Abstract
Respiratory infection by Actinobacillus pleuropneumoniae causes a highly pathogenic necrotizing pleuropneumonia with severe edema, hemorrhage and fever. Acute infection is characterized by expression of inflammatory cytokines, including interleukin-1 (IL-1), IL-6 and IL-8. To determine if high level production of inflammatory cytokines contributed to disease pathogenesis, we investigated if inhibiting macrophage activation with adenovirus type 5-expressed IL-10 (Ad-5/IL-10) reduced the severity of acute disease. Porcine tracheal epithelial cells infected with Ad-5/IL-10 produced bioactive human IL-10. When pigs were intratracheally infected with A. pleuropneumoniae, pigs pretreated with Ad-5/IL-10 showed a significant reduction in the amount of lung damage when compared to adenovirus type 5-expressing β-galactosidase (Ad-5/β-Gal)-treated and untreated pigs. In addition, serum zinc levels were unchanged, the lung weight/body weight ratio (an indicator of vascular leakage) was significantly reduced, and lung pathology scores were reduced. Myeloperoxidase activity in lung lavage fluid samples, an indicator of neutrophil invasion, was decreased to levels similar to that seen in pigs not infected with A. pleuropneumoniae. Reduction in inflammatory cytokine levels in lung lavage fluid samples correlated with the clinical observations in that pigs pretreated with Ad-5/IL-10 showed a corresponding reduction of IL-1 and tumor necrosis factor (TNF) compared with untreated and Ad-5/β-Gal-treated pigs. IL-6 levels were unaffected by pretreatment with Ad-5/IL-10, consistent with observations that IL-6 was not derived from alveolar macrophages. Since inflammatory cytokines are expressed at high levels in acute bacterial pleuropneumonia, these results indicate that macrophage activation, involving overproduction of IL-1 and TNF, is a prime factor in infection-related cases of massive lung injury.
Overproduction of inflammatory cytokines has been suggested to be a major factor in several diseases that are associated with tissue damage. This is especially true in various models of pulmonary disease, such as sepsis-induced lung injury and acute respiratory distress syndrome (34, 35, 38). In pigs, one such disease is pleuropneumonia caused by the gram-negative bacterium Actinobacillus pleuropneumoniae (25). This disease is characterized by high fever and respiratory distress, with 10 to 30% of infected animals dying within 24 to 48 h and the remainder obtaining a chronic persistent infection (10, 30). Lungs from pigs infected with A. pleuropneumoniae show necrosis, fibrin, hemorrhagic lesions, and pleurisy (25, 27, 30). Lesioned areas of the lungs have high numbers of neutrophils in the alveolar sacs (19, 20, 25). These neutrophils are thought to be responsible for the lung damage associated with pleuropneumonia (34).
In pigs experimentally challenged with A. pleuropneumoniae, high levels of inflammatory cytokines are present in lung lavages. The primary source of tumor necrosis factor (TNF), interleukin-1 (IL-1), and IL-8 is the alveolar macrophage, which is the predominant immune cell in the lung (4, 21, 29). IL-6 is produced in lung tissue (4). IL-1 and IL-6 bioactivities in lung lavage fluid samples of A. pleuropneumoniae-infected pigs are elevated 1,000-fold (4, 16). TNF alpha (TNF-α) levels also may be profoundly elevated but show greater variation (4, 16). Because TNF and IL-1 induce vascular permeability, injure endothelial cells, and cause death in sepsis-induced lung injury (11, 34), we hypothesize that the overproduction of inflammatory cytokines plays a key role in the lung damage associated with pleuropneumonia.
IL-10 is an anti-inflammatory cytokine that is produced by T cells, B cells, monocytes, and macrophages and which functions generally to suppress immune and inflammatory reactions (26, 32). It not only inhibits TNF-α and IL-1β expression directly in alveolar macrophages but also up-regulates other anti-inflammatory cytokines, including IL-1Ra from fibroblasts and sTNF-RI (12, 13, 36). The anti-inflammatory and immunoregulatory properties of IL-10 are consistent with a therapeutic role for IL-10 in diseases characterized by exuberant production of inflammatory cytokines (17).
To test the hypothesis that macrophage activation, resulting in the excessive production of inflammatory cytokines, exacerbates the pathogenesis of bacterial lung infection, IL-10 was administered to the lung via a recombinant, replication-deficient adenovirus which expressed IL-10. The results showed that IL-10 expression in the lung substantially reduced inflammatory cytokine expression and pathological signs of bacterial pleuropneumonia.
MATERIALS AND METHODS
Pigs.
Four- to six-week-old (8 to 10 kg) male pigs were obtained from a pleuropneumonia-negative commercial herd. Animals were anesthetized with telazole (10 mg/kg of body weight) (Fort Dodge Laboratories, Fort Dodge, Iowa) and treatments were administered intratracheally via the CO2 port of an ETCO2 tracheal tube (Sheridan Catheter Corp., Argyle, N.Y.) as previously described (3).
Pigs were randomly assigned to six groups (Table 1). Pigs were intratracheally instilled with 5 ml of 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (control pigs) or 5 ml of 5% BSA in PBS containing 1010 PFU of either nonreplicating adenovirus type 5 containing the lacZ gene (Ad-5/β-galactosidase [Ad-5/β-Gal]) or the human IL-10 gene (Ad-5/IL-10) per pig. Pigs were monitored for clinical signs daily for 6 days. On the sixth day, 5 ml of 5% BSA in PBS (control pigs) or 5 ml of 5% BSA in PBS containing 104 CFU of A. pleuropneumoniae was instilled intratracheally. Twenty-four hours after instillation of the bacteria, pigs were sacrificed. Euthanasia was performed with Beuthanasia-D Special (Schering-Plough Animal Health, Kenilworth, N.J.). Treatment groups were housed in separate isolation rooms throughout the experiment. All animal protocols were approved by the Institutional Animal Care and Use Committee, University of Minnesota.
