Abstract
The ability of intracellular pathogens to sense and adapt to the hostile environment of the host is an important factor governing virulence. We have sequenced the operon encoding the major heat shock proteins GroES and GroEL in the gram-positive food-borne pathogen Listeria monocytogenes. The operon has a conserved orientation in the order groES groEL. Upstream of groES and in the opposite orientation is a gene encoding a homologue of the Bacillus subtilis protein YdiL, while downstream of groEL is a gene encoding a putative bile hydrolase. We used both reverse transcriptase-PCR (RT-PCR) and transcriptional fusions to the UV-optimized Aequorea victoria green fluorescent protein (GFPUV) to analyze expression of groESL under various environmental stress conditions, including heat shock, ethanol stress, and acid shock, and during infection of J774 mouse macrophage cells. Strains harboring GFPUV transcriptional fusions to the promoter region of groESL demonstrated a significant increase in fluorescence following heat shock that was detected by both fluorimetry and fluorescence microscopy. Using both RT-PCR and GFP technology we detected expression of groESL following internalization by J774 cells. Increased intracellular expression of dnaK was also determined using RT-PCR. We have recently described a system which utilizes L. monocytogenes hemolysin as an in vivo reporter of gene expression within the host cell phagosome (C. G. M. Gahan and C. Hill, Mol. Microbiol. 36:498–507, 2000). In this study a strain was constructed in which hemolysin expression was placed under the control of the groESL promoter. In this strain hemolysin expression during infection also confirms transcription from the groESL promoter during J774 and murine infection, albeit at lower levels than the known virulence factor plcA.
Listeria monocytogenes is a gram-positive food-borne pathogen which poses a significant threat to the health of susceptible individuals (21). The ability of the pathogen to detect and react to adverse environmental conditions is central to its capacity to cause disease. Listeria cells may encounter environmental stresses such as heat, elevated osmolarity, and low pH while residing in foods, during survival of gastric passage and growth in the small intestine, and during intracellular pathogenesis (13). It has been demonstrated that an ability to adapt to environmental stress is essential for the realization of full virulence potential in L. monocytogenes and in other intracellular pathogens, such as Salmonella enterica serovar Typhimurium (12, 23, 43, 48). In addition, for intracellular pathogens, perturbations in host microenvironments, such as the low pH, oxidative stress, and low Mg2+ of the host cell phagosome, are necessary for triggering the synthesis of essential virulence factors (16, 29). Environmental stimuli therefore function as signals allowing sequential induction and repression of appropriate virulence factors during pathogenesis.
Following penetration of host cells, the production of listeriolysin by L. monocytogenes causes lysis of the host cell phagosome, allowing escape of the bacterium into the host cell cytoplasm. It has been demonstrated that prior to lysis the phagosome becomes acidified and that this process is necessary for optimal listeriolysin functionality (5). During this period the phagosome most likely represents a suboptimal environment for the bacterium. Indeed, two-dimensional gel electrophoresis of L. monocytogenes grown in cultured mammalian cells has revealed a pattern of protein synthesis that is distinct from that of cells grown under optimal conditions, suggesting bacterial adaptation to the host environment (25). Two-dimensional gel electrophoresis studies have also revealed a significant shift in protein synthesis in L. monocytogenes following an increase in the acidity of the growth media to levels approximating the pH of the host cell phagosome (39, 41, 42). The data indicate that Listeria cells are capable of reacting to the environment through upregulation in the synthesis of a number of proteins. It is now evident that this process of bacterial adaptation is required for optimal virulence potential (14, 37, 43).
Production of the heat shock proteins DnaK, DnaJ, GroES, and GroEL by bacteria is associated with exposure to environmental stress conditions. The molecular chaperonin proteins GroES and GroEL are synthesized at elevated levels by bacteria exposed to various environmental stressors. GroEL is among the most highly conserved proteins in nature (46) and together with GroES functions to maintain protein integrity under abusive environmental conditions (30). Studies using a variety of bacterial genera have also demonstrated elevated synthesis of GroEL following exposure to low pH, ethanol, salt, and bile salts, suggesting a role for this protein in the general stress response (27, 32, 44). Indeed, evidence suggests a function for GroEL in bacterial growth at all temperatures, indicating a role in protein folding even under optimal growth conditions (17). Previous studies have utilized two-dimensional gel electrophoresis to demonstrate the appearance of the GroEL protein following heat shock induction of L. monocytogenes, and partial sequencing of the protein has been carried out (25). However, despite studies with other organisms, the groESL operon of L. monocytogenes has not previously been characterized.
Here we describe the sequence of groESL in L. monocytogenes strain LO28. We also compare two reporter systems which utilize transcriptional fusions of genes to either gfpUV or hly in a single copy in situ in the listerial chromosome to allow in vivo monitoring of transcription from the groESL promoter region. Both systems indicate increased expression of groESL during infection of mice and within cultured mouse macrophages.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains and plasmids used in this study are listed in Table 1. L. monocytogenes strain LO28 (serotype 1/2c) was obtained from P. Cossart, Institut Pasteur, Paris, France. All Listeria strains were cultured in brain heart infusion (BHI) broth or tryptic soy broth-yeast extract (0.6%) (TSB-YE) with added antibiotics (Sigma Chemical Company, St. Louis, Mo.) when appropriate: erythromycin (5 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (10 μg/ml). Escherichia coli strains were grown in Luria-Bertani broth with 150 μg of erythromycin/ml added when appropriate.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli EC1000 | Kmr, MC1000 derivative carrying a single copy of pWV01 repA in glgB | 45 |
| L. monocytogenes LO28 | Wild-type strain, serovar 1/2c | P. Cossart |
| L. monocytogenes LO28Δhly | Δhly, L. monocytogenes LO28 | 24 |
| L. monocytogenes gro-gfp | Emr, gro-gfp transcriptional fusion in LO28 | This study |
| L. monocytogenes plc-gfp | Emr, plcA-gfp transcriptional fusion in LO28 | This study |
| L. monocytogenes gro-hly | Emr, gro-hly transcriptional fusion in LO28Δhly | This study |
| L. monocytogenes plc-hly | Emr, plcA-hly transcriptional fusion in LO28Δhly (previously designated L. monocytogenes PLC1) | 24 |
| L. monocytogenes pGFP-Int | Emr Cmr, LO28 containing pGFP-Int and pVE6007 | This study |
| L. monocytogenes CGNeg | Emr, hly fusion strain, negative hemolysis under all conditions tested | 24 |
| Plasmids | 45 | |
| pORIX | Emr, lacZ derivative of pORI13 (REF) with single BlnI site downstream of multiple cloning site; Ori+ Rep− | 24 |
| pGFP-Int | Emr, derivative of pORIX containing promoterless gfpuv downstream of MCS | This study |
| pCOR2 | Emr, derivative of pORIX containing promoterless hly downstream of MCS | 24 |
| pVE6007 | Cmr, temperature sensitive derivative of pWV01 | 36 |
| pNF579 | pKSV7 plasmid containing gfpuv | N. E. Freitag |
Generation and screening of a Tn917 mutant bank.
