Abstract
Background
We experienced a pseudo-outbreak of aspergillosis in a newly constructed COVID-19 ward. Within the first 3 months from the commencement of the ward, six intubated patients of COVID-19 developed probable or possible pulmonary aspergillosis. We suspected an outbreak of pulmonary aspergillosis associated with ward construction and launched air sampling for the investigation of the relationship between these.
Methods
The samples were collected at 13 locations in the prefabricated ward and three in the general wards, not under construction, as a control.
Results
The results from samples revealed different species of Aspergillus from those detected by the patients. Aspergillus sp. was detected not only from the air samples in the prefabricated ward but also in the general ward.
Discussion
In this investigation, we could not find evidence of the outbreak that links the construction of the prefabricated ward with the occurrence of pulmonary aspergillosis. It might suggest that this series of aspergillosis was more likely occurred from fungi that inherently colonized patients, and was associated with patient factors such as severe COVID-19 rather than environmental factors. Once an outbreak originating from building construction is suspected, it is important to conduct an environmental investigation including an air sampling.
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, aspergillosis, outbreak, air sampling
Background
The association between COVID-19 and pulmonary aspergillosis is well documented (Koehler et al., 2021); the prevalence of COVID-19 associated pulmonary aspergillosis (CAPA) has been reported to be 5–15% (Borman et al., 2020). It is more common in intubated patients and has a mortality rate of about 50% (Salmanton-García et al., 2021). Environmental factors might be also associated with the incidence of nosocomial aspergillosis, such as the construction of the treating areas, including inflow of unfiltered outside air, air conditioners, and duct systems in healthcare settings (Kanamori et al., 2015). However, confirmation of environmental factors leading to the outbreak of aspergillosis at any given wards can be challenging (Lai, 2001), since no standardized methods are established to be compared for data collection and analysis such as types of air sampler, sampling locations, air volume, and environmental conditions (Chang et al., 2008).
We experienced a potential outbreak of aspergillosis at a ward designated to take care of COVID-19 patients in the first 3 months of utilizing a prefabricated ward. However, we determined that it was not a true outbreak but rather a pseudo-outbreak, by utilizing air sampling of the environment of the COVID-19 ward. Here, we report the utilities and limitations of air sampling when nosocomial outbreaks derived from the environment were suspected.
Setting and methods
Our hospital is a tertiary medical institution designated to care for both moderate and severe COVID-19 patients. We began to see COVID-19 patients in February 2020 and started to use a newly built prefabricated ward on 9 November 2020. Aspergillus sp. was isolated from the sputum of six intubated patients within the first 3 months from the commencement of the ward, which had not occurred previously since the hospitalization of COVID-19 patients.
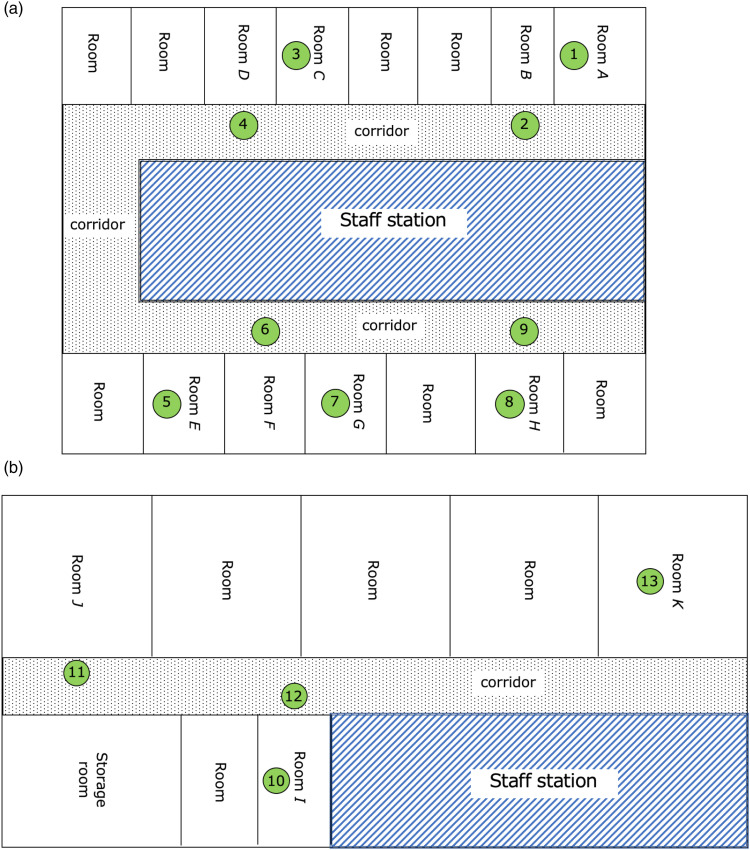
The prefabricated ward had 14 intensive care unit (ICU) beds and 22 high care unit (HCU) beds (Figure1(a) and (a)). All rooms in the ward were under negative pressure, but corridors were not. The patient room did not have a double door. The negative pressure environment was established by attaching the air supply port at the ceiling of the corridor and the exhaust port in each hospital room. The air supply system of the ward provides outside air into the hospital room through a medium-performance filter that removes 60% of dust less than 0.5 μm diameter.
