Abstract
Chlamydia pneumoniae infection induces inflammatory changes in blood vessels in normocholesterolemic rabbits, but it is not known whether the same phenomenon occurs in other animal models. Thus, in this study, C57BL/6J mice were inoculated with C. pneumoniae. Inflammatory changes in the heart or aorta were observed in a small number of chronically infected mice. No evidence of atherosclerotic lesions was found in any of the mice. These findings suggest that chronic C. pneumoniae infection can induce inflammatory changes in the heart and aorta of C57BL/6J mice, but does not initiate definitive atherosclerosis.
There is considerable seroepidemiological evidence of an association between Chlamydia pneumoniae serum antibody titers or circulating immune complexes (CICs) and cardiovascular disease (12, 18, 19). The presence of the organism has been demonstrated by means of isolation, PCR, immunohistochemistry, and electron microscopy in atherosclerotic lesions, but rarely within normal arteries (8, 9, 17, 20). Recently, animal models have been used to determine whether C. pneumoniae infection plays a causative role in the development of atherosclerosis. These studies have demonstrated that chronic C. pneumoniae infection accelerates development of fatty streaks in hyperlipidemic rabbits (16) and mice (6, 14). Furthermore, C. pneumoniae infection induces inflammatory changes in blood vessels of normocholesterolemic rabbits (5, 10). In C57BL/6J mice, C. pneumoniae disseminates to the aorta following single or repeated intranasal inoculations, but only persists following repeated infections (2, 15). However, it is not yet known whether persistent infection will initiate the atherosclerotic process under normolipidemic conditions in this model. In the present study, we examined the heart and aorta of normocholesterolemic C57BL/6J mice to determine whether there were any histopathologic changes following C. pneumoniae infection.
Eight-week-old male C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). The mice were kept in filter-top cages (four per cage) and fed with a regular chow diet and water ad libitum throughout the study. Mice were mildly sedated by intraperitoneal injections of a mixture of ketamine (Fort Dodge Laboratories, Shenandoah, Iowa) and xylazine (Lloyd Laboratories, Shenandoah, Iowa) and inoculated intranasally with 3 × 107 inclusion-forming units of C. pneumoniae strain AR-39 either once at 8 weeks of age or three times at 8, 9, and 10 weeks of age with density gradient-purified organisms as described previously (15). Control mice were sham inoculated with sterile phosphate-buffered saline. Mice were heavily sedated (Avertin; 2,2,2-tribromoethanol; Aldrich, Milwaukee, Wis.); sacrificed by exsanguination from the femoral arteries at 1, 4, and 8 weeks after the last inoculation; and blood was collected. Serum samples from each mouse were frozen at −75°C for later serology and cholesterol measurements. The mice were perfusion fixed with 10% buffered formalin administered through the left ventricle. The lungs, heart, and thoracic aorta with its main branches attached were dissected out intact. Each aorta was separated from the heart, and the heart was embedded in paraffin and serially sectioned at the aortic sinus. Each thoracic aorta was also embedded and sectioned longitudinally. Four to five sections of the heart and three to four sections of the aorta were mounted per slide and stained with hematoxylin-eosin. Every 10th slide was examined. Where intimal changes were found in the aorta, adjacent sections were then stained with van Gieson stain to determine whether there were disruptions of the elastic laminae. In a separate group of mice of a longer-term follow-up, frozen sections of the aortic sinuses were obtained to perform oil red O staining to detect early lesion formation.
C. pneumoniae-specific antibody titers were determined by the microimmunofluorescence test with formalin-fixed C. pneumoniae elementary bodies (AR-39) as the antigen (23). Total blood cholesterol was measured with a commercial enzymatic test kit (Sigma, St. Louis, Mo.). Cholesterol levels were determined in triplicate and averaged.
