Abstract
Pancreatic β-cell dysfunction has been demonstrated to mediate key roles in the pathogenesis of gestational diabetes mellitus (GDM). Accumulating evidence has supported the functional involvement of microRNAs (miRNAs) in various types of diabetes, including GDM. However, the detailed biological effect of miRNAs in pancreatic β-cell dysfunction remains poorly understood. In the present study, microarray data of miRNAs in the blood plasma of patients with GDM were retrieved from the Gene Expression Omnibus dataset under the accession number GSE98043. Reverse transcription-quantitative PCR (RT-qPCR) was performed to measure the expression levels of miR-143-3p in the blood plasma isolated from 30 female patients with GDM women and 30 healthy female individuals. Subsequently, murine pancreatic β-cell line, MIN6 cells were treated with high glucose (HG) to construct in vitro cell models of GDM. miR-143-3p in HG-treated MIN6 cells was overexpressed or knocked down using miR-143-3p mimics and miR-143-3p inhibitor. Cell viability, insulin secretion and proinflammatory cytokine production were examined using CCK-8 and ELISA, respectively Cell apoptosis was measured by flow cytometry assay. The protein expression levels of proteins involved in the TAK1/NF-κB pathway were also assessed using western blot. The levels of miR-143-3p were found to be markedly lower in samples from patients with GDM, which were in turn negatively correlated with blood glucose levels. Overexpression of miR-143-3p in MIN6 cells significantly reversed HG-induced cell apoptosis and impairments in cell viability and insulin secretion. In addition, miR-143-3p overexpression attenuated HG-induced proinflammatory cytokine production by MIN6 cells. Subsequently, TGFβ-activated kinase 1 (TAK1), an upstream regulator of the NF-κB pathway, was found to be a direct target of miR-143-3p in pancreatic β cells through luciferase assays and western blot. Overexpression of TAK1 was revealed to abolish the curative effects of miR-143-3p on insulin secretion, cell viability and inflammatory response in HG-treated MIN6 cells. In addition, miR-143-3p could inactivate the NF-κB pathway by inhibiting TAK1 expression. Collectively, these results suggest that miR-143-3p levels are downregulated in the peripheral blood of patients with GDM. Therefore, miR-143-3p overexpression may serve as a method for preventing pancreatic β cell dysfunction by inhibiting the TAK1/NF-κB pathway.
Keywords: gestational diabetes mellitus, microRNA-143-3p, pancreatic β-cells, TGFβ-activated kinase 1, NF-κB pathway
Introduction
Gestational diabetes mellitus (GDM) is one of the most common complications associated with pregnancy, which affects ~14% of pregnancies worldwide (1). It is a risk for mothers and their children because GDM can cause either short- or long-term adverse health effects (2). Although several clinical symptomatic supportive treatment options are available, such as lifestyle intervention (diet and exercise) and insulin therapy, all have demonstrated limited efficacy (3). Therefore, it is necessary to identify novel prognostic markers and molecular therapeutic targets for improving the diagnosis and treatment of GDM.
GDM is characterized by a transitory form of diabetes induced by insulin resistance and pancreatic β-cell dysfunction during pregnancy (4,5). The former is a common feature of GDM, where alleviating insulin resistance using pioglitazone clinically has become an effective approach for treating this condition (6,7). Pancreatic β-cells is a unique population of cells that store and produce insulin in response to metabolic demand, and dysfunction in this cell type is a central component of the pathogenesis of GDM (8). When the quantity of insulin secreted by pancreatic β-cells is insufficient to compensate for the reduced insulin sensitivity of tissues, glucose homeostasis is disrupted, resulting in GDM (9). Responsible for impoverished insulin synthesis and secretion, increased cell apoptosis and proinflammatory cytokine production in pancreatic β-cells are frequently reported in GDM (10-13), which attracts extensive attention to decipher the underlying molecular mechanisms behind the phenotypes. However, the molecular effectors that regulate pancreatic β-cell function in spite of multitudinous efforts especially inflammation and apoptosis, remain elusive.
MicroRNAs (miRNAs or miRs) is a family of short, non-coding RNAs that are ~22 nucleotides in length and typically regulate gene expression by binding to the 3'-untranslated region (3'-UTR) of target gene mRNAs, which causes translational repression or degradation (14,15). Accumulating evidence suggests that miRNAs can exert key roles in the pathogenesis and development of diabetes, including GDM (16,17). Several miRNAs have been previously demonstrated to be differently expressed in GDM, including miR-132, miR-181a and miR-29c, implicating their potential diagnostic value in GDM (18,19). Furthermore, miRNAs have been reported to regulate β-cell development and function. miR-320a was found to target musculoaponeurotic fibrosarcoma oncogene homolog F to suppress insulin secretion whilst inducing apoptosis in pancreatic β cells (20). In addition, the expression of miR-221/222 was significantly upregulated in β-cells from the high-fat diet (HFD)-fed mice and db/db mice, and overexpression of miR-221/222 impaired the insulin production and secretion of β-cells and resulted in glucose intolerance in vivo (21). In GDM, miRNA dysregulation can contribute to the pathogenesis of GDM by regulating the inflammatory response in pancreatic β cells (22,23). miR-101a has been demonstrated to impair the glucose-induced inflammatory response by decreasing the expression of the transcription factor One Cut homeobox2(24). Another previous study showed that silencing miRNA-222 suppressed the inflammatory response in GDM mice by promoting C-X-C motif chemokine 4 receptor expression (25). Therefore, identifying additional miRNAs as biomarkers for clinical diagnosis and pathogenesis would be beneficial for exploring novel and prospective strategies for GDM treatment.
In the present study, dataset from the GSE98043 mRNA expression profiling microarray were analyzed to identify core miRNAs and pathways that can contribute to GDM. Subsequently, the effect and mechanism of any miRNAs found in this screen in the regulation of pancreatic β cell proliferation, apoptosis and insulin secretion, in addition to the inflammatory response, were investigated in MIN6 cells under high glucose (HG) conditions. It is hoped that results of these experiments can provide a theoretical basis for the investigation into the pathogenesis and potential drug targets for GDM.
Materials and methods
MicroRNA expression profile data from Gene Expression Omnibus (GEO)
MicroRNA array expression profile data (GSE98043) was downloaded from GEO database (https://www.ncbi.nlm.nih.gov/geo/) (26). GSE98043 was analyzed using Agilent-070156 Human_miRNA_V21.0_Microarray 046064 (Feature Number version) and consisted of 4 plasma samples from pregnant women (2 from normal controls and 2 from GDM patients). Differentially expressed miRNAs (DEmiRNA) were identified through the R ‘limma’ package (Version 4.2), which is a widely used tool that can be used to analyze data from any GEO series and significance analysis of microarray (SAM), to determine the differential expression of microRNAs among groups (27). miRNAs were considered to be differentially expressed according to the P<0.05 threshold from the limma analysis and median false discovery rate <0.05 from SAM. Data were visualized as heat maps using the online tool Morpheus (https://software.broadinstitute.org/morpheus/).
