Highlights
-
•
HomeCageScan analysis of Fabry rats reveals behavioral changes that are sex and genotype dependent.
-
•
Hierarchical clustering reveals unique groupings of behaviors.
-
•
Gabapentin administration restores depressed sniffing behavior in Fabry rats.
Keywords: Fabry disease, HomeCageScan, Pain, Non-evoked behavior
Abstract
HomeCageScan (HCS) is an automated behavioral scoring system that can be used to classify and quantify rodent behaviors in the home cage. Although HCS has been used for a number of inducible models of severe pain, little has been done to test this system in clinically relevant genetic disease models associated with chronic pain such as Fabry disease. Rats with Fabry disease exhibit mechanical hypersensitivity, however, it is unclear if these rodents also exhibit ongoing non-evoked pain. Therefore, we analyzed HCS data from male and female rats with Fabry disease. Using hierarchical clustering and principal component analysis, we found both sex and genotype differences in several home cage behaviors. Additionally, we used hierarchical clustering to derive behavioral clusters in an unbiased manner. Analysis of these behavioral clusters showed that primarily female Fabry animals moved less, spent less time caring for themselves (e.g., less time spent grooming and drinking), explored less, and slept more; changes that are similar to lifestyle changes observed in patients with long lasting chronic pain. We also show that sniffing, one of the exploratory behaviors that is depressed in Fabry animals, can be partly restored with the analgesic gabapentin, suggesting that depressed sniffing may reflect ongoing pain. Therefore, this approach to HCS data analysis can be used to assess drug efficacy in Fabry disease and potentially other genetic and inducible rodent models associated with persistent pain.
Introduction
Measuring ongoing pain behaviors in rodents can be time consuming, resource intensive, and difficult to quantify. Therefore, assays of experimenter evoked behaviors (e.g. withdrawal responses elicited by stimulation with von Frey probes, needles, hot or cold thermal stimuli, etc.) are commonly used to measure pain in rodents (Allchorne et al., 2005, Brenner et al., 2012, Deuis et al., 2017, Hargreaves et al., 1988). However, there are numerous drawbacks to these tests, including discrete time of administration, confounding influence of the often-novel testing environments, measurement of reflexive behaviors instead of affective behaviors (Loeser, 2012, Vierck et al., 2008) (persistent pain in humans is commonly non-reflexive (Forstenpointner et al., 2021, Schmelz, 2021)), and the subjectivity of experimenter scoring (Clark, 2016, Fisher et al., 2021, Fried et al., 2020, Sadler et al., 2021, Sorge et al., 2014). To better understand how persistent pain affects non-evoked behaviors, we can use the HomeCageScan (HCS) automated behavioral scoring system to evaluate spontaneous and ongoing pain behaviors in the more natural environment of the home cage (Roughan et al., 2009). HCS is an automated video-based behavioral scoring system developed to continuously measure rodent behavior in the home cage environment. It has been shown to have high concordance with human scoring and advantageously allows for behavioral measurements over long periods of time (Melo-Carrillo and Lopez-Avila, 2013, Roughan et al., 2009, Yamamoto et al., 2018, Zhang et al., 2021). Previous literature has shown that HCS can detect behavioral differences in post-operative pain, neuropathic injury models, lipopolysaccharide (LPS) sepsis models, and inflammatory models of pain (Lehmann et al., 2013, Roughan et al., 2009; V Gris and K., Yamamoto, K., Gharagozloo, M., Mahmoud, S., Simard, C., Gris, P., Gris, D., , 2018, Wright-Williams et al., 2013, Zhang et al., 2021). However, analysis of the large dataset obtained is non-trivial and can make interpretation of the results difficult (e.g., what does it mean to have an animal that “turns” less). Therefore, tools to reduce the data set size are important in improving data interpretation. Additionally, to our knowledge, no genetic models of human disease associated with persistent or chronic wide-spread pain have been evaluated with HCS. Developing and characterizing methods to analyze ongoing pain are important for development of pain therapeutics using rodent pain models.
