Abstract
Neuroimaging studies have demonstrated that migraine is accompanied by spontaneous brain activity alterations in specific regions. However, these findings are inconsistent, thus hindering our understanding of the potential neuropathology. Hence, we performed a quantitative whole‐brain meta‐analysis of relevant resting‐state functional imaging studies to identify brain regions consistently involved in migraine. A systematic search of studies that investigated the differences in spontaneous brain activity patterns between migraineurs and healthy controls up to April 2022 was conducted. We then performed a whole‐brain voxel‐wise meta‐analysis using the anisotropic effect size version of seed‐based d mapping software. Complementary analyses including jackknife sensitivity analysis, heterogeneity test, publication bias test, subgroup analysis, and meta‐regression analysis were conducted as well. In total, 24 studies that reported 31 datasets were finally eligible for our meta‐analysis, including 748 patients and 690 controls. In contrast to healthy controls, migraineurs demonstrated consistent and robust decreased spontaneous brain activity in the angular gyrus, visual cortex, and cerebellum, while increased activity in the caudate, thalamus, pons, and prefrontal cortex. Results were robust and highly replicable in the following jackknife sensitivity analysis and subgroup analysis. Meta‐regression analyses revealed that a higher visual analog scale score in the patient sample was associated with increased spontaneous brain activity in the left thalamus. These findings provided not only a comprehensive overview of spontaneous brain activity patterns impairments, but also useful insights into the pathophysiology of dysfunction in migraine.
Keywords: functional neuroimaging, meta‐analysis, migraine, resting‐state, spontaneous brain activity
In this article, we performed the first quantitative voxel‐wise meta‐analysis of spontaneous brain activity abnormalities in patients with migraine, and found several brain regions associated with migraine, including the angular gyrus, visual cortex, cerebellum, caudate, thalamus, pons, and prefrontal cortex.
1. INTRODUCTION
Migraine is a prevalent neurovascular disorder in the general population characterized by disabling attacks, usually unilateral, of moderate‐to‐severe intensity headache. It is often accompanied by nausea, vomiting, and extreme sensitivities to visual, auditory, olfactory, and somatosensory stimuli. Additionally, migraineurs may have a variety of other neurological symptoms, like vertigo, dizziness, tinnitus, and cognitive impairment (Dodick, 2018). Migraine severely interferes with people's quality of life and leads to substantial social and economic burdens. Current theories of migraine pathophysiology predominantly involve the activation and sensitization of the trigeminovascular system and clinical manifestation of cortical spreading depression in migraine aura (Ashina et al., 2019; Noseda & Burstein, 2013). Nevertheless, the potential mechanisms remain elusive, limiting the development of effective treatments for this prevalent disorder.
In recent years, neuroimaging approaches hold promise for investigating intrinsic brain activity abnormalities and providing new information on the pathogenesis of neuropsychiatric disorders. Thereinto, as a noninvasive imaging technique based on blood‐oxygen‐level‐dependent (BOLD) signal, resting‐state functional magnetic resonance imaging (rs‐fMRI) has been widely used to measure spontaneous brain activity, thus potentially elucidating the neural mechanisms of migraine (Chen & Glover, 2015; Fox & Raichle, 2007; Gusnard et al., 2001; Schwedt et al., 2015). There are several analytic approaches to depict the characteristics of BOLD signals in rs‐fMRI, such as amplitude of low‐frequency fluctuations (ALFF), fractional amplitude of low‐frequency fluctuations (fALFF), and regional homogeneity (ReHo). As reliable and reproducible data‐driven approaches, ALFF estimates the total power of a given time course within the low‐frequency range (e.g., 0.01–0.1 Hz), whereas fALFF represents the relative contribution of specific low‐frequency oscillations to the whole frequency range (Zang et al., 2007; Zou et al., 2008). ReHo evaluates the similarity or synchronization of the time series between a given voxel and its nearest neighbors (Zang et al., 2004). An alternative imaging approach with radiotracer techniques like positron emission tomography (PET) or single‐photon emission computed tomography could be used to measure regional cerebral blood flow (rCBF) or cerebral glucose metabolism, which can also reflect spontaneous neuronal activity (Hannawi et al., 2015; Schytz et al., 2019). Besides, arterial spin labeling (ASL), a technique utilizing magnetically labeled arterial blood water protons as endogenous tracer, is able to provide reliable absolute quantification of rCBF as well (Petcharunpaisan et al., 2010).
Numerous studies have identified extensive spontaneous brain activity changes in multiple brain regions in migraineurs compared with healthy controls (HCs), such as the frontal cortex (Lisicki et al., 2019; Wei et al., 2022; Zhang et al., 2021), cerebellum (Liu et al., 2021; Wang et al., 2016; Zhao et al., 2014), and middle temporal gyrus (Michels et al., 2019; Ning et al., 2017; Zhao et al., 2013). The affected brain regions found in these studies vary considerably, and even conflicting findings exist in some studies. For example, some studies found increased ReHo in the thalamus in migraineurs (Chen et al., 2019; Meylakh et al., 2018), whereas another study observed decreased ReHo in the same region (Zhao et al., 2014). In addition, increased ALFF and decreased ReHo in the putamen were also detected in distinct studies (Chen et al., 2019; Li et al., 2017). The reasons for these inconsistent results come from multiple aspects. The neuroimaging approaches mentioned above rely on different underlying theoretical assumptions, and although they can all be used to measure intrinsic neuronal activity, these differences might lead to divergent results. The differences in demographic and clinical characteristics of subjects, and analytic protocols (e.g., data preprocessing and statistical analysis) are also possible reasons. In addition, individual studies with small sample sizes have low statistical power and higher probability of false positives, which could affect the generalizability of obtained results (Müller et al., 2018; Tahmasian et al., 2019). This inconsistency hindered us from understanding the pathophysiological mechanisms of migraine, and further exploration is really warranted to advance the field. Neuroimaging meta‐analysis method enables unbiased synthesis of results from numerous studies. Early neuroimaging meta‐analyses in migraine mostly summarized brain morphological alterations (Masson et al., 2021; Sheng et al., 2020; Wang, Wang, et al., 2020). To our knowledge, there has not yet been a quantitative meta‐analysis targeting the resting‐state local dysfunction of specialized brain regions in migraineurs. Hence, with the anisotropic effect size version of seed‐based d mapping (AES‐SDM) software, we performed a whole‐brain voxel‐wise meta‐analysis to unify various findings of previous functional neuroimaging studies into consistent patterns of impairments in migraine.
AES‐SDM is a coordinate‐based meta‐analytic tool that quantitatively integrates published studies using reported peak coordinates and statistical parametric maps. With strict selection of brain regions at the whole‐brain level and unbiased inclusion of null findings, AES‐SDM has high sensitivity and a low rate of false positives (Radua et al., 2014; Radua & Mataix‐Cols, 2012). Furthermore, it has the advantage over activation likelihood estimate or multilevel kernel density analysis methods for combining both positive and negative coordinates in the same map to prevent opposite directions of findings in a particular voxel at the same time (Radua & Mataix‐Cols, 2009). Hitherto, AES‐SDM has proven to be a powerful tool and is widely used in neuroimaging meta‐analyses (Li et al., 2022; Pan et al., 2021). In the present meta‐analysis, the aims were mainly twofold. First, we sought to obtain consistent and robust results of spontaneous brain activity alterations in patients with migraine by integrating existing eligible studies about resting‐state brain activity; second, we aimed to explore the underlying roles of different demographic, clinical, or methodological variables in main results through subgroup meta‐analyses and meta‐regression analyses.
2. METHODS
2.1. Search strategy and selection criteria
A systematic search was conducted in PubMed, Web of Science, and Embase databases to retrieve studies published before April 2022 with the following search terms: “migraine” and (“neuroimaging” or “fMRI” or “functional magnetic resonance imaging” or “ALFF” or “amplitude of low‐frequency fluctuations” or “fALFF” or “fractional amplitude of low‐frequency fluctuations” or “ReHo” or “regional homogeneity” or “ASL” or “arterial spin labeling” or “PET” or “positron emission tomography” or “SPECT” or “single photon emission computed tomography”) and (“resting state” or “rest”). The reference lists of included studies and relevant scholarly reviews were also searched for additional studies. To be included, the studies needed to satisfy the following criteria: (1) the studies were original research and published in English‐language journals with peer review; (2) enrolled adult patients with migraine according to established diagnostic criteria; (3) conducted a whole‐brain voxel‐wise analysis to compare regional spontaneous brain activity of migraineurs with that of HCs; (4) provided three‐dimensional coordinates of significant clusters in Montreal Neurological Institute (MNI) or Talairach space, or reported null findings; (5) applied consistent statistical thresholds across the whole brain. The exclusion criteria were as follows: (1) the studies concerned other types of headache (e.g., cluster headache, medication overuse headache or tension headache) or a special subtype of migraine (e.g., vestibular migraine or pediatric migraine); (2) the studies only reported results obtained from the region of interest analysis or small volume correction; (3) the number of participants was less than seven in either the migraine group or control group (Tahmasian et al., 2019); (4) sufficient data for the meta‐analysis could not be obtained from original articles or after contacting the authors. If one patient group overlapped with another study, the study with larger sample size was retained. If an article reported multiple independent patient samples or neuroimaging metrics, they were treated as separate datasets. Moreover, in case of a longitudinal design, we only included the baseline comparison between patients and HCs. The preferred reporting items for systematic reviews and meta‐analyses guidelines were followed in our study (Moher et al., 2009), and the detailed research screening process is presented in Figure 1.