TABLE 1.
Effect of IL-10 treatment on serum zinc levels in pigs infected with A. pleuropneumoniae
| Group | Treatmenta | Zinc level in serum (ppm) ate:
|
||
|---|---|---|---|---|
| Adenovirus treatment | A. pleuropneumoniae instillation | Harvest | ||
| 1 | None | 1.02 ± 0.14 | 1.03 ± 0.01b | 1.01 ± 0.09 |
| 2 | A. pleuropneumoniae | 1.12 ± 0.01 | 1.02 ± 0.12 | 0.66 ± 0.08c |
| 3 | Ad-5/β-Gal | 1.07 ± 0.07 | 0.93 ± 0.10b | 0.91 ± 0.05 |
| 4 | Ad-5/β-Gal and A. pleuropneumoniae | 1.02 ± 0.28 | 1.02 ± 0.13 | 0.65 ± 0.12c |
| 5 | Ad-5/IL-10 | 1.20 ± 0.23 | 0.96 ± 0.11b | 0.92 ± 0.07d |
| 6 | Ad-5/IL-10 and A. pleuropneumoniae | 0.93 ± 0.11 | 1.07 ± 0.37 | 0.81 ± 0.17d |
Blood samples were taken prior to the indicated treatment.
No statistical difference among the three groups (P > 0.05, one-tailed, unpaired t-test).
Statistically lower serum zinc (P < 0.05 [one-tailed, unpaired t test]) than the corresponding non-A. pleuropneumoniae treated group.
No statistical difference between these two groups (P > 0.05, one-tailed, unpaired t test).
At 6 days after adenovirus treatment, animals in groups 2, 4, and 6 were challenged with A. pleuropneumoniae. Lungs were harvested 24 h later. Results are means ± standard errors of the means.
Adenoviruses, A. pleuropneumoniae inoculation, and cell culture.
Nonreplicating Ad-5/β-Gal and Ad-5/IL-10 driven by the Rous sarcoma virus early promoter were supplied by Beverly Davidson (Vector Core Laboratory, University of Iowa, Iowa City). Virus titers were determined with a limiting-dilution assay on 293 cells (23). Porcine tracheal epithelial (PTE) cells were supplied by Lance Bouen (University of Minnesota, St. Paul, Minn.) and were maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS).
In vivo inoculations utilized A. pleuropneumoniae, serotype 1, strain L91-2, a field strain isolated from a disease outbreak in Sioux City, Iowa. Bacteria were streaked onto Casman's agar plates containing 0.5% β-NAD. Bacteria were grown overnight at 37°C in 5% CO2. A single colony was picked and used to inoculate 3% tryptic soy broth containing 1% NZ amine, 0.1% yeast extract, and 0.001% β-NAD. Log-phase growing bacteria were washed one time with PBS and resuspended in PBS containing 5% BSA. Animals received 5 × 104 CFU in a volume of 5 ml per inoculation. Bacterial plate counts were performed immediately after instillation to verify the number of viable bacteria.
PTE cells were treated with Ad-5/IL-10 at a multiplicity of infection of 10:1 in six-well plates (1.5 × 105 cells/well) in a total volume of 0.5 ml DMEM–10% FBS for 2 h at 37°C. Control wells were treated with medium only. After 2 h, the cells were washed three times with DMEM–10% FBS and overlaid with 1 ml of DMEM–10% FBS. Day 0 samples were removed immediately after addition of medium (day 0) and at daily intervals up to 10 days postinfection. After removal, the medium was frozen in dry ice-ethanol and stored at −70°C. RNA was processed immediately after removal of the medium (see below).
Clinical and gross pathology.
Pigs were observed for signs of sickness throughout the experiment. Signs included diarrhea, coughing, nasal discharge, respiratory distress, clinical depression, inappetence, and inactivity.
Percent lung weight/body weight ratio (%L/B) was determined from heart and lung plucks. Lungs were scored using a method described by Bertram (4, 6). Lung pathology scores were confirmed by two different individuals according to the following scale: 0, no lesions; 1, not more than two lesions, each less than 15 mm in diameter; 2, not more than two lesions, each less than 50 mm in diameter, or a few scattered loci; 3, two or more lesions greater than 50 mm in diameter or numerous scattered loci but not involving more that one-third of the lung; 4, more lesions or lung involvement than described for 0 to 3.
Serum zinc level determinations and A. pleuropneumoniae reisolation from lung tissue were performed by the Minnesota Diagnostic Laboratory.
RNA isolation, semiquantitative reverse transcription (RT)-PCR, and Northern blotting.
RNA was isolated from cultured cells (9 × 106 cells) using Trizol (Gibco BRL) and from 108 lung lavage macrophages or 0.5 g of lung tissue using acid-guanidinium extraction (9). RNA was quantitated by UV spectroscopy at 260 nm.
Semiquantitative RT-PCR was performed using 1 μg of RNA. The RNA was reverse-transcribed using a 1 mM concentration of each deoxynucleoside triphosphate, 5 mM MgCl2, and Superscript reverse transcriptase (2.5 U/μl; Life Technologies). Incubations were at 25°C for 10 min, 42°C for 15 min, 99°C for 5 min, and 5°C for 5 min. Control reactions to check for DNA contamination were run without reverse transcriptase.
PCRs were performed with 2.5 μl of the cDNA products in a final volume of 25 μl with an annealing temperature of 55°C (30 s), an elongation temperature of 72°C (45 s), and a melting temperature of 93°C (45 s) for 30 cycles (β-actin) or 35 cycles (IL-10). Amplification from mRNA and not DNA was achieved by using primers which spanned introns in genomic DNA. Reaction products were electrophoresed in 1.5% agarose gels and quantitated using NIH Image software. Relative IL-10 levels were determined after normalization to β-actin.
