Abstract
Epigallocatechin gallate (EGCg) is a major form of tea catechin and has a variety of biological activities, including antitumor as well as antimicrobial activity against some pathogens. Although the biological activities of EGCg have been extensively studied, its immunological effects are not well known. In the present study, the ability of EGCg to modulate macrophage immune functions in an in vitro Legionella pneumophila infection model of macrophages was examined. The study showed that EGCg inhibited the growth of L. pneumophila in macrophages at a concentration as low as 0.5 μg/ml without any direct antibacterial effect on the organisms. The EGCg selectively upregulated the production of interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) and downregulated IL-10 production of macrophages induced by L. pneumophila infection in a dose-dependent manner, but did not alter IL-6 production even at a high dose. The upregulation of the levels of macrophage gamma interferon (IFN-γ) mRNA by EGCg was also demonstrated. Treatment of macrophage cultures with anti-TNF-α and anti-IFN-γ monoclonal antibodies markedly abolished the anti-L. pneumophila activity of macrophages induced by the EGCg treatment. These results indicate that EGCg selectively alters the immune responses of macrophages to L. pneumophila and leads to an enhanced anti-L. pneumophila activity of macrophages mediated by enhanced production of both TNF-α and IFN-γ. However, the enhancement of in vitro anti-L. pneumophila activity by EGCg may not be directly mediated by IL-10 and IL-12 production modulation. Thus, the results of this study revealed the immunomodulatory effect of EGCg on macrophages, which have a critical role in infections.
Tea (Camellia sinensis) has been used as a daily beverage for several thousand years since it was introduced as a beverage in China and is now the second most common beverage consumed by humans (37). Even though it has been known traditionally that tea may have some beneficial health effects, such effects were not demonstrated by well-controlled laboratory studies until the 1970s (37). However, current studies have revealed the biological effects of tea, such as antitumor as well as antimicrobial effects, even at the molecular level. The active components of tea responsible for such biological effects are now known to be catechins (also known as polyphenols), which constitute seven forms, including epigallocatechin gallate (EGCg).
EGCg is a major catechin compound in tea extracts and is also the most active form among the tea catechins in a variety of biological activities. For instance, EGCg has anticarciogenic (14, 43), antioxidant (9, 14), as well as antimicrobial activities (12, 34, 35, 38, 39). Although the mechanism of antimicrobial activity of EGCg has been studied (12), it is still unclear. However, the immunomodulatory effect of EGCg has been increasingly recognized, since the bioavailability of EGCg in plasma after drinking tea is known to be high (24, 25, 44). In fact, it is known that EGCg potently stimulates the production of interleukin-1 alpha (IL-1α), IL-1β, and tumor necrosis factor alpha (TNF-α) by cultured human peripheral blood mononuclear cells (31). Furthermore, EGCg protects against UV radiation-induced immunosuppression and tolerance induction by reducing IL-10 production and increasing IL-12 production in epidermal and dermal cells (13). However, the detailed immunomodulatory effects of EGCg on immune cells has not been investigated.
Legionella pneumophila, a gram-negative facultative intracellular pathogen, is the causative agent of Legionnaires' disease in immunocompromised patients. Even though there are extensive studies on L. pneumophila infection, particularly the mechanism of infection, how L. pneumophila infection of the lung is controlled is not yet clear. Nevertheless, it is widely accepted that the activation of macrophages to suppress intracellular bacterial growth is an essential effector mechanism in the resolution of legionellosis (10). It is known that the Th1 cytokine gamma interferon (IFN-γ) can activate macrophages and monocytes to inhibit L. pneumophila growth (2, 27). Besides the direct effect of the Th1 cytokine IFN-γ to activate macrophages, Th1 cells play an essential role in the development of cell-mediated immunity to pathogens (11). Both IFN-γ and IL-12, which has a major role in the differentiation of the Th1 cell phenotype, are produced by macrophages. In addition, it has been reported that the inflammatory cytokine TNF-α is required for the prompt resolution of pneumonic legionellosis and points to a direct role for TNF-α in the activation of phagocytes (33). Other inflammatory cytokines, such as IL-6, are also known to control infections (5, 18). In contrast, Th2 cytokines, particularly IL-10, may facilitate growth of L. pneumophila in permissive mononuclear phagocytes due in part to IL-10-mediated inhibition of TNF-α secretion and IFN-γ-mediated mononuclear phagocyte activation (28). Nevertheless, all of these cytokines, IL-6, IL-10, IL-12, TNF-α, and even IFN-γ, are known to be produced by macrophages in response to bacterial infections and may be involved in the regulation of infection. Therefore, the modulation of production of such key cytokines from macrophages may eventually affect the outcome of the infection. Therefore, in the present study, a macrophage infection model with L. pneumophila (20) was used to determine the ability of EGCg to modulate macrophage immune functions.
MATERIALS AND METHODS
Macrophages.
The MH-S murine alveolar macrophage cell line, purchased from the American Type Culture Collection (Manassas, Va.), was used in this study. The cells were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS; Hyclone Laboratories, Logan, Utah). The MH-S cells were allowed to adhere to 24-well tissue culture plates at a concentration of 5 × 105 cells/ml for 2 h in 5% CO2 at 37°C. The resulting cell monolayers were washed with Hanks' balanced salt solution, supplied with 10% FCS–RPMI 1640 medium without antibiotics, and then used for experiments.