A Tn917 mutant bank was created using the temperature-sensitive plasmid pTV1-OK as described previously (14). Isolated transformants were grown overnight in TSB-YE containing kanamycin at 30°C, subcultured into TSB-YE containing erythromycin (0.04 μg/ml) at 42°C, and selected for kanamycin-sensitive Tn917 integrants on tryptic soy agar (TSA)-YE containing erythromycin. To isolate acid-sensitive Tn917 mutants, 2,000 integrants were replica plated onto TSA-YE plates at pH 7 and TSA-YE plates adjusted to pH 5 with 3 M lactic acid. Two integrants demonstrated restricted growth on pH 5 plates, and one of them was selected for further study.
Sequence analysis of groESL.
Inverse PCR and sequence analysis of the acid-sensitive Tn917 mutant of L. monocytogenes revealed insertion of the transposon downstream of a gene with homology to ydiL in B. subtilis (data not shown). Further sequencing of the inverse PCR product revealed the presence of groES and groEL genes. Another inverse PCR was carried out to generate a PCR product for further sequencing of groEL. Briefly, genomic DNA from wild-type L. monocytogenes LO28 was digested with AvrII, and 5 μl was used in a ligation mix with a total volume of 50 μl. PCR was carried out using the primers InEL-IN (5′-GGAATCGCCTGCTCCTTCTAC-3′) and InEL-OUT (5′-CTCTACTCGCGCAGCTGTA-3′) with Expand long-template Taq polymerase (Roche, Mannheim, Germany) and appropriate conditions. The resulting 2.1-kb fragment was sequenced using a Beckman CEQ 2000 DNA analysis system. Confirmatory sequencing of the entire groESL operon was subsequently carried out. Homology searches were performed against the GenBank database using the BLAST program.
Transcriptional analysis of groESL expression.
L. monocytogenes LO28 was grown to an optical density at 600 nm (OD600) of 0.15 at 37°C. Cells were heat shocked at 45°C for various times. Total RNA was isolated using a hot acid phenol procedure (38). Briefly, 1-ml aliquots of culture were pelleted by centrifugation in an Eppendorf centrifuge model 5415C (12,000 rpm for 30 s) and immediately frozen by immersion in a liquid N2 bath followed by storage at −70°C. Pellets were thawed slowly on ice and resuspended in 500 μl of ice cold lysis buffer (20 mM sodium acetate [pH 5.2], 1 mM EDTA, 1% sodium dodecyl sulfate). Cell suspensions were added to 500 μl of preheated (65°C) acid phenol-chloroform-isoamylalcohol (Sigma) and 200 mg of 425 to 600-μm glass beads (Sigma) and placed on a heating block (65°C) for 10 min with frequent vortexing. Suspensions were centrifuged for 10 min at 14,000 rpm, and the aqueous phase was extracted with 500 μl of hot acid phenol-chloroform-isoamylalcohol and precipitated in 2.5 volumes of ice cold ethanol for 1 h. Suspensions were pelleted by centrifugation in an Eppendorf centrifuge model 5415C (14,000 rpm for 15 min), washed with 1 ml 70% ethanol, and resuspended in 100 μl of buffer containing 10 mM MgCl2, 1 mM dithiothreitol, 10 mM Tris (pH 7), 1 mM EDTA, 5 U of DNAse I (Roche), and 5 U of RNasin (Roche). The RNA was stored at −70°C until required for analysis.
For slot blot analysis, 1 μg of total RNA from each time point was denatured in diethyl pyrocarbonate-treated water containing 1% dimethyl sulfoxide (Sigma) at 65°C for 15 min. The denatured RNA was then loaded onto a nylon membrane using a vacuum slot blot manifold (Bio-Rad Laboratories, Hercules, Calif.) and fixed using a UV Stratalinker (Stratagene). The primers GSRA (5′-GTAGAGCGGAACGTGTTAC-3′) and GSF2 (5′-GTAGTAGCCGTGAAAGC-3′) were used to generate a 400-bp fragment of groEL using standard PCR. This fragment was labeled using the Dig Hi Prime digoxigenin labeling kit (Roche). Ten nanograms of labeled probe was used to hybridize with the immobilized RNA at 42°C overnight. The membrane was subsequently blocked, washed, and treated with alkaline phosphatase-conjugated antidigoxigenin antibody as instructed by the manufacturers (Roche). The membrane was subsequently exposed to Kodak XR-Omat film, and signal intensities were compared using densitometry analysis (Phoretix, Newcastle upon Tyne, United Kingdom).
For reverse transcriptase PCR (RT-PCR) analysis cDNA synthesis was carried out by adding 1 μg of total RNA to 4 μl of 5× RT buffer (Roche), 2 μl of 100 mM dithiothreitol, 0.5 μl of a deoxynucleoside triphosphate mix (dATP, dCTP, dGTP, and dTTP; each 10 mM), 1 μl (40 U) of RNasin, 100 ng of the random primer p(dN)6, and 1 μl of Expand reverse transcriptase (Roche). The reaction mixture was incubated at 37°C for 2 h. PCR was carried out using the following primers: for groEL, GSRA and GSF2; for dnaK, DNAK-F (5′-GCTGGTCTTGAAGTAGAAC-3′) and DNAK-R (5′-GTTCATCAAATTTAGCACGAGT-3′); for plcA, PLCA-X (5′-TTCGGGGAAGTCCATGATTAG-3′) and PLCA-Y (5′-CACTACTCCCAGAACAGACACG-3′); and for 16S RNA, 16sRNA-E (5′-TTAGCTAGTTGGTAGGGT-3′) and 16sRNA-B (5′-AATCCGGACAACGCTTGC-3′). PCRs were carried out for 16, 22, or 30 cycles to allow optimal quantitation of PCR products. cDNA was added to PCRs for groEL, plcA, or dnaK at levels which gave similar band intensities for 16S RNA (control) reactions.
Construction of gfpuv transcriptional gene fusion strains.
The RepA− pORI system for generating single plasmid insertions into the chromosome has been described previously (34, 36). We have created a pORIX derivative which contains a single BlnI cut site downstream of a multiple cloning site (24). In order to create a plasmid with UV-optimized green fluorescent protein (GFPUV) as a reporter, we amplified gfpuv from plasmid pNF579 (a gift from N. E. Freitag, Detroit, Mich.) using primers CGFP-1 (5′-CCCCTAGGAGGAGGAAAAATATGAGTAAAGGAGAAGAAC-3′) and CGFP-2 (5′-GCCCTAGGTTATTTGTAGAGCTCATCCATG-3′) designed to contain BlnI cut sites (underlined). High-fidelity Vent DNA polymerase (New England Biolabs, Beverly, Mass.) was used for PCRs. The resulting fragment was digested with BlnI and cloned into similarly digested pORIX in E. coli EC1000 (45) to create the plasmid pGFP-Int. The orientation of the gfpuv ligation was checked using PCR. Plasmids containing promoter regions upstream of gfpuv in pGFP-Int were created by amplifying appropriate regions from L. monocytogenes LO28 genomic DNA and cloning into the multiple cloning site using E. coli EC1000 as the host. Primers GRO1 (5′-CTTCTTATAGATCTCGTTATGAAGCTT-3′), containing a BglII cut site (underlined), and GRO2 (5′-CTTTGGCAGAGTCTAGAAATCCAATCC-3′), containing an XbaI cut site, were used to amplify a region containing the putative promoter region of groESL including the CIRCE (for “controlling inverted repeat of chaperone expression”) element. Primers PLC1A (5′-GGTTGGATCCGATAATCTAGACTATCG-3′) (XbaI cut site) and PLC3 (5′-TTCGCTTCTGCAGATGAAACGC-3′) (PstI cut site) were used to amplify a region containing the plcA promoter (24).