For Northern blotting, RNA (20 μg/lane) was electrophoresed in a 1% agarose–2.2 M formaldehyde gel and transferred onto a nylon membrane in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Probes were produced with the Prime-It II kit (Stratagene) and 25 ng of the appropriate cDNA insert as a template and purified using the Qiagen nucleotide removal kit. Overnight hybridizations were performed in Quik-Hyb (Stratagene) at 68°C. The membranes were washed once with 2× SSC containing 1% sodium dodecyl sulfate at room temperature and then with 0.2× SSC containing 1% sodium dodecyl sulfate at 42°C. Membranes were quantified by phosphorimaging (Molecular Dynamics, Sunnyvale, Calif.).
Cytokine quantitation.
IL-10 was quantitated using an enzyme-linked immunosorbent assay (ELISA) (Biosource International) which recognized human IL-10 (hIL-10) but not porcine IL-10 (unpublished observation). TNF-α levels were determined by ELISA specific for porcine TNF-α (Endogen). IL-6 was quantitated using a B-9 cell bioassay (1), and IL-1 was quantitated using a D-10S cell (American Type Culture Collection) bioassay (15). IL-1Ra was quantitated using a hIL-1Ra ELISA (R & D Systems).
IL-10 bioactivity.
IL-10 activity was determined by measuring inhibition of lipopolysaccharide (LPS)-induced TNF-α expression from porcine alveolar lavage fluid sample cells. Lavage fluid sample cells were plated at a density of 2 × 105 cells/well in a 96-well plate, washed, and incubated overnight at 37°C. The medium was removed and 100 μl of fresh medium containing recombinant hIL-10 (R & D Systems) or medium from untreated or Ad-5/IL-10-treated PTE cells was added to each well. Cells were incubated for 1 h, and then LPS was added to a final concentration of 0.16 μg/ml for 6 h at 37°C. TNF in the medium was quantitated by ELISA.
MPO activity.
Neutrophils were quantified by myeloperoxidase (MPO) activity (8). Briefly, 50 μl of lung lavage fluid diluted in PBS was placed in wells of a 96-well plate and enzymatic activity was determined with tetramethylbenzidine and H2O2 (Kirkegaard & Perry Laboratories, Inc.).
Statistical analysis.
Group differences were analyzed for significance by unpaired t tests or the Mann-Whitney test (see Fig. 5A) using Prism software (GraphPad Software, San Diego, Calif.).
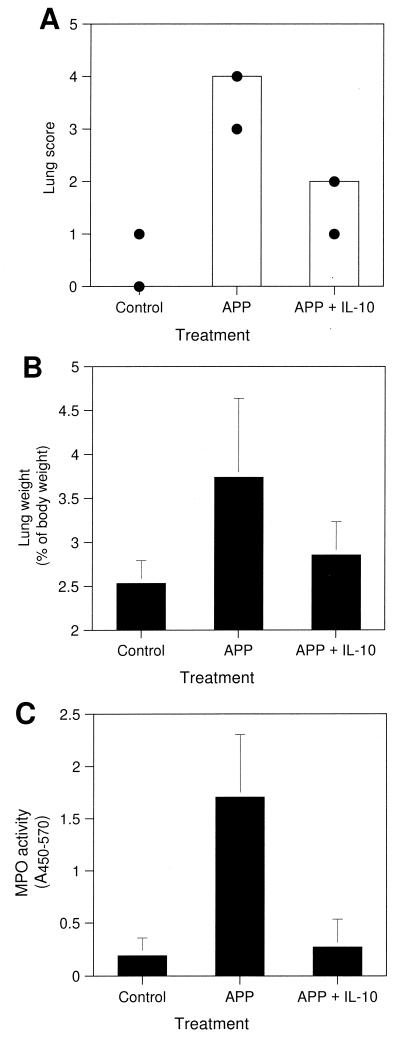
FIG. 5.
Effect of Ad-5/IL-10 on A. pleuropneumoniae-induced lung pathology. Pigs were treated as described in the legend to Fig. 4, and pathological changes were assessed. (A) Gross lung lesions were scored on a scale from 1 to 4 as described in Materials and Methods. (B) Relative %L/B. (C) Lung lavage fluid samples were analyzed for MPO activity with tetramethylbenzidine substrate. Data represent median (bar) and minimum and maximum (circles) of each group (A) or means ± standard deviations (error bars) of triplicate determinations (B and C) from eight control animals (not infected with A. pleuropneumoniae), five A. pleuropneumoniae-infected animals (APP), or three A. pleuropneumoniae-infected animals pretreated with Ad-5/IL-10 (APP + IL-10).
RESULTS
In vitro expression of IL-10.
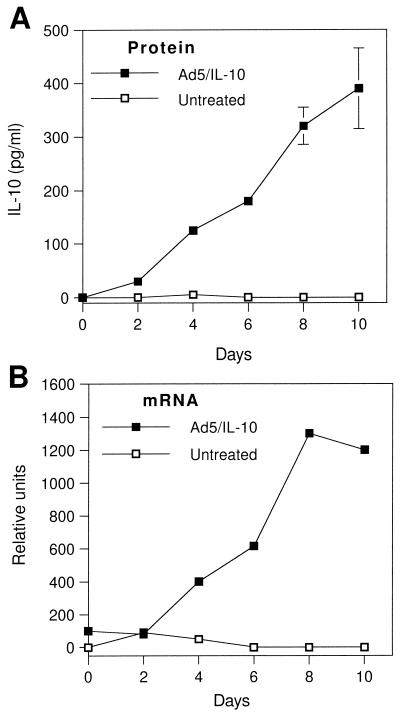
PTE cells infected with Ad-5/IL-10 showed no difference in gross morphology from normal untreated cells during 10 days of incubation. Untreated PTE cells secreted no detectable IL-10 over a 10-day time course, while those treated with Ad-5/IL-10 secreted IL-10 throughout, reaching a final concentration of 390 ± 75 pg/ml (Fig. 1A). RNA isolated from these cells was also analyzed by semiquantitative RT-PCR to determine the time course of IL-10 mRNA expression (Fig. 1B). IL-10 mRNA was detectable at low levels 2 h after treatment with Ad-5/IL-10 and peaked at 8 days.
