Abstract
Earlier pubertal development appears to be one pathway through which childhood trauma contributes to psychopathology in adolescence. Puberty-related changes in neural networks involved in emotion processing, namely the amygdala-medial prefrontal (mPFC) circuit, may be a potential mechanism linking trauma and adolescent psychopathology. Our participants were 227 youth between 10 and 13 years of age who completed assessments of threat and deprivation-related experiences of adversity, pubertal stage, and internalizing and externalizing symptoms. A subset (n = 149) also underwent a functional MRI scan while passively viewing fearful and calm faces. Potential mechanisms linking childhood trauma with psychopathology, encompassing earlier pubertal timing and neural response to aversive stimuli were explored. Earlier pubertal development was associated with childhood trauma as well as increased externalizing symptoms in boys only. Earlier pubertal timing in males and females was negatively associated with activation in bilateral amygdala, hippocampal, and fusiform regions when comparing fearful and calm faces. However, amygdala-mPFC connectivity showed no association with pubertal timing or psychopathology symptoms. These findings do not support accelerated amygdala-mPFC development as a mechanism linking childhood trauma and psychopathology, but instead provide support for the role of pubertal development in normative decreases in limbic activation across development.
Keywords: Adolescence, Trauma, Pubertal Timing, Amygdala, Connectivity, Externalizing Symptoms
Highlights
-
•
Earlier puberty mediates the link between trauma and externalizing problems in males.
-
•
Earlier puberty relates to reduced activation in amygdala, hippocampus, and fusiform gyrus to aversive stimuli.
-
•
Pubertal timing was not related to amygdala-mPFC connectivity.
-
•
These neural patterns are not a mechanism linking trauma and psychopathology.
Exposure to early life adversity (ELA) is associated with elevated risk for numerous forms of psychopathology across the lifespan, including depression, anxiety, substance abuse and externalizing problems (Green et al., 2010, McLaughlin et al., 2012). Adolescence is a period of heightened vulnerability for psychopathology (Lee et al., 2014), and ELA is strongly associated with adolescent-onset psychopathology (McLaughlin et al., 2012). Despite the powerful links between ELA and adolescent psychopathology, the mechanisms underlying these associations remain poorly understood (Gee, 2021, McLaughlin et al., 2020).
One potential mechanism linking ELA and psychopathology in adolescence is earlier pubertal onset. Most conceptual models on the link between ELA and earlier pubertal onset are rooted in life history theory (Belsky et al., 1991, Ellis et al., 2009, Ellis et al., 2022) and are based on the idea that experiences in early-life can program an individual’s developmental trajectory in order to respond most effectively to the environmental demands they are likely to encounter later in life. Recently, conceptual models have focused on how different dimensions of adverse early environments may have differing consequences for the pace of development across different neurobiological systems (Ellis et al., 2022, McLaughlin et al., 2021). We have argued that early environments characterized by a high degree of threat may be particularly likely to accelerate pubertal onset, as they signal that the environment is dangerous and that morbidity and mortality risk is high (Colich et al., 2020). In contrast, environments characterized by deprivation may lead to delayed pubertal onset, as they signal that environmental resources may not be adequate to support reproduction. Indeed, across two independent samples, we found that children exposed to trauma exhibited earlier pubertal timing, whereas children who experienced deprivation did not, after controlling for co-occurring ELA (Colich et al., 2020, Sumner et al., 2019). Similarly, in a meta-analysis spanning 43 studies and over 100,000 participants, ELA experiences characterized by threat were associated with earlier pubertal timing, but no association with pubertal timing was observed for poverty or experiences characterized by deprivation (i.e., neglect or institutional rearing; Colich et al., 2020). These findings support the idea that trauma in childhood and adolescence may alter the pace of development, resulting in faster aging of physiological systems and an earlier onset of puberty (Ellis and Giudice, 2019, Ellis et al., 2022). Earlier pubertal timing, in turn, is consistently linked with elevated risk for psychopathology in both male and female adolescents (Ullsperger and Nikolas, 2017). Indeed, earlier pubertal timing to appears to be one pathway through which trauma contributes to risk for psychopathology in adolescence (Colich et al., 2020, Negriff et al., 2015, Negriff et al., 2015, Sumner et al., 2019). Altogether, this work supports early pubertal timing as a mechanism linking trauma and adolescent psychopathology.
Numerous mechanisms might explain why earlier pubertal development is associated with elevated risk for adolescent psychopathology. Early work focused on discrepancies in the pace of development among physical, emotional, and cognitive systems (Brooks-Gunn et al., 1985, Ge and Natsuaki, 2009). This work emphasized how more physically developed adolescents (i.e. those who experienced earlier onset of puberty) may not be emotionally or cognitively as advanced as others expect them to be based on their physical appearance, which may lead to psychological distress and risk for psychopathology. These discrepancies reflect that puberty may play a role in the development of some neurobiological systems but not others. Specifically, Ladouceur (2012) has suggested that puberty has a bigger impact on the development of neural networks involved in emotion processing relative to networks underlying cognitive control, such as the frontoparietal control network, and that increasing sex steroids at the start of pubertal development decrease connectivity between the prefrontal cortex and subcortical limbic regions. This altered fronto-limbic connectivity may be one pathway through which early pubertal timing increases risk for psychopathology in early adolescence (Ladouceur, 2012).
Despite substantial interest in how puberty shapes neurodevelopment, evidence for the impact of early pubertal timing on brain development is sparse. Some studies have found associations of early adrenarche on changes in neural responses to emotional faces in the salience network and ventromedial prefrontal cortex (Whittle et al., 2015), and altered amygdala-prefrontal cortical (PFC) connectivity (Barendse et al., 2019, Whittle et al., 2015). More specifically, Whittle and colleagues (2015) found that males and females who experienced early pubertal timing, as defined by high DHEA levels independent of age, showed reduced cingulate cortex activation in response to viewing emotional faces. Females with higher DHEA levels also showed reduced activation in the insula, striatum and dorso-lateral PFC regions, but increased activation in subgenual cingulate and ventro-lateral PFC regions in response to emotional faces. In this same sample (Barendse et al., 2019) found that earlier pubertal timing was associated with decreased amygdala-inferior frontal gyrus (IFG) connectivity in males, and increased connectivity in females across two timepoints in early adolescence. Earlier pubertal timing was indirectly associated with increasing anxiety symptoms through increases in positive amygdala-IFG connectivity over time. This limited work suggests that pubertal timing, measured by DHEA levels independent of age, may be associated with both amygdala-PFC circuitry and adolescent psychopathology.
