
| c.625C > T(p.Arg209Cys) | 132 | Dystonia/choreoathetosis/spasticity | Elective | 15 | BFMDRS-m 101 > 54, BFMDRS-d 30 > 24 (improvement: 46%, 20%) |
– | ||
| c.709G > A,g.563707758G > A(p.Glu237Lys) | Infant | Dystonia/hyperkinesia | Elective | 6 | Almost complete remission | – | ||
| c.709G > A(p.E237K) | 96 | Dystonia | Elective | 10.5 | Death 4.5 years after DBS implantation | 4.5 y | ||
| 2018 | Honey | 56,370,675 G > T(p.Arg209Leu) | 18 | Choreoathetosis/dystonia | Elective | 10 | PBADS 27 > 5, CPCHILD 2 > 60 | 6 mo |
| 2018 | Marecos 27 | c.626G > A(p.Arg209His) | 15 | Dystonia/chorea | Elective | 11 | Decrease of intensity and frequency | – |
| 2019 | Benato | c.736G > A(p.Glu246Lys) | 13 | Dystonia/chorea | Emergency (Status dystonicus) | 5 | Return to baseline, before status dystonicus. Two exacerbations | 4.5 y |
| 2018 | Marecos 27 | c.736G > A(p.Glu246Lys) | 48 | Chorea/ballism | Emergency (Status dystonicus) | 13 | Return to baseline, before status dystonicus. No exacerbations | 8 y |
| 2020 | Yamashita | c.620C > T(p.S207F) | 36 | Dystonia/choreoathetosis | Elective | 17 | Attenuation of hyperkinesia, induction of bradykinesia. Barthel index 40 > 75, GMFM 45% |
– |
| 2021 | Danhofer | c.625 C > T; p. (Arg209Cys) | 36 | Ballism/dystonia | Emergency (Status dystonicus) | 12 | Return to baseline, before status dystonicus | 3 mo |
Abbreviations: BFMDRS, Burke-Fahn-Marsden dystonia rating scale; -d, disability subscore; CPCHILD, Caregiver Priorities and Child Health Index of Life with Disabilities; DBS, deep brain stimulation; GMFM, Gross Motor Function Measure; GPi, globus pallidus internus; -m, motor subscore; mo, months: PBADS, Pediatric Barry Albright Dystonia Scale; y, years.
GPi-DBS was performed in all patients. “Age presentation” is expressed in months, referring to the first symptom presentation of the movement disorder(s). “Age DBS” refers to the age in years at DBS treatment.
References can be found in Supplementary File I >. NB: superscript numbers 23 24 27 refer to the corresponding references cited in the main text.
One patient reported by Marecos et al 27 presented with the same exact mutation (c.626G > A, p.Arg209His) as our second patient, with the difference that their patient received an elective DBS, while ours was an emergency DBS due to status dystonicus. In GNAO1 -related movement disorders, GPi-DBS has been considered as “potentially life-saving” 14 28 due to the tendency of these patients to develop status dystonicus. 29 So far, seven patients in the literature have undergone successful emergency DBS ( Table 2 ), making our patient the eighth successful case in the current literature. Based on these positive effects, GPi-DBS should be considered early in the treatment of children with severe GNAO1 -related dystonia and as an early intervention in the case of a status dystonicus, possibly even with the aim of preventing it.
To be able to evaluate the result of DBS treatment in the individual patient, it is very important to preoperatively discuss expectations and set goals with the patient and the parents in a multidisciplinary team and examine these at set intervals postoperatively. In addition to quantifying the severity of dystonia with dystonia rating scales, subjective outcomes, such as comfort and quality of life, should also be evaluated. Even subtle changes can be meaningful in patients with complex movement disorders, especially when dystonia is not the only disabling symptom, such as in the patients we described above. Dystonia rating scales, in fact, may not fully capture the effects of DBS. 30 Different measurement scales have been developed over the years to serve these purposes. For example, individual perception of performance and satisfaction can be measured with the Canadian Occupational Performance Measure scale, which has proven to be efficacious in measuring functional outcomes and improvements after DBS in both primary and secondary pediatric dystonia. 31 32 The BFMDRS is often employed to quantify pre- and postoperative dystonia severity in the literature, although it is not specific to pediatric dystonia. 30 Other outcome scales, like the Barry-Albright Dystonia Scale, the Movement Disorder-Childhood Rating Scale or the Dyskinesia Impairment Scale, are mainly designed to evaluate pediatric acquired dystonia presenting with concomitant movement disorders. 30
Furthermore, measurement scales to evaluate quality of life, mood, eating and drinking, and speech are also important. No international consensus exists yet on the choice of measurement tools for this purpose. Additional scoring systems need to be developed and validated to properly assess the severity of combined movement disorders as well as nonmotor symptoms and DLA in inherited childhood dystonia, and the efficacy of DBS in children.
