Abstract
Previous studies showed that decorin-binding protein A (DbpA) of Borrelia burgdorferi was a protective immunogen in the murine model of Lyme borreliosis when mice were challenged (needle inoculated) intradermally with in vitro-cultivated spirochetes. In the present study, DbpA-immunized C3H/HeJ mice were not protected from infection when infested with Ixodes scapularis nymphs harboring virulent B. burgdorferi 297. This lack of protection correlated with the failure to detect DbpA on B. burgdorferi in ticks, suggesting that DbpA is not available as a target for bactericidal antibodies in serum when B. burgdorferi-infected ticks take their blood meal from an immunized host. The failure of DbpA immunization to protect tick-challenged mice contradicts the results of earlier needle inoculation vaccination experiments and suggests that DbpA may not be suitable as a Lyme disease vaccine.
The new outer surface protein A (OspA) Lyme disease vaccine, recently approved for human use by a panel of the United States Food and Drug Administration, represents a significant medical advance (21, 22). However, there are a number of unresolved issues concerning this first-generation monovalent vaccine (23), including (i) uncertainty about its efficacy in children under the age of 15 (a group with a particularly high risk of contracting Lyme disease), (ii) heterogeneity among (and even absence of) ospA genes within United States isolates of B. burgdorferi (4, 17), and (iii) the fact that protective levels of anti-OspA antibodies in humans can wane after vaccination (18). Of equal potential importance is the fact that OspA expression by B. burgdorferi is sharply downregulated as the tick vector takes its blood meal and the spirochetes migrate from the midgut to the salivary glands of the tick (9, 20). OspA vaccination thus is efficacious via the killing of B. burgdorferi in tick midguts by OspA antibodies present in the blood meal (9, 11). One way of potentially enhancing the efficacy of the current OspA Lyme disease vaccine would be to expand the number of vaccinogens to include one or more also expressed during the mammalian phase of infection; such a multivalent vaccine would provide immune targets during both phases of the zoonotic life cycle of B. burgdorferi.
To this end, at least four studies demonstrated that decorin-binding protein A (DbpA), which is expressed by B. burgdorferi during the mammalian phase of infection, was protective in the murine model of Lyme borreliosis when immunized mice were challenged (needle inoculated) intradermally or subcutaneously with borreliae cultivated in vitro (5, 10, 14, 15). As such, DbpA has emerged as a leading new candidate vaccinogen for Lyme disease. In view of the importance of DbpA as a potential human Lyme disease vaccinogen, we sought to extend our previous vaccination studies (14) to confirm that mice immunized with DbpA also were protected from infection when infested with Ixodes scapularis nymphs harboring B. burgdorferi 297, a challenge condition which mimics the natural mode of B. burgdorferi transmission.
Preparation of recombinant antigens (DbpA, OspA, and glutathione S-transferase [GST]) and immunization of C3H/HeJ mice were described previously (14). All mice achieved serum antibody titers against each respective immunogen of greater than 1:10,000, as determined by testing 10-fold serial dilutions of sera via immunoblotting (14) (data not shown). Infection of I. scapularis nymphs with B. burgdorferi 297 and infestation of mice for challenge were performed essentially as described previously (27); each mouse was infested with 10 infected nymphs which fed for 4 days. B. burgdorferi infections of mice were confirmed by culture of heart, urinary bladder, and punch biopsy specimens from ear pinna in BSK-H medium (Sigma Chemical Co., St. Louis, Mo.) (14). Two separate vaccination experiments, using different lots of antigens prepared on different days, were performed. As predicted, the combined results of the two separate vaccination experiments showed that immunization with OspA protected 100% of mice (Table 1). In contrast, immunization with DbpA had virtually no protective effect (8.3%) (Table 1). GST, used as a negative control, also did not confer protection (Table 1).
TABLE 1.