Further support for the influence of pubertal development on cortico-limbic circuitry comes from work suggesting that the amygdala-PFC circuit undergoes significant changes across late childhood and adolescence, such that connectivity between the amygdala and the medial prefrontal cortex (mPFC) becomes increasingly negative when performing an emotional processing task as individuals move from childhood to adolescence (the developmental period coinciding with the onset and progression of puberty (Gee et al., 2013, Silvers et al., 2016, Wu et al., 2016). However, it is important to note that this pattern is not found consistently in patterns of resting-state functional connectivity between amygdala and mPFC (i.e. Brieant et al., 2021; Gabard-Durnam et al., 2014; Thijssen et al., 2021; van Duijvenvoorde et al., 2019). Building on this work, some evidence suggests that ELA may accelerate this pattern of development, resulting in an earlier emergence of a negative amygdala-mPFC pattern of connectivity (Callaghan and Tottenham, 2016, Colich et al., 2017, Gee et al., 2013, Keding and Herringa, 2016, Peverill et al., 2019). Furthermore, recent evidence suggests that pubertal development may mediate the association between adverse experiences in the home and amygdala-cingular-opercular network connectivity at rest (Demidenko et al., 2022, Thijssen et al., 2020, Thijssen et al., 2022). However, this finding has not emerged consistently across studies (see Colich et al., 2020 for a review), perhaps in part because the development shift of amygdala-mPFC connectivity is not a reliable marker of maturation (Bloom et al., 2021). Thus, amygdala-mPFC connectivity may be one aspect of neurodevelopment that is particularly likely to be influenced by the timing of puberty.
Here we examine how different dimensions of ELA are associated with pubertal timing, neural response and amygdala-mPFC connectivity to aversive stimuli, and adolescent psychopathology. We examine these associations in a longitudinal community sample of children and adolescents followed from age 3 into adolescence. First we asked whether earlier pubertal timing mediated the association between childhood trauma and symptoms of psychopathology in adolescence. Given evidence suggesting dense sex hormone receptors in the amygdala and hippocampus (Ahmed et al., 2008), and puberty-specific changes in brain regions involved in face processing such as the fusiform cortex (Scherf et al., 2012), we examine the association of pubertal timing with neural response in these regions to aversive stimuli to understand the mechanisms through which earlier pubertal timing places adolescents at heightened risk for psychopathology. We also examined associations with neural responses in basic visual processing region V1, a region we hypothesized would not be associated with pubertal timing. Finally, given earlier work suggesting adolescent shifts in amygdala-PFC connectivity, we evaluated whether earlier pubertal timing was associated with a more mature negative pattern of amygdala-mPFC connectivity that might mediate the association between earlier pubertal timing and psychopathology in adolescence.
1. Materials and methods
1.1. Participants
Participants in this study were drawn from a longitudinal study of children with the initial aim of understanding the development of self-regulation (Lengua et al., 2015). Participants were recruited at 36–40 months of age and were followed into adolescence, across multiple assessments. Families were recruited to achieve equal representation across income levels, where: 29 % of the sample were at or near poverty (below 150 % of the federal poverty threshold), 28 % of the sample had low income (between 150 % and the local median income of $58 K), 25 % were middle-income and 18 % were upper-income. Families were required to be proficient in English in order to understand study procedures, and families with children diagnosed with a developmental disability were excluded. At age 10–13 years, a total of 227 children and a parent or guardian participated in a series of assessments examining mechanisms linking adverse childhood experiences with psychopathology. A subset of these children (n = 183) underwent an MRI session which included a structural T1-weighted scan and functional MRI scan while passively viewing fearful and neutral faces. All subjects were cleared for any MRI contraindications. Of those, 14 were excluded from MRI analysis: 2 subjects due to task administration errors, 6 due to poor fMRI data quality, 3 due to poor behavioral performance (accuracy <50 % ), 1 due to a scanner error, 1 due to an incidental finding during the scan, and 1 did not complete the task. An additional 20 subjects did not complete the pubertal assessment and were excluded. A total of 149 subjects were included in all fMRI analyses. All procedures were approved by the Institutional Review Board at the University of Washington. Written informed consent was obtained from legal guardian, and children provided written assent.
1.2. Measures
1.2.1. Threat experiences
To quantify the severity and frequency of experiences of threat, we created a dimensional measure comprised of three components. First, we created an indicator of the number of distinct types of violence experienced by the child. To do so we used a count of exposure to 5 types of interpersonal violence: physical abuse, sexual abuse, domestic violence, witnessing a violent crime, or being a victim of a violent crime. Each exposure was counted if it was endorsed by the parent or child on the UCLA PTSD Reactions Index (Anon, 2004). We additionally coded physical abuse, sexual abuse, and domestic violence as present if they were endorsed by the child on the CECA Interview (Bifulco et al., 1994) or by the parent on the Juvenile Victimization Questionnaire (JVQ; Finkelhor et al., 2005). Second, we created a measure of frequency of violence exposure by using the summed frequency ratings of witnessed and experienced violence on the Violence Exposure Scale for Children-Revised (VEX-R; Fox and Leavitt, 1995). Third, we created a measure of violence severity by summing the severity scores of the CTQ Physical and Sexual Abuse subscales (Bernstein et al., 1997). To create the composite for threat, we first standardized each of these three sub-scales and then averaged them together. This approach to creating the threat composite in this dataset was pre-registered (https://osf.io/6yf4p/). See Weissman et al. (2022) for more detail on this measure. Upon visual inspection, one outlier was detected. To ensure this outlier did not drive any effects, we winsorized their value on the threat composite.
1.2.2. Deprivation experiences
To quantify the severity and frequency of deprivation experiences, we created a dimensional metric comprised of three types of deprivation: cognitive, emotional, and physical. Cognitive deprivation was assessed using the Home Observation Measurement of the Environment – Short Form (HOME-SF; Mott, 2004). This measure assesses numerous forms of cognitive stimulation, including the presence of learning materials in the home, the child’s engagement with activities outside the home, the degree of parent-child interaction, and parent scaffolding of child learning. The original scoring assesses the degree of cognitive stimulation. Because we were interested in quantifying cognitive deprivation, we reversed scored the measure. The HOME items are scored dichotomously such that the presence of a stimulating activity or experience is coded as 1 and the absence is coded as 0. To create a cognitive deprivation measure, we created a binary score of the 19 cognitive stimulation items such that the presence of each item reflecting cognitive stimulation was scored as a 0 and the absence was scored as a 1. We then z-scored this variable to create a final cognitive deprivation variable. Emotional deprivation was quantified by creating a composite of several scales assessing emotional neglect of the child by caregivers. These included the emotional neglect items from the CECA as well as the emotional neglect subscale of the Multidimensional Neglectful Behavior Scale (MNBS; Kantor et al., 2004). The CECA neglect scale includes items that assess both emotional and physical neglect. We included only items assessing emotional neglect (items 2, 3, 5, 8, 11, 12, 13, and 14). We created a total sum score for each of these scales, standardized each scale (i.e., created a z-score), and averaged these two z-scores together to create the final composite score of emotional deprivation. Physical deprivation was quantified using the physical needs subscale of the MNBS, the 4-item Household Food Insecurity Scale, and the physical neglect subscale of the CTQ. Because each of these items are on similar scales and had a nearly identical range in our dataset, we took the mean of these three scales and then created a z-score from this average to create a composite score of physical deprivation. To create a composite reflecting all three types of deprivation, we took the mean of the cognitive, emotional, and physical deprivation standardized scores. This approach to creating the deprivation composite in this dataset was pre-registered (https://osf.io/6yf4p/).