FIGURE 1.
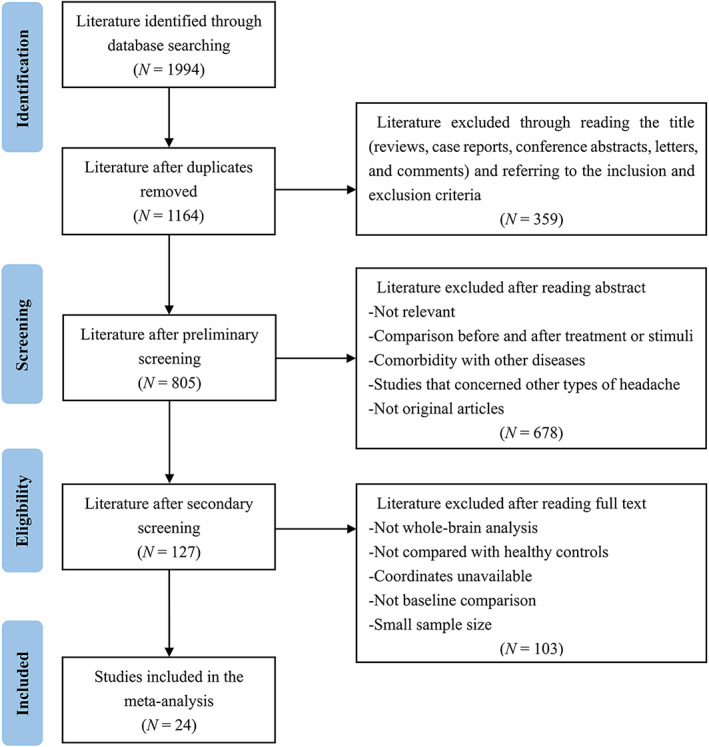
The flow diagram of the search strategy and retrieved studies according to the PRISMA guidelines. N, number; PRISMA, preferred reporting items for systematic reviews and meta‐analyses
2.2. Data extraction
The following information was extracted from the retrieved studies: demographic (e.g., sample size, mean age and gender) and clinical characteristics (e.g., illness duration, medication status, and pain intensity), methodological features, peak coordinates and statistics (e.g., t‐values or other equivalents). We were unable to extract any peaks or statistics from studies reporting null findings, and an “NA” was used instead. In order to reduce the MNI/Talairach coordinate disparity, reported Talairach coordinates should be transformed into MNI coordinates for analysis (Lancaster et al., 2007). Two authors independently searched the literature, extracted and crosschecked the data, and any disagreements were resolved by consensus.
2.3. Quality assessment
The quality of selected studies was assessed with a 10‐point checklist based on previous meta‐analyses, mainly including the quality of demographic and clinical characterization of subjects, image acquisition and analysis methods, and the quality of reported results and conclusions (Lan et al., 2021; Shepherd et al., 2012). Each item received a score of 1, 0.5, or 0 if the criteria were fully, partially, or not met, respectively. The aim of this rating was to describe the completeness of published studies with a numeric score to aid readers, and it is not intended to critique the investigators or the work itself. The detailed checklist and scores of included studies are shown in Tables S1 and S2.
2.4. Meta‐analysis
A voxel‐wise meta‐analysis of spontaneous brain activity differences between migraineurs and HCs was performed by means of the AES‐SDM software (version 5.15, https://www.sdmproject.com/). The SDM method has been described in detail elsewhere (Radua et al., 2014; Radua & Mataix‐Cols, 2009). In brief, peak MNI coordinates (Talairach coordinates were converted to MNI coordinates by the SDM online converter, https://www.sdmproject.com/utilities/?show=Coordinates) and t‐values or their equivalents (Z‐values or p‐values, which were converted to t‐statistics by the SDM online converter, http://www.sdmproject.com/utilities/?show=Statistics) of regional spontaneous brain activity differences between migraineurs and HCs were extracted from each dataset. In case of studies not reporting any statistics, a “p” was used for positive peaks, and an “n” for negative peaks. Subsequently, the maps of whole‐brain effect size and variance were recreated for each study with an anisotropic Gaussian kernel (full‐width at half‐maximum = 20 mm). Finally, the mean map was generated by combining individual maps using random‐effects meta‐analytic model, weighted by the sample size, intrastudy variance, and interstudy heterogeneity. To optimally balance false positives and negatives, the statistical significance level was set at a voxel‐wise p < .005 with peak height Z > 1 and a cluster extent of more than 100 voxels (Tang et al., 2018).
2.5. Heterogeneity test and publication bias
For the purpose of estimating the between‐study variability in our results, a heterogeneity test was performed based on Cochran's Q statistic, and the percentage of total variation due to heterogeneity was measured with I 2 statistics. Q is distributed as a χ 2 distribution with k − 1 (k is the number of datasets included in meta‐analysis) degrees of freedom, and p Cochran's Q < .05 indicates significant between‐study heterogeneity; I 2 index has a range of values from 0% to 100%, with percentages around 25%, 50%, and 75% representing small, moderate, and large amounts of heterogeneity, respectively (Higgins et al., 2003). Additionally, publication bias for significant findings was examined with funnel plots and Egger's tests by extracting the values from relevant peaks. A visually asymmetric funnel plot and p < .05 in Egger's test suggest the existence of publication bias in a specific region.
2.6. Jackknife sensitivity analysis
In order to assess the robustness and replicability of main results, we performed a whole‐brain voxel‐wise jackknife sensitivity analysis. The approach was to repeat the same meta‐analysis over and over but discarding one different dataset each time. If a previously significant brain region remains significant in all or most of the combinations of datasets, then it was regarded as robust. In previous neuroimaging meta‐analyses, we found that there is currently no consensus on threshold selection for jackknife sensitivity analysis to determine the robustness of main results (Long et al., 2022; Wang, Gao, et al., 2020), and a threshold of 80% was used in this study.
2.7. Subgroup analysis
Subgroup meta‐analyses were performed to establish the consistency of findings and ascertain latent factors affecting main results, including only those studies that were clinically or methodologically homogenous. Specifically, we conducted subgroup meta‐analyses of (1) patients with migraine without aura, (2) drug‐free patients, (3) studies applying corrected thresholds for multiple comparison, (4) BOLD‐fMRI studies, (5) ALFF/fALFF studies, and (6) ReHo studies. Given the insufficient datasets, we did not perform additional subgroup analyses. The same statistical significance level was set as in the main analysis (voxel‐wise p < .005, peak height Z > 1, and cluster extent >100 voxels).
2.8. Meta‐regression analysis
Meta‐regression analyses were performed to examine the underlying effects of relevant demographic, clinical, and methodological variables on between‐group differences if they were reported in at least 10 datasets. To minimize the detection of spurious relationships, a more conservative threshold of p < .0005 was adopted (Radua et al., 2012). We required that findings be detected in both the slope and one of the extremes of the regressor, and kept results in regions that were significant in the primary meta‐analysis.
3. RESULTS
3.1. Included studies and sample characteristics
After duplicate removal, 1164 articles were identified, of which 24 studies that reported 31 datasets were finally eligible for our meta‐analysis, including a total of 748 migraineurs and 690 HCs (Chen et al., 2018, 2019; Kassab et al., 2009; Kim et al., 2010; 2021; Lei & Zhang, 2021; Li et al., 2017; Li, Zhou, Cheng, et al., 2020; Li, Zhou, Lan, et al., 2020; Lisicki et al., 2019; Liu et al., 2021; Magis et al., 2017; Meylakh et al., 2018, 2020; Michels et al., 2019; Ning et al., 2017; Wang et al., 2016; Wei et al., 2022; Yang et al., 2022; Zhang et al., 2016, 2017, 2021; Zhao et al., 2013, 2014). Sample size weighted t‐tests revealed that the patient groups and control groups were matched by age (p = .219) and female ratio (p = .195). The demographic and clinical characteristics of included studies are summarized in Table 1, and a summary of the neuroimaging methodological parameters is shown in Table 2.
TABLE 1.
