Abstract
Background
Cerebral arterial stenosis (CAS), in the absence of a structural lesion, can result in cognitive impairment that represents an ongoing contention among studies. Accordingly, we investigated cognitive functions in asymptomatic patients with CAS, using P300 which is a neurophysiological tool. We also compared cognition in intracranial stenosis (ICS) and extracranial stenosis (ECS).
Methods
Asymptomatic patients with CAS (≥ 70%) in the absence of structural brain lesions were categorized into ICS and ECS groups of 15 patients each, in addition to 15 normal controls. MRI, MRA, CT angiography, P300 analysis, Mini-Mental State examination (MMSE), Wisconsin Card Sorting Test (WCST), and Wechsler Memory Scale Test-Revised (WMST) were performed to all patients.
Results
Impairment on all cognitive scales ranged from 70 up to 100% among CAS group. Prolonged p300 latency and reaction time correlated with worse performance on WMST (p = 0.02), while lower amplitude and decreased accuracy correlated with more errors on WCST (p = 0.01). ICS scores on WCTS were lower than those of ECS group (p = 0.001), while ECS had a longer reaction time (p = 0.02) and lower scores on MMS and WMST than those of ICS group (p = 0.03).
Conclusion
Patients with asymptomatic CAS had a high prevalence of cognitive dysfunction which places them at risk of higher morbidity. ICS group showed impairment on executive functions, while the ECS group showed predilection to memory and information processing dysfunction.
Keywords: Cerebral arterial stenosis, Asymptomatic cerebral arterial stenosis, Structural brain lesions, Cognitive dysfunction, Intracranial stenosis
Introduction
Cerebral arterial stenosis (CAS) is known to impact the brain through various mechanisms, whether thromboembolic, luminal narrowing with impaired perfusion, or watershed infarction [1]. This might result in strokes that can either be overt and cause disability or silent so as to elude medical attention [2], 3. Moreover, the impact of CAS on the brain can even be more subtle and result in variable degrees of cognitive impairment, the consequences of which, as well as their management, are still controversial.
Zhao et al. [4] showed that the incidence of cognitive impairment in patients with moderate-to-severe stenosis was markedly higher than controls and could reach 79.7%.
Several other studies have investigated the influence of CAS on cognitive functions in patients with a history of overt as well as in silent cerebral infarctions where, in addition to stroke, the patients were found to suffer from impaired executive function and processing speed [2, 3, 5].
However, in patients with previous strokes, global ischemia due to CAS might not be the main player in cognitive decline since one cannot exclude the direct effect of a strategic infarction on cognition [5].
On the other hand, a systematic review revealed an ongoing debate about the prevalence of cognitive decline in cases of asymptomatic carotid stenosis [6].
Therefore, it seems that a contention still holds concerning the frequency and severity of cognitive decline as a consequence of CAS in the absence of a structural lesion. While some argue whether the benefit of revascularization outweighs its risks [7, 8], others claim that subclinical cognitive dysfunction in patients with CAS places them at risk of mobility problems leading to a 2.86 times greater liability to falls [9]. Thus, early identification of covert cognitive impairment is essential to reduce the risk of institutionalization, morbidity, and mortality in elderly people with asymptomatic CAS. Also, in order to justify any form of intervention for CAS in asymptomatic subjects, we need to document cognitive impairment prior to intervention and furthermore to prove reversibility of impairment following revascularization.
Consequently, in the current study, we recruited patients having severe CAS with no structural brain lesions. All cases were either asymptomatic or had a TIA. Mental functions were assessed by psychometric tests. And since psychometric tests have shown disparity among studies, we also applied a visual odd ball test which is believed to have less practice effect and negligible test–retest variability than traditional scales [10].
Taghavy and Kiigler [11] (need PDF) showed that P300 is related to task processing and is thus an endogenous potential that is more biological and reproducible, with nearly no test–retest variability. We also investigated if intracranial arterial stenosis (ICAS) and extracranial arterial stenosis (ECAS) had a differential effect on cognitive functions.
Methods
This is a cross-sectional case-controlled study where 30 consecutive participants were included. All patients had no history of cognitive impairment or enduring neurological deficit. Twenty-four cases presented with cardiac or peripheral vascular disorders and were referred for carotid duplex, and 6 patients gave a history of TIA. All of the patients had a normal MRI brain. All subjects gave an informed consent for participation, and the approval of the local IRB was obtained.
Patients
The total number of the study population was 45, 15 with intracranial stenosis and 15 with extracranial stenosis and 15 age-matched healthy controls.