Bacteria.
L. pneumophila M124, serogroup 1, was originally obtained from a case of fatal legionellosis (6). The bacteria were cultured on buffered charcoal yeast extract (BCYE) medium (Becton Dickinson, Sparks, Md.) for 3 days at 37°C. The bacterial suspensions were prepared in pyrogen-free saline, and the concentration of bacteria was determined by spectrophotometry.
Macrophage infection.
The macrophage monolayers were infected with L. pneumophila (infectivity ratio, 10 bacteria per cell) for 30 min, washed to remove nonphagocytized bacteria, and incubated in RPMI 1640 medium containing 10% FCS with no antibiotics. The cultures were then incubated for up to 48 h at 37°C in 5% CO2.
Macrophage treatment.
The macrophage cultures infected with bacteria were treated with various concentrations (0, 0.5, 5, and 50 μg/ml) of EGCg (Calbiochem, San Diego, Calif.) for up to 48 h at 37°C in 5% CO2. In some experiments, macrophage cultures infected with bacteria and treated or not with EGCg were incubated with either anti-mouse TNF-α immunoglobulin G (IgG) (20 μg/ml), anti-mouse IFN-γ IgG (10 μg/ml), or control hamster IgG (Pharmingen, San Diego, Calif.). The antibody concentrations used were previously confirmed to show complete neutralization of cytokines produced in cultures (21).
Viable bacteria in cell cultures (CFU assay).
The number of viable bacteria (CFU) in cell lysates was determined by standard plate counts on BCYE medium, as described previously (42). After incubation, the cell monolayers were lysed with 0.1% saponin, and the number of viable bacteria in the lysates was determined.
Direct antimicrobial activity.
To evaluate direct anti-L. pneumophila activity of EGCg, culture of L. pneumophila in bacterial medium with EGCg was performed. In brief, AYE broth medium (7) with or without EGCg at various concentrations was dispensed into culture flasks and then inoculated with L. pneumophila at a final concentration of 5 × 103 bacteria/ml. After incubation for 24 or 48 h at 37°C, the number of viable bacteria (CFU) in the culture broth was determined by standard plate counts on BCYE medium.
ELISA.
The amount of IL-6, IL-10, IL-12 p40/p70, TNF-α, and IFN-γ in the culture supernatants of macrophage cultures were determined by sandwich enzyme-linked immunosorbent assay (ELISA) using matched antibody pairs and protein standard for ELISA (BD Pharmingen) (IL-6, IL-10, IL-12, and IFN-γ) and Duoset ELISA development system (R & D Systems, Minneapolis, Minn.) (TNF-α). Concentrations were calculated from the standard curve performed for each plate.
RT-PCR.
Total RNA was extracted from cells by the microspin technique with the Rneasy minikit (Qiagen, Valencia, Calif.) in accordance with the manufacturer's manual. Reverse transcription (RT) of total RNA (1 μg) was performed with avian myeloblastosis virus transcriptase in a commercial reaction mixture (Reverse Transcription System; Promega, Madison, Wis.). The resulting cDNA, equivalent to 0.1 μg of starting RNA, was subjected to PCR with primers for β2-microglobulin (BMG) and IFN-γ, deoxynucleoside triphosphate mixtures, and Ampli Taq Gold DNA polymerase (Perkin Elmer, Norwalk, Conn.). The primer sequences for BMG were described previously (40). The sequences of the primers for IFN-γ were 5′-CAT TGA AAG CCT AGA AAG TCT-3′ (sense) and 5′-CTC ATG GAA TGC ATC CTT TTT CG-3′ (antisense). The PCR was performed in a Minicycler (MJ Research, Watertown, Mass.) for either 25 cycles and 60°C annealing temperature (BMG) or 40 cycles and 55°C annealing temperature (IFN-γ). The first cycle consisted of 5 min of denaturation at 94°C, a 5-min annealing at either 60 or 55°C, and then 25 to 40 cycles each of 1.5 min at 72°C, 45 s at 94°C, and 45 s at the annealing temperature for each primer, with a final extension of 10 min at 72°C. The PCR products were analyzed on an ethidium bromide-stained 2% agarose gel.
Statistical analysis.
Statistical analysis was performed using a repeated-measures analysis of variance.
RESULTS
Effect of EGCg on L. pneumophila growth in macrophages.
