Summary
Tertiary lymphoid structures (TLSs) are ectopic lymphoid aggregates that can develop within or adjacent to tumors, but protocols that can accurately identify and characterize TLSs are lacking. Here, we present a protocol for the in situ interrogation and characterization of TLSs in human and murine tissue sections using Opal™-tyramide signal amplification multiplex immunohistochemistry. This protocol enables simultaneous detection of up to 7 markers (6 antigens and a DAPI counterstain). We also describe a grading system to identify immature and mature TLSs.
Subject areas: Cancer, Immunology, Microscopy
Graphical abstract
Highlights
-
•
In situ characterization of tertiary lymphoid structures in human and murine tissue
-
•
Opal™-TSA multiplex immunohistochemistry-based approach
-
•
Grading system for immature and mature tertiary lymphoid structures
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Tertiary lymphoid structures (TLSs) are ectopic lymphoid aggregates that can develop within or adjacent to tumors, but protocols that can accurately identify and characterize TLSs are lacking. Here, we present a protocol for the in situ interrogation and characterization of TLSs in human and murine tissue sections using Opal™-tyramide signal amplification multiplex immunohistochemistry. This protocol enables simultaneous detection of up to 7 markers (6 antigens and a DAPI counterstain). We also describe a grading system to identify immature and mature TLSs.
Before you begin
Opal™-TSA 7-marker panel (CD4, CD8, CD19, CD21, DC-LAMP, PNAd and DAPI) for human or 6-marker panel (CD4, CD8, CD19, CD21, PNAd and DAPI) for murine fixed tissue sections
The protocol below enables the in-situ interrogation of TLSs using an Opal™-TSA 7-marker panel (CD4, CD8, CD19, CD21, DC-LAMP, PNAd and DAPI) for human formalin-fixed paraffin-embedded (FFPE) tissue sections or an Opal™-TSA 6-marker panel (CD4, CD8, CD19, CD21, PNAd and DAPI) panel for murine tissue sections fixed in formalin-free zinc salt fixative (zinc fixation is used in the murine protocol to avoid epitope loss and accommodate for the anti-CD21 antibody which does not perform well in FFPE murine tissue in a multiplex setting). Individual antibodies, their Opal™ fluorophore pairing, and their sequence within this panel have been manually optimized using human lymph node or murine gastric submucosal tissue sections, using manufacturer recommendations.
The following considerations are crucial for the successful optimization of Opal™-TSA multiplex immunohistochemistry (mIHC)-based panels:
-
1.
Pairing with Opal™ fluorophores:
Opal™ brightness ranking from brightest to dimmest ranges from Opal™ 620 (high), Opal™ 520 (high) to Opal™ 570 (medium), Opal™ 540 (medium), Opal™ 650 (medium) and Opal™ 690 (low). When investigating co-expression of markers on specific cell types, ensure that paired Opal™ fluorophores are not spectrally adjacent (e.g., CD3-Opal™ 540 should not be followed by CD8-Opal™ 570). Low abundance markers should be paired with brighter fluorophores (i.e., Opal™ 620 or Opal™ 520), while high abundance markers should be paired with dimmer fluorophores (i.e., Opal™ 690).
-
2.
Primary antibody optimization:
Individual primary antibody optimization requires determination of the antigen retrieval buffer, titration of the antibody concentration, and titration of the Opal™ fluorophore. These determinants are best achieved using positive control tissue for each marker of interest (e.g., anti-human CD8 antibody on healthy human tonsil or lymph node tissues). All comparisons made during individual primary antibody optimization (i.e., heat-induced epitope retrieval (HIER) method, heat stress testing, antibody concentration, and Opal™ fluorophore dilution) should be done so using serial sections cut from the same tissue block. For low abundance markers of interest, it is advised to use brighter fluorophores (e.g., Opal™ 520 or Opal™ 620) for optimization.
Optimal HIER and primary antibody concentration can be determined simultaneously by performing a titration assay. Starting from the vendor recommended antibody concentration, proceed with a 1:2 and 1:4 dilution factor of the antibody across both antigen retrieval buffers (AR6 and AR9) using a total of 6 serial positive control tissue sections. Tweaking of fluorescent intensities following primary antibody concentration optimization can be achieved by increasing or decreasing the concentration of paired Opal™ fluorophores (Opal™ 690 is recommended at a dilution of 1:50 due to its dim properties).
-
3.
mHC panel staining order:
Once optimal conditions have been determined for the primary antibody of interest, a heat-stress test should be performed to determine the best position of the marker in the multiplex panel.1 The exposure of specific epitopes may be altered following subsequent rounds of HIER. Successive rounds of HIER can either improve the exposure of an epitope and therefore would fare better later in the mIHC panel or degrade an epitope and hence would fare better earlier on. Similarly, the intensity of some Opal fluorophores (i.e., Opal™ 520 and Opal™ 570) can be attenuated by multiple rounds of HIER. This test stains 6 serial positive control tissue sections after 1, 2, 3, 4 ,5 or 6 rounds of HIER using the optimized antibody conditions. Variability in staining intensity using fixed exposure times between slides is an indicator of epitope exposure or degradation across different HIER rounds. The number of HIER rounds that produces the most intense and specific staining will inform the position and order of the primary antibody in the mIHC panel. Similarly, determining the optimal time of HIER prior to adding each primary antibody is also critical in enhancing the result of any mIHC stain.
Finally, it is best to arrange the order of antibodies such that sequential antibodies do not co-localize in the same cellular compartments within the same cells. This will prevent issues that may arise due to incomplete stripping between HIER rounds, which make it hard to discern between co-expressed markers.
Institutional permissions
All experiments using specimens derived from animals were performed in compliance with protocols approved by the Austin Health Animal Ethics Committee (A2018/05583), and those derived from humans by the Austin Health Human Research Ethics Committee (HREC/14/Austin/425 and HREC H2008/03230), and the La Trobe University Human Ethics Committee (HEC19147).
Ethical approval from relevant Institutional regulatory human or animal committees is mandatory before conducting experiments using specimens derived from humans or animals, and all experiments must conform to the relevant regulatory standards.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-human CD4 [EPR6855] antibody | Abcam | Cat#ab133616 (1:1000 dilution) |
| Rat monoclonal anti-mouse CD4 [4SM95] antibody | Invitrogen | Cat#14-9766-82 (1:1000 dilution) |
| Mouse monoclonal anti-human CD8 alpha [C8/144B] antibody | Invitrogen | Cat#MA5-13473 (1:50 dilution) |
| Rat monoclonal anti-mouse CD8 [4SM15] antibody | Invitrogen | Cat#14-0808-82 (1:2000 dilution) |
| Rabbit monoclonal anti-human CD19 [EPR5906] antibody | Abcam | Cat#ab134114 (1:300 dilution) |
| Rabbit polyclonal anti-mouse CD19 antibody | Cell Signaling Technology | Cat#3574 (1:200 dilution) |
| Mouse monoclonal anti-human CD21 [1F8] antibody | Agilent Technologies | Cat#M0784 (1:10 dilution) |
| Rabbit monoclonal anti-mouse CD21 [EP3093] antibody | Abcam | Cat#75985 (1:1500 dilution) |
| Rabbit polyclonal anti-human CD208/DC-LAMP antibody | Abcam | Cat#ab111090 (1:100 dilution) |