1.2.3. Pubertal development
Pubertal stage was determined using self-report Tanner Staging (Marshall and Tanner, 1969b, Marshall and Tanner, 1969a, Morris and Udry, 1980). Using drawings of two secondary sex characteristics (pubic hair and breast/testes development), participants reported their development on a scale of 1–5. We computed an average score of these ratings. A Tanner stage of 1 signifies no pubertal development has begun; a stage of 5 signifies adult levels of maturity. Self-report Tanner stage scores correlate with physicians’ physical examination of pubertal development (Coleman and Coleman, 2002, Shirtcliff et al., 2009).
To create a metric of pubertal timing, we used the residuals from a regression model in which we regressed chronological age onto Tanner stage. Positive residuals (>0) reflect greater pubertal maturation than is typical for that chronological age. Given significant sex differences in the sample (including significant sex differences in Tanner stage: t(189) = −3.94, p < 0.01) we calculated this pubertal timing variable separately for boys and girls.
1.2.4. Internalizing and externalizing symptoms
Children and caregivers completed the Youth Self-Report (YSR) and Child Behavioral Checklist (CBCL; Achenbach, 1991). These widely used scales utilize normative data to generate age-standardized estimates of symptom severity. We used the highest internalizing/externalizing problems T-score from child or caregiver as our metric of internalizing and externalizing symptoms.
1.2.5. Covariates
All models were adjusted for age. Models including threat or deprivation exposure were also adjusted for socioeconomic status (income to needs ratio).
1.3. Mediation analyses
We first used linear regression analyses to estimate the associations of threat and deprivation experiences with internalizing and externalizing psychopathology. Second, we used linear regression to estimate associations between ELA and pubertal timing. Third, we used linear regression to estimate the association between pubertal timing and internalizing and externalizing symptoms. These models were estimated separately for boys and girls given sex differences in ELA experiences, pubertal timing, and symptoms of psychopathology. We tested a mediation model only when there was a significant association between ELA and pubertal timing and pubertal timing and internalizing or externalizing symptoms. Mediation models with bootstrapped confidence intervals (10,000 iterations) were tested using version 4.1 of the PROCESS macro (Hayes, 2013) in R, version 3.6.2 (R Core Team, 2019).
1.4. fMRI
1.4.1. Emotional faces task
The emotional face task utilized a block design that consisted of two runs of six 18-second blocks during which participant passively viewed fearful, calm, perceptually matched scrambled face stimuli or fixation blocks displayed in a pseudorandom order that ensured no block type was displayed twice in a row. During each block, 36 faces of different actors expressing the same emotion were displayed for 300 ms each, with 200 ms between each face (based on prior face processing tasks designed to elimit strong amygdala response; (Somerville et al., 2004). Faces were drawn from the NimStim stimulus set (Tottenham et al., 2009). For this study, we focused on fearful faces, which elicit a strong amygdala response (Fusar-Poli et al., 2009) and signal the presence of a potential threat in the environment. Calm faces were used as our neutral face comparison, as neutral faces can be perceived as threatening, especially to children and adolescents (Lobaugh et al., 2006, Thomas et al., 2001, Tottenham et al., 2014). As an attention check, participants were prompted once per block to indicate by an index or middle finger button press whether the last face they saw was male or female (or whether a dot appeared on the left or right side of the screen for the scrambled face blocks). Three participants were excluded from analyses for nonresponses to these cues. See (Cuartas et al., 2021, Weissman et al., 2022) for more details on the task.
1.4.2. fMRI data acquisition
Data were collected with a 3 T Philips Achieva scanner at the University of Washington Integrated Brain Imaging Center using a 32-channel head coil. Functional data were acquired using a gradient-echo T2 * -weighted EPI sequence of 37 3-mm-thick slices acquired sequentially and parallel to the AC-PC line with repetition time (TR)= 2000 ms, echo time (TE)= 25 ms, flip angle= 79 degrees, interslice gap = 0 mm, field of view = 224 × 224 x 132.6 mm, matrix size 76 × 74, voxel size = 2.8 × 2.8 × 3.6 mm. For coregistration of functional images, a structural T1-weighted (T1w) multi-echo MPRAGE image was acquired using the following parameters: TR= 2530 ms, TE= 1640–7040 μs, flip angle= 7 degrees, field of view= 256 mm2, 176 slices, in-plane voxel size= 1 mm3.
1.4.3. fMRI analysis
Preprocessing and statistical analysis of fMRI data was performed in a pipeline using Make, a software development tool optimized for neuroimaging analyses that rely on multiple software packages (Askren et al., 2016). Motion and slice-time correction and skull-stripping were performed in FRIB Software Library (FSL). Despiking was performed using AFNI’s 3dDespike tool. Spatial smoothing was performed with a Gaussian kernel (6-mm full width at half maximum) using the Smallest Univalue Segment Assimilating Nucleus (SUSAN) noise reduction technique from FSL (Jenkinson et al., 2012, Smith and Brady, 1997). Outlier volumes were defined as having a framewise displacement > 1 mm, or the derivative of variance in BOLD signal across the brain exceeded the upper fence (above 75th percentile + 1.5 X interquartile range), or signal intensity was more than 3 SDs from the mean. These volumes (TRs) were regressed out of within-person models. Six rigid-body motion regressors and the time series extracted from white matter and ventricles were included in within-person models to reduce noise associated with motion and physiological fluctuations. Participants with > 20 % of volumes in any run marked as outliers as described above were excluded from analyses (n = 6). Within-person and between-person models were estimated using FEAT in FSL (FMRIB's Software Library; (Woolrich et al., 2004; Woolrich, Ripley et al., 2001). Following estimation of within-person models, the resulting functional contrast images were normalized into standard space (Montreal Neurological Institute, 2 mm 152 Atlas Brain), and coregistrated to native structural T1w space using Advanced Normalization Tools (ANTs) software (Avants et al., 2011).
The time course of each of the three block types (fearful, neutral, scrambled) was convolved with a double-gamma hemodynamic response function (HRF), and regressed on each participant’s preprocessed data using FSL’s FEAT (Woolrich et al., 2001). Group analyses were carried out using FMRIB's Local Analysis of Mixed Effects (FLAME1) modelling and estimations. We applied cluster-level correction in FSL using a threshold that is not associated with elevation in Type 1 error (z > 3.1, p < 0.01)(Eklund et al., 2019; Eklund et al., 2016). We examined differences in BOLD response for the contrast of Fear > Neutral face conditions for its associations with pubertal timing. This was done by examining associations with Tanner Stage controlling for chronological age in whole-brain analyses. We also included sex and a sex-by-pubertal timing interaction to ensure that there were no significant sex differences in brain activation, given sex differences in adversity experiences, pubertal stage, and psychopathology.