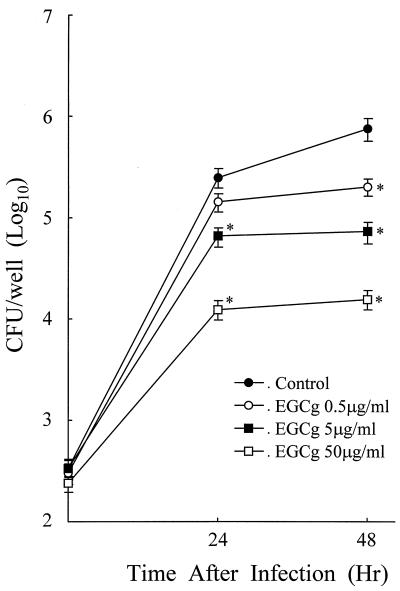
Since it is known that the growth of L. pneumophila in macrophages is dependent on the activation of host macrophages (41), treatment of macrophages with EGCg may alter the growth of L. pneumophila in cells if EGCg has any immunomodulatory activity related to the antibacterial activity of macrophages. As evident in Fig. 1, treatment of macrophages with EGCg after infection with bacteria induced an inhibition of the growth of L. pneumophila in the cells in a dose-dependent manner. A marked inhibitory effect of EGCg for L. pneumophila growth occurred at a dose of 5 μg/ml at 24 h after infection. Even as little as 0.5 μg/ml significantly inhibited L. pneumophila growth at 48 h after infection. However, the inhibitions observed were not due to a killing effect, since the number of viable bacteria in EGCg-treated macrophages was substantial even with an EGCg concentration as high as 50 μg/ml.
FIG. 1.
Effect of EGCg on L. pneumophila growth in MH-S macrophage cells. Macrophage monolayers were infected with L. pneumophila for 30 min, and then various concentrations of EGCg were added. The number of viable bacteria in macrophages was determined by the standard plate count method. Data represent the mean CFU ± standard deviation (SD) for triplicate macrophage cultures. The data presented are representative of three experiments. ∗, P < 0.05, significantly different from the control group at the same time point.
Direct antibacterial effect of EGCg on L. pneumophila.
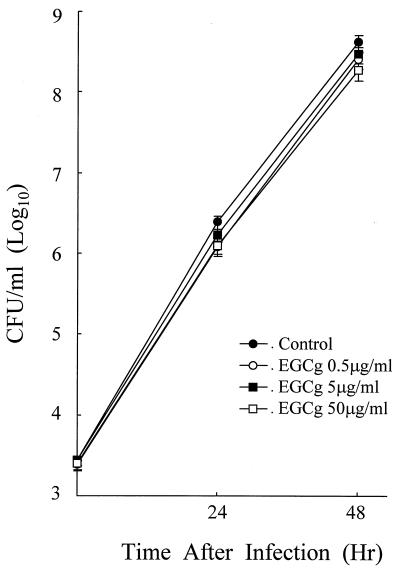
In order to determine whether EGCg has direct antibacterial activity on L. pneumophila, the growth of L. pneumophila in liquid bacterial medium without host cells in the presence and absence of EGCg (0.5 to 50 μg/ml) was examined. As shown in Fig. 2, EGCg could not alter L. pneumophila growth in the bacterial medium regardless of the concentration tested. Even as much as 500 μg of EGCg/ml did not result in any alteration of L. pneumophila growth in the liquid medium (data not shown). Thus, these results indicate that EGCg does not have any direct anti-L. pneumophila activity at the concentrations tested.
FIG. 2.
Direct antibacterial effect of EGCg on L. pneumophila growth. AYE broth medium with various concentrations of EGCg was dispensed into culture flasks, and L. pneumophila (5 × 103/ml) was added. After incubation, the number of viable bacteria in broth was determined by the standard plate count method. Data represent the mean CFU ± SD for triplicate macrophage cultures. The data presented are representative of three experiments.
Effect of EGCg on macrophage cytokine production induced by L. pneumophila infection.
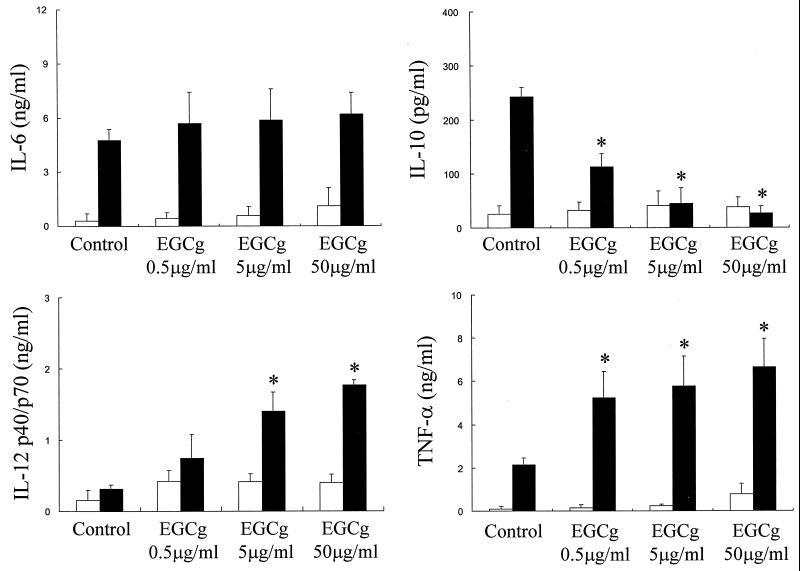
From the previous results, it seemed likely that EGCg could inhibit L. pneumophila growth due to macrophage activation. In order to determine such a possibility, the effect of EGCg on the production of macrophage cytokines was examined. As shown in Fig. 3, treatment of macrophages with EGCg alone slightly induced macrophage IL-6 and TNF-α protein production at a high EGCg concentration, such as 50 μg/ml, but this was not significant. EGCg treatment of macrophages also did not induce any macrophage IL-10 and IL-12 production. However, EGCg markedly upregulated the production of TNF-α and IL-12 induced by L. pneumophila infection, even at a concentration as low as 0.5 μg/ml in the case of TNF-α. In contrast, IL-10 production induced by L. pneumophila infection was strongly downregulated by EGCg in a dose-dependent manner. On the other hand, the production of IL-6 induced by L. pneumophila infection was not affected by EGCg, even at a concentration as high as 50 μg/ml. EGCg at 50 μg/ml also did not induce any detectable IFN-γ, as determined by ELISA (lower detection limit, 32 pg/ml) in the culture supernatants of macrophages uninfected or infected with L. pneumophila.