Patients were included if they were neurologically asymptomatic or had a history of TIA dating at least 3 months prior to recruitment. All patients had either intra- or extra-cranial severe arterial stenosis (≥ 70%) as diagnosed by MRA and confirmed by CT angiography, and all the patients and controls received at least 12 years of formal education. They were excluded if they or their relatives reported symptoms suggestive of cognitive impairment, concomitant neurological or metabolic disorders that could compromise mental functions, or if they had a history of a symptomatic infarction. Also, patients were excluded if MRI showed silent infarctions or small vessel disease.
Procedures
Neurological history and examination were performed to verify inclusion and exclusion criteria. Laboratory investigations were done for vascular risk factors and echocardiography to exclude patients with low ejection fraction ≤ 50%.
-
Neuroimaging included magnetic resonance imaging (MRI) the brain including FLAIR sequence to ensure the absence of major infarctions and 3D TOF magnetic resonance angiography (MRA) to screen for the presence of intracranial stenosis. Carotid duplex of both carotids and vertebrobasilar system to detect extracranial stenosis.
Patients showing arterial stenosis performed CT angiography for both extracranial and intracranial carotid and vertebrobasilar systems to confirm the presence and the degree of stenosis. CTA was conducted using a 64-slice CT scanner (Light Speed VCT 64- slice Scanner; General Electric, Milwaukee, WI). The degree of stenosis was calculated using the method published for the Warfarin-Aspirin Symptomatic Intracranial Disease Study: percent stenosis = [(1 − (D stenosis/D normal)] × 100, where D stenosis = the diameter of the artery at the site of the most severe stenosis and D normal = the diameter of the proximal normal artery [12].
Neuropsychological assessment included Mini-Mental State examination (MMSE), Wisconsin Card Sorting Test (WCST): to assess prefrontal lobe dysfunction [13], Wechsler Memory Scale Test-Revised (WMST) to assess different memory functions [14].
P300 recording and analysis
Advanced Neuro’s EEGO sports amplifier and caps were used for EEG recording. Advanced Neuro-Technology e-evoked stimulus presentation software was used for stimulus presentation. Matlab — EEGlab/ERP lab (“ERPLAB Toolbox — ERPinfo.ORG,” n.d.) toolbox was used for ERP analysis [15].
EEG recording was performed using a 64-electrode cap with linked mastoid electrodes as a reference. The low-pass filter was 70 Hz, and the high-pass filter was 0.01 Hz with a sample rate 500 Hz, and impedance values were kept below 10 kΩ.
Visual oddball paradigm consisted of 200 trials where either of the two images was randomly presented (a man and a shark); one of the images was the frequent stimulus representing 85% of stimuli while the other was the rare (odd) stimulus representing 15% of stimuli. Each image was displayed on screen for 250 ms with an inter-stimulus interval randomly ranging from 1000 to 1100 ms.
Subjects were instructed to maintain fixation on the screen during the presentation of the stream and to press a button in a hand-held controller whenever they saw the rare stimulus. The timing of each stimulus and button presses was automatically marked and coded on the EEG by the stimulus presenting software.
P300 analysis
The EEG was segmented into epochs of 1200 ms with 200-ms pre-stimulus interval and 1000-ms post-stimulus interval. Baseline correction was applied to the pre-stimulus interval. Moving window technique was used for artifact rejection with a window size of 200 ms and a rejection threshold of 100 µv in all electrodes.
Independent component analysis (ICA) was done in all cases. Individual components were examined to remove eye, muscle, and movement artifacts. This was followed by averaging of all stimuli, and the waves for frequent and rare trials were subtracted, and the resultant wave was studied (frequent wave − rare wave = ERP wave).
P300 wave was identified as the largest positive peak occurring after the N1, P2, and N2 components in frontal to parietal scalp areas. In recordings with a bifurcated peak of Pa and Pb, Pb was considered to represent the P300 wave [16].
Definitions of wave components
Amplitude (μV) is defined as the difference between the mean pre-stimulus baseline voltage and the largest positive-going peak of the ERP waveform within a time window of 200–600 ms. Latency (ms) is defined as the time from stimulus onset to the point of maximum positive amplitude within the time window. Reaction time is calculated since the appearance of the odd-stimulus till controller button press within range of 200–1000 ms. Accuracy is defined as the percent of correct responses to the total number of odd stimuli. These wave components were automatically identified through ERP LAb toolbox to avoid human bias [17].
Patients’ results on cognitive scales were compared to normative data of normal elderly controls [13, 18–20]. And results of P300 odd ball test were compared to age-matched controls performed in the study.