Demographic and clinical characteristics of the studies included in meta‐analysis
| Study | Modality/ analysis | Migraine type | Sample size (female) | Mean age (y) | Education (y) | Age at onset (y) | Duration (y) | VAS | Medication (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | |||||||
| Li et al. (2017) | fMRI/ALFF | MwoA | 62 (48) | 42 (34) | 21.29 | 21.21 | NA | NA | NA | 5.56 | 5.48 | Drug free |
| Ning et al. (2017) | fMRI/ALFF | MwoA | 16 (13) | 16 (13) | 28.30 | 27.10 | 15.10 | 14.60 | NA | 4.78 | 5.40 | Drug free |
| Wang et al. (2016) | fMRI/ALFF | NA | 30 (NA) | 24 (NA) | NA | NA | NA | NA | NA | NA | NA | NA |
| Wei et al. (2022) | fMRI/ALFF | MwoA | 55 (48) | 50 (44) | 33.58 | 37.26 | 12.56 | 12.20 | NA | 7.22 | 6.49 | Drug free |
| Zhang et al. (2017) | fMRI/ALFF | MwoA | 30 (22) | 31 (22) | 41.00 | 40.20 | NA | NA | NA | 9.60 | 7.20 | Drug free |
| Kim et al. (2021) | fMRI/fALFF | MwoA | 44 (44) | 31 (31) | 36.20 | 35.20 | 14.30 | 14.50 | 22.60 | 13.60 | 7.50 | Drug free |
| Li et al. (2020) | fMRI/fALFF | MwoA | 70 (56) | 43 (34) | 21.51 | 21.23 | NA | NA | NA | 5.30 | 5.49 | Drug free |
| Wang et al. (2016) | fMRI/fALFF | NA | 30 (NA) | 24 (NA) | NA | NA | NA | NA | NA | NA | NA | NA |
| Yang et al. (2022) | fMRI/fALFF | MwoA | 25 (18) | 23 (19) | 31.36 | 32.74 | 13.92 | 14.09 | NA | 8.7 | 6.16 | Drug free |
| Chen et al. (2019) | fMRI/ReHo | MwoA | 17 (8) | 31 (18) | 49.59 | 49.77 | 8.82 | 13.06 | NA | 7.41 | 7.24 | NA |
| Chen et al. (2019) | fMRI/ReHo | MwoA | 20 (16) | 31 (18) | 38.00 | 49.77 | 11.40 | 13.06 | NA | 9.80 | 7.35 | NA |
| Chen et al. (2019) | fMRI/ReHo | MwoA | 19 (14) | 31 (18) | 42.00 | 49.77 | 10.94 | 13.06 | NA | 9.37 | 6.63 | NA |
| Lei et al. (2021) | fMRI/ReHo | NA | 22 (17) | 22 (16) | 33.32 | 34.59 | 12.41 | 16.36 | NA | NA | NA | NA |
| Li et al. (2020) | fMRI/ReHo | MwoA | 72 (57) | 46 (34) | 21.30 | 21.24 | NA | NA | NA | 5.56 | 5.55 | Drug free |
| Liu et al. (2021) | fMRI/ReHo | MwoA | 37 (31) | 15 (13) | 37.97 | 34.88 | 15.03 | 15.94 | NA | 16.19 | 7.73 | Drug free |
| Meylakh et al. (2018) | fMRI/ReHo | NA | 8 (NA) | 78 (66) | NA | 30.70 | NA | NA | NA | NA | NA | NA |
| Wei et al. (2022) | fMRI/ReHo | MwoA | 55 (48) | 50 (44) | 33.58 | 37.26 | 12.56 | 12.20 | NA | 7.22 | 6.49 | Drug free |
| Zhang et al. (2016) | fMRI/ReHo | MwoA | 22 (13) | 22 (13) | 41.80 | 42.00 | NA | NA | NA | 9.80 | 7.70 | NA |
| Zhang et al. (2017) | fMRI/ReHo | MwoA | 30 (22) | 31 (22) | 41.00 | 40.20 | NA | NA | NA | 9.60 | 7.20 | Drug free |
| Zhao et al. (2013) | fMRI/ReHo | MwoA | 20 (13) | 20 (15) | 37.52 | 28.40 | 13.80 | 14.20 | NA | 16.25 | 5.00 | Drug free |
| Zhao et al. (2013) | fMRI/ReHo | MwoA | 20 (15) | 20 (15) | 27.12 | 28.40 | 13.20 | 14.20 | NA | 4.05 | 5.37 | Drug free |
| Zhao et al. (2014) | fMRI/ReHo | MwoA | 19 (19) | 20 (20) | 21.80 | 22.40 | 14.70 | NA | NA | 9.10 | 5.00 | NA |
| Chen et al. (2018) | ASL/rCBF | MwoA | 15 (11) | 15 (11) | 32.00 | 38.00 | NA | NA | NA | 10.00 | 8.00 | Drug free |
| Meylakh et al. (2020) | ASL/rCBF | MwA/MwoA | 7 (5) | 26 (22) | 32.00 | 32.30 | NA | NA | NA | 10.29 | NA | 42.86 |
| Michels et al. (2019) | ASL/rCBF | MwA/MwoA | 17 (13) | 19 (11) | 32.70 | 31.00 | NA | NA | NA | 12.00 | NA | 5.85 |
| Michels et al. (2019) | ASL/rCBF | MwA | 12 (9) | 19 (11) | 33.49 | 31.00 | NA | NA | NA | 11.92 | NA | 8.33 |
| Zhang et al. (2021) | ASL/rCBF | MwoA | 40 (30) | 42 (27) | 35.10 | 41.05 | 14.18 | 13.18 | NA | 9.20 | 5.03 | Drug free |
| Kassab et al. (2009) | PET/18FDG | MwA/MwoA | 11 (8) | 14 (4) | 37.00 | 36.75 | NA | NA | NA | NA | NA | Drug free |
| Kim et al. (2010) | PET/18FDG | MwA/MwoA | 20 (3) | 20 (3) | 34.00 | 33.70 | NA | NA | 24.10 | 9.90 | NA | Drug free |
| Lisicki et al. (2019) | PET/18FDG | MwoA | 19 (15) | 20 (15) | 34.37 | 36.10 | NA | NA | NA | 15.20 | NA | Drug free |
| Magis et al. (2017) | PET/18FDG | MwoA | 11 (10) | 20 (15) | 37.09 | 36.00 | NA | NA | NA | NA | NA | Drug free |
Abbreviations: ALFF, amplitude of low‐frequency fluctuations; ASL, arterial spin labeling; fALFF, fractional amplitude of low‐frequency fluctuations; FDG, fluorodeoxyglucose; fMRI, functional magnetic resonance imaging; MwA, migraine with aura; MwoA, migraine without aura; NA, not available; PET, positron emission tomography; rCBF, regional cerebral blood flow; ReHo, regional homogeneity; VAS, visual analog scale; y, year.
TABLE 2.
Technique details of the studies included in the meta‐analysis
| Study | Scanner | Head coil | Sequence | TR/TE (ms) | ST (mm) | FWHM (mm) | Threshold | Number of coordinates |
|---|---|---|---|---|---|---|---|---|
| Li et al. (2017) | Siemens (3.0 T) MRI | 8‐channel phase‐array | EPI | 2000/30 | 5 | 6 | p < .05 (corrected) | 5 |
| Ning et al. (2017) | Siemens (3.0 T) MRI | NA | EPI | 2000/30 | 3.50 | NA | p < .05 (corrected) | 5 |
| Wang et al. (2016) | GE (3.0 T) MRI | NA | EPI | 2000/40 | 4 | 4 | p < .05 (corrected) | 16 |
| Wei et al. (2022) | Philips (3.0 T) MRI | 8‐channel | EPI | 2000/30 | 3.50 | 6 | p < .001 (uncorrected) | 5 |
| Zhang et al. (2017) | Siemens (3.0 T) MRI | 12‐channel | GRE‐EPI | 2000/30 | 3.50 | 6 | p < .05 (corrected) | 2 |
| Kim et al. (2021) | Siemens (3.0 T) MRI | 12‐channel | EPI | 2000/30 | 3.75 | 6 | p < .05 (corrected) | 3 |
| Li et al. (2020) | Siemens (3.0 T) MRI | 8‐channel phase‐array | EPI | 2000/30 | 5 | 8 | p < .05 (corrected) | 4 |
| Wang et al. (2016) | GE (3.0 T) MRI | NA | EPI | 2000/40 | 4 | 4 | p < .05 (corrected) | 6 |
| Yang et al. (2022) | GE (3.0 T) MRI | 24‐channel | GRE‐SS‐EPI | 2400/30 | 3 | 6 | p < .05 (corrected) | 2 |
| Chen et al. (2019) | Siemens (3.0 T) MRI | NA | NA | 2000/30 | 4 | 8 | p < .05 (corrected) | 14 |
| Chen et al. (2019) | Siemens (3.0 T) MRI | NA | NA | 2000/30 | 4 | 8 | p < .05 (corrected) | 10 |
| Chen et al. (2019) | Siemens (3.0 T) MRI | NA | NA | 2000/30 | 4 | 8 | p < .05 (corrected) | 7 |
| Lei et al. (2021) | Siemens (3.0 T) MRI | NA | NA | 2300/30 | 3.70 | 6 | p < .01 (corrected) | 2 |
| Li et al. (2020) | Siemens (3.0 T) MRI | 8‐channel phase‐array | EPI | 2000/30 | 5 | 8 | p < .05 (corrected) | 4 |
| Liu et al. (2021) | United Imaging (3.0 T) MRI | 12‐channel | EPI | 2000/30 | 3.50 | 6 | p < .05 (corrected) | 2 |
| Meylakh et al. (2018) | Philips (3.0 T) MRI | NA | EPI | 2000/30 | 4 | 3 | p < .05 (corrected) | 3 |
| Wei et al. (2022) | Philips (3.0 T) MRI | 8‐channel | EPI | 2000/30 | 3.50 | 6 | p < .001 (uncorrected) | 1 |
| Zhang et al. (2016) | Siemens (3.0 T) MRI | 12‐channel | GRE‐EPI | 2000/30 | 3.50 | 8 | p < .05 (corrected) | 2 |
| Zhang et al. (2017) | Siemens (3.0 T) MRI | 12‐channel | GRE‐EPI | 2000/30 | 3.50 | 6 | p < .05 (corrected) | 3 |
| Zhao et al. (2013) | Siemens (3.0 T) MRI | 8‐channel phase‐array | EPI | 2000/30 | 5 | 4 | p < .01 (corrected) | 59 |
| Zhao et al. (2013) | Siemens (3.0 T) MRI | 8‐channel phase‐array | EPI | 2000/30 | 5 | 4 | p < .01 (corrected) | 23 |
| Zhao et al. (2014) | GE (3.0 T) MRI | 8‐channel phase‐array | EPI | 2000/30 | 5 | 4 | p < .05 (corrected) | 22 |
| Chen et al. (2018) | GE (3.0 T) MRI | 8‐channel quadrature | pCASL | 5128/15.9 | 3 | 6 | p < .05 (uncorrected) | 1 |
| Meylakh et al. (2020) | Philips (3.0 T) MRI | NA | pCASL | 5310/12.7 | 3 | 6 | p < .05 (corrected) | 3 |
| Michels et al. (2019) | Philips (3.0 T) MRI | 15‐element | 2D pCASL | 4200/16 | 6 | 6 | p < .05 (corrected) | 1 |
| Michels et al. (2019) | Philips (3.0 T) MRI | 15‐element | 2D pCASL | 4200/16 | 6 | 6 | p < .05 (corrected) | 2 |
| Zhang et al. (2021) | Philips (3.0 T) MRI | 8‐channel digital | pCASL | 4000/11 | 4 | 8 | p < .05 (corrected) | 3 |
| Kassab et al. (2009) | Siemens PET | — | — | — | NA | NA | p < .001 (uncorrected) | 6 |
| Kim et al. (2010) | Philips PET | — | — | — | 2 | 12 | p < .001 (uncorrected) | 15 |
| Lisicki et al. (2019) | Philips PET | — | — | — | NA | 8 | p < .001 (uncorrected) | 40 |
| Magis et al. (2017) | Philips PET | — | — | — | NA | 8 | p < .001 (uncorrected) | 15 |
Abbreviations: D, dimensional; EPI, echo planar imaging; FWHM, full‐width at half‐maximum; GRE, gradient echo; MRI, magnetic resonance imaging; NA, not available; pCASL, pseudo‐continuous arterial spin labeling; PET, positron emission tomography; SS, single shot; ST, slice thickness; T, Tesla; TE, echo time; TR, repetition time.
