Abstract
Streptococcus intermedius is a beta‐hemolytic, non‐motile, catalase‐negative, gram‐positive member of the Streptococcus anginosus group. When compared to other members of this group like S. anginosus and Streptococcus constellatus, S. intermedius infections are more substantial. In this case, we present a 47‐year‐old male patient who was found to have S. intermedius abscesses in his lungs, liver, and brain. The treatment of choice for these abscesses is a combination of drainage, surgery, and antibiotic therapy.
Keywords: abscesses, pathology, Streptococcus infection
Short abstract
Streptococcus intermedius has a unique tropism in the brain, liver, and lungs leading to abscess formation. When compared to other members of this group like Streptococcus anginosus and Streptococcus constellatus, S. intermedius infections are more substantial. S. intermedius is unique to the other bacteria in SAG due to the expression of intermedilysin (ILY), allowing it to cause cell necrosis with membrane bleb formation. The mortality rate for SAG‐associated bacteremia is 10%–16%. Early recognition and timely intervention are important for successful treatment and improved outcomes. There are currently no screening guidelines when S. intermedius is found in culture data; however, clinicians should have a low threshold to consider imaging for indolent infections.
1. INTRODUCTION
Streptococcus intermedius is a beta‐hemolytic, non‐motile, catalase‐negative, gram‐positive member of the Streptococcus anginosus group (SAG), along with S. anginosus and Streptococcus constellatus. 1 , 2 This group is part of the normal human microbiota in the respiratory, gastrointestinal, and genitourinary tracts. 1 They can cause purulent infections and abscesses in the heart, brain, liver, lungs, spleen, peritoneum, and appendix. 1 , 3 S. intermedius is the most pathogenic of the group, having significantly longer hospital stays compared to S. anginosus and significantly higher mortality rates than S. constellatus. 1 , 4 Its high‐grade pathogenicity allows it to cause an abscess in multiple sites, particularly the brain and liver due to its apparent tropism to these sites. 5 , 6 A 2020 study that collected data on patients with culture‐positive SAG from the bacterial laboratory database of the Affiliated Hospital of Jining Medical University Hospital and Jining No. 1 People's Hospital showed that the three different organisms in this group were predominantly found in different age groups and different locations. 7 S. intermedius was mostly found in patients aged 65 years old and above and was more likely to produce cranial infections. 7 It is more commonly seen in males, with a male:female ratio of about 2:1, and the highest number of reported cases are in the United States. 1 S. constellatus was mostly found in patients aged 35–54 years old and was more likely to produce chest infections, and S. anginosus was mostly found in patients aged 18–34 years old and was more likely to produce perianal abscesses. 7
2. CASE PRESENTATION