Complications of DBS for pediatric dystonia may affect the recovery phase and need close monitoring during the follow-up period. Surgical wound infections and hardware-related complications (electrode, extension, or battery, with electrode fracture seen in 4.3%) are the most frequently encountered problems, occurring respectively in 10.5 to 12.5% and in 18.5 to 29.5% of pediatric patients. 33 34 Although DBS is generally considered a safe procedure, potential complications must be openly discussed with patients and parents. Furthermore, when considering DBS for pediatric dystonia, it is important to carefully estimate the burden of patient's morbidity and to clarify whether this can be explained by the dystonic symptoms only, or if there are comorbid symptoms (i.e., spasticity) that can still result in limitations to patient's quality of life after surgery.
To optimize the effect of DBS in pediatric dystonia cases, several issues should be addressed in the coming years. The first is the systematic and unbiased collection of outcomes using international databases such as the Pedi-DBS registry. 35 Another promising avenue for different indications of DBS is the use of so-called “physiomarkers,” patterns of neural activity that are associated with symptom severity and can be used as feedback parameters to optimally titrate stimulation. 8 36 In dystonia, the presence of low-frequency oscillations appears to be the most promising physiomarker for DBS to date. 9 Finally, so-called “connectomic targeting,” using diffusion tensor imaging-based tractography, can help find the optimal site of stimulation in the GPi to modulate cortico-basal ganglia thalamocortical circuits. 37
In conclusion, GPi-DBS is an effective therapeutic option in children with pharmacotherapeutic resistant dystonia, especially in monogenetic forms, and should be considered early in the disease course. To be able to optimally benefit from DBS, it is important to set individual patient goals and use rating scales that capture the full effect on the child's physical and psychosocial functioning. Because of the relative rarity of DBS in children and the heterogeneity of childhood dystonia, it is crucial to perform long-term follow-up and to use standardized outcome measures in future studies to be able to combine international data.
Acknowledgments
We would like to thank the patients and their families for allowing us to write this scientific report.
Funding Statement
Funding This research received no external funding.
Conflict of Interest M.B. was awarded the TKI-PPP grant in 2021. A.I.B. is the chair of the board of the Dutch Academy of Childhood Disability. J.M.D. was awarded the Netherlands Organization for Health Research and Development grant and a Medtronic grant. J.M.D. is the president of the Netherlands workgroup for Movement Disorders. P.R.S. was awarded consulting fees from Boston Scientific, Medtronic, Elekta and lecture fees from Boston Scientific and Elekta. P.R.S. is the treasurer of the European Society for Stereotactic and Functional Neurosurgery, an associate editor of the Journal of Parkinson's Disease and is in the advisory board of Boston Scientific and Insightec.
Authors' Contributions
LAvdP, MG, and MB contributed to conceptualization, investigation, methodology, and writing of the original draft. MG, LAvP, MB, JMD, LAB, AIB, JG, RP, and PRS helped in writing—,reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Patient Consent
Patients and parents' consent for publication was obtained.
