Abstract
A woman in her 80s was brought to the emergency department for acute onset of generalised weakness, lethargy and altered mental state. The emergency medical service found her to have symptomatic bradycardia, and transcutaneous pacing was done. Medical history was notable for hypertension, hyperlipidaemia, type 2 diabetes, and a recently diagnosed SARS-CoV-2 (COVID-19) infection for which she was prescribed ritonavir-boosted nirmatrelvir (Paxlovid) two days before the presentation. On arrival at the hospital, she was found to have marked bradycardia with widened QRS, hyperglycaemia and metabolic acidosis. Transvenous pacing along with pressor support and insulin were initiated, and she was admitted to the intensive care unit. Drug interaction between ritonavir-boosted nirmatrelvir and verapamil leading to verapamil toxicity was suspected of causing her symptoms, and both drugs were withheld. She reverted to sinus rhythm on the fourth day, and the pacemaker was discontinued.
Keywords: Arrhythmias, COVID-19, Drug interactions
Background
Verapamil acts by blocking the rapid influx of calcium into the cardiac conduction system, cardiac myocytes and vascular smooth muscle, resulting in prolongation of the conduction time, decrease in myocardial contractility and vascular relaxation. Metabolic studies carried out in vitro indicate that verapamil is metabolised by cytochrome P450 (CYP) enzymes, including CYP3A4, CYP1A2, CYP2C8, CYP2C9 and CYP2C18.1 2 Ritonavir, which strongly inhibits CYP P450 3A4 and P-glycoprotein (P-gp) enzymes, results in increased concentrations of drugs metabolised by those enzymes. Ritonavir is coformulated with nirmatrelvir to raise the blood concentration of nirmatrelvir to increase its effectiveness against SARS-CoV-2.3 Most reported verapamil toxicity cases are due to massive, intentional overdoses. Herein, we describe an unusual case of a woman who presented with a complete heart block, hyperglycaemia and metabolic acidosis resulting from verapamil toxicity due to a drug interaction with ritonavir-boosted nirmatrelvir.
Case presentation
A woman in her early 80s was admitted to the emergency department (ED) for an acute onset of generalised weakness, lethargy and altered mental status. Before presenting to the ED, she received transcutaneous pacing for severe bradycardia from the emergency medical service. She had generalised weakness and dizziness symptoms after being diagnosed with COVID-19 two days prior to presentation, for which she was started on ritonavir-boosted nirmatrelvir. She was fully vaccinated against COVID-19 including a booster dose. The medical history was significant for hyperlipidaemia, hypertension and type 2 diabetes mellitus. She was on hydrochlorothiazide 50 mg and verapamil extended-release 240 mg for hypertension and metformin for type 2 diabetes mellitus. The vital signs on presentation showed a heart rate of 28 per minute, blood pressure of 58/35 mm Hg, oxygen saturation of 81% on room air, oral temperature of 36.3°C and respiratory rate of 30 /min. The physical examination showed lethargy and bradycardia. The laboratory investigations were significant for hyperglycaemia and high anion gap metabolic acidosis with elevated serum lactate suspected to be from cardiogenic shock (table 1). The ECG revealed marked bradycardia with widened QRS complex and absent p-waves, consistent with sinus arrest with ventricular escape at a rate of 29 beats per minute (bpm) (figure 1).
Table 1.
Laboratory investigations on admission and discharge (day 5) from the ICU
| Laboratory reference range | On admission | Day 5 | |
| Haemoglobin (g/L) | 120-160 | 75 | 96 |
| Total leucocyte count (10ˆ9/L) | 4.0–10.5 | 4.0 | 8.0 |
| Platelet count (10ˆ9/L) | 130–400 | 371 | 542 |
| Blood glucose (mg/dL) | 70–99 | 514 | 129 |
| Haemoglobin A1c (%) | <5.7% | 5.8 | – |
| Blood urea nitrogen (mg/dL) | 6–20 | 36 | 22 |
| Serum creatinine (mg/dL) | 0.5–1.2 | 2.79 | 1.2 |
| Serum lactate (mmol/L) | 0.5–2.2 | 9.1 | 1.3 |
| Serum Na+ (mmol/L) | 135–145 | 129 (135 corrected) | 142 |
| Serum K+ (mmol/L) | 3.5–5.3 | 4.0 | 4.1 |
| Serum Cl− (mmol/L) | 96–106 | 108 | 107 |
| pH (arterial) | 7.35–7.45 | 7.21 | 7.38 |
| pCO2 (arterial) | 35–45 mm Hg | 22 | 39 |
| Serum bicarbonate (mEq/L) | 22–29 | 8.8 | 23 |
| Urine ketones (dipstick) | – | Negative | – |
| Serum beta-hydroxybutyrate (mmol/L) | 0.01–0.25 | 0.17 | – |
| Anion gap (mmol/L) | 8–12 | 18.2 | 12 |
| AST (IU/L) | 8–48 | 60 | 58 |
| ALT (IU/L) | 7–55 | 33 | 45 |
| Total bilirubin (mg/dL) | 0.1–1.2 | 0.50 | 0.50 |
ALT, alanine aminotransaminase; AST, aspartate aminotransferase; ICU, intensive care unit.