A 47‐year‐old male patient presented with a 5‐day history of nonproductive cough, fever, chills, shortness of breath, and pleuritic chest pain. He has a medical history of non‐insulin‐dependent diabetes mellitus type 2. He denied nausea, vomiting, diarrhea, abdominal pain, headache, recent travel, recent dental procedures, alcohol use, smoking, and recreational drug use. Vital signs were stable on admission. On a physical examination, he was ill appearing, diaphoretic, tachypnic (respiratory rate 34–36 breaths/min), and in respiratory distress using accessory muscles. Initial neurological examination was with GCS 15, right side 4+/5 except 3+/5 for shoulder extension, abduction and flexion and left side 5+/5. On follow‐up examination, GCS 15 with bilaterally 5+/5 with normal gait. Laboratory values were significant for these elevated values: HgA1C 7.2 (4.0%–5.6%), WBC 27,930 (reference range 4800–10,800/μl), CRP 208 (reference range 0–5.0 mg/L), Procalcitonin 1.11 (reference range 0.02–0.10 ng/ml), and Ferritin 2568 (reference range 30–400 ng/ml). Initial chest X‐ray revealed multilobar pneumonia and large right‐sided pleural effusion (Figure 1). Further investigation with a CT of chest/abdomen/pelvis with contrast revealed a large empyema and multiple hepatic abscesses (Figures 2 and 3). Before starting broad‐spectrum antibiotics, the empyema and hepatic abscess were drained and cultures grew S. intermedius (ceftriaxone and penicillin sensitive; detection of the organism was through the 16S genetic sequencing and biochemical tests). Pleural fluid studies demonstrated glucose 116 mg/dl, LDH 560 U/L, Protein 0.9 g/dl, TG 17 mg/dl. AFS negative, no cell count. The patient continued on 2 g of IV ceftriaxone daily. On the day of discharge, the patient lost balance while walking and sustained a fall after which a non‐contrast head CT was obtained and identified multiple basal ganglia and right occipital lesions, with a follow‐up MRI brain that confirmed ring‐enhancing lesions concerning brain abscesses (Figure 4). These lesions were deemed inaccessible by neurosurgery and then treated as a hematogenous spread of S. intermedius in the setting of recent bacteremia with 2 g of IV ceftriaxone twice a day, 500 mg of metronidazole every 8 h, and 1000 mg of vancomycin twice a day for 10 weeks. Follow‐up imaging confirmed the lesions to have decreased in size before discharge after 10 weeks of antibiotics. He was discharged on 875 mg of Augmentin twice daily for 2 months prior to first repeat MRI; antibiotics were continued for a total of 5 months with subsequent MRI demonstrating improvement of right occipital and left thalamic region.
FIGURE 1.
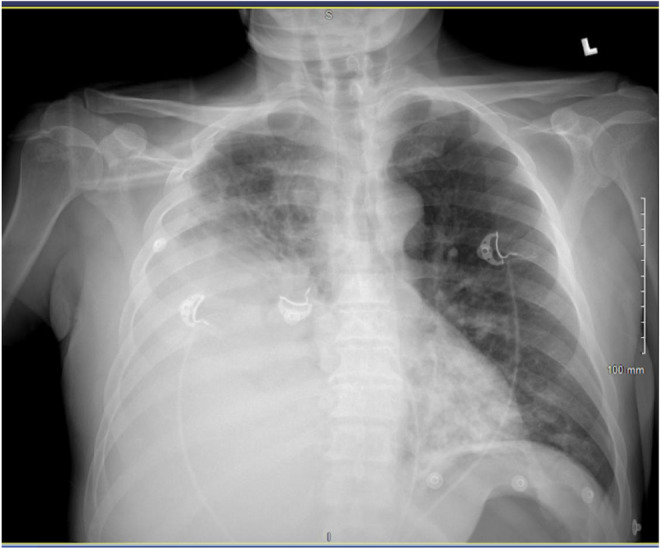
Chest X‐ray: Bilateral pneumonia and large right pleural effusion
FIGURE 2.
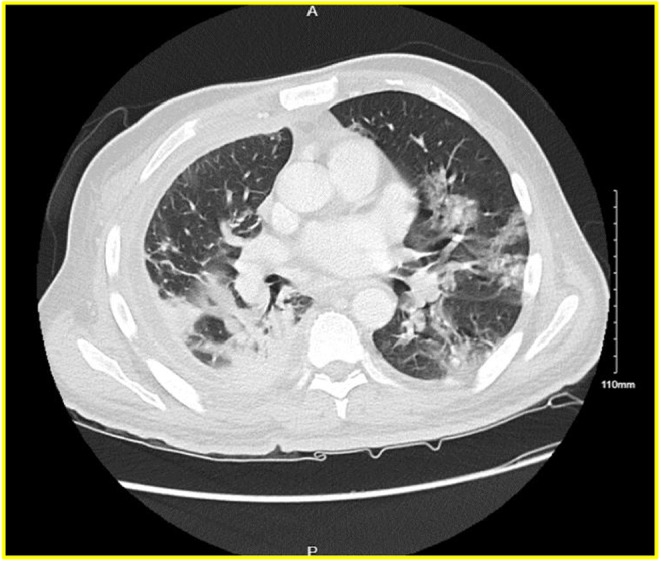
CT chest/abdomen/pelvis with contrast—Interval progression of bilateral diffuse ground‐glass infiltrates suspicious for COVID‐19 infection. Bibasilar consolidations are right worse than left.