Statistical analysis
Statistical analysis was done using SPSS version 19th version Statistics (SPSS Inc., Chicago). To test for normality of continuous data distribution, the Shapiro-Wilks test was used. Mean and standard deviation were used for normally distributed data, while median and interquartile range (IQR) were used for skewed data. Categorical data were presented as frequencies. An independent samples t-test and one-way ANOVA (with Bonferroni post hoc test) were used to compare normally distributed continuous variable with nominal independent variables. The chi-square test was used for comparison of nominal data. The Pearson correlation coefficient was used to determine the relationship between two quantitative variables and the degree to which the two variables coincide with one another. For comparing patients with normative values for cognitive scales: the scores lying outside normal mean ± 2SD were considered abnormal.
Results
A total of thirty patients were recruited, the mean age was 58 years, and males constituted 36.7%. The commonest risk factors were hypertension and diabetes, followed by dyslipidemia, smoking, and ischemic heart disease as shown in (Table 1).
Table 1.
Descriptive results and clinical characteristics of the patient group
| Variables | Patients (N = 30) | Controls (N = 15) | p value | |
| ICS (N = 15) | ECS (N = 15) | |||
| Age, median (IQR) | 62 (55–72) | 55 (42–65) | 63 (62–65) | 0.09 |
| Male, n. (%) | 33.3% | 40% | 46.7% | 0.8 |
| Hypertension, n. (%) | 73.3% | 73.3% | 80% | 0.9 |
| Diabetes, n. (%) | 73.3% | 73.3% | 66.7% | 0.9 |
| Dyslipidemia, n. (%) | 46.7% | 13.3% | 26.7% | 0.1 |
| Smoking, n. (%) | 13.3% | 26.7% | 33.3% | 0.4 |
| Patients (N = 30) |
Normative values Mean (SD) |
% patients deviated from norm | ||
| MMSE | 24 (3.3) | 27 | 70 | |
| WCST | ||||
| Total error | 46.8 (12.4) | 25.62 ± 20.87 | 83.3 | |
| Perseverative error | 51.6 (12.7) | 14.05 ± 13.43 | 100 | |
| WMST | ||||
| Total score | 39.4 (8.7) | 63 ± 12.6 | 100 | |
| Information | 4.5 (1.4) | 13.4 ± 0.6 | 100 | |
| Orientation | 4.7 (1.3) | |||
| Mental control | 1.9 (1.6) | 4.9 ± 1.2 | 93.3 | |
| Logical memory | 7 (2.8) | 22.5 ± 6.3 | 100 | |
| Digit span total | 7.6 (2) | 14.9 ± 3.3 | 90 | |
| Associate learning | 3.9 (1) | 6.9 ± 1.2 | 100 | |
| P300 latency (ms) | 458.5 (89.6) | 324 (87.1) | 80 | |
| P300 amplitude (µv) | 8.38 (4) | 9.8 (2.8) | 36.6 | |
| P300 reaction time (ms) | 517.8 (50.3) | 447.4 (26.4) | 69.5 | |
| P300 accuracy (%) | 86.9 (9.9) | 95.5 (6.8) | 47.8 | |
ICS intracranial stenosis, ECS extracranial stenosis, IQR interquartile range, MMSE mini mental state examination, WCST Wisconsin card sorting test, WMST Wechsler memory scale test
The mean percentage of arterial stenosis was 88.3% with a predominance of anterior circulation stenosis representing 90% of the whole group.
The patients showed variable degrees of impairment on different cognitive scales and subscales ranging from 70 up to 100% (Table 1).
Eighty percent of patients showed delayed peak latency, and 69.5% showed delayed reaction time of P300, while P300 amplitude and accuracy were relatively less impaired (36.6% and 47.8%; respectively).
Comparison of the whole CAS group with controls and further comparison of ICS with ECS group showed non-significant difference as regards age, gender, degree of stenosis, and risk factors except for dyslipidemia that was more frequent in the ECS group; p: 0.04 (Table 2).
Table 2.