| Rat monoclonal anti-human PNAd [MECA-79] antibody | Novus Biologicals | Cat#NB100-77673 (1:100 dilution) |
| Rat monoclonal anti-mouse PNAd [MECA-79] antibody | BD Pharmingen | Cat#553863 (1:5000 dilution) |
| Mouse monoclonal anti-human MHC Class I [HC-10] antibody | ONJCRI | N/A (1:3000 dilution) |
| ImmPRESS® HRP goat anti-rat IgG mouse adsorbed Polymer Detection Kit, Peroxidase | Vector Laboratories | Cat#VEMP744415 |
| ImmPRESS® HRP horse anti-rabbit IgG Polymer Detection Kit, Peroxidase | Vector Laboratories | Cat#VEMP740150 |
| Biological samples | ||
| Healthy human lymph node FFPE blocks, male and female (unknown ages) | N/A | N/A |
| Human prostate cancer FFPE blocks, male (48–78 years old) | N/A | N/A |
| Human melanoma FFPE blocks, male and female (19–92 years old) | N/A | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| 1× Phosphate-buffered saline (PBS), no calcium, no magnesium | Gibco | Cat#14190144 |
| Calcium acetate monohydrate | Sigma-Aldrich | Cat#402850 |
| Zinc acetate dihydrate | Sigma-Aldrich | Cat#Z0625 |
| Zinc chloride | Sigma-Aldrich | Cat#208086 |
| Neutral buffered formalin (NBF) 10% Green | Australian Biostain | Cat#029-ANBFG.2.5L |
| Ethanol Absolute 100% | POCD Scientific | Cat#ETHABS100/5P |
| Xylene (Sulfur free) | POCD Scientific | Cat#XYL2.5P |
| TRIS - Ultra Pure Grade | Astral Scientific | Cat#BIO3094T |
| Sodium chloride | Sigma-Aldrich | Cat#793566 |
| TWEEN® 20 | Sigma-Aldrich | Cat#P1379 |
| Hydrogen peroxide (H2O2) 30% w/w | ChemSupply | Cat#HA154-500M |
| PeroxAbolish | Biocare Medical | Cat#BIC-PXA969L |
| Glycine | Astral Scientific | Cat#BIOGB0235 |
| Ethylenediaminetetraacetic acid (EDTA) | Sigma-Aldrich | Cat#798681 |
| Deionized H2O (dH2O) | N/A | N/A |
| VECTASHIELD Vibrance™ | Abacus dx | Cat#VEH170010 |
| ProLong™ Diamond Antifade Mountant | Invitrogen | Cat#P36970 |
| Critical commercial assays | ||
| Opal™ 7-Color manual IHC Kit∗ | Akoya Biosciences | Cat#NEL811001KT |
| Experimental models: Organisms/strains | ||
| GP130F/F C57BL6 mice, male and female (14–16 weeks old) | ONJCRI | N/A |
| Software and algorithms | ||
| Phenochart Whole Slide Viewer | Akoya Biosciences | https://www.akoyabio.com/support/software/phenochart-whole-slide-viewer/ |
| inForm® Tissue Analysis Software | Akoya Biosciences | https://www.akoyabio.com/phenoimager/software/inform-tissue-finder/ |
| HALO® Image Analysis Platform | Indica Labs | https://indicalab.com/halo/ |
| Other | ||
| Surgipath Paraplast | Leica Biosystems | Cat#39601006 |
| Tissue embedding cassettes with lid | Techno Plas | Cat#CASLID-03 |
| Wax heater pot | N/A | N/A |
| Tissue embedding center | N/A | N/A |
| Thermal console | N/A | N/A |
| Cryo console | N/A | N/A |
| Rotary microtome | N/A | N/A |
| Blade Microtome Patho Cutter R 35° Pink 80 mm | Erma | Cat#08-636-0 |
| SuperFrost Plus™ Adhesion slides | Thermo Scientific | Cat#11950657 |
| Cover glasses (22 × 50 mm) | Marienfeld | Cat#0101142 |
| Tissue-Tek® slide staining set (includes vertical slide rack and staining dishes) | ProSciTech | Cat#H4451 |
| StainTray™ (Black Lid) | Thermo Scientific | Cat#44-0404-10 |
| ImmEdge Hydrophobic Barrier PAP Pen | Vector Laboratories | Cat#H-4000 |
| Mini PAP Pen | Life Technologies | Cat#008877 |
| Magnetic stirrer | N/A | N/A |
| pH meter | N/A | N/A |
| Orbital shaker (with heating) | N/A | N/A |
| Standard microwave oven with carousel, rated at 1,000 W or higher with 10 or more power settings | N/A | N/A |
| Vectra® 3 Automated Quantitative Pathology Imaging System | Akoya Biosciences | N/A |
| BOND RX® Automated Research Stainer | Leica Biosystems | N/A |
∗Includes 10× antigen retrieval (AR) AR6 buffer, 10× AR9 buffer, Blocking/Antibody diluent, Opal™ Polymer HRP Mouse and Rabbit (Ms + Rb), 1× Plus Amplification diluent, Opal™ 520, 540, 570, 620, 650 and 690 fluorophores, and spectral DAPI.
Materials and equipment
Zinc fixative
| Reagent | Final concentration | Amount |
|---|---|---|
| TRIS base | 99.9 mM | 12.1 g |
| Calcium acetate | 2.84 mM | 0.5 g |
| Zinc acetate | 22.8 mM | 5.0 g |
| Zinc chloride | 36.7 mM | 5.0 g |
| ddH2O | N/A | 1.0 L |
| Total | N/A | 1.0 L |
Dissolve 12.1 g of Tris base in 1 L of dH2O using a magnetic stirrer and adjust the pH to 7.4. Add 0.5 g of calcium acetate, 5 g of zinc acetate and 5 g of zinc chloride. The final pH will be approximately 6.5–7.0, do not readjust pH as this may cause the zinc to precipitate. Store at room temperature (RT, 15°C–30°C), for a maximum of 24 months.
10× TBST
| Reagent | Final concentration | Amount |
|---|---|---|
| TRIS base | 0.2 M | 24.3 g |
| Sodium chloride | 1.5 M | 87.7 g |
| ddH2O | N/A | 1.0 L |
| Total | 10× | 1.0 L |
Dissolve 24.3 g of Tris base with 87.7 g of Sodium chloride in 1 L of dH2O using a magnetic stirrer. Store at RT (15°C–25°C), for a maximum of 24 months.
Tris-EDTA buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| TRIS base | 99.9 mM | 12.1 g |
| EDTA | 12.7 mM | 3.7 g |
| ddH2O | N/A | 1.0 L |
| Total | N/A | 1.0 L |
Dissolve 12.1 g of Tris and 3.7 g of EDTA in 1 L of dH2O using a magnetic stirrer and adjust the pH to 9.0. Store at RT (15°C–25°C), for a maximum of 3 months.
-
•
70% Ethanol: dilute 100% Ethanol at 1:1.4 with dH2O. Store at RT (15°C–25°C), for a maximum of 24 months.
-
•
90% Ethanol: dilute 100% Ethanol at 1:1.1 with dH2O. Store at RT (15°C–25°C), for a maximum of 24 months.
In a fume hood, pre-fill 9 glass jars with 70% Ethanol, 90% Ethanol, 100% Ethanol, 100% Ethanol, 100% Ethanol, 100% Ethanol, Xylene, Xylene and Xylene in preparation for sequential immersion (dehydration and clearing).
-
•
3% H2O2: dilute 30% H2O2 at 1:10 with dH2O. Store at 2°C–8°C, for a maximum of 3 months.
-
•
1× TBST: dilute 500 mL of 10× TBS in 4.5 L of ddH2O and adjust to a pH of 7.4–7.6. Add 2.5 mL of TWEEN 20. Store at RT (15°C–25°C), for a maximum of 3 months.
-
•
1× AR6 buffer: dilute 10× AR6 buffer at 1:10 with dH2O. Store at 2°C–8°C, for a maximum of 3 months.
-
•
1× AR9 buffer: dilute 10× AR9 buffer at 1:10 with dH2O. Store at 2°C–8°C, for a maximum of 3 months.
-
•
Low pH glycine-based buffer: dissolve 3.75 g of glycine in 1 L of dH2O using a magnetic stirrer and adjust the pH to 2.2. Store at RT (15°C–25°C), for a maximum of 3 months.
-
•
Heated elution buffer preparation: pre-heat the low pH glycine-based buffer in a light-protective jar covered with a lid in a 50°C in an orbital shaker at least an hour before elution. Use a thermometer to ensure the buffer reaches a temperature of 50°C. This method of antibody stripping has been shown to effectively remove antibody complexes from tissue sections.2
-
•
Opal™ fluorophores: reconstitute in 75 μL of DMSO. Store at 2°C–8°C, for a maximum of 3 months.
-
•
Opal™ fluorophore working solution: dilute Opal™ fluorophore stock solution at 1:100 in 1× Plus Amplification diluent. Freshly prepare this solution just before use, keep on ice and protect from light.
-
•
DAPI working solution: dilute 2 drops of DAPI in 1 mL of 1× TBST. Freshly prepare this solution just before use.
-
•
Spectral library: A spectral library requires monoplex staining of positive control tissue sections with a marker that is abundant and uniformly expressed. This will enable accurate spectral unmixing during image analysis and should be performed each time a new Opal™ kit or new Opal™ fluorophores are used. For this protocol, we stained a HeLa cell block with anti-MHC Class I antibodies or murine spleen tissue sections with anti-CD8 antibodies paired with each Opal™ fluorophore.
-
•
Autofluorescence control: An unstained study tissue slide that has undergone every staining step other than incubation with Opal™ fluorophores and DAPI should be used be included to subtract background autofluorescence.