CICs were isolated from sera of 19 C. pneumoniae-infected and 9 sham-inoculated mice by polyethylene glycol precipitation (PEG) as described previously (12). Briefly, 100 μl of the serum and an equal volume of 7% PEG in sodium borate buffer (pH 8.4) were mixed and incubated overnight at 4°C. CICs were recovered by centrifugation at 5,000 × g for 30 min. Precipitates were washed twice with 3.5% PEG–borate, and CICs were dissociated with 100 μl of sodium borate buffer (pH 12). The presence of C. pneumoniae-specific antibodies was determined by dot blot analysis with C. pneumoniae (AR-39) elementary bodies as antigen and detected with peroxidase-conjugated anti-mouse immunoglobulin G (IgG). Samples that were positive by dot blotting were further analyzed by Western blotting.
PCR analysis was performed for all hearts and aortas in which histopathologic changes were observed. Slide-mounted tissue sections of hearts and aortas were scraped off of glass slides with a clean, new razor blade for each sample. Approximately 40 μg of tissue was obtained per specimen and placed in a microcentrifuge tube. Paraffin was removed by being dissolved with xylene. Residual xylene was removed by being washed with absolute ethanol. Samples were air dried. DNA was extracted from tissues as previously described (15). C. pneumoniae DNA was amplified by using the C. pneumoniae-specific primer set HL-1 and HR-1. Mouse β-globin DNA, a housekeeping gene, was also amplified with mouse β-globin primers to confirm the presence of amplified DNA (3).
In a separate group of mice, the lungs, heart, and aorta were obtained 1 week after repeated inoculations without prior fixation in order to localize chlamydial antigen within the tissues. Frozen sections were reacted with a Chlamydia genus-specific mouse monoclonal antibody (CF-2), which is directed against chlamydial lipopolysaccharide. To reduce cross-reactivity of the antibody, sections were treated with Histomouse blocking reagents (Zymed Laboratories, San Francisco, Calif.) and then stained by the indirect method with CF-2 at a 1:2,000 dilution with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.). Sections were counterstained with hematoxylin. Duplicate tissue sections were incubated with normal mouse ascitic fluid as negative controls.
Clinical signs following inoculations with C. pneumoniae included increased respiratory rate, decreased body weight, and nasoocular discharge. The discharges were most severe the day after the first and second inoculations and resolved within 2 weeks after the third inoculation. No mortality was observed. All infected mice seroconverted after the repeated inoculations. Serum IgG titers against C. pneumoniae at 1, 4, and 8 weeks postinoculation ranged from 1:128 to 1:1,024. Serum IgM titers ranging from 1:32 to 1:256 were found in three animals (25%) 1 week after the third inoculation. All of the control sera remained antibody negative.
Circulating immune complexes in the sera were found in 8 (42%) of 19 infected animals tested. Sham-inoculated controls did not develop immune complexes. Circulating immune complexes were not found in a separate group of mice infected with Chlamydia trachomatis strain E/UW-5/6x (eight serum samples tested). Therefore, formation of CICs appears to be a specific finding for C. pneumoniae infection in this model. Infection with C. pneumoniae did not result in a significant increase in serum cholesterol levels at 1 week (an average of 99 versus 84 mg/dl), 4 weeks (101 versus 74 mg/dl), or 8 weeks (88 versus 74 mg/dl), respectively, for infected versus controls. Therefore, the observed histologic changes were unrelated to blood cholesterol levels.