Plasmids were electroporated into L. monocytogenes LO28 containing the RepA+ helper plasmid pVE6007 (Cmr) using a standard protocol (40) and incubated at 30°C. Plasmid integration resulted following growth at 42°C in antibiotic-free BHI broth and plating onto prewarmed BHI plates containing erythromycin at 42°C. Individual colonies were replica plated onto BHI-chloramphenicol and BHI-erythromycin plates followed by incubation at 30°C to determine loss of pVE6007 (Cms) and integration of pGFP-Int (Emr).
Quantitation of fluorescence.
gfpUV fusion strains or controls were grown overnight in BHI broth containing 5 μg of erythromycin/ml at 30°C, and 500 μl was used to inoculate 10 ml of antibiotic-free BHI broth at 30°C. For growth with fluorescence analysis, cells were immediately incubated at 43°C. For analysis of various stresses, fresh inocula were grown at 30°C for 1 h before the addition of ethanol (4% [vol/vol] final concentration), hydrogen peroxide (0.1% [vol/vol] final concentration), or bile salts no. 3 (Oxoid) (0.08% [wt/vol] final concentration, pH 6.8), reduction in pH to pH 5.0 (with HCl), or shift in temperature to 43°C. Cells were subjected to various stresses (except heat stress) at 30°C. At appropriate time points, the OD600 of cultures was measured, cells were resuspended to an OD600 of 0.2, and 1 ml of cells was washed once in phosphate-buffered saline (PBS) and resuspended in 200 μl of PBS. Cultures were diluted 1:2 in PBS, and 100 μl was used to measure fluorescence. Fluorescence of strains was measured using a Wallac 1420 multilabel counter (Perkin Elmer Life Sciences, Wellesley, Mass.) fitted with a 395-nm excitation filter and a 535-nm emission filter. Specific fluorescence intensity is the raw fluorescence intensity divided by the OD600. Relative fluorescence intensity is the specific fluorescence intensity test value minus the intensity for the negative control (L. monocytogenes LO28, no plasmid) for each time point.
J774 infection with gfpUV fusion strains.
J774 mouse macrophage cells were grown on coverslips in antibiotic-free Dulbecco's modified Eagle medium (DMEM) (Gibco Laboratories, Grand Island, N.Y.) in tissue culture petri dishes (Gibco). Monolayers were infected with appropriate gfpUV fusion strains or a negative control (L. monocytogenes containing pGFP-Int) at a multiplicity of infection of 100 CFU/cell. After 1 h, monolayers were washed once with DMEM and subsequently incubated with DMEM containing 15 μg of gentamicin/ml for a further 6 h. Cells were then washed three times with PBS and mounted on slides for microscopic analysis. Tandem samples were also assayed for bacterial numbers by the addition of an infected coverslip to 10 ml of ice-cold sterile distilled water, serial dilution, and plating onto BHI agar plates. For microscopic analysis, samples were fixed by the addition of a drop of 3.7% formaldehyde in PBS for 5 min at room temperature, washed with PBS, covered with Micromount mounting medium (Surgipath Medical Industries, Richmond, Ill.), and allowed to set overnight. Slides were examined by confocal microscopy (Bio-Rad). The parameters used for each confocal examination were identical.
RT-PCR from L. monocytogenes infecting J774 cells.
J774 cells were grown in antibiotic-free DMEM in tissue culture petri dishes and were infected at a multiplicity of infection of approximately 100 bacteria per cell with L. monocytogenes LO28. Infected cells were incubated for 30 min, and following this period gentamicin was added to a final concentration of 15 μg/ml. After a further defined incubation time, monolayers were washed twice in PBS, and internalized bacteria were released by lysis of J774 cells with ice-cold sterile distilled water. Bacteria were pelleted by centrifugation, and RNA extraction was carried out as detailed above. Control bacterial suspensions were grown overnight in BHI broth, washed twice in PBS, and resuspended in antibiotic-free DMEM for 90 min under the same conditions as J774 cells.
Creation of a hly-groESL promoter fusion strain.
The pCOR2-based system for creating transcriptional fusions to hly has been described previously (24). In this study we cloned the putative promoter region from groESL, generated using GRO1 and GRO2 primers, into pCOR2 and forced integration of the plasmid into the chromosome of the hly mutant strain L. monocytogenes LO28Δhly. The resulting strain is referred to as the L. monocytogenes gro-hly strain.
Both L. monocytogenes gro-hly and L. monocytogenes plc-hly (24) were analyzed for virulence in a murine model of infection and compared to a hemolysin-negative fusion strain, L. monocytogenes CGNeg (24). Eight- to twelve-week-old female BALB/c mice were inoculated intraperitoneally with 2 × 108 CFU of the appropriate inoculum in 200 μl of PBS. Numbers of bacteria surviving in mouse livers and spleens were determined 2 days postinfection (24).
Survival and growth of fusion strains was determined in J774 cells using a modification of a previously described procedure (22). J774 cells were grown in 24-well tissue culture plates (Gibco) in antibiotic-free DMEM. Washed bacterial suspensions were added to individual wells at a multiplicity of infection of 5 bacteria per cultured cell, and plates were centrifuged at 150 × g for 10 min to increase contact between bacteria and macrophages. Plates were incubated for 1 h to allow uptake of Listeria cells, and growth medium was removed and replaced with DMEM containing gentamicin (15 μg/ml). Plates were incubated for 30 min, and at this stage (T0) and at various intervals, wells were washed twice with PBS and monolayers were lysed with ice-cold sterile distilled water. Bacterial counts were determined by serial dilution and plating onto BHI agar plates.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this study have been submitted to GenBank and assigned accession number AF335323.
RESULTS
Sequence analysis of groESL in L. monocytogenes.