Step-by-step method details
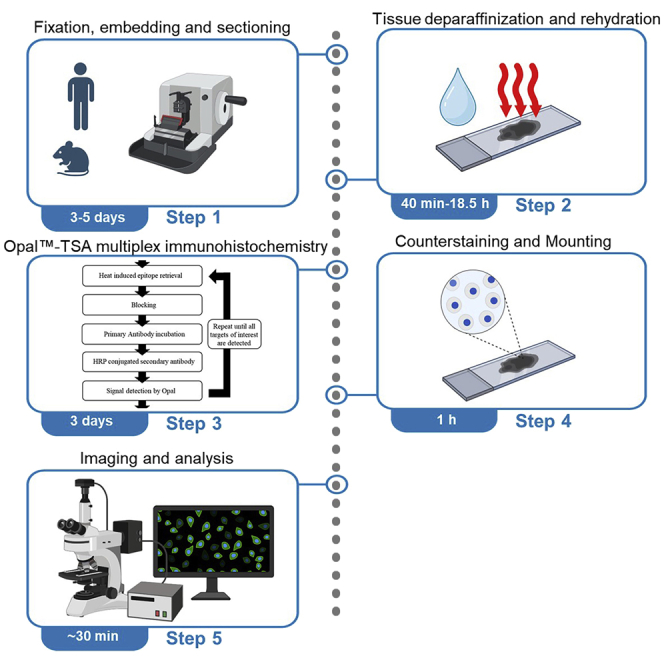
Fixation, embedding and sectioning of human or murine tissue
Timing: 3–5 days
This step fixes human tissue specimens in formalin or murine tissue in formalin-free zinc salt fixative, dehydrates, clears, and embeds it in paraffin to preserve tissue structures and enable subsequent sectioning and long-term storage.
-
1.
Obtain fresh human or murine tissue specimens (≤20 × 20 mm) in accordance with the institution’s human or animal ethical approval and store the specimen in a container with 1× PBS on ice prior to fixation.
-
2.
Transfer the specimen into an embedding cassette labeled with a lead/graphite pencil using a sterile pipette tip.
-
3.
Fix human tissue by submersion in a container with 10% NBF solution for 1–16 h in a fume hood or fix murine tissue in a container with zinc-salt fixative overnight (16–18 h) in a fume hood.
Note: A P2 mask should be worn when working with NBF.
Note: Human tissue specimens can be left in 10% NBF overnight (16–18 h).
Note: Zinc fixation is used for murine tissue to avoid epitope masking and accommodate for the anti-CD21 antibody which does not perform well in FFPE murine tissue in a multiplex setting.
-
4.Dehydrate tissue using gradual ethanol immersions in pre-filled glass jars in a fume hood.
-
a.70% Ethanol for 15 min.
-
b.90% Ethanol for 15 min.
-
c.100% Ethanol for 15 min.
-
d.100% Ethanol for 30 min.
-
e.100% Ethanol for 30 min.
-
f.100% Ethanol for 30 min.
-
a.
-
5.Clear tissue to displace the Ethanol and allow for wax infiltration using gradual xylene immersions in pre-filled glass jars in a fume hood.
-
a.Xylene for 30 min.
-
b.Xylene for 30 min.
-
c.Xylene for 60 min.
-
a.
Note: A P2 mask should be worn when working with Xylene.
Note: Specimens can be left in Xylene overnight (16–18 h).
Note: After repeated use, Xylene should be disposed using appropriate labelled waste container.
-
6.Infiltrate the tissue with paraffin using gradual paraffin wax immersions in pre-filled and warmed wax heater pots in a fume hood.
-
a.Paraffin wax at 56°C for 1 h.
-
b.Paraffin wax at 56°C for 1 h.
-
a.
Note: After repeated use, allow wax to cool before disposing using appropriate biohazard bag and bin.
-
7.Embed the tissue in paraffin using a paraffin embedding center.
-
a.Pre-warm a metal mold at 23°C in the warming tray of a wax-free thermal console.
-
b.Transfer warm mold using sterile warm forceps to the hot plate of a paraffin embedding center, fill the base of the mold with melted paraffin and let settle.
-
c.Remove the specimen from the embedding cassette using sterile forceps and place it at the center of the mold.
-
d.Transfer the mold to the cold spot using forceps and position the specimen with the assistance of the magnifier and light.
-
e.Fill the mold with melted paraffin and place the labeled embedding cassette without a lid on top of the mold, base downwards, filling the cassette with wax if necessary to avoid trapped air bubbles between the mold and the cassette frame.
-
f.Place the mold/cassette assemble in the cryo console at 4°C overnight (16–18 h) to set.
-
g.Remove the set tissue block from the mold and store at RT (15°C–25°C) or at 4°C until use.
-
a.
-
8.
Cut 4-micron sections from tissue blocks containing specimen of interest using a rotary microtome with a new blade, and place individual sections in a pre-filled container with dH2O.
Note: FFPE blocks can be placed on ice for 30 min prior to sectioning to prevent curling or folding of sections.
-
9.
Transfer sections onto blank pre-labeled SuperFrost Plus™ Adhesion slides by placing a slide underneath an individual section in the water and lifting the slide diagonally to capture it.
-
10.
Melt sections onto slides by gently submerging each slide on an angle into a pre-filled water bath at 37°C.
-
11.
Place FFPE human tissue slides on a slide rack and dry in a 37°C incubator for 24 h or formalin free zinc salt-fixed murine tissue slides on a slide rack and dry at RT (15°C–25°C) overnight (16–18 h) to preserve immune epitopes.
-
12.
Store slides in a slide box and keep at RT (15°C–25°C) until use.
Pause point: Sectioned tissue slides can be stored at RT (15°C–25°C) for at least several months until use.
Tissue deparaffinization and rehydration
Timing: 40 min to 18.5 h
This step removes the paraffin and rehydrates the tissue.
-
13.
Place sectioned FFPE human tissue slides on a slide rack (oven-safe) and bake in a 60°C oven for at least 1 h or overnight (16–18 h).
Note: Overnight baking is recommended for less adherent tissue types.
Note: Skip this step for formalin free zinc salt-fixed murine tissue slides to preserve immune epitopes.
-
14.Transfer slides to vertical slide rack (Tissue-Tek® Vertical slide rack) and deparaffinize tissue sections using gradual Xylene immersions in pre-filled glass jars (Tissue-Tek® staining dish) in a fume hood.
-
a.Xylene for 10 min.
-
b.Xylene for 10 min.
-
c.Xylene for 10 min.
-
a.
Note: A P2 mask should be worn when working with Xylene.
-
15.Rehydrate tissue sections using gradual ethanol immersions in pre-filled glass jars in a fume hood.
-
a.100% Ethanol for 5 min.
-
b.100% Ethanol for 5 min.
-
c.70% Ethanol for 5 min.
-
d.Rinse in dH2O.
-
a.
-
16.
Block endogenous peroxidases by immersing tissue sections in 3% H2O2 for 5 min, and rinse in dH2O.
Note: For human FFPE tissue sections proceed to steps 17–107, and for murine formalin free zinc salt-fixed tissue sections proceed to steps 108–173.
Opal™-TSA multiplex immunohistochemistry 7-marker (CD4, CD8, CD19, CD21, DC-LAMP, PNAd and DAPI) manual staining for human FFPE tissue sections
Timing: 3 days
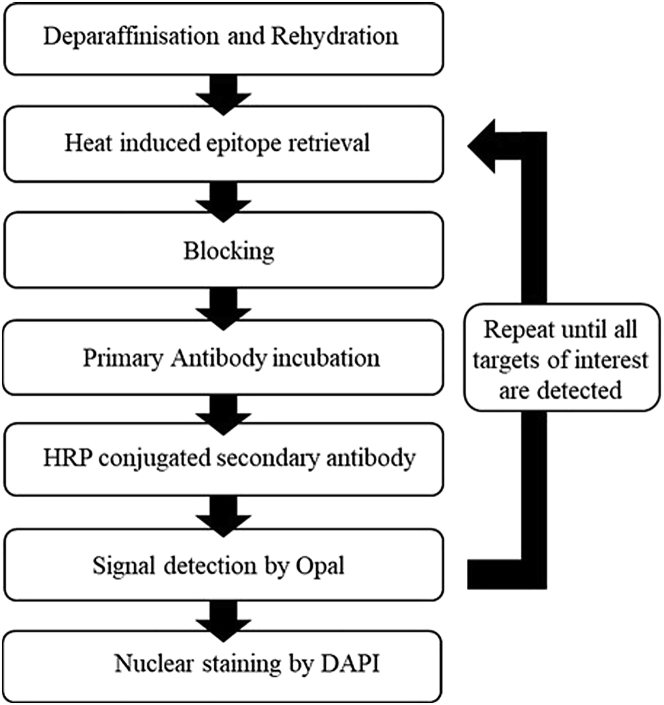
This step sequentially stains human FFPE tissue sections with CD21, DC-LAMP, CD4, PNAd, CD8 and CD19 by repetition of HIER, blocking, incubation of primary antibody, secondary antibody, and Opal™ fluorophores, and elution of bound antibodies, as per the Opal™-TSA mIHC flow chart in Figure 1.