In addition to whole-brain analyses, we also extracted data from the contrast of Fear > Neutral faces within four apriori regions of interest (ROI): amygdala, hippocampus, fusiform gyrus, and V1. The Harvard Oxford subcortical probabilistic structural atlas was used to define amygdala, hippocampus, and fusiform regions, while the Juelich atlas was used to define the V1 ROI. All ROI masks were thresholded at 50 % probably, binarized and registered to each subjects’ functional native space using ANTs. The bilateral amygdala ROI was also used as the seed for perform functional connectivity analyses. For each subject, a functionally-weighted estimates for the contrast Fear > Neutral faces was extracted for each ROI. Regression analyses in R (R Core Team, 2019) were conducted to evaluate associations with pubertal timing and internalizing and externalizing symptoms. Again, we explored main effects of sex and a sex-by-pubertal timing interaction to ensure there were no significant sex differences in ROI activation.
1.4.4. Task-based functional connectivity
To examine whole-brain functional connectivity associated with bilateral amygdala activity during the fearful > neutral faces conditions, we employed generalized psychophysiological interaction modelling (gPPI; McLaren et al., 2012). In each within-person model, the psychological condition was defined as (Fear > Neutral task contrast), and the physiological condition was defined as bilateral amygdala activity across the task (seed was defined previously). Whole-brain functional connectivity was explored as the interaction between amygdala activity and task conditions (amygdala x (Fear > Neutral) task contrast). In other words, this analysis was designed to reveal which regions of the brain (in a whole-brain analysis) are functionally associated with the amygdala activity during fearful > neutral face conditions.
We further examined amygdala-mPFC connectivity using 3 mPFC spherical ROIs (radius = 5 mm) from Bloom et al. (2021) rather than the original mPFC ROIs from Gee et al. (2013) due to the original ROI containing a high proportion of white matter voxels relative to cortical voxels. The 3 spherical ROIs defined by Bloom et al., include: one centered at the peak coordinates of the original ROI, the second slightly anterior and the third shifted slightly ventral relative to the second (see Bloom, [ et al., 2021] for more detail about ROI creation).
2. Results
2.1. Demographic and clinical characteristics
Participant demographic and clinical characteristics and intercorrelations are presented in Table 1 and 2. As expected in an age-matched adolescent sample, boys and girls differed significantly in self-reported pubertal stage, with girls having significantly higher Tanner staging scores than boys. Not expected however, was that boys and girls differed significantly in our measures of adversity experiences, with boys having experienced higher levels of both threat- and deprivation-related adversity. Finally, boys had significantly higher levels of internalizing symptoms and marginally higher levels of externalizing symptoms than girls.
Table 1.
Participant characteristics.
| Boys (n = 117) | Girls (n = 110) | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | % (n) | Mean (SD) | Range | % (n) | ||
| Demographics | |||||||
| Age, years | 11.48 (0.48) | 10–13 | 11.47(0.47) | 10–13 | t(225) = 0.15, p = 0.88 | ||
| Tanner Stage | 1.98 (0.76) | 1–4 | 2.45 (0.89) | 1–5 | t(191) = −3.98, p < 0.001 | ||
| Race/ethnicity, % | χ2(4) = 2.87, p = 0.58 | ||||||
| White | 63.25 (74) | 64.55 (71) | |||||
| Black | 10.26 (12) | 14.55 (16) | |||||
| Latino | 11.97 (14) | 10 (11) | |||||
| Asian | 10.26 (12) | 5.45 (6) | |||||
| Other | 4.27 (5) | 5.45 (6) | |||||
| Parent Income to Needs Ratio | 3.44 (1.85) | 0–8 | 3.73 (1.76) | 0–8 | t(223) = −1.20, p = 0.23 | ||
| Early-life adversity exposure | |||||||
| Threat exposure composite | 0.11 (0.74) | -0.59–3 | -0.12 (0.62) | -0.59–3 | t(225) = 2.46, p = 0.01 | ||
| Deprivation exposure composite | 0.12 (0.73) | -1.22–2.34 | -0.11 (0.66) | -1.17–2.48 | t(225) = 2.49, p = 0.01 | ||
| Psychopathology | |||||||
| Internalizing Problems | 59.56 (9.05) | 41–79 | 54.86 (9.63) | 38–80 | t(225) = 3.78, p < 0.001 | ||
| Externalizing Problems | 53.89 (7.99) | 33–80 | 51.78 (8.92) | 34–73 | t(225) = 1.88, p = 0.06 |
Note. SD=standard deviation
Table 2.
Correlation matrix.
| Age | Tanner Stage | Income to Needs Ratio | Threat Exposure | Deprivation Exposure | Internalizing Problems | Externalizing Problems | |
|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||
| Tanner Stage | 0.32 *** | 1 | |||||
| Income to Needs Ratio | 0.003 | -0.029 | 1 | ||||
| Threat Exposure | -0.07 | 0.027 | -0.29 *** | 1 | |||
| Deprivation Exposure | 0.009 | -0.03 | -0.51 *** | 0.32 *** | 1 | ||
| Internalizing Problems | -0.01 | -0.11 | -0.19 ** | 0.29 *** | 0.32 *** | 1 | |
| Externalizing Problems | 0.0002 | 0.23 ** | -0.25 *** | 0.35 *** | 0.30 *** | 0.51 *** | 1 |
*p < 0.05
**p < 0.01
***p < 0.001
2.2. Associations among early life adversity, pubertal timing and psychopathology
2.2.1. Early life adversity and psychopathology
Greater threat-related adversity experiences were associated with higher levels of internalizing symptoms in girls only (Boys: β = 0.119, p = 0.231; Girls: β = 0.298, p = 0.003) and with higher levels of externalizing symptoms in boys only (Boys: β = 0.393, p < 0.001; Girls: β = 0.121, p = 0.225). Greater deprivation experiences were also associated with higher levels of internalizing symptoms (Boys: β = 0.306, p = 0.006; Girls: β = 0.177, p = 0.122) and externalizing symptoms in boys only (Boys: β = 0.337, p < 0.001; Girls: β = 0.042, p = 0.719).
2.2.2. Early life adversity and pubertal timing
Greater threat-related adversity experiences were associated with earlier pubertal timing in boys only (Boys: β = 0.245, p = 0.010; Girls: β = −0.089, p = 0.436). Greater deprivation experiences were not associated with earlier pubertal timing in boys or girls (Boys: β = −0.028, p = 0.797; Girls: β = −0.069, p = 0.608).
2.2.3. Pubertal timing and psychopathology
Earlier pubertal timing was associated with higher levels of externalizing symptoms in both boys and girls (Boys: β = 0.337, p = 0.006; Girls: β = 0.301, p = 0.007). Earlier pubertal timing was not associated with higher levels of internalizing symptoms in either boys or girls (Boys: β = −0.197, p = 0.098; Girls: β = 0.087, p = 0.437).
2.3. Mediation models
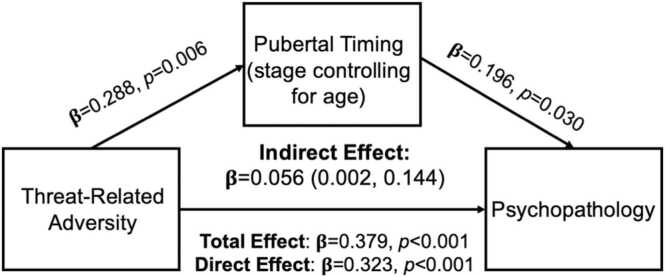
Given the pattern of associations above, we tested whether pubertal timing mediated the association between threat-related ELA and externalizing symptoms for boys only. We observed a significant indirect effect of threat experiences on externalizing symptoms through pubertal timing (β = 0.056, 95 % CI=0.002, 0.144; Fig. 1), suggesting that earlier pubertal timing mediates the association between exposure to threat-related adversity and externalizing symptoms, controlling for deprivation experiences.