FIG. 3.
Effect of EGCg on macrophage cytokine production induced by L. pneumophila infection. The production of IL-6, IL-10, IL-12 p40/p70, and TNF-α protein in the supernatants obtained from the macrophage cultures at 24 h after infection was measured by ELISA. Open columns, noninfected control group; solid columns, L. pneumophila-infected group. Results are expressed as means ± SD for three independent experiments. ∗, P < 0.05, significantly different from the non-EGCg-treated L. pneumophila infection group.
Involvement of enhanced cytokine production in inhibition of L. pneumophila growth by EGCg.
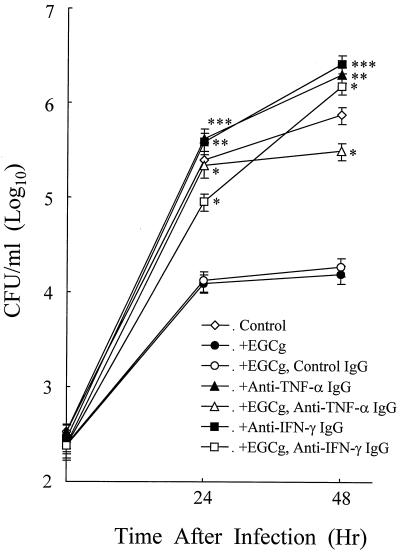
Since both endogenous and exogenous TNF-α and IFN-γ are known to activate macrophages to inhibit L. pneumophila growth (16, 19, 21, 27), the involvement of enhanced TNF-α and IFN-γ, which was not detected in the culture supernatants by ELISA, in EGCg-induced L. pneumophila growth inhibition was examined by neutralization with specific antibodies to these cytokines. Macrophages infected and treated with EGCg (50 μg/ml) were incubated with either anti-TNF-α antibody, anti-IFN-γ antibody, or control IgG, and the number of viable bacteria in the cultures was then determined at 24 and 48 h after incubation. As shown in Fig. 4, treatment of macrophages with anti-TNFα antibody almost completely abolished the growth-inhibitory effect of EGCg at 24 h after infection. However, this abolishment effect of anti-TNF-α antibody decreased at 48 h after infection, since the number of viable bacteria in the infection-only control cultures increased but not in the anti-TNF-α antibody-treated macrophage cultures. Furthermore, the control anti-TNF-α-treated macrophages without EGCg showed more viable bacteria in the cultures at 48 h after infection. This enhancement of bacterial growth in anti-TNF-α antibody-treated macrophages has been observed previously as the result of neutralization of self-produced cytokines during infection (21). Therefore, the abolishment of the EGCg-induced growth-inhibitory effect by anti-TNF-α antibody was partial at 48 h after infection compared with the control anti-TNF-α antibody-treated macrophages. In contrast, anti-IFN-γ treatment of macrophages showed a partial abolishment of the EGCg-induced growth inhibition at 24 h after infection (Fig. 4). However, at 48 h after infection, anti-IFN-γ antibody treatment showed almost complete abolishment of the EGCg-induced growth inhibition. As observed in the control anti-TNF-α antibody-treated macrophages, the control anti-IFN-γ antibody treated-macrophages also showed an increase in the number of viable bacteria in the cells at 48 h after infection. The control IgG-treated macrophages did not show alteration of the EGCg-induced growth inhibition.
FIG. 4.
Effect of TNF-α and IFN-γ neutralization treatment on EGCg-induced anti-L. pneumophila growth activity of macrophages. See the legend to Fig. 1. Infected macrophages were untreated or treated with EGCg (50 μg/ml), and either control IgG (20 μg/ml), anti-mouse TNF-α IgG (20 μg/ml), or anti-mouse IFN-γ IgG (10 μg/ml) was added. The data represent the mean CFU ± SD for triplicate macrophage cultures. The data presented are representative of three experiments. ∗, P < 0.05, significantly different from the EGCg-alone group at the same time point. ∗∗, P < 0.05, significantly different from the EGCg- and anti-mouse TNF-α IgG-treated group at the same time point. ∗∗∗, P < 0.05, significantly different from the EGCg- and anti-mouse IFN-γ IgG-treated group at the same time point.