-
17.
Wash slides in 1× TBST thrice on an orbital shaker at 2 min intervals.
-
18.
Pre-fill Hellendahl staining jars with AR6 buffer, place slides vertically and add lids.
-
19.
Unmask antigen epitopes by microwaving jars at 100% power until the buffer begins to boil, and then immediately reduce power to 20% and microwave for 20 min.
Note: HIER and stripping enables antigen epitopes to be unmasked and removes bound antibody-HRP complexes (only Opal™ fluorophore deposits remain), thereby allowing addition of the next primary antibody.
-
20.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 20 min.
-
21.
Wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
22.
Dry slides by flicking and carefully remove residual buffer surrounding the tissue using lint-free paper.
-
23.
Fill a light-protected staining tray (StainTray™ Black lid) to create a humidity chamber, and then lay slides flat side-by-side.
CRITICAL: Ensure that the tissue always remains hydrated and avoid staining more than 20 slides at a time. Once the first fluorophore is added, all incubation steps must be performed in a light-protected humidity chamber.
-
24.
To conserve reagents, circle tissue with a hydrophobic barrier pen (ImmEdge Hydrophobic Barrier PAP Pen), making sure to leave an adequate margin, and let the barrier dry.
-
25.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:10 dilution of the anti-CD21 antibody (final concentration: 199 μg/mL) in Blocking/Antibody diluent and keep on ice until use.
-
a.
Note: This step reduces the likelihood of obtaining background and/or non-specific signal.
-
26.
Remove the blocking buffer from each slide, add diluted anti-CD21 antibody to the entire tissue, and incubate at 4°C overnight (16–18 h).
Note: This step allows the primary antibody to bind to its cellular target.
-
27.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
28.
Dry slides and place these side-by-side in the humidity chamber.
-
29.
Add Opal™ Polymer HRP Ms + Rb secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 40 min.
Note: In this step, the HRP conjugate of the secondary antibody reacts with the mouse/rabbit backbone of the tissue-bound primary antibody.
-
30.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:100 dilution of Opal™ 520 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
31.
Dry slides and place these side-by-side in the humidity chamber.
-
32.
Add diluted Opal™ 520 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
Note: In this step, the bound HRP conjugate converts tyramide into free radicals that form covalent bonds with tyrosine residues at the corresponding antigenic site, thereby enabling signal amplification.
-
33.
Rinse slides in 1× TBST, wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
34.
Pre-fill jars with AR9 buffer, place slides vertically and add lids.
-
35.
Unmask antigen epitopes by microwaving jars at 100% power until the buffer begins to boil, and then immediately reduce power to 20% and microwave for 15 min.
-
36.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 20 min.
-
37.
Wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
38.
Dry slides and place these side-by-side in the humidity chamber.
-
39.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:100 dilution of the anti-DC-LAMP antibody (final concentration: 10 μg/mL) in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
40.
Remove the blocking buffer from each slide, add diluted anti-DC-LAMP antibody to the entire tissue, and incubate at RT (15°C–25°C) for 1 h.
-
41.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
42.
Dry slides and place these side-by-side in the humidity chamber.
-
43.
Add Opal™ Polymer HRP Ms + Rb secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 20 min.
-
44.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:100 dilution of Opal™ 540 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
45.
Dry slides and place these side-by-side in the humidity chamber.
-
46.
Add diluted Opal™ 540 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
47.
Rinse slides in 1× TBST, wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
48.
Pre-fill jars with AR9 buffer, place slides vertically and add lid.
-
49.
Unmask antigen epitopes by microwaving jars at 100% power until the buffer begins to boil, and then immediately reduce power to 20% and microwave for 15 min.
-
50.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 20 min.
-
51.
Wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
52.
Dry slides and place these side-by-side in the humidity chamber.
-
53.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:1000 dilution of the anti-CD4 antibody (final concentration: 0.143 μg/mL) in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
54.
Remove the blocking buffer from each slide, add diluted anti-CD4 antibody to the entire tissue, and incubate at RT (15°C–25°C) for 1 h.
-
55.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
56.
Dry slides and place these side-by-side in the humidity chamber.
-
57.
Add Opal™ Polymer HRP Ms + Rb secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 20 min.
-
58.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:100 dilution of Opal™ 570 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
59.
Dry slides and place these side-by-side in the humidity chamber.
-
60.
Add diluted Opal™ 570 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
61.
Rinse slides in 1× TBST, wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
62.
Pre-fill jars with AR9 buffer, place slides vertically and add lid.
-
63.
Unmask antigen epitopes by microwaving jars at 100% power until the buffer begins to boil, and then immediately reduce power to 20% and microwave for 15 min.
-
64.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 20 min.
-
65.
Wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
66.
Dry slides and place these side-by-side in the humidity chamber.
-
67.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:100 dilution of the anti-PNAd antibody (final concentration: 10 μg/mL) in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
68.
Remove the blocking buffer from each slide, add diluted anti-PNAd antibody to the entire tissue, and incubate at 4°C overnight (16–18 h).
-
69.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
70.
Dry slides and place these side-by-side in the humidity chamber.
-
71.
Add Opal™ Polymer HRP Ms + Rb secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 20 min.
-
72.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:100 dilution of Opal™ 620 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
73.
Dry slides and place these side-by-side in the humidity chamber.
-
74.
Add diluted Opal™ 620 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
75.
Rinse slides in 1× TBST, wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
76.
Pre-fill jars with AR9 buffer, place slides vertically and add lid.
-
77.
Unmask antigen epitopes by microwaving jars at 100% power until the buffer begins to boil, and then immediately reduce power to 20% and microwave for 15 min.
-
78.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 20 min.
-
79.
Wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
80.
Dry slides and place these side-by-side in the humidity chamber.
-
81.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:50 dilution of the anti-CD8 antibody (final concentration: 10 μg/mL) in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
82.
Remove the blocking buffer from each slide, add diluted anti-CD8 antibody to the entire tissue, and incubate at RT (15°C–25°C) for 1 h.
-
83.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
84.
Dry slides and place these side-by-side in the humidity chamber.
-
85.
Add Opal™ Polymer HRP Ms + Rb secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 20 min.
-
86.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:100 dilution of Opal™ 650 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
87.
Dry slides and place these side-by-side in the humidity chamber.
-
88.
Add diluted Opal™ 650 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
89.
Rinse slides in 1× TBST, wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
90.
Pre-fill jars with AR9 buffer, place slides vertically and add lid.
-
91.
Unmask antigen epitopes by microwaving jars at 100% power until the buffer begins to boil, and then immediately reduce power to 20% and microwave for 15 min.
-
92.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 20 min.
-
93.
Wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
-
94.
Dry slides and place these side-by-side in the humidity chamber.
-
95.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:300 dilution of the anti-CD19 antibody (final concentration: 6.12 μg/mL) in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
96.
Remove the blocking buffer from each slide, add diluted anti-CD19 antibody to the entire tissue, and incubate at RT (15°C–25°C) for 1 h.
-
97.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
98.
Dry slides and place these side-by-side in the humidity chamber.
-
99.
Add Opal™ Polymer HRP Ms + Rb secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 20 min.
-
100.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:100 dilution of Opal™ 690 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
101.
Dry slides and place these side-by-side in the humidity chamber.
-
102.
Add diluted Opal™ 690 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
103.
Rinse slides in 1× TBST, wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
Note: The step can be automated to substantially reduce timing and minimize user-error. This was successfully done using the BOND RX® Automated Research Stainer, with minor modifications. Briefly, endogenous peroxidase blocking was performed for 10 min, all HIER steps for 10 min at 95°C, all blocking for 15 min, all primary antibodies incubated for 30 min, anti-CD21 antibody used at a 1:50 dilution after HIER with AR9 buffer, and all secondary antibodies incubated for 15 min.
-
104.
Pre-fill jars with AR6 buffer, place slides vertically and add lid.
-
105.
Microwaving jars at 100% power until the buffer begins to boil, and then immediately reduce power to 20% and microwave for 15 min.
-
106.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 20 min.
-
107.
Wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals, and rinse in dH2O.