3.2. Meta‐analysis
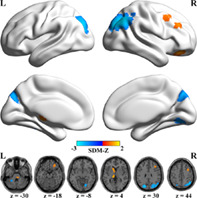
As illustrated in Table 3 and Figure 2, in contrast to HCs, migraineurs exhibited decreased spontaneous brain activity in the right angular gyrus (ANG) extending to middle occipital gyrus (MOG) and superior occipital gyrus (SOG), left MOG extending to SOG, right lingual gyrus (LING), and left cerebellum, whereas increased functional activity in the left caudate, left thalamus, right part of the pons, right middle frontal gyrus (MFG), right orbital part of middle frontal gyrus (ORBmid) extending to superior frontal gyrus (ORBsup) and inferior frontal gyrus (ORBinf), and right MFG extending to triangular part of inferior frontal gyrus (IFGtriang).
TABLE 3.
Meta‐analysis results of differences in resting‐state brain activity between migraineurs and controls
| Brain regions | SDM‐Z | p‐value | Peak MNI coordinates | Cluster size (voxels) | Heterogeneity test | Egger's test | |||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Q (p‐value) | I 2 (%) | p‐value | ||||
| Migraineurs < HCs | |||||||||
| Right ANG/MOG/SOG | −2.684 | ~0 | 44 | −66 | 40 | 1866 | 19.935 (.918) | 0 | .016 |
| Left MOG/SOG | −2.763 | ~0 | −24 | −74 | 24 | 855 | 28.585 (.539) | 0 | .408 |
| Right LING | −2.147 | .0001 | 18 | −74 | −6 | 245 | 30.051 (.463) | 0.170 | .051 |
| Left cerebellum | −1.680 | .0024 | −28 | −46 | −28 | 103 | 27.311 (.607) | 0 | .131 |
| Migraineurs > HCs | |||||||||
| Left caudate | 1.343 | .0004 | −8 | 6 | 6 | 414 | 16.795 (.975) | 0 | .571 |
| Left thalamus | 1.499 | .0002 | −16 | −26 | 4 | 303 | 29.906 (.470) | 0 | .626 |
| Right ORBmid/ORBsup/ORBinf | 1.284 | .0005 | 26 | 34 | −18 | 183 | 27.009 (.623) | 0 | .810 |
| Right part of the pons | 1.335 | .0004 | 6 | −28 | −32 | 150 | 17.469 (.967) | 0 | .323 |
| Right MFG | 1.227 | .0008 | 36 | 12 | 44 | 125 | 23.710 (.785) | 0 | .257 |
| Right MFG/IFGtriang | 1.245 | .0007 | 34 | 28 | 28 | 112 | 26.715 (.638) | 0 | .230 |
Abbreviations: ANG, angular gyrus; HCs, healthy controls, IFGtriang, triangular part of inferior frontal gyrus; LING, lingual gyrus; MFG, middle frontal gyrus; MNI, Montreal Neurological Institute; MOG, middle occipital gyrus; ORBinf, orbital part of inferior frontal gyrus; ORBmid, orbital part of middle frontal gyrus; ORBsup, orbital part of superior frontal gyrus; Q, Cochran's Q statistic; SDM, seed‐based d mapping; SOG, superior occipital gyrus.
FIGURE 2.
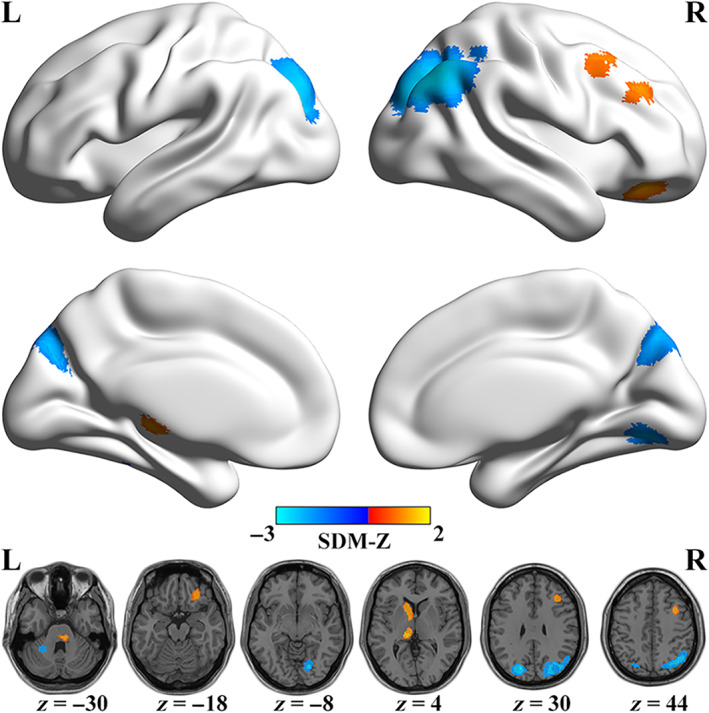
Regions of significantly increased (warm color) and decreased (cold color) spontaneous brain activity in migraineurs in the pooled meta‐analysis. L, left; PRISMA, preferred reporting items for systematic reviews and meta‐analyses; R, right; SDM, seed‐based d mapping
3.3. Heterogeneity test and publication bias
For all clusters reported above, no significant between‐study heterogeneity was observed (Table 3, all p‐values > .4 and I 2s < 1%), but Egger's tests indicated that there was publication bias in the right ANG/MOG/SOG (p = .016, Table 3 and Figure S1).
3.4. Jackknife sensitivity analysis
In jackknife analysis, the probability map indicated that all aforementioned regions were robust to be found in more than 80% iterations, with the right ANG/MOG/SOG and left MOG/SOG being preserved in all iterations (Figure 3). Results of the pooled meta‐analysis thus exhibited high replicability and reliability.
FIGURE 3.