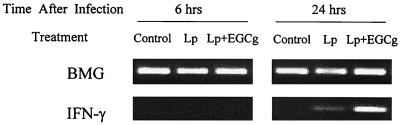
Effect of EGCg on IFN-γ mRNA expression induced by L. pneumophila infection.
Since treatment with anti-IFN-γ antibody induced a marked abolishment of the EGCg-induced growth inhibition, steady-state levels of IFN-γ mRNA in macrophages infected with L. pneumophila unstimulated and stimulated with EGCg were analyzed by RT-PCR. As shown in Fig. 5, mRNA for IFN-γ was detected in L. pneumophila-infected cells at 24 h after infection, but not at 6 h. Moreover, when the macrophages were infected and treated with EGCg (50 μg/ml), a remarkable enhancement of IFN-γ mRNA accumulation was observed. EGCg (50 μg/ml) alone did not induce any IFN-γ mRNA in macrophages at either 6 or 24 h after treatment (data not shown).
FIG. 5.
Effect of EGCg on IFN-γ mRNA expression induced by L. pneumophila infection. Macrophage monolayers were infected with L. pneumophila (Lp) and incubated in the presence or absence of EGCg (50 μg/ml). The expression of mRNA for BMG and IFN-γ was determined by RT-PCR, as described in Materials and Methods.
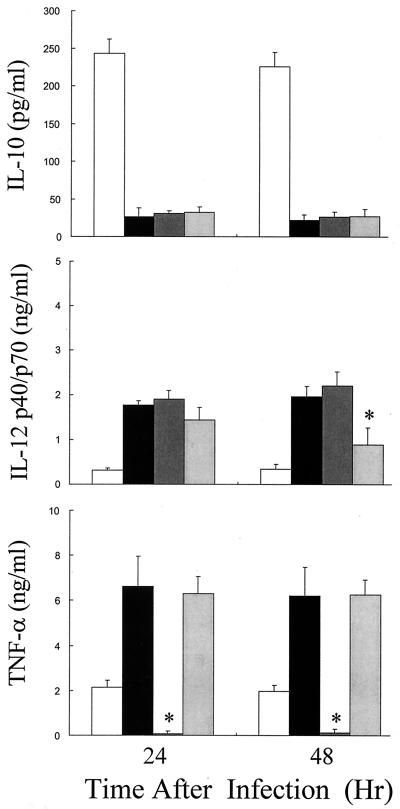
Effect of cytokine neutralization on immunomodulatory activity of EGCg on cytokine production induced by L. pneumophila.
In order to analyze the effect of TNF-α and IFN-γ neutralization by antibodies on the EGCg (50 μg/ml)-induced cytokine modulation of L. pneumophila-infected macrophages, IL-10, IL-12, and TNF-α production in antibody-treated macrophages was analyzed. As shown in Fig. 6, treatment of macrophages with anti-TNF-α antibody almost completely neutralized TNF-α that was detected only minimally by ELISA. However, neither the upregulation of IL-12 nor the downregulation of IL-10 by EGCg was altered by anti-TNF-α antibody treatment at any time tested. The anti-IFN-γ antibody treatment did not affect the upregulation of TNF-α production at any time or of IL-12 production at 24 h after infection, but significantly reduced the IL-12 production upregulated by EGCg treatment at 48 h after infection. The downregulated IL-10 production was not affected by treatment with anti-IFN-γ antibody.
FIG. 6.
Effect of TNF-α and IFN-γ neutralization treatment on macrophage cytokine production induced by L. pneumophila infection under stimulation with EGCg (50 μg/ml). See the legend to Fig. 4. The production of IL-10, IL-12 p40/p70, and TNF-α protein in the supernatants obtained from the macrophage cultures at 24 h after infection was measured by ELISA. Open columns, L. pneumophila infection control group; solid columns, L. pneumophila infection and EGCg stimulation group; dark shaded columns, L. pneumophila infection, EGCg stimulation, and TNF-α neutralization group; light shaded columns, L. pneumophila infection, EGCg stimulation, and IFN-γ neutralization group. Results are expressed as means ± SD for three independent experiments. ∗, P < 0.05, significantly different from the L. pneumophila infection and EGCg stimulation group.
DISCUSSION
In the present study, the ability of EGCg to modulate macrophage immune functions was examined in vitro using L. pneumophila-infected MH-S murine alveolar macrophage cells. The results clearly indicated that EGCg strongly inhibits L. pneumophila growth in macrophages at a concentration as low as 0.5 μg/ml. Previous studies have shown that EGCg has direct antimicrobial activity against a variety of pathogens (12, 34, 35, 38), but the inhibitory activity required relatively high concentrations. For example, the MICs of EGCg for Staphylococcus aureus and Escherichia coli were 73 and 183 μg/ml, respectively (12). In the present study, L. pneumophila did not show any susceptibility to EGCg in bacterial culture medium, even at a concentration as high as 500 μg/ml. However, a possible direct effect of EGCg on the growth of L. pneumophila may not be completely ruled out due to the limited experimental conditions used in this study. Nevertheless, it seems very likely that L. pneumophila may be resistant to the direct antibacterial effect of EGCg. In contrast, EGCg induced a strong indirect anti-L. pneumophila activity mediated by host cell activation. Nakayama et al. reported that EGCg has a strong anti-influenza virus activity at relatively low concentrations, such as 0.5 μg/ml, showing a significant inhibition of plaque formation induced by influenza virus (26). The mechanism proposed for the anti-influenza virus activity of EGCg was inhibition of virus adsorption to host cells by EGCg, because EGCg bound to the hemagglutinin of the influenza virus, followed by inhibition of hemagglutination by viruses (26). Therefore, the inhibition mechanism of L. pneumophila growth in macrophages by EGCg demonstrated in this study seems to be different from the anti-influenza virus activity reported for EGCg.