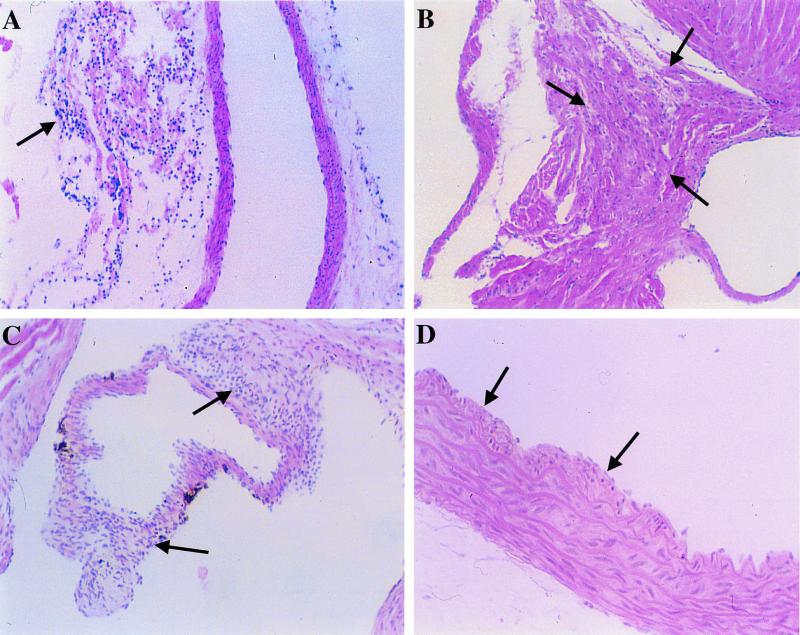
Mild to moderate inflammatory infiltrates were noted in the lungs of all mice 1 week after single or repeated inoculations. However, inflammatory changes persisted in only 4 (31%) of 13 animals at 4 weeks and resolved in all but one mouse at 8 weeks postinoculation. Inflammatory changes in the heart were observed in 6 (15%) of 40 mice which received multiple inoculations. Specifically, mononuclear infiltrates were observed in the adventitia of the coronary arteries in four animals, within the myocardium in one animal, and on aortic valvular leaflets, suggestive of florid endocarditis, in another animal. Examination of the thoracic aorta also revealed mononuclear infiltrates in the adventitia in four animals and thickening of the intima in another two infected animals (Fig. 1). However, disruptions of the elastic laminae at the lesion sites were not observed by van Gieson stain in any of the aortas. Foam cells or more advanced atherosclerotic lesions were not observed in any of the mice. In summary, inflammatory changes in the heart and/or aorta were noted in 8 (20%) of 40 animals, and intimal changes were noted in 2 (5%) of the 40 infected animals. A single inoculation at 8 weeks of age did not induce any inflammatory changes in the heart and aorta (n = 15). None of the sham-inoculated animals (n = 24) showed any abnormal morphology in the lung, heart, or aorta.
FIG. 1.
Photomicrographs of tissue sections of the heart and aorta from mice inoculated intranasally with C. pneumoniae (strain AR-39) at 8, 9, and 10 weeks of age and sacrificed 1 (D), 4 (B), or 8 (A and C) weeks after the last inoculation. Mononuclear infiltrates (arrows) were found in the adventitia of coronary arteries (A), within the myocardium (B), and on leaflets of an aortic valve (C), suggestive of endocarditis. Intimal thickening (arrows) was noted in aortas of two of the infected mice (D). Hematoxylin-eosin stain. Original magnifications, ×100 for A, B, and C and ×200 for D.
No atherosclerotic changes were observed in the aortic sinus, the site of the earliest lesion formation, in mice evaluated 20 weeks after the third inoculation (infected, n = 7; control, n = 8).
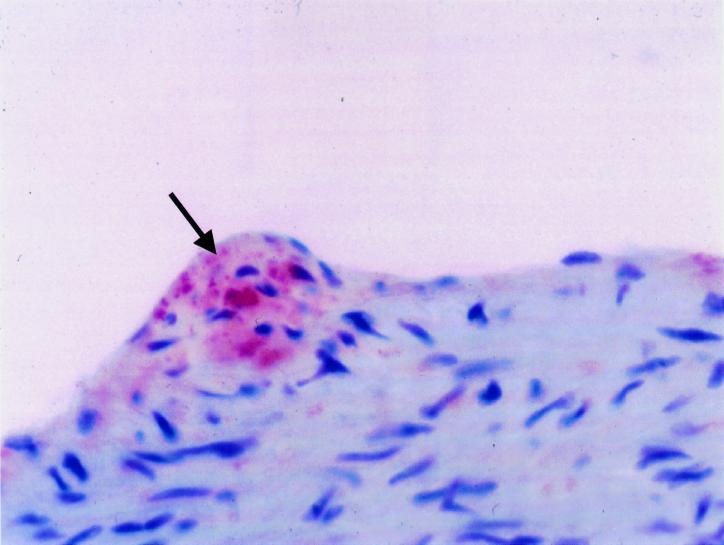
PCR was positive for C. pneumoniae DNA in one of the hearts where histopathologic changes were observed and colocalized with the site of inflammation (endocarditis of the aortic valve). Adjacent tissue sections from areas without histopathologic changes in the same mouse and in the hearts and aortas of the remaining infected animals were negative for chlamydial DNA by PCR. Chlamydial antigen was detected in the aorta by immunohistochemistry in one of the three mice tested. Staining was localized to the endothelial layer within the aortic sinus (Fig. 2). All sham-inoculated mice were negative by immunohistochemistry in all of the aortas and other tissues.