Fig. 1.
Mediation Model Figure for threat experiences on externalizing symptoms through pubertal timing (Tanner Stage controlling for chronological age) in boys only. Model adjusted for co-occurring deprivation experiences.
3.3. fMRI.
For whole-brain maps of task-evoked activation for fear versus calm faces in the full sample (n = 149) see Fig. S1.
There was no association of pubertal timing (Tanner Stage controlling for chronological age), sex, or a pubertal timing by sex interaction with activation to fear > neutral faces in whole-brain analysis. Given the absence of sex differences in activation across the whole brain for our contrast of interest, we conducted the remaining fMRI analyses in the full sample as opposed to separately for boys and girls.
2.3.1. ROI analyses
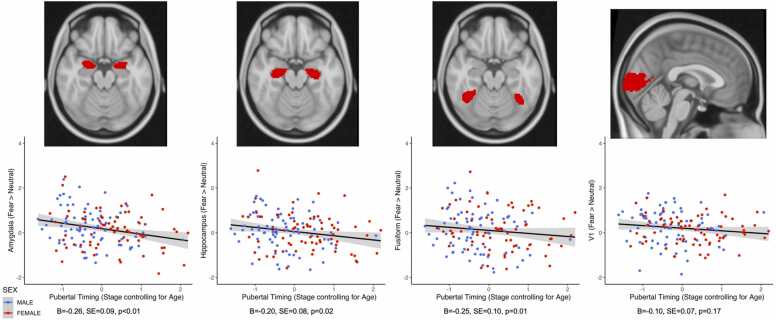
Earlier pubertal timing was negatively associated with activation in bilateral amygdala (β = −0.287, p = 0.002), bilateral hippocampus (β = −0.235, p = 0.010), and fusiform gyrus (β = −0.193, p = 0.032) to fear > neutral faces. There was no association between pubertal timing and activation in our V1 control region (β = −0.137, p = 0.136). These results suggest that earlier pubertal timing is associated with decreased activation in the amygdala, hippocampus, and fusiform gyrus to fear > neutral faces. There was no association of sex or pubertal timing by sex interactions in these ROIs (βs<0.098, ps > 0.220).
Given the associations between pubertal timing and activation in these ROIs, we also examined associations between activation within these ROIs and internalizing and externalizing symptoms. Activation in these regions was unrelated to internalizing or externalizing symptoms (Bs<0.142, ps > 0.416).(Fig. 2).
Fig. 2.
Earlier pubertal timing is negatively associated with activity in bilateral amygdala, bilateral hippocampus, and fusiform regions in response to Fear > Neutral faces. There was no significant association between pubertal timing and activation in the V1 control region. Figure displays z-statistics extracted from each structural ROI.
2.3.2. gPPI functional connectivity analyses
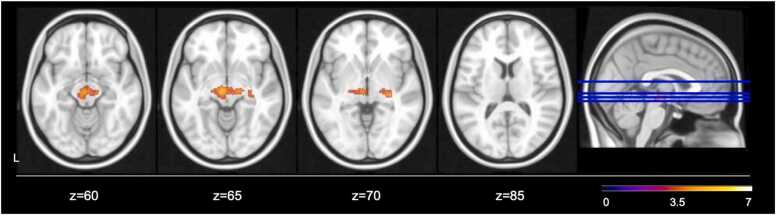
Finally, generalized psychophysiological interaction modellings was used to examine whole-brain functional connectivity of bilateral amygdala with other regions during fear > neutral faces task conditions. Across the whole sample, greater activation in bilateral amygdala was associated with greater activation in a cluster spanning the midbrain and posterior inferior thalamic nuclei (k = 521 voxels, P = 0.0017, Zmax=4.6, z = −6, y = −18, z = −8; Fig. 3). There was no significant main effect of pubertal timing, sex, or pubertal timing by sex interaction on functional connectivity of the bilateral amygdala with any other region across the whole-brain analyses.
Fig. 3.
Whole-brain functional connectivity of bilateral amygdala with other regions of Fear > Neutral task contrast (n = 149, Z > 3.1, p < 0.01).
We then followed up these analyses with ROI analyses to explore associations between bilateral amygdala and mPFC activity that have formed the basis of prior work on the developmental shift in amygdala-mPFC connectivity with age (Bloom et al. (2021). Again, we observed no associations of pubertal timing, sex, or a pubertal timing -by-sex interaction between bilateral amygdala and any of the 3 mPFC ROIs. These results suggest that pubertal timing is not associated with amygdala-mPFC connectivity as hypothesized. There was no significant association between age alone and amygdala-mPFC connectivity in any of the 3 mPFC ROIs examined.
3. Discussion
Earlier pubertal timing is one potential mechanism linking childhood trauma with the emergence of psychopathology in adolescence (Colich and McLaughlin, 2022, McLaughlin et al., 2020). In this study, we explore whether earlier pubertal timing is associated with alterations in neural function—specifically in amygdala-mPFC circuitry—and whether these alterations in neural function may be a mechanism through which earlier pubertal timing is associated with adolescent psychopathology. We explored these associations in a large community sample of 10–13-year-old adolescents. Earlier pubertal timing mediated the association between threat exposure and externalizing symptoms in boys only; deprivation experiences were unrelated to pubertal timing. Contrary to our hypotheses that earlier pubertal timing would be associated with heightened limbic activation to threat cues, earlier pubertal timing was associated with decreased activation in the amygdala, hippocampus, and fusiform gyrus to fear relative to neutral faces. We found no associations between pubertal timing and whole-brain amygdala connectivity or amygdala-mPFC connectivity. These findings support a role for pubertal timing in developmentally normative decreases in limbic activation across development (Gee et al., 2013, Silvers et al., 2017, Vink et al., 2014), but do not support a role for alterations in amygdala-PFC function or connectivity as a link between pubertal timing and psychopathology in adolescence.
In this sample, we found support for earlier pubertal timing as a mechanism linking trauma exposure and externalizing psychopathology in boys only. Given mixed evidence supporting sex differences in the association between trauma and pubertal timing, and the consistent associations including from a large meta-analysis of earlier pubertal timing with both internalizing and externalizing psychopathology (Hamlat et al., 2019, Platt et al., 2017, Ullsperger and Nikolas, 2017), we interpret this finding to be sample-specific rather than suggesting meaningful sex differences in these associations. Boys in our sample experienced both higher levels of adversity and psychopathology relative to girls, and we most likely observed this sex difference because of the higher levels of trauma exposure and psychopathology in boys. Similarly, these findings may be specific to externalizing symptoms only given that boys tend to show higher levels of externalizing symptoms in childhood and adolescence relative to girls, and experience greater increases in externalizing symptoms from childhood to adolescence (Boeldt et al., 2012, Bongers et al., 2004). These findings replicate and extend our earlier work suggesting earlier pubertal timing as a mechanism linking trauma exposure and psychopathology (Colich et al., 2020, Sumner et al., 2019).