Comparison of demographic data and risk factors of both test groups
| Groups | p value | ||
|---|---|---|---|
| ICS N = 15 |
ECS N = 15 |
||
| Gender (male), % | 6 (40%) | 5 (50%) | 1 |
| Age (years), mean ± SD | 54.06 ± 10.32 | 61.93 ± 10.78 | 0.05 |
| Smoking, % | 4 (26.7%) | 2 (13.3%) | 0.361 |
| DM, % | 11 (73.3%) | 11 (73.3%) | 1 |
| HTN, % | 11 (73.3%) | 11 (73.3%) | 1 |
| ISHD, % | 1 (6.7%) | 5 (33.3%) | 0.068 |
| Dyslipidemia, % | 2 (13.3%) | 7 (46.7%) | 0.04 |
| Degree of stenosis, mean ± SD | 87.2 ± 6.2 | 89.3 ± 5.9 | 0.359 |
ICS Intracranial stenosis, ECS, extracranial stenosis, DM diabetes mellitus, HTN hypertension, ISHD ischemic heart disease
Assessment of cognitive functions in both test groups
The results of all cognitive scales were lower than the cut off values for normal elderly population. It is notable that the ICS group showed relatively worse scores than the ECS group on WCST that was only significant for total errors and perseverative errors (p ≤ 0.001 for each). The group of ECS displayed more decline on MMS, and on all categories of WMST, with a significant difference in mental control and total digital span scores (p = 0.03 for each) (Table 3).
Table 3.
Assessment of cognitive functions in both groups
| Cognitive Scales | ICS (N = 15) | ECS (N = 15) | p value | Normal cut off values |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| MMSE | 24.9 ± 3.7 | 23.1 ± 2.6 | 0.14 | 27 |
| WCST | ||||
| Categories completed | 2.2 ± 1.7 | 2.6 ± 1.6 | 0.52 | 5.07 ± 1.63 |
| Total error | 54.6 ± 8 | 38.4 ± 10.9 | < 0.001 | 25.62 ± 20.87 |
| Perseverative errors | 53.2 ± 13.6 | 49.9 ± 11.9 | < 0.001 | 14.05 ± 13.43 |
| Non-perseverative errors | 11.6 ± 6.6 | 13.1 ± 3.1 | 0.4 | 11.57 ± 9.79 |
| WMST | ||||
| Total score | 32.1 ± 9.3 | 27.7 ± 4.3 | 0.1 | 63 ± 12.6 |
| Information | 4.8 ± 1.5 | 4.3 ± 1.3 | 0.32 | 13.4 ± 0.6 |
| Orientation | 5.0 ± 1. 2 | 4.6 ± 1.2 | 0.38 | |
| Mental control | 2.5 ± 1.7 | 1.3 ± 1.4 | 0.03 | 4.9 ± 1.2 |
| Logical memory | 7.3 ± 3.2 | 6.6 ± 2.4 | 0.51 | 22.5 ± 6.3 |
| Digit Span total score | 8.4 ± 2.5 | 6.8 ± 1.0 | 0.037 | 14.9 ± 3.3 |
| Associate learning | 4.0 ± 1.3 | 3.8 ± 0.4 | 0.65 | 6.9 ± 1.2 |
ICS intracranial stenosis, ECS extracranial stenosis, MMSE mini mental state examination, WCST Wisconsin card sorting test, WMST Wechsler memory scale test
P300 analysis
Comparison of test groups with control group showed significantly prolonged latency, lower accuracy and longer reaction time (p ≤ 0.001, 0.006, and < 0.001; respectively) (Fig. 1). Post hoc analysis to compare each test group to normal showed that ECS group had a significantly longer latency, less percentage of accuracy, and longer reaction time (p ≤ 0.001, 0.005, and < 0.001 respectively). The ICS group showed significantly longer latency and reaction time than the control (p = 0.004 and 0.005 respectively).
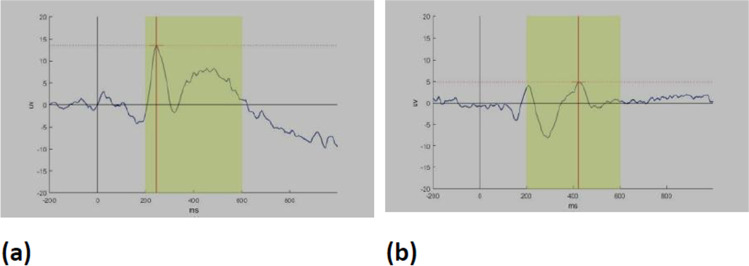
Fig. 1.
a P300 wave in a normal participant. b P300 wave in a patient with CAS showing delayed latency and diminished amplitude
Comparison of both test groups showed that ECS group had a significantly longer reaction time (p = 0.02), with a relatively longer latency and lower accuracy than ICS group (Table 4).
Table 4.