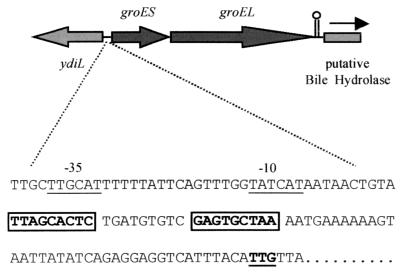
We initially located the groESL operon through genetic analysis of an acid-sensitive transposon mutant of L. monocytogenes LO28. This mutant was one of two mutants, isolated from a bank of 2,000 tested, which demonstrated slow growth at pH 5 but not at a neutral pH (data not shown). Initial sequencing revealed the presence of the transposon in a region downstream of a gene which is a homologue of the Bacillus subtilis ydiL gene. The transposon does not disrupt ydiL in L. monocytogenes, and further genetic analysis of this mutant is ongoing to determine the exact effect of the mutation. However, since groESL is upstream of ydiL in B. subtilis, we continued sequencing this region in L. monocytogenes. Further analysis determined that, as in B. subtilis, the L. monocytogenes groESL operon lies upstream of ydiL.
Analysis of this region revealed two open reading frames encoding proteins with significant homologies to GroES and GroEL in gram-positive bacteria. Protein homology searches showed that the first open reading frame encoded a protein that is 77% identical to the GroES protein of B. subtilis and 71% identical to the GroES protein of Bacillus stearothermophilus. The downstream region encodes a protein with high identity (86%) with GroEL in B. subtilis and significant identities with GroEL (Hsp60) in a number of bacterial species. Further downstream in L. monocytogenes is an open reading frame which differs from the corresponding region in B. subtilis and encodes a putative protein with significant identity (52% over 105 amino acids) with bile salt hydrolase in Lactobacillus plantarum (Fig. 1).
FIG. 1.
Molecular organization of groES and groEL in L. monocytogenes LO28. CIRCE tandem repeat elements are boxed. The putative start codon, based upon BLAST homology searches, is underlined. Possible promoter regions (−10 and −35 sites) are indicated. ydiL was identified on the basis of homology with B. subtilis ydiL, a gene encoding a putative transmembrane protein. The arrow indicates the direction of the putative bile acid hydrolase gene based upon homology with a gene encoding a conjugated bile salt hydrolase (M96175) in L. plantarum.
Downstream of the putative promoter region of groESL in L. monocytogenes is a conserved CIRCE element. These regulatory elements are highly conserved in heat shock genes (dnaK and groESL) of gram-positive bacteria (28, 46). In L. monocytogenes, as in all other gram-positive bacteria, the two inverted repeats are separated by a 9-bp spacer region (28). As in B. subtilis, Lactobacillus helveticus, and Lactobacillus zeae, the putative initiation codon is UUG rather than AUG, and this may serve to limit expression of the gene at the level of translation (47). Between the end of the groEL gene and the start of the putative bile hydrolase is a likely rho-independent transcriptional terminator (ΔG = −13.4 kcal/mol).
Heat shock and transcriptional analysis of groESL in L. monocytogenes.
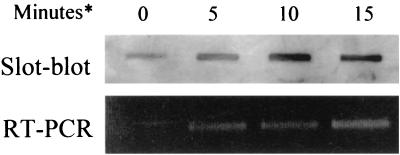
Initial experiments showed that L. monocytogenes cells subjected to heat shock at 45°C for 30 min develop increased heat resistance (to 55°C) relative to nonadapted controls (data not shown). This demonstrates the presence in L. monocytogenes of an adaptive heat shock response which in other organisms involves the induction of GroES and GroEL. To examine transcriptional induction of groEL, we isolated total RNA from bacterial cultures subjected to a shift in temperature from 37 to 45°C. Cells in this study were demonstrably more resistant to lethal heat treatment than nonshocked controls (data not shown). Total RNA concentrations examined for each sample were identical based upon gel visualization and UV spectrophotometric (Genequant) analysis. RNA was analyzed for specific groEL mRNA using either RNA slot blotting or RT-PCR (Fig. 2). The results indicate the presence of transcript at low levels at 37°C prior to heat shock. However, 5 min following heat shock there is a clear increase in transcription of groEL, reaching high levels after 15 min. Densitometry analysis of slot blots suggested a 4.6-fold increase in transcription 10 min following heat shock, with a 4.9-fold increase after 15 min relative to control (nonshocked) cells. Finally, RT-PCR using a forward primer on groES and a reverse primer on groEL produced a product of the predicted size, indicating that both groES and groEL can be transcribed as a single mRNA (data not shown).
FIG. 2.
Analysis of the transcription of L. monocytogenes groEL following heat shock using slot blot analysis or RT-PCR. L. monocytogenes RNA was isolated prior to heat shock (0 min) or at 5, 10, or 15 min (∗) following a shift from 37 to 45°C. Total bacterial RNA concentrations were determined by Genequant analysis, and identical concentrations of RNA were analyzed for specific groEL RNA in each case. RT-PCR represents 16 cycles of PCR.
Design of the gfpUV-based integrating plasmid pGFP-Int.
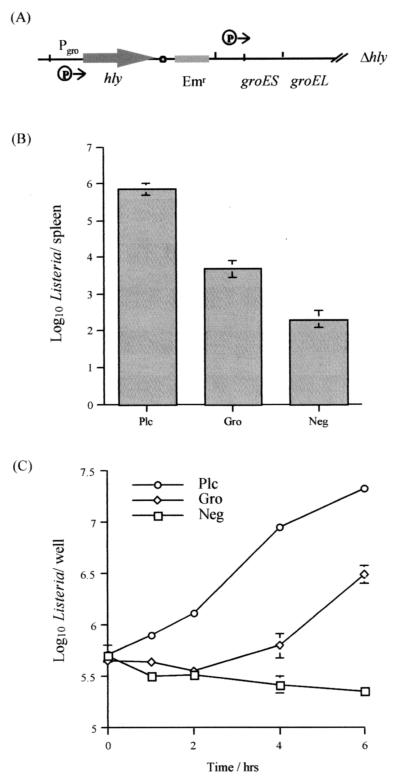
We have previously described a system to create single plasmid insertions into the chromosome of L. monocytogenes to allow monitoring of gene expression using hemolysin (24). The system was originally described for Lactococcus lactis and utilizes a RepA− integrating plasmid in which the desired promoter element is cloned upstream of a promoterless reporter gene. The RepA− plasmid can then be introduced into a host strain harboring a temperature-sensitive RepA+ helper plasmid (pVE6007) (34). Growth of the strain at the permissive temperature (30°C) allows stable replication of both plasmids due to in trans complementation from helper to pORI. However, an increase in growth temperature results in curing of the helper plasmid, leading to the loss of the RepA+ phenotype and forced integration of the RepA− plasmid at a site of homology provided by cloned host DNA (Fig. 3A).