Blood sample collection
Whole blood samples were collected from 30 women who underwent pregnancies with GDM and 30 healthy women who underwent healthy pregnancies (control) at the Department of Gynecology, Handan Central Hospital (Handan, China) between November, 2019 and November, 2020. GDM was diagnosed according to the World Health Organization/International Association of the Diabetes and Pregnancy Study Groups criteria (28,29). The GDM patients with pre-gestational diabetes, multiple gestations accompanied with further complications and those taking medications were all excluded and pregnancies with GDM who did not fulfill any of the exclusion criteria were recruited in the current study. The control participants without GDM, pre-eclampsia, low birthweight, or preterm delivery were selected in this study. The main clinicopathological parameters are reported and summarized in Table I, and data are presented as means ± SD for both control and GDM groups. The present study was approved by the Ethics Committee of Handan Central Hospital. Briefly, the whole blood samples were obtained from all individuals through venous blood collection prior to breakfast, before they were put into a heparin sodium-containing anticoagulation tube. All blood samples were obtained with informed consent. The blood glucose levels were measured using a glucose assay kit (cat. no. ab65333; Abcam).
Table I.
Clinicopathological characteristics of individuals with GDM and healthy controls.
| Characteristics | Control (n=30) | GDM (n=30) | P-value |
|---|---|---|---|
| Age, years | 31.32±2.45 | 32.02±2.18 | 0.529 |
| Pregnancy period, weeks | 39.84±0.94 | 39.67±0.86 | 0.635 |
| Birth weight, kg | 3.41±0.27 | 3.99±0.42 | 0.020 |
| BMI, kg/m2 | 23.22±1.23 | 24.54±1.86 | 0.029 |
Data are presented as means ± SD; BMI, Body mass index; GDM, gestational diabetes mellitus.
Cell culture and culture conditions
The MIN6 murine pancreatic β-cell line, a well-established cellular model for studying islet β-cell function, was purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. They were cultured with DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 12% FBS (Gibco; Thermo Fisher Scientific, Inc.), 50 µM β-mercaptoethanol, penicillin (100 U/ml) and streptomycin (100 U/ml; Sigma-Aldrich; Merck KGaA) at 37˚C in a humidified atmosphere under 5% CO2.
In the present study, normal glucose (3.3 mM glucose) was set as the control group. MIN6 cells were incubated in complete medium containing 16.7 mM glucose (final concentration in the medium) for 24 h at 37˚C, which was set as the HG group (30). By contrast, cells in the HG + miR-143-3p mimics or mimic-negative control (NC) group were transfected with miR-143-3p mimics or mimics NC for 24 h at 37˚C, before being cultured in 16.7 mM glucose for another 24 h at 37˚C. In addition, cells in the HG + miR-143-3p mimics + pcDNA-TAK1/pcDNA-empty group were co-transfected with the miR-143-3p mimic and the pcDNA-TAK1 plasmid or pcDNA-empty vector at 37˚C for 6 h, and subsequently the transfection reagent was removed and the medium containing 16.7 mM glucose was added for 48 h at 37˚C.
Cell transfection
miR-143-3p mimics, mimics negative control (NC), miR-143-3p inhibitor, inhibitor NC and pcDNA-TAK1 were designed and synthesized by Shanghai GenePharma Co., Ltd. and the pcDNA 3.1 vector (pcDNA empty) was used as a control for the overexpression vector pcDNA-TAK1. The miR-143-3p mimics sequence was 5'-UGAGAUGAAGCACUGUAGCUC-3'; whereas the mimics NC sequence was 5'-ACUGAGCUCGUAGUAUGCAAG-3'. The miR-143-3p inhibitor sequence was 5'-GAGCUACAGUGCUUCAUCUCA-3', whilst the inhibitor NC sequence was 5'-AGUCUCGCGCUUACUGAAUCA-3'.
When the MIN6 cell confluency reached 75%, miR-143-3p mimics (50 nM) or inhibitor (100 nM) and/or pcDNA-TAK1/pcDNA empty vector (2 µg; accession no. NM_172688.3) were transfected or co-transfected into the cells using the Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) by following the manufacturer's protocol. After 48 h in total, the cells were collected for further experiments.
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from whole blood and cultured cells were extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. For the reverse transcription of miRNA and mRNA, cDNA was synthesized using an miScript II RT kit (Qiagen GmbH) at 37˚C for 60 min and PrimeScript™ RT reagent kit (Takara Bio, Inc.) at 42˚C for 30 min, respectively. Subsequent qPCR was performed using the SYBR® Premix Ex Taq™ (Takara Bio, Inc.) on an ABI Prism 7500 Sequence Detection System (Thermo Fisher Scientific, Inc.). The following thermocycling conditions were used: Pre-denaturation at 95˚C for 10 sec; followed by 40 cycles of denaturation at 95˚C for 15 sec and annealing at 55˚C for 15 sec, and extension at 72˚C for 40 sec; and a final extension of 10 min at 72˚C. The following primer sequences were used: miR-143-3p forward, 5'-CTGGCGTTGAGATGAAGCAC-3' and reverse, 5'-CAGAGCAGGGTCCGAGGTA-3'; U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'; TGFβ-activated kinase 1 (TAK1) forward, 5'-AGCTTGATGACACGCTGTTG-3' and reverse, 5'-GAGTTGCTCTGCCCTTCATC-3'; GAPDH forward, 5'-CTCATGACCACAGTCCATGCCATCACTG-3' and reverse, 5'-CATGAGGTCCACCACCCTGTTGCTGTA-3'. miR-143-3p expression was normalized to that of U6, whereas TAK1 expression was normalized to that of GAPDH. Data were analyzed by using the 2-ΔΔCq method (31).
Detection of glucose-stimulated insulin secretion
Cells were incubated in DMEM containing 3.3 or 16.7 mM glucose at 37˚C for 24 h, respectively. After cell sonication as below: 3 sec On and 5 sec OFF on ice for 10 min, the supernatant was collected after centrifugation at 12,000 x g for 10 min at 4˚C. Glucose-stimulated insulin secretion into supernatant was measured using an insulin ELISA kit (Cat no. P01325) from RayBiotech, Inc. according to the manufacturer's protocols.
Cell viability assay
Cells were seeded into a 96-well plate with 1x104 cells per well. When the MIN6 cell confluency reached 75%, the cells were transfected at 37˚C for 24 h with the miRNA mimics/inhibitor or plasmids using the Lipofectamine 2000 transfection reagent, before being treated with 16.7 mM glucose. After 0, 12, 24 and 48 h treatment, 10 µl CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to each well, before the cells were continuously incubated for another 2 h at 37˚C and the absorbance was read using a microplate reader (BioTek Instruments, Inc.) at 450 nm according to the manufacturer's protocols.
Apoptosis assay
Cells were seeded into six-well plates at 1x106 cells per well. When the MIN6 cell confluency reached 75%, the cells were transfected at 37˚C for 24 h with the miRNA mimics/inhibitor or plasmids using the Lipofectamine 2000 transfection reagent, before they were treated with 16.7 mM glucose. After 24 h treatment, MIN6 β-cells in each group were detached by trypsinization and collected by centrifugation at 12,000 x g for 10 min at 4˚C. To measure apoptosis, the Annexin V-FITC Apoptosis Staining/Detection (Abcam) was used according to the manufacturer's instructions. The cells (1x105 cells) were then resuspended in pre-cooled 500 µl binding buffer and incubated with 5 µl Annexin V-FITC and 1 µl propidium iodide (PI). After incubation at room temperature in the dark for 15 min, cell apoptosis was analyzed using a FACScan flow cytometer (BD Biosciences). The MultiCycle Software version 5.0 (Phoenix Flow Systems, San Diego, CA, USA) for Windows 7 (Microsoft Corporation, Redmond, WA, USA) was used to analyze the experimental data.