Analysis of P300 in both test groups and control group
| Intracranial stenosis N = 15 |
Extracranial stenosis N = 15 |
Control group N = 15 |
p value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Latency (ms) | 434.8 | 98.0 | 482.2 | 79.8 | 324.0 | 87.1 | < 0.001 |
| Amplitude (µv) | 7.4 | 2.9 | 12.1 | 11.0 | 9.8 | 2.8 | 0.182 |
| Accuracy (%) | 90.0 | 4.9 | 85.2 | 11.1 | 95.5 | 6.8 | 0.006 |
| Reaction time (ms) | 493.1 | 39.6 | 530.9 | 43.4 | 447.4 | 26.4 | < 0.001 |
|
Post hoc Bonferroni |
ICS vs ECS p values |
ICS vs control p values |
ECS vs control p values |
||||
| Latency (ms) | 0.45 | 0.004 | < 0.001 | ||||
| Accuracy (%) | 0.35 | 0.22 | 0.005 | ||||
| Reaction time(ms) | 0.02 | 0.005 | < 0.001 | ||||
ICS intracranial stenosis, ECS extracranial stenosis
Correlation between P300 parameters and neuropsychological scales showed that P300 latency negatively correlated with total Wechsler Memory Score, logical memory, and digital span: p = 0.02, 0.02 and 0.04; respectively. P300 amplitude negatively correlated with perseverative errors: p = 0.01. P300 reaction time negatively correlated with total Wechsler memory score, mental control, and digital span: p = 0.007, 0.002, and 0.02. P300 accuracy negatively correlated with non-perseverative errors (Table 5).
Table 5.
Correlation between P300 parameters and neuropsychological scales (WMST, WCST)
| Cognitive scales | P300 Latency | P300 amplitude | P300 reaction time | P300 accuracy | |
|---|---|---|---|---|---|
| MMSE | Pearson | − .299 | − .279 | − .238 | − .216 |
| Sig | 0.1 | 0.13 | 0.2 | .252 | |
| Categories completed | Pearson | − .117 | .155 | − .098 | − .258 |
| Sig | 0.53 | 0.4 | 0.6 | .169 | |
| Perseverative errors | Pearson | − .128 | − .455 | − .002 | − .014 |
| Sig | 0.5 | 0.01 | 0.9 | .941 | |
| Non-perseverative errors | Pearson | − .188 | .026 | .311 | − .410 |
| Sig | 0.32 | 0.89 | 0.09 | 0.025 | |
| Total score (WMST) | Pearson | − .414 | − .155 | − .486 | .071 |
| Sig | 0.02 | 0.4 | 0.007 | 0.7 | |
| Information | Pearson | − .283 | .020 | − .272 | − .139 |
| Sig | 0.12 | 0.9 | 0.14 | 0.46 | |
| Orientation | Pearson | − .248 | .220 | − .302 | .112 |
| Sig | 0.18 | 0.24 | 0.1 | 0.55 | |
| Mental control | Pearson | − .250 | − .324 | − .536 | .079 |
| Sig | 0.18 | 0.08 | 0.002 | 0.67 | |
| Logical memory | Pearson | − .415 | − .200 | − .361 | .067 |
| Sig | 0.02 | 0.28 | 0.05 | 0.72 | |
| Digit Span total score | Pearson | − .369 | − .107 | − .424 | .106 |
| Sig | 0.045 | 0.57 | 0.02 | 0.57 | |
| Associate learning | Pearson | − .098 | − .200 | − .097 | .004 |
| Sig | 0.6 | 0.29 | 0.6 | 0.98 | |
MMSE mini mental state examination, WCST Wisconsin card sorting test, WMST Wechsler memory scale test
To sum up:
Prolonged latency and prolonged reaction time correlated with worse performance on WMST.
Lower amplitude and decreased accuracy correlated with more errors on WCST.
Discussion
Previous research has shown discordant results concerning the impact of CAS on cognition. While various studies showed that CAS was significantly associated with bad performance on executive functions and memory [21–23], others found no such association [24, 25].
In the current study, all the patients had severe stenosis, and their cognitive scores were markedly inferior to normative scores, despite that none of them complained of cognitive impairment. Similar findings were described by Jackson and colleagues [26] and others [22]. Liu et al. reported that up to 79.7% of his cohort of ICS had cognitive dysfunction [27].
The controversial results for cognitive affection with CAS could be explained in the context of variation in cerebral vasomotor reactivity among patients with asymptomatic stenosis. Patients with impaired reactivity were more liable to have cognitive impairment [28]. Moreover, those with reduced perfusion had a worse performance on MMSE test. However, others attributed cognitive deterioration in patients with CAS to non-disabling strokes [5].