Immunization of C3H/HeJ mice with recombinant proteins and subsequent challenge by infestation with I. scapularis nymphs harboring B. burgdorferi 297
| Expt. | Vaccinogen | No. of mice challenged | No. of mice protected |
|---|---|---|---|
| 1 | GST | 4 | 0 |
| OspA | 5 | 5 | |
| DbpA | 3 | 1 | |
| 2 | GST | 5 | 0 |
| OspA | 5 | 5 | |
| DbpA | 9 | 0 |
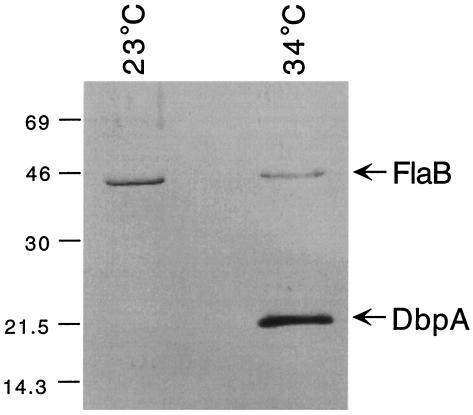
In view of the previous reports of immunoprotection conferred by DbpA (5, 10, 14, 15), the failure of DbpA to protect immunized mice challenged via tick infestation was surprising. Therefore, additional experiments were conducted in an attempt to explain these unanticipated results. In this regard, temperature regulation appears to be important for a number of B. burgdorferi lipoproteins, such as OspC, OspE, OspF, Mlp8, Mlp9, and Mlp10 (1, 24, 27). Of note, Cassatt et al. (5) reported that B. burgdorferi B31 sharply downregulates its expression of DbpA when cultivated at 23°C, a temperature corresponding more closely with that of the tick's natural habitat when it is not feeding. To examine whether DbpA expression also is temperature regulated in B. burgdorferi 297, low-passage-number spirochetes were first cultivated at 34°C to exponential phase in BSK-H medium; an aliquot of the culture was stored at −70°C for future use. The remainder was diluted to a final concentration of 103 spirochetes per ml in fresh BSK-H medium and incubated at 23°C for about 14 days, or until the density of the culture reached about 107 spirochetes per ml. Culture volumes containing 107 34°C-cultivated B. burgdorferi or 2 × 107 23°C-cultivated B. burgdorferi were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analyses. In these analyses, anti-FlaB monoclonal antibody 8H3-33, which has the same specificity as 1H6-33 (1), was used to monitor the different protein levels within the two gel lanes. The rat polyclonal antiserum utilized, which was directed specifically against DbpA, was described previously (14). Spirochetes cultivated at 34°C expressed large amounts of DbpA, whereas DbpA was undetectable in even twice the number of organisms cultivated at 23°C (Fig. 1).
FIG. 1.
SDS-PAGE and immunoblot analysis of DbpA expression in B. burgdorferi 297 cultivated at either 23°C (2 × 107 spirochetes) or 34°C (107 spirochetes). Polyclonal rat anti-DbpA antiserum and monoclonal antibody 8H3-33, directed against FlaB, were used as the respective antibody probes. Numbers at the left denote protein apparent molecular weights (in thousands).
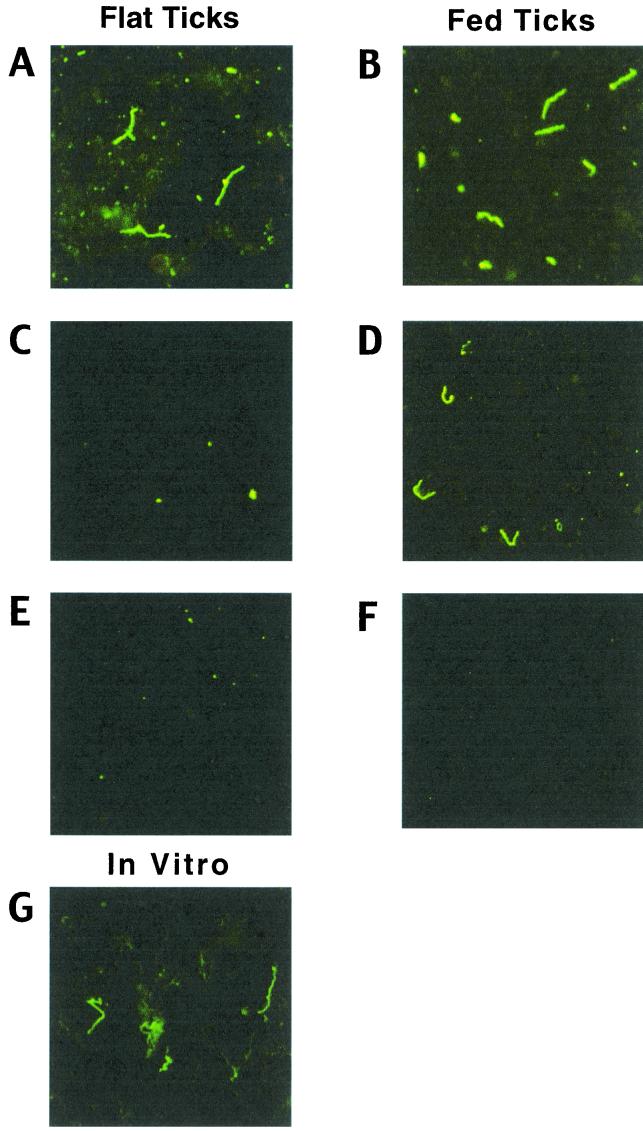
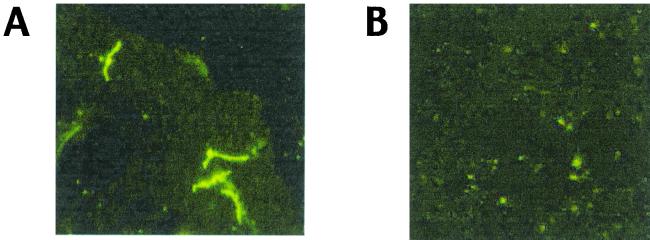