Caspase 3 activity assay
Caspase-3 activity was measured using a Caspase-3 Activity kit (Cat no. C1115, Beyotime Institute of Biotechnology) according to the manufacturer's protocol. Briefly, cells were harvested 24 h after 16.7 mM glucose treatment at 37˚C and lysed in RIPA buffer (cat no. P0013B, Beyotime Institute of Biotechnology). Caspase-3 activity assay was performed in 96-well plates by incubating 10 µl of each cell lysate sample in 80 µl reaction buffer containing 10 µl of the caspase-3 substrate Ac-DEVD-pNA; (2 mM) at 37˚C for 2 h. The samples were measured with a microplate reader (Model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at an absorbance of 405 nm.
ELISA
In the culture supernatants, TNF-α (cat no. MTA00B), IL-8 (cat no. DY442) and IL-6 (cat no. M6000B) levels were measured using ELISA kits (R&D Systems, Inc.) according to the manufacturer's protocols. Absorbance was detected using an automatic multi-well spectrophotometer (Bio-Rad Laboratories, Inc.) at 450 nm.
Luciferase reporter assay
TargetScan's bioinformatics prediction (version 7.2; www.targetscan.org/vert_72) was used to predict gene targets for miR-143-3p. Gene ontology enrichment analysis and alternative analyses were carried out using EnrichR (version: January 23rd). An adjusted P-value cut-off of below 0.05 was used to filter results. The fragment of the 3'-untranslated region (UTR) of TAK1 [wild-type (wt) or mutant (mut)] was amplified and cloned into the pGL-control vector (Promega). Site-directed mutagenesis of the TAK1 3'-UTR at the putative miR-143-3p binding site was performed using a QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA, USA). MIN6 cells at a density of 1x105 per well were seeded into a 24-well plate until they reached ~60% confluence. In total, 2.5 µg wild-type (wt)-TAK1-UTR-pGL3 or mutant (mut)-TAK1-UTR-pGL3 plasmids was co-transfected with 100 nM miR-143-3p mimics or inhibitors using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). Following 48 h transfection, the luciferase activity was measured using a Dual-luciferase reporter assay system (Promega Corporation). pRL-TK plasmid, containing the Renilla luciferase activity, was transfected as an internal control.
Western blotting
Total cellular proteins were extracted using RIPA buffer (Beyotime Institute of Biotechnology) and protein concentration was determined using the BCA kit (Pierce; Thermo Fisher Scientific, Inc.). Protein isolation and immunoblotting were performed as previously described (32). Briefly, total protein (40 µg/lane) was separated by 8% SDS-PAGE and electrophoretically transferred onto a PVDF membrane (MilliporeSigma). Subsequently, membranes were blocked with 5% skim milk for 1 h at room temperature. The membranes were incubated with specific primary antibodies against TAK1 (cat no. 5206, 1:1,000), cleaved caspase 3 (cat. no. 9661, 1:1,000), inhibitor of NF-κB (IκB) kinase subunit β (IKKβ; cat. no. 8943; 1:1,000), phosphorylated (p-)-IKKβ (cat. no. 2697; 1:1,000), anti-IκBα (cat. no. 4812; 1:1,000), p-IκBα (cat. no. 2859; 1:1,000) or β-actin (cat. no. 4970; 1:1,000) at 4˚C overnight. Following incubation with HRP-conjugated anti-rabbit IgG secondary antibody (cat. no. 7074; 1:2,000) for 1 h at room temperature, the bands were detected using ECL Prime Western Blotting Detection Reagent(Cytiva). All antibodies were purchased from Cell Signaling Technology, Inc. The intensities of the bands of interest were analyzed using the ImageJ software (version 1.46; National Institutes of Health).
Statistical analysis
Data are represented as the mean ± SD from ≥ three replicates and analyzed using the SPSS 22.0 software package (IBM Corp.). Significance was determined using unpaired Student's t-test between two groups whereas for multiple comparisons among > two groups one-way ANOVA followed by Tukey's post hoc test was used. P<0.05 was considered to indicate a statistically significant difference. Correlation analysis was performed using Pearson's correlation coefficient analysis.
Results
miR-143-3p is downregulated in the peripheral blood of patients with GDM
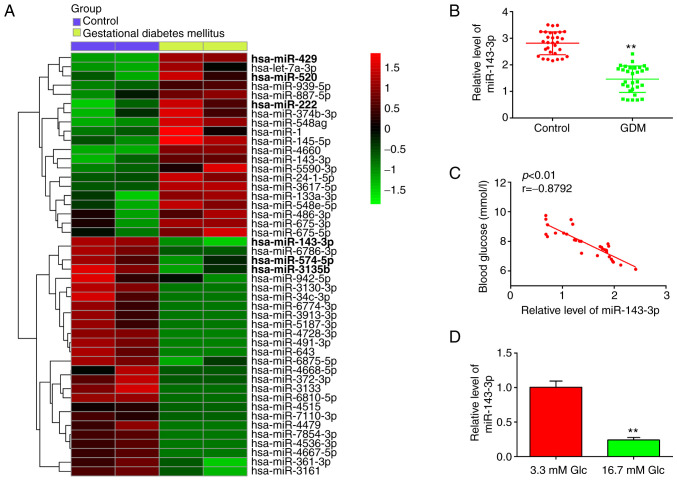
Firstly, 46 miRNAs, including 20 upregulated and 26 downregulated miRNAs, were found from the GEO dataset with the accession number GSE98043 (Fig. 1A). In this list, 20 miRNAs were upregulated and 26 miRNAs were downregulated in the blood samples between the GDM and Control group. Notably, miR-143-3p, miR-574-5p and miR-3135b were found to be downregulated, whereas miR-429, miR-520 and miR-222 were particularly upregulated, compared with those in the control group (Fig. 1A). Association between miRNAs miR-574-5p, miR-3135b, miR-429, miR-520, miR-222 and GDM has been previously reported (25,26,33,34), suggesting the experimental validity of our microarray findings. Notably, miR-143-3p showed the highest degree of downregulation among other miRNAs screened in this dataset, consistent with previous studies (35,36). Furthermore, miR-143-3p has been previously reported to serve an important role in the inflammatory response and apoptosis in several diseases, such as mycoplasmal pneumonia and autoimmune hepatitis (37,38). However, its role in the development of inflammatory response and apoptosis in GDM remains unclear. Therefore, miR-143-3p was selected for further study.