FIG. 1.
Expression of IL-10 by PTE cells. PTE cells were incubated without or with Ad-5/IL-10 at a 10:1 multiplicity of infection. (A) Medium samples were removed at the indicated times, and IL-10 was detected by ELISA. Data are the means ± standard deviations (error bars) of triplicate determinations. (B) RNA was isolated using Trizol at the times indicated, and IL-10 mRNA levels were determined by semiquantitative RT-PCR as described in Materials and Methods. Gel bands were quantitated using NIH Image software.
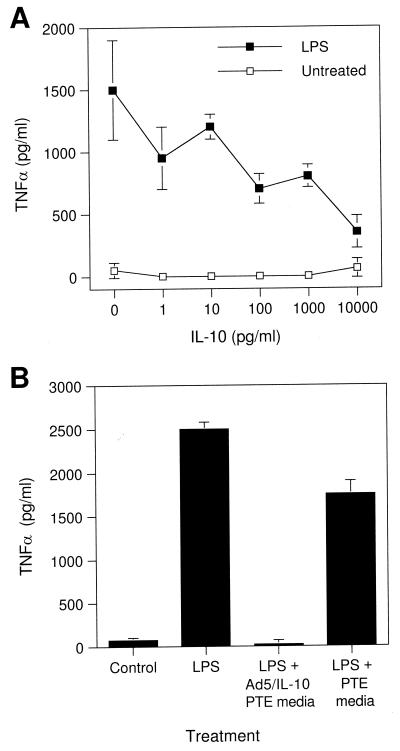
The bioactivity of IL-10 secreted from Ad-5/IL-10-treated cells was confirmed by its ability to inhibit the LPS-induced expression of TNF-α from porcine alveolar macrophages. Pretreatment of alveolar macrophages with IL-10 at concentrations from 10 pg/ml to 10 ng/ml inhibited TNF-α secretion in a dose-dependent manner (Fig. 2A). Culture supernatants from Ad-5/IL-10-treated PTE cells, but not untreated ones, also inhibited LPS-induced TNF-α secretion by porcine alveolar macrophages (Fig. 2B).
FIG. 2.
Bioactivity of IL-10 on porcine cells. (A) Inhibition of LPS-stimulated TNF-α expression from porcine alveolar lavage fluid cells by IL-10. Alveolar macrophages were treated with various concentrations of IL-10 for 1 h and then stimulated with LPS (0.16 μg/ml) for 6 h. Media were analyzed by ELISA for porcine TNF-α. (B) Inhibition of LPS-stimulated TNF-α expression from porcine alveolar lavage fluid cells by media from Ad-5/IL-10-infected PTE cells. Alveolar macrophages were cultured alone or with LPS and treated with medium from Ad-5/IL-10-treated PTE or untreated PTE cells. Data are the means ± standard deviations (error bars) of triplicate determinations.
In vivo expression of Ad-5/β-Gal and Ad-5/IL-10.
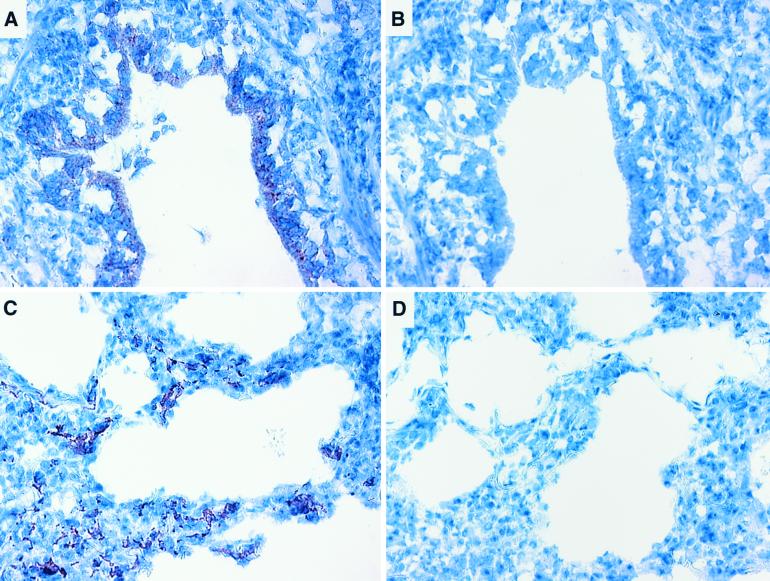
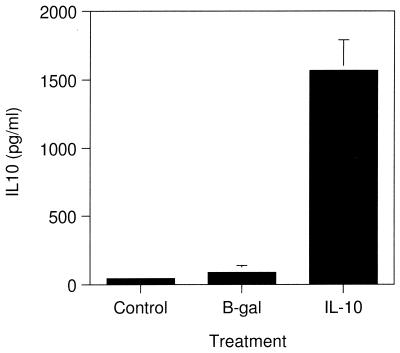
The distribution of Ad-5-delivered proteins in the lungs of pigs following intratracheal administration was determined by immunohistochemical labeling of β-Gal in lung tissue isolated from pigs treated with 1010 PFU of Ad-5/β-Gal (Fig. 3). β-Gal labeling was detected in lung tissue sections taken from the caudal and middle lobes of the right lung and caudal lobe of the left lung but not from the cranial lobe of either lung. Labeling was evident in the epithelial cells of the alveoli and bronchioles (Fig. 3). No labeling was seen in tissue sections incubated with nonimmune antisera (Fig. 3B and D). The production of IL-10 in the lungs of Ad-5/IL-10-treated pigs was confirmed by ELISA. Lung lavage fluid from animals pretreated with Ad-5/IL-10 and challenged with A. pleuropneumoniae contained significant levels of IL-10, while that from Ad-5/β-Gal-treated or nontreated animals did not (Fig. 4).