The results shown in Fig. 1, in conjunction with those of Cassatt et al. (5), imply that temperature is one important regulator of DbpA expression in B. burgdorferi. Given this, we hypothesized that DbpA may not be expressed by B. burgdorferi in tick midguts, at least those of flat ticks. To examine this possibility, indirect immunofluorescence assays (IFAs) were performed (1) on spirochetes harvested from the midguts of both flat and engorged (4-day) nymphs. Spirochetes were readily detected in both flat- and fed-tick midgut smears with a rat polyclonal antiserum to B. burgdorferi 297, although, as expected, spirochetes were less numerous in flat ticks (Fig. 2A and B). OspC expression was virtually undetectable in borreliae from flat ticks (Fig. 2C), whereas OspC was upregulated in fed ticks (Fig. 2D), as predicted from previous studies of OspC expression (1, 20). Consistent with the immunoblotting data (Fig. 1), DbpA was readily detected by IFA among borreliae cultivated in vitro at 34°C (Fig. 2G). In contrast, DbpA was not detectable in borreliae from either flat (Fig. 2E) or 4-day-fed (Fig. 2F) ticks. Moreover, although borreliae were readily detectable by IFA (using antiserum to B. burgdorferi 297) within the salivary glands of ticks fed for 48 to 72 h (Fig. 3A), the time of maximal spirochete load (8), no borreliae expressing DbpA were found in the same sample of tick salivary glands (Fig. 3B). These results imply that the expression of DbpA by B. burgdorferi does not occur to any appreciable degree within either flat or fed ticks. That DbpA is not expressed by B. burgdorferi in ticks but is expressed on the surfaces of in vitro-cultivated spirochetes (5, 10, 12, 14, 15) suggests that such expression of DbpA, in essence, may be an artifact of culturing B. burgdorferi under the artificial growth conditions provided by BSK medium.
FIG. 2.
(A to F) IFAs of borreliae harvested from the midguts of either flat or 4-day-fed I. scapularis nymphs harboring B. burgdorferi 297. Nymph midgut contents were expressed onto microscope slides, dried, fixed with acetone, and then probed with rat antiserum directed against either whole-cell lysates of B. burgdorferi 297 (A and B), OspC (C and D), or DbpA (E to G). (G) Smear of B. burgdorferi 297 cultivated in vitro at 34°C and probed with anti-DbpA antiserum. The secondary antibody probe was fluorescein isothiocyanate-conjugated goat anti-rat immunoglobulin G. Spirochetes were observed under a 40× objective; data were recorded via a charge-coupled device camera mounted on an Olympus dark-field and fluorescence microscope. Panels shown are representative of at least 30 microscope fields examined in each of two separate experiments.
FIG. 3.
IFAs of borreliae harvested from the salivary glands of B. burgdorferi-infected I. scapularis nymphs. Nymphs were allowed to feed on normal C3H/HeJ mice for 48 to 72 h, at which time the ticks were removed. Tick salivary glands were then dissected out (with caution to exclude midgut contents) and homogenized (by repeated gentle pipeting) in 10-μl aliquots of phosphate-buffered saline. The samples were then divided into two equal portions on microscope slides, dried, fixed with acetone, and probed with rat antiserum directed against either a whole-cell lysate of B. burgdorferi 297 (A) or DbpA (B). All other procedures were as described for Fig. 2. The panels shown are representative of at least 30 microscope fields examined in each of five separate salivary gland smears probed with each antiserum.
