Abstract
Objective
Our purpose was to explore the relationship between triglyceride glucose (TyG) index and the risk of new-onset hypertension in Chinese individuals aged ≥45 years.
Methods
From 2011 to 2018, data from the China Health and Retirement Longitudinal Survey (CHARLS) were analyzed. The relationship between TyG index and hypertension was assessed utilizing Cox regression and restricted cubic spline (RCS) plot, and the importance of the TyG index in hypertension development was demonstrated by a random forest machine learning model. Finally, subgroup analysis was conducted to test for potential interactions on hypertension development between the TyG index and subgroups.
Results
19.7% of the 4755 individuals who were involved in this survey developed hypertension over an average follow-up period of 5.22 years. Compared with the first quartile of albumin, the multivariate HR (95% CI) for the risk of new-onset hypertension across the TyG index quartiles was 1.09 (0.89, 1.33), 1.09 (0.89, 1.33), and 1.29 (1.06, 1.58), respectively (P for trend <0.001). The RCS plot revealed a linear relationship (P for nonlinear = 0.322), and the random forest machine learning model illustrated that the TyG index was a significant hazard factor on hypertension development. There was no interaction between subgroups and the relationships of the TyG index with the prevalence of hypertension (all P-value >0.05).
Conclusion
TyG index was an independent hazard indicator for new-onset hypertension, and routine measurement and control of TyG index level might be great for preventing hypertension development.
Keywords: triglyceride glucose index, hypertension, risk, CHARLS
Introduction
Hypertension is strongly related to high mortality and morbidity of cardiovascular disease,1–3 which places a substantial burden on public health.4 Moreover, the aggravation tendency of population aging and unfavorable behavior of impairing health spread among people have significantly increased the morbidity of hypertension, which renders the development of hypertension an increasing health concern.3,5,6 To alleviate the medical burden and reduce the incidence of hypertension, we should focus on hypertension management. In addition, the prevention of hypertension is the top public-health priority for hypertension management.3 Therefore, the most effective method for preventing hypertension development may be to address the risk factors that contribute to it.
The triglyceride glucose (TyG) index value is computed utilizing fasting blood-glucose (FBG) and triglycerides (TG), and the equation is as follows: TyG index = Ln [fasting plasma glucose (mg/dL) × fasting triglyceride level (mg/dL)/2].7 Furthermore, the TyG index has been confirmed to be a marker of insulin resistance (IR).8 However, recent studies confirmed that the TyG index could increase cardiovascular disease hazard and result in atherosclerosis and arterial stiffness,9–12 which might be linked to IR.13–15 Moreover, among the many risk factors of hypertension, atherosclerosis and arterial stiffness play a significant role in hypertension development,16–18 and their ability to contribute to development of hypertension may increase with age-related physiological alterations. In addition, researches have also confirmed that the TyG index is strongly correlated with new-onset hypertension.19,20 Accordingly, the TyG index may play a significant part in hypertension development. However, few studies were reported on the relationship between the TyG index and hypertension development among Chinese individuals aged ≥45 years.
Therefore, by analyzing data from the China Health and Retirement Longitudinal Survey (CHARLS), the purpose of the present study is to determine the correlation between the TyG index and hypertension development among Chinese individuals aged ≥45 years.
Methods
Populations
CHARLS, collecting information on social demographics, economic, health status, and blood examination of community residents, is an ongoing large-scale longitudinal survey that enrolls Chinese adults aged ≥45 years or above.21 From June 2011 to March 2012, the baseline data for CHARLS was done. The first wave (W1, 2011–2012) included 10,257 households and 17,708 people from 28 provinces in China, and participants were followed up on every two years. The present study investigated data from a baseline data in 2011 and three follow-up waves, including the second follow-up wave in 2013, the third follow-up wave in 2015, and the fourth follow-up wave in 2018.
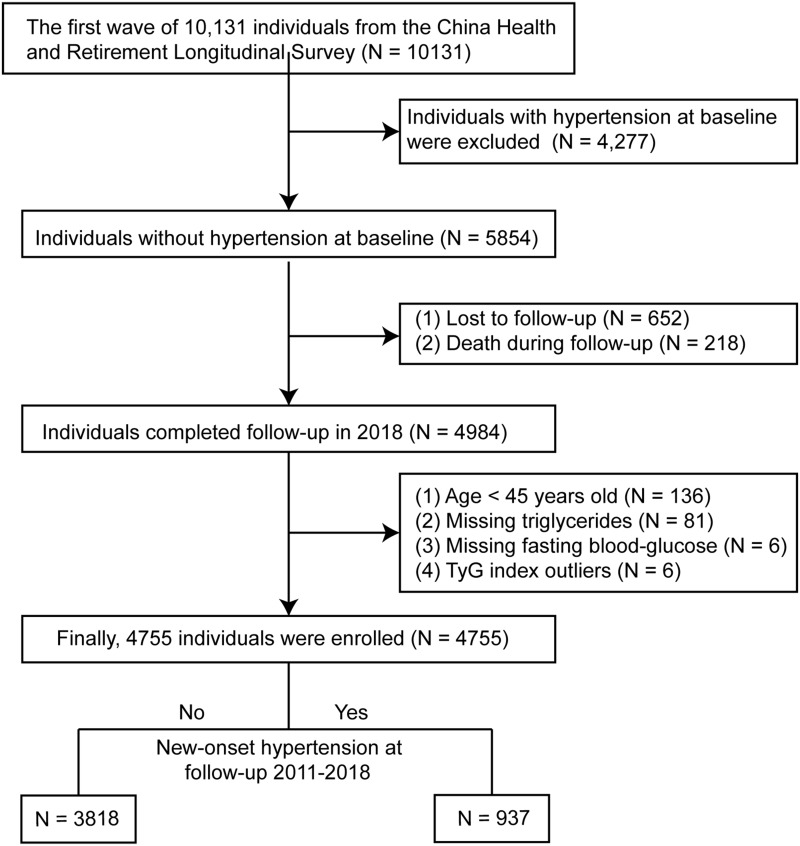
As shown in the flowchart (Figure 1), 10,131 individuals in the W1 were enrolled in the present study, of which 4277 participants have hypertension at baseline. In addition, ofthe remaining 5854 participants, 870 participants were excluded because of loss of follow-up (n = 652) and death (n = 218). After further excluding 229 participants at baseline with age <45 years old, TyG index outliers, and missing data on triglyceride and fasting blood glucose, 4755 people were finally enrolled when followed up to 2018, including 937 participants with hypertension and 3818 participants without hypertension, establishing a hypertension incidence of 19.7% in the present study. The Ethics Review Committee of Peking University approved the CHARLS study (IRB00001052-11015), and all participants have provided informed consent before involvement.