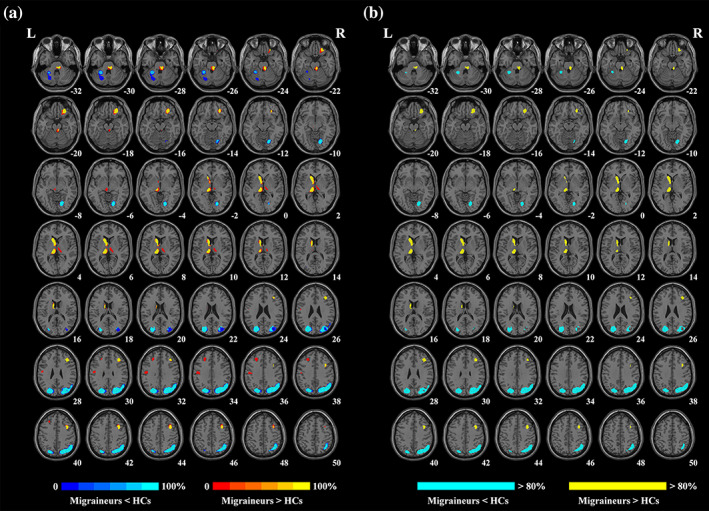
Results of the jackknife sensitivity analysis. (a) The voxel‐wise probability map presents significant clusters in jackknife analysis, and the value in each voxel represents the probability of occurrence in all iterations. (b) Regions that survived more than 80% iterations. HCs, healthy controls; L, left; R, right
3.5. Subgroup analysis
In subgroup analysis, findings in the patients of migraine without aura subgroup (22 datasets, Table S3 and Figure 4a) and drug‐free patients subgroup (19 datasets, Table S4 and Figure 4b) were largely consistent with the pooled meta‐analysis. The threshold correction subgroup (24 datasets, Table S5 and Figure 4c) showed decreased spontaneous brain activity in the right SOG extending to MOG and ANG, left MOG extending to SOG, and left cerebellum, whereas increased functional activity in the left caudate, left thalamus, right middle temporal gyrus, right ORBsup extending to ORBmid and ORBinf, left postcentral gyrus (PoCG), and right part of the pons. Findings in the subgroup of BOLD‐fMRI studies (22 datasets, Table S6 and Figure 4d) were similar to the main results, while increased functional activity was also found in the left PoCG and left MFG extending to dorsolateral part of superior frontal gyrus (SFGdor). In the ALFF/fALFF studies subgroup (9 datasets, Table S7 and Figure 4e), we found decreased spontaneous brain activity in the right ANG extending to MOG, inferior parietal (excluding supramarginal and angular) gyri and SOG, right cerebellum extending to LING, left SOG, and left cerebellum, whereas increased functional activity in the bilateral insula, left caudate, right MFG extending to IFGtriang, left MFG extending to SFGdor, right MFG, and left thalamus. In the ReHo studies subgroup (13 datasets, Table S8 and Figure 4f), we found decreased spontaneous brain activity in the right SOG extending to cuneus, precuneus, MOG, and ANG, and left MOG extending to SOG, whereas increased functional activity in the left caudate extending to thalamus, left PoCG, right thalamus, and right part of the pons. To sum up, the main results were broadly unchanged in different subgroups.
FIGURE 4.
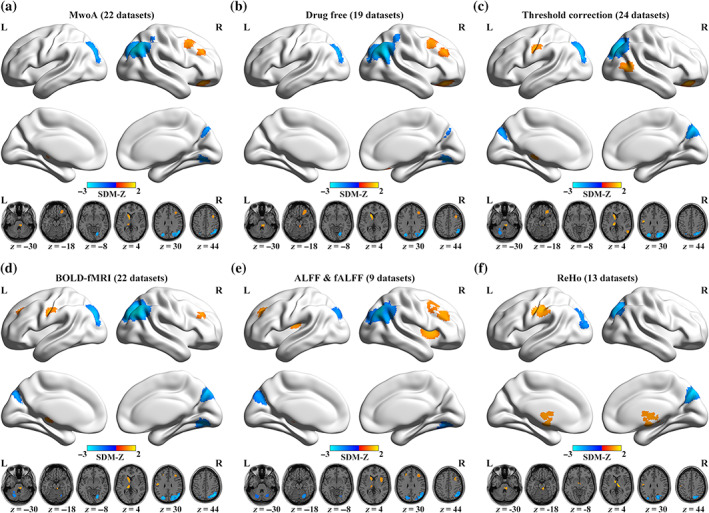
Regions of significantly altered spontaneous brain activity in six specific subgroups: (a) MwoA patients, (b) drug‐free patients, (c) threshold correction, (d) BOLD‐fMRI studies, (e) ALFF/fALFF studies, (f) ReHo studies. ALFF, amplitude of low‐frequency fluctuations; BOLD, blood‐oxygen‐level‐dependent; fALFF, fractional amplitude of low‐frequency fluctuations; fMRI, functional magnetic resonance imaging; L, left; MwoA, migraine without aura; R, right; ReHo, regional homogeneity; SDM, seed‐based d mapping
3.6. Meta‐regression analysis
In meta‐regression analyses, we separately examined the associations between spontaneous brain activity alterations and mean age (available in 28 datasets), percentage of female patients (available in 28 datasets), illness duration (available in 25 datasets), and visual analog scale (VAS) scores (available in 20 datasets). The results indicated that the VAS score in migraineurs was positively associated with altered spontaneous brain activity in the left thalamus (peak MNI coordinate: x = −12, y = −22, z = 4, 140 voxels, SDM‐Z = 2.168, p < .0001), shown in Figure 5. The mean age, percentage of female patients, and illness duration were not moderators that influenced the brain activity measures. Since insufficient datasets included, we were unable to perform meta‐regression analyses for other continuous variables.
FIGURE 5.
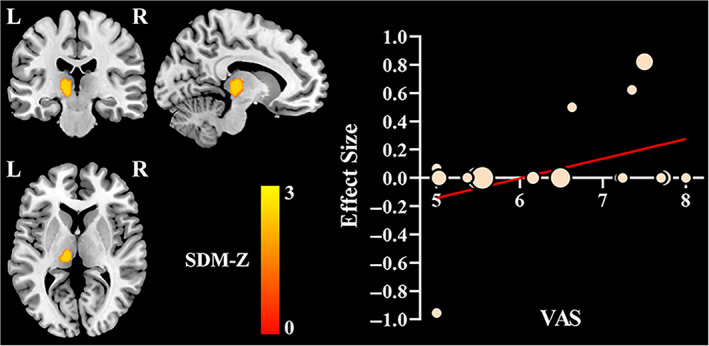
Significant result of meta‐regression analyses. The VAS score was positively associated with resting‐state brain activity alterations in the left thalamus. In the scatter plot, each dot represents a dataset, with larger dots representing greater sample sizes. L, left; R, right; SDM, seed‐based d mapping; VAS, visual analog scale
4. DISCUSSION
To the best of our knowledge, this is the first quantitative meta‐analysis to investigate spontaneous brain activity alterations in migraineurs. Our results revealed that decreased spontaneous brain activity was located in the right ANG, bilateral MOG, bilateral SOG, right LING, and left cerebellum, whereas increased brain activity was in the left caudate, left thalamus, right part of the pons, right MFG, right ORBmid/ORBsup/ORBinf, and right IFGtriang. Jackknife sensitivity analysis and subgroup analyses suggested that the main findings were largely unchanged. Furthermore, significant modulation effect of the VAS score on spontaneous brain activity increasement in the left thalamus was found in patients with migraine, as revealed by meta‐regression analyses.
The ANG, located in the posterior part of inferior parietal lobule and within the default mode network, is mainly responsible for the sensory information processing, pain chronicalization, cognitive, emotional, and other advanced functions of human brain in the resting state. Due to the location at the junction of the occipital, temporal, and parietal lobes, ANG functions to convey and integrate information between different modalities and processing subsystems (Buckner & DiNicola, 2019; Lo Buono et al., 2017; Seghier, 2013). In our research, migraine patients had lower spontaneous brain activity in the right ANG in contrast to control groups. This change might affect the transmission and processing of pain information and cognitive behavior.
In our study, we observed significantly lower activation in the bilateral MOG, bilateral SOG, and right LING. Regions above are all in the occipital lobe, which is a major part of the visual cortex and is involved in the reception, segmentation, and integration of visual information (Baker et al., 2018). By far, visual aura is the most common type of migraine aura, and causes the disturbance of vision and photophobia, suggesting an abnormal neuronal activity in the visual cortex. Therefore, it is speculated that alterations of these regions might be connected with visual aura in patients with migraine (Hayne & Martin, 2019). In this research, we included migraine patients both with and without aura, and identified consistent spontaneous brain activity abnormalities in the visual cortex, our findings are in line with a previous neuroimaging research (Puledda et al., 2019). As a result, we can conclude that the dysfunction of visual processing is present in migraineurs, with or without aura, and is considered an important pathological feature of them.
The cerebellum is primarily involved in coordination, motor control, and sensory perception. Moreover, it has anatomical connections with multiple areas of the frontal cortex and limbic regions, which are critical for its response to nociceptive stimuli and involvement in pain modulation (Moulton et al., 2010; Wang et al., 2016). As shown in the main results, the significantly decreased spontaneous brain activity reflected the dysfunction of cerebellum in migraineurs, possibly due to the nociceptive stimuli caused by frequent episodes of migraine. Functional deficits in the cerebellum may have implications for trigeminal nociception and multimodal information integration, and contribute to susceptibility to migraine attacks (Russo et al., 2019).
We found an increased brain activity in the left caudate in migraineurs. The caudate plays a key role in both sensory processing and suppression of pain (Wunderlich et al., 2011). We thus infer that increased intrinsic brain activity in it may represent an adaptive response to migraine attacks. Migraine patients had relatively higher intrinsic brain activity in the left thalamus in our meta‐analysis. The thalamus is a critical center for relaying ascending nociceptive information from the peripheral nervous system to the cortex. It is not only involved in pain processing, but also responsible for fundamental roles in sensory hypersensitivity of the auditory, visual and somatosensory systems in migraine patients. The thalamus also plays an additional role in the development of migraine and migraine‐related symptoms (Niddam et al., 2018; Younis et al., 2019). Increased neuronal activity in the thalamus suggests dysfunctional pain processing in migraine, and we speculate that it could be implicated in long‐term ongoing transmission of nociceptive information induced by frequent migraine attacks.
The descending pain system of brainstem is the major site of trigeminal pain processing and modulation. Within brainstem, the periaqueductal gray is thought to be a key area in migraine. As a relay station between cortical and brainstem structures, the periaqueductal gray plays a vital part in the modulation of pain by providing an antinociceptive effect on the primary afferent system as well as influencing autonomic and defensive behavioral responses (Akerman et al., 2011; Gee et al., 2005). Our current research showed an abnormally increased brain activity in the right part of the pons, providing new evidence for the functional impairments in brainstem that contributes to the neural pathophysiology of migraine.