Supplementary Material
References
- 1.Sanger T D, Chen D, Fehlings D L. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538–1549. doi: 10.1002/mds.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Egmond M E, Kuiper A, Eggink H. Dystonia in children and adolescents: a systematic review and a new diagnostic algorithm. J Neurol Neurosurg Psychiatry. 2015;86(07):774–781. doi: 10.1136/jnnp-2014-309106. [DOI] [PubMed] [Google Scholar]
- 3.Allen N M, Lin J P, Lynch T, King M D. Status dystonicus: a practice guide. Dev Med Child Neurol. 2014;56(02):105–112. doi: 10.1111/dmcn.12339. [DOI] [PubMed] [Google Scholar]
- 4.Eggink H, Coenen M A, de Jong R. Motor and non-motor determinants of health-related quality of life in young dystonia patients. Parkinsonism Relat Disord. 2019;58:50–55. doi: 10.1016/j.parkreldis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Zorzi G, Carecchio M, Zibordi F, Garavaglia B, Nardocci N. Diagnosis and treatment of pediatric onset isolated dystonia. Eur J Paediatr Neurol. 2018;22(02):238–244. doi: 10.1016/j.ejpn.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 6.IDYS Study Group . Bonouvrié L A, Becher J G, Vles J SH, Vermeulen R J, Buizer A I. The effect of intrathecal baclofen in dyskinetic cerebral palsy: the IDYS trial. Ann Neurol. 2019;86(01):79–90. doi: 10.1002/ana.25498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkaim L M, De Vloo P, Kalia S K, Lozano A M, Ibrahim G M. Deep brain stimulation for childhood dystonia: current evidence and emerging practice. Expert Rev Neurother. 2018;18(10):773–784. doi: 10.1080/14737175.2018.1523721. [DOI] [PubMed] [Google Scholar]
- 8.Piña-Fuentes D, Beudel M, Van Zijl J C. Low-frequency oscillation suppression in dystonia: implications for adaptive deep brain stimulation. Parkinsonism Relat Disord. 2020;79:105–109. doi: 10.1016/j.parkreldis.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Larsh T, Wu S W, Vadivelu S, Grant G A, O'Malley J A. Deep brain stimulation for pediatric dystonia. Semin Pediatr Neurol. 2021;38:100896. doi: 10.1016/j.spen.2021.100896. [DOI] [PubMed] [Google Scholar]
- 10.Tisch S, Kumar K R. Pallidal deep brain stimulation for monogenic dystonia: the effect of gene on outcome. Front Neurol. 2021;11:630391. doi: 10.3389/fneur.2020.630391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks W A, Acord S, Bailey L, Honeycutt J. Neuromodulation in childhood onset dystonia: evolving role of deep brain stimulation. Curr Phys Med Rehabil Rep. 2020;8(02):37–43. [Google Scholar]
- 12.Air E L, Ostrem J L, Sanger T D, Starr P A. Deep brain stimulation in children: experience and technical pearls. J Neurosurg Pediatr. 2011;8(06):566–574. doi: 10.3171/2011.8.PEDS11153. [DOI] [PubMed] [Google Scholar]
- 13.DiFrancesco M F, Halpern C H, Hurtig H H, Baltuch G H, Heuer G G. Pediatric indications for deep brain stimulation. Childs Nerv Syst. 2012;28(10):1701–1714. doi: 10.1007/s00381-012-1861-2. [DOI] [PubMed] [Google Scholar]
- 14.North American Pediatric DBS Collaboration . Elkaim L M, Alotaibi N M, Sigal A. Deep brain stimulation for pediatric dystonia: a meta-analysis with individual participant data. Dev Med Child Neurol. 2019;61(01):49–56. doi: 10.1111/dmcn.14063. [DOI] [PubMed] [Google Scholar]
- 15.Isaias I U, Alterman R L, Tagliati M.Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration Brain 2008131(Pt 7):1895–1902. [DOI] [PubMed] [Google Scholar]
- 16.Hale A T, Monsour M A, Rolston J D, Naftel R P, Englot D J. Deep brain stimulation in pediatric dystonia: a systematic review. Neurosurg Rev. 2020;43(03):873–880. doi: 10.1007/s10143-018-1047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tisch S, Rothwell J C, Limousin P, Hariz M I, Corcos D M. The physiological effects of pallidal deep brain stimulation in dystonia. IEEE Trans Neural Syst Rehabil Eng. 2007;15(02):166–172. doi: 10.1109/TNSRE.2007.896994. [DOI] [PubMed] [Google Scholar]
- 19.Barow E, Neumann W J, Brücke C.Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements Brain 2014137(Pt 11):3012–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D H, Méneret A, Friedman J R. ADCY5-related dyskinesia: broader spectrum and genotype-phenotype correlations. Neurology. 2015;85(23):2026–2035. doi: 10.1212/WNL.0000000000002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a20.Méneret A, Gras D, McGovern E, Roze E. Caffeine and the dyskinesia related to mutations in the ADCY5 gene. Ann Intern Med. 2019;171(06):439. doi: 10.7326/L19-0038. [DOI] [PubMed] [Google Scholar]