Figure 1.
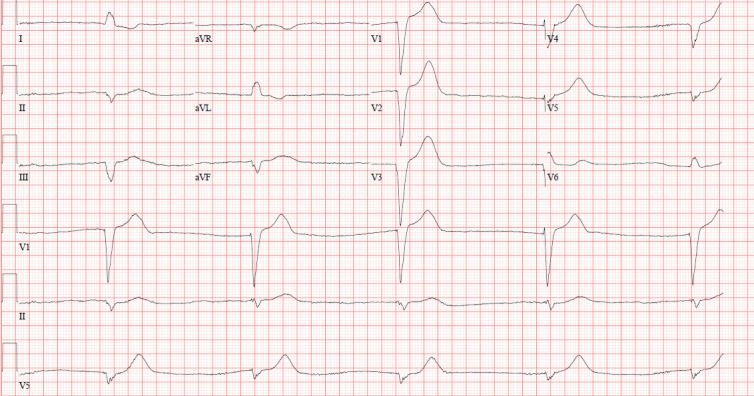
A 12-lead ECG showing bradycardia with widened QRS duration and no atrial electrical activity, consistent with sinus arrest with ventricular escape at a rate of 29 bpm on the day of presentation.
Investigations
Laboratory results are summarised in table 1.
Differential diagnosis
Diabetic ketoacidosis was initially considered as a cause of hyperglycaemia and acidosis in the setting of type 2 diabetes mellitus but was ruled out after the dipstick test for urine ketones returned negative, and serum beta-hydroxybutyrate levels were found to be within the normal range.
Treatment
Shortly after the presentation, a transvenous pacer was placed with a set rate of 70 bpm, and she was admitted to the intensive care unit. Intravenous epinephrine (4 µg/min) and norepinephrine (4 µg/min) along with correctional insulin were started, and the blood glucose levels were monitored every 6 hours. The aetiology of the patient’s severe, symptomatic bradycardia and hyperglycaemia was determined to be a drug interaction between verapamil and ritonavir-boosted nirmatrelvir, which were withheld, and she was placed under close monitoring. Over the next 24 hours, she continued with a paced rhythm of 70 per minute, and her haemodynamic parameters stabilised (figure 2). Her blood pressure on day 2 was 166/100, for which she was started on intravenous hydralazine. Forty-eight hours after admission, the pacer was turned down to 40 bpm, and she had partially recovered to a junctional rhythm at 55 bpm (figure 3). The metabolic picture (including severe lactic acidosis and hyperglycaemia) improved.
Figure 2.
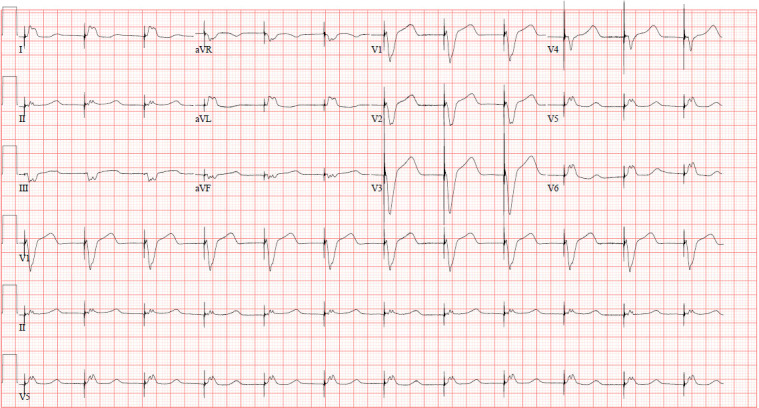
A 12-lead ECG after transvenous pacing showing ventricular paced rhythm at 70 beats per minute on day 2 of presentation.
Figure 3.
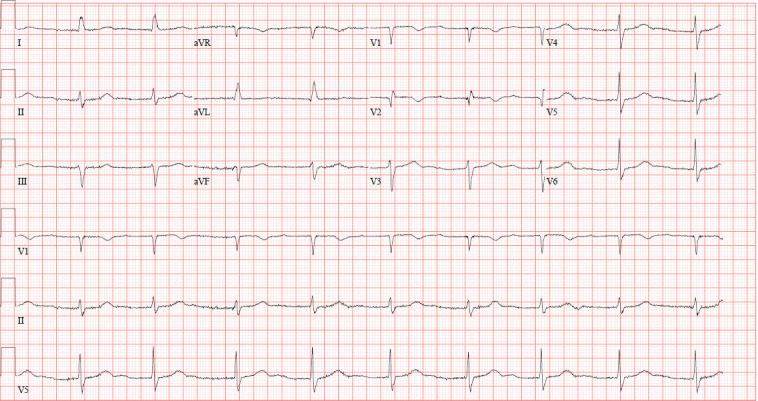
A 12-lead ECG with pacemaker turned down, showing junctional rhythm, with a ventricular rate of 55 beats per minute, left axis deviation and incomplete right bundle branch block, on day 3 of presentation.