Figure 1.
Flowchart.
Abbreviation: TyG index, triglyceride glucose index.
Collection of Relevant Covariates
Relevant sociodemographic variables include age, gender, health-related behaviors (the presence or absence of current smoking and drinking), marital status (the presence or absence of current marriage), and education level. Anthropometric indicators consisted of systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, height, and weight. In addition, previous medical history was collected, including dyslipidemia, diabetes mellitus (DM), CVD, kidney disease, and chronic lung diseases. The medicine use history was collected, including glucose-lowering drugs and lipid-lowering drugs. In addition, trained qualified investigators collected related sociodemographic variables, anthropometric indicators, previous medical history, and drug use history utilizing standardized questionnaires.
Blood samples were shipped to the Chinese Center for Disease Control and Prevention (China CDC) after collection and placed at −80°C. Determination of laboratory examination indicators, including fasting blood-glucose (FBG), triglycerides (TG), white blood cell (WBC), platelets, blood urea nitrogen (BUN), creatinine, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum uric acid, total cholesterol, glycosylated hemoglobin (HbA1c), hematocrit, high-sensitivity C-reactive protein (hs-CRP), and hemoglobin, was examined by trained qualified researchers.
The Definition of TyG Index and Hypertension
The TyG index was the exposure variable of the present study, computed utilizing FBG and TG, and the formula is as follows: TyG index = Ln [fasting plasma glucose (mg/dL)×fasting triglyceride level (mg/dL)/2].7 Additionally, SBP and DBP were measured by a qualified investigator utilizing an automated sphygmomanometer (Omron HEM-7200 Monitor) after approximately 30 minutes of rest in a chair, with the bottom edge of the cuff approximately 0.5 inches above the elbow. Each measurement was separated by 45–60 seconds, for a total of three measurements. Consequently, SBP and DBP were considered as the average of three measurements. Hypertension was identified by any one of the following three criteria:1 mean SBP ≥140 mmHg or DBP ≥90 mmHg;2 Doctor-diagnosed hypertension;3 Presently taking blood pressure medications.22,23
Statistical Analysis
Because CHARLS is a longitudinal study of sampling, sample weights were taken into account during statistical analysis. We used the “mice” package in R Version 4.1.3 to perform weighted multiple imputation for missing data, and the number of imputations was selected to be 5 times. In addition, in order to ensure the stability of the data after imputation, variables with missing values greater than 10% will be deleted. Baseline characteristics were group in accordance with the TyG index quartiles (quartile 1, quartile 2, quartiles 3, quartile 4) as well as new-onset hypertension presence or absence. Continuous variables were shown by mean with standard deviation. If the test of normality and the homogeneity test of variance are performed, the T-test is appropriate; otherwise, the Wilcoxon test is appropriate, and Kruskal–Wallis test is used for comparison of three or more groups. Categorical variables, shown by the percentage, were compared using the X2 test.
Restricted cubic spline (RCS) plots were made to figure out the dose–response relationship between both the TyG index level and hypertension development, and three knots were chosen at the 25th, 50th, and 75th quartiles. Machine learning could accurately analyze and fit data in the form of mathematical equations without making linear assumptions. Therefore, the importance of the TyG index in hypertension development was analyzed using a random forest machine learning model. Mean decrease accuracy represented the variable importance contributing to new-onset hypertension in the random forest machine learning model, with a greater value corresponding to a greater weight.
Meanwhile, unadjusted and adjusted Cox regression analyses with P for trend were applied to analyze the relationship between both the TyG index level and hypertension development, as well as to assess the association between various interquartile of the TyG index and hypertension development. Model 1 examined the association between both the TyG index and hypertension development without adjusting for confounding variables. Model 2 adjusted to take into account the most common confounding biases, which include age and gender. In model 3, we adjusted for smoke, drink, heart rate, marital status, gender, height, platelets, total cholesterol, LDL-C, HbA1c, and hemoglobin, which were screened out by unadjusted Cox regression analysis with a P-value <0.05 (Supplementary Material 1).