Note: A summary of the antibody dilutions, opal combinations and sequence of staining sequence of primary antibody and Opal™ fluorophores is listed in Table 1.
Note: Proceed to step 174 for Counterstaining and Mounting.
Figure 1.
Flowchart of the general Opal™-TSA mIHC assay method
Following tissue deparaffinization and rehydration, tissue sections undergo sequential target staining through repetition of HIER, blocking, incubation of primary antibody, secondary antibody, and Opal™ fluorophores, and elution of bound antibodies.
Table 1.
Detailed staining conditions and order of the Opal™-TSA mIHC 7-marker panel for human FFPE tissue sections
| Primary antibody | Antibody dilution (final concentration) | Antibody incubation time | Opal™ fluorophore (dilution) |
|---|---|---|---|
| Anti-human CD21 [1F8] | 1:10 (199 μg/mL) | Overnight (16–18 h) | 520 (1:100) |
| Anti-human CD208/DC-LAMP | 1:100 (10 μg/mL) | 1 h | 540 (1:100) |
| Anti-human CD4 [EPR6855] | 1:1000 (0.143 μg/mL) | 1 h | 570 (1:100) |
| Anti-human PNAd [MECA-79] | 1:100 (10 μg/mL) | Overnight (16–18 h) | 620 (1:100) |
| Anti-human CD8 alpha [C8/144B] | 1:50 (10 μg/mL) | 1 h | 650 (1:100) |
| Anti-human CD19 [EPR5906] | 1:300 (6.12 μg/mL) | 1 h | 690 (1:100) |
Opal™-TSA multiplex immunohistochemistry 6-marker (CD4, CD8, CD19, CD21, PNAd and DAPI) manual staining for murine formalin free zinc salt-fixed tissue sections
Timing: 3 days, overnight (16–18 h) for primary antibody incubation
This step sequentially stains murine formalin free zinc salt-fixed tissue sections with CD21, CD4, PNAd, CD8 and CD19 by repetition of HIER, blocking, incubation of primary antibody, secondary antibody, and Opal™ fluorophores, and elution of bound antibodies, as per the Opal™-TSA mIHC flow chart in Figure 1 above.
-
108.
Wash slides in 1× TBST thrice on an orbital shaker at 2 min intervals.
Note: Antigen retrieval is not required and omitted for zinc-fixed tissue sections here.
-
109.
Dry slides by flicking and carefully remove residual buffer surrounding the tissue using lint-free paper. Fill a light-protected staining tray (StainTray™ Black lid) to create a humidity chamber, and then lay slides flat side-by-side.
CRITICAL: Ensure that the tissue always remains hydrated and avoid staining more than 20 slides at a time. Once the first fluorophore is added, all incubation steps must be performed in a light-protected humidity chamber.
-
110.
To conserve reagents, circle tissue with a hydrophobic barrier pen (Mini PAP Pen), making sure to leave an adequate margin, and let the barrier dry.
-
111.
Block endogenous peroxidase by covering tissue with PeroxAbolish and incubate at RT (15°C–25°C) for 30 min.
Note: Typically, a 1.5 cm × 1.5 cm tissue section requires at least 150 μL of the relevant solution (i.e., primary, and secondary antibodies, TSA fluorophores).
-
112.
Wash slides in jars pre-filled with dH2O once, and with 1× TBST twice on an orbital shaker at 2 min intervals.
-
113.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:5000 dilution of the anti-PNAd antibody in Blocking/Antibody diluent and keep on ice until use.
-
a.
Note: This step reduces the likelihood of obtaining background and/or non-specific signal.
-
114.Remove the blocking buffer from each slide, add diluted anti-PNAd antibody to the entire tissue, and incubate at RT (15°C–25°C) for 1 h.
-
a.During this step, pre-heat the low pH glycine-based buffer on a 50°C orbital shaker.
-
a.
Note: This step allows the primary antibody to bind to its cellular target.
-
115.
Rinse slides in 1× TBST, transfer to Hellendahl staining jars, and wash in 1× TBST thrice on an orbital shaker at 2 min intervals.
-
116.
Dry slides and place these side-by-side in the humidity chamber.
-
117.
Add ImmPRESS® Polymer HRP anti-rat secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
Note: In this step, the HRP conjugate of the secondary antibody reacts with the rat backbone of the tissue-bound primary antibody.
-
118.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:150 dilution of Opal™ 690 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
119.
Dry slides and place these side-by-side in the humidity chamber.
-
120.
Add diluted Opal™ 690 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
Note: In this step, the bound HRP conjugate converts tyramide into free radicals that form covalent bonds with tyrosine residues at the corresponding antigenic site, thereby enabling signal amplification.
-
121.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
122.
Pre-fill jars with low pH glycine-based buffer and then add slides vertically.
-
123.
Elute bound antibodies by shaking at 50°C on an orbital shaker at 100 rpm for 30 min.
-
124.
Wash slides in jars pre-filled with dH2O once, and with 1× TBST twice on an orbital shaker at 2 min intervals.
-
125.
Dry slides and place these side-by-side in the humidity chamber.
-
126.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:1000 dilution of the anti-CD4 antibody in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
127.
Remove the blocking buffer from each slide, add diluted anti-CD4 antibody to the entire tissue, and incubate at 4°C overnight (16–18 h).
-
128.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, pre-heat the low pH glycine-based buffer on a 50°C orbital shaker.
-
a.
-
129.
Dry slides and place these side-by-side in the humidity chamber.
-
130.
Add ImmPRESS® Polymer HRP anti-rat secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
131.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:150 dilution of Opal™ 620 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
132.
Dry slides and place these side-by-side in the humidity chamber.
-
133.
Add diluted Opal™ 620 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
134.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
135.
Pre-fill jars with low pH glycine-based buffer and then add slides vertically.
-
136.
Elute bound antibodies by shaking at 50°C on an orbital shaker at 100 rpm for 30 min.
-
137.
Wash slides in jars pre-filled with dH2O once, and in 1× TBST twice on an orbital shaker at 2 min intervals.
-
138.
Dry slides and place these side-by-side in the humidity chamber.
-
139.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:2000 dilution of the anti-CD8 antibody in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
140.Remove the blocking buffer from each slide, add diluted anti-CD8 antibody to the entire tissue, and incubate at RT (15°C–25°C) for 1 h.
-
a.During this step, allow Tris-EDTA buffer to equilibrate to RT (15°C–25°C) prior to use.
-
a.
-
141.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
142.
Dry slides and place these side-by-side in the humidity chamber.
-
143.
Add ImmPRESS® Polymer HRP anti-rat secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
144.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:150 dilution of Opal™ 570 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
145.
Dry slides and place these side-by-side in the humidity chamber.
-
146.
Add diluted Opal™ 570 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
147.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
148.
Pre-fill jars with tris-EDTA buffer and then add slides vertically.
-
149.
Unmask antigen epitopes by microwaving jars with lids on at 100% power until the buffer begins to boil, and then immediately reduce power to 10% and microwave for 7.5 min.
-
150.
Remove jar lids and allow slides to equilibrate to RT (15°C–25°C) for at least 15 min.
-
151.
Wash slides in jars pre-filled with dH2O once, and with 1× TBST twice on an orbital shaker at 2 min intervals.
-
152.
Dry slides and place these side-by-side in the humidity chamber.
-
153.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:200 dilution of the anti-CD19 antibody in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
154.
Remove the blocking buffer from each slide, add diluted anti-CD19 antibody to the entire tissue, and incubate at 4°C overnight (16–18 h).
-
155.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, pre-heat the low pH glycine-based buffer on a 50°C orbital shaker.
-
a.
-
156.
Dry slides and place these side-by-side in the humidity chamber.
-
157.
Add ImmPRESS® Polymer HRP anti-rabbit secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
158.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:150 dilution of Opal™ 650 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
159.
Dry slides and place these side-by-side in the humidity chamber.
-
160.
Add diluted Opal™ 650 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
161.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
162.
Pre-fill jars with low pH glycine-based buffer and then add slides vertically.
-
163.
Elute bound antibodies by shaking at 50°C on an orbital shaker at 100 rpm for 30 min.
-
164.
Wash slides in jars pre-filled with dH2O once, and with 1× TBST twice on an orbital shaker at 2 min intervals.
-
165.Add Blocking/Antibody Diluent to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
a.While blocking, prepare a 1:1500 dilution of the anti-CD21 antibody in Blocking/Antibody diluent and keep on ice until use.
-
a.
-
166.
Remove the blocking buffer from each slide, add diluted anti-CD21 antibody to the entire tissue, and incubate at RT (15°C–25°C) for 1 h.