Significantly increased local activity was found in the prefrontal cortex (PFC), including the right MFG, right ORBmid/ORBsup/ORBinf, and right IFGtriang. Existing studies have highlighted frontal lobe‐related cognitive impairments in migraineurs, including working memory and executive function deficits, and identified abnormalities within these regions, especially the PFC, which is essential to executive control of pain‐related stimuli, and act as a hub of the descending pain modulatory system (Lorenz et al., 2003; Schmitz et al., 2008; Wager et al., 2004). We found an excessive increase in the correlation and synchronization of local spontaneous brain activity, thus suggesting impaired cognitive and emotion processing of pain in the PFC.
According to the results of meta‐regression analyses, only the VAS, which is a unidimensional measure of pain intensity and used to record the pain progression of patients (the higher scores mean more pain intensity), was found to be positively associated with altered spontaneous brain activity in the left thalamus. The more intense the pain, the abnormally higher spontaneous brain activity would be observed in this region. Therefore, we infer that clinical symptoms are associated with neuronal activation abnormalities and pain processing dysfunction in the left thalamus. In addition, Egger's tests indicated that the right ANG/MOG/SOG were subject to publication bias. This may be related to the fact that small studies have lower statistical power, and consequently, their effect sizes are not capable of reaching statistical significance and imputed as null effect sizes. Samples heterogeneity, the tendency to publish studies with positive rather than negative results, and incomplete studies inclusion (for the studies were limited to those published in English), are also possible causes of publication bias (Müller et al., 2018; Tahmasian et al., 2019). Verification is needed in further studies.
There are several limitations in our meta‐analysis. First, we included studies using a variety of neuroimaging approaches to investigate resting‐state abnormalities in migraine. All these imaging approaches could reflect intrinsic brain activity, but their different theoretical bases and methodologies may have implications for the meta‐analysis. To address this issue, we performed three subgroup analyses, including only the BOLD‐fMRI studies, fALFF/ALFF studies, or ReHo studies. We did not perform subgroup analyses of ASL studies or PET studies due to insufficient datasets. Despite the fact that relevant subgroup meta‐analyses were performed in this study, the influence may not be fully eliminated. Second, of the 31 datasets included in our study, the sample sizes range from 7 to 72 in the migraine groups and from 14 to 78 in the HC groups. Studies with small sample sizes have a higher probability of false positives that affected the generalizability of the obtained results. It is highly required to increase the sample size (and therefore statistical power) in future research. Third, migraine is a neurological disease with heterogeneous clinical conditions among patients. We have performed meta‐analyses for the subgroup of migraine without aura and unmedicated patients. Given a lack of data, it was unavailable to carry out other subgroup meta‐analyses, such as studies with female or male subjects, studies with medicated patients, and studies with migraine patients with aura. Finally, the coordinate‐based meta‐analysis only summarizes reported local peak coordinates rather than working with raw data, which may lead to less precise results (Salimi‐Khorshidi et al., 2009).
5. CONCLUSION
We performed the first quantitative voxel‐wise meta‐analysis of whole‐brain resting‐state neuroimaging studies for migraine that employed more than one imaging metric, with the aim of providing the most comprehensive overview of spontaneous brain activity patterns impairments in migraineurs. Our findings indicated that migraineurs demonstrated a decreased spontaneous brain activity in the ANG, visual cortex, and cerebellum, whereas increased activity in the caudate, thalamus, pons, and PFC. Meta‐regression analyses revealed that a higher VAS score in the patient sample was associated with increased spontaneous brain activity in the left thalamus. These findings could provide useful insights into the underlying pathophysiology of brain dysfunction in migraine and guide further research.
AUTHOR CONTRIBUTIONS
Feng Liu, Lining Guo, and Qiang Xu contributed to the study design. Mengjing Cai, Yao Zhao, He Wang, Dianxun Fu, and Lin Ma prepared and managed the data. Mengjing Cai, Jiawei Liu, Xuexiang Wang, Juanwei Ma, and Mengge Liu performed data analysis and interpretation. Mengjing Cai, Jiawei Liu, and Xuexiang Wang wrote the article. Feng Liu, Lining Guo, Qiang Xu, and Wenqin Wang critically reviewed the article. All authors read and approved the final article.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting information
Appendix S1 Supporting Information
Cai, M. , Liu, J. , Wang, X. , Ma, J. , Ma, L. , Liu, M. , Zhao, Y. , Wang, H. , Fu, D. , Wang, W. , Xu, Q. , Guo, L. , & Liu, F. (2023). Spontaneous brain activity abnormalities in migraine: A meta‐analysis of functional neuroimaging. Human Brain Mapping, 44(2), 571–584. 10.1002/hbm.26085
Mengjing Cai, Jiawei Liu, and Xuexiang Wang contributed equally to this study.
Funding information Science & Technology Development Fund of Tianjin Education Commission for Higher Education, Grant/Award Number: 2017KJ096
Contributor Information
Qiang Xu, Email: xuqiang9042@gmail.com.
Lining Guo, Email: 18334721302@163.com.
Feng Liu, Email: fengliu@tmu.edu.cn.
DATA AVAILABILITY STATEMENT
The input datasets and result files for the current study are publicly available in figshare at https://doi.org/10.6084/m9.figshare.20522805.v1.
REFERENCES
- Akerman, S. , Holland, P. R. , & Goadsby, P. J. (2011). Diencephalic and brainstem mechanisms in migraine. Nature Reviews. Neuroscience, 12(10), 570–584. 10.1038/nrn3057 [DOI] [PubMed] [Google Scholar]
- Ashina, M. , Hansen, J. M. , Do, T. P. , Melo‐Carrillo, A. , Burstein, R. , & Moskowitz, M. A. (2019). Migraine and the trigeminovascular system‐40 years and counting. Lancet Neurology, 18(8), 795–804. 10.1016/s1474-4422(19)30185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C. M. , Burks, J. D. , Briggs, R. G. , Stafford, J. , Conner, A. K. , Glenn, C. A. , Sali, G. , McCoy, T. , Battiste, J. D. , O'Donoghue, D. L. , & Sughrue, M. E. (2018). A Connectomic atlas of the human cerebrum‐chapter 9: The occipital lobe. Operative Neurosurgery (Hagerstown), 15(suppl_1), S372–S406. 10.1093/ons/opy263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , & DiNicola, L. M. (2019). The brain's default network: Updated anatomy, physiology and evolving insights. Nature Reviews. Neuroscience, 20(10), 593–608. 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Yan, M. Y. , Yu, Y. , Ke, J. , Xu, C. Y. , Guo, X. N. , Lu, H. , Wang, X. , Hu, L. , Wang, J. , Ni, J. , & Zhao, H. R. (2019). Alterations in regional homogeneity assessed by fMRI in patients with migraine without Aura. Journal of Medical Systems, 43(9), 298. 10.1007/s10916-019-1425-z [DOI] [PubMed] [Google Scholar]
- Chen, J. E. , & Glover, G. H. (2015). Functional magnetic resonance imaging methods. Neuropsychology Review, 25(3), 289–313. 10.1007/s11065-015-9294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Chen, X. , Liu, M. , Liu, M. , Ma, L. , & Yu, S. (2018). Evaluation of gray matter perfusion in episodic migraine using voxel‐wise comparison of 3D pseudo‐continuous arterial spin labeling. The Journal of Headache and Pain, 19(1), 36. 10.1186/s10194-018-0866-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick, D. W. (2018). Migraine. Lancet, 391(10127), 1315–1330. 10.1016/s0140-6736(18)30478-1 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience, 8(9), 700–711. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Gee, J. R. , Chang, J. , Dublin, A. B. , & Vijayan, N. (2005). The association of brainstem lesions with migraine‐like headache: An imaging study of multiple sclerosis. Headache, 45(6), 670–677. 10.1111/j.1526-4610.2005.05136.x [DOI] [PubMed] [Google Scholar]
- Gusnard, D. A. , Raichle, M. E. , & Raichle, M. E. (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews. Neuroscience, 2(10), 685–694. 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Hannawi, Y. , Lindquist, M. A. , Caffo, B. S. , Sair, H. I. , & Stevens, R. D. (2015). Resting brain activity in disorders of consciousness: A systematic review and meta‐analysis. Neurology, 84(12), 1272–1280. 10.1212/wnl.0000000000001404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayne, D. P. , & Martin, P. R. (2019). Relating photophobia, visual Aura, and visual triggers of headache and migraine. Headache, 59(3), 430–442. 10.1111/head.13486 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassab, M. , Bakhtar, O. , Wack, D. , & Bednarczyk, E. (2009). Resting brain glucose uptake in headache‐free migraineurs. Headache, 49(1), 90–97. 10.1111/j.1526-4610.2008.01206.x [DOI] [PubMed] [Google Scholar]