Finally, subgroup analysis of Cox regression was conducted to assess the relationship between TyG index and hypertension development by age, gender, education, the level of FBG and TG, the presence or absence of DM, dyslipidemia, and CVD. The software Stata 15 Version (https://www.stata.com/) and R Studio (Version 4.1.3, https://www.Rproject.org) were conducted to analyze the data, and all P-value <0.05 were statistically significant.
Results
Participants
Finally, 4755 individuals had been enrolled in the present study. The mean age of 4755 participants was 57.20 ± 8.30 years old, and female made up 54.0% of the total population. The incidence of hypertension was 19.7%, and the average value of the TyG index in baseline characteristics was 8.56 ± 0.54. Table 1 shows the baseline characteristics of all participants in accordance with the quartiles of TyG index (quartile 1, quartile 2, quartiles 3, quartile 4). Significant difference was found in the comparison of baseline characteristics based on the TyG index quartiles, except for age, education, marital status, creatinine, chronic lung diseases, cardiovascular disease, and kidney disease. In addition, the prevalence of hypertension in the quartile 1, quartile 2, quartile 3, and quartile 4 groups was 15.5%, 17.8%, 20.1%, and 25.4%, respectively (Table 1).
Table 1.
Baseline Characteristics by the Quartiles of Triglyceride Glucose Index
| Variables | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|
| N = 1189 | N = 1189 | N = 1189 | N = 1188 | ||
| TyG index | 7.90 ± 0.21 | 8.34 ± 0.10 | 8.70 ± 0.12 | 9.29 ± 0.25 | <0.001 |
| Age, years | 57.25 ± 8.61 | 57.55 ± 8.61 | 57.25 ± 8.07 | 56.74 ± 7.86 | 0.125 |
| Female, (%) | 564 (47.4) | 621 (52.2) | 693 (58.3) | 688 (57.9) | <0.001 |
| Height, cm | 158.51 ± 8.28 | 157.92 ± 8.37 | 157.58 ± 8.49 | 158.19 ± 8.25 | 0.044 |
| Weight, kg | 54.94 ± 9.09 | 56.30 ± 9.92 | 57.51 ± 10.26 | 60.76 ± 10.35 | <0.001 |
| Smoke (%) | 507 (42.6) | 456 (38.4) | 432 (36.3) | 426 (35.9) | 0.002 |
| Drink (%) | 357 (30.0) | 316 (26.6) | 266 (22.4) | 299 (25.2) | <0.001 |
| SBP, mmHg | 116.36 ± 11.60 | 117.53 ± 11.40 | 118.60 ± 11.50 | 120.43 ± 11.16 | <0.001 |
| DBP, mmHg | 69.03 ± 8.62 | 70.01 ± 8.93 | 70.93 ± 8.89 | 72.05 ± 8.47 | <0.001 |
| Heart rate, times/min | 70.22 ± 9.73 | 71.46 ± 9.41 | 72.64 ± 9.77 | 73.06 ± 9.70 | <0.001 |
| Education, (%) | 0.726 | ||||
| Primary school or lower | 794 (66.8) | 813 (68.4) | 823 (69.2) | 785 (66.1) | |
| Secondary school | 363 (30.5) | 343 (28.8) | 337 (28.3) | 368 (31.0) | |
| Higher | 32 (2.7) | 33 (2.8) | 29 (2.4) | 35 (2.9) | |
| Marital status, (%) | 1034 (87.0) | 1026 (86.3) | 1039 (87.4) | 1053 (88.6) | 0.371 |
| WBC, ×109 | 5.84 ± 1.62 | 5.94 ± 1.67 | 6.15 ± 1.66 | 6.42 ± 1.72 | <0.001 |
| Platelets, ×109 | 208.28 ± 64.80 | 206.22 ± 67.37 | 210.10 ± 69.67 | 214.00 ± 67.21 | 0.035 |
| BUN, mg/dl | 16.11 ± 4.30 | 15.78 ± 4.13 | 15.11 ± 3.95 | 15.21 ± 3.96 | <0.001 |
| FBG, mg/dL | 95.17 ± 12.03 | 100.62 ± 12.80 | 104.42 ± 14.37 | 113.59 ± 15.33 | <0.001 |
| Creatinine, mg/dl | 0.76 ± 0.16 | 0.76 ± 0.16 | 0.76 ± 0.17 | 0.77 ± 0.17 | 0.369 |
| Total cholesterol, mg/dl | 178.22 ± 32.74 | 187.52 ± 32.83 | 193.15 ± 35.56 | 203.79 ± 38.17 | <0.001 |
| Triglycerides, mg/dl | 58.34 ± 12.08 | 85.26 ± 13.10 | 118.23 ± 20.38 | 196.99 ± 43.50 | <0.001 |
| HDL-C, mg/dL | 60.58 ± 14.07 | 55.28 ± 13.19 | 50.45 ± 12.72 | 42.03 ± 11.90 | <0.001 |
| LDL-C, mg/dL | 107.68 ± 28.26 | 117.04 ± 29.45 | 120.67 ± 31.84 | 114.15 ± 37.99 | <0.001 |