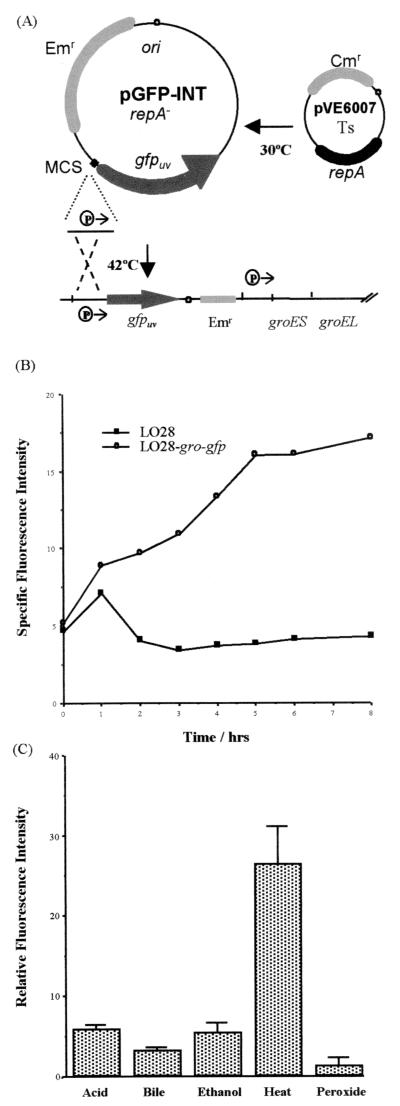
FIG. 3.
(A) Site-directed integration of pGFP-Int into the chromosome of L. monocytogenes LO28. The promoter region of groESL was cloned into the multiple cloning site of the RepA− plasmid pGFP-Int (Emr). This construct was used to transform L. monocytogenes containing the temperature-sensitive, RepA+ plasmid pVE6007 (Cmr) and incubated at 30°C. Upon temperature upshift, the pVE6007 plasmid no longer supports replication of pGFP-Int, which integrates into the chromosome at the point of homology provided by the cloned L. monocytogenes DNA. Integrants are Cms and Emr. (B) Specific fluorescence intensity of L. monocytogenes gro-gfp and L. monocytogenes LO28 (wild type) during growth at 43°C. Data are representative of duplicate experiments. (C) Fluorescence intensity of L. monocytogenes gro-gfp exposed to acid (HCl, pH 5.0), bile salts (0.08% [wt/vol]), ethanol (4% [vol/vol]), heat (43°C), and hydrogen peroxide (0.1% [vol/vol]). Fluorescence data are relative to the negative control (L. monocytogenes LO28) and are the means plus standard deviations for triplicate experiments.
We have created a RepA− plasmid (pGFP-Int) based on the lactococcal plasmid pORI13, which contains a promoterless copy of gfpuv (15) downstream of the multiple cloning site (see Materials and Methods). Cloning the promoter regions of either groESL (Pgro) or plcA (Pplc) into pGFP-Int permits the creation of single-copy chromosomal fusions to gfpuv in the L. monocytogenes chromosome (Fig. 3A).
In order to determine the efficacy of the system we measured the specific fluorescence intensity of L. monocytogenes gro-gfp during growth at an elevated temperature (43°C). Specific fluorescence intensity represents raw fluorescence data normalised for cell density (OD600). Growth at this temperature resulted in a fourfold increase in specific fluorescence intensity relative to the wild type, indicative of expression of GFP from Pgro (Fig. 3B). The fluorescence intensity reached a peak between 6 and 8 h of growth. No significant increase in fluorescence was seen in L. monocytogenes gro-gfp grown at 30 or 37°C for 8 h (data not shown).
Previous studies of various bacteria have shown increased expression of GroEL following exposure to low pH, ethanol, and bile salts (27, 44). We used the gro-gfp strain to determine transcription from Pgro under various stress conditions. Cells were exposed to various stressors for 8 h, and fluorescence intensity was determined using a fluorimeter (see Materials and Methods). Data is presented as fluorescence intensity relative to that of unlabeled wild-type cells subjected to each stress. While ethanol, acid, and bile salts were capable of stimulating significant (P < 0.05) increases in fluorescence, the levels of expression never reached those of heat-shocked cells under the conditions examined (Fig. 3C). Peroxide stress did not result in significant induction of GFP under the conditions used (Fig. 3C). The overall results are similar to those of a recent study utilizing gfpuv fusions to analyze dnaK expression in E. coli (11).
groEL expression within J774 mouse macrophage cells.
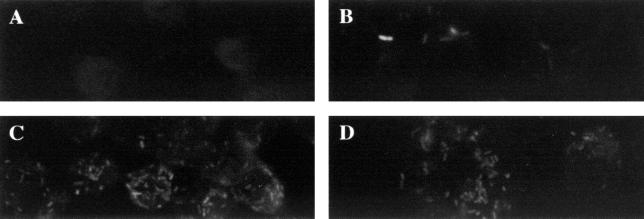
We have used a number of approaches to analyze transcription of groEL within the cultured mouse macrophage cell line J774. J774 cells were grown on coverslips and infected with either L. monocytogenes gro-gfp, L. monocytogenes plc-gfp, or L. monocytogenes(pGFP-Int) (negative control). Cells were grown for 6 h and then analyzed using confocal laser microscopy for fluorescent bacterial cells. In random microscopic fields, fluorescent bacteria were clearly visualized within J774 cells infected with gro-gfp and plc-gfp strains but not with the negative control (Fig. 4). This is consistent with expression of GFPUV from both Pgro and Pplc during infection. Similar numbers of fluorescent bacteria were seen in macrophages infected with L. monocytogenes gro-gfp and L. monocytogenes plc-gfp. Fluorescent bacteria could be visualized in directly adjacent fields of J774 cells infected with gro-gfp and plc-gfp strains but not with the negative control. J774 cells infected with unstimulated gfpUV fusion strains for 1 h were negative for fluorescent bacteria, suggesting that longer time periods are required for expression of GFPUV (data not shown). Bacterial plate counts from duplicate coverslips demonstrated that all coverslips, including those with L. monocytogenes(pGFP-Int)-infected cells, contained similarly high numbers of bacteria (∼106 CFU/coverslip).
FIG. 4.
Fluorescence of L. monocytogenes cells incubated with J774 mouse macrophages. Monolayers were grown on glass coverslips and infected with L. monocytogenes pGFP-Int for 6 h (A), with heat-shocked (43°C) L. monocytogenes gro-gfp for 1 h (B), with L. monocytogenes gro-gfp for 6 h (C), or with L. monocytogenes plc-gfp for 6 h (D). Coverslips were prepared as described in Materials and Methods and examined by confocal microscopy.
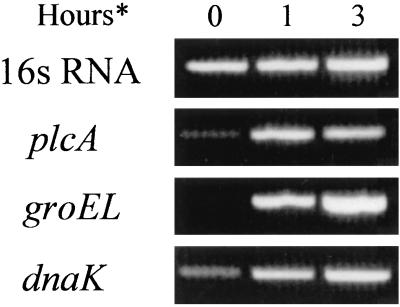
A previous study used RT-PCR to determine virulence gene expression by L. monocytogenes infecting cultured mammalian cells (8). Here we used RT-PCR to detect groEL mRNA produced by wild-type L. monocytogenes during infection of J774 cells. Total RNA was isolated from L. monocytogenes-infected J774 cells at 1 and 3 h postinfection. Total cDNA was subsequently analyzed for groEL, dnaK, plcA, and 16S RNA (control). cDNA was added such that PCR products for 16S RNA were of similar intensity for all samples after 16 cycles of PCR, indicating similar amounts of total bacterial RNA in each sample (Fig. 5). This allowed the analysis of the same cDNA with different primer pairs. The data indicate a clear and substantial increase in expression of groEL during J774 infection. Similarly, there is a clear increase in expression of dnaK under these conditions. There is also an increase in transcription of the virulence factor plcA during infection relative to the control (T0). A repeat experiment demonstrated similar results.
