Abstract
Introduction
Identification of advanced hepatic fibrosis in non-alcoholic fatty liver disease (NAFLD) is important as this may progress to cirrhosis and hepatocellular carcinoma. The risk of hepatic fibrosis is especially high among patients with diabetes with NAFLD. Annual screening of patients with diabetes for fatty liver and calculation of Fibrosis-4 (FIB-4) score and exclusion of significant fibrosis with vibration-controlled transient elastography (VCTE) have been recommended. However, VCTE is expensive and may not be freely available in resource-limited settings. We aim to identify predictors of significant liver fibrosis who are at increased risk of progression to advanced liver fibrosis and to develop a prediction model to prioritise referral of patients with diabetes and NAFLD for VCTE.
Methods and analysis
This cross-sectional study is conducted among all consenting adults with type 2 diabetes mellitus with NAFLD at the Colombo North Teaching Hospital, Ragama, Sri Lanka. All patients get the FIB-4 score calculated. Those with FIB-4 ≥1.3 undergo VCTE (with FibroScan by Echosens). Risk associations for progression to advanced liver fibrosis/cirrhosis will be identified by comparing patients with significant fibrosis (liver stiffness measure (LSM) ≥8 kPa) and without significant fibrosis (LSM <8 kPa). A model to predict significant liver fibrosis will be developed using logistic regression.
Ethics and dissemination
Ethical approval has been obtained from the Ethics Committee of the Faculty of Medicine, University of Kelaniya (P/66/07/2021). Results of the study will be disseminated as scientific publications in reputable journals.
Keywords: General diabetes, General endocrinology, Hepatology, Gastroenterology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Individual data of patients are used. Advanced fibrosis is confirmed with vibration-controlled transient elastography (with FibroScan by Echosens).
Risk factors for advanced fibrosis out of over 100 variables will be selected.
All possible risk factors will be studied without prejudice and the risk factors with the highest predictive values will be used in the prognostic model.
This is limited to a Sri Lankan cohort and therefore is not generalisable to all other nations.
This cohort is of a community of very low seroprevalence of hepatitis B and C. However, we have not studied hepatitis B and C serology in individual patients.
Introduction
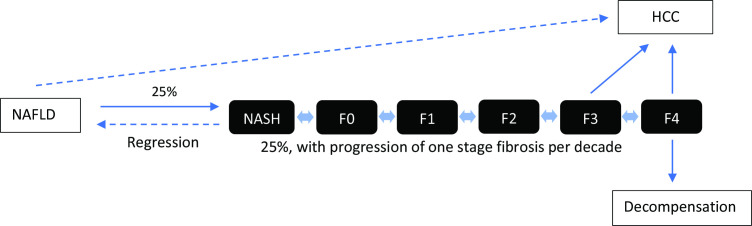
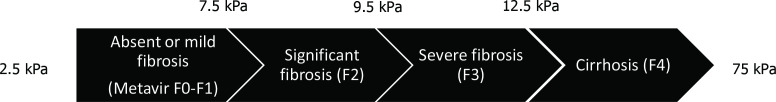
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in the world with a global prevalence of 25%, with the highest rates in the Middle East and South America and the lowest in Africa.1–3 NAFLD is a spectrum ranging from simple steatosis, non-alcoholic steatohepatitis (NASH) to cirrhosis (figure 1).4 NASH is strongly associated with liver fibrosis and is defined histologically on a scale ranging from F0 to F4. Stages F0–F1 have no or minimal fibrosis, F2 is significant fibrosis with risk of progression to advanced fibrosis, F3 is advanced fibrosis and F4 cirrhosis (figure 2).5 Fibrosis of the liver can be measured non-invasively with vibration-controlled transient elastography (VCTE) with FibroScan by Echosens, which measures liver stiffness. A liver stiffness measure (LSM) between 2.5 and 7 kPa is suggestive of absent or mild fibrosis, ≥7.1 kPa significant fibrosis (≥F2), ≥9.5 kPa advanced fibrosis (≥F3) and ≥12.5 kPa cirrhosis (F4).5 6
Figure 1.