FIGURE 3.
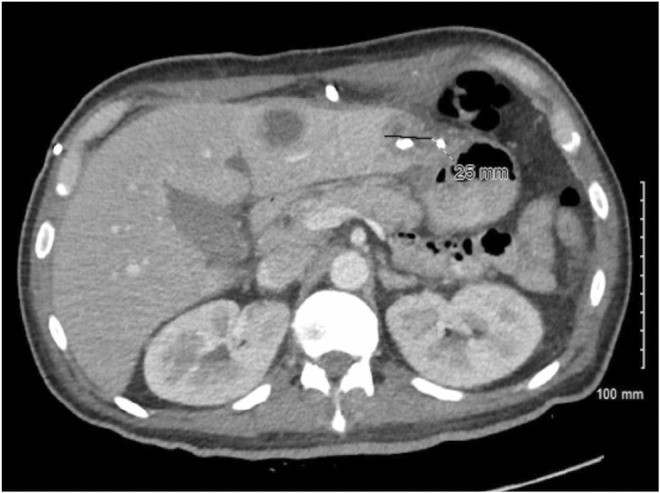
CT chest/abdomen/pelvis with contrast—Interval progression of bilateral diffuse ground‐glass infiltrates suspicious for COVID‐19 infection. Bibasilar consolidations are right worse than left. Interval decrease in small loculated right pleural effusion compatible with empyema. Trace left pleural effusion
FIGURE 4.
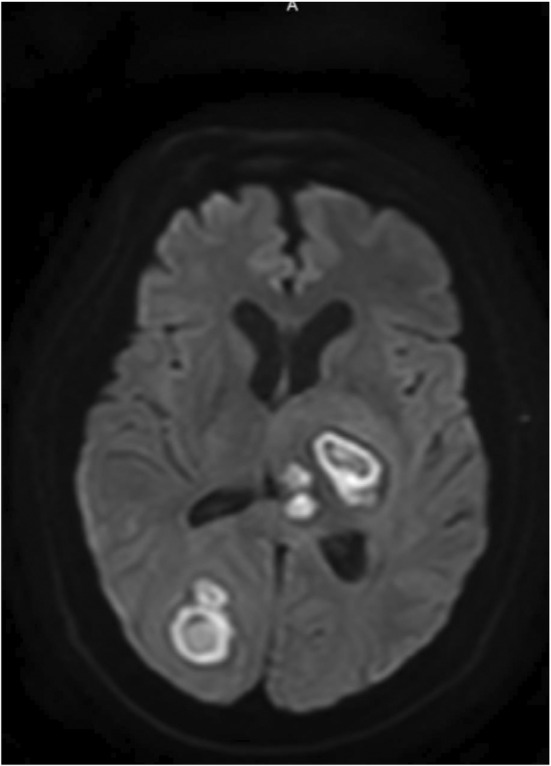
MRI head—Brain abscesses in the left thalamus, right parieto‐occipital region, and right frontal lobe. The largest of these in the left thalamic and right parieto‐occipital regions measure 3.1 and 3.4 cm, respectively. There is moderate associated mass effect and mild hydrocephalus.