As evident in this study, EGCg markedly modulated the immune response of macrophages to L. pneumophila infection and resulted in the inhibition of bacterial growth in the cells. The main effector molecules responsible for the inhibition may be TNF-α and IFN-γ produced by macrophages. This conclusion is consistent with previous reports that both TNF-α and IFN-γ are strong activators for macrophages to induce anti-L. pneumophila activity (2, 16, 19, 21, 27). However, the neutralization experiments with anti-TNF-α antibody did not result in complete abolishment of the EGCg-induced anti-L. pneumophila activity at 48 h after infection. It can be conjectured, therefore, that TNF-α may only partially be involved in the anti-L. pneumophila activity of macrophages induced by EGCg in the late phase of infection, such as 48 h after infection. In contrast, IFN-γ may be effective mainly in the late phase of infection, such as 48 h after infection, rather than the early phase (24 h) of infection, because neutralization experiments with anti-IFN-γ antibody indicated that the partial abolishment of the EGCg-induced antibacterial activity was observed at the early infection phase but almost complete abolishment was observed at 48 h after infection. Such different involvement of TNF-α and IFN-γ in the anti-L. pneumophila activity in the different infection phases seems likely to be linked to the limited effect of EGCg on bacterial growth in macrophages, which showed that the antibacterial activity of EGCg plateaus at 24 h (Fig. 1). Nevertheless, these results suggest that both TNF-α and IFN-γ might be involved in the anti-L. pneumophila activity of macrophages induced by EGCg.
The production of IFN-γ in the culture supernatants of macrophages infected with L. pneumophila and stimulated with EGCg could not be detected by ELISA. However, the enhanced expression of mRNA for the IFN-γ gene in the macrophages stimulated with EGCg was observed. Furthermore, treatment of macrophages with anti-IFN-γ antibody inhibited the EGCg-induced anti-L. pneumophila activity. From these results, it seems likely that even the amount of IFN-γ induced by EGCg in response to L. pneumophila, although low and not detected by ELISA, may be involved in the EGCg-induced anti-L. pneumophila activity. While it is generally considered that NK cells and activated T lymphocytes are the major source for IFN-γ, current studies indicate that macrophages also produce IFN-γ (4, 8, 23, 29). Therefore, IFN-γ produced by macrophages may have an important role in the course of infection.
The EGCg treatment also modulated the production of IL-10 (downregulation) and IL-12 (upregulation) of L. pneumophila-infected macrophages. The cytokine IL-10 has been shown to exhibit important deactivating effects on macrophages in murine models of L. pneumophila (28), leishmanial (1), and mycobacterial (3) infections. Moreover, it is known that IL-10 is secreted by L. pneumophila-infected monocytes and alveolar macrophages, enhances bacterial growth, reverses the protective effect of IFN-γ, and blocks the secretion of TNF-α by infected cells (28). Therefore, downregulation of IL-10 by EGCg is likely to be involved in the EGCg-induced anti-L pneumophila activity of macrophages. However, such modulation may not be directly involved in the EGCg-induced anti-L. pneumophila activity of macrophages in vitro, since the diminution in anti-L. pneumophila activity was not associated with a change in IL-10 production.
IL-12 plays a key role in the development of Th1 responses, leading to IFN-γ production (36). Therefore, the suppression of IL-12 production by the IFN-γ neutralization treatment observed at 48 h after infection seems likely to indicate an involvement of IL-12 in the EGCg-induced anti-L. pneumophila activity. However, such a possibility is also less likely due to the absence of other immune cells in the in vitro experimental system used. Nevertheless, it is obvious that EGCg could modulate the IL-10 and IL-12 production of infected macrophages and may have the potential to cause further immunomodulation in the immune system.
It has been reported that EGCg itself induces TNF-α from peripheral blood mononuclear cells (31). However, in the present study, EGCg alone did not induce any significant induction of cytokines, including TNF-α. This discrepancy may be due to the different cells used. The modulation of cytokine production of L. pneumophila-infected macrophages by EGCg was specific for certain cytokines, such as TNF-α, IFN-γ, IL-10, and IL-12, but not IL-6. Similar observations regarding modulation of cytokine production by EGCg was also seen in macrophages stimulated with bacterial lipopolysaccharide (unpublished data). Therefore, the immunomodulatory effect of EGCg on macrophages may not be unique for infected macrophages. Further study to elucidate the mechanism(s) of the immunoregulation of macrophages by EGCg is under way.