FIG. 3.
Expression of Ad-5/β-Gal in porcine lungs. Lung tissue of a pig treated with Ad-5/β-Gal was collected and fixed in formalin, and β-Gal was localized ([A and B] bronchiole; [C and D] alveolar spaces by immunohistochemistry [A and C] anti-β-Gal primary antibody; [B and D] irrelevant primary antibody).
FIG. 4.
Expression of Ad-5/IL-10 in porcine lungs. Pigs were treated with vehicle only (Control [n = 4]), Ad-5/β-Gal (B-gal [n = 6]), or Ad-5/IL-10 (IL-10 [n = 6]) and 6 days later were infected intratracheally with 5 × 104 CFU of A. pleuropneumoniae. After 24 h, pigs were sacrificed and lung lavage fluid samples were analyzed for hIL-10 (y axis) by ELISA. Data are the means ± standard deviations (error bars) of triplicate determinations.
Alteration of A. pleuropneumoniae pathogenesis by Ad-5/IL-10: clinical observations, lung pathology, and serum zinc levels.
In order to determine if IL-10 delivered to the lung by Ad-5 prior to infection with A. pleuropneumoniae would ameliorate the resulting disease, we pretreated pigs with either Ad-5/IL-10, Ad-5/β-Gal, or vehicle. After 6 days, half of each group was challenged with A. pleuropneumoniae. All pigs appeared generally healthy prior to and after Ad-5 treatment up to the time of A. pleuropneumoniae instillation. Twenty-four hours after challenge, pigs pretreated with vehicle or pretreated with Ad-5/β-Gal exhibited nasal discharge, labored breathing, depression, and listlessness. In marked contrast, A. pleuropneumoniae-infected pigs pretreated with Ad-5/IL-10 showed no clinical signs of disease. A. pleuropneumoniae was reisolated from the lungs of all pigs that were inoculated, including those pretreated with Ad-5/IL-10 which did not show clinical signs. A. pleuropneumoniae was not isolated from any of the animals pretreated with vehicle.
Pretreatment with Ad-5/IL-10 significantly reduced the pathologic changes induced by A. pleuropneumoniae (Fig. 5). The lungs of pigs not infected with A. pleuropneumoniae were normal in appearance. A. pleuropneumoniae-infected pigs pretreated with vehicle or pretreated with Ad-5/β-Gal had extensive fibrin deposits on the visceral and parietal pleura and extensive consolidation, edema, and hemorrhage of the lungs. A. pleuropneumoniae-infected pigs pretreated with Ad-5/IL-10 had no fibrin deposits, and lung lesions were far less severe than those in the lungs from the other A. pleuropneumoniae-infected pigs. Pretreatment with Ad-5/IL-10 significantly reduced the median lung score from 4 (with a range of 3 to 4) to 2 (with a range of 1 to 2) (P = 0.018) (Fig. 5A).
Relative lung weight, an indicator of tissue edema, for A. pleuropneumoniae-infected pigs that were pretreated with vehicle or pretreated with Ad-5/β-Gal averaged 3.7 ± 0.89% of body weight, compared to 2.5 ± 0.26% for non-A. pleuropneumoniae-infected pigs (P = 0.02) (Fig. 5B). Pretreatment with Ad-5/IL-10 tended to reduce the effect of A. pleuropneumoniae on lung weight (2.8 ± 0.38%; P = 0.055).
Neutrophil invasion.
The influx of neutrophils, a common response to bacterial infection, was substantial in pigs infected with A. pleuropneumoniae without exposure to adenovirus or treated with Ad-5/β-Gal. MPO activity, a measure of neutrophilia (8), was approximately 8.5-fold higher in infected pigs than in noninfected pigs (Fig. 5C) (P = 0.003). By contrast, pretreatment with Ad-5/IL-10 completely suppressed neutrophil influx, since MPO activity was the same as that in noninfected pigs (Fig. 5C).
Serum zinc levels.
Zinc levels in serum taken prior to and immediately before the introduction of A. pleuropneumoniae in virus-treated pigs (groups 3 to 6) were comparable to that seen in pigs receiving no virus; approximately 1 ppm (Table 1). Twenty-four hours following A. pleuropneumoniae instillation, serum zinc levels in pigs pretreated with vehicle (groups 1 and 2) dropped significantly from 1.01 ± 0.01 ppm in pigs not infected with A. pleuropneumoniae (group 1) to 0.66 ± 0.08 ppm in A. pleuropneumoniae-infected pigs (group 2) (P = 0.027). Similarly, pigs pretreated with Ad-5/β-Gal (groups 3 and 4) exhibited a significant drop in serum zinc from 0.93 ± 0.05 ppm in pigs not infected with A. pleuropneumoniae to 0.65 ± 0.12 ppm in A. pleuropneumoniae-infected pigs (P = 0.013). However, in pigs pretreated with Ad-5/IL-10 (groups 5 and 6) serum zinc did not decrease significantly when infected with A. pleuropneumoniae (0.92 ± 0.07 ppm in pigs not infected with A. pleuropneumoniae to 0.81 ± 0.17 ppm in A. pleuropneumoniae-infected pigs; P = 0.180).
Alteration of A. pleuropneumoniae pathogenesis by Ad-5/IL-10: effects on lung cytokines.