Fabry disease is an X-linked lysosomal storage disease that presents with life-long ongoing pain (Miller et al., 2020, Miller et al., 2018, Waltz et al., 2021). This disease presents with many symptoms including renal disease, cardiovascular disease, stroke, angiokeratomas, digestive problems, reduction in vision and hearing, and pain (Miller et al., 2020). The pain that patients experience significantly impacts their quality of life and daily function (Biegstraaten et al., 2012, Hopkin et al., 2008, Üçeyler et al., 2014). Although there are several preclinical studies exploring evoked pain withdrawal responses, there is a lack of study regarding ongoing pain-related behavior in preclinical rodent models. Herein, we utilized HCS analysis to assess ongoing pain in rats with Fabry disease. We describe use of principle component analysis (PCA) and hierarchical clustering approaches to analyze the large HCS data sets and screen for relevant behaviors and unbiased behavioral groupings in the Fabry disease rat model. This unbiased approach to analyzing HCS data will facilitate clustering of behaviors into relevant groups based on behavioral data rather than experimenter presumptions. Additionally, we investigated how HCS behaviors were affected by both genotype and sex of the animals. Therefore, measuring ongoing rodent behavior in their home cages can provide insight into persistent pain. Such analysis that may be minimally affected by experimenter intrusion or variables is critical for understanding the full picture of a disease associated with chronic pain in preclinical models and provides new information on how therapeutics can alleviate pain symptoms that persist across the lifespan.
Materials and methods
Animals
The Fabry rat line (Rat Genome Database symbol: Glaem2Mcwi) was created at the Medical College of Wisconsin (MCW) on a Dark Aguti background as previously described (Miller et al., 2018). The CRISPR deletion in the Gla gene leads to a loss of α-galactosidase A (α-Gal) activity in the hemizygous male and homozygous female rats (KO). The heterozygous females (Het) maintain partial activity of this enzyme compared to wildtype animals (WT) similar to some classical Fabry mutations in patients (Miller et al., 2018). Animals generated from this colony were used between 40 and 80 weeks of age; the age we have previously seen mechanical sensitization due to Fabry disease (Miller et al., 2018). Food and water were provided ad libitum while animals were housed in the main colony or in the HCS apparatus. Animals were kept on a 12-hour light/dark cycle during all experiments. All animal housing and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at MCW.
HomeCageScan
During the HCS video analysis, animals were singly housed in standard clear cages measuring 42 cm × 18 cm × 24 cm. Animals were free to consume chow and water during their time in the recording cage. Water was provided through a water bottle with a long lick spout (6 cm) to allow for better detection of drinking behaviors by the video analysis software. Additionally, since the rats are a similar color to the brown chip bedding, white Alpha Dri paper bedding was used during the HCS experiments to aid the software from distinguishing them from the cage background. Animals were then placed on the Home Cage Rack (Clever Systems, #CSI-ENV-HCR-R). Rats were acclimated to the cage rack overnight prior to the start of video recording the following dark phase. All video recording was performed during the dark cycle when the rats are most active. Infrared backlighting was used to illuminate animals during the dark cycle. Analysis of the video feed was performed using the HomeCageScan High-Throughput Real-Time Option v3.00 software (Clever Systems). Rats were not removed from their cages during the 3-dark phase recording periods. After the recordings concluded, animals were returned to their home cages in the colony.
Behaviors (Supplemental Table 1) for the recording period during the dark phase were exported in 1-minute bins from the HCS software and converted into fractions of the dark phase spent doing each behavior. Since the rats did not exhibit the behaviors substantial hanging, digging, urinate, arousal, jumping, or circling, these behaviors were excluded from our final analyses (Supplemental Table 2). We relied on the software validation of behavior scoring previously published (Roughan et al., 2009) and did not manually score our videos other than to anecdotally check that the software correctly classifying the behaviors at the beginning of each recording. At times the software is not able to classify a behavior and labels it as “unknown”. The average fraction of the night across all rats (male/female, all genotypes) is reported in Supplemental Table 3.
In experiments where gabapentin was administered, rats were acclimated overnight in the home cage rack as described above. All rats were weighed immediately prior to being placed on the rack. Gabapentin was made up fresh in saline at 60 mg/mL prior to injecting the rats to yield an injection volume of ∼ 0.5 mL or less for each rat. Rats were then given a i.p. injection of saline 30 min prior to the start of the acclimation dark phase to acclimate them to injections prior to the start of the experiment. During the subsequent three recording dark phases, all rats received a saline injection 30 min prior to the start of the dark cycle. Following the saline injection dark phases, rats were then given 100 mg/kg gabapentin for the following three dark phases. Behavior data was extracted from the first four hours of the dark cycle, when the gabapentin has previously been shown to have the highest efficacy (Radulovic et al., 1995, Vollmer et al., 1986). We ran a dose response to gabapentin and we found that a 75–200 mg/kg dose in our rats does not induce a reduction of movement in male rats in open field measurements (Supplemental Fig. 1). Due to the small effect size and high variability in the WT and KO behavior we chose this dose to give us the best chance to detect a difference. Additionally, since male patients often have the most severe form of Fabry disease, we decided to perform this experiment in male rats. Data processing was performed similarly as above for the sniffing behavior.
