Abstract
Penile squamous cell carcinoma (PSCC) remains a worldwide healthcare concern with poor outcomes and inadequate therapeutic options. Molecular characterization continues to describe the intricacies of PSCC biology, which vary by human papillomavirus (HPV) infection. Despite these advancements in our understanding, utilization of targeted therapies remains limited and underexplored. In this study, we evaluated the transcript and protein expression of Nectin-4 (PVRL4) in PSCC tumors and evaluated whether this is related to tumor features or clinical outcomes. Using two separate PSCC cohorts, we demonstrate that the majority of tumors have active transcription of Nectin-4. We then validated our findings using immunohistochemistry in a tissue microarray representing 57 patients with PSCC. We identified that Nectin-4 was expressed at higher levels in patients with high-risk HPV infection. No significant differences were identified in tumor characteristics or various clinical endpoints when comparing tumors with high and low Nectin-4 expression. This study demonstrates that Nectin-4 is expressed in PSCC and may represent a novel therapeutic target.
Patient summary
In this study, we evaluated the expression of Nectin-4, a cell surface protein, in tumors from patients with nonmetastatic penile squamous cell carcinoma (PSCC). To our knowledge, this is the first study to describe elevated expression of Nectin-4 in PSCC, which may suggest its utility as a therapeutic target.
Keywords: Human papillomavirus, Nectin-4, Penile cancer
Penile cancer is a rare malignancy in most developed countries, but may contribute up to 10% of the cancer burden in men within some Asian, African, and South American regions. Most penile cancers have squamous cell histology (penile squamous cell carcinoma [PSCC]), and a recent meta-analysis identified that approximately 50% are associated with human papillomavirus (HPV) infection. Although localized disease may be managed well with surgical resection or penile-sparing radiotherapy, patients with locally advanced or metastatic disease continue to pose a clinical challenge [1].
At present, systemic therapy options have been limited to common chemotherapies (eg, cisplatin, paclitaxel, ifosfamide, and docetaxel), and when tumors are refractory to combination systemic therapy (eg, paclitaxel, ifosfamide, and cisplatin), the median survival is poor with no effective therapies. Targeted monoclonal antibody–based therapies (eg, cetuximab and panitumumab) and various immune checkpoint inhibitors are being assessed either alone or in combination with other agents, although mostly in the setting of grouped histology basket trials [2], [3]. Recently, promising tumor control was described in 17 patients with stage IV PSCC treated with combined platinum-based chemotherapy, epidermal growth factor receptor blockade, and anti–programmed death receptor-1 (anti–PD-1) antibody [4]. Despite these efforts, there remains a great need to identify novel therapeutic targets in this patient population.
Nectin-4, also known as poliovirus receptor-like 4 (PVRL4), is an immunoglobulin superfamily member that orchestrates cell-cell junction formation and maintenance, as well as cytoskeletal dynamics. Depending on the cellular context, Nectin-4 may form homophilic/heterophilic receptor interactions and elicit diverse signaling pathways (eg, PI3K/Akt) by interaction with ErbB2 or within a Rac-1 module [5]. In addition to cancer cell signaling, Nectin-4 may further modulate the tumor immune response by acting as a ligand for TIGIT, an immune checkpoint receptor on various immune cells [6].
Nectin-4 has been shown to have weak-to-moderate expression in select nonmalignant adult tissues but is overexpressed in several cancer types, including those without squamous differentiation. Notably, urothelial cancers have elevated Nectin-4 expression, which has formed the basis for antibody-drug conjugate (ADC) therapy with enfortumab vedotin [7]. Given the success and clinical accessibility of Nectin-4–targeted therapy, we evaluated whether PSCC tumors express Nectin-4 and whether this is related to HPV infection status, tumor characteristics, or clinical outcomes.