-
167.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
168.
Dry slides and place these side-by-side in the humidity chamber.
-
169.
Add ImmPRESS® Polymer HRP anti-rabbit secondary antibody to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
170.Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST thrice on an orbital shaker at 2 min intervals.
-
a.During this step, prepare a 1:150 dilution of Opal™ 540 in 1× Plus Amplification Diluent and keep on ice until use.
-
a.
-
171.
Dry slides and place these side-by-side in the humidity chamber.
-
172.
Add diluted Opal™ 540 to the entire tissue, and incubate at RT (15°C–25°C) for 10 min.
-
173.
Rinse slides in 1× TBST, wash slides in jars pre-filled with 1× TBST thrice, and with dH2O once on an orbital shaker at 2 min intervals.
Note: A summary of the antibody dilutions, opal combinations and sequence of staining sequence of primary antibody and Opal™ fluorophores is listed in Table 2.
Note: Proceed to step 174 for Counterstaining and Mounting.
Table 2.
Detailed staining conditions and order of the Opal™-TSA mIHC 6-marker panel for formalin free zinc salt-fixed tissue sections
| Primary antibody | Antibody dilution | Antibody incubation time | Opal™ fluorophore (dilution) |
|---|---|---|---|
| Anti-mouse PNAd antibody [MECA-79] | 1:5000 | 1 h | 690 (1:150) |
| Anti-mouse CD4 antibody [4SM95] | 1:1000 | Overnight (16–18 h) | 620 (1:150) |
| Anti-mouse CD8 antibody [4SM15] | 1:2000 | 1 h | 570 (1:150) |
| Anti-mouse CD19 antibody | 1:200 | Overnight (16–18 h) | 650 (1:150) |
| Anti-mouse CD21 antibody [EP3093] | 1:1500 | 1 h | 540 (1:150) |
Counterstaining and mounting
Timing: 1 h
This step counterstains individual cell nuclei and mounts slides for subsequent imaging.
-
174.
Dry slides and place these side-by-side in the humidity chamber.
-
175.
Stain cell nuclei by adding DAPI (1 drop per 500 μL of 1× TBST) to the entire tissue, and incubate at RT (15°C–25°C) for 5 min.
-
176.
Rinse slides in 1× TBST, and wash slides in jars pre-filled with 1× TBST for 2 min, and with dH2O for 2 min on an orbital shaker.
-
177.
Dry slides, pipette 40 μL of VECTASHIELD Vibrance mounting medium onto FFPE slides or 10–15 μL of ProLong™ Diamond Antifade Mountant (requires curing at RT (15°C–25°C) overnight (16–18 h) in the dark) onto formalin free zinc salt-fixed slides and apply coverslips, avoiding the formation of bubbles.
-
178.
Slides are now ready for imaging using the Vectra automated quantitative pathology imaging system.
Pause point: Coverslipped slides can be stored in a slide folder at RT (15°C–25°C) until use. However, imaging is recommended within 48 h.
Imaging and analysis
Timing: ∼30 min per slide
This step determines the optimal imaging conditions (exposure times), details subsequent whole slide scan and multispectral imaging and analyses processes.
-
179.
For each slide, create an individual protocol and manually set optimal exposure time settings across fluorescent filter cubes (including DAPI, FITC, Cy3, Texas red and Cy5) for whole slide scans (WSSs, 10×) and multispectral images (MSIs, 20×), using the Vectra® 3 Automated Quantitative Pathology Imaging System.
Note: Exposure times around 150 milliseconds (ms) for each filter cube are recommended.
-
180.
Perform WSSs after loading all slides into a slide cassette.
-
181.
View captured WSSs using Phenochart, select areas for MSI acquisition, and proceed with automated imaging.
-
182.
Spectrally unmix all MSIs using custom spectral libraries on the inForm® Cell Analysis software.
-
183.
Unmixed MSIs can be analyzed and cell types of interest phenotyped and quantified, as per user requirements.
Note: An optimal signal intensity is between 10–30 (determined using the signal intensity indicator tool), and signal-to-noise ratio is greater than 10.
-
184.
Alternatively, these MSIs can be exported (multi-image TIFF) and analyzed using the HALO® Image Analysis Platform.
Expected outcomes
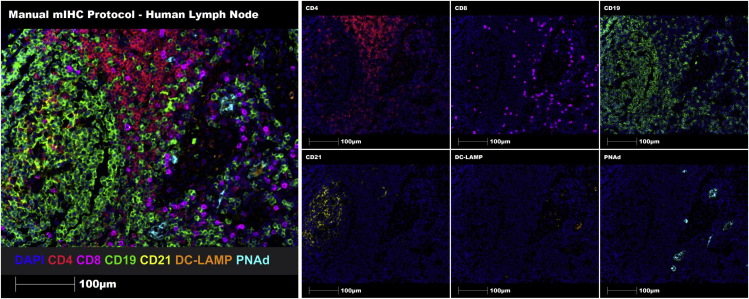
TLSs are ectopic, specialized lymphoid aggregates that resemble those seen in secondary lymphoid organs (e.g., lymph nodes). To date, IHC-based TLS identification often relies on standard H&E staining of tumor tissues or lacks the incorporation of adequate cellular and structural components deemed essential to accurately identify and characterize TLSs. As a result, immune cell aggregates are often misclassified as TLSs, and TLS maturity is not considered. Moreover, while genomic methods can be used to accurately identify TLSs, these are often expensive, have sample requirements, don’t always reflect the tumor microenvironment entirely,3 and don’t distinguish between areas of immune cell infiltrates, immune cell aggregates or TLSs in specimens. The Opal™-TSA mIHC approach enables the simultaneous detection of up to 6 antigens.4,5,6 Hence, a 7-marker mIHC panel (CD4, CD8, CD19, CD21, DC-LAMP, PNAd and DAPI) was manually optimized (Figure 2) and subsequently automated (Figure 3) for the detection of TLSs in human FFPE solid tumor tissue sections using healthy human lymph nodes as positive control tissue. This tissue is ideal for the identification of mature fully functional specialized lymphoid aggregates composed of B cells (CD19+ cells), helper and killer T cells (CD4+ and CD8+ cells, respectively), follicular and mature dendritic cells (CD21+ and DC-LAMP+, respectively), and high endothelial venules (HEVs, PNAd+).
Figure 2.
Multiplex manual staining of a representative human lymph node FFPE tissue section using the 7-marker TLS panel
Representative region of interest (ROI) showing merge (on left) and single staining (on right): CD4 (red), CD8 (magenta), CD19 (green), CD21 (yellow), DC-LAMP (orange), PNAd (cyan), and DAPI (blue). Images were taken at 20× magnification, and scale bars indicate 100 μm.
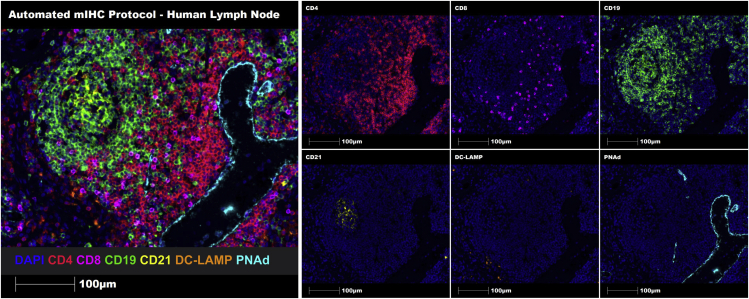
Figure 3.
Multiplex automated staining of a representative human lymph node FFPE tissue section using the 7-marker TLS panel
Representative region of interest (ROI) showing merge (on left) and single staining (on right): CD4 (red), CD8 (magenta), CD19 (green), CD21 (yellow), DC-LAMP (orange), PNAd (cyan), and DAPI (blue). Images were taken at 20× magnification, and scale bars indicate 100 μm.