Principal component analysis and hierarchical clustering analysis
In order to analyze the data from naïve rats, we used principal component analysis (PCA). PCA calculates vectors which align with the largest sources of variation in the multidimensional data (Supplemental Fig. 2). These vectors form the principal components of the data, which are unique (i.e., orthogonal) and are derived from different weighted combinations of the original measured behaviors (Supplemental Table 4). The larger the coefficient weight, the more that behavior contributes to the principal component. Therefore, the original behavior data can be transformed into principal components to pull out the greatest variation in the data while reducing the number of parameters needed to visualize at once. For instance, in Supplemental Fig. 2, the measured behaviors sleep, walk, and rear can be transformed to principal components (PC1 and PC2) through the linear combinations of each behavior. This allows for visualization of the data in two dimensions where the two groups of animals can be clearly separated along PC1. Although PCA easily pulls out variation in the data and simplifies visualization, dimension reduction results in < 100 % of the variation in the original data being represented. Despite this, dimensionality reduction in data is highly useful for understanding broad changes in the animal population. Parallel analysis was used to identify principal components that were distinguishable Monte Carlo simulated random data (>95th percentile, 1000 simulations). This insured that the selected principal components used to analyze the data were not explainable by random noise.
In order to better understand the relationship between behaviors, we created a correlation matrix for all behaviors. Hierarchical clustering was then used to determine what behavior correlation profiles grouped together. Hierarchical clustering calculates the difference between the time spent doing each behavior and converts that into a “distance” (calculated using Euclidian methods) between each data point (all behavior correlations) from every other data point, then pairs the two behaviors that have the most similar correlation profiles. The behavioral correlation profiles are averaged to combine and create a new data point that represent the average of both behavior correlation profiles. This process of calculating distances between data points, pairing the most similar data points, and combining points repeats until every data point is connected to the other data points in a hierarchical tree (Supplemental Fig. 3). This tree, termed a dendrogram, contains the information on how closely related each behavioral profile is to the rest. Therefore, in the dendrogram the behaviors that have the most similar correlation profiles have the shortest connecting lines, while the behaviors that had the least similar correlation profiles are connected by the tallest lines. By choosing lines of similar heights, clusters of animals that perform behaviors to a similar degree can be grouped together. Characteristic behaviors were determined from normalized behavioral data (mean = 0, stdev = 1).
Whole body plethysmography
Animals were place in a custom-made calibrated whole-body plethysmograph chamber designed for long-term housing to record breathing as described previously (Manis et al., 2021). Briefly, animals were placed in the plexiglass chamber and allowed to acclimate overnight prior to initiating recording 24 h later. Breathing was recorded from animals for the subsequent three days (∼72 h). Data was digitally filtered in LabChart 8 with a lowpass 12 Hz cutoff frequency (transition width 7.691 Hz) to remove electrical noise. Breath rate was then calculated by the software using the respiratory belt detection with a minimum peak height of 1 SD from baseline. Since respiratory frequency during sniffing is at a much higher frequency than tidal breathing (Carnevali et al., 2013, Sirotin et al., 2014, Strohl et al., 1997, Wesson et al., 2009), breaths taken at a frequency>250 BPM (∼4Hz) were considered sniffing (Supplemental Fig. 4). Time spent sniffing was normalized per hour.
Open field
Open field testing was used to measure total distance travelled. Animals were acclimated in the testing room for 1 h, and 30 min with experimenter present in the room. Animals received i.p. injection of saline or the specified gabapentin dose and then returned to their home cage. After 30 min, animals were placed into an open field apparatus with ambient lighting and white noise. Animals were tested between 2 and 6 pm. Animals were recorded for 15 min total via an overhead camera, and total distance traveled was analyzed with ANY‐maze software (Stoelting Co., Wood Dale, IL, USA).