In the current study, the impact of stroke or any structural lesion on cognition has been precluded by history and by MRI. Thus, any degree of impairment could be ascribed to the global effect of CAS.
All the patients showed variable degrees of impairment of executive as well as memory functions. We should however be conservative while interpreting low scores on memory tests that are observed in the presence of impaired mental control. Mental control measures attentional abilities; thus, the defective memory performance could be ascribed to inadequate information acquisition [14].
This uncertainty could be verified by the P300 study which is a more objective electrophysiological test, less dependent on patients’ motivation, and less liable to learning effect. P300 reflects information processing which, according to the information processing theory, is an integral part of memory function, whether sensory, working, or long-term memory [29]. Similarly, other researchers employed different neurophysiological tools as transcranial magnetic stimulation to study the therapeutic effect of pharmacological intervention on cognitive functions in patients with mild cognitive impairment [30].
We included an age-matched normal control group to avoid the reported variability of P300 parameters according to age and literacy, in addition to the paucity of research that targets healthy elderly [31]. Compared to normal controls, patients with CAS showed prolonged P300 latency and reaction time which reflect delayed information processing, while accuracy and amplitude, which refer to restricted attention and concentration, were less impaired. This discrepancy might denote actual impairment of information processing rather than attention. Similar findings were reported where clinically non-demented patients with high-grade stenosis had longer latency and lower amplitude [32] that correlated with cognitive testing [10].
Delayed P300 latency and prolonged reaction time, in our patients, were correlated with bad performance on WMST. P300 latency is known to represent information processing, and delayed elements of this endogenous wave are related to memory dysfunction [10].
On the other hand, reduced amplitude and accuracy were correlated with WCST. Amplitude and accuracy reflect impaired attention which is an integral part of executive functions [33]. These findings were similarly reported showing that different parameters of P300 wave coincided with cognitive testing [33, 34].
Consequently, we can assume that in our patients, both processes of mental control and memory function are defective.
When the patients were compared according to the site of stenosis, the ICS group had more impairment on executive functions, while the ECS group showed more memory dysfunction. Also, while both groups were defective on visual oddball testing, ECS had a significantly worse performance. It has been previously demonstrated that severe ECS can compromise the development of collaterals that are required to compensate for hypoperfusion [8] and might result in a state of global dys-autoregulation [35].
On the other hand, ICS being more distal, allows for such collaterals to develop from the patent proximal sites [36]. Thus, ECS is expected to result in more cognitive dysfunction than ICS.
Finally, we would like to highlight that in order to explore the reversibility of cognitive dysfunction in this group of patients, they receive revascularization by angioplasty. The impact of this intervention on cognitive functions will be reported in another article.
Conclusion
Patients with asymptomatic CAS have a high prevalence of cognitive dysfunction which places them at risk of higher morbidity. ICS group had more impairment on executive functions, while the ECS group showed more memory and information processing dysfunction. Early detection of stenosis with significant effect on cognition, even before the incidence of stroke, would maximize the clinical benefit of vascular interventions in these patients.
Study limitations
Among the limitations of this research is the lack of perfusion studies for cases. This would have added valuable information about the state of global brain perfusion in asymptomatic patients and its correlation with cognitive impairment.
Author contribution
Nevine El Nahas: design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content. Amr Zaki: data collection and analysis including statistical analysis, drafting and revision of manuscript. Magd Zakaria: design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content. Azza Abd El Naser: data analysis, drafting and revision of manuscript. Ahmed El Bassiony: data collection and analysis including statistical analysis, drafting and revision of manuscript. Eman Abdeldayem: design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content. Hossam Shokri: design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content. Ahmed El Bokl: design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Conflict of interest
None.
Ethical approval
Ethical approval was obtained from Research Ethics Committee, Faculty of Medicine, Ain Shams University. No. of ethical approval is FWA 000017585.
Informed consent
Consent was obtained from all patients and controls to participate in the study.
Sponsor’s role
N/A.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nevine El Nahas, Email: nevine_elnahas@med.asu.edu.eg.
Amr Zaki, Email: dr_amrsaeed_2012@hotmail.com.
Magd Zakaria, Email: magdzakaria@live.com.
Azza Abd El Naser, Email: dr-azzasaeed@outlook.com.
Ahmed El Bassiony, Email: ahmedelbassiony@gmail.com.
Eman Abdeldayem, Email: Eman.abdeldayem84@gmail.com.
Hossam Shokri, Email: hossam.shokri@med.asu.edu.eg.