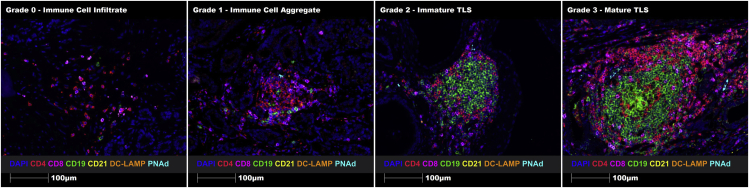
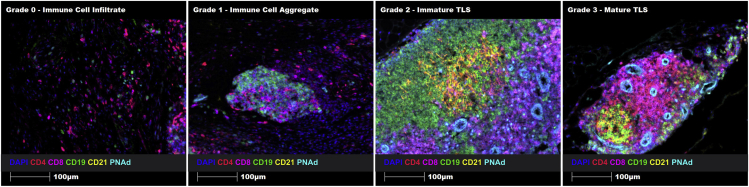
Following optimization, this panel was used to successfully identify and characterize immune cell aggregates and TLSs on full-face melanoma and prostate cancer FFPE tissue sections. In solid tumors, functional and mature TLSs exhibit a B cell zone with follicular dendritic cells, a T cell zone with mature dendritic cells, and surrounding or central high endothelial venules (HEVs),7 similar to what is seen in secondary lymphoid organs. After staining many solid tumors with this panel, it was apparent that not all immune cell aggregates were in fact TLSs, and that TLSs can be detected while forming (immature) and when fully formed and functional (mature) (Figure 4). Moreover, mature TLSs can generate tumor antigen-specific CD8+ killer T cells and antibody-producing B cells (plasmablasts and plasma cells).8,9 Hence, we propose the following grading system to accurately classify the formation of these specialized structures, including an immune cell infiltrate (Grade 0: sparse T and/or B cells), an immune cell aggregate (Grade 1: T or B cell aggregate without defined B or T cell zones, follicular DC network, mature DCs or HEVs.), an immature TLS (Grade 2: poorly-defined T and B cell zones with HEVs, but without a follicular DC network or mature DCs), and a mature TLS (Grade 3: well-defined T and B cell zones with HEVs, a follicular DC network and mature DCs).
Figure 4.
Proposed grading system for the accurate classification of immune cell infiltrates, immune cell aggregates, immature TLSs and mature TLSs using the 7-marker TLS panel on human prostate cancer FFPE tissue sections
Representative ROIs showing merge staining: CD4 (red), CD8 (magenta), CD19 (green), CD21 (yellow), DC-LAMP (orange), PNAd (cyan), and DAPI (blue). Images were taken at 20× magnification, and scale bars indicate 100 μm.
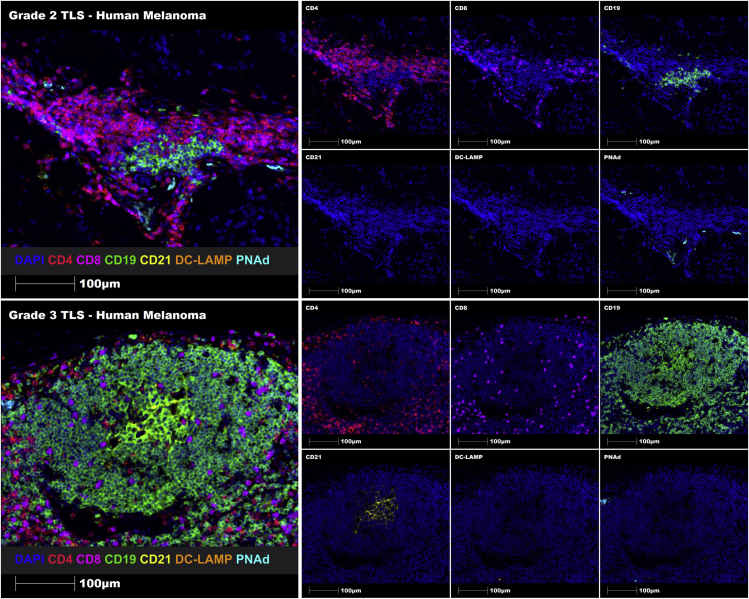
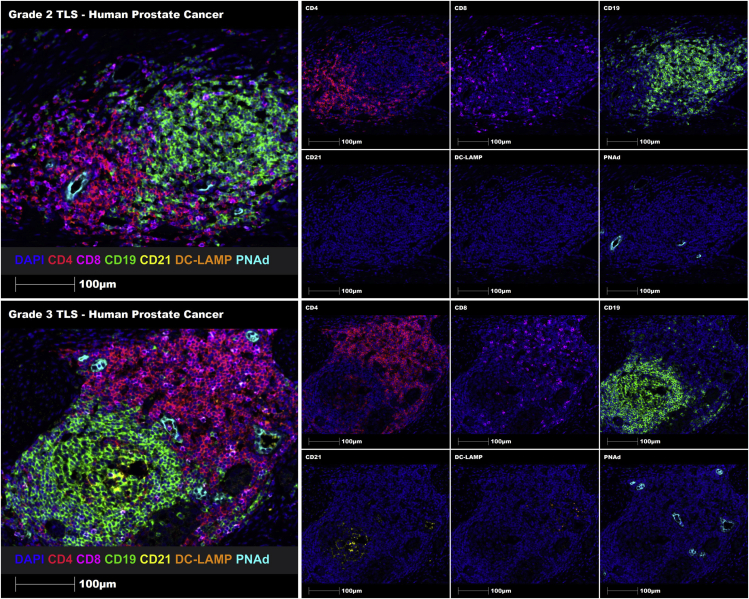
Using this 7-plex TLS panel and the proposed grading system, both Grade 2 and Grade 3 TLSs were successfully identified in melanoma FFPE tissue sections using manual staining (Figure 5), and in prostate cancer FFPE tissue sections using automated staining (Figure 6). Hence, this comprehensive protocol enables the identification and grading of immune cell aggregates, immature TLSs and mature TLSs using human FFPE tumor tissue sections.
Figure 5.
Multiplex manual staining of a Grade 2 immature TLS (upper panel) and a Grade 3 mature TLS (lower panel) in human melanoma tissue sections using the 7-marker TLS panel
Representative ROIs showing merge (on left) and single staining (on right): CD4 (red), CD8 (magenta), CD19 (green), CD21 (yellow), DC-LAMP (orange), PNAd (cyan), and DAPI (blue). Images were taken at 20× magnification, and scale bars indicate 100 μm.
Figure 6.
Multiplex automated staining of a Grade 2 immature TLS (upper panel) and a Grade 3 mature TLS (lower panel) in human prostate cancer tissue sections using the 7-marker TLS panel
Representative ROIs showing merge (on left) and single staining (on right): CD4 (red), CD8 (magenta), CD19 (green), CD21 (yellow), DC-LAMP (orange), PNAd (cyan), and DAPI (blue). Images were taken at 20× magnification, and scale bars indicate 100 μm.
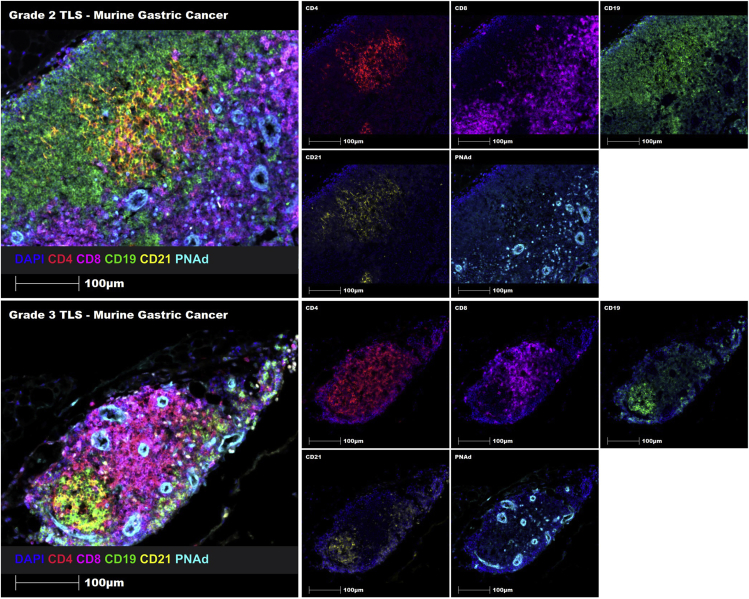
Similarly, a 6-marker mIHC panel (CD4, CD8, CD19, CD21, PNAd and DAPI) was manually optimized for the detection of TLSs in murine tissue sections fixed in formalin-free zinc salt fixative using murine gastric submucosal tissue sections, which have been previously established to harbor TLSs.10 Following optimization, this panel was used to successfully identify and characterize immune cell aggregates and TLSs on murine gastric cancer tissue sections.
We propose the following grading system to accurately identify and classify the maturity of TLSs in murine solid tumors, including an immune cell infiltrate (Grade 0: sparse T and/or B cells), an immune cell aggregate (Grade 1: T or B cell aggregate without defined B or T cell zones, follicular DC network or HEVs.), an immature TLS (Grade 2: poorly-defined T and B cell zones with HEVs, but without a follicular DC network), and a mature TLS (Grade 3: well-defined T and B cell zones with HEVs and a follicular DC network) (Figure 7).