Statistics
Statistics, principal component analysis (PCA), and correlation analysis were performed in GraphPad Prism 9. For behavioral data, two- or three-way ANOVAs were performed as described in the Fig. legends. p-values were corrected for multiple comparisons (e.g., WT dark phase 1 vs KO dark phase 1) within each behavior and for multiple simultaneous behavior comparisons (e.g., behavior 1 vs behavior 2) using the Holm-Šidák post-hoc correction. Corrected p-values<0.05 were considered significant. Behaviors were standardized (mean = 0, stdev = 1) prior to PCA analysis. Hierarchical clustering was done in MATLAB 2019b using behavior data or the correlation matrix derived from behaviors. No formal power analysis was used to calculate the number of animals needed to detect a set effect size as the effect size differed for each behavioral output. Therefore, we used numbers of animals consistent with our previous behavior studies (Miller et al., 2018).
Results
PCA analysis of Fabry rat home cage behaviors separate animals by genotype and sex
We used video recording coupled with HomeCageScan automated behavioral analysis to quantify 41 different non-evoked behaviors in Fabry rats (Yamamoto et al., 2018). We selected 28 behaviors that were observed in the majority (>50 %) of rats in the HCS setup (Supplemental Tables 1 and 2). This selection process removed primarily hanging behaviors that we have not observed in rats while they are in their home cages. Rats are most active during the dark phase (Stenvers et al., 2016, Stephan and Zucker, 1972, Usui et al., 2003). Therefore we calculated the fraction of the dark phase that rats spent doing each behavior over 3 consecutive recording dark phases. We hypothesized that rats may engage in a different frequency of activities on any given dark phase; thus, performing recordings over multiple dark phases ensured that we captured a variety of behavioral profiles.
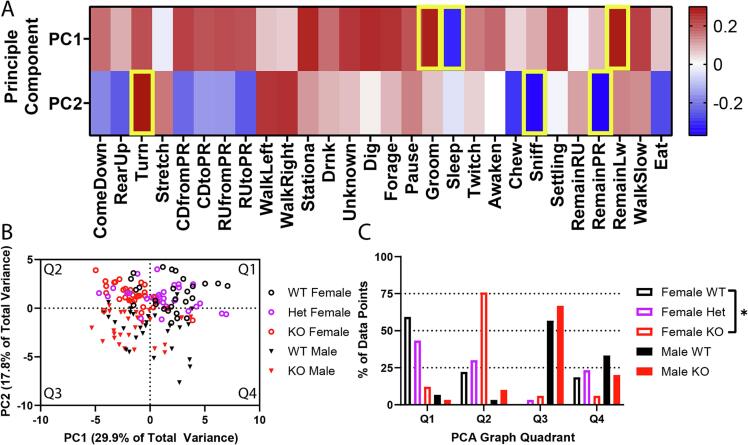
We wanted to determine if there were differences between WT and KO rats. However, analyzing all 28 behaviors would increase the likelihood of false positive differences between genotypes, making it difficult to interpret if these behavioral differences were meaningful (Supplemental Table 5). Additionally, simultaneous visualization of all behaviors across multiple days, sexes, and genotypes of rats is impractical. Therefore, we first used principal component analysis (PCA) to analyze differences in behavioral profiles between Fabry KO rats (Supplemental Fig. 2, also see Methods). We calculated the first two principal components (Fig. 1A) and transformed our data into the principal component space and found that the rats clustered into distinct groups (Fig. 1B). Quantifying the percentage of rats for each sex and genotype in the 4 PCA quadrants, we found that male and female rats separated from each other along the PC2 axis (Female: y > 0, Male: y < 0) (Fig. 1C). Additionally, WT and KO animals were split across the PC1 axis (WT: x > 0, KO: x < 0) (Fig. 1C). The heterozygous female rats had a similar distribution across the 4 quadrants to the WT female rats (Chi-square p = 0.8129) in their clustering on the PCA plot (Fig. 1B,C). Separation of animal groups indicates that the behaviors with the highest weights in PC1 and PC2 may uniquely separate the genotypes and sexes, respectively. We also analyzed PC3 and PC4, that explain only 12.4 % and 7.5 % of the variance in the data and found that these principal components were able to separate genotypes (Supplemental Fig. 5). These components were less able to distinguish between animal sexes (Supplemental Fig. 5). Analysis of the principal component weights (Supplemental Table 4) show that the top three behaviors that influence the genotype separation (along PC1) were grooming (7.5 %), sleeping (9.6 %), and remaining low (8.0 %) (Fig. 1A). The three highest weighted behaviors for separating the two sexes (along PC2) were turning (8.9 %), sniffing (12.4 %), and remaining partially reared (13.7 %) (Fig. 1A). Therefore, PCA analysis broadly shows us that our rodent model exhibit both sex and genotype differences in non-evoked behaviors.