- 21.Vijiaratnam N, Bhatia K P, Lang A E, Raskind W H, Espay A J. ADCY5–related dyskinesia: improving clinical detection of an evolving disorder. Mov Disord Clin Pract (Hoboken) 2019;6(07):512–520. doi: 10.1002/mdc3.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng H, Khalil S, Neubig R R, Sidiropoulos C. A mechanistic review on GNAO1-associated movement disorder. Neurobiol Dis. 2018;116:131–141. doi: 10.1016/j.nbd.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Waak M, Mohammad S S, Coman D. GNAO1-related movement disorder with life-threatening exacerbations: movement phenomenology and response to DBS. J Neurol Neurosurg Psychiatry. 2018;89(02):221–222. doi: 10.1136/jnnp-2017-315653. [DOI] [PubMed] [Google Scholar]
- 24.Koy A, Cirak S, Gonzalez V. Deep brain stimulation is effective in pediatric patients with GNAO1 associated severe hyperkinesia. J Neurol Sci. 2018;391:31–39. doi: 10.1016/j.jns.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Malaquias M J, Fineza I, Loureiro L, Cardoso L, Alonso I, Magalhães M. GNAO1 mutation presenting as dyskinetic cerebral palsy. Neurol Sci. 2019;40(10):2213–2216. doi: 10.1007/s10072-019-03964-7. [DOI] [PubMed] [Google Scholar]
- 26.Axeen E, Bell E, Robichaux Viehoever A, Schreiber J M, Sidiropoulos C, Goodkin H P. Results of the first GNAO1-related neurodevelopmental disorders caregiver survey. Pediatr Neurol. 2021;121:28–32. doi: 10.1016/j.pediatrneurol.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Marecos C, Duarte S, Alonso I, Calado E, Moreira A.[GNAO1: a new gene to consider on early-onset childhood dystonia]. [Article in Spanish]Rev Neurol 20186609321–322. [PubMed] [Google Scholar]
- 28.Nerrant E, Gonzalez V, Milesi C. Deep brain stimulation treated dystonia-trajectory via status dystonicus. Mov Disord. 2018;33(07):1168–1173. doi: 10.1002/mds.27357. [DOI] [PubMed] [Google Scholar]
- 29.Fasano A, Ricciardi L, Bentivoglio A R. Status dystonicus: predictors of outcome and progression patterns of underlying disease. Mov Disord. 2012;27(06):783–788. doi: 10.1002/mds.24981. [DOI] [PubMed] [Google Scholar]
- 30.Albanese A, Sorbo F D, Comella C. Dystonia rating scales: critique and recommendations. Mov Disord. 2013;28(07):874–883. doi: 10.1002/mds.25579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimeno H, Tustin K, Lumsden D, Ashkan K, Selway R, Lin J P. Evaluation of functional goal outcomes using the Canadian Occupational Performance Measure (COPM) following Deep Brain Stimulation (DBS) in childhood dystonia. Eur J Paediatr Neurol. 2014;18(03):308–316. doi: 10.1016/j.ejpn.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Eggink H, Toonen R F, van Zijl J C. The effectiveness of deep brain stimulation in dystonia: a patient-centered approach. Tremor Other Hyperkinet Mov (N Y) 2020;10:2. doi: 10.5334/tohm.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaminska M, Perides S, Lumsden D E. Complications of deep brain stimulation (DBS) for dystonia in children - the challenges and 10 year experience in a large paediatric cohort. Eur J Paediatr Neurol. 2017;21(01):168–175. doi: 10.1016/j.ejpn.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 34.GEPESTIM consortium . Koy A, Bockhorn N, Kühn A A. Adverse events associated with deep brain stimulation in patients with childhood-onset dystonia. Brain Stimul. 2019;12(05):1111–1120. doi: 10.1016/j.brs.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Marks W, Bailey L, Sanger T D. PEDiDBS: The pediatric international deep brain stimulation registry project. Eur J Paediatr Neurol. 2017;21(01):218–222. doi: 10.1016/j.ejpn.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Piña-Fuentes D, Beudel M, Little S. Toward adaptive deep brain stimulation for dystonia. Neurosurg Focus. 2018;45(02):E3. doi: 10.3171/2018.5.FOCUS18155. [DOI] [PubMed] [Google Scholar]
- 37.Lumsden D E, Ashmore J, Ball G. Fractional anisotropy in children with dystonia or spasticity correlates with the selection for DBS or ITB movement disorder surgery. Neuroradiology. 2016;58(04):401–408. doi: 10.1007/s00234-015-1639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