Ahmed El Bokl, Email: ahmed.elbokl@med.asu.edu.eg.
References
- 1.Yaghi S, Prabhakaran S, Khatri P, Liebeskind DS. Intracranial atherosclerotic disease: mechanisms and therapeutic implications. Stroke. 2019;50(5):1286–1293. doi: 10.1161/STROKEAHA.118.024147. [DOI] [PubMed] [Google Scholar]
- 2.Gong L, Wang H, Dong Q, et al. Intracranial atherosclerotic stenosis is related to post-stroke cognitive impairment: a cross-sectional study of minor stroke. Curr Alzheimer Res. 2020;17(2):177–184. doi: 10.2174/1567205017666200303141920. [DOI] [PubMed] [Google Scholar]
- 3.Azeem F, Durrani R, Zerna C, Smith EE. Silent brain infarctions and cognition decline: systematic review and meta-analysis. J Neurol. 2020;267(2):502–512. doi: 10.1007/s00415-019-09534-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhao M, Liu M, Nie Z, Lu Z, Jin L, Li Y. Correlation of moderate-to-severe intracranial arterial stenosis load with low cerebral perfusion, white matter hyperintensity, and cognitive dysfunction in patients without strokes. Int J Clin Exp Med. 2019;12(5):5051–5059. [Google Scholar]
- 5.Luo RT, Wang PJ, Deng XF, et al. An integrated analysis of risk factors of cognitive impairment in patients with severe carotid artery stenosis. Biomed Environ Sci. 2018;31(11):797–804. doi: 10.3967/bes2018.107. [DOI] [PubMed] [Google Scholar]
- 6.Chang XL, Zhou HQ, Lei CY, et al. Association between asymptomatic carotid stenosis and cognitive function: a systematic review. Neurosci Biobehav Rev. 2013;37(8):1493–1499. doi: 10.1016/j.neubiorev.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Kolb B, Fadel H, Rajah G, Saber H, Luqman A, Rangel-Castilla L. Effect of revascularization on cognitive outcomes in intracranial steno-occlusive disease: a systematic review. Neurosurg Focus. 2019;46(2):1–9. doi: 10.3171/2018.11.FOCUS18517. [DOI] [PubMed] [Google Scholar]
- 8.Schröder J, Heinze M, Günther M, et al (2019) Dynamics of brain perfusion and cognitive performance in revascularization of carotid artery stenosis. NeuroImage Clin. 22(October 2018). 10.1016/j.nicl.2019.101779 [DOI] [PMC free article] [PubMed]
- 9.Gray VL, Goldberg AP, Rogers MW, et al. Asymptomatic carotid stenosis is associated with mobility and cognitive dysfunction and heightens falls in older adults. J Vasc Surg. 2020;71(6):1930–1937. doi: 10.1016/j.jvs.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghavy A, Hamer H. Symptomatic and asymptomatic high-grade unilateral internal carotid artery stenosis: scalp topography of event-related potentials (P300) and psychometric testing. Electroencephalogr Clin Neurophysiol. 1995;94(3):163–174. doi: 10.1016/0013-4694(94)00241-C. [DOI] [PubMed] [Google Scholar]
- 11.Taghavy A, Kügler CFA. The pattern flash elicited p300-complex (PF-p300): a new method for studying cognitive processes of the brain. Int J Neurosci. 1988;38(1–2):179–188. doi: 10.3109/00207458809000495. [DOI] [PubMed] [Google Scholar]
- 12.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. Am J Neuroradiol. 2000;21(4):643–646. [PMC free article] [PubMed] [Google Scholar]
- 13.Tombaugh TN, McIntyre NJ (1992) The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc 40(9):922–935 [DOI] [PubMed]
- 14.Tulsky DS, Chiaravalloti ND, Palmer B, Chelunge GJ (2003) The Wechsler Memory Scale, Third Edition. Clinical Interpretation of the WAIS-III and WMS-III. Published online. 93–139
- 15.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Elting JW, Van Weerden TW, Van der Naalt J, De Keyser JHA, Maurits NM. P300 component identification using source analysis techniques: reduced latency variability. J Clin Neurophysiol. 2003;20(1):26–34. doi: 10.1097/00004691-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Calderon J, Luck SJ (2014) ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. Published online.10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed]
- 18.Yang C, Sun X, Tao W, et al. Multistage grading of amnestic mild cognitive impairment: the associated brain gray matter volume and cognitive behavior characterization. Front Aging Neurosci. 2017;8(JAN):1–8. doi: 10.3389/fnagi.2016.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- 20.Elwood RW. The Wechsler Memory Scale—Revised: Psychometric characteristics and clinical application. Neuropsychol Rev. 1991;2:179–201. doi: 10.1007/BF01109053. [DOI] [PubMed] [Google Scholar]