| hs-CRP, mg/dl | 1.22 ± 1.11 | 1.25 ± 1.09 | 1.35 ± 1.13 | 1.50 ± 1.13 | <0.001 |
| HbA1c, % | 5.07 ± 0.38 | 5.10 ± 0.41 | 5.16 ± 0.43 | 5.27 ± 0.48 | <0.001 |
| Serum uric acid, mg/dl | 4.13 ± 1.05 | 4.23 ± 1.12 | 4.26 ± 1.12 | 4.48 ± 1.17 | <0.001 |
| Hematocrit, % | 40.93 ± 6.06 | 40.83 ± 5.94 | 41.11 ± 5.83 | 41.60 ± 5.81 | 0.007 |
| Hemoglobin, g/dl | 13.99 ± 1.93 | 14.07 ± 1.90 | 14.16 ± 1.92 | 14.42 ± 1.91 | <0.001 |
| Dyslipidemia, (%) | 37 (3.1) | 49 (4.1) | 70 (5.9) | 96 (8.1) | <0.001 |
| DM, (%) | 25 (2.1) | 31 (2.6) | 45 (3.8) | 71 (6.0) | <0.001 |
| Chronic lung diseases, (%) | 120 (10.1) | 109 (9.2) | 128 (10.8) | 102 (8.6) | 0.281 |
| Cardiovascular disease, (%) | 82 (6.9) | 90 (7.6) | 92 (7.7) | 110 (9.3) | 0.180 |
| Kidney disease, (%) | 77 (6.5) | 88 (7.4) | 73 (6.1) | 63 (5.3) | 0.210 |
| Lipid-lowering drugs, (%) | 38 (3.2) | 49 (4.1) | 70 (5.9) | 96 (8.1) | <0.001 |
| DM-lowering drugs, (%) | 25 (2.1) | 33 (2.8) | 45 (3.8) | 71 (6.0) | <0.001 |
| New-onset hypertension, (%) | 184 (15.5) | 212 (17.8) | 239 (20.1) | 302 (25.4) | <0.001 |
Note: Data are present as MD ± SD or N (%).
Abbreviations: Q, quartiles; TyG index, triglyceride glucose index; glucose index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; BUN, blood urea nitrogen; FBG, fasting blood-glucose; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; HbA1c, glycosylated hemoglobin; DM, diabetes mellitus; MD, mean deviation; SD, standard deviation.
In addition, compared with the group without new-onset hypertension, individuals of the new-onset hypertension group were older and higher weight and were more likely to have dyslipidemia, DM, CVD, and a higher use ratio of glucose-lowering drugs and lipid-lowering drugs. Besides, the new-onset hypertension group had increased levels of SBP, DBP, heart rate, platelets, FBG, TG, LDL-C, total cholesterol, HbA1c, serum uric acid, hematocrit, hemoglobin, hs-CRP, and TyG index, and decreased HDL-C level (all P-value <0.05). Except for the above variables, all variables were not significant (all P-value >0.05) (Table 2).
Table 2.
Comparison of Baseline Characteristics by the Presence or Absence of New-Onset Hypertension
| Variables | Overall (N = 4755) | Not New-Onset Hypertension (N = 3818) | New-Onset Hypertension (N = 937) | P-value |
|---|---|---|---|---|
| Age, years | 57.20 ± 8.30 | 56.90 ± 8.25 | 58.43 ± 8.37 | <0.001 |
| Female, (%) | 2566 (54.0) | 2063 (54.0) | 503 (53.7) | 0.875 |
| Height, cm | 158.05 ± 8.35 | 158.02 ± 8.28 | 158.16 ± 8.65 | 0.649 |
| Weight, kg | 57.38 ± 10.14 | 56.82 ± 9.99 | 59.65 ± 10.47 | <0.001 |
| Smoke, (%) | 1821 (38.3) | 1467 (38.4) | 354 (37.8) | 0.745 |
| Drink, (%) | 1238 (26.0) | 979 (25.6) | 259 (27.6) | 0.227 |
| SBP, mmHg | 118.23 ± 11.51 | 116.78 ± 11.34 | 124.13 ± 10.25 | <0.001 |
| DBP, mmHg | 70.51 ± 8.80 | 69.71 ± 8.69 | 73.76 ± 8.49 | <0.001 |
| Heart rate, times/min | 71.85 ± 9.71 | 71.58 ± 9.62 | 72.94 ± 10.01 | <0.001 |
| Education, (%) | 0.099 | |||
| Primary school or lower | 3215 (67.6) | 2554 (66.9) | 661 (70.5) | |
| Secondary school | 1411 (29.7) | 1159 (30.4) | 252 (26.9) | |
| Higher | 129 (2.7) | 105 (2.8) | 24 (2.6) | |
| Marital status, (%) | 4152 (87.3) | 3350 (87.7) | 802 (85.6) | 0.086 |
| WBC, ×10^9 | 6.09 ± 1.68 | 6.07 ± 1.66 | 6.14 ± 1.75 | 0.286 |
| Platelets, ×10^9 | 209.65 ± 67.32 | 208.46 ± 66.69 | 214.50 ± 69.68 | 0.014 |
| BUN, mg/dl | 15.55 ± 4.10 | 15.54 ± 4.09 | 15.62 ± 4.16 | 0.573 |
| FBG, mg/dL | 103.45 ± 15.25 | 102.58 ± 14.85 | 106.99 ± 16.31 | <0.001 |
| Creatinine, mg/dl | 0.76 ± 0.16 | 0.76 ± 0.16 | 0.77 ± 0.17 | 0.059 |