Natural history of non-alcoholic fatty liver disease (NAFLD). HCC, hepatocellular carcinoma; NASH, non-alcoholic steatohepatitis.
Figure 2.
Liver stiffness cut-offs in chronic liver diseases.
NAFLD is a dynamic condition that can regress to simple steatosis with liver-directed therapy, smoulder at a relatively constant level of activity, or cause progressive fibrosis and lead to cirrhosis. However, the majority of patients with NAFLD have a benign course and only 10% develop progressive fibrosis leading to cirrhosis and hepatocellular carcinoma (HCC).7 8 There is no widely accepted pharmacological treatment for established cirrhosis and the only curative treatment is liver transplantation. However, progressive NASH and its complications such as cirrhosis could be delayed or prevented if people with increased risk of progressive fibrosis are detected early in the course of the disease (at F2–3 stage or LSM ≤8 kPa) and liver-directed therapies are initiated. This is the key to reducing the liver-related burden of NAFLD in both high-income and low-income countries.9
NAFLD is the hepatic manifestation of insulin resistance, which is the hallmark of type 2 diabetes mellitus (T2DM).10 It is three times more prevalent among patients with diabetes than in the general population.11 Globally, 55.5% of patients with T2DM and 62% of Asians with T2DM have NAFLD.12 13 On the other hand, NAFLD is considered a risk factor for T2DM,14 15 and patients with NAFLD have a twofold increased risk of developing T2DM compared with those without NAFLD.16 Diabetes is independently associated with the degree of steatosis, NAFLD progression to NASH, advanced fibrosis and the development of HCC.17–20 The progression of NAFLD to advanced fibrosis is two times higher among patients with diabetes than in the general population. Around 20% of patients with T2DM with NAFLD progress to advanced liver fibrosis over a mean of 5.9 years.9 21 Therefore, both the American Diabetes Association (2020)22 and the European Association for the Study of the Liver (2016)23 recommend screening of all patients with T2DM for NAFLD to prevent liver-related complications of NAFLD. There are two proposed algorithms for screening patients with diabetes for NAFLD, and both propose annual screening of patients with diabetes with NAFLD but using two different screening tools: one using the Fibrosis-4 score (FIB-4)24 25 and the other using the NAFLD fibrosis score (NFS).26 27 The proposed algorithm involves two steps. The first is an annual screening of patients with diabetes with NAFLD and the second step is LSM using transient elastography26 for those with FIB-4 ≥1.325 or NFS between −1.455 and 0.676.26 The patients at high risk of advanced fibrosis/cirrhosis with an LSM ≥8 kPa are advised referral to specialised liver centres for further assessment and management.25 28–31
Diabetes has become an epidemic in low/middle-income countries including those in Asia. Of the world’s population with diabetes, 60% are Asians.32 Diabetes is on the rise among Asians due to urbanisation, changing to Western lifestyles and dietary habits in addition to a relatively high genetic predisposition.33 34 Asians are at higher risk of developing T2DM compared with people of European ancestry with genetic predisposition through PNPLA3 SNPs and polymorphisms in apolipoprotein.32 However, VCTE is expensive and is not freely available for all patients with diabetes in low/middle-income countries.
Therefore, we aimed to identify clinical predictors of significant liver fibrosis with risk of progression to advanced liver fibrosis (ie, LSM ≥8 kPa)29) in patients with type 2 diabetes who are at risk of progression to advanced liver fibrosis and cirrhosis, and develop a model to identify patients at high risk of progression to advanced fibrosis. This we hope will help to guide clinicians in primary and secondary care in resource-poor settings to prioritise referrals for VCTE and to specialised liver centres.