3. DISCUSSION
Streptococcus intermedius abscesses have two presentations one of which being monomicrobial associated with hematogenous spread or are deep‐seated and extensive compared with the other two species within its group which were polymicrobial and often more superficial. 4 However, it has been seen that when S. intermedius is found in conjunction with anaerobes in an abscess, there is synergy between the organisms causing enhanced growth. 8 SAG abscesses overall have a longer duration of symptoms than other organisms and the majority of patients need surgical or percutaneous drainage for cure. 6 Interestingly, there appear to be no differences in mortality, duration of antibiotics, or complications compared to non‐SAG organisms. 6 Infection with S. intermedius is very rare in previously healthy individuals without any identifiable risk factors. 9 The two most common risk factors are dental manipulation and sinusitis. Others include a history of diabetes, heavy alcohol consumption, congenital heart disease, heart‐related conditions, malignancy, periodontal disease, preceding pneumonia, neurological diseases, and COPD. 1 , 5 , 9 In this case, the patient was noted to have dental caries; however, upon evaluation by oral maxillofacial surgery, there was low concern for dental abscesses. It was likely the source was aspiration in the setting of dental caries.
Infection and abscess formation are started with tissue damage and are then followed by colonization. 1 S. intermedius has surface proteins called Antigens I/II that bind to human lactoferrin, fibronectin, and laminin components that subsequently induce monocytes to release IL8, activating neutrophils leading to a proinflammatory state that induces tissue damage and abscess formation. 1 , 8 Hydrolytic enzymes are then released to induce tissue liquefaction and form pus. 8 Hyaluronidase, one of the hydrolytic enzymes released by S. intermedius, has numerous effects. It decreases the viscosity of ground substance and increases the permeability of connective tissue allowing bacterial and toxins to spread while, along with α‐N‐acetylmuramidase (sialidase), creating a source of nutrients for the bacteria from the degradation products. 1 , 8 It has been shown that the higher the concentration of hyaluronidase, the deeper the lesion. 8 It is also involved in biofilm formation, which decreases the organism's antibiotic susceptibility and protects it from host immunological defenses. 1 It has signal molecules called autoinducers that give it the ability to monitor the concentration of bacterial signals and biofilm formation, allowing it to regulate its pathogenic capabilities like proteolytic and hemolytic enzyme activities, iron acquisition, antibiotic production, carbohydrate metabolism, and biofilm formation. 8 S. intermedius thrives in acidic environments causing the release of iron from host transferrin to facilitate bacterial growth and infectious spread. 8 It also can move to distant sites and rapidly decrease the pH, facilitating the release of iron at this new location for its growth. 8 Some S. intermedius have a polysaccharide capsule, which inhibits phagocytosis and increases its tendency to form abscesses. 1 , 8 Encapsulated strains have larger abscesses, earlier spontaneous drainage, and a higher lethality. 8 Superantigens also play an important role in the S. intermedius's virulence by stimulating the release of proinflammatory mediators by activating glial cells and peripheral immune cells, causing a positive feedback loop to recruit and activate new inflammatory cells and glia to create a vicious cycle allowing extensive damage to normal brain tissue. 8 S. intermedius is unique to the other bacteria in SAG due to the expression of intermedilysin (ILY), allowing it to cause cell necrosis with membrane bleb formation. 1 , 8 It is an important virulence factor in causing deep‐seated abscesses, and its expression correlates with strain pathogenicity or with the severity of infections. 1 , 8 Isolates found in deep‐seated brain abscesses had a 6.2‐ to 10.2‐fold higher expression of ILY than those found in normal habitats like dental plaques, and ily knockout strains showed greatly decreased adherence, invasion, and cytotoxicity of human liver cells. 8 Because of its specificity to S. intermedius, the ILY gene is being used as a specific marker detector via PCR. 6 , 8