| Total Cholesterol, mg/dl | 190.67 ± 36.09 | 189.68 ± 35.53 | 194.67 ± 38.05 | <0.001 |
| Triglycerides, mg/dl | 114.69 ± 57.98 | 112.25 ± 56.75 | 124.63 ± 61.80 | <0.001 |
| HDL-C, mg/dL | 52.09 ± 14.67 | 52.46 ± 14.64 | 50.59 ± 14.72 | <0.001 |
| LDL-C, mg/dL | 114.89 ± 32.44 | 114.21 ± 32.03 | 117.64 ± 33.95 | 0.004 |
| hs-CRP, mg/dl | 1.33 ± 1.12 | 1.30 ± 1.11 | 1.45 ± 1.15 | <0.001 |
| HbA1c, % | 5.15 ± 0.43 | 5.14 ± 0.42 | 5.21 ± 0.47 | <0.001 |
| Serum uric acid, mg/dl | 4.28 ± 1.12 | 4.25 ± 1.11 | 4.38 ± 1.16 | 0.002 |
| Hematocrit, % | 41.12 ± 5.92 | 41.00 ± 5.89 | 41.59 ± 6.00 | 0.007 |
| Hemoglobin, g/dl | 14.16 ± 1.92 | 14.13 ± 1.92 | 14.29 ± 1.92 | 0.025 |
| Dyslipidemia, (%) | 252 (5.3) | 186 (4.9) | 66 (7.0) | 0.010 |
| DM, (%) | 172 (3.6) | 121 (3.2) | 51 (5.4) | 0.001 |
| Chronic lung diseases, (%) | 459 (9.7) | 354 (9.3) | 105 (11.2) | 0.083 |
| Cardiovascular disease, (%) | 374 (7.9) | 281 (7.4) | 93 (9.9) | 0.011 |
| Kidney disease, (%) | 301 (6.3) | 236 (6.2) | 65 (6.9) | 0.437 |
| Lipid-lowering drugs, (%) | 253 (5.3) | 187 (4.9) | 66 (7.0) | 0.011 |
| DM-lowering drugs, (%) | 174 (3.7) | 123 (3.2) | 51 (5.4) | 0.002 |
| TyG index | 8.56 (0.54) | 8.53 (0.53) | 8.67 (0.55) | <0.001 |
Note: Data are present as MD ± SD or N (%).
Abbreviations: TyG index, triglyceride glucose index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; BUN, blood urea nitrogen; FBG, fasting blood-glucose; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; HbA1c, glycosylated hemoglobin; DM, diabetes mellitus; MD, mean deviation; SD, standard deviation.
Association Between Risk of New-Onset Hypertension and TyG Index
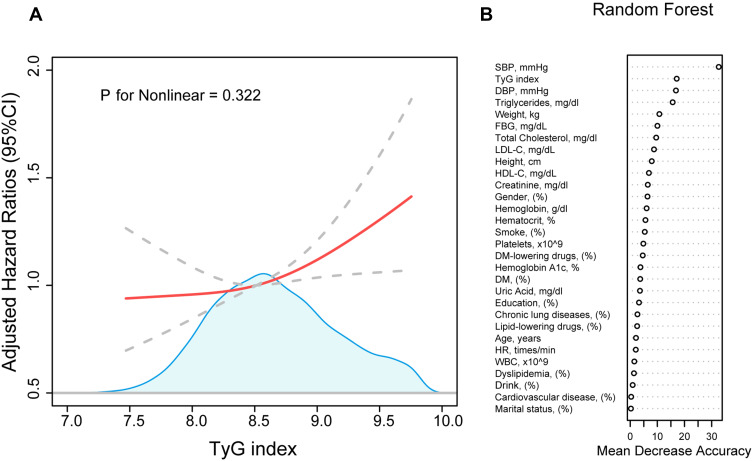
937 participants (19.7%) developed hypertension in 2011–2018, with an average follow-up of 5.22 years. A linear relationship illustrated by the RCS plot was presented between the TyG index level and hypertension development (P for nonlinear = 0.322) (Figure 2A). In addition, the random forest model showed that the TyG index is an important factor for leading to the development of hypertension, and the importance was second only to SBP (Figure 2B).
Figure 2.
The association between TyG index and the development of hypertension. (A) represent a cubic spline model of the relationship between TyG index and the risk of new-onset hypertension after adjustment for smoke, drink, heart rate, marital status, gender, height, platelets, total cholesterol, LDL-C, HbA1c, hemoglobin. (B) represent the importance of TyG index in the development of hypertension by random forest machine learning model.
Abbreviations: TyG index, triglyceride glucose index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood-glucose; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BUN, blood urea nitrogen; WBC, white blood cell; DM, diabetes mellitus.
In addition, when TyG index was analyzed as a continuous variable, TyG index could increase the risk of hypertension development in Model 1, Model 2, and Model 3 with HR of 1.19 (1.06–1.33), 1.17 (1.04, 1.31), and 1.21 (1.05, 1.38), respectively (Table 3). Furthermore, when the TyG index was analyzed as categorical variable (TyG index quartiles), the incidence of hypertension increased with the elevated categories in all the three models (P for trend <0.05). In addition, the adjusted HR (95% CI) for risk of new-onset hypertension across the TyG index quartile was 1.09 (0.89, 1.33), 1.09 (0.89, 1.33), and 1.29 (1.06, 1.58), compared with the first quartile of TyG index (Model 3).