Methods and analysis
Study design and setting
This study is an ongoing cross-sectional study to identify patients with diabetes with NAFLD who are at risk of progression to advanced liver fibrosis and/or cirrhosis. We compare the risk associations of patients with advanced liver fibrosis diagnosed by an LSM ≥8 kPa by transient elastography and patients with no or minimal liver fibrosis diagnosed by FIB-4 score <1.3. The study is being conducted at the Colombo North Teaching Hospital, Ragama, Sri Lanka.
Study objectives
To identify patients with diabetes with NAFLD who are at increased risk of progression to advanced liver fibrosis/cirrhosis and to develop a prediction model for the same. We further plan to validate the FIB-4 score among Sri Lankans.
Study population and eligibility criteria
All adult patients with T2DM attending medical/diabetes clinics of three consultants at three private sector hospitals of the Gampaha District of Sri Lanka over 12 consecutive months who have ultrasonographic evidence of NAFLD will be the study population.
Inclusion criteria
Patients with confirmed T2DM.
Patients who are aged over 18 years.
Patients who have ultrasonic (US) evidence of fatty liver in a US scan done within the previous 3 months of recruitment.
Exclusion criteria
Patients without consent.
Patients with established cirrhosis on US scan.
Males consuming alcohol >14 units/week and females consuming alcohol >7 units/week.
Evidence of hepatitis B or C infection.
Patients with diagnosed liver diseases of known aetiology other than NAFLD.
Patients on medications known to cause fatty liver or liver fibrosis, for example, tamoxifen, methotrexate, etc.
Sample size
The sample size was calculated for developing a clinical prediction model35 36 assuming an R2 value of 0.2 for the logistic model, 10 parameters in the model, and 0.05 acceptable difference in apparent and adjusted R2, 0.05 margin of error in the estimation of intercept and an anticipated prevalence of advanced liver fibrosis of 15% among T2DM with NAFLD.37
The calculated minimum sample size is 398 patients with T2DM with NAFLD (among whom 60 are expected to have significant fibrosis, that is, LSM ≥8 kPa). This is likely to provide six events per predictor parameter.
Patient recruitment
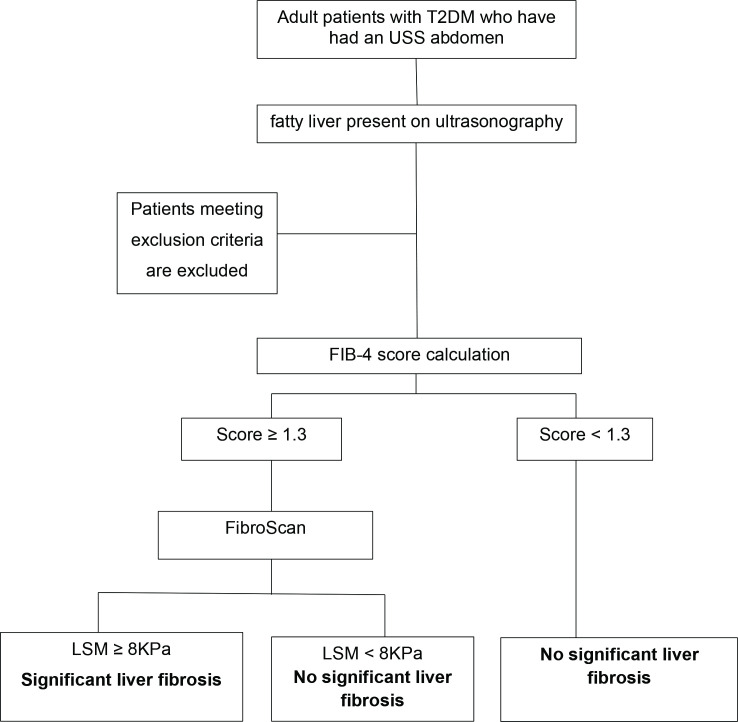
Consecutive adult patients (>18 years) with T2DM attending medical/diabetes clinics of three consultants at three private sector hospitals of the Gampaha District of Sri Lanka over 12 consecutive months starting from November 2021 are being screened, and the eligible patients are recruited to this study after being referred to the gastroenterology and hepatology clinic of the Colombo North Teaching Hospital, Ragama, until the sample size is achieved (figure 3). All eligible patients are given a patient information sheet to read and time to clarify doubts with investigators before consenting. The participants will be informed about the study including the data collection procedure and that a subgroup of participants will undergo a non-invasive VCTE of the liver free of charge. The patients will be made aware of the ability to withdraw consent at any point without having to give reasons. Informed written consent from all participants will be obtained before recruiting into the study. Permission has been obtained from the director of the North Colombo Teaching Hospital and the consultant in charge of the diabetic and endocrine clinics to carry out the study.