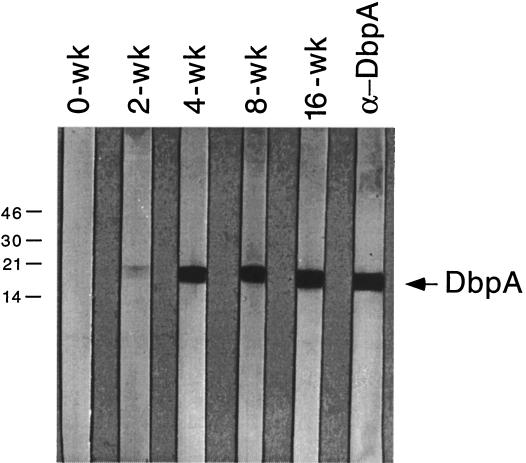
The lack of detectable DbpA in B. burgdorferi within either flat or fed ticks also suggests that DbpA is not available as a B. burgdorferi target for bactericidal antibody in tick midguts during tick feeding, consistent with our finding that DbpA-immunized mice were not protected when challenged via tick infestation (Table 1). However, we also found that antibodies reactive with recombinant DbpA were detectable in the serum of tick-challenged mice as early as 2 weeks after tick infestation (Fig. 4), comparable to what was reported previously after low-dose needle inoculation of mice with in vitro-cultivated B. burgdorferi (14), suggesting that DbpA expression is upregulated relatively soon after B. burgdorferi is first introduced into mammalian tissues.
FIG. 4.
Appearance of antibodies against DbpA during the course of tick-transmitted B. burgdorferi infection of C3H/HeJ mice. Groups of 10 mice were infested with I. scapularis nymphs harboring B. burgdorferi 297 (27); at various intervals postinfestation (denoted in weeks [wk] at the tops of the immunoblot strips), blood from mice within each group was collected and sera were then obtained and pooled. Recombinant DbpA was subjected to SDS-PAGE as previously described (14). Nitrocellulose strips were immunoblotted with 1:500 dilutions of the respective pooled sera. The lane at the right is a strip probed with rat antiserum against DbpA (α-DbpA). Numbers at the left denote protein apparent molecular weights (in thousands).
If DbpA is expressed relatively early in the course of mammalian infection by B. burgdorferi, it is reasonable to assume that antibody elicited by immunization with DbpA still should be bactericidal to B. burgdorferi just after tick transmission; thus, the data of Table 1 raise an interesting paradox. One plausible explanation for the inability of DbpA antibodies to kill B. burgdorferi transmitted by tick bite (Table 1) is that although DbpA is expressed sometime after B. burgdorferi enters mammalian tissue, this lipoprotein is not sufficiently shuttled to the surface of B. burgdorferi, where it can serve as an immune target. Another possibility is that changes in the expression of other outer surface lipoproteins during mammalian infection by B. burgdorferi somehow limit antibody access to DbpA, as has been proposed for P66 (3). Interestingly, in passive immunization experiments in which rabbit anti-DbpA antiserum was administered to mice at various times after challenge with in vitro-cultivated B. burgdorferi B31, Hanson et al. (15) showed that needle inoculation of mice with B. burgdorferi could be aborted only up to 4 days postinfection, suggesting that either DbpA expression or its membrane topology is altered relatively quickly so that the protein becomes refractory to bactericidal antibody. Nonetheless, normal antigen processing and presentation of subsurface DbpA, on the other hand, would still account for the appearance of antibodies early in the course of infection (Fig. 4); the subsurface localization of other B. burgdorferi lipoproteins, even some known to have surface topologies under certain conditions, has been well documented (6, 7, 16, 26). That DbpA is expressed but may not be surface exposed during the mammalian phase of infection by B. burgdorferi is consistent with the fact that DbpA antibodies do not clear an endogenous, chronic B. burgdorferi infection. This notion is inconsistent, however, with the findings of Cassatt et al. (5), who found that B. burgdorferi B31 within the plasma of spirochetemic mice were killed when exposed to DbpA antisera in vitro, suggesting that DbpA is surface exposed in B. burgdorferi during the mammalian spirochetemic phase. The reasons for these opposing results are unclear, but it is known that in vitro killing assays with spirochetes are prone to misinterpretation due to technical limitations. Finally, the notion that DbpA is not expressed on the surface of B. burgdorferi during mammalian infection calls into question whether DbpA really does serve as a surface ligand for the adherence of B. burgdorferi to tissue matrix proteins (e.g., decorin), as has been proposed elsewhere (2, 12, 13).