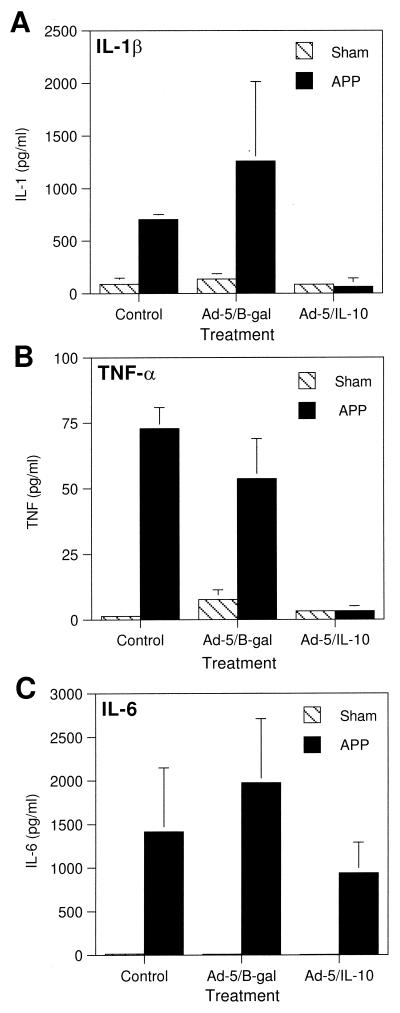
IL-1, TNF-α, and IL-6 levels were examined in lung lavage fluid samples to correlate the clinical results with cytokine production (Fig. 6). Both IL-1 and TNF-α were elevated upon infection with A. pleuropneumoniae in pigs pretreated with vehicle or pretreated with Ad-5/β-Gal. IL-1 levels were 88 ± 57 pg/ml in pigs not infected with A. pleuropneumoniae and 702 ± 47 pg/ml in A. pleuropneumoniae-infected pigs (Fig. 6A). Similarly, TNF-α levels were 1.3 ± 1.3 and 73 ± 8.2 pg/ml in noninfected and A. pleuropneumoniae-infected pigs, respectively (Fig. 6B). In contrast, pretreatment of infected pigs with Ad-5/IL-10 resulted in levels of IL-1 and TNF-α that were the same as in pigs not infected with A. pleuropneumoniae (85 ± 25 pg/ml versus 60 ± 83 pg/ml for IL-1 and 3.2 ± 0.1 pg/ml versus 3.2 ± 1.9 pg/ml for TNF-α, respectively). In contrast to IL-1β and TNF-α, IL-6 levels were not significantly altered by Ad-5/IL-10 pretreatment (Fig. 6C).
FIG. 6.
Effect of Ad-5/IL-10 on A. pleuropneumoniae-induced lung lavage fluid sample cytokine profiles. Pigs were treated as described in the legend to Fig. 4 and lung lavage fluids were analyzed for IL-1β, TNF-α, and IL-6 as described in Materials and Methods. Each bar represents the results from two (control) or three (treatment) individual animals (error bars, standard deviations).
DISCUSSION
Excessive inflammatory reactions triggered by expression of proinflammatory cytokines contribute to the severity of bacterial infections of the lung, which may result in life-threatening disease (4, 5, 7, 41). Marked elevations of IL-1, IL-6, and TNF occurred acutely after A. pleuropneumoniae infection in swine and were coincident with the onset of clinical disease (4, 16). Similarly, murine models of bacterial pneumonia indicate that proinflammatory cytokine expression is amplified acutely following infection and that overexpression of TNF exacerbates disease (22, 33). While high levels of inflammatory cytokines are associated with increased disease severity, moderate levels of these cytokines, especially TNF, are implicated in effective control of lung infection. Endogenous TNF appears to be required for murine defense to acute Klebsiella pneumoniae infection, whereas high levels of expression were not beneficial (18, 33).
In order to determine if excessive proinflammatory cytokine production exacerbates the pathogenesis of bacterial lung infection in pigs, we used in vivo gene therapy with recombinant Ad-5/IL-10 to suppress the production of cytokines in macrophages. IL-10 inhibits TNF and IL-1 production in macrophages and monocytes and also up-regulates other anti-inflammatory cytokines, including sTNF-R1 and IL-1Ra from fibroblasts (2, 12, 13, 36). hIL-10 was also demonstrated to attenuate TNF expression in porcine monocytes (14).
Pretreatment of pigs by instillation of Ad-5/IL-10 into the lungs reduced the severity of pleuropneumonia. Lung damage (lung pathology scores), lung edema (%L/B), acute phase response (serum zinc), and neutrophil influx (MPO) were all significantly reduced. The amelioration of disease severity was associated with reduced expression of IL-1 and TNF in pigs treated with Ad-5/IL-10 but not with Ad-5/β-Gal. We showed that IL-10 blocks the expression of inflammatory cytokines in macrophages induced by LPS, that Ad-5/IL-10 infects porcine endothelial cells, and that Ad-5/IL-10 expresses biologically active protein.
The inhibition of LPS-stimulated TNF-α expression from porcine alveolar macrophages by IL-10 showed dose dependence similar to that observed in human and mouse cells. Recombinant hIL-10 has a 50% effective dose (ED50) of 0.5 to 1.0 ng/ml using a murine mast cell proliferation assay (37). Similarly, hIL-10 inhibits LPS-stimulated TNF-α expression in human macrophages by 40% (36) and in synovial fluid macrophages and monocytes by 75% (12, 13) at a concentration of 10 ng/ml. hIL-10 also inhibited porcine TNF-α expression by 70% at a concentration of 10 ng/ml with an ED50 of ∼1.2 ng/ml.
The lack of effect of Ad-5/IL-10 on IL-6 expression in lungs of swine acutely infected with A. pleuropneumoniae was consistent with previous observations that IL-6 is not produced to a significant degree by macrophages in lung (4). IL-10 also does not suppress LPS-induced expression of IL-6 by human alveolar macrophages (42). Thus, it appears that IL-10 reduces the severity of acute pneumonia by preventing activation of macrophages, the expression of proinflammatory cytokines, and the recruitment of neutrophils.