Table 3.
Association Between Triglyceride Glucose Index and the Risk of New-Onset Hypertension
| Case/total | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| HR (95% CI) P | HR (95% CI) P | HR (95% CI) P | ||
| TyG index (per SD increase) | 1.19 (1.06–1.33) 0.003 | 1.17 (1.04, 1.31) 0.008 | 1.21 (1.05, 1.38) 0.006 | |
| Categories | ||||
| Q1 | 184/1189 | Reference | Reference | Reference |
| Q2 | 212/1189 | 1.13 (0.93, 1.38) 0.212 | 1.09 (0.90, 1.33) 0.374 | 1.09 (0.89, 1.33) 0.389 |
| Q3 | 239/1189 | 1.16 (0.96, 1.41) 0.122 | 1.12 (0.92, 1.36) 0.252 | 1.09 (0.89, 1.33) 0.394 |
| Q4 | 302/1188 | 1.32 (1.10, 1.58) 0.003 | 1.27 (1.06, 1.53) 0.011 | 1.29 (1.06, 1.58) 0.011 |
| P for trend | <0.001 | <0.001 | <0.001 | |
Notes: Model 1: none was adjusted. Model 2: age and gender were adjusted. Model 3: smoke, drink, heart rate, marital status, gender, height, platelets, total cholesterol, LDL-C, HbA1c, and hemoglobin were adjusted.
Abbreviations: TyG index, triglyceride glucose index; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood-glucose; HbA1c, glycosylated hemoglobin; HR, hazard ratio; CI, confidence interval; SD, standard deviation; Q, quartiles.
Subgroup Analysis
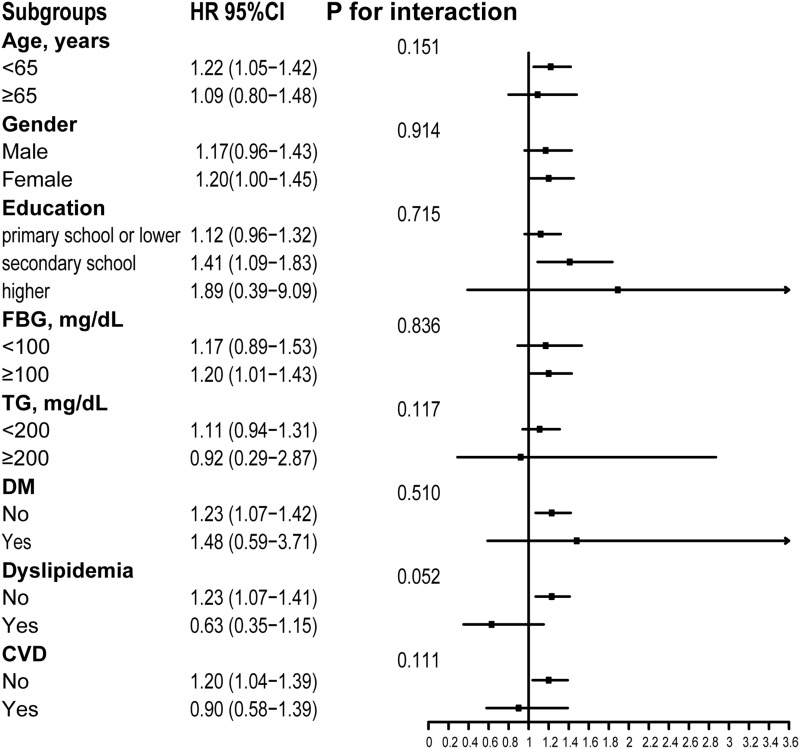
Figure 3 shows that subgroup analyses were utilized to stratify the association between TyG index and new-onset hypertension by age (<65 or ≥65 years), gender, education, the level of FBG (<100 or ≥100 mg/dL) and TG (<200 or ≥200 mg/dL), and the presence or absence of DM, dyslipidemia, and CVD. No interaction was significantly observed between subgroup variables and relationships of TyG index with prevalence of hypertension (all P > 0.05), which indicated that subgroup variables, including age, gender, the level of FBG and TG, and DM, dyslipidemia, CVD or not at baseline, do not affect the relationship between TyG index and hypertension development.
Figure 3.
Subgroups analyses of the relationship between TyG index and new-onset hypertension after adjustment for smoke, drink, heart rate, marital status, gender, height, platelets, total cholesterol, LDL-C, HbA1c, hemoglobin.
Abbreviations: TyG index, triglyceride glucose index; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; CVD, cardiovascular diseases.
Discussion
Currently, hypertension is a major risk factor for CVD mortality and morbidity. Despite the widespread popularization of hypertension treatment,24 the incidence of hypertension continues to rise.6 Regarding the public health management of hypertension, hypertension prevention may be more meaningful than hypertension treatment. In addition, the improvement of public health management regarding cardiovascular risk factors has resulted in a substantial decline in heart disease mortality and morbidity over the past decade.25 Therefore, the improvement of risk factors leading to hypertension development, which may be a crucial step in the public health management of hypertension, could result in a decline in the prevalence of hypertension and contribute to a decrease in medical costs. In addition, we discovered that the TyG index, a marker of IR, was an independent hazard factor for hypertension development after analyzing data from the CHARLS in 2011–2018. Therefore, it may be necessary to observe the prevalence of hypertension by measuring the TyG index routinely.