- Kim, J. H. , Kim, S. , Suh, S. I. , Koh, S. B. , Park, K. W. , & Oh, K. (2010). Interictal metabolic changes in episodic migraine: A voxel‐based FDG‐PET study. Cephalalgia, 30(1), 53–61. 10.1111/j.1468-2982.2009.01890.x [DOI] [PubMed] [Google Scholar]
- Kim, Y. E. , Kim, M. K. , Suh, S. I. , & Kim, J. H. (2021). Altered trigeminothalamic spontaneous low‐frequency oscillations in migraine without aura: A resting‐state fMRI study. BMC Neurology, 21(1), 342. 10.1186/s12883-021-02374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, H. , Suo, X. , Li, W. , Li, N. , Li, J. , Peng, J. , Lei, D. , Sweeney, J. A. , Kemp, G. J. , Peng, R. , & Gong, Q. (2021). Abnormalities of intrinsic brain activity in essential tremor: A meta‐analysis of resting‐state functional imaging. Human Brain Mapping, 42(10), 3156–3167. 10.1002/hbm.25425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, J. L. , Tordesillas‐Gutiérrez, D. , Martinez, M. , Salinas, F. , Evans, A. , Zilles, K. , Mazziotta, J. C. , & Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapping, 28(11), 1194–1205. 10.1002/hbm.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, M. , & Zhang, J. J. (2021). Brain function state in different phases and its relationship with clinical symptoms of migraine: An fMRI study based on regional homogeneity (ReHo). Annals of Translational Medicine, 9(11), 928. 10.21037/atm-21-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Zhang, Y. , Zhao, Y. , Li, Z. , Kemp, G. J. , Wu, M. , & Gong, Q. (2022). Cortical thickness abnormalities in patients with post‐traumatic stress disorder: A vertex‐based meta‐analysis. Neuroscience and Biobehavioral Reviews, 134, 104519. 10.1016/j.neubiorev.2021.104519 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Zhou, J. , Cheng, S. , Lan, L. , Sun, R. , Liu, M. , Yang, J. , Gao, Y. , Guo, T. , Gong, Q. , Zeng, F. , & Liang, F. (2020). Cerebral fractional amplitude of low‐frequency fluctuations may predict headache intensity improvement following acupuncture treatment in migraine patients. Journal of Traditional Chinese Medicine, 40(6), 1041–1051. [DOI] [PubMed] [Google Scholar]
- Li, Z. J. , Zeng, F. , Yin, T. , Lan, L. , Makris, N. , Jorgenson, K. , Guo, T. , Wu, F. , Gao, Y. , Dong, M. , Liu, M. , Yang, J. , Li, Y. , Gong, Q. , Linag, F. , & Kong, J. (2017). Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. Neuroimage Clinical, 15, 367–375. 10.1016/j.nicl.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. J. , Zhou, J. , Lan, L. , Cheng, S. R. , Sun, R. R. , Gong, Q. Y. , Wintemark, M. , Zeng, F. , & Liang, F. R. (2020). Concurrent brain structural and functional alterations in patients with migraine without aura: An fMRI study. The Journal of Headache and Pain, 21(1), 141. 10.1186/s10194-020-01203-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisicki, M. , D'Ostilio, K. , Coppola, G. , Parisi, V. , de Noordhout, A. M. , Magis, D. , Schoenen, J. , Scholtes, F. , & Versijpt, J. (2019). Age related metabolic modifications in the migraine brain. Cephalalgia, 39(8), 978–987. 10.1177/0333102419828984 [DOI] [PubMed] [Google Scholar]
- Liu, S. S. , Luo, S. L. , Yan, T. W. , Ma, W. , Wei, X. Y. , Chen, Y. L. , Zhan, S. , & Wang, B. (2021). Differential modulating effect of acupuncture in patients with migraine without Aura: A resting functional magnetic resonance study. Frontiers in Neurology, 12, 680896. 10.3389/fneur.2021.680896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Buono, V. , Bonanno, L. , Corallo, F. , Pisani, L. R. , Lo Presti, R. , Grugno, R. , Di Lorenzo, G. , Bramanti, P. , & Marino, S. (2017). Functional connectivity and cognitive impairment in migraine with and without aura. The Journal of Headache and Pain, 18(1), 72. 10.1186/s10194-017-0782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J. , Qin, K. , Wu, Y. , Li, L. , & Zhou, J. (2022). Gray matter abnormalities and associated familial risk endophenotype in individuals with first‐episode bipolar disorder: Evidence from whole‐brain voxel‐wise meta‐analysis. Asian Journal of Psychiatry, 74, 103179. 10.1016/j.ajp.2022.103179 [DOI] [PubMed] [Google Scholar]
- Lorenz, J. , Minoshima, S. , & Casey, K. L. (2003). Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain, 126(Pt 5), 1079–1091. 10.1093/brain/awg102 [DOI] [PubMed] [Google Scholar]
- Magis, D. , D'Ostilio, K. , Thibaut, A. , De Pasqua, V. , Gerard, P. , Hustinx, R. , Laureys, S. , & Schoenen, J. (2017). Cerebral metabolism before and after external trigeminal nerve stimulation in episodic migraine. Cephalalgia, 37(9), 881–891. 10.1177/0333102416656118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson, R. , Demarquay, G. , Meunier, D. , Lévêque, Y. , Hannoun, S. , Bidet‐Caulet, A. , & Caclin, A. (2021). Is migraine associated to brain anatomical alterations? New data and coordinate‐based meta‐analysis. Brain Topography, 34(3), 384–401. 10.1007/s10548-021-00824-6 [DOI] [PubMed] [Google Scholar]
- Meylakh, N. , Marciszewski, K. K. , Di Pietro, F. , Macefield, V. G. , Macey, P. M. , & Henderson, L. A. (2018). Deep in the brain: Changes in subcortical function immediately preceding a migraine attack. Human Brain Mapping, 39(6), 2651–2663. 10.1002/hbm.24030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylakh, N. , Marciszewski, K. K. , Di Pietro, F. , Macefield, V. G. , Macey, P. M. , & Henderson, L. A. (2020). Altered regional cerebral blood flow and hypothalamic connectivity immediately prior to a migraine headache. Cephalalgia, 40(5), 448–460. 10.1177/0333102420911623 [DOI] [PubMed] [Google Scholar]
- Michels, L. , Villanueva, J. , O'Gorman, R. , Muthuraman, M. , Koirala, N. , Buchler, R. , Gantenbein, A. R. , Sandor, P. S. , Luechinger, R. , Kollias, S. , & Riederer, F. (2019). Interictal Hyperperfusion in the higher visual cortex in patients with episodic migraine. Headache, 59(10), 1808–1820. 10.1111/head.13646 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton, E. A. , Schmahmann, J. D. , Becerra, L. , & Borsook, D. (2010). The cerebellum and pain: Passive integrator or active participator? Brain Research Reviews, 65(1), 14–27. 10.1016/j.brainresrev.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, V. I. , Cieslik, E. C. , Laird, A. R. , Fox, P. T. , Radua, J. , Mataix‐Cols, D. , Tench, C. R. , Yarkoni, T. , Nichols, T. E. , Turkeltaub, P. E. , Wager, T. D. , & Eickhoff, S. B. (2018). Ten simple rules for neuroimaging meta‐analysis. Neuroscience and Biobehavioral Reviews, 84, 151–161. 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niddam, D. M. , Lai, K. L. , Tsai, S. Y. , Lin, Y. R. , Chen, W. T. , Fuh, J. L. , & Wang, S. J. (2018). Neurochemical changes in the medial wall of the brain in chronic migraine. Brain, 141(2), 377–390. 10.1093/brain/awx331 [DOI] [PubMed] [Google Scholar]
- Ning, Y. Z. , Li, K. S. , Zhang, Y. , Liu, H. W. , Fu, C. H. , Han, X. , Ren, Y. , & Zou, Y. H. (2017). Effect of acupuncture at Zulinqi (GB41) on the amplitude of low frequency fluctuations in migraine without aura patients: A resting‐state functional magnetic resonance imaging study. International Journal of Clinical and Experimental Medicine, 10(2), 3038–3048. [Google Scholar]
- Noseda, R. , & Burstein, R. (2013). Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain, 154(Suppl 1), S44–S53. 10.1016/j.pain.2013.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, N. , Wang, S. , Zhao, Y. , Lai, H. , Qin, K. , Li, J. , Biswal, B. B. , Sweeney, J. A. , & Gong, Q. (2021). Brain gray matter structures associated with trait impulsivity: A systematic review and voxel‐based meta‐analysis. Human Brain Mapping, 42(7), 2214–2235. 10.1002/hbm.25361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcharunpaisan, S. , Ramalho, J. , & Castillo, M. (2010). Arterial spin labeling in neuroimaging. World Journal of Radiology, 2(10), 384–398. 10.4329/wjr.v2.i10.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puledda, F. , Ffytche, D. , O'Daly, O. , & Goadsby, P. J. (2019). Imaging the visual network in the migraine Spectrum. Frontiers in Neurology, 10, 1325. 10.3389/fneur.2019.01325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J. , Borgwardt, S. , Crescini, A. , Mataix‐Cols, D. , Meyer‐Lindenberg, A. , McGuire, P. K. , & Fusar‐Poli, P. (2012). Multimodal meta‐analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neuroscience and Biobehavioral Reviews, 36(10), 2325–2333. 10.1016/j.neubiorev.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Radua, J. , & Mataix‐Cols, D. (2009). Voxel‐wise meta‐analysis of grey matter changes in obsessive‐compulsive disorder. The British Journal of Psychiatry, 195(5), 393–402. 10.1192/bjp.bp.108.055046 [DOI] [PubMed] [Google Scholar]