Brain abscesses typically present with intermittent fever, constant headaches, and/or mental status changes with elevated CRP and WBC count. 1 , 6 It can occur due to spread from the contiguous focus of infection (sinus, teeth, or middle ear), hematogenous spread from distant focus, or as a result of head trauma or neurosurgery. 6 , 8 An important primary source of S. intermedius is from a liver abscess, but most patients have multiple risk factors for developing an invasive intracerebral abscess including congenital heart disease, sinusitis, otitis media, and dental caries. 8 , 10 The majority of brain abscesses are solitary and occur in the frontal lobe. 6 Due to newer antibiotics and earlier detection with CT scans and MRIs, morbidity and mortality have decreased. 6 Pleuro‐pulmonary infections are uncommon and usually present with fever, cough, chest pain, and dyspnea with elevated CRP and WBC count. 10 These infections can present as pulmonary abscesses, pneumonia, pleural effusions, pyopneumothorax, and pleural fistula. 11 It occurs due to aspiration of oral secretions, which might be why it is more frequent in the elderly. 10 Aspirate of the pleural fluid would show exudate, with elevated protein, LDH, and WBC count. 10 The prognosis of pleuro‐pulmonary infections is generally favorable. 10 Liver abscesses are generally associated with underlying hepatobiliary or pancreatic disease, diabetes, liver transplantation, and colorectal neoplasm. 2 The probable origin is a dental plaque. 2 Symptoms depend on the size of abscesses and the general health of the patient, but often include upper abdominal quadrant pain, high fevers, nausea, and vomiting, 11 and most patients have an elevated WBC count and liver function tests. 2 Less common symptoms include loss of appetite, jaundice, ascites, pleural effusion, and respiratory symptoms. 11 If liver abscesses are untreated, major complications include sepsis and multiorgan dysfunction/failure, rupture of abscess into peritoneal cavity, thrombosis of the portal or hepatic veins, IVC occlusion of the pseudoaneurysm of the hepatic artery, hemophilia, and fistula to the portal vein or suprahepatic veins, 2 , 11 with a mortality rate of 100%. 11 When treated, these numbers go as low as 2.5%–14%. 12 Other possible infection sites include the mouth, female genital tract, appendix, and blood stream. 10
Patients with suspected infection by S. intermedius should have the abscess drained and cultured along with blood cultures if there is suspicion of hematogenous spread. 4 , 6 Abscess culture can be negative due to antibiotic therapy. If there is a negative culture and suspicion is high, gene amplification and sequencing tests may be used to aid in diagnosis and allow for more targeted antibiotic therapy. 4 S. intermedius can also be difficult to detect with conventional microbiology, so a broad range PCR method targeting the 16S ribosomal RNA genes is typically used. 12 Since the ILY gene is specific to S. intermedius, this is the gene sequenced with PCR to detect these S. intermedius infections. 6 , 8 Brain abscesses can be evaluated with an MRI which would show ring‐enhancing lesions. 8 A lumbar puncture should not be done as CSF analysis does not contribute to diagnosis and there is a risk of brain herniation with the increased ICP caused by abscesses. 4 Pleuro‐pulmonary infections should get cultures of the pleural effusion via percutaneous lung needle aspiration or transthoracic needle aspiration. 10 , 13 If pleural effusion is negative, a lung sample can be taken for microscopy and culture. 14
Management and treatment of these abscesses include drainage, surgery, and antibiotics. 1 Antibiotic therapy is based on susceptibility. All SAG are generally susceptible to the usual dose of penicillin, amoxicillin, cefotaxime, or ceftriaxone and have variable susceptibility to tetracycline, clindamycin, and erythromycin. 12 Of the beta‐lactams, ceftriaxone is preferred due to its excellent activity and tissue penetration. 10 If the patient has a penicillin allergy, vancomycin is typically used. 10 However, recent penicillin strains have been reported and in cases of high risk of antibiotic resistance, carbapenems, especially ertapenem, are very effective. 12 , 13 Most strains are resistant to aminoglycosides, and resistance to macrolides and clindamycin are increasing. 9 , 10 Empiric therapy should depend based on patients' current condition and whether they have been exposed to antimicrobials within the past 3 months 9 Fluoroquinolones have also been shown to be efficacious against SAG, but resistance develops quickly so these are not appropriate as empiric therapy. 10 The most commonly used antibiotic regimens currently used are a combination of ceftriaxone and metronidazole or a combination of ceftriaxone, metronidazole, and vancomycin. 1 However, with the use of multiple antibiotic regimens in patients, there is a concern for the emergence of drug‐resistant strains, especially with vancomycin and carbapenems. 1 There also have been cases of clinical failure with antibiotics that showed susceptibility in vitro studies. Potential causes of clinical failure can include the inappropriate selection of empiric therapy, inability to penetrate tissue spaces, and inadequate dosing to reach therapeutic concentrations in infected areas. 9