Figure 1.
miR-143-3p is significantly downregulated in the blood samples of patients with GDM. (A) Heatmap of miRNAs that are significantly up/downregulated. The color coding in the heat maps is linear, with green is the lowest whereas red is the highest. Data was retrieved from Gene Expression Omnibus dataset, accession no. GSE98043. (B) The expression of miR-143-3p was measured by RT-qPCR in the peripheral blood samples of 30 patients with GDM and healthy individuals. **P<0.01 vs. Control. (C) Correlation between miR-143-3p levels and blood glucose levels was determined by Pearson correlation analysis. (D) miR-143-3p expression was measured by RT-qPCR in glucose-stimulated (3.3 or 16.7 mmol glucose) MIN6 cells. Data are presented as the means ± SD from three individual experiments. **P<0.01 vs. 3.3 mM Glc. miR, microRNA; GDM, gestational diabetes mellitus; RT-qPCR, reverse transcription-quantitative PCR; Glc, glucose.
To validate the expression levels of miR-143-3p, RT-qPCR was performed in blood samples isolated from 30 pregnant patients with GDM and 30 healthy pregnant individuals. As shown in Fig. 1B, miR-143-3p levels were significantly lower in the peripheral blood of patients with GDM compared with those in healthy individuals. Pearson correlation analysis revealed an inverse correlation between the blood glucose concentration and miR-143-3p levels among the patients with GDM (r=-0.8792; Fig. 1C). Subsequently, HG-induced MIN6 cell model was established as previously described (28). RT-qPCR results demonstrated that miR-143-3p expression was significantly decreased in the HG group compared with that in the control group (Fig. 1D). These data suggest that miR-143-3p may be involved in the pathogenesis of GDM.
Overexpression of miR-143-3p promotes insulin secretion and inhibites cell apoptosis in HG-stimulated MIN6 cells
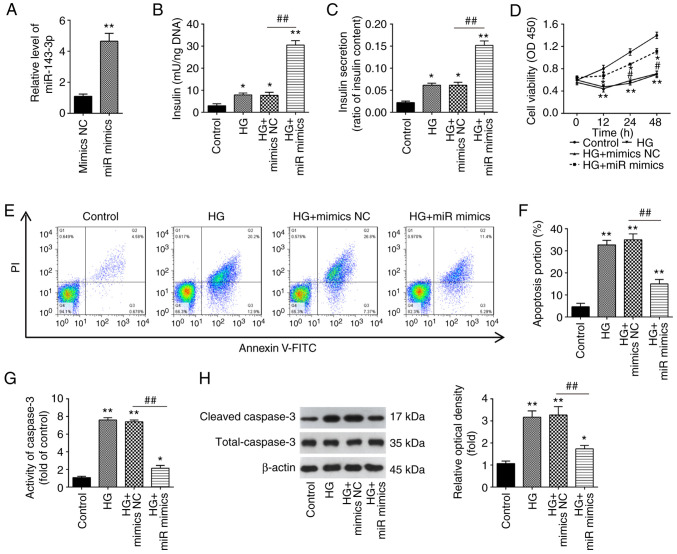
To investigate the function of miR-143-3p in GDM, MIN6 cells were transfected with the miR-143-3p mimics or mimics NC, before being cultured under 16.7 mM glucose (HG). According to the results of RT-qPCR, miR-143-3p was found to be significantly increased after miR-143-3p transfection compared with that in cells transfected with mimics NC, suggesting that the transfection of miR-143-3p mimics was successful (Fig. 2A). As shown in Fig. 2B and C, HG resulted in a significant increase in insulin concentration and secretion in MIN6 cells compared with those in the control group, which was significantly prevented by miR-143-3p overexpression beforehand. Furthermore, the effects of miR-143-3p on MIN6 cell viability and apoptosis was next assessed. A significant decrease in cell viability in HG-stimulated MIN6 cells was detected, but this inhibitory effect was reversed after the overexpression of miR-143-3p (Fig. 2D). In addition, HG-induced cell apoptosis was significantly reversed by miR-143-3p overexpression in MIN6 cells (Fig. 2E). Similarly, caspase 3 activity assay also revealed that caspase 3 activity was significantly increased by HG, which was likewise significantly reversed by the overexpression of miR-143-3p in MIN6 cells (Fig. 2F and G). The caspase 3 activity assay data were supported by those from western blotting, which revealed significantly increased caspase 3 cleavage in response to HG but was in turn significantly reversed by miR-143-3p overexpression (Fig. 2H). These data indicate that miR-143-3p overexpression can promote insulin secretion whilst inhibiting cell apoptosis in HG-treated MIN6 cells.
Figure 2.
Overexpression of miR-143-3p promotes insulin secretion and inhibits cell apoptosis in HG-treated MIN6 cells. (A) Reverse transcription-quantitative PCR was used to measure miR-143-3p expression in MIN6 cells after transient transfection with miR-143-3p mimics. Data are presented as the means ± SD from three individual experiments. **P<0.01 vs. mimics NC. (B-H) MIN6 cells were transfected with the miR-143-3p mimics or mimics NC for 24 h before being cultured in 16.7 mM HG for additional 24 h. The effects of miR-143-3p on (B) insulin content and (C) insulin secretion were determined using an insulin ELISA kit. (D) Cell Counting Kit-8 assay was performed to detect cell viability. (E) Apoptosis was detected using flow cytometry (F) and was quantified. (G) The activity of caspase 3 was measured using a Caspase-3 Activity kit. (H) The expression of cleaved caspase 3 was detected by western blotting. Data are presented as the means ± SD from three individual experiments. *P<0.05 and **P<0.01 vs. Control; #P<0.05, ##P<0.01. vs HG + mimics NC group. miR, microRNA; HG, high glucose; NC, negative control; OD, optical density.
Overexpression of miR-143-3p protects MIN6 cells against HG-induced inflammation
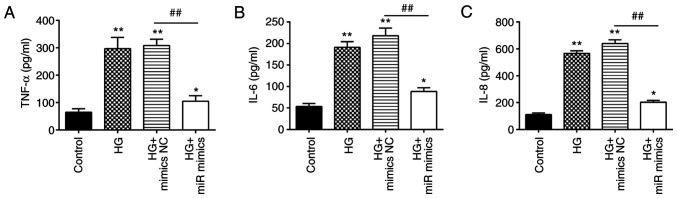
Apart from insulin resistance, excessive inflammation is also one of the key factors involved in the pathogenesis of GDM (39,40). Therefore, the effects of miR-143-3p on the inflammatory response in MIN6 cells after HG treatment were next investigated. HG stimulation led to a significant increase in the levels of proinflammatory factors TNF-α, IL-6, and IL-8 (Fig. 3A-C). However, overexpression of miR-143-3p significantly decreased the levels of these proinflammatory factors previously raised by HG in MIN6 cells. These data suggest that the overexpression of miR-143-3p can suppress the HG-induced inflammatory response in MIN6 cells.
Figure 3.
Overexpression of miR-143-3p inhibits the HG-induced inflammatory response in MIN6 cells. MIN6 cells were transfected with miR-143-3p mimics or mimics NC for 24 h, before they were cultured in 16.7 mM HG for additional 24 h. The levels of (A) TNF-α, (B) IL-6 and (C) IL-8 were then measured by ELISA. Data are presented as the means ± SD from three individual experiments. *P<0.05 and **P<0.01 vs. Control; ##P<0.01 vs. HG + mimics NC group. miR, microRNA; HG, high glucose; NC, negative control.