FIG. 5.
Analysis of the transcription of L. monocytogenes genes following growth in J774 macrophage cells. J774 cells were infected with wild-type L. monocytogenes LO28 for 1 or 3 h (∗), cells were lysed with sterile distilled water, and total RNA was isolated from intracellular bacterial cells. Controls (T0) were exposed to DMEM for 90 min and washed in sterile distilled water prior to RNA isolation. Total cDNA was used for PCRs (30 cycles) at levels which gave similar intensities for 16S RNA reactions (16 cycles).
Evidence for in vivo expression of groESL using a hemolysin fusion strain.
We have previously described a system for isolation of in vivo-expressed promoters in L. monocytogenes which relies upon expression of the hemolysin gene (hly) from random promoter elements (24). Here we describe the use of this system for the analysis of in vivo expression from a specific promoter, Pgro.
Hemolysin is expressed optimally from its own promoter within the phagosome of infected cells and functions to allow phagosomal lysis and escape of the bacterium into the host cell cytoplasm where bacterial division can take place. In this study, we uncoupled hemolysin from its normal regulation by placing a promoterless copy of hly under the influence of Pgro in an hly mutant host strain (Fig. 6A). Hemolysin expression is low from this fusion strain as measured on blood agar plates at 37°C and is similar to fusions to previously described in vivo-induced genes (24). The L. monocytogenes gro-hly strain is capable of reaching high numbers in the spleens of infected mice relative to the L. monocytogenes CGNeg control strain (Fig. 6B). This is indicative of in vivo expression of hly from Pgro. However, in the spleens of infected mice, the fusion strain does not reach the same high levels as the L. monocytogenes plc-hly strain or the wild-type L. monocytogenes LO28, which causes lethality when administered at similar levels (24). This indicates that while some expression from Pgro does take place in vivo, the levels of expression and/or the time course of expression is not sufficient for a full restoration of virulence potential. Data from infected livers demonstrated identical trends (data not shown).
FIG. 6.
Analysis of promoter-hly fusion strains for growth in J774 cells and during murine infection. (A) Representation of the hly fusion to the groESL promoter region in L. monocytogenes gro-hly. (B) Analysis of levels of L. monocytogenes strains in the spleens of infected mice. Mice were infected intraperitoneally with L. monocytogenes gro-hly, L. monocytogenes plc-hly, or L. monocytogenes CGNeg, and bacterial numbers in the spleens were determined at 2 days postinfection. Data are means ± standard deviations for four mice per group. (C) Growth of fusion strains in J774 cells. J774 cells were infected with L. monocytogenes gro-hly, L. monocytogenes plc-hly, or L. monocytogenes CGNeg, and numbers of intracellular bacteria were determined at specific time points postinfection. Data are means ± standard deviations for three wells per time point.
We have further analyzed growth of these fusion strains during infection of J774 cells (Fig. 6C). Analysis of cell numbers at various time points during infection demonstrates that hemolysin-negative cells are incapable of growth in this cell line under the conditions used. The L. monocytogenes plc-hly fusion strain grows rapidly in J774 cells at rates similar to those of wild-type Listeria cells (data for the wild type not shown). This is most likely due to rapid production of hemolysin from the PrfA-regulated plcA promoter, resulting in escape from the phagosome and growth of the strain in the cytoplasm. In contrast, growth of the L. monocytogenes gro-hly strain occurs at a later stage, suggesting a lag in expression of hemolysin from the groESL promoter in strains confined within the phagosome.
DISCUSSION
We have sequenced the groESL operon in L. monocytogenes LO28 and have analyzed expression of groEL under various stress conditions, including infection of mouse macrophages. The groESL operon of L. monocytogenes demonstrates a conserved organization, with groES followed by groEL. The region upstream of the operon contains a homologue of the B. subtilis gene ydiL. However, in contrast to the gene organization in B. subtilis, downstream of groEL in L. monocytogenes is a gene encoding a putative bile hydrolase (33). In addition, the regulatory region of the listerial groESL contains a distinctive CIRCE element, an inverted repeat which in other organisms acts as a negative cis element preventing excessive expression under normal growth conditions and facilitating heat shock induction of the operon (46). Analysis of putative open reading frames suggests that the GroES and GroEL proteins demonstrate highest identity to B. subtilis proteins.
Previous studies have demonstrated that L. monocytogenes exhibits a typical heat shock response to mildly elevated growth temperatures, which serves to protect cells against normally lethal temperatures (10, 18). This adaptive response involves the induction of both GroEL and DnaK heat shock proteins, which can be detected at 30 min after heat shock (45°C) (25). Here we show that mild heat shock (45°C) results in increased transcription of groEL as rapidly as 5 min following temperature upshift and reaches high levels at 15 min following heat shock. This finding reflects a rapid response to the environmental insult and is similar to data obtained with other gram-positive organisms (4, 7).
In order to facilitate analysis of expression from the groESL promoter and other promoter regions, we have developed a system which utilizes the UV-optimized GFPUV from Aequoria victoria as a marker of gene expression. The system employs the stable integration of a promoter probe vector in single copy in the chromosome and thereby overcomes possible artifacts associated with plasmid copy number, which may be encountered especially under environmental stress conditions. A significant increase in fluorescence intensity was evident following growth of the L. monocytogenes gro-gfp fusion strain at a high temperature (43°C). Levels of fluorescence were similar to those in a recent study of heat shock gene expression in E. coli using a gfpuv fusion (11). The delay in fluorescent signal noted in the present study is due to the time necessary for chromophore formation and has been documented previously (3, 11). However, the data indicate that the transcriptional fusion of the groESL promoter to gfpuv is a useful marker of gene expression.
Previous studies have demonstrated increased expression of groEL under a range of environmental stress conditions. Stress conditions encountered during infection, such as low pH, bile salts, and oxidative stress, have been shown to elicit groESL expression in a variety of organisms (19, 27, 32). In addition, ethanol has been shown to efficiently induce groESL expression (44). We have used the L. monocytogenes gro-gfp strain to demonstrate expression following exposure to low pH, bile salts, and ethanol, suggesting that these environmental insults have the potential to induce gene expression. However, as noted in similar studies, heat shock remains the most potent inducer of groESL expression under the conditions analyzed (6, 19, 27). Given the acidity of the macrophage phagosome following infection (5), it is notable that exposure of L. monocytogenes to pH 5.0 is capable of eliciting groESL expression.