Although we originally hypothesized that earlier pubertal timing would be associated with increased limbic activation, we found the opposite – earlier pubertal timing was associated with decreased activation in the amygdala, hippocampus, and fusiform gyrus in response to aversive stimuli. Our original hypotheses were based upon older theoretical models of adolescent brain development (Chein et al., 2011, Ladouceur, 2012, Steinberg, 2010) suggesting that disparities in maturational trajectories of the amygdala and PFC across adolescence contribute to heightened risk for internalizing and externalizing symptoms in adolescence. Similarly, given associations between childhood trauma and heightened amygdala reactivity to threat cues and reduced activation in prefrontal regions that modulate amygdala reactivity to negative cues (McCrory et al., 2013, McCrory et al., 2011, McLaughlin et al., 2015, Peverill et al., 2019), we hypothesized heightened amygdala activation as a mechanism linking earlier pubertal timing and psychopathology in adolescence. Despite these empirically-driven hypotheses, in this study we found the opposite pattern. Earlier maturational disparity theories of neural development have been challenged (Pfeifer and Allen, 2012) and a more complicated picture of how pubertal development impacts neural development has emerged (see Byrne et al., 2016 and Vijayakumar, Op de Macks, Shirtcliff, & Pfeifer, 2018 for a review). Similarly, the limited body of work exploring the impact of pubertal timing on neural development reveals an inconsistent pattern of results, with early puberty (as defined by high DHEA levels independent of chronological age) associated with reduced activity in multiple nodes of the salience network (anterior cingulate, insula, and striatum) to emotional stimuli (Whittle et al., 2015). These patterns are consistent with our findings and together provide preliminary support for the idea that earlier pubertal timing accelerates the developmentally normative pattern of decreasing activation with maturation in regions involved in salience processing (Gee et al., 2013, Silvers et al., 2017, Vink et al., 2014). These findings shed additional light on this developmental pattern of decreasing activation in these regions to suggest that functional development of these regions may be driven by factors associated with pubertal development. However, future studies using a smaller age range and including precise hormonal metrics of pubertal development are needed to further evaluate this interpretation.
Contrary to our original hypotheses, we found no association between pubertal timing and amygdala-mPFC connectivity. It has been proposed that in humans, the pattern of functional connectivity between the amygdala and the mPFC shifts from positive to negative across development in the context of emotional processing tasks (Gee et al., 2013, Silvers et al., 2016, Wu et al., 2016). We hypothesized that this shift in connectivity from childhood to adolescence is related to pubertal development, and thus, earlier pubertal timing would be associated with greater negative connectivity in this circuit. In contrast, we found no associations of either pubertal timing or chronological age with connectivity within this circuit. These results add to a growing body of research suggesting that patterns of neurodevelopmental maturation in amygdala-mPFC connectivity have not been reliably established, including from authors of the original work on this topic (Bloom et al., 2021, Brieant et al., 2021). Similarly, it is possible that heterogeneity across task design (passive viewing vs. emotion labeling; event-related vs. block-design) and task contrasts used in analyses may contribute to differences in results seen across studies (see (Bloom et al., 2021 for a deeper discussion of this issue). For instance, original studies that found a shift from positive to negative connectivity used the contrast of fearful faces relative to implicit baseline (Gee et al., 2013), rather than a contrast that specifically isolated responses to fearful affect (i.e., neutral or scrambled faces). Similarly, there is evidence to suggest accelerated pubertal development is associated with a more mature pattern of amygdala-cingulate functional connectivity at rest (Thijssen et al., 2020, Thijssen et al., 2022). Further work is necessary to disentangle the impact of task and analysis decisions on discrepant results across the literature (Bloom et al., 2021, Demidenko et al., 2022). Similarly, future longitudinal work with a range of emotional processing tasks will be needed to establish the developmental trajectory of amygdala-mPFC connectivity to determine the reliability of using this pattern as a robust metric of neural development.
Several limitations of this study highlight key directions for future research in this area. This sample was ideal for our research questions in many ways. Sampling participants across a wide range of socioeconomic backgrounds and including only a restricted age range allowed for the investigation of associations with pubertal stage, independent from chronological age, and produced wide variation in adversity experiences. However, the sample was not recruited specifically for adversity exposure, and there were unexpected sex differences in adversity experiences that we believe contributed to sex differences in our results. Given that we do not believe these sex differences in exposure to adversity nor sex differences in associations between adversity, pubertal timing, and psychopathology to be representative of sex differences in the general population, we are careful to not draw unwarranted conclusions about sex differences from these findings. Although these data do not support a normative developmental trajectory of positive to negative amygdala-mPFC connectivity in humans, careful consideration must be given to the restricted age range of the sample. Although we would expect to see significant effects of chronological age from ages 10–13 on amygdala-PFC connectivity given earlier studies, it is possible that a wider age-range is necessary to capture this effect. Regardless, this work highlights the need to better establish markers of neurodevelopment, which may highlight alternative mechanisms linking early pubertal maturation and psychopathology. More global metrics, such as “BrainAGE,” may do a better job at distinguishing departure from typical development, and allows for the investigation into whether earlier pubertal timing accelerates development of the brain globally or within specific networks associated with emotion processing. Finally, in this study we sought to explore potential mechanisms through which early adversity exposure and pubertal timing contribute to risk for psychopathology in adolescence. Given the cross-sectional nature of our study design, we cannot imply causality from these data. Although we found evidence for statistical mediation in these data, we cannot conclude that trauma exposure leads to externalizing problems through accelerated pubertal timing. Replicating these findings in longitudinal studies is an important next step.
4. Conclusions
Our findings replicate earlier work suggesting that earlier pubertal timing may be a mechanism linking early trauma exposure and psychopathology, though we found this association only in boys in this sample. We also found associations between earlier pubertal timing and decreased activation in regions involved in salience processing and the fusiform gyrus. These findings suggest that typical development decreases in salience network activation may be driven by pubertal development. Furthermore, our findings are consistent with other recent work in suggesting that positive to negative amygdala-mPFC connectivity may not be a consistent developmental pattern, and as such, should not be used as a marker of brain maturation (Bloom et al., 2021). These results highlight the need to continue to explore markers of neurodevelopmental maturation in order to identify deviations from typical trajectories of development, as potential neurobiological mechanisms linking early-life adversity, pubertal timing, and psychopathology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101187.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
Data will be made available on request.