Figure 7.
Proposed grading system for the accurate classification of immune cell infiltrates, immune cell aggregates, immature TLSs and mature TLSs using the 6-marker TLS panel on murine gastric cancer tissue sections
Representative ROIs showing merge staining: CD4 (red), CD8 (magenta), CD19 (green), CD21 (yellow), PNAd (cyan), and DAPI (blue). Images were taken at 20× magnification, and scale bars indicate 100 μm.
Using this 6-plex TLS panel and the proposed grading system, both Grade 2 and Grade 3 TLSs were also successfully identified in murine gastric cancer tissue sections using manual staining (Figure 8). Hence, this comprehensive protocol enables the identification and grading of immune cell aggregates, immature TLSs and mature TLSs using formalin-free zinc salt fixed murine tumor tissue sections.
Figure 8.
Multiplex manual staining of a Grade 2 immature TLS (upper panel) and a Grade 3 mature TLS (lower panel) in murine gastric cancer tissue sections using the 6-marker TLS panel
Representative ROIs showing merge (on left) and single staining (on right): CD4 (red), CD8 (magenta), CD19 (green), CD21 (yellow), PNAd (cyan), and DAPI (blue). Images were taken at a 20× magnification, and scale bars indicate 100 μm.
Limitations
The human tissue protocol was optimized for use with human FFPE tissue sections; accordingly, the protocol might require further optimizations with fresh frozen tissue sections. Although the protocol accommodates up to 7 markers (6 antigens and DAPI), individual antigen targets may be replaced and subsequently re-optimized. However, we caution against this, because all 6 antigen targets are required to adequately identify mature TLSs. Moreover, while it is recommended that users start with fresh tissues and perform the fixation, embedding and sectioning as described here, archival FFPE tissue blocks and/or sections can also be used. Finally, this protocol is intended for manual multiplex staining, but adaptation to an automated system has also been performed using the BOND RX® Automated Research Stainer.
Similarly, the murine tissue protocol was optimized for use with murine tissue that has been fixed in zinc. Zinc-salt fixative avoids the loss of epitopes as observed in tissue processed in aldehyde-based fixatives such as formalin,11,12 and its application to FFPE tissue sections would require further optimizations. Zinc fixation is used in this protocol to accommodate for the anti-CD21 antibody which does not perform well in FFPE tissue in a multiplex setting and enhances signal detection for CD4 and CD8. Finally, this protocol is intended for manual multiplex staining, and its adaptation to an automated system (e.g., BOND RX® Automated Research Stainer) would require further optimization.
Troubleshooting
Problem 1
Tissue loss due to repeated HIER and stripping steps by microwaving (steps 19, 35, 49, 63, 77, 91 and 105) or repeated elution (steps 123, 136, 149 and 163).
Potential solution
This often occurs for less adherent tissue types and can mainly be avoided by increasing slide baking from 1 h to overnight (16–18 h, step 13). If tissue loss is still observed consider altering the method of HIER or transition to automated staining, if possible, or use Trajan series 3 adhesive microscope slides (Trajan; Cat# 473042491).
Problem 2
Heterogenous staining or signal intensity across a single tissue section (step 181).
Potential solution
This can occur due to a compromised hydrophobic barrier that does not prevent leakage during blocking, primary antibody, secondary antibody, or Opal™ fluorophore incubation steps. This can be avoided by reapplying hydrophobic barrier in between multiplexing rounds. This can also be caused by the incomplete removal of residual buffer (TBST or dH2O) between incubation steps, or insufficient mixing of primary antibody or Opal™ fluorophore dilutions. If heterogenous staining or signal intensity remains, consider performing incubation steps on an orbital shaker.
Problem 3
Low signal for individual antibodies using different solid tumor FFPE tissue (steps 183 and 184).
Potential solution
This can arise due to differential fixation kinetics across different types of solid tumor tissues. Substituting Opal™ Polymer HRP Ms + Rb secondary antibody with ImmPRESS Universal Antibody (anti-mouse IgG/anti-rabbit IgG, Peroxidase) Polymer Reagent (Vector Laboratories, Cat#MP-7500) should substantially resolve any low intensity signals. If this substitution does not resolve the problem, individual primary antibodies may require further optimization using the specific study tissue type.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jessica Da Gama Duarte (jessica.duarte@onjcri.org.au).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
The Olivia Newton-John Cancer Research Institute acknowledges the support of the Victorian Government Operational Infrastructure Support Program. We acknowledge The Ian Potter Foundation for providing funds to purchase the Vectra System and BondRX.
This project was partly funded by the National Medical Health and Research Council (NHMRC) of Australia (GNT1125951), by the Austin Medical Research Foundation, and by Tour de Cure.
L.P. is supported by the La Trobe University Full-Fee Research Scholarship and the La Trobe University Postgraduate Research Scholarship. J.D.G.D. was supported by Cure Cancer Australia through the Cancer Australia Priority-Driven Cancer Research Scheme (#1187815). The contents of the published material are solely the responsibility of La Trobe University and do not reflect the views of Cancer Australia. A.B. is the recipient of a Fellowship from the Victorian Government Department of Health and Human Services acting through the Victorian Cancer Agency.
The graphical abstract was created with BioRender.com.
Author contributions
Methodology, L.T.Q., L.P., E.T., J.H., J.D.G.D.; Writing—original draft preparation, L.P., J.D.G.D.; Writing—review and editing, L.T.Q., L.P., E.T., M.E., A.B., J.H., J.D.G.D; Supervision, M.E., A.B., J.H., J.D.G.D; Funding acquisition, M.E., A.B., J.H., J.D.G.D. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate/analyse datasets/code.
References
- 1.Syed J., Ashton J., Joseph J., Jones G.N., Slater C., Sharpe A., Ashton G., Howat W., Byers R., Angell H.K. Multiplex immunohistochemistry: the importance of staining order when producing a validated protocol. Immunotherapy. 2019;5:1000157. doi: 10.35248/2471-9552.19.5.157. [DOI] [Google Scholar]
- 2.Pang L., Ernst M., Huynh J. Development of a multiplex immunohistochemistry workflow to investigate the immune microenvironment in mouse models of inflammatory bowel disease and colon cancer. Int. J. Mol. Sci. 2021;22:11001. doi: 10.3390/ijms222011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Y.C., Hsu C.L., Jeng Y.M., Ho M.C., Ho C.M., Yeh C.P., Yeh C.Y., Hsu M.C., Hu R.H., Cheng A.L. Reliability of a single-region sample to evaluate tumor immune microenvironment in hepatocellular carcinoma. J. Hepatol. 2020;72:489–497. doi: 10.1016/j.jhep.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Hunyady B., Krempels K., Harta G., Mezey É. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J. Histochem. Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 5.Tóth Z.E., Mezey É. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J. Histochem. Cytochem. 2007;55:545–554. doi: 10.1369/jhc.6A7134.2007. [DOI] [PubMed] [Google Scholar]
- 6.Stack E.C., Wang C., Roman K.A., Hoyt C.C. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70:46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Dieu-Nosjean M.C., Goc J., Giraldo N.A., Sautès-Fridman C., Fridman W.H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Teillaud J.L., Dieu-Nosjean M.C. Tertiary lymphoid structures: an anti-tumor school for adaptive immune cells and an antibody factory to fight cancer? Front. Immunol. 2017;8:830–836. doi: 10.3389/fimmu.2017.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meylan M., Petitprez F., Becht E., Bougoüin A., Pupier G., Calvez A., Giglioli I., Verkarre V., Lacroix G., Verneau J., et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022;55:527–541.e5. doi: 10.1016/j.immuni.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Hill D.G., Yu L., Gao H., Balic J.J., West A., Oshima H., McLeod L., Oshima M., Gallimore A., D’Costa K., et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int. J. Cancer. 2018;143:167–178. doi: 10.1002/ijc.31298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks D.J., Johnson L., Mitchell S.M., Gough J., Cooley W.A., La Ragione R.M., Spencer Y.I., Wangoo A. Evaluation of zinc salt based fixatives for preserving antigenic determinants for immunohistochemical demonstration of murine immune system cell markers. Biotech. Histochem. 2006;81:23–30. doi: 10.1080/10520290600725375. [DOI] [PubMed] [Google Scholar]
- 12.Mori H., Soonsawad P., Schuetter L., Chen Q., Hubbard N.E., Cardiff R.D., Borowsky A.D. Introduction of zinc-salt fixation for effective detection of immune cell-related markers by immunohistochemistry. Toxicol. Pathol. 2015;43:883–889. doi: 10.1177/0192623315587593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyse datasets/code.