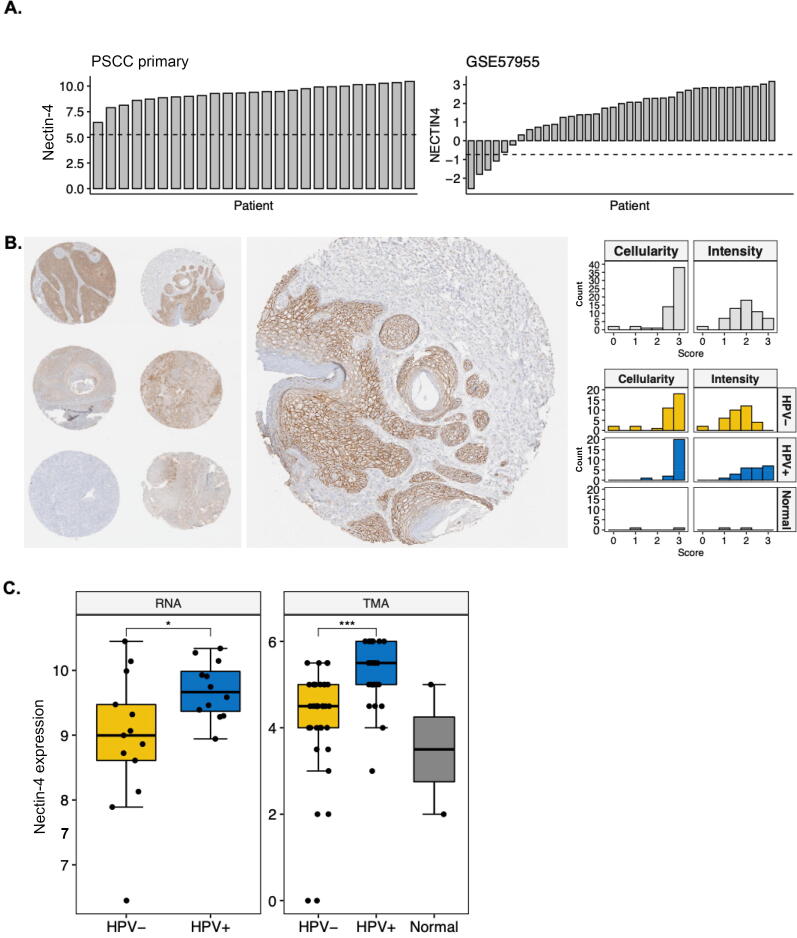
We first evaluated Nectin-4 gene expression using an Affymetrix U133 2.0 microarray chip in 25 surgically resected primary PSCC tissues [8], as well as an independent cohort of PSCC tumors with gene expression normalized to that in normal glans (n = 39; only 35 samples were evaluable for Nectin-4 in GSE57955) [9]. An analysis of gene expression demonstrated that Nectin-4 was highly expressed in both datasets (Fig. 1A). In the independent cohort, 70.3% and 48.7% of tumors had, respectively, two- and four-fold higher Nectin-4 expression than normal glans. Next, we evaluated Nectin-4 protein expression with standard immunohistochemical (IHC) methods using a tissue microarray (TMA) consisting of tumor cores from 57 patients who were treated between 2000 and 2013 at our institution for invasive PSCC; two patients had available normal penile tissue for comparison. Patient demographics, as well as tumor and treatment characteristics, have been described previously [10] and are in updated summary in Supplementary Table 1. An IHC analysis demonstrated that 89.4% (n = 51) of samples had >25% of cells with positive Nectin-4 staining and the intensity was moderate to strong in 61.4% (n = 35/57; Fig. 1B). Notably, no nonmalignant penile tissue had high Nectin-4 stain intensity.
Fig. 1.
Nectin-4 is differentially expressed at the gene and protein levels in invasive PSCC. (A) Waterfall plots demonstrating variability in Nectin-4 gene expression in internal (left panel) and external (right panel; GSE57955) tumor cohorts; the external cohort gene expression is relative to normal glans tissue. The dashed line indicates 25th quantile of global gene expression. (B) Representative image of subsetted TMA cores stained for Nectin-4 protein with an enlarged view of a single core (left panel) and histogram demonstrating IHC scoring (cell counts positive and intensity of stain) stratified by HPV infection status and normal penile tissue (right panel). (C) Boxplots comparing Nectin-4 gene (left panel) and protein (right panel) expression based on HPV infection and normal penile tissue. HPV = human papillomavirus; IHC = immunohistochemical; PSCC = penile squamous cell carcinoma; TMA = tissue microarray. * p < 0.05. *** p < 0.001.