Figure 3.
Study design and participant flow through the study FIB-4 score. FIB-4, Fibrosis-4; LSM, liver stiffness measure; T2DM, type 2 diabetes mellitus; USS, ultra sound scan.
Study procedure
Patients with diabetes mellitus and complying with inclusion and exclusion criteria will be interviewed and medical records will be assessed. Data will be collected using an interviewer-administered questionnaire. Information on demography, history of metabolic risk factors, medications, diet and exercise will be recorded. Haematological and biochemical investigations done within 3 months before recruitment will be extracted from clinic records. Anthropometry: height, weight and waist circumference will be measured at recruitment. FIB-4 score will be calculated for all patients using age, sex, and the most recent AST, ALT and platelet count done within 3 months of recruitment to the study. All patients with a FIB-4 score ≥1.3 will undergo VCTE of the liver free of charge by a single, trained medical officer. LSM and Controlled Attenuation Parameter (CAP) measures will be recorded. A subset of patients with a FIB-4 score <1.3 will also undergo VCTE to confirm the validity of the FIB-4 score to exclude significant liver fibrosis.
Statistical analysis
IBM SPSS V.22.0 software will be used for data management and analyses. Multiple logistic regression will be used to identify factors associated with significant and beyond liver fibrosis. A model will be developed using the identified risk factors to predict ‘significant liver fibrosis’ according to their weighted scores (β-coefficient). Cut-off points and the sensitivity and specificity of predictions will be determined using receiver operating characteristic curves. The predictions of the new model will be compared with the predictions of the simple FIB-4 score.
Data management and monitoring
All completed questionnaires and VCTE reports will be stored securely. An electronic screening log and database will be maintained as a password-protected file.
Ethical considerations
This is not an interventional study and is associated with no risks to the patients. Selected patients will undergo non-invasive VCTE of the liver free of charge at the North Colombo Teaching Hospital, which is the only state hospital with a fibro scanner in Sri Lanka. There are no risks associated with this scan. The results of the scan will only be divulged to the treating physician of the patients for initiation of relevant treatment options. The findings of the study could be beneficial to all patients with diabetes in the early diagnosis/prediction of significant liver fibrosis in individuals. Participants will have the right to withdraw from the study at any point without providing explanations. Ethical approval for the study has been obtained from the Ethics Committee of the Faculty of Medicine, University of Kelaniya, Sri Lanka (ref. P/66/07/2021).
Termination of the study
The study will be terminated if:
A new and cost-effective tool to predict significant liver fibrosis that changes current guidelines becomes available.
Significant violation of good clinical practice that compromises the ability to achieve study objectives or compromises subject safety occurs.
Study status
The trial commenced in November 2021 according to the protocol version 2.0, 06 August 2021, and is currently open for recruitment. We have recruited 220 patients for the study so far.
Patient and public involvement
Reports of the VCTE (LSM) done as part of the trial will be available to all participants who request it and will be used in the standard management when required. All patients are given a health education leaflet on fatty liver and secondary prevention. Results of the VCTE are notified to the treating physician for necessary action. The results of the study will be disseminated to study participants and other patients with diabetes and fatty liver using patient education leaflets and lectures after completion of the study.