TAK1 is a direct target of miR-143-3p in MIN6 cells
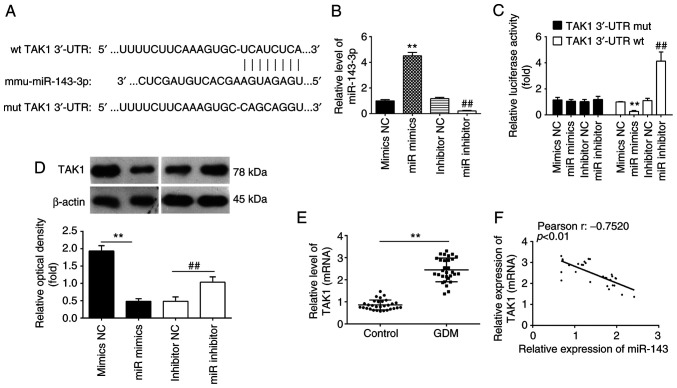
To investigate the underlying molecular mechanism involved in the miR-143-3p-mediated suppression of inflammation and apoptosis, the TargetScan software was next used to analyze the putative target genes of miR-143-3p. Bioinformatics analyses predicted that 3'-UTR regions of the TAK1 gene contains a potential binding site for miR-143-3p (Fig. 4A). Therefore, MIN6 cells were next transfected with either the miR-143-3p mimics or miR-143-3p inhibitor. The expression levels of miR-143-3p were significantly increased and decreased after transfection with the miR-143-3p mimics and miR-143-3p inhibitor, respectively, compared with those in their corresponding NC (Fig. 4B). To verify if TAK1 is indeed a direct target of miR-143-3p, a dual-luciferase reporter assay was performed. Compared with that in their corresponding NCs, overexpression of miR-143-3p was found to significantly decrease the luciferase activities of the 3'-UTR segment of TAK1, whilst luciferase activities of the same construct was significantly increased by miR-143-3p knockdown (Fig. 4C). Subsequently, the potential effects of miR-143-3p on the expression of TAK1 was measured on a protein level in MIN6 cells by western blot analysis. As shown in Fig. 4D, compared with that in their corresponding NCs, the expression of TAK1 proteins was significantly reduced following the overexpression of miR-143-3p, but was significantly upregulated following the knockdown of miR-143-3p, in the MIN6 cells. Since the expression levels of miR-143-3p were lower in the whole blood samples of patients with GDM, the expression levels of TAK1 were also detected in the samples from 30 pregnant individuals with GDM and 30 healthy pregnant individuals. As shown in Fig. 4E, TAK1 expression was significantly upregulated in whole blood samples of patients with GDM compared with that of healthy individuals. In addition, Pearson correlation analysis revealed an inverse correlation between TAK1 and miR-143-3p expression among the GDM patients (r=-0.7520; Fig. 4F). These results suggest that TAK1 is a direct functional target of miR-143-3p in MIN6 cells.
Figure 4.
TAK1 is a direct target of miR-143-3p in MIN6 cells. (A) Scheme of the luciferase reporter constructs for TAK1, showing the potential interaction sites between miR-143-3p and the wt and mut 3'-UTR of TAK1. (B) RT-qPCR was used to measure miR-143-3p expression after transient transfection with the miR-143-3p mimics or inhibitor in MIN6 cells. (C) Luciferase reporter assay was performed to verify the binding between miR-143-3p and TAK1. (D) The expression of TAK1 was measured by western blotting after transfection with the miR-143-3p mimics or inhibitor in MIN6 cells. Data are presented as the means ± SD from three individual experiments. **P<0.01 vs. mimics NC and ##P<0.01 vs. inhibitor NC. (E) The expression of TAK1 was measured by RT-qPCR in the peripheral blood samples from 30 patients with GDM and healthy individuals. **P<0.01. (F) Correlation between miR-143-3p and TAK1 expression was determined by Pearson correlation analysis. TAK1, TGFβ-activated kinase 1; miR, microRNA; 3'UTR, 3' untranslated region; GDM, gestational diabetes mellitus; wt, wild-type; mut, mutant.
Overexpression of miR-143-3p alleviates HG-induced pancreatic β-cell dysfunction by targeting TAK1
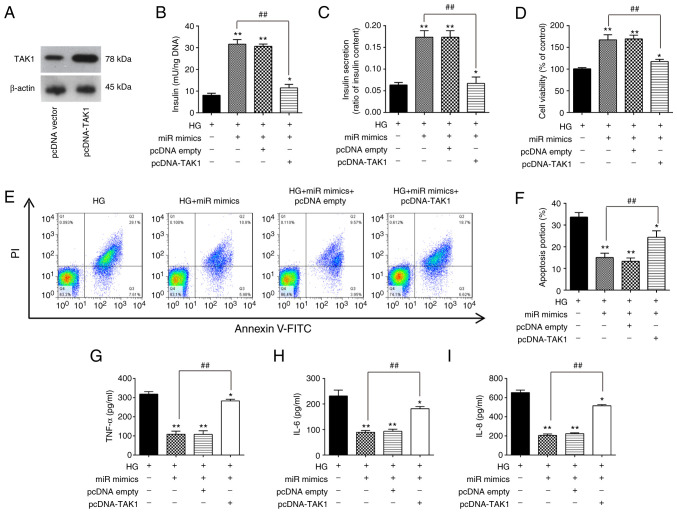
To clarify if TAK1 is involved the inhibitory effects of miR-143-3p on HG-induced inflammation and apoptosis in MIN6 cells, the TAK1 expression vector pcDNA-TAK1 and miR-143-3p mimics were co-transfected into MIN6 cells followed by HG treatment. First, the expression of the TAK1 protein was markedly increased in MIN6 cells after transfection with the pcDNA-TAK1 plasmid compared with that in cells transfected with the empty plasmid (Fig. 5A). Insulin concentration and insulin secretion were next measured, both of which were significantly increased by miR-143-3p overexpression in HG-treated MIN6 cells (Fig. 5B and C). However, these miR-143-3p overexpression-mediated increases were in turn significantly reversed by TAK1 overexpression (Fig. 5B and C). In addition, the increased cell viability induced by miR-143-3p mimic transfection was also significantly reversed by TAK1 overexpression in HG-treated MIN6 cells (Fig. 5D). Subsequently, apoptosis and inflammatory response in HG-treated MIN6 cells were measured. As shown in Fig. 5E and F, the inhibitory effects of miR-143-3p overexpression on the apoptosis in HG-treated MIN6 cells were also significantly reversed by TAK1 overexpression. Similarly, the reduction of proinflammatory cytokine secretion caused by miR-143-3p overexpression were all significantly reversed by TAK1 overexpression (Fig. 5G-I). These data therefore strongly supports the notion that miR-143-3p overexpression can protect MIN6 cell function by negatively targeting TAK1.
Figure 5.