Fig. 1.
PCA reveals inherent differences between Fabry rat genotypes and sexes. A) Contributions of each behavior to principal components PC1 and PC2. Yellow boxes indicate the top 3 highest contributing behaviors to each principal component. C) PCA plot of KO and WT male and female rats. Each point represents one animal on a particular dark cycle. D) Quantification of percentage of each genotype in each of the PCA quadrants. Chi squared test used to test for differences between genotype profiles. N = 10 wt-Male, 10 KO-Male, 11 wt-Female, 10 Het-Female, and 9 KO-Female. *p < 0.05.
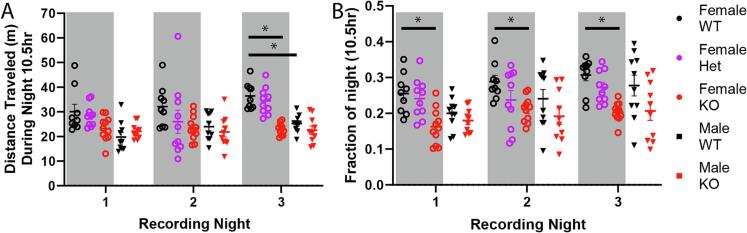
Although PCA methods point out larger shifts in the data between genotype and sex of animals, they do not provide much detail on what specific behaviors or groups of behaviors distinguish animals. We observed that KO animals were generally characterized by sleeping and resting behaviors while WT were characterized by active behaviors. Previous studies have shown that decreased movement is a common observation in other pain conditions (Cho et al., 2013, Stevenson et al., 2009). Therefore, we grouped behaviors that required animal activity together into a global activity metric (Supplemental Table 6), then compared both global activity and total distance travelled between genotypes and sexes. There was a significant decrease in KO female rat movement and activity (Fig. 2, Supplemental Tables 7 and 8) which was not observed in male KO rats. Additionally, WT female rats moved around more compared to WT males, despite male and female KO animals exhibiting the same amount of total activity. This indicates that female, but not male, movement and activity is significantly affected in the Fabry disease rodent model.
Fig. 2.
Female but not male Fabry animals show decreases in movement in their home cage. A) Total distance traveled during the dark phase for each of 3 days of recording. Grey boxes indicate female animals. B) Fraction of the dark phase rats were engaged in active movement. Blue text represents the results of the 3-way ANOVA (Het Female rats were not included in the statistical analysis). N = 10 wt-Male, 10 KO-Male, 11 wt-Female, 10 Het-Female, and 9 KO-Female. *p < 0.05.