Discussion
In this study, we aim to develop a practical, cost-effective model to predict patients with diabetes with NAFLD who are at increased risk of progressing to advanced liver fibrosis and/or cirrhosis to target those who require transient elastography. Furthermore, even though there are non-invasive markers of liver fibrosis such as FIB-4,24 BRAD38 and NFS,27 none of these has been developed specifically for patients with diabetes and has been validated in Asians except among Japanese.39
Apart from a few studies from North India40 and Vietnam,41 there are no reports of VCTE data in patients with diabetes from Asia. There are no data on VCTE from Sri Lanka. Furthermore, the FIB-4 score has not been validated among Sri Lankans. Through our study, we hope to provide a low-cost, practical model to identify the patients with type 2 diabetes and NAFLD who are at increased risk of progression to advanced fibrosis/cirrhosis.
Limitations
Liver biopsy is the gold standard for staging liver fibrosis but in our study, VCTE will be used to diagnose significant liver fibrosis.42 However, liver biopsy is an invasive procedure and current practice guidelines recommend VCTE as a surrogate to exclude advanced fibrosis, with liver biopsy reserved for those with equivocal VCTE results.25 26
Supplementary Material
Acknowledgments
Jayani Manchanayake and Dileepa Ediriweera for helping in developing the protocol.
Footnotes
Twitter: @pathmes
Contributors: CM conceived the study. CM, TE, CD, RF, NL, LR, CR, DK, AP HJdS and ASD contributed to the study design. CM drafted the manuscript, while ASD and HJdS significantly edited the manuscript. All authors assisted in developing the protocol and have read, reviewed, edited and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 3.Amarapurkar DN, Hashimoto E, Lesmana LA, et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol 2007;22:788–93. 10.1111/j.1440-1746.2007.05042.x [DOI] [PubMed] [Google Scholar]
- 4.Diehl AM, Day C, Cause DC. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med Overseas Ed 2017;377:2063–72. 10.1056/NEJMra1503519 [DOI] [PubMed] [Google Scholar]
- 5.Castera L, Forns X, Alberti A. Non-Invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835–47. 10.1016/j.jhep.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 6.Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, apri, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343–50. 10.1053/j.gastro.2004.11.018 [DOI] [PubMed] [Google Scholar]
- 7.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467–74. 10.1111/j.1572-0241.1999.01377.x [DOI] [PubMed] [Google Scholar]
- 8.Diehl AM, Day C, Cause DC. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063–72. 10.1056/NEJMra1503519 [DOI] [PubMed] [Google Scholar]
- 9.Eslam M, Sanyal AJ, George J, et al. MAFLD: a Consensus-Driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999–2014. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 10.Alkhouri N, Poordad F, Lawitz E. Management of nonalcoholic fatty liver disease: lessons learned from type 2 diabetes. Hepatol Commun 2018;2:778–85. 10.1002/hep4.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diab Rep 2015;15:607. 10.1007/s11892-015-0607-4 [DOI] [PubMed] [Google Scholar]
- 12.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793–801. 10.1016/j.jhep.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 13.Herath HMM, Kodikara I, Weerarathna TP, et al. Prevalence and associations of non-alcoholic fatty liver disease (NAFLD) in Sri Lankan patients with type 2 diabetes: A single center study. Diabetes Metab Syndr 2019;13:246–50. 10.1016/j.dsx.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Adams LA, Waters OR, Knuiman MW, et al. Nafld as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 2009;104:861–7. 10.1038/ajg.2009.67 [DOI] [PubMed] [Google Scholar]
- 15.Shibata M, Kihara Y, Taguchi M, et al. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 2007;30:2940–4. 10.2337/dc07-0792 [DOI] [PubMed] [Google Scholar]
- 16.Kasturiratne A, Weerasinghe S, Dassanayake AS, et al. Influence of non-alcoholic fatty liver disease on the development of diabetes mellitus. J Gastroenterol Hepatol 2013;28:142–7. 10.1111/j.1440-1746.2012.07264.x [DOI] [PubMed] [Google Scholar]