The effect of IL-10 treatment on outcome of bacterial pneumonia in mice may be similar to or different from the protective effect in pigs. Systemic IL-10 decreased lung injury in mice given cytotoxic strains of Pseudomonas aeruginosa, an effect which required the presence of gamma interferon (28). Local expression of IL-10 in lung also improved survival against K. pneumoniae infection, enhanced lung clearance, and reduced dissemination (33). By contrast, IL-10 impaired the murine host defense against streptococcal pneumonia, including attenuation of proinflammatory cytokine expression (39). The variability in effect of IL-10 on disease severity and outcome is likely dependent on the pathogen. For example, A. pleuropneumoniae in our experimental model induces an extremely acute pleuropneumonia, whereas P. aeruginosa in mice is a model of chronic infection. Since resolution of infection requires a balance between proinflammatory and anti-inflammatory responses, differences in the model organism and its interaction with the host might affect treatment outcomes (22). IL-10 also may have somewhat different functions in swine and mice similar to other differences in innate immune responses to acute bacterial infection. Pigs readily express the principal neutrophil chemoattractant, IL-8, in alveolar macrophages and in the lungs (4, 16, 21), whereas mice lack the IL-8 gene. Swine do not express inducible nitric oxidase synthase activity in the lung, whereas inducible nitric oxidase synthase is an important innate immune component in mice (24).
The presence of adenovirus alone did not adversely affect lung pathology or induce an inflammatory response. The dose of virus was in the range which caused little or no response in baboons or humans (31, 40). Our results show that immunologic manipulation of cytokine expression can be an important adjunct therapy in the treatment of serious lung infections. The anti-inflammatory and immunoregulatory properties of IL-10 are consistent with a therapeutic role in diseases characterized by exuberant production of inflammatory cytokines, including bacterial infections and autoimmune inflammatory diseases (17).
ACKNOWLEDGMENTS
This work was supported by United States Department of Agriculture grant 96-35204-3389 (M.P.M.).
We thank Beverly Davidson for providing Ad-5/IL-10 and Ad-5/β-Gal viral stocks.
REFERENCES
- 1.Aarden L A, De Groot E R, Schaap O L, Lansdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong L, Jordan N, Millar A. Interleukin 10 (IL-10) regulation of tumour necrosis factor alpha (TNF-alpha) from human alveolar macrophages and peripheral blood monocytes. Thorax. 1996;51:143–149. doi: 10.1136/thx.51.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baarsch, M. J., D. L. Foss, and M. P. Murtaugh. Pathophysiological correlates of acute porcine pleuropneumonia. Am. J. Vet. Res., in press. [DOI] [PubMed]
- 4.Baarsch M J, Scamurra R W, Burger K, Foss D L, Maheswaran S K, Murtaugh M P. Inflammatory cytokine expression in swine experimentally infected with Actinobacillus pleuropneumoniae. Infect Immun. 1995;63:3587–3594. doi: 10.1128/iai.63.9.3587-3594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Reduction by cefodizime of the pulmonary inflammatory response induced by heat-killed Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:2527–2533. doi: 10.1128/aac.42.10.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram T A. Quantitative morphology of peracute pulmonary lesions in swine induced by Haemophilus pleuropneumoniae. Vet Pathol. 1985;22:598–609. doi: 10.1177/030098588502200615. [DOI] [PubMed] [Google Scholar]
- 7.Braciak T A, Mittal S K, Graham F L, Richards C D, Gauldie J. Construction of recombinant human type 5 adenoviruses expressing rodent IL-6 genes. An approach to investigate in vivo cytokine function. J Immunol. 1993;151:5145–5153. [PubMed] [Google Scholar]
- 8.Bradley P P, Priebat D A, Christensen R D, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Investig Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers R. Therapeutic control and economic aspect of porcine pleuropneumonia in finishing units. Vet Rec. 1986;119:89–90. doi: 10.1136/vr.119.4.89. [DOI] [PubMed] [Google Scholar]
- 11.Goldblum S E, Hennig B, Jay M, Yoneda K, McClain C J. Tumor necrosis factor alpha-induced pulmonary vascular endothelial injury. Infect Immun. 1989;57:1218–1226. doi: 10.1128/iai.57.4.1218-1226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart P H, Ahern M J, Smith M D, Finlay-Jones J J. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84:536–542. [PMC free article] [PubMed] [Google Scholar]
- 13.Hart P H, Jones C A, Finlay-Jones J J. Monocytes cultured in cytokine-defined environments differ from freshly isolated monocytes in their responses to IL-4 and IL-10. J Leukoc Biol. 1995;57:909–918. doi: 10.1002/jlb.57.6.909. [DOI] [PubMed] [Google Scholar]
- 14.Hofstetter C, Kleen M, Habler O, Allmeling A M, Krombach F, Zwissler B. Recombinant human interleukin-10 attenuates TNFα production by porcine monocytes. Eur J Med Res. 1998;3:299–303. [PubMed] [Google Scholar]
- 15.Hopkins S J, Humphreys M. Simple, sensitive and specific bioassay of interleukin-1. J Immunol Methods. 1989;120:271–276. doi: 10.1016/0022-1759(89)90252-4. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Potter A A, Campos M, Leighton F A, Willson P J, Haines D M, Yates W D. Pathogenesis of porcine Actinobacillus pleuropneumonia, part II: roles of proinflammatory cytokines. Can J Vet Res. 1999;63:69–78. [PMC free article] [PubMed] [Google Scholar]