- Radua, J. , & Mataix‐Cols, D. (2012). Meta‐analytic methods for neuroimaging data explained. Biology of Mood & Anxiety Disorders, 2, 6. 10.1186/2045-5380-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J. , Rubia, K. , Canales‐Rodríguez, E. J. , Pomarol‐Clotet, E. , Fusar‐Poli, P. , & Mataix‐Cols, D. (2014). Anisotropic kernels for coordinate‐based meta‐analyses of neuroimaging studies. Frontiers in Psychiatry, 5, 13. 10.3389/fpsyt.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, A. , Tessitore, A. , Silvestro, M. , Di Nardo, F. , Trojsi, F. , Del Santo, T. , De Micco, R. , Esposito, F. , & Tedeschi, G. (2019). Advanced visual network and cerebellar hyperresponsiveness to trigeminal nociception in migraine with aura. The Journal of Headache and Pain, 20(1), 46. 10.1186/s10194-019-1002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi‐Khorshidi, G. , Smith, S. M. , Keltner, J. R. , Wager, T. D. , & Nichols, T. E. (2009). Meta‐analysis of neuroimaging data: A comparison of image‐based and coordinate‐based pooling of studies. NeuroImage, 45(3), 810–823. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Schmitz, N. , Arkink, E. B. , Mulder, M. , Rubia, K. , Admiraal‐Behloul, F. , Schoonman, G. G. , Kruit, M. C. , Ferrari, M. D. , & van Buchem, M. A. (2008). Frontal lobe structure and executive function in migraine patients. Neuroscience Letters, 440(2), 92–96. 10.1016/j.neulet.2008.05.033 [DOI] [PubMed] [Google Scholar]
- Schwedt, T. J. , Chiang, C. C. , Chong, C. D. , & Dodick, D. W. (2015). Functional MRI of migraine. Lancet Neurology, 14(1), 81–91. 10.1016/s1474-4422(14)70193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schytz, H. W. , Amin, F. M. , Selb, J. , & Boas, D. A. (2019). Non‐invasive methods for measuring vascular changes in neurovascular headaches. Journal of Cerebral Blood Flow and Metabolism, 39(4), 633–649. 10.1177/0271678x17724138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier, M. L. (2013). The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist, 19(1), 43–61. 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, L. , Ma, H. , Shi, Y. , Dai, Z. , Zhong, J. , Chen, F. , & Pan, P. (2020). Cortical thickness in migraine: A coordinate‐based meta‐analysis. Frontiers in Neuroscience, 14, 600423. 10.3389/fnins.2020.600423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, A. M. , Laurens, K. R. , Matheson, S. L. , Carr, V. J. , & Green, M. J. (2012). Systematic meta‐review and quality assessment of the structural brain alterations in schizophrenia. Neuroscience and Biobehavioral Reviews, 36(4), 1342–1356. 10.1016/j.neubiorev.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Tahmasian, M. , Sepehry, A. A. , Samea, F. , Khodadadifar, T. , Soltaninejad, Z. , Javaheripour, N. , Khazaie, H. , Zarei, M. , Eickhoff, S. B. , & Eickhoff, C. R. (2019). Practical recommendations to conduct a neuroimaging meta‐analysis for neuropsychiatric disorders. Human Brain Mapping, 40(17), 5142–5154. 10.1002/hbm.24746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, S. , Lu, L. , Zhang, L. , Hu, X. , Bu, X. , Li, H. , Hu, X. , Gao, Y. , Zeng, Z. , Gong, Q. , & Huang, X. (2018). Abnormal amygdala resting‐state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta‐analysis. eBioMedicine, 36, 436–445. 10.1016/j.ebiom.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager, T. D. , Rilling, J. K. , Smith, E. E. , Sokolik, A. , Casey, K. L. , Davidson, R. J. , Kosslyn, S. M. , Rose, R. M. , & Cohen, J. D. (2004). Placebo‐induced changes in FMRI in the anticipation and experience of pain. Science, 303(5661), 1162–1167. 10.1126/science.1093065 [DOI] [PubMed] [Google Scholar]
- Wang, H. Z. , Wang, W. H. , Shi, H. C. , & Yuan, C. H. (2020). Is there a reliable brain morphological signature for migraine? The Journal of Headache and Pain, 21(1), 89. 10.1186/s10194-020-01158-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. J. , Chen, X. , Sah, S. K. , Zeng, C. , Li, Y. M. , Li, N. , Liu, M.‐Q. , & Du, S. L. (2016). Amplitude of low‐frequency fluctuation (ALFF) and fractional ALFF in migraine patients: A resting‐state functional MRI study. Clinical Radiology, 71(6), 558–564. 10.1016/j.crad.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Gao, Y. , Tang, S. , Lu, L. , Zhang, L. , Bu, X. , Li, H. , Hu, X. , Hu, X. , Jiang, P. , Jia, Z. , Gong, Q. , & Huang, X. (2020). Large‐scale network dysfunction in the acute state compared to the remitted state of bipolar disorder: A meta‐analysis of resting‐state functional connectivity. eBioMedicine, 54, 102742. 10.1016/j.ebiom.2020.102742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H. L. , Tian, T. , Zhou, G. P. , Wang, J. J. , Guo, X. , Chen, Y. C. , Yu, Y.‐S. , Yin, X. , Li, J. , & Zhang, H. (2022). Disrupted dynamic functional connectivity of the visual network in episodic patients with migraine without Aura. Neural Plasticity, 2022, 9941832. 10.1155/2022/9941832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich, A. P. , Klug, R. , Stuber, G. , Landwehrmeyer, B. , Weber, F. , & Freund, W. (2011). Caudate nucleus and insular activation during a pain suppression paradigm comparing thermal and electrical stimulation. Open Neuroimaging Journal, 5, 1–8. 10.2174/1874440001105010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Wei, K. , Zhang, H. , Hu, H. , Yan, L. , Gui, W. , Liu, Y. , & Chen, X. (2022). Identifying functional brain abnormalities in migraine and depression comorbidity. Quantitative Imaging in Medicine and Surgery, 12(4), 2288–2302. 10.21037/qims-21-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis, S. , Hougaard, A. , Noseda, R. , & Ashina, M. (2019). Current understanding of thalamic structure and function in migraine. Cephalalgia, 39(13), 1675–1682. 10.1177/0333102418791595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, Y. , Jiang, T. , Lu, Y. , He, Y. , & Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. NeuroImage, 22(1), 394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Zang, Y. F. , He, Y. , Zhu, C. Z. , Cao, Q. J. , Sui, M. Q. , Liang, M. , Tian, L.‐X. , Jinag, T.‐Z. , & Wang, Y. F. (2007). Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain and Development, 29(2), 83–91. 10.1016/j.braindev.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Huang, X. , Mao, C. , Chen, Y. , Miao, Z. , Liu, C. , Xu, C. , Wu, X. , & Yin, X. (2021). Assessment of normalized cerebral blood flow and its connectivity with migraines without aura during interictal periods by arterial spin labeling. The Journal of Headache and Pain, 22(1), 72. 10.1186/s10194-021-01282-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. L. , Su, J. J. , Wang, M. X. , Zhao, Y. , Yao, Q. , Zhang, Q. T. , Lu, H. , Zhang, H. , Wang, S. , Li, G.‐F. , Wu, Y.‐L. , Liu, F.‐D. , Shi, Y.‐H. , Li, J. , Liu, J.‐R. , & Du, X. X. (2016). Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. The Journal of Headache and Pain, 17(1), 98. 10.1186/s10194-016-0692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. L. , Su, J. J. , Wang, M. X. , Zhao, Y. , Zhang, Q. T. , Yao, Q. , Lu, H. , Zhang, H. , Li, G.‐F. , Wu, Y.‐L. , Liu, Y.‐S. , Liu, F.‐D. , Zhuang, M.‐T. , Shi, Y.‐H. , Hou, T.‐Y. , Zhao, R. , Qiao, Y. , Li, J. , Liu, J.‐R. , & Du, X. X. (2017). The sensorimotor network dysfunction in migraineurs without aura: A resting‐state fMRI study. Journal of Neurology, 264(4), 654–663. 10.1007/s00415-017-8404-4 [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Liu, J. X. , Dong, X. L. , Peng, Y. L. , Yuan, K. , Wu, F. M. , Sun, J. , Gong, Q. , Qin, W. , & Liang, F. R. (2013). Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. The Journal of Headache and Pain, 14(1), 85. 10.1186/1129-2377-14-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Liu, J. X. , Yan, X. M. , Dun, W. H. , Yang, J. , Huang, L. Y. , Kai, Y. , Yu, D. , Qin, W. , Jie, T. , & Liang, F. R. (2014). Abnormal brain activity changes in patients with migraine: A short‐term longitudinal study. Journal of Clinical Neurology, 10(3), 229–235. 10.3988/jcn.2014.10.3.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, Q. H. , Zhu, C. Z. , Yang, Y. , Zuo, X. N. , Long, X. Y. , Cao, Q. J. , Wang, Y.‐F. , & Zang, Y. F. (2008). An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF) for resting‐state fMRI: Fractional ALFF. Journal of Neuroscience Methods, 172(1), 137–141. 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The input datasets and result files for the current study are publicly available in figshare at https://doi.org/10.6084/m9.figshare.20522805.v1.