References
- Achenbach T. YSR, and TRP Profiles. University of Vermont, Research Center for Children, Youth, & Families,; Burlington, VT: 1991. Integrative Guide to the 1991 CBCL/4-18. [Google Scholar]
- Ahmed E.I., Zehr J.L., Schulz K.M., Lorenz B.H., DonCarlos L.L., Sisk C.L. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 2008;11(9):995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon Steinberg, A.M. , Brymer, M.J. , Decker, K.B. , & Pynoos, R.S. (Eds.). The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index, 6 § (2004). [DOI] [PubMed]
- Askren M.K., McAllister-Day T.K., Koh N., Mestre Z., Dines J.N., Korman B.A., Madhyastha T.M. Using make for reproducible and parallel neuroimaging workflow and quality-assurance. Front. Neuroinform. 2016;10(February):1–16. doi: 10.3389/fninf.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse M.E.A., Simmons J.G., Patton G., Mundy L., Byrne M.L., Seal M.L., Whittle S. Adrenarcheal timing longitudinally predicts anxiety symptoms via amygdala connectivity during emotion processing. J. Am. Acad. Child Adolesc. Psychiat. 2019 doi: 10.1016/j.jaac.2019.04.018. [DOI] [PubMed] [Google Scholar]
- Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62(4):647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Ahluvalia T., Pogge D., Handelsman L. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bifulco A., Brown G.W., Harris T.O. Childhood experience of care and abuse (CECA): a retrospective interview measure. J. Child Psychol. Psychiatry. 1994;35(8):1419–1435. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Bloom, P.A., Vantieghem, M., Gabard-durnam, L., Gee, D.G., Flannery, J., Caldera, C., Bloom, P.A. , 2021. Age-related change in task-evoked amygdala-prefrontal circuitry: a multiverse approach with an accelerated longitudinal cohort aged 4–22 years. [DOI] [PMC free article] [PubMed]
- Boeldt D.L., Rhee S.H., DiLalla L.F., Mullineaux P.Y., Schulz-Heik R.J., Corley R.P., Hewitt J.K. The association between positive parenting and externalizing behaviour. Infant Child Dev. 2012;21(1):85–106. doi: 10.1002/icd.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers I.L., Koot H.M., Van Der Ende J., Verhulst F.C. Developmental trajectories of externalizing behaviors in childhood and adolescence. Child Dev. 2004;75(5):1523–1537. doi: 10.1111/j.1467-8624.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- Brieant A.E., Sisk L.M., Gee D.G. Associations among negative life events, changes in cortico-limbic connectivity, and psychopathology in the ABCD study. Dev. Cogn. Neurosci. 2021 doi: 10.1016/j.dcn.2021.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J., Petersen A.C., Eichorn D. The study of maturational timing effects in adolescence. J. Youth Adolesc. 1985;14(3):149–161. doi: 10.1007/BF02090316. [DOI] [PubMed] [Google Scholar]
- Byrne M.L., Whittle S.L., Vijayakumar N., Dennison M.J., Simmons J.G., Allen N.B. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev. Cogn. Neurosci. 2016 doi: 10.1016/j.dcn.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B., Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L.D., O’Brien L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L., Coleman J. The measurement of puberty: a review. J. Adolesc. 2002;25:535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Colich N.L., McLaughlin K.A. Accelerated pubertal development as a mechanism linking trauma exposure with depression and anxiety in adolescence. Curr. Opin. Psychol. 2022 doi: 10.1016/j.copsyc.2022.101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., Williams E.S., Ho T.C., King L.S., Humphreys K.L., Price A.N., Gotlib I.H. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Dev. Psychopathol. 2017;29(05):1851–1864. doi: 10.1017/S0954579417001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., Rosen M.L., Williams E.S., McLaughlin K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol. Bull. 2020;August doi: 10.1037/bul0000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., Platt J.M., Keyes K.M., Sumner J.A., Allen N.B., McLaughlin K.A. Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychol. Med. 2020;50(7):1090–1098. doi: 10.1017/S0033291719000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartas J., Weissman D.G., Sheridan M.A., Lengua L., McLaughlin K.A. Corporal punishment and elevated neural response to threat in children. Child Dev. 2021;92(3):821–832. doi: 10.1111/cdev.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko, M.I., Kelly, D.P., Hardi, F.A., Ip, K.I., Lee, S., Becker, H., Keating, D.P. , 2022. Neuroimage: Reports Mediating effect of pubertal stages on the family environment and neurodevelopment: An open-data replication and multiverse analysis of an ABCD Study ®, 2(September). https://doi.org/10.1016/j.ynirp.2022.100133. [DOI] [PMC free article] [PubMed]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund, A., Knutsson, H., Nichols, T.E. 2019. Cluster failure revisited: Impact of first level design and physiological noise on cluster false positive rates, (March 2018), 2017–2032. https://doi.org/10.1002/hbm.24350. [DOI] [PMC free article] [PubMed]
- Ellis B.J., Giudice M.Del. Developmental adaptation to stress: an evolutionary perspective. Annu. Rev. Psychol. 2019;70:111–139. doi: 10.1146/annurev-psych-122216-011732. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Figueredo A.J., Brumbach B.H., Schlomer G.L. Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Hum. Nat. 2009;Vol. 20 doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Sheridan M.A., Belsky J., Mclaughlin K.A. Why and how does early adversity influence development? toward an integrated model of dimensions of environmental experience. Dev. Psychopathol. 2022:1–25. doi: 10.1017/S0954579421001838. [DOI] [PubMed] [Google Scholar]
- Finkelhor D., Hamby S.L., Ormrod R., Turner H. The juvenile victimization questionnaire: reliability, validity, and national norms. Child Abus. Negl. 2005 doi: 10.1016/j.chiabu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Fox, N.A., Leavitt, L.A. , 1995. The Violence Exposure Scale for Children-Revised (VEX-R). College Park, MD: University of Maryland.