Behavior correlations and hierarchical clustering can be used to define groups of behaviors in an unbiased way
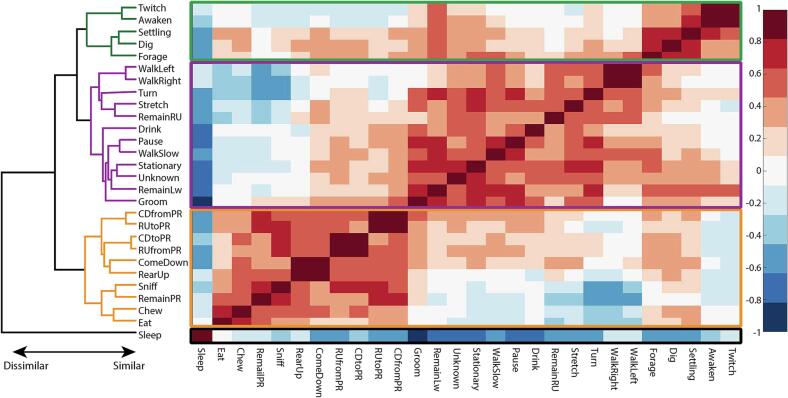
Although we able to identify some broad changes in behaviors, we wanted to identify behaviors that group together regardless of sex or genotype. We therefore used correlation analysis paired with hierarchical clustering to understand what behaviors group together in an unbiased fashion (Fig. 3). We used an unbiased grouping approach as to not make assumptions on what behaviors grouped together. The correlation analysis indicated that nearly 70 % (194/276 correlations) of the behaviors showed positive correlations with other behaviors. Behaviors that had strong positive correlations (r > 0.7, p < 0.05) between each other included RU with CD, RUfromPR with CDtoPR, RUtoPR with CDfromPR, walking right with walking left, awaken with twitch, remainPR with RUtoPR and sniffing, and remaining low with turning, being stationary, and grooming. Strong positive correlations between behaviors indicate that those behaviors tend to vary together between rats. Additionally, these positive correlations could indicate that animals perform these sequences of behaviors as part of more complex behaviors such as eating, drinking, sniffing, and other movement around the home cage. For instance, animals that sniff may be partly rearing or fully rearing and then remain reared in order to sniff their cage. The most distinct behavior was the sleeping behavior, which was negatively correlated to every other behavior and had strong negative correlations (r < -0.7, p < 0.05) with being stationary and grooming. Therefore, as expected, sleeping was the least similar to all other behaviors.
Fig. 3.
Unbiased behavioral groupings emerge after hierarchacal clustering of behavioral correlations. Correlation matrix showing Spearman correlation coefficent was calculated from all naïve rats measured in HCS. Hierarchacal clustering of correlation coefficents with different behavioral groupings indicated by color in the dendrogram. N = 10 wt-Male, 10 KO-Male, 11 wt-Female, 10 Het-Female, and 9 KO-Female.
Although individual behaviors can be analyzed for each animal, differences in individual behaviors can be difficult to interpret due to some behaviors potentially being involved with multiple other more complex behaviors. Therefore, we grouped behaviors based on their similarities in correlation profile using hierarchical clustering (Fig. 3). Using this method, we identified 4 groups of behaviors that had similar correlation profiles: sleep, twitching/foraging, locomotion/self-care, and exploratory (Supplemental Table 9). Movement behaviors including walking, stretching, and remaining reared up had similar profiles. Additionally, low movement/self-care behaviors like grooming, drinking, pausing, being stationary, walking slow, turning, and remaining low had grouped together. Although sleep associated behaviors twitching and awakening had similar profiles, they were more similar to the movement and low-movement behaviors than the sleep behavior. Finally, we found that exploratory behaviors such as sniffing, rearing, and eating all grouped together. Grouping of these behaviors in an unbiased fashion revealed that several behaviors such as drinking and eating, which would be expected to be in the same behavior group, were not associated. Therefore, this unbiased quantitative approach revealed novel groupings of behaviors for the Fabry rat model that would not have been detected with an a priori subjective grouping.