In a number of other facultative intracellular pathogens, GroEL is clearly expressed following penetration of host cells (2, 9, 35, 49). However, examinations of heat shock gene expression in S. enterica serovar Typhimurium suggest that GroEL expression is increased during growth in J774 cells (9) but not in U937 cells (1). Two previous studies have failed to detect an increase in synthesis of GroEL by L. monocytogenes infecting J774 cells (26) or peritoneal macrophages (31). Both studies utilized pulse-labeling of bacterial proteins following macrophage uptake and two-dimensional gel electrophoresis for protein analysis. In contrast, we have adopted a genetic approach to study the possible role of groESL in pathogenesis. Since GroEL may play a role in cellular physiology even under optimal growth conditions (17), a deletion of groEL would most likely have pleiotropic effects. Instead, we examined transcription of groEL during growth of L. monocytogenes in J774 mouse macrophage cells using two approaches, RT-PCR and GFP technology, that have been used previously to study expression of virulence genes during infection (8, 20). Both approaches show a clear and significant increase in expression of groESL following uptake by macrophages. Infection of J774 cells with the gro-gfp fusion strain results in induction of fluorescence and is comparable to the fluorescence of a plc-gfp control strain as visualized using confocal laser microscopy. Using RT-PCR, a significant increase in bacterial groEL mRNA is evident following infection of J774 cells. Increased expression of the bacterial virulence gene plcA is also apparent. Interestingly, the heat shock gene dnaK is also expressed at elevated levels postinfection. This may reflect a recent finding that DnaK is required for efficient entry into macrophages (26).
While we provide clear evidence for increased transcription of groESL during macrophage infection, as mentioned previously another study failed to detect an increase in GroEL protein in L. monocytogenes infecting the same mouse cell line (25). This may simply be a result of the differences in approaches used. RT-PCR is a sensitive technique capable of detecting slight changes in levels of transcript, whereas larger increases may be necessary for visualization on protein gels. Alternatively, the increased transcription of groESL seen in our study may not result in increased GroES or GroEL translation. In L. monocytogenes, as in L. helveticus, L. zeae, and B. subtilis, the putative start codon for groES is UUG rather than AUG. In other bacterial genera it is likely that non-AUG initiation codons function to limit translation (47). In addition, other mechanisms exist to limit protein expression at the translational level and may play a role during infection. The significant increase in transcription of groESL during infection may not therefore result in a detectable increase in protein levels. This hypothesis will require further investigation.
Utilization of a recently described promoter probe vector system (24) has provided further evidence for increased transcription of groESL during infection. We have constructed a L. monocytogenes strain in which hemolysin is expressed from the promoter region of groESL but not from its native promoter. During infection, hemolysin can therefore function as a reporter of groESL expression. Indeed, the L. monocytogenes gro-hly fusion strain demonstrated increased survival potential in mice relative to a negative control, indicative of expression of hemolysin from the groESL promoter during infection. However, the strain did not reach the same high levels in mouse tissues as a positive control in which hemolysin is expressed from the PrfA-dependent plcA promoter. Similarly, the gro-hly strain demonstrated a significantly increased growth potential in J774 cells relative to the negative control but did not reach the same levels as the plc-hly strain. However, the gro-hly fusion strain exhibited a lag period during which growth did not take place, most likely due to delayed expression of hemolysin from the groESL promoter in bacteria residing within the phagosome. It is envisaged that the application of hemolysin as an in vivo reporter of expression from known promoter regions will provide a useful tool for analysis of in vivo-induced loci.
Our results describe the gene sequence and organization of the groESL operon in L. monocytogenes. The data demonstrate expression from the groESL promoter during infection but possibly at lower levels than specialized virulence factors, such as plcA, which are part of the PrfA regulon. The data suggest that infection represents a significant stress for the bacterium and results in expression of general stress proteins.
ACKNOWLEDGMENTS
We thank Nancy Freitag for providing plasmid pNF579 and Bernice Rea for assistance with the confocal microscopy study.
C.G.M.G. is supported by a Health Research Board Postdoctoral Research Fellowship and by BioResearch Ireland. This work has also been funded by the Food Sub-Programme administered by the Department of Agriculture, Food and Forestry, and is supported by national and EU funds.
REFERENCES
- 1.Abshire K Z, Neidhardt F C. Analysis of proteins synthesized by Salmonella typhimurium during growth within a host macrophage. J Bacteriol. 1993;175:3734–3743. doi: 10.1128/jb.175.12.3734-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albano C R, Randers-Eichhorn L, Chang Q, Bentley W E, Rao G. Quantitative measurement of green fluorescent protein expression. Biotechnol Tech. 1996;10:953–958. [Google Scholar]
- 4.Arnau J, Sørensen K I, Appel K F, Vogensen F K, Hammer K. Analysis of heat shock gene expression in Lactococcus lactis MG1363. Microbiology. 1996;142:1685–1691. doi: 10.1099/13500872-142-7-1685. [DOI] [PubMed] [Google Scholar]
- 5.Beauregard K E, Kyung-Dall L, Collier R J, Swanson J A. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutibonnes P, Giard J C, Hartke A, Thammavongs B, Auffray Y. Characterization of the heat shock response in Enterococcus faecalis. Antonie Leeuwenhoek. 1993;64:47–55. doi: 10.1007/BF00870921. [DOI] [PubMed] [Google Scholar]
- 7.Broadbent J R, Oberg C J, Wei L. Characterization of the Lactobacillus helveticus groESL operon. Res Microbiol. 1998;149:247–253. doi: 10.1016/s0923-2508(98)80300-8. [DOI] [PubMed] [Google Scholar]
- 8.Bubert A, Sokolovic Z, Chun S-K, Papatheodorou L, Simm A, Goebel W. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol Gen Genet. 1999;261:323–336. doi: 10.1007/pl00008633. [DOI] [PubMed] [Google Scholar]
- 9.Buchmeier N A, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- 10.Bunning V K, Crawford R G, Tierney J T, Peeler J T. Thermotolerance of Listeria monocytogenes and Salmonella typhimurium after sublethal heat shock. Appl Environ Microbiol. 1990;56:3216–3219. doi: 10.1128/aem.56.10.3216-3219.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha H J, Srivastava R, Vakharia V N, Rao G, Bentley W E. Green fluorescent protein as a noninvasive stress probe in resting Escherichia coli cells. Appl Environ Microbiol. 1999;65:409–414. doi: 10.1128/aem.65.2.409-414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatfield S N, Strahan K, Pickard D, Charles I, Hormaeche C, Dougan G. Evaluation of Salmonella typhimurium strains harboring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury R, Sahu G K, Das J. Stress response in pathogenic bacteria. J Biosci. 1996;21:149–160. [Google Scholar]