Overexpression of miR-143-3p attenuates HG-induced inflammatory response and apoptosis by targeting TAK1. (A) Western blotting was used to measure TAK1 expression after transient transfection with pcDNA-TAK1 in MIN6 cells. (B-I) MIN6 cells were co-transfected with the miR-143-3p mimics and pcDNA-TAK1 for 24 h, before they were cultured in 16.7 mM HG for an additional 24 h. (B) Insulin content and (C) insulin secretion were measured using an insulin ELISA kit. (D) Cell Counting Kit-8 assay was performed to detect cell viability. (E) Apoptosis was detected by flow cytometry and (F) was quantified. The levels of (G) TNF-α, (H) IL-6 and (I) IL-8 were measured using ELISA kits. Data are presented as the means ± SD from three individual experiments. *P<0.05 and **P<0.01 vs. HG; ##P<0.01 vs. HG + miR mimics group. miR, microRNA; TAK1, TGFβ-activated kinase 1; HG, high glucose.
Overexpression of miR-143-3p blocks the TAK1/NF-κB pathway in MIN6 cells
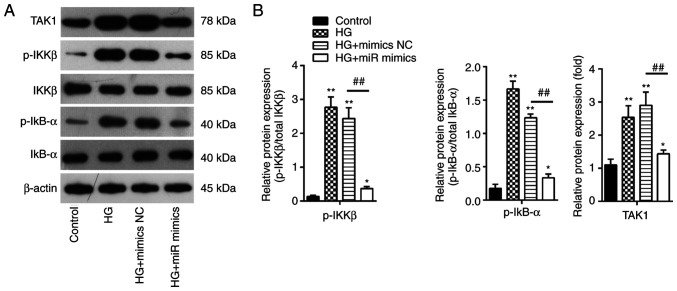
It has been extensively reported that NF-κB functions as a key regulator in the inflammatory process in a wide range of diseases, particularly inflammation-related diseases including GDM (41,42). The present study showed that miR-143-3p overexpression can negatively regulate the expression of TAK1 in MIN6 cells, which is an important upstream activator of the NF-κB pathway. Therefore, the potential effects of miR-143-3p on the activation of TAK1/NF-κB signaling in vitro were next examined. The protein expression levels of TAK1, p-IKKβ and p-IκBα were detected by western blotting. It was found that HG treatment led to a significant increase in IKKβ and IκBα phosphorylation in addition to increasing TAK1 expression in MIN6 cells compared with those in the control group (Fig. 6A). However, these effects were significantly reversed by miR-143-3p overexpression in MIN6 cells (Fig. 6B). These results suggest that miR-143-3p overexpression blocked the TAK1/NF-κB pathway in MIN6 cells under HG conditions.
Figure 6.
Overexpression of miR-143-3p blocks the TAK1/NF-κB pathway in MIN6 cells. MIN6 cells were transfected with the miR-143-3p mimics or mimics NC for 24 h, before being cultured in 16.7 mM HG for an additional 24 h. (A) Protein expression levels of TAK1, phosphorylation levels IKKβ and IκBα were all measured by western blotting. (B) The bands were semi-quantitatively analyzed using ImageJ software. Data are presented as the means ± SD from three individual experiments. *P<0.05 and **P<0.01 vs. control; ##P<0.01 vs HG + mimics NC group. miR, microRNA; TAK1, TGFβ-activated kinase 1; HG, high glucose; p-, phosphorylated; IKKβ, inhibitor of NF-κB kinase subunit β; IκBα, inhibitor of NF-κB α.
Discussion
In the present study, miR-143-3p levels were found to be lower in the whole blood from patients with GDM, which were in turn inversely correlated with blood glucose concentrations. Furthermore, overexpression of miR-143-3p promoted cell viability and insulin secretion, inhibited apoptosis and the inflammatory response in pancreatic β cells cultured under high-glucose conditions. It was also found that the TAK1/NF-κB signaling pathway may be involved. These findings suggest that miR-143-3p can regulate the pathogenesis of GDM and may serve as a novel therapeutic target for GDM.
Several studies have reported that GDM can alter the expression profile of miRNAs, such that they can be exploited as diagnostic targets for GDM that regulate cell physiology (30,43). Chen et al (44) previously demonstrated that high expression levels of miR-351 in liver tissues can negatively regulate the PI3K/AKT pathway by interacting with flotillin 2, thereby acting as an inhibitor of GDM. In another study, Pfeiffer et al (45) reported miR-330-3p as being significantly upregulated in women with GDM compared to nondiabetic controls and miR-330-3p to be significantly related to the percentage of caesarean deliveries. This strong novel association of circulating miR-330-3p with risk of primary caesarean delivery as a pregnancy outcome linked with poor maternal glycaemic control. In addition, Fu et al (46) reported that miR-875-5p silencing notably reduced expression levels of pro-inflammatory markers by targeting thioredoxin reductase 1 cytoplasmic in rats with GDM. By contrast, Zhang et al (25) revealed that silencing miR-222 expression can suppress the inflammatory response in mice with GDM by promoting CXCR4 whist inactivating NOD-, LRR- and pyrin domain-containing protein 3 inflammasomes. In the present study, after retrieving a miRNA microarray dataset (GSE98043), large numbers of miRNAs were found to be aberrantly expressed in the whole blood of patients with GDM, including miR-143-3p, which was downregulated. In addition, there was a significant inverse correlation between miR-143-3p levels and the blood glucose concentrations. These data suggest that miR-143-3p may be involved in GDM, implying its potential role in GDM.
The role of miR-143-3p in GDM has been studied previously (35,47). Decreased miR-143-3p placental expression was reported in macrosomia, which may be associated with maternal and infant health problems (36). These previous studies suggest a potential role for miR-143-3p in GDM. Several studies have shown that miR-143-3p is involved in the initiation of the inflammatory response and apoptosis in various disease settings (48). miR-143-3p overexpression attenuated the Derp1-induced inflammatory response and apoptosis of BEAS-2B cells by downregulating high mobility group box 1 (HMGB1) expression in Bronchial asthma (49). In addition, miR-143-3p overexpression has been demonstrated to alleviate the pulmonary inflammatory response and suppress alveolar epithelial cell apoptosis in mice with mycoplasmal pneumonia (37). miR-143-3p was also found to at least partially reverse obesity-induced insulin resistance by downregulating insulin-like growth factor 2 receptor expression in differentiated hADSCs (50). However, the role of miR-143-3p in GDM remains elusive and requires further investigation. In the present study, miR-143-3p overexpression was shown to enhance cell viability and insulin secretion, inhibited the apoptosis and inflammatory response in pancreatic β cells following HG stimulation. This suggests that miR-143-3p may be involved in the pathogenesis of GDM by regulating pancreatic β-cell function.
Although miR-143-3p is emerging as an important functional component in GDM development, the molecular mechanisms remain poorly understood. In the present study, TAK1, also known as MAP3K7, was found to be a target of miR-143-3p that can be targeted by miR-143-3p, suggesting that the development of GDM may be associated with the TAK1 signaling pathway. Furthermore, the seemingly therapeutic effects induced by miR-143-3p overexpression on the pancreatic β cells treated with HG were reversed by TAK1 overexpression. As a member of the MAPK kinase kinase family, TAK1 can be activated by various inflammatory mediators, such as IL-1β (51-53). Once activated, TAK1 phosphorylates and activates the IKK complex, especially IKKβ, leading to the degradation of IκBα and triggers the activation of the transcription factor NF-κB (54). This activated NF-κB then promotes the transcription of genes associated with pathways controlling inflammatory responses, cell proliferation and apoptosis, impairing β-cell survival and function (55). After transfection with the miR-143-3p mimics, the expression levels of p-IKKβ and p-IκBα were significantly decreased in HG-treated MIN6 cells, suggesting the inactivation of NF-κB pathway. Combing the results from previous studies and those from the present study that the inflammatory responses can be relieved by suppressing TAK1/NF-κB activity, where miR-143-3p may be a potential therapeutic target for GDM treatment (56,57).