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34:6. [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J.L.J., Flannery J., Goff B., Gee D.G.D.G., Humphreys K.L., Telzer E., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectinal study. Neuroimage. 2014;95(95):193–207. doi: 10.1016/j.neuroimage.2014.03.038.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Natsuaki M.N. In search of explanations for early pubertal timing effects on developmental psychopathology. Curr. Dir. Psychol. Sci. 2009;18(6):327–331. doi: 10.1111/j.1467-8721.2009.01661.x. [DOI] [Google Scholar]
- Gee D.G. Early adversity and development: parsing heterogeneity and identifying pathways of risk and resilience. Am. J. Psychiatry. 2021;178(11):998–1013. doi: 10.1176/appi.ajp.2021.21090944. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci.: Off. J. Soc. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlat E.J., Snyder H.R., Young J.F., Hankin B.L. Pubertal timing as a transdiagnostic risk for psychopathology in youth. Clin. Psychol. Sci. 2019;7(3):411–429. doi: 10.1177/2167702618810518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A.F. , 2013). Introduction to Mediation, Moderation, and Conditional Process Analysis. [DOI] [PubMed]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kantor G., Holt M., Mebert C., Straus M., Drach K., Ricci L., Brown W. Development and preliminary psychometric properties of the multidimensional neglectful behavior scale-child report. Child Maltreatment. 2004;9(4):409–428. doi: 10.1177/1077559504269530. [DOI] [PubMed] [Google Scholar]
- Keding T.J., Herringa R.J. Paradoxical prefrontal–amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 2016;41(12):2903–2912. doi: 10.1038/npp.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front. Integr. Neurosci. 2012;6(August):1–11. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F.S., Heimer H., Giedd J.N., Lein E.S., Estan N., Weinberger D.R., Casey B. Adolescent mental health--Opportunity and obligation. Science. 2014;346(6209):547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua L.J., Moran L., Zalewski M., Ruberry E., Kiff C., Thompson S. Relations of growth in effortful control to family income, cumulative risk, and adjustment in preschool-age children. J. Abnorm. Child Psychol. 2015;43(4):705–720. doi: 10.1007/s10802-014-9941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh N.J., Gibson E., Taylor M.J. Children recruit distinct neural systems for implicit emotional face processing. Neuroreport. 2006;17(2):215–219. doi: 10.1097/01.wnr.0000198946.00445.2f. [DOI] [PubMed] [Google Scholar]
- Marshall W., Tanner J. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1969;45(239):291–303. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W., Tanner J. Variations in the pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44(291):291–303. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A.S.A., Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front. Psychiatry. 2011;2(JUL):1–14. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Kelly P.A., Bird G., Sebastian C.L., Mechelli A., Viding E. Amygdala activation in maltreated children during pre-attentive emotional processing. Br. J. Psychiatry. 2013;202(4):269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry. 2012;69(11):1151. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Peverill M., Gold A.L., Alves S., Sheridan M.A., KA., M, Sheridan M.A. Child maltreatment and neural systems underlying emotion regulation. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Colich N.L., Rodman A.M., Weissman D.G. Mechanisms linking childhood trauma exposure and psychopathology: a transdiagnostic model of risk and resilience. BMC Med. 2020;18(1):1–11. doi: 10.1186/s12916-020-01561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Humphreys K.L., Belsky J., Ellis B.J. The value of dimensional models of early experience: thinking clearly about concepts and categories. Perspect. Psychol. Sci. 2021 doi: 10.1177/1745691621992346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N.M., Udry J.R. Validation of a self-administered instrument to assess stage of adolescent development I. J. Youth Adolesc. 1980;9:3. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Mott F.L. The utility of the HOME-SF scale for child development research in a large national longitudinal survey: the national longitudinal survey of youth 1979 cohort. Parenting. 2004;4(2–3):259–270. doi: 10.1080/15295192.2004.9681273. [DOI] [Google Scholar]
- Negriff S., Blankson A.N., Trickett P.K. Pubertal timing and tempo: associations with childhood maltreatment. J. Res. Adolesc. 2015;25(2):201–213. doi: 10.1111/jora.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negriff S., Saxbe D.E., Trickett P.K. Childhood maltreatment, pubertal development, HPA axis functioning, and psychosocial outcomes: an integrative biopsychosocial model. Dev. Psychobiol. 2015;57(8):984–993. doi: 10.1002/dev.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverill M., Sheridan M.A., Busso D.S., McLaughlin K.A. Atypical prefrontal–amygdala circuitry following childhood exposure to abuse: links with adolescent psychopathology. Child Maltreatment. 2019 doi: 10.1177/1077559519852676. 107755951985267–107755951985267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn. Sci. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J.M., Colich N.L., McLaughlin K.A., Gary D., Keyes K.M. Transdiagnostic psychiatric disorder risk associated with early age of menarche: a latent modeling approach. Compr. Psychiatry. 2017;79:70–79. doi: 10.1016/j.comppsych.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Scherf K.S., Behrmann M., Dahl R.E. Facing changes and changing faces in adolescence: a new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev. Cogn. Neurosci. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Dahl R.E., Pollak S.D. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Lumian D.S., Gabard-Durnam L., Gee D.G., Goff B., Fareri D.S., Tottenham N. Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J. Neurosci. 2016;36(24):6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R., Ochsner K.N. The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Dev. Cogn. Neurosci. 2017;25:128–137. doi: 10.1016/j.dcn.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Brady J.M. SUSAN—a new approach to low level image processing. Int. J. Comput. Vis. 1997;23(1):45–78. doi: 10.1023/A:1007963824710. [DOI] [Google Scholar]
- Somerville L.H., Kim H., Johnstone T., Alexander A.L., Whalen P.J. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biol. Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Steinberg L.D. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Sumner J.A., Colich N.L., Uddin M., Armstrong D., McLaughlin K.A. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psychiatry. 2019;85(3):268–278. doi: 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Collins P.F., Luciana M. Pubertal development mediates the association between family environment and brain structure and function in childhood. Dev. Psychopathol. 2020;32(2):687–702. doi: 10.1017/S0954579419000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Collins P.F., Weiss H., Luciana M. The longitudinal association between externalizing behavior and frontoamygdalar resting-state functional connectivity in late adolescence and young adulthood. J. Child Psychol. Psychiatry Allied Discip. 2021;62(7):857–867. doi: 10.1111/jcpp.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Collins P.F., Luciana M. Does pubertal stage mediate the association between family environment and structure and function of the amygdala-mPFC circuit? A replication study of the longitudinal ABCD cohort. Dev. Cogn. Neurosci. 2022;56(June) doi: 10.1016/j.dcn.2022.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Whalen P.J., Eccard C.H., Dahl R.E., Ryan N.D., Casey B.J. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49(4):309–316. doi: 10.1016/S0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.J.W., Leon A.C.A., McCarry T., Nurse M., Hare T.A., Nelson C.A. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Phuong J., Flannery J., Gabard-Durnam L., Goff B. A negativity bias for ambiguous facial expression valence during childhood: converging evidence from behavior facial corrugator muscle responses. Emotion. 2014;13(1):92–103. doi: 10.1037/a0029431.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger J.M., Nikolas M.A. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: are there sex differences in risk. Psychol. Bull. 2017;143(9):903–938. doi: 10.1037/bul0000106. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde A.C.K., Westhoff B., de Vos F., Wierenga L.M., Crone E.A. A three-wave longitudinal study of subcortical–cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum. Brain Mapp. 2019;40(13):3769–3783. doi: 10.1002/hbm.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., de Macks Op, Shirtcliff Z., Pfeifer, J. H E.A., &. Puberty and the human brain: insights into adolescent development. Neurosci. Biobehav. Rev. 2018;92(July):417–436. doi: 10.1016/j.neubiorev.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Derks J.M., Hoogendam J.M., Hillegers M., Kahn R.S. Functional differences in emotion processing during adolescence and early adulthood. NeuroImage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Weissman D.G., Rosen M.L., Colich N.L., Sambrook K.A., Lengua L.J., Sheridan M.A., McLaughlin K.A. Exposure to violence as an environmental pathway linking low socioeconomic status with altered neural processing of threat and adolescent psychopathology. J. Cogn. Neurosci. 2022:1–14. doi: 10.1162/jocn_a_01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S.L., Simmons J.G., Byrne M.L., Strikwerda-Brown C., Kerestes R., Seal M.L., Allen N.B. Associations between early adrenarche, affective brain function and mental health in children. Soc. Cogn. Affect. Neurosci. 2015;10(9):1282–1290. doi: 10.1093/scan/nsv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M., Ripley B., Brady M., Smith S. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich M., Behrens T., Beckmann C.F., Jenkinson M., Smith S. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D., Phan K.L. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 2016;37(5):1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.