We then evaluated whether Nectin-4 expression was associated with HPV infection. Our institutional PSCC samples were previously evaluated for the presence of high-risk HPV (hrHPV) with in situ hybridization (ISH) and p16 IHC with scoring by an expert genitourinary pathologist [10]. Thirteen (52%) of the tumors profiled for gene expression and 23 (40.3%) in the TMA were infected by hrHPV; there was a discordance in HPV ISH and p16 in three TMA samples (5.3%). Interestingly, we found that Nectin-4 expression was significantly elevated in hrHPV-infected tumors at both the gene (p = 0.03; Fig. 1C, left panel) and the protein (p < 0.001; Fig. 1C, right panel) level. Of interest, among the nine cores with zero-to-low Nectin-4 staining, only one was infected with hrHPV.
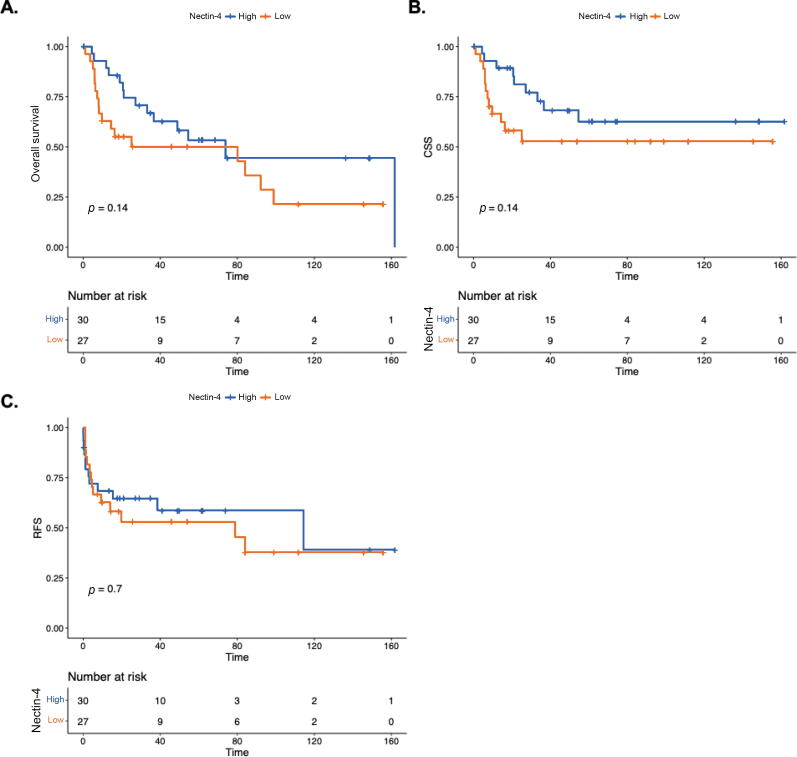
Last, we evaluated whether Nectin-4 protein expression was associated with tumor features or outcomes (Fig. 2). The median follow-up time for this cohort was 61.9 mo (range: 45.6–78.2 mo). Infection with hrHPV was associated with improved cancer-specific survival (CSS; p = 0.026), but not overall survival (OS) or relapse-free survival (RFS). In contrast, Nectin-4 expression (dichotomized at the median to categorize low and high expression) was not associated with CSS, OS, or RFS (p > 0.05). In addition, Nectin-4 was not associated with T or N category (p = 0.45), tumor grade (p = 0.23), or presence of lymphovascular invasion (p = 0.64).
Fig. 2.
Association of Nectin-4 with clinical outcomes: (A) overall survival, (B) cancer-specific survival (CSS), and (C) relapse-free survival (RFS) related to Nectin-4 protein expression (dichotomized into low and high based on median values).
This study demonstrates that Nectin-4 is expressed at a significant level in PSCC. Of interest, our observation of differential expression was based on hrHPV infection, although evaluation of the boxplots in Figure 1C demonstrates that some noninfected tumors and normal penile tissue express Nectin-4 at levels similar to those infected by hrHPV. Although it requires further validation, it is plausible that HPV instigates Nectin-4 expression in some tumors via E6/7 oncogenic activity, which may be modulated further by underlying molecular repertoires.