There remain to be some limitations to the present study. Although it investigated the cellular function of miR-143-3p and its underlying mechanism in GDM, in vivo studies are required to validate these preliminary in vitro results obtained. In addition, only one cell line, MIN6, was used in the present study. Therefore, further study with other cell lines, such as INS-1 cells, is necessary for verification. For the screening of differentially expressed miRNAs in the present study, only the GSE98043 dataset was used. For bioinformatics analysis, ≥ two datasets are required to screen differentially expressed microRNAs to increase reliability. Therefore, new datasets and a more in-depth analysis are required in any future studies.
In conclusion, results from the present study provided novel findings that the overexpression of miR-143-3p can ameliorate pancreatic β cell dysfunction by inhibiting the TAK1/NF-κB pathway. These data provide novel insights into the pathogenesis of GDM, which may facilitate the identification of novel diagnostic or therapeutic targets for GDM.
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was supported by the Mandatory Planning Projects of Handan Science and Technology Bureau (grant no. 19422083009-9).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CRL and LPY conceived and designed the experiments. CRL, HQF and LNZ performed the experiments. CRL, HQF, LNZ, YRG and JJM analyzed the data. LPY and CRL confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Handan Central Hospital (Approval number: 2019014 Handan, China). All blood samples were obtained with informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Boulton A. Strengthening the international diabetes federation (IDF) Diabetes Res Clin Pract. 2020;160(108029) doi: 10.1016/j.diabres.2020.108029. [DOI] [PubMed] [Google Scholar]
- 2.Zhu WW, Yang HX, Wang C, Su RN, Feng H, Kapur A. High prevalence of gestational diabetes mellitus in beijing: Effect of maternal birth weight and other risk factors. Chin Medical J (Engl) 2017;130:1019–1025. doi: 10.4103/0366-6999.204930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 4.Alejandro EU, Mamerto TP, Chung G, Villavieja A, Gaus NL, Morgan E, Pineda-Cortel MRB. Gestational diabetes mellitus: A harbinger of the vicious cycle of diabetes. Int J Mol Sci. 2020;21(5003) doi: 10.3390/ijms21145003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341–363. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebner M, Lucic I, Leonard TA, Yudushkin I. PI(3,4,5)P3 Engagement restricts Akt activity to cellular membranes. Mol Cell. 2017;65:416–431.e6. doi: 10.1016/j.molcel.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: The last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieto D, Contreras C, Sanchez A. Endothelial dysfunction, obesity and insulin resistance. Curr Vasc Pharmacol. 2014;12:412–426. doi: 10.2174/1570161112666140423221008. [DOI] [PubMed] [Google Scholar]
- 10.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–138. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 11.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: From metabolic stress to therapy. Diabetes Care. 2008;31 (Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 12.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab. 2009;11 (Suppl 4):S82–S90. doi: 10.1111/j.1463-1326.2009.01113.x. [DOI] [PubMed] [Google Scholar]
- 13.Busch AK, Cordery D, Denyer GS, Biden TJ. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic beta-cell function. Diabetes. 2002;51:977–987. doi: 10.2337/diabetes.51.4.977. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Wang J, Liu Y, Wang C, Duan D, Lu N, Wang K, Zhang L, Gu K, Chen S, et al. Comparisons of serum miRNA expression profiles in patients with diabetic retinopathy and type 2 diabetes mellitus. Clinics (Sao Paulo) 2017;72:111–115. doi: 10.6061/clinics/2017(02)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastiani G, Guarino E, Grieco GE, Formichi C, Poggi CD, Ceccarelli E, Dotta F. Circulating microRNA (miRNA) expression profiling in plasma of patients with gestational diabetes mellitus reveals upregulation of miRNA miR-330-3p. Front Endocrinol (Lausanne) 2017;8(345) doi: 10.3389/fendo.2017.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu Y, Chen D, Xu J, Huo R, Dai J, et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS One. 2011;6(e23925) doi: 10.1371/journal.pone.0023925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collares CV, Evangelista AF, Xavier DJ, Rassi DM, Arns T, Foss-Freitas MC, Foss MC, Puthier D, Sakamoto-Hojo ET, Passos GA, Donadi EA. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res Notes. 2013;6(491) doi: 10.1186/1756-0500-6-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du H, Yin Z, Zhao Y, Li H, Dai B, Fan J, He M, Nie X, Wang CY, Wang DW, Chen C. miR-320a induces pancreatic beta cells dysfunction in diabetes by inhibiting MafF. Mol Ther Nucleic Acids. 2021;26:444–457. doi: 10.1016/j.omtn.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan L, Shan A, Su Y, Cheng Y, Ji H, Yang Q, Lei Y, Liu B, Wang W, Ning G, et al. MiR-221/222 inhibit insulin production of pancreatic beta-cells in mice. Endocrinology. 2020;161(bqz027) doi: 10.1210/endocr/bqz027. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Wang H, Li C, Zhang X, Xiu X, Teng P, Wang Z. Dysregulation of microRNA-657 influences inflammatory response via targeting interleukin-37 in gestational diabetes mellitus. J Cell Physiol. 2019;234:7141–7148. doi: 10.1002/jcp.27468. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, Wang Z, Liu G, Jin C, Zhang Q, Man S, Wang Z. MiR-657 promotes macrophage polarization toward M1 by targeting FAM46C in gestational diabetes mellitus. Mediators Inflamma. 2019;2019(4851214) doi: 10.1155/2019/4851214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Wang Z, Tu Y, Shen H, Dai Z, Lin J, Zhou Z. miR-101a and miR-30b contribute to inflammatory cytokine-mediated beta-cell dysfunction. Lab Invest. 2015;95:1387–1397. doi: 10.1038/labinvest.2015.112. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Luan S, Xiao X, Lin L, Zhao X, Liu X. Silenced microRNA-222 suppresses inflammatory response in gestational diabetes mellitus mice by promoting CXCR4. Life Sci. 2021;266(118850) doi: 10.1016/j.lfs.2020.118850. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Li Z, Zhao M, Ye W, Wu H, Liao Q, Bu S, Zhang Y. Circulating miRNAs miR-574-5p and miR-3135b are potential metabolic regulators for serum lipids and blood glucose in gestational diabetes mellitus. Gynecol Endocrinol. 2021;37:665–671. doi: 10.1080/09513590.2021.1908990. [DOI] [PubMed] [Google Scholar]