Figure 1.
The prefabricated ward map and locations of air sampling. The circled number represents the location of the air sampling. The staff station is enclosed in diagonal lines and the corridor of the ward is the dotted area. A. Intensive care unit for COVID-19 in the prefabricated ward. Samples No. 1–9 were collected. B. High care unit for COVID-19 in the prefabricated ward. Samples No. 10–13 were collected.
After detecting Aspergillus sp. from 6 patients in the ward, we began air samplings using BIOSAMP MBS-1000N (Transtech Inc, Tokyo, Japan) with the sampler placed at a height of 1.0 m above the floor. Five hundred liters of air were sampled each time at 13 locations in the prefabricated ward and three locations in a general ward including non-COVID-19 ICU as a control. Sabouraud agar medium was used for culturing the sampled air. The medium was incubated at 25°C for up to 2 weeks.
The diagnosis and classification of CAPA were made according to a recent European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) definitions (Koehler et al., 2021).
Ethical considerations
This study was approved by the ethics committee of our hospital. The ethical principle of confidentiality was upheld throughout the process. Consent was obtained by uploading the information disclosure document to the hospital website. Data from this evaluation will be stored in a password-protected computer for a minimum of 5 years.
Results
There were 6 patients who developed pulmonary aspergillosis (1 probable and 5 possible) in the prefabricated ward from 9 November 2020 to 31 January 2021. Air sampling was conducted on 10 February 2021.
The characteristics of the patients are shown in Table 1. The mean age of these patients was 71.5-year-old. All of them had been intubated, hospitalized in ICU, and treated with 6 mg/day of dexamethasone. Tocilizumab, an agent of the anti-interleukin-6 monoclonal antibody, was used for patients Ⅳ and Ⅵ. None of them had undergone neither bronchoalveolar lavage (BAL) nor histopathological examination. Using the classification of CAPA proposed by ECMM/ISHAM (Koehler et al., 2021), patient Ⅳ had probable pulmonary aspergillosis due to positive serum galactomannan antigen. Others were classified as possible pulmonary aspergillosis due to their positive result of the sputum culture. Patient Ⅴ had Aspergillus sp. detected in her sputum on the day when she was admitted to our hospital from her home, and we assumed that she was a carrier of Aspergillus sp, and could have developed the disease by this inherent organism. All patients were treated with an anti-fungal agent as CAPA. Patients Ⅰ, Ⅱ, and Ⅵ died the during hospitalization period (3/6, 50%).
Table 1.
Patients list whose sputum Aspergillus sp. was isolated from.