- 21.Lim MJR, Tan CS, Gyanwali B, Chen C, Hilal S. The effect of intracranial stenosis on cognitive decline in a memory clinic cohort. Eur J Neurol. 2021;28(6):1829–1839. doi: 10.1111/ene.14788. [DOI] [PubMed] [Google Scholar]
- 22.Paraskevas KI, Faggioli G, Ancetti S, Naylor AR. Editor’s choice – asymptomatic carotid stenosis and cognitive impairment: a systematic review. Eur J Vasc Endovasc Surg. 2021;61(6):888–899. doi: 10.1016/j.ejvs.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Yan Z, Liang Y, Shi J, et al. Carotid stenosis and cognitive impairment amongst older Chinese adults living in a rural area: a population-based study. Eur J Neurol. 2016;23(1):201–204. doi: 10.1111/ene.12906. [DOI] [PubMed] [Google Scholar]
- 24.Zwartbol MHT, van der Kolk AG, Ghaznawi R, van der Graaf Y, Hendrikse J, Geerlings MI. Intracranial atherosclerosis on 7T MRI and cognitive functioning: the SMART-MR study. Neurology. 2020;95(10):e1351–e1361. doi: 10.1212/WNL.0000000000010199. [DOI] [PubMed] [Google Scholar]
- 25.Lal BK, Dux MC, Sikdar S, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg. 2017;66(4):1083–1092. doi: 10.1016/j.jvs.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Jackson DC, Sandoval-Garcia C, Rocque BG, et al. Cognitive deficits in symptomatic and asymptomatic carotid endarterectomy surgical candidates. Arch Clin Neuropsychol. 2016;31(1):1–7. doi: 10.1093/arclin/acv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M, Nie ZY, Li RR, et al. Correlation of brain perfusion with white matter hyperintensity, brain atrophy, and cognition in patients with posterior cerebral artery stenosis and subjective cognitive decline. Med Sci Monit. 2018;24:5729–5738. doi: 10.12659/MSM.909188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buratti L, Balucani C, Viticchi G, et al. Cognitive deterioration in bilateral asymptomatic severe carotid stenosis. Stroke. 2014;45(7):2072–2077. doi: 10.1161/STROKEAHA.114.005645. [DOI] [PubMed] [Google Scholar]
- 29.Çeliköz N, Erişen Y, Şahin M (2019) Cognitive learning theories with emphasis on latent learning, gestalt and information processing theories. Journal of Educational and Instructional Studies in the World 9(3).
- 30.Martorana A, Di Lorenzo F, Manenti G, Semprini R, Koch G. Homotaurine induces measurable changes of short latency afferent inhibition in a group of mild cognitive impairment individuals. Front Aging Neurosci. 2014;6(SEP):1–7. doi: 10.3389/fnagi.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavarini SCI, Brigola AG, Luchesi BM, et al. On the use of the P300 as a tool for cognitive processing assessment in healthy aging a review. Dement Neuropsychol. 2018;12(1):1–11. doi: 10.1590/1980-57642018dn12-010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiigler CFA, Vlajic P, Funk H, Raithel D, Platt D. The event-related P300 potential approach to cognitive functions of nondemented patients with cerebral and peripheral arteriosclerosis. J Am Geriatr Soc. 1995;43(11):1228–1236. doi: 10.1111/j.1532-5415.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 33.Sachs G, Anderer P, Margreiter N, Semlitsch H, Saletu B, Katschnig H. P300 event-related potentials and cognitive function in social phobia. Psychiatry Res - Neuroimaging. 2004;131(3):249–261. doi: 10.1016/j.pscychresns.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Q, Dong X, Ruan C, et al. Cognitive impairment in Chinese IIDDs revealed by MoCA and P300. Mult Scler Relat Disord. 2017;16(May):1–7. doi: 10.1016/j.msard.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Reinhard M, Gerds TA, Grabiak D, et al. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol. 2008;255(8):1182–1189. doi: 10.1007/s00415-008-0865-z. [DOI] [PubMed] [Google Scholar]
- 36.Chen YF, Tang SC, Wu WC, Kao HL, Kuo YS, Yang SC. Alterations of cerebral perfusion in asymptomatic internal carotid artery steno-occlusive disease. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-02094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]