The immunomodulatory effect of antimicrobial agents on immune cells, such as monocytes and lymphocytes, has been increasingly highlighted. In particular, the effects of macrolide as well as fluoroquinolone antibiotics on immune responses have been extensively studied (15, 17, 22, 30, 32). However, the most important immunomodulatory activity of these antibiotics is an anti-inflammatory activity, such as suppressed cytokine production by immune cells. In this regard, the finding of anti-L. pneumophila activity of macrophages mediated by enhanced production of some cytokines by EGCg, which is not a chemotherapeutic agent or an antibiotic, may introduce a possible new alternative direction for treatment of bacterial infections.
In summary, we demonstrated that EGCg induces an anti-L. pneumophila activity of macrophages at a low dose without direct antimicrobial effect. EGCg selectively upregulated the production of IL-12 and TNF-α and downregulated IL-10 production, but did not modulate IL-6 production induced by L. pneumophila infection. Upregulation of the production of IFN-γ by EGCg was also conjectured. Moreover, neutralization treatment of both TNF-α and IFN-γ significantly abolished the anti-L. pneumophila activity of EGCg. These findings indicate that macrophages may be activated by EGCg to inhibit L. pneumophila growth in the cells by upregulated TNF-α and IFN-γ production. This enhancement of the anti-L. pneumophila activity of EGCg may not involve modulation of IL-10 and IL-12 production.
ACKNOWLEDGMENTS
We thank Tadakatsu Shimamura, Showa University School of Medicine, Tokyo, Japan, for critical reading of the manuscript.
This work was supported by grant AI45169 from the National Institute of Allergy and Infectious Diseases and a grant from the American Lung Association of Florida.
REFERENCES
- 1.Barral-Netto M, Barral A, Brownwell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–547. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj N, Nash T W, Horwitz D M. IFN-γ-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986;137:2662–2669. [PubMed] [Google Scholar]
- 3.Denis M, Ghadirian E. II-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–5430. [PubMed] [Google Scholar]
- 4.Fenton M J, Vermeulen M W, Kim S, Burdick M, Strieter R M, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flesch I E, Kaufmann S H. Stimulation of antibacterial macrophage activities by B-cell stimulatory factor 2 (interleukin-6) Infect Immun. 1990;58:269–271. doi: 10.1128/iai.58.1.269-271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman H, Widen R, Klein T W, Searls L, Cabrian K. Legionella pneumophila-induced blastogenesis of murine lymphoid cells in vitro. Infect Immun. 1984;43:314–319. doi: 10.1128/iai.43.1.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebran S J, Newton C A, Yamamoto Y, Klein T W, Friedman H. A rapid colorimetric assay for evaluating Legionella pneumophila growth in macrophages in vitro. J Clin Microbiol. 1994;32:127–130. doi: 10.1128/jcm.32.1.127-130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gessani S, Belardelli F. IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998;9:117–123. doi: 10.1016/s1359-6101(98)00007-0. [DOI] [PubMed] [Google Scholar]
- 9.Ho C T, Chen Q, Shi H, Zhang K Q, Rosen R T. Antioxidative effect of polyphenol extract prepared from various Chinese teas. Prev Med. 1992;21:520–525. doi: 10.1016/0091-7435(92)90059-q. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz M A. Cell mediated immunity in Legionnaires' disease. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 12.Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- 13.Katiyar S, Challa A, McCormick T S, Cooper K D, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by the green tea polyphenol (−)-epigallocatechin-3-gallate may be associated with alternations in IL-10 and IL-12 production. Carciogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 14.Katiyar S, Kagarwal R, Zain M T, Mukntar H. Protection against N-nitrosodiethylamine and benzo[a]-pyrene-induced forestomach and lung tumorigenesis in A/J mice by green tea. Carcinogenesis. 1993;14:849–855. doi: 10.1093/carcin/14.5.849. [DOI] [PubMed] [Google Scholar]
- 15.Khan A A, Slifer T R, Remington J S. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob Agents Chemother. 1998;42:1713–1717. doi: 10.1128/aac.42.7.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein T W, Yamamoto Y, Brown H K, Friedman H. Interferon-gamma induced resistance to Legionella pneumophila in susceptible A/J mouse macrophages. J Leukocyte Biol. 1991;49:98–103. doi: 10.1002/jlb.49.1.98. [DOI] [PubMed] [Google Scholar]
- 17.Labro M T. Anti-inflammatory activity of macrolides: a new therapeutic potential? J Antimicrob Chemother. 1998;41:37–46. doi: 10.1093/jac/41.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Simpson R J, Cheers C. Recombinant interleukin-6 protects mice against experimental bacterial infection. Infect Immun. 1992;60:4402–4406. doi: 10.1128/iai.60.10.4402-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsiota-Bernard P, Lefebre C, Sedqui M, Cornillet P, Guenounou M. Involvement of tumor necrosis factor alpha in intracellular multiplication of Legionella pneumophila in human monocytes. Infect Immun. 1993;61:4980–4983. doi: 10.1128/iai.61.12.4980-4983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsunaga K, Klein T W, Friedman H, Yamamoto Y. The alveolar macrophage cell line MH-S is valuable as in vitro model for Legionella pneumophila infection. Am J Respir Cell Mol Biol. 2001;24:326–331. doi: 10.1165/ajrcmb.24.3.4359. [DOI] [PubMed] [Google Scholar]