FIG. 2.
Detection of chlamydial antigen (arrow) by immunohistochemistry with a Chlamydia genus-specific mouse monoclonal antibody (CF-2). The mouse was sacrificed 3 days after three intranasal inoculations with C. pneumoniae (strain AR-39) at 8, 9, and 10 weeks of age. Staining was localized to the endothelial layer within the aortic sinus. Counterstaining was with hematoxylin-eosin. Magnification, ×400.
The present study demonstrates that chronic C. pneumoniae infection induced by multiple inoculations is capable of inducing inflammatory changes in the heart and aorta of a small number of C57BL/6J mice fed a regular chow diet. A single inoculation caused pathologic changes in the lungs, but failed to induce any inflammatory reaction in cardiovascular tissue. This is consistent with our previous finding demonstrating that infection disseminates to the aorta following a single inoculation, but persistent infection is not established. In contrast, repeated inoculations establish persistent infection in the aorta (2).
Despite the establishment of persistent infection of the aorta following multiple inoculations, there was no foam cell formation or other changes characteristic of atherosclerosis. In a small number of animals, there were areas of intimal thickening in the aorta at 1 week following the inoculations. Areas of intimal thickening may be a precursor to the development of atherosclerosis because it occurs at sites prone to develop lesions in several animal models (7, 21, 22). However, the presence of foam cells, the hallmark of the initial stage of atherosclerotic lesion development in all animal models, was not observed in the present study. Similar observations have been made by Fan et al. with normolipidemic mice following respiratory tract infection of the mouse pneumonitis strain of C. trachomatis (4). This is in contrast to observations made in normocholesterolemic rabbits, where foam cell formation and “atherosclerotic-like” changes were noted in a significant number of infected animals (5, 10). Why there are species-specific differences in the response to C. pneumoniae infections has yet to be determined. It is possible that mice are more resistant than rabbits to develop atherosclerosis after inoculations with C. pneumoniae, possibly due to their favorable high-density lipoprotein/low-density lipoprotein ratio (1). Another possible factor is that mice may require a longer time to develop atherosclerotic changes after C. pneumoniae infection. However, Hu et al. chronically infected LDL-deficient mice over a period of 9 months with C. pneumoniae and did not observe any histopathologic change in the aortas of mice fed a regular diet (6): neither did we in the present study in normolipidemic C57BL/6J mice 20 weeks after the last inoculation.
C. pneumoniae-specific CICs were demonstrated following chronic infection. Interestingly, C. pneumoniae-specific CICs have been associated with coronary heart disease and are an additional risk factor for coronary artery disease (11, 12). Immune complexes are known to induce endothelial injury (13). However, we did not observe any correlation of arterial pathology with CICs.
The present study suggests that chronic chlamydial infection by itself does not initiate atherosclerotic lesions and thus must act in concert with other cardiovascular risk factors to promote induction and progression of atherosclerosis. To date, accelerations of plaque formation by C. pneumoniae have only been demonstrated in hyperlipidemic rabbits (16) and mice (6, 14). In the C57BL/6J mouse model, we were able to demonstrate that chronic C. pneumoniae infection accelerates atherosclerotic lesion development in diet-induced hyperlipidemic mice (E. Blessing et al., submitted for publication), but not in normolipidemic mice. In the future, it would be worthwhile to evaluate whether chronic C. pneumoniae infection promotes induction and progression of atherosclerosis in conjunction with other cardiovascular risk factors, such as cigarette smoking, hypertension, or diabetes.
In conclusion, the present study is the first report of C. pneumoniae-induced inflammatory changes in the heart and aorta of normocholesterolemic mice. Although the establishment of persistent infection induces inflammation in cardiovascular tissue, it does not appear to induce definite atherosclerosis.