| No | Age | Sex | Admission date | Collection date of the sputum culture a | Species | Admission room | Intubation date | Comorbidities | Serum GM antigen (titer) | Treatment | In-hospital mortality b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ⅰ | 65 | M | 10/11/2020 | 4/12/2020 | A. terreus | Room F | 25/112020 | DM | Negative | VCZ | Death (Day 112) |
| Ⅱ | 88 | F | 8/12/2020 | 19/12/2020 | A. niger | Room G | 8/12/2020 | CKD | No data | L-AmB | Death (Day 7) |
| Ⅲ | 73 | M | 27/12/2020 | 12/1/2021 | A. fumigatus | Room B | 1/1/2021 | Schizophrenia | No data | VRCZ→MFG | Survive (discharged on day 81) |
| Ⅳ | 72 | M | 10/1/2021 | 15/1/2021 | A. terreus | Room B | 11/1/2021 | DM | Positive (0.7) | VCZ | Survive (discharged on day 26) |
| Ⅴ | 72 | F | 17/1/2021 | 17/1/2021 | A. fumigatus | Room G | 17/1/2021 | DM | No data | MFG→VCZ | Survive (discharged on day 59) |
| Ⅵ | 59 | M | 18/1/2021 | 26/1/2021 | A. flavus | Room D | 29/1/2021 | CKD (PD) | No data | MFG→L-AmB→VCZ | Death (Day 99) |
Abbreviation: GM, galactomannan; DM, diabetes mellitus; CKD, chronic kidney disease; PD, peritoneal dialysis; VCZ, voriconazole; L-AmB, liposomal amphotericin B; MFG, micafungin.
aThe sputum culture which Aspergillus sp. was first detected.
bDays since the index sputum culture was collected.
Figure 1 shows a ward map and air sampling locations, and the results of air sampling are listed in Table 2. The most frequently detected fungus was Aspergillus versicolor, which grew in all specimens except for sample No. 2, 5, or 13. A. versicolor was detected not only from the air samples of the prefabricated ward but also the general ward including non-COVID-19 ICU. Aspergillus terreus was detected in sample No. 1, which was the same species detected in patients Ⅰ and Ⅳ. However, they did not use the room of sample No. 1, and they never shared the same utensils between them.
Table 2.
The results of air sampling.
| Sample no | Locations | Results | Colony count |
|---|---|---|---|
| 1 | In room A, ICU | A. terreus | 1 colony |
| 2 | At the corridor in front of room B, ICU | Negative | — |
| 3 | In room C, ICU | A. versicolor | 50 colonies |
| 4 | At the corridor in front of the room D, near the air supply port, ICU | A. versicolor | 13 colonies |
| Cladosporium sp. | 1 colony | ||
| 5 | In room E, ICU | Negative | — |
| 6 | At the corridor in front of the room F, near the air supply port, ICU | A. versicolor | 19 colonies |
| Curvularia verruculosa | 1 colony | ||
| 7 | In room G, ICU | A. versicolor | 1 colony |
| 8 | In room H, ICU | A. versicolor | 2 colonies |
| Rhodotorula sp. | 1 colony | ||
| 9 | At the corridor in front of the room H, ICU | A. versicolor | 4 colonies |
| Drepanoconis sp. | 1 colony | ||
| 10 | In room I, HCU | A. versicolor | 1 colony |
| 11 | At the corridor in front of the room J, near the air supply port, HCU | A. versicolor | 2 colonies |
| Candida albicans | 1 colony | ||
| 12 | At the corridor in front of room I, near the air supply port, HCU | A. versicolor | 5 colonies |
| 13 | In room K, HCU | Negative | — |
| 14 (control) | Under the air supply port of ICU | A. versicolor | 7 colonies |
| 15 (control) | In room of ICU | A. versicolor | 2 colonies |
| 16 (control) | In the room of the general ward | A. versicolor | 9 colonies |
Sample numbers of 1–13 were collected at the prefabricated ward, in which all rooms were under negative pressure while the corridors were not.
Discussion
Outbreaks of nosocomial aspergillosis associated with hospital construction, renovation, and demolition have been reported (Kanamori et al., 2015). Our observation of multiple CAPA cases at the same ward is, however, not likely to be associated with a nosocomial outbreak, and it was rather likely to be coincidental episodes of pulmonary aspergillosis occurring independently, possibly due to severe respiratory condition of COVID-19.
In the results of our air sampling, almost all of Aspergillus sp. were different from those isolated from the patients. A. versicolor, one of the most common indoor molds (Ezeonu et al., 1995), was detected in most of the environmental samples, and it was not detected from the patients. It means that there was no nosocomial transmission of aspergillosis, and the cases of aspergillosis that occurred in our hospital are more likely to be from organisms inherently colonized. The only A. terreus detected from patients Ⅰ and Ⅳ was consistent with that detected from air sample No. 1. However, the patients never used that room, and there were no utensils shared among those patients, suggesting that nosocomial transmission of A. terreus is not likely to have occurred in the wards. On the other hand, Aspergillus sp. was detected from sample No. 14 to 16, collected from the air supply port in the general ward and the ICU, but they did not appear to have caused pulmonary aspergillosis inside the ward. This implies that the multiple cases of aspergillosis that occurred in our hospital may have been related to some patient factors rather than to environmental ones.