ADC therapy with enfortumab-vedotin has demonstrated efficacy in patients with metastatic urothelial carcinoma, which is supported by prior analyses describing moderate-to-strong Nectin-4 stain intensity in approximately 60% of samples [7]. Notably, our analysis demonstrates a similar intensity of expression (61%) in PSCC. This may suggest that targeting Nectin-4 in PSCC, irrespective of HPV status, may represent an actionable therapeutic target. Further, given the cooperative role of Nectin-4 in regulating tumor immune responses [6], additional studies are warranted to evaluate this facet in PSCC. Research in PSCC continues to burgeon with cooperative global efforts, which are anticipated to deliver novel therapeutic options for this challenging malignancy.
Author contributions: Philippe E. Spiess had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Grass, Chahoud.
Acquisition of data: Grass, Chahoud, Eschrich, Dhillon, Spiess.
Analysis and interpretation of data: Grass, Chahoud, Eschrich, Dhillon, Lopez.
Drafting of the manuscript: Grass, Chahoud, Eschrich, Johnstone, Spiess.
Critical revision of the manuscript for important intellectual content: Grass, Chahoud, Eschrich, Johnstone, Spiess.
Statistical analysis: Grass, Eschrich.
Obtaining funding: Grass, Chahoud.
Administrative, technical, or material support: Grass, Johnstone, Spiess.
Supervision: Spiess.
Other: None.
Financial disclosures: Philippe E. Spiess certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jad Chahoud has received advisory board fees from Pfizer, Exelixis, and Aveo, none in relation to this work. Philippe E. Spiess has no financial disclosures to report and only leadership disclosures: President of Global Society of Rare Genitourinary Tumors, NCCN bladder and penile cancer vice-chair, Member of ASCO/EAU penile cancer panel. The other authors have no relevant disclosures related to this work.
Funding/Support and role of the sponsor: G. Daniel Grass reports funding from the state of Florida via the Center for Immunization and Infection Research in Cancer at H. Lee Moffitt Cancer Center and Research Institute as well as funding from the Congressionally Directed Medical Research Programs: Rare Cancers Research Program.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.12.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Thomas A., Necchi A., Muneer A., et al. Penile cancer. Nat Rev Dis Primers. 2021;7:11. doi: 10.1038/s41572-021-00246-5. [DOI] [PubMed] [Google Scholar]
- 2.Joshi V.B., Spiess P.E., Necchi A., Pettaway C.A., Chahoud J. Immune-based therapies in penile cancer. Nat Rev Urol. 2022;19:457–474. doi: 10.1038/s41585-022-00617-x. [DOI] [PubMed] [Google Scholar]
- 3.Joshi V.B., Chadha J., Chahoud J. Penile cancer: updates in systemic therapy. Asian J Urol. 2022;9:374–388. doi: 10.1016/j.ajur.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan R., Ma H., Jiang L., et al. First-line programmed death receptor-1 (PD-1) inhibitor and epidermal growth factor receptor (EGFR) blockade, combined with platinum-based chemotherapy, for stage IV penile cancer. BJU Int. 2022 doi: 10.1111/bju.15828. In press. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Han X., Li L., et al. Role of Nectin-4 protein in cancer. Int J Oncol. 2021;59:93. doi: 10.3892/ijo.2021.5273. [DOI] [PubMed] [Google Scholar]
- 6.Reches A., Ophir Y., Stein N., Kol I., et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J Immunother Cancer. 2020;8:e000266. doi: 10.1136/jitc-2019-000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath E.I., Rosenberg J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol. 2021;18:93–103. doi: 10.1038/s41585-020-00394-5. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Z., Grass G.D., Azizi M., et al. Intrinsic radiosensitivity, genomic-based radiation dose and patterns of failure of penile cancer in response to adjuvant radiation therapy. Rep Pract Oncol Radiother. 2019;24:593–599. doi: 10.1016/j.rpor.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuasne H., Cólus I.M. de S., Busso A.F., et al. Genome-wide methylation and transcriptome analysis in penile carcinoma: uncovering new molecular markers. Clin Epigenet. 2015;7:46. doi: 10.1186/s13148-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azizi M., Tang D.H., Verduzco D., et al. Impact of PI3K-AKT-mTOR signaling pathway up-regulation on prognosis of penile squamous-cell carcinoma: results from a tissue microarray study and review of the literature. Clin Genitourin Cancer. 2019;17:e80–e91. doi: 10.1016/j.clgc.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.