Acknowledgments
This work was supported in part by National Institutes of Health grants HL-56036 and AI-43060.
We thank Alison Cappuccio, Jerry Ricks, Chunmei Fu, and Anne Tecklenburg for expert technical assistance.
REFERENCES
- 1.Breslow J L. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 2.Campbell L A, Moazed T C, Kuo C C, Grayston J T. Preclinical models for Chlamydia pneumoniae and cardiovascular disease: hypercholesterolemic mice. Clin Microbiol Infect. 1998;4:4S23–4S32. [PubMed] [Google Scholar]
- 3.Ellison J, Dean M, Goldman D. Efficacy of fluorescence-based PCR-SSCP for detection of point mutations. BioTechniques. 1993;4:684–691. [PubMed] [Google Scholar]
- 4.Fan Y, Wang S, Yang X. Chlamydia trachomatis (mouse pneumonitis strain) induces cardiovascular pathology following respiratory tract infection. Infect Immun. 1999;67:6145–6151. doi: 10.1128/iai.67.11.6145-6151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong I W, Chiu B, Viira E, Jang D, Mahony J B. De novo induction of atherosclerosis by Chlamydia pneumoniae in a rabbit model. Infect Immun. 1999;67:6048–6055. doi: 10.1128/iai.67.11.6048-6055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu H, Pierce G N, Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Investig. 1999;103:747–753. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D N, Schmee J, Lee K T, Thomas W A. Atherosclerotic lesions in the coronary arteries of hyperlipidemic swine. I. Cell increases, divisions, losses and cells of origin in first 90 days of diet. Atherosclerosis. 1987;64:231–242. doi: 10.1016/0021-9150(87)90251-6. [DOI] [PubMed] [Google Scholar]
- 8.Kuo C C, Grayston J T, Campbell L A, Goo Y A, Wissler R W, Benditt E P. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old) Proc Natl Acad Sci USA. 1995;92:6911–6914. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo C C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 10.Laitinen K, Laurila A, Pyhälä L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leinonen M, Linnanmaki E, Mattila K, Nieminen M S, Valtonen V, Leirisalo-Repo M, Saikku P. Circulating immune complexes containing chlamydial lipopolysaccharide in acute myocardial infarction. Microb Pathog. 1990;1:67–73. doi: 10.1016/0882-4010(90)90042-o. [DOI] [PubMed] [Google Scholar]
- 12.Linnanmaki E, Leinonen M, Mattila K, Nieminen M S, Valtonen V, Saikku P. Chlamydia pneumoniae-specific circulating immune complexes in patients with chronic coronary heart disease. Circulation. 1993;4:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Virella M F, Virella G. Immunological and microbiological factors in the pathogenesis of atherosclerosis. Clin Immunol Immunopathol. 1985;37:377–386. doi: 10.1016/0090-1229(85)90107-2. [DOI] [PubMed] [Google Scholar]
- 14.Moazed T, Campbell L A, Rosenfeld M E, Grayston J T, Kuo C C. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- 15.Moazed T, Kuo C C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 16.Muhlestein J B, Anderson J L, Hammond E H, Zhao L, Trehan S, Schwobe E, Carlquist J F. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–636. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez J the Chlamydia pneumoniae/Atherosclerosis Study Group. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Saikku P, Leinonen M, Tenkanen L, Linnanmäki E, Ekman M R, Manninen V, Manttari M, Frick M H, Huttunen J K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 19.Saikku P, Leinonen M, Mattila K, Ekman M R, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 20.Shor A, Kuo C C, Patton D L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992;82:158–160. [PubMed] [Google Scholar]
- 21.Thomas W A, Lee K T, Kim D N. Cell population kinetics in atherogenesis. Cell births and losses in intimal cell mass-derived lesions in the abdominal aorta of swine. Ann N Y Acad Sci. 1985;454:305–315. doi: 10.1111/j.1749-6632.1985.tb11870.x. [DOI] [PubMed] [Google Scholar]
- 22.Velican D, Velican C. Intimal thickening in developing coronary arteries and its relevance to atherosclerotic involvement. Atherosclerosis. 1976;23:345–355. [Google Scholar]
- 23.Wang S P, Grayston J T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970;70:367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]