COVID-19 associated pulmonary aspergillosis is reported to be associated with older age, long-term immunosuppression, underlying chronic obstructive pulmonary disease (COPD), and critical conditions but not with comorbidities of diabetes, initial corticosteroid, or tocilizumab treatment (Chong et al., 2021). Although all of our patients were aged, intubated for COVID-19, and treated with corticosteroids, none was under a long period of immunosuppression or had COPD. In other words, they did not have any obvious factors other than critical conditions of COVID-19 and their age for developing pulmonary aspergillosis. These patient factors could have contributed to the development of CAPA in our ward.
Our study has several limitations. First, this is a single-center observational study and may not apply externally. Second, despite air sampling has been conducted in many studies, standard air sampling methods for data collection and analysis have not been established (Chang et al., 2008). This means that the reliability of the culture results for each study is not constant and the results of air sampling do not always indicate the presence or absence of nosocomial infections. In this investigation, different species, except for A. terreus, had been detected from the patients. Since molecular testings of A. terreus detected from the air sampling and the sputum culture were not conducted, we cannot completely deny the relevance of the development of pulmonary aspergillosis to ward construction. Third, to reduce aerosol exposure to medical personnel, we could not perform confirmatory testing such as bronchoscopy. Because of the low sensitivity of serum galactomannan antigen in non-neutropenic patients, BAL galactomannan antigen measurement is preferred for diagnosing pulmonary aspergillosis. In addition, histopathological examination and/or BAL culture are needed for the diagnosis of proven pulmonary aspergillosis (Koehler et al., 2021). Therefore, we could not distinguish between infection from colonization of Aspergillus sp. strictly. However, we treated them as probable or possible pulmonary aspergillosis because they all had severe respiratory failure.
Conclusions
In our investigation on a series of the possible occurrence of CAPA in association with the construction of prefabricated wards, we could not identify the obvious relation between these. These multiple developments of pulmonary aspergillosis inside our ward are likely to be independent and occurred from inherently colonized organisms, and that is more likely to be a pseudo-outbreak associated with the factors belonging to the patients such as severe COVID-19 and aged. Therefore, it is important to conduct an environmental investigation to confirm the presence or absence of true-outbreak.
Acknowledgements
We thank the members of the Department of Clinical Laboratory, and members of staff in the COVID-19 ward.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Kohei Hasegawa https://orcid.org/0000-0002-0980-609X
References
- Borman AM, Palmer MD, Fraser M, et al. (2020) COVID-19-associated invasive aspergillosis: Data from the UK national mycology reference laboratory. Journal of Clinical Microbiology 59(1): e021366-e021420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Athan E, Morrissey CO, et al. (2008) Preventing invasive fungal infection during hospital building works. Internal Medicine Journal 38(6b): 538–541. [DOI] [PubMed] [Google Scholar]
- Chong WH, Saha BK, Neu KP. (2021) Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): a systematic review and meta-analysis. Infection 50, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeonu IM, Price DL, Crow SA, et al. (1995) Effects of extracts of fiberglass insulations on the growth of Aspergillus fumigatus and A. versicolor. Mycopathologia 132(2): 65–69. [DOI] [PubMed] [Google Scholar]
- Kanamori H, Rutala WA, Sickbert-Bennett EE, et al. (2015) Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clinical Infectious Diseases 61(3): 433–444. [DOI] [PubMed] [Google Scholar]
- Koehler P, Bassetti M, Chakrabarti A, et al. (2021) Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. The Lancet Infectious Diseases 21(6): e149-e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K. (2001) A cluster of invasive aspergillosis in a bone marrow transplant unit related to construction and the utility of air sampling. American Journal of Infection Control 29(5): 333–337. [DOI] [PubMed] [Google Scholar]
- Salmanton-García J, Sprute R, Stemler J, et al. (2021) COVID-19–associated pulmonary aspergillosis, March–August 2020. Emerging Infectious Diseases 27(4): 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]