Outcome and follow-up
On day 4 of the presentation, she reverted to sinus rhythm, so the pacemaker was turned off after a trial of successfully not requiring pacing at a low backup rate with continuous cardiac monitoring. She maintained a normal sinus rhythm with nonspecific T wave abnormality and prolonged QT (figure 4). The blood glucose, anion gap and lactate levels normalised. By day 5 after the initial presentation, she had remained in sinus rhythm with the pacer turned off and maintained a heart rate of 70 bpm for over 24 hours. The transvenous pacing leads were removed, and she was discharged from the ICU on oral hydrochlorothiazide and metoprolol.
Figure 4.
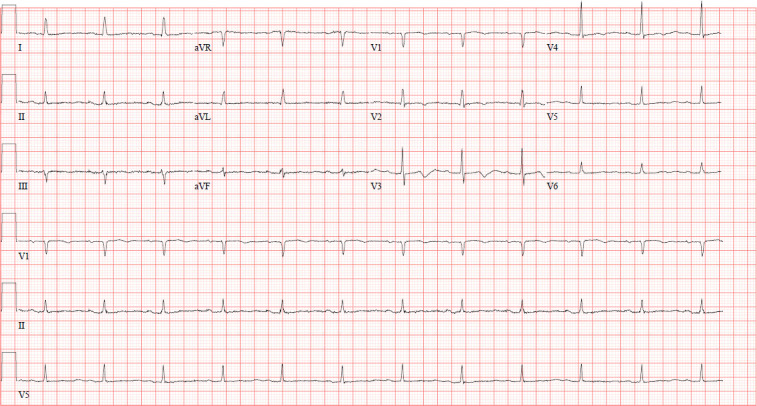
A 12-Lead ECG showing normal sinus rhythm with non-specific T wave abnormalities and long QT on day 4 with the pacemaker turned off.
Discussion
The cardiovascular adverse effects of verapamil toxicity are well documented in the literature and include bradycardia, hypotension and conduction abnormalities (with complete atrioventricular block as the most severe sequela).1 2 Verapamil and other calcium channel blockers (CCBs) inhibit L-type calcium channels in pancreatic Islet cells, reducing insulin secretion leading to hyperglycaemia.4 In addition, metabolic acidosis due to increased lactate production in response to hypoperfusion, and serum potassium disturbances may also be seen.4 5 In this report, we analysed a drug–drug interaction between verapamil and ritonavir-boosted nirmatrelvir, which resulted in heart block, cardiogenic shock and hyperglycaemia due to verapamil toxicity.
Verapamil toxicity was first suspected because of the unexplained hypotension and cardiac conduction abnormalities, which began a few days after the initiation of ritonavir-boosted nirmatrelvir. The patient had been taking an extended-release formulation of verapamil for hypertension for more than 10 years and did not report any overdose. Furthermore, the liver and renal functions were normal. In the literature, serious adverse effects from verapamil are mostly reported in patients with hepatic or renal impairment, which decrease the drug metabolism or excretion, and with accidental overdoses or very high therapeutic doses (>240 mg/day).6 7
The mainstay of management of CCB toxicity is high-dose insulin euglycaemia therapy (intravenous bolus of 1 unit/kg followed by a one unit/kg/hour infusion) with as-needed dextrose infusion to maintain euglycaemia and catecholamine infusions to improve inotropy and chronotropy.8–10 In addition, temporary transvenous pacing provides immediate improvement in haemodynamic instability resulting from cardiac conduction abnormalities.10 The CYP3A4 is involved in the metabolism of other CCBs (diltiazem, amlodipine, felodipine, nicardipine and nifedipine) and one angiotensin-receptor blocker, losartan.10 There is a significant list of drug interactions listed within the ritonavir-boosted nirmatrelvir drug information package insert, however, verapamil is not explicitly stated (whereas other CCBs have been mentioned).11 To prevent potential drug interactions, on instituting therapy with ritonavir-boosted nirmatrelvir in patients on verapamil or other drugs metabolised by the CYP3A4 enzyme, one should consider close monitoring or temporary discontinuation of the drug.