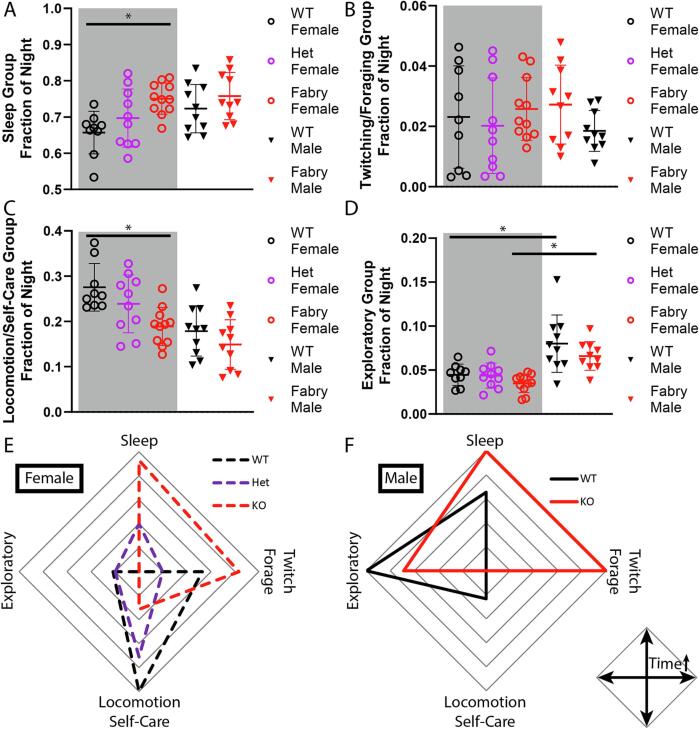
Primarily female Fabry animals show significantly increased sleep and decreased locomotion/self-care behaviors and trending decreases in exploratory behaviors
Using the behavioral groups defined by hierarchical clustering, we were able simplify the 28 individual behaviors derived from HCS data into 4 behavioral parameters: sleep (Fig. 4A, Supplemental Table 10), twitch/awaken/forage (Fig. 4B, Supplemental Table 11), movement/self-care (Fig. 4C, Supplemental Table 12), and exploratory (Fig. 4D, Supplemental Table 13). The behavior groups were determined in an unbiased fashion, but the behavior group terms (e.g., movement/self-care and exploratory) are subjective terms to describe the type of behaviors found in those groups. There was significant heterogeneity in rat behavior over the dark cycles, therefore, to simplify analysis we averaged the recordings prior to grouping behaviors as previously done (Zhang et al., 2021). There were significant genotype differences in 2/4 behavioral group (Fig. 4A,C) and sex differences in 2/4 of the behavioral parameters (Fig. 4C,D). Consistent with the activity data, female KO animals spent more time sleeping compared to female WT while the male animals exhibited a trending difference in sleeping (Fig. 4A). Analysis of twitching/foraging related behaviors revealed no effect of sex, consistent with PCA and hierarchical clustering analysis which indicated that these behaviors are not highly contributing to either sex or genotype differences (Fig. 4B). In addition to reduction in both locomotion and self-care behaviors in KO animals, male rats (both WT and KO) performed these behaviors less compared to their female counterparts (Fig. 4C). A similar difference between genotypes was observed with exploratory behaviors although male rats explored more than female animals (Fig. 4D). When overall average behavioral profiles are assessed, both male and female KO animals moved less, performed less self-care behaviors, explored less, and slept more than WT as shown by their distinct behavioral profile (Fig. 4E,F). Although WT male and female animals favor different groups of behaviors, the KO animals have similar behavioral profiles, indicating Fabry animals, regardless of male or female sex, behave similarly to an extent.
Fig. 4.
Primarily female Fabry rats display reduced locomotion, self-care, and exploration while increasing time spent sleeping. Sleeping (A), twitching/foraging group (B), locomotion/self-care group (C), and exploratory group (D) defined by hierarchacal clustering. Fraction of time spent for each behavior group (average of days 1–2). Data normalized to the minimum and maximum means for each behavior group is plotted in a radial graph for both female (E) and male (F) rats. Mean behaviors are normalized and plotted. N = 10 wt-Male, 10 KO-Male, 11 wt-Female, 10 Het-Female, and 9 KO-Female. *p < 0.05.
Sniffing is reduced in male and female Fabry animals and can be reversed in male rats with administration of gabapentin
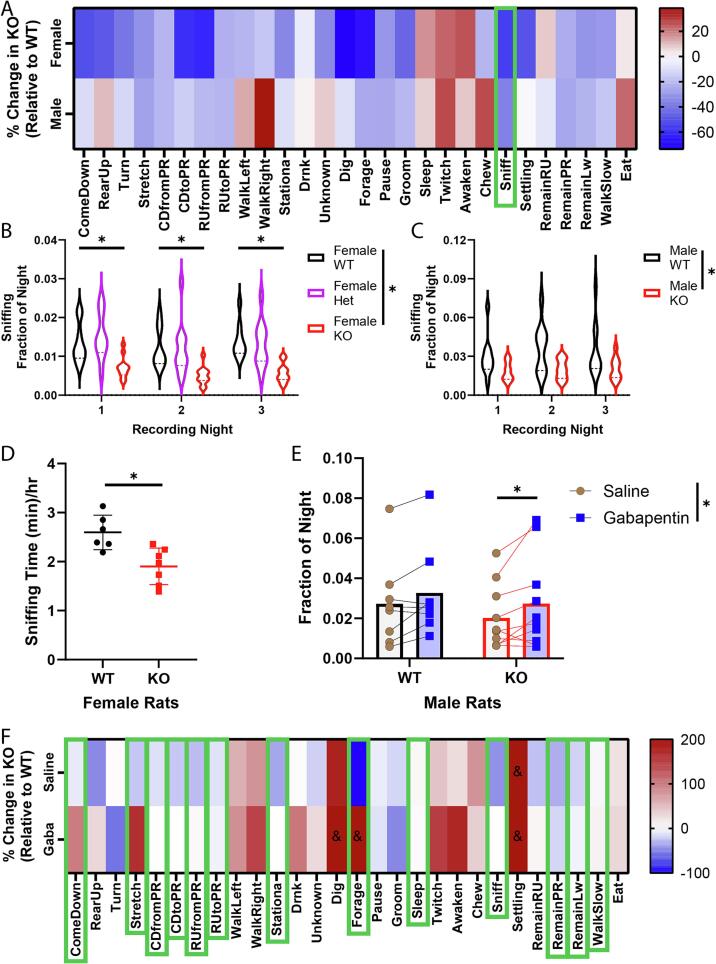