In addition, the TyG index is a trustworthy replacement to IR and computed using FBG and TG.7,8 Chen et al's study confirmed that TyG could predict independently the development of DM, and the risk of DM was elevated by 22% with an SD of the TyG index.26 The high TyG index level could heighten the risk of atherosclerotic cardiovascular disease.12 In addition, the study discovered that high TyG index level could increase the risk of CVD in DM patients.9 The report indicated that the prevalence of CVD caused by the high level of the TyG index was 1.25-fold that of the low TyG index level.27 Similarly, our study discovered that the TyG index was an independent hazard factor for new-onset hypertension. With each SD increase in the TyG index, the risk of new-onset hypertension increased by 21%. In addition, the random forest model showed the importance of the TyG index in contributing to hypertension development was second only to SBP and DBP. The above statement could explain that the TyG index might play a key part in the pathogenesis of CVD.
Moreover, a longitudinal study conducted by Zheng et al enrolled 4686 individuals aged 20–80 years old to assess the relationship between TyG index and hypertension development, and confirmed that a high TyG index was related to a rising risk of hypertension development.19 However, the findings obtained by the inclusion of their study from a single hospital population were not representative of the prevalence of hypertension in the whole population of China, and subgroup analysis about the relationship between TyG index and hypertension had not been performed, which might affect the stability of the conclusions. In contrast to their study, the data for the present study came from CHARLS, which covered Chinese individuals aged ≥45 years from 28 provinces and cities across the country.21 Moreover, subgroup studies of the correlation between TyG index and hypertension development were conducted to further determine the potential interaction factors. Finally, we determined that age, gender, FBG level, TG level, and the presence or absence of DM, dyslipidemia, and CVD did not influence the relationship either. However, our study might also have some bias. Since we could not obtain whether the lost and dead populations had new-onset hypertension during the follow-up period, this part of the population was excluded. However, the exclusion of lost and dead populations might have introduced a potential and important bias to our study. This might be one of the limitations of the present study.
When it comes to the mechanism, IR might be a major reason for the development of hypertension caused by the TyG index.12,28 Hyperinsulinemia related to IR might contribute to sympathetic excitation, stimulate the release of catecholamine, and subsequently result in an increase in peripheral resistance and cardiac output.29 Also, thickening of vascular smooth muscle caused by high concentrations of catecholamines could contribute to lumen narrowing or hypertension.30 In addition, an increase in the renin-angiotensin-aldosterone system (RAAS) activity caused by IR could lead to water-sodium retention indirectly, which might cause the development of hypertension eventually.31,32 In addition, a greater TyG index indicated faster arterial stiffness development and a rising hazard of vascular damage.10,33 Therefore, from the preceding statement, we could deduce that the TyG index could rise the incidence of hypertension.
In the end, the present study concluded that the TyG index might be an effective predictor of new-onset hypertension in Chinese individuals aged ≥45 years old and that its level should be monitored regularly to prevent hypertension from developing prematurely.
Limitations
The present study was a nationally representative sample. However, some limitations should be found. Firstly, the population of the present study was middle-aged and elderly individuals aged ≥45 years old, and the relationship might apply to adults aged <45 years old. Secondly, daily physical activity might influence blood pressure measurements and primary and secondary hypertension could not differentiate in the present study. Thirdly, 652 patients were lost to follow-up and 218 patients passed away in the present study, which might have introduced a potential and important bias to our study. Fourthly, the potential confounders may not have been adjusted. Therefore, further research is necessary.
Conclusion
The results of the present study demonstrated that the TyG index was an independent risk factor for new-onset hypertension in Chinese middle-aged and elderly individuals aged ≥45 years old. Therefore, the effective management of the TyG index level might be helpful for the prevention of hypertension.
Acknowledgments
Thanks to the National Development Research Institute of Peking University and the Chinese Social Science Research Center of Peking University for providing CHARLS data.
Funding Statement
Social Development Fund of the Jiangsu Provincial Science and Technology Department (Grant number: BE2019639), Research and Practice Innovation Plan for Postgraduates (Grant number: SJCX22_1267), Development Plan Project for traditional Chinese medicine science and technology in Jiangsu (Grant number: YB201988), and Project Fund for the Jiangsu Provincial Health Commission (Grant number: M2020015) supported the present study.
Ethical Approval
The data for this research was obtained from CHARLS database and conform to the Declaration of Helsinki, and this survey had been approved to be conducted by the Peking University’s Ethical Review Committee (Ethical Number: IRB00001052-11015). In addition, this study was also approved by the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Ethical Number: XYFY2022-KL375-01).
Disclosure
All authors declare no conflicting interests in this work.