As of 2nd December 2022, the ongoing COVID-19 pandemic has affected more than 600 million patients worldwide, leading to an unprecedented public health crisis.12 Clinical manifestation of SARS-CoV-2 varies in the general population from asymptomatic carrier state to severe pneumonia, respiratory distress and multiorgan failure.13 Age remains the strongest risk factor for severe COVID-19 disease, and people aged 65 years or older accounted for more than 80% of COVID-19-related deaths in the USA.14 Furthermore, patients with underlying medical conditions such as cardiovascular disease, chronic kidney disease, diabetes, chronic lung disease, smoking, cancer, solid organ or haematopoietic stem cell transplant have an increased risk of developing severe COVID-19 infection.13 The patient discussed in this report had several risk factors for developing severe COVID-19 disease (old age, hypertension, and diabetes); however, it is worth noting that her admission to the ICU was due to a drug interaction in an otherwise uncomplicated COVID-19 infection.
Patient’s perspective.
I have been feeling weak and dizzy for the past few days. I don’t remember the events of the day I was admitted to the hospital except the feeling of black-out. I am thankful to the team of emergency medical professionals who attended to me. I feel a lot better knowing my diagnosis.
learning points.
Low-dose ritonavir is a very potent inhibitor of cytochrome P450 and P-glycoprotein enzymes. The cytochrome P450 enzyme system is involved in the metabolism of many drugs, including verapamil, so there is an increased likelihood of drug interactions, and prescribers should carefully review the patient’s complete medication regimen before starting it.
Verapamil toxicity should be suspected in the setting of new onset of cardiac conduction defects or shock, even with patients on regular doses.
High-dose insulin euglycaemia therapy, catecholamine infusions and transvenous pacing form the mainstay of management in calcium channel blocker toxicity to improve inotropy and chronotropy.
Footnotes
Twitter: @obaidimtiyaz
Contributors: OIH contributed to draft preparation, acquiring images, preparing figures 1–4, editing and submission and revision of the manuscript; SM contributed to the drug interaction detection, and editing of the manuscript; SH contributed to case management and editing of the manuscript; PS: reviewed, edited, and revised the manuscript and provided inputs as an expert consultant.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Elliott WJ, Ram CVS. Calcium channel blockers. J Clin Hypertens 2011;13:687–9. 10.1111/j.1751-7176.2011.00513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fravel MA, Ernst M. Drug interactions with antihypertensives. Curr Hypertens Rep 2021;23:14. 10.1007/s11906-021-01131-y [DOI] [PubMed] [Google Scholar]
- 3.Ritonavir-Boosted Nirmatrelvir (Paxlovid) . COVID-19 treatment guidelines. Available: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir-paxlovid-/ [Accessed 22 Aug 2022].
- 4.Graudins A, Lee HM, Druda D. Calcium channel antagonist and beta-blocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol 2016;81:453–61. 10.1111/bcp.12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine M, Boyer EW, Pozner CN, et al. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit Care Med 2007;35:2071–5. 10.1097/01.CCM.0000278916.04569.23 [DOI] [PubMed] [Google Scholar]
- 6.Hegazi MO, Aldabie G, Al-Mutairi S, et al. Junctional bradycardia with verapamil in renal failure--care required even with mild hyperkalaemia. J Clin Pharm Ther 2012;37:726–8. 10.1111/j.1365-2710.2012.01352.x [DOI] [PubMed] [Google Scholar]
- 7.Cohen AS, Matharu MS, Goadsby PJ. Electrocardiographic abnormalities in patients with cluster headache on verapamil therapy. Neurology 2007;69:668–75. 10.1212/01.wnl.0000267319.18123.d3 [DOI] [PubMed] [Google Scholar]
- 8.Espinoza TR, Bryant SM, Aks SE. Hyperinsulin therapy for calcium channel antagonist poisoning: a seven-year retrospective study. Am J Ther 2013;20:29–31. 10.1097/MJT.0b013e31824d5fbd [DOI] [PubMed] [Google Scholar]
- 9.St-Onge M, Anseeuw K, Cantrell FL, et al. Experts consensus recommendations for the management of calcium channel blocker poisoning in adults. Crit Care Med 2017;45:e306–15. 10.1097/CCM.0000000000002087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Onge M, Dubé P-A, Gosselin S, et al. Treatment for calcium channel blocker poisoning: a systematic review. Clin Toxicol 2014;52:926–44. 10.3109/15563650.2014.965827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PAXLOVIDTM . Paxlovid (nirmatrelvir and ritonavir). Kirkland, Quebec, Canada: Pfizer Canada ULC; 2022. [Accessed 5 December 2022]. [Google Scholar]
- 12.WHO coronavirus (COVID-19) Dashboard. Available: https://covid19.who.int [Accessed 5 December 2022].
- 13.Cascella M, Rajnik M, Aleem A. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2022. http://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 14.CDC . People with certain medical conditions. Centers for Disease Control and Prevention, 2022. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]