- 14.Cotter P D, Emerson N, Gahan C G M, Hill C. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J Bacteriol. 1999;181:6840–6843. doi: 10.1128/jb.181.21.6840-6843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crameri A, Whitehorn E A, Tate E, Stemmer W P C. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol. 1996;14:315–319. doi: 10.1038/nbt0396-315. [DOI] [PubMed] [Google Scholar]
- 16.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 17.Fayet O, Ziegelhoffer T, Georgopoulos C. The groES and groEL heat shock products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171:1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedio W M, Jackson H. Effect of tempering on the heat resistance of Listeria monocytogenes. Lett Appl Microbiol. 1989;9:157–160. [Google Scholar]
- 19.Flahaut S, Hartke A, Giard J C, Benachour A, Boutibonnes P, Auffray Y. Relationship between stress response toward bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol Lett. 1996;138:49–54. doi: 10.1111/j.1574-6968.1996.tb08133.x. [DOI] [PubMed] [Google Scholar]
- 20.Freitag N E, Jacobs K E. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect Immun. 1999;67:1844–1852. doi: 10.1128/iai.67.4.1844-1852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gahan C G M, Collins J K. Listeriosis: biology and implications for the food industry. Trends Food Sci Technol. 1991;2:89–93. [Google Scholar]
- 22.Gahan C G M, Collins J K. Non-dystrophic 129 REJ mice are susceptible to i.p. infection with Listeria monocytogenes despite an ability to recruit inflammatory neutrophils to the peritoneal cavity. Microb Pathog. 1995;18:355–364. doi: 10.1006/mpat.1995.0032. [DOI] [PubMed] [Google Scholar]
- 23.Gahan C G M, Hill C. The relationship between acid stress responses and virulence in Salmonella typhimurium and Listeria monocytogenes. Int J Food Microbiol. 1999;50:93–100. doi: 10.1016/s0168-1605(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 24.Gahan C G M, Hill C. The use of listeriolysin to identify in vivo induced genes in the Gram-positive intracellular pathogen Listeria monocytogenes. Mol Microbiol. 2000;36:498–507. doi: 10.1046/j.1365-2958.2000.01869.x. [DOI] [PubMed] [Google Scholar]
- 25.Hanawa T, Yamamoto T, Kamiya S. Listeria monocytogenes can grow in macrophages without the aid of proteins induced by environmental stresses. Infect Immun. 1995;63:4595–4599. doi: 10.1128/iai.63.12.4595-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanawa T, Fukuda M, Kawakami H, Hirano H, Kamiya S, Yamamoto T. The Listeria monocytogenes DnaK chaperone is required for stress tolerance and efficient phagocytosis with macrophages. Cell Stress Chaperones. 1999;4:118–128. [PMC free article] [PubMed] [Google Scholar]
- 27.Hartke A, Frère J, Boutibonnes P, Auffray Y. Differential induction of the chaperonin GroEL and the co-chaperonin GroES by heat, acid, and UV-irradiation in Lactococcus lactis subsp. lactis. Curr Microbiol. 1997;34:23–26. doi: 10.1007/s002849900138. [DOI] [PubMed] [Google Scholar]
- 28.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 29.Heithoff D M, Conner C P, Hentschel U, Govantes F, Hanna P C, Mahan M J. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol. 1999;181:799–807. doi: 10.1128/jb.181.3.799-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendrick J P, Hartl F-U. Molecular chaperone functions of heat shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 31.Hévin B, Morange M, Fauve R M. Absence of an early detectable increase in heat-shock protein synthesis by Listeria monocytogenes within mouse mononuclear phagocytes. Res Immunol. 1993;144:679–689. doi: 10.1016/s0923-2494(93)80051-y. [DOI] [PubMed] [Google Scholar]
- 32.Kilstrup M, Jacobsen S, Hammer K, Vogensen F K. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl Environ Microbiol. 1997;63:1826–1837. doi: 10.1128/aem.63.5.1826-1837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 34.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–7018. doi: 10.1128/jb.177.24.7011-7018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin J, Ficht T A. Protein synthesis in Brucella abortus induced during macrophage infection. Infect Immun. 1995;63:1409–1414. doi: 10.1128/iai.63.4.1409-1414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A. New thermostable plasmid for gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marron L, Emerson N, Gahan C G M, Hill C. A mutant of Listeria monocytogenes LO28 unable to induce an acid tolerance response displays diminished virulence in a murine model. Appl Environ Microbiol. 1997;63:4945–4947. doi: 10.1128/aem.63.12.4945-4947.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrath S, Fitzgerald G, van Sinderen D. Improvement and optimization of two engineered phage resistance mechanisms in Lactococcus lactis. Appl Environ Microbiol. 2001;67:608–616. doi: 10.1128/AEM.67.2.608-616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Driscoll B, Gahan C G M, Hill C. Two-dimensional polyacrylamide gel electrophoresis analysis of the acid tolerance response in Listeria monocytogenes LO28. Appl Environ Microbiol. 1997;63:2679–2685. doi: 10.1128/aem.63.7.2679-2685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S F, Stewart G S A B. High efficiency transformation of Listeria monocytogenes by electroporation of penicillin treated cells. Gene. 1990;94:129–132. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 41.Phan-Thanh L, Mahouin F. A proteomic approach to study acid response in Listeria monocytogenes. Electrophoresis. 1999;20:2214–2224. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2214::AID-ELPS2214>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.Phan-Thanh L, Mahouin F, Alige S. Acid responses of Listeria monocytogenes. Int J Food Microbiol. 2000;55:121–126. doi: 10.1016/s0168-1605(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 43.Rouquette C, Ripio M-T, Pellegrini E, Bolla J M, Tascon R I, Vazquez-Boland J A, Berche P. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol Microbiol. 1996;21:977–987. doi: 10.1046/j.1365-2958.1996.641432.x. [DOI] [PubMed] [Google Scholar]
- 44.Salotra P, Singh D K, Seal K P, Krishna N, Jaffe H, Bhatnagar R. Expression of DnaK and GroEL homologs in Leuconostoc esenteroides [sic] in response to heat shock, cold shock or chemical stress. FEMS Microbiol Lett. 1995;131:57–62. doi: 10.1111/j.1574-6968.1995.tb07754.x. [DOI] [PubMed] [Google Scholar]
- 45.Sanders J W, Venema G, Kok J, Leenhouts K. Identification of a sodium chloride-regulated promoter in Lactococcus lactis by single-copy chromosomal fusion with a reporter gene. Mol Gen Genet. 1998;27:299–310. doi: 10.1007/s004380050697. [DOI] [PubMed] [Google Scholar]
- 46.Segal G, Ron E Z. Regulation and organisation of the groE and dnaK operons in Eubacteria. FEMS Microbiol Lett. 1996;138:1–10. doi: 10.1111/j.1574-6968.1996.tb08126.x. [DOI] [PubMed] [Google Scholar]
- 47.Vellanoweth R L. Translation and its regulation. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 699–711. [Google Scholar]
- 48.Wilmes-Riesenberg M R, Bearson B, Foster J W, Curtiss R., III Role of the acid tolerance response in virulence of Salmonella typhimurium. Infect Immun. 1996;64:1085–1092. doi: 10.1128/iai.64.4.1085-1092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto T, Hanawa T, Ogata S. Induction of Yersinia enterocolitica stress proteins by phagocytosis with macrophage. Microbiol Immunol. 1994;38:295–300. doi: 10.1111/j.1348-0421.1994.tb01779.x. [DOI] [PubMed] [Google Scholar]