Brain abscesses are managed with a combination of neurosurgical intervention, antibiotics, and eradication of any primary foci. 8 Patients with significant risk factors for surgery or small cerebral lesions <5 mm on CT should undergo conservative management with antibiotics. 4 , 8 In those who can tolerate surgery, CT‐guided stereotactic aspiration of abscess can be performed as it is minimally invasive, rapid, and effective in surgical drainage especially if an abscess is small and deep‐seated. 4 CT‐guided stereotactic aspiration is associated with lower morbidity and mortality and has a better overall prognosis. 4 A more superficial abscess can be treated with craniotomy and excision of abscess. 4 The working group of the British Society of Antimicrobial Chemotherapy recommended that brain abscesses should be treated with a combination of beta‐lactam and metronidazole for 3–4 weeks if surgically resected or 4–6 weeks if aspirated. 8 Pleuro‐pulmonary infections are managed via drainage of pleural fluid, pleurectomy, thoracotomy, decortication, and debridement. 10 , 11 Reasons for clinical failure seen in pneumonia complicated by empyema by S. intermedius species include the inability to penetrate pleural space and the inability to reach therapeutic levels in desired areas. 9 Early recognition and timely intervention important for successful treatment and better prognosis. 12 Liver abscesses are treated with IV broad‐spectrum antibiotics, most commonly fluoroquinolones or third‐generation cephalosporin in combination with metronidazole, and percutaneous drainage. 11 For abscesses less than 5 cm, antibiotics alone can be sufficient; for abscess between 5 and 7.3 cm with sustains fevers for more than 23–48 h or suggestion of perforation, a combination of percutaneous drainage and systemic antibiotics should be used, and for abscesses that are larger than 7.3 cm should be considered for surgical drainable due to the risk of being multiloculated and spontaneous rupture. 11
4. CONCLUSION
Streptococcus intermedius has a unique tropism in the brain, liver, and lungs leading to abscess formation. When compared to other members of this group like S. anginosus and S. constellatus, S. intermedius infections are more substantial. S. intermedius is unique to the other bacteria in SAG due to the expression of intermedilysin (ILY), allowing it to cause cell necrosis with membrane bleb formation. The mortality rate for SAG‐associated bacteremia is 10%–16%. Early recognition and timely intervention are important for successful treatment and improved outcomes. There are currently no screening guidelines when S. intermedius is found in culture data; however, clinicians should have a low threshold to consider imaging for indolent infections.
AUTHOR CONTRIBUTIONS
Jonathan Vincent M. Reyes: Conceptualization; investigation; project administration; writing – original draft. Manasa Dondapati: Investigation; methodology; project administration; resources; writing – original draft; writing – review and editing. Saad Ahmad: Validation; visualization; writing – original draft; writing – review and editing. David Song: Supervision; validation; visualization; writing – original draft; writing – review and editing. Vikash Jaiswal: Supervision; validation; visualization; writing – review and editing. Nishan Babu Pokhrel: Validation; visualization; writing – review and editing. Joseph L. Lieber: Supervision; validation; visualization; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVAL
Our institution does not require ethical approval for reporting individual cases or case series.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENT
None declared by authors.
Reyes JVM, Dondapati M, Ahmad S, et al. A case report of multiple abscesses caused by Streptococcus intermedius . Clin Case Rep. 2023;11:e06813. doi: 10.1002/ccr3.6813
DATA AVAILABILITY STATEMENT
Available upon request from corresponding author.