- 21.McHugh S L, Newton C A, Yamamoto Y, Klein T W, Friedman H. Tumor necrosis factor induces resistance of macrophages to Legionella pneumophila infection. Proc Soc Exp Biol Med. 2000;224:191–196. doi: 10.1046/j.1525-1373.2000.22420.x. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa K, Oseko F, Morikawa S, Iwamoto K. Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro. Antimicrob Agents Chemother. 1994;38:2643–2647. doi: 10.1128/aac.38.11.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa K, Miyazawa T. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate, in the rat. J Nutr Sci Vitaminol (Tokyo) 1997;43:679–684. doi: 10.3177/jnsv.43.679. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa K, Okuda S, Miyazawa T. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci Biotechnol Biochem. 1997;61:1981–1985. doi: 10.1271/bbb.61.1981. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993;21:289–299. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- 27.Nash T W, Libby D M, Horwitz M A. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988;140:3978–3981. [PubMed] [Google Scholar]
- 28.Park D R, Skerrett S J. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ. J Immunol. 1996;157:2528–2538. [PubMed] [Google Scholar]
- 29.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf S F, Belardelli F, Gessani S. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 30.Riesbeck K, Forsgren A, Henriksson A, Bredberg A. Ciprofloxacin induces an immunomodulatory stress response in human T lymphocytes. Antimicrob Agents Chemother. 1998;42:1923–1930. doi: 10.1128/aac.42.8.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakagami H, Takeda M, Sugaya K, Omata T, Takahashi H, Yamamura M, Hara Y, Shimamura T. Stimulation by epigallocatechin gallate of interleukin-1 production by human peripheral blood mononuclear cells. Anticancer Res. 1995;15:971–974. [PubMed] [Google Scholar]
- 32.Scaglione F, Ferrara F, Dugnani S, Demartini G, Triscari F, Fraschini F. Immunostimulation by clarithromycin in healthy volunteers and chronic bronchitis patients. J Chemother. 1993;5:228–232. doi: 10.1080/1120009x.1993.11739237. [DOI] [PubMed] [Google Scholar]
- 33.Skerrett S J, Bagby G J, Schmidt R A, Nelson S. Antibody-mediated depletion of tumor necrosis factor-α impairs pulmonary host defenses to Legionella pneumophila. J Infect Dis. 1997;176:1019–1028. doi: 10.1086/516530. [DOI] [PubMed] [Google Scholar]
- 34.Toda M, Okubo S, Hara Y, Shimamura T. Antibacterial and bactericidal activities of tea extracts and catechins against methicillin-resistant Staphylococcus aureus. Jpn J Bacteriol. 1991;46:839–844. doi: 10.3412/jsb.46.839. [DOI] [PubMed] [Google Scholar]
- 35.Toda M, Okubo S, Hiyoshi R, Shimamura T. The bactericidal activity of tea and coffee. Lett Appl Microbiol. 1989;8:123–125. [Google Scholar]
- 36.Trinchieri G. Interleukin-12 and its role in the generation of Th1 cells. Immunol Today. 1993;14:335–337. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 37.Weisburger J H. Second international scientific symposium on tea and human health: an introduction. Proc Soc Exp Biol Med. 1999;220:193–194. doi: 10.1046/j.1525-1373.1999.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 38.Yam T S, Shah J M, Hamilton-Miller T. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol Lett. 1997;152:169–174. doi: 10.1111/j.1574-6968.1997.tb10424.x. [DOI] [PubMed] [Google Scholar]
- 39.Yam T S, Shah J M, Hamilton-Miller T. The effect of component of tea (Camellia sinensis) on methicillin resistance, PBP2′ synthesis, and β-lactamase production in Staphylococcus aureus. J Antimicrob Chemother. 1998;42:211–216. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Retzlaff C, He P, Klein T W, Friedman H. Quantitative reverse transcription-PCR analysis of Legionella pneumophila-induced cytokine mRNA in different macrophage populations by high-performance liquid chromatography. Clin Diagn Lab Immunol. 1995;2:18–24. doi: 10.1128/cdli.2.1.18-24.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Klein T W, Friedman H. Legionella and macrophages. In: Zwilling B S, Eisenstein T K, editors. Macrophage-pathogen interactions. New York, N.Y: Marcel Dekker, Inc; 1994. pp. 329–348. [Google Scholar]
- 42.Yamamoto Y, Klein T W, Newton C A, Widen R, Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988;56:370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamane T, Takahashi T, Kuwata K, Oya K, Inagake M, Kitao Y, Suganuma M, Fujiki H. Incorporation of 5-bromo-2′-deoxyuridine into colorectal liver metastases and liver in patients receiving a 7-day hepatic arterial infusion. Cancer Res. 1995;5:2081–2084. [Google Scholar]
- 44.Yang C S, Chen L, Lee M J, Balentine D, Kuo M C, Schantz S P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–354. [PubMed] [Google Scholar]