- 27.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(Article3) doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 28.Weinert LS. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Comment to the international association of diabetes and pregnancy study groups consensus panel. Diabetes Care. 2010;33(e97) doi: 10.2337/dc10-0544. author reply e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, de Leiva A, Hod M, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H, Tao S. MiRNA-221 protects islet beta cell function in gestational diabetes mellitus by targeting PAK1. Biochem Biophys Res Commun. 2019;520:218–224. doi: 10.1016/j.bbrc.2019.09.139. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Wen J, Han S, Cui M, Wang Y. [Retracted] long noncoding RNA MCM3APAS1 drives ovarian cancer progression via the microRNA1433p/TAK1 axis. Oncol Rep. 2021;46(178) doi: 10.3892/or.2021.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei L, Cao C, Ma X, Wang X, Wang M, Zhang P. Elevated serum and urine MiR-429 contributes to the progression of gestational diabetes mellitus. Clin Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.200909. 10.7754/Clin.Lab.2020.200909. [DOI] [PubMed] [Google Scholar]
- 34.Wen J, Bai X. miR-520h Inhibits cell survival by targeting mTOR in gestational diabetes mellitus. Acta Biochim Pol. 2021;68:65–70. doi: 10.18388/abp.2020_5389. [DOI] [PubMed] [Google Scholar]
- 35.Muralimanoharan S, Maloyan A, Myatt L. Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: Role of miR-143. Clin Sci (Lond) 2016;130:931–941. doi: 10.1042/CS20160076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JT, Cai QY, Ji SS, Zhang HX, Wang YH, Yan HT, Yang XJ. Decreased miR-143 and increased miR-21 placental expression levels are associated with macrosomia. Mol Med Rep. 2016;13:3273–3280. doi: 10.3892/mmr.2016.4892. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Li H, Shi Y, Wang S, Xu Y, Li H, Liu D. miR-143-3p impacts on pulmonary inflammatory factors and cell apoptosis in mice with mycoplasmal pneumonia by regulating TLR4/MyD88/NF-kappaB pathway. Biosci Rep. 2020;40(BSR20193419) doi: 10.1042/BSR20193419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu H, Chen D, Cai C, Du Q, Lin H, Pan T, Sheng L, Xu Y, Teng T, Tu J, et al. microRNA-143-3p attenuated development of hepatic fibrosis in autoimmune hepatitis through regulation of TAK1 phosphorylation. J Cell Mol Med. 2020;24:1256–1267. doi: 10.1111/jcmm.14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajifaraji M, Jahanjou F, Abbasalizadeh F, Aghamohammadzadeh N, Abbasi MM, Dolatkhah N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac J Clin Nutr. 2018;27:581–591. doi: 10.6133/apjcn.082017.03. [DOI] [PubMed] [Google Scholar]
- 40.Sha H, Zeng H, Zhao J, Jin H. Mangiferin ameliorates gestational diabetes mellitus-induced placental oxidative stress, inflammation and endoplasmic reticulum stress and improves fetal outcomes in mice. Eur J Pharmacol. 2019;859(172522) doi: 10.1016/j.ejphar.2019.172522. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Qu L, Ni H, Wang Y, Li L, Yang X, Wang X, Hou Y. Expression and function of lncRNA MALAT1 in gestational diabetes mellitus. Adv Clin Exp Med. 2020;29:903–910. doi: 10.17219/acem/121524. [DOI] [PubMed] [Google Scholar]
- 42.Kuzmicki M, Telejko B, Wawrusiewicz-Kurylonek N, Lipinska D, Pliszka J, Wilk J, Zielinska A, Skibicka J, Szamatowicz J, Kretowski A, Gorska M. The expression of genes involved in NF-kappaB activation in peripheral blood mononuclear cells of patients with gestational diabetes. Eur J Endocrinol. 2013;168:419–427. doi: 10.1530/EJE-12-0654. [DOI] [PubMed] [Google Scholar]
- 43.Elliott HR, Sharp GC, Relton CL, Lawlor DA. Epigenetics and gestational diabetes: A review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia. 2019;62:2171–2178. doi: 10.1007/s00125-019-05011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen SH, Liu XN, Peng Y. MicroRNA-351 eases insulin resistance and liver gluconeogenesis via the PI3K/AKT pathway by inhibiting FLOT2 in mice of gestational diabetes mellitus. J Cell Mol Med. 2019;23:5895–5906. doi: 10.1111/jcmm.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeiffer S, Sanchez-Lechuga B, Donovan P, Halang L, Prehn JHM, Campos-Caro A, Byrne MM, López-Tinoco C. Circulating miR-330-3p in late pregnancy is associated with pregnancy outcomes among lean women with GDM. Sci Rep. 2020;10(908) doi: 10.1038/s41598-020-57838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu S, Fu S, Ma X, Yang X, Ling J. miR8755p regulates IR and inflammation via targeting TXNRD1 in gestational diabetes rats. Mol Med Rep. 2021;23(303) doi: 10.3892/mmr.2021.11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hromadnikova I, Kotlabova K, Dvorakova L, Krofta L. Diabetes mellitus and cardiovascular risk assessment in mothers with a history of gestational diabetes mellitus based on postpartal expression profile of microRNAs associated with diabetes mellitus and cardiovascular and cerebrovascular diseases. Int J Mol Sci. 2020;21(2437) doi: 10.3390/ijms21072437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu B, Zhao Y, Zhang H, Xie D, Nie W, Shi K. Inhibition of microRNA-143-3p attenuates myocardial hypertrophy by inhibiting inflammatory response. Cell Biol Int. 2018;42:1584–1593. doi: 10.1002/cbin.11053. [DOI] [PubMed] [Google Scholar]
- 49.Cai XJ, Huang LH, Zhu YK, Huang YJ. LncRNA OIP5AS1 aggravates house dust miteinduced inflammatory responses in human bronchial epithelial cells via the miR1433p/HMGB1 axis. Mol Med Rep. 2020;22:4509–4518. doi: 10.3892/mmr.2020.11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin X, Du Y, Lu W, Gui W, Sun S, Zhu Y, Wang G, Eserberg DT, Zheng F, Zhou J, et al. CircRNF111 protects against insulin resistance and lipid deposition via regulating miR-143-3p/IGF2R axis in metabolic syndrome. Front Cell Dev Biol. 2021;9(663148) doi: 10.3389/fcell.2021.663148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ajibade AA, Wang HY, Wang RF. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34:307–316. doi: 10.1016/j.it.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Delaney JR, Mlodzik M. TGF-beta activated kinase-1: New insights into the diverse roles of TAK1 in development and immunity. Cell Cycle. 2006;5:2852–2855. doi: 10.4161/cc.5.24.3558. [DOI] [PubMed] [Google Scholar]
- 53.Fan Y, Yu Y, Mao R, Zhang H, Yang J. TAK1 Lys-158 but not Lys-209 is required for IL-1beta-induced Lys63-linked TAK1 polyubiquitination and IKK/NF-κB activation. Cell Signal. 2011;23:660–665. doi: 10.1016/j.cellsig.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Ann Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Lee JH, Rane SG. TGF-β signaling in pancreatic islet β cell development and function. Endocrinology. 2021;162(bqaa233) doi: 10.1210/endocr/bqaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ardestani A, Maedler K. The hippo signaling pathway in pancreatic β-cells: Functions and regulations. Endocr Rev. 2018;39:21–35. doi: 10.1210/er.2017-00167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.