REFERENCES
- 1. Issa E, Salloum T, Tokajian S. From normal flora to brain abscesses: a review of Streptococcus intermedius . Front Microbiol. 2020;11:826. doi: 10.3389/fmicb.2020.00826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ioannou A, Xenophontos E, Karatsi A, Petrides C, Kleridou M, Zintilis C. Insidious manifestation of pyogenic liver abscess caused by Streptococcus intermedius and Micrococcus luteus: a case report. Oxf Med Case Reports. 2016;2016(1):1‐3. doi: 10.1093/omcr/omv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parthvi R, Amin M, Mehra S. Antimicrobial therapy for pyogenic liver abscess secondary to Streptococcus intermedius bacteremia. Am J Ther. 2017;24(6):e770‐e771. doi: 10.1097/MJT.0000000000000537 [DOI] [PubMed] [Google Scholar]
- 4. Maliyil J, Caire W, Nair R, Bridges D. Splenic abscess and multiple brain abscesses caused by Streptococcus intermedius in a young healthy man. Proc (Bayl Univ Med Cent). 2011;24(3):195‐199. doi: 10.1080/08998280.2011.11928714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catalya S, Komal B, Tulpule S, Raoof N, Sen S. Isolated Streptococcus intermedius pulmonary nodules. IDCases. 2017;8:48‐49. doi: 10.1016/j.idcr.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tran MP, Caldwell‐McMillan M, Khalife W, Young VB. Streptococcus intermedius causing infective endocarditis and abscesses: a report of three cases and review of the literature. BMC Infect Dis. 2008;8:154. doi: 10.1186/1471-2334-8-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang S, Li M, Fu T, Shan F, Jiang L, Shao Z. Clinical characteristics of infections caused by Streptococcus anginosus group. Sci Rep. 2020;10(1):9032. doi: 10.1038/s41598-020-65977-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mishra AK, Fournier PE. The role of Streptococcus intermedius in brain abscess. Eur J Clin Microbiol Infect Dis. 2013;32(4):477‐483. doi: 10.1007/s10096-012-1782-8 [DOI] [PubMed] [Google Scholar]
- 9. Wargo KA, McConnell VJ, Higginbotham SA. A case of Streptococcus intermedius empyema. Ann Pharmacother. 2006;40(6):1208‐1210. doi: 10.1345/aph.1G704 [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto M, Fukushima T, Ohshiro S, et al. Brain abscess caused by Streptococcus intermedius: two case reports. Surg Neurol. 1999;51(2):219‐222. doi: 10.1016/s0090-3019(97)00505-3 [DOI] [PubMed] [Google Scholar]
- 11. Sotto Mayor J, Robalo MM, Pacheco AP, Esperança S. Pyogenic liver abscess: uncommon presentation. BMJ Case Rep. 2016;2016:bcr2016214841. doi: 10.1136/bcr-2016-214841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Millichap JJ, McKendrick AI, Drelichman VS. Streptococcus intermedius liver abscesses and colon cancer: a case report. West Indian Med J. 2005;54(5):341‐342. doi: 10.1590/s0043-31442005000500014 [DOI] [PubMed] [Google Scholar]
- 13. Noguchi S, Yatera K, Kawanami T, et al. Pneumonia and empyema caused by Streptococcus intermedius that shows the diagnostic importance of evaluating the microbiota in the lower respiratory tract. Intern Med. 2014;53(1):47‐50. doi: 10.2169/internalmedicine.53.0971 [DOI] [PubMed] [Google Scholar]
- 14. Hannoodi F, Ali I, Sabbagh H, Kumar S. Streptococcus intermedius causing necrotizing pneumonia in an immune competent female: a case report and literature review. Case Rep Pulmonol. 2016;2016:7452161. doi: 10.1155/2016/7452161 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request from corresponding author.