References
- 1.Zeng XY, Liu SW, Wang LJ, et al. [Mortality and life expectancy that attributable to high blood pressure in Chinese people in 2013]. Zhonghua Liu xing Bing Xue za zhi. 2017;38(8):1011–1016. Chinese. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Lacey B, Clarke R, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176(4):524–532. doi: 10.1001/jamainternmed.2016.0190 [DOI] [PubMed] [Google Scholar]
- 3.He J, Gu D, Chen J, et al. Premature deaths attributable to blood pressure in China: a prospective cohort study. Lancet. 2009;374(9703):1765–1772. doi: 10.1016/S0140-6736(09)61199-5 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Peng X, Nie X, et al. Burden of hypertension in China over the past decades: systematic analysis of prevalence, treatment and control of hypertension. Eur J Prev Cardiol. 2016;23(8):792–800. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Barber RM, Foreman KJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. [DOI] [PubMed] [Google Scholar]
- 7.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. [DOI] [PubMed] [Google Scholar]
- 8.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Z, Xie E, Jiao S, et al. Triglyceride glucose index exacerbates the risk of future cardiovascular disease due to diabetes: evidence from the China Health and Retirement Longitudinal Survey (CHARLS). BMC Cardiovasc Disord. 2022;22(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20(1):146. doi: 10.1186/s12933-021-01342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Zhan A, Huang X, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type hypertension registry study. Cardiovasc Diabetol. 2020;19(1):139. doi: 10.1186/s12933-020-01124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361. doi: 10.1186/s12916-020-01824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108. doi: 10.1186/s12933-017-0589-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SB, Ahn CW, Lee BK, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. doi: 10.1186/s12933-018-0692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva A, Caldas APS, Hermsdorff HHM, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. doi: 10.1186/s12933-019-0893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett DK, Boland LL, Evans GW, et al. Hypertension and arterial stiffness: the atherosclerosis risk in communities study. ARIC investigators. Am J Hypertens. 2000;13(4 Pt 1):317–323. doi: 10.1016/S0895-7061(99)00281-2 [DOI] [PubMed] [Google Scholar]
- 17.Polipanov AG, Mamasaidov ZA, Geleskhanova YN, Cheskidova NB, Romanova TA, Dzhumagulova AS. [Evaluation of arterial stiffness and possibility to predict carotid atherosclerosis in patients with essential hypertension based on an outpatient facility]. Klin Med. 2016;94(3):211–217. Russian. [PubMed] [Google Scholar]
- 18.Pewowaruk RJ, Korcarz C, Tedla Y, et al. Carotid artery stiffness mechanisms associated with cardiovascular disease events and incident hypertension: the multi-ethnic study of atherosclerosis (MESA). Hypertension. 2022;79(3):659–666. doi: 10.1161/HYPERTENSIONAHA.121.18772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16(1):175. doi: 10.1186/s12944-017-0562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jian S, Su-Mei N, Xue C, Jie Z, Xue-Sen W. Association and interaction between triglyceride-glucose index and obesity on risk of hypertension in middle-aged and elderly adults. Clin Exp Hypertens. 2017;39(8):732–739. doi: 10.1080/10641963.2017.1324477 [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004. doi: 10.1097/HJH.0000000000002453 [DOI] [PubMed] [Google Scholar]
- 23.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 24.Goldman L, Cook EF. The decline in ischemic heart disease mortality rates. An analysis of the comparative effects of medical interventions and changes in lifestyle. Ann Intern Med. 1984;101(6):825–836. doi: 10.7326/0003-4819-101-6-825 [DOI] [PubMed] [Google Scholar]
- 25.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874. doi: 10.1001/jama.293.15.1868 [DOI] [PubMed] [Google Scholar]
- 26.Chen CL, Liu L, Lo K, et al. Association between triglyceride glucose index and risk of new-onset diabetes among Chinese adults: findings from the China health and retirement longitudinal study. Front Cardiovasc Med. 2020;7:610322. doi: 10.3389/fcvm.2020.610322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zuo Y, Qian F, et al. Triglyceride-glucose index variability and incident cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):105. doi: 10.1186/s12933-022-01541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lytsy P, Ingelsson E, Lind L, Arnlöv J, Sundström J. Interplay of overweight and insulin resistance on hypertension development. J Hypertens. 2014;32(4):834–839. doi: 10.1097/HJH.0000000000000081 [DOI] [PubMed] [Google Scholar]
- 29.Tack CJ, Smits P, Willemsen JJ, Lenders JW, Thien T, Lutterman JA. Effects of insulin on vascular tone and sympathetic nervous system in NIDDM. Diabetes. 1996;45(1):15–22. doi: 10.2337/diab.45.1.15 [DOI] [PubMed] [Google Scholar]
- 30.Takagi M, Tanaka Y, Yamasaki Y, et al. Responsiveness of insulin-induced cardiac sympathetic nerve activation associates with blood pressure regulation in diabetics. Am J Physiol Endocrinol Metab. 2003;284(5):E1022–6. doi: 10.1152/ajpendo.00169.2002 [DOI] [PubMed] [Google Scholar]
- 31.Saitoh S. [Insulin resistance and renin-angiotensin-aldosterone system]. Nihon Rinsho. 2009;67(4):729–734. Japanese. [PubMed] [Google Scholar]
- 32.Zemel MB. Insulin resistance vs. hyperinsulinemia in hypertension: insulin regulation of Ca2+ transport and Ca(2+)-regulation of insulin sensitivity. J Nutr. 1995;125(6Suppl):1738s–43s. doi: 10.1093/jn/125.suppl_6.1738S [DOI] [PubMed] [Google Scholar]
- 33.Zhao S, Yu S, Chi C, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai study. Cardiovasc Diabetol. 2019;18(1):95. doi: 10.1186/s12933-019-0898-x [DOI] [PMC free article] [PubMed] [Google Scholar]