Both OspC (20) and DbpA (5) (Fig. 1) of B. burgdorferi are upregulated by elevated temperatures under in vitro growth conditions. However, it is noteworthy that whereas OspC expression is induced by the process of tick feeding, presumably due to both temperature and blood effects (20), this same phenomenon was not observed for DbpA. It is thus tempting to speculate that some other environmental factor(s) may be involved in the suppression of DbpA expression during tick feeding. In this regard, preliminary studies from our laboratory suggest that changes in the pH of tick midgut contents during feeding favor the upregulation of OspC but suppress DbpA expression (X. Yang, M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, and M. V. Norgard, unpublished data). This does not preclude the possibility that other stimuli are involved in the induction of DbpA as B. burgdorferi enters mammalian tissue. The pattern of DbpA expression thus may represent a model system for determining how factors of mammalian tissue (other than blood) modulate (upregulate) differential antigen expression in B. burgdorferi.
There is much that is not yet understood about the factors that influence the regulation, expression, and membrane topology of DbpA, but it seems that there is something particularly enigmatic about the manner in which DbpA is expressed and shuttled to its membrane location(s). Regardless of the precise mechanism(s) involved in the control of DbpA expression, the combined findings of this study suggest that DbpA may not be suitable as a human Lyme disease vaccinogen. They also sound a cautionary note with regard to attempting to extrapolate the results of mouse vaccination experiments using needle inoculation as the challenge route for B. burgdorferi to what normally occurs during natural tick transmission. This notion seems to be consistent with a growing body of evidence that needle inoculation is not tantamount to the tick delivery of arthropod-borne B. burgdorferi (19, 25). Although needle inoculation of mice remains a mainstay of Lyme disease vaccine research, results of such experiments should not be taken as definitive evidence for immunoprotection. Implicit in this tenet is that tick challenges of immunized mice should become the “gold standard” prior to performance of human clinical trials with a candidate Lyme disease vaccinogen.
Acknowledgments
We thank Taissia Popova for excellent technical assistance and Anette Huebner for critical reading of the manuscript.
We gratefully acknowledge funding for this work provided by grants AI-45538 and AI-29735 from the Lyme disease program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown E L, Guo B P, O'Neal P, Höök M. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J Biol Chem. 1999;274:26272–26278. doi: 10.1074/jbc.274.37.26272. [DOI] [PubMed] [Google Scholar]
- 3.Bunikis J, Barbour A G. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caporale D A, Kocher T D. Sequence variation in the outer-surface-protein genes of Borrelia burgdorferi. Mol Biol Evol. 1994;11:51–64. doi: 10.1093/oxfordjournals.molbev.a040092. [DOI] [PubMed] [Google Scholar]
- 5.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman J L, Benach J L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987;155:756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- 7.Cox D L, Akins D R, Bourell K W, Lahdenne P, Norgard M V, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Silva A M, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 9.de Silva A M, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fikrig E, Telford III S R, Barthold S W, Kantor F S, Spielman A, Flavell R A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci USA. 1992;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo B P, Brown E L, Dorward D W, Rosenberg L C, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 13.Guo B P, Norris S J, Rosenberg L C, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagman K E, Lahdenne P, Popova T G, Porcella S F, Akins D R, Radolf J D, Norgard M V. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Höök M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson M, Bergstrom S. Transcriptional and translational regulation of the expression of the major outer surface proteins in Lyme disease Borrelia strains. Microbiology. 1995;141:1321–1329. doi: 10.1099/13500872-141-6-1321. [DOI] [PubMed] [Google Scholar]
- 17.Mathiesen D A, Oliver J H, Kolbert C P, Tullson E D, Johnson B J B, Campbell G L, Mitchell P D, Reed K D, Telford S R, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 18.Padilla M L, Callister S M, Schell R F, Bryant G L, Jobe D A, Lovrich S D, DuChateau B K, Jensen J R. Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with Borrelia burgdorferi outer surface protein A vaccine. J Infect Dis. 1996;174:739–746. doi: 10.1093/infdis/174.4.739. [DOI] [PubMed] [Google Scholar]
- 19.Schwan T G. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis. 1996;5:167–181. [PubMed] [Google Scholar]
- 20.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E The Recombinant Outer-Surface Protein A Lyme Disease Study Consortium. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 22.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S The Lyme Disease Vaccine Study Group. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 23.Steigbigel R T, Benach J L. Immunization against Lyme disease—an important first step. N Engl J Med. 1998;339:263–264. doi: 10.1056/NEJM199807233390409. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wikel S K. Tick modulation of host immunity: an important factor in pathogen transmission. Int J Pathol. 1999;29:851–859. doi: 10.1016/s0020-7519(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 26.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Popova T G, Hagman K E, Wikel S K, Schoeler G B, Caimano M J, Radolf J D, Norgard M V. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect Immun. 1999;67:6008–6018. doi: 10.1128/iai.67.11.6008-6018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]