- 17.Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1224–9. 10.1016/j.cgh.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 18.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 19.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56:943–51. 10.1002/hep.25772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 2008;48:792–8. 10.1002/hep.22429 [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–54. 10.1016/j.cgh.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Association AD . 4. comprehensive medical evaluation and assessment of comorbidities: standards of medical care in Diabetes—2020. Diabetes Care 2019;43:S37–47. 10.2337/dc20-S004 [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 24.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–12. 10.1016/j.cgh.2009.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira Barbosa J, Lai M. Nonalcoholic fatty liver disease screening in type 2 diabetes mellitus patients in the primary care setting. Hepatol Commun 2021;5:158–67. 10.1002/hep4.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol 2013;10:666–75. 10.1038/nrgastro.2013.175 [DOI] [PubMed] [Google Scholar]
- 27.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 28.Petroni ML, Brodosi L, Bugianesi E, et al. Management of non-alcoholic fatty liver disease. BMJ 2021;72:m4747. 10.1136/bmj.m4747 [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Huang C, Xu W, et al. Accuracy of FibroScan in analysis of liver fibrosis in patients with concomitant chronic hepatitis B and nonalcoholic fatty liver disease. Medicine 2020;99:e20616. 10.1097/MD.0000000000020616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cusi K, Isaacs S, Barb D, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: Co-Sponsored by the American association for the study of liver diseases (AASLD). Endocr Pract 2022;28:528–62. 10.1016/j.eprac.2022.03.010 [DOI] [PubMed] [Google Scholar]
- 31.Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006–19. 10.1136/gutjnl-2021-324243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan JCN, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–40. 10.1001/jama.2009.726 [DOI] [PubMed] [Google Scholar]
- 33.Dassanayake AS. Nonalcoholic fatty liver disease: identifying the disease burden in Sri Lanka. Euroasian J Hepatogastroenterol 2018;8:69–72. 10.5005/jp-journals-10018-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niriella MA, Pathmeswaran A, De Silva ST, et al. Incidence and risk factors for non-alcoholic fatty liver disease: a 7-year follow-up study among urban, adult Sri Lankans. Liver Int 2017;37:1715–22. 10.1111/liv.13478 [DOI] [PubMed] [Google Scholar]
- 35.Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ 2020;368:m441. 10.1136/bmj.m441 [DOI] [PubMed] [Google Scholar]
- 36. Joie Ensor [aut c, Emma C, Martin [aut]. pmsampsize: Calculates the minimum sample size required for developing a multivariable prediction model, 2022. Available: https://cran.r-project.org/web/packages/pmsampsize/index.html
- 37.Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021;44:399–406. 10.2337/dc20-1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441–7. 10.1136/gut.2007.146019 [DOI] [PubMed] [Google Scholar]
- 39.Sumida Y, Yoneda M, Hyogo H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol 2012;12:2. 10.1186/1471-230X-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta A, Anoop S, Ansari IA, et al. High prevalence of hepatic steatosis and hepatic fibrosis in patients with type 2 diabetes mellitus. Clin Nutr ESPEN 2021;46:519–26. 10.1016/j.clnesp.2021.08.028 [DOI] [PubMed] [Google Scholar]
- 41.Tuong TTK, Tran DK, Phu PQT, et al. Non-Alcoholic fatty liver disease in patients with type 2 diabetes: evaluation of hepatic fibrosis and steatosis using Fibroscan. Diagnostics 2020;10:159. 10.3390/diagnostics10030159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong VW-S, Chan W-K, Chitturi S, et al. Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018;33:70–85. 10.1111/jgh.13857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.