- 17.Keystone E C, Wherry J C, Grint P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritus. Rheum Dis Clin N Am. 1998;24:629–639. doi: 10.1016/s0889-857x(05)70030-2. [DOI] [PubMed] [Google Scholar]
- 18.Laichalk L L, Kunkel S L, Strieter R M, Danforth J M, Bailie M B, Standiford T J. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect Immun. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liggett A D, Harrison L R, Farrell R L. Acute inflammatory effects of intratracheally instilled Escherichia coli endotoxin and sonicated suspension of Haemophilus pleuropneumoniae in swine. Can J Vet Res. 1986;50:526–531. [PMC free article] [PubMed] [Google Scholar]
- 20.Liggett A D, Harrison L R, Farrell R L. Sequential study of lesion development in experimental Haemophilus pleuropneumonia. Res Vet Sci. 1987;42:204–212. [PubMed] [Google Scholar]
- 21.Lin G, Pearson A E, Scamurra R W, Zhou Y, Baarsch M J, Weiss D J, Murtaugh M P. Regulation of interleukin-8 expression in porcine alveolar macrophages by bacterial lipopolysaccharide. J Biol Chem. 1994;269:77–85. [PubMed] [Google Scholar]
- 22.Moore T A, Standiford T J. The role of cytokines in bacterial pneumonia: an inflammatory balancing act. Proc Assoc Am Phys. 1998;110:297–305. [PubMed] [Google Scholar]
- 23.O'Reilly D R, Miller L K, Luckow V A. Baculovirus expression vectors: a laboratory manual. W. H. New York, N.Y: Freeman and Co.; 1992. [Google Scholar]
- 24.Pampusch M S, Bennaars A M, Harsch S, Murtaugh M P. Inducible nitric oxide synthase expression in porcine immune cells. Vet Immunol Immunopathol. 1998;61:279–289. doi: 10.1016/s0165-2427(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 25.Pattison I H, Howell D G, Elliot J. A haemophilus-like organism isolated from pig lung and the associated pneumonic lesion. J Comp Pathol. 1957;67:320–324. doi: 10.1016/s0368-1742(57)80031-9. [DOI] [PubMed] [Google Scholar]
- 26.Rennick D M, Fort M M, Davidson N J. Studies with IL-10 −/− mice: an overview. J Leukoc Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 27.Rosendal S, Miniats O P, Sinclair P. Protective efficacy of capsule extracts of Haemophilus pleuropneumoniae in pigs and mice. Vet Microbiol. 1986;12:229–240. doi: 10.1016/0378-1135(86)90052-0. [DOI] [PubMed] [Google Scholar]
- 28.Sawa T, Corry D B, Gropper M A, Ohara M, Kurahashi K, Wiener K, Kronish J P. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol. 1997;159:2858–2866. [PubMed] [Google Scholar]
- 29.Scamurra R W, Arriaga C, Sprunger L, Baarsch M J, Murtaugh M P. Regulation of interleukin-6 expression in porcine immune cells. J Interferon Cytokine Res. 1996;16:289–296. doi: 10.1089/jir.1996.16.289. [DOI] [PubMed] [Google Scholar]
- 30.Sebunya T N, Saunders J R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983;182:1331–1337. [PubMed] [Google Scholar]
- 31.Simon R H, Engelhardt J F, Yang Y, Zepeda M, Weber-Pendleton S, Grossman M, Wilson J M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum Gene Ther. 1993;4:771–780. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- 32.Spits H, de Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99:8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- 33.Standiford T J, Wilkowski J M, Sisson T H, Hattori N, Mehrad B, Bucknell K A, Moore T A. Intrapulmonary tumor necrosis factor gene therapy increases bacterial clearance and survival in murine gram-negative pneumonia. Hum Gene Ther. 1999;10:899–909. doi: 10.1089/10430349950018300. [DOI] [PubMed] [Google Scholar]
- 34.Stephens K E, Ishizaka A, Wu Z H, Larrick J W, Raffin T A. Granulocyte depletion prevents tumor necrosis factor-mediated acute lung injury in guinea pigs. Am Rev Respir Dis. 1988;138:1300–1307. doi: 10.1164/ajrccm/138.5.1300. [DOI] [PubMed] [Google Scholar]
- 35.Suter P M, Suter S, Girardin E, Roux-Lombard P, Grau G E, Dayer J M. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 36.Thomassen M J, Divis L T, Fisher C J. Regulation of human alveolar macrophage inflammatory cytokine production by interleukin-10. Clin Immunol Immunopathol. 1996;80:321–324. doi: 10.1006/clin.1996.0130. [DOI] [PubMed] [Google Scholar]
- 37.Thompson-Snipes L, Dhar V, Bond M W, Mosmann T R, Moore K W, Rennick D M. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran V N, Misset B, Lebargy F, Carlet J, Bernaudin J F. Expression of tumor necrosis factor-alpha gene in alveolar macrophages from patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;147:1585–1589. doi: 10.1164/ajrccm/147.6_Pt_1.1585. [DOI] [PubMed] [Google Scholar]
- 39.Van der Poll T, Marchant A, Keogh C V, Goldman M, Lowry S F. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 40.Wilson J M, Engelhardt J F, Grossman M, Simon R H, Yang Y. Gene therapy of cystic fibrosis lung disease using E1 deleted adenoviruses: a phase I trial. Hum Gene Ther. 1994;5:501–519. doi: 10.1089/hum.1994.5.4-501. [DOI] [PubMed] [Google Scholar]
- 41.Xing Z, Braciak T, Jordana M, Croitoru K, Graham F L, Gauldie J. Adenovirus-mediated cytokine gene transfer at tissue sites. Overexpression of IL-6 induces lymphocytic hyperplasia in the lung. J Immunol. 1994;153:4059–4069. [PubMed] [Google Scholar]
- 42.Zissel G, Schlaak J, Schlaak M, Muller-Quernheim J. Regulation of cytokine release by alveolar macrophages treated with interleukin-4, interleukin-10, or transforming growth factor beta. Eur Cytokine Netw. 1996;7:59–66. [PubMed] [Google Scholar]