Since locomotion, self-care, and exploration were decreased in KO animals, we screened the behaviors in these groups to determine if any of the individual behaviors were more robustly affected in both male and female Fabry rats. We observed several behaviors that were different between male and female Fabry animals compared to WT, and found that sniffing had the greatest reduction in both male and female Fabry animals (Fig. 5A). This finding was consistently seen across the three recording days, further indicating that a reduced sniffing behavior is a robust finding in KO animals (Fig. 5B,C, Supplemental Tables 14 and 15). HCS measures behaviors based on video modeling of rat movement, therefore, sniffing behavior recorded from HCS is not a direct measurement of sniffing. We confirmed this change in sniffing behavior by using whole body plethysmography to measure high frequency sniffing (Fig. 5D). Consistent with the group behavior results (Fig. 4C,D), KO rats spent significantly less time sniffing than WT rats (Female: 55 % decrease; Male: 41 % decrease). Next, we determined if reduced sniffing behavior may be a pain-related behavior by administering the analgesic gabapentin to rats and subsequently recording sniffing behavior with HCS. Gabapentin is a first-line analgesic given to Fabry patients for pain management (Politei et al., 2016). Animals were administered gabapentin and sniffing behavior was recorded for the first 4 h of the dark phase, when gabapentin is most effective (Radulovic et al., 1995, Vollmer et al., 1986). Baseline sniffing behaviors were established with saline injection the two dark phases prior. We found that sniffing was significantly increased in the KO animals following gabapentin injection (99 % restored to WT), supporting the idea that decreased sniffing may be pain-related (Fig. 5E, Supplemental Table 16). Furthermore, we noticed that several rearing, walking, and resting behaviors in Fabry male rats were also altered by gabapentin administration (Fig. 5F).
Fig. 5.
Gabapentin partly reverses decreased sniffing behaviors in Fabry rats. (A) Percentage change in KO animals relative to WT for exploratory, self-care, and locomotion related behaviors. Green box represents the behavior with the greatest consistent change in both male and female rats. Fraction of the dark phase spent sniffing for female (B) or male (C) rats. (D) Whole-body plethysmography of female WT or KO rats. Sniffing (breaths > 250 BPM) were recorded continuously over three days and average sniffing time/hour calculated. (E) Male rats were given i.p. saline injections for 2 days followed by gabapentin (100 mg/kg) injections for 2 days. Fraction of the dark phase spent sniffing was calculated over 4 h following injection. Sniffing data was averaged over 2 days. (F) Heatmap of exploratory, self-care, and locomotion related behaviors after saline or gabapentin injection. Green boxes indicate behaviors that were changed after gabapentin administration towards WT levels. Behaviors marked with “&” are > 200 % increase/decrease due to low WT saline average for that behavior. (A-C): N = 10 wt-Male, 10 KO-Male, 11 wt-Female, 10 Het-Female, and 9 KO-Female. (D): N = 6 wt-Females, 7-KO Females, (E-F): N = 8 wt-Males, 10 KO-Males. *p < 0.05.
Discussion
Video-based monitoring and scoring systems have allowed researchers to explore how animals behave in their home cage. Monitoring animals in their home cage removes artifacts that arise from a novel testing environment, presence of experimenters, and presence of novel stimuli. Minimizing these potential confounding behavioral artifacts improves our interpretation of animal behavioral outputs (Sadler et al., 2021). HCS also allows for observation of multiple behaviors for hours or days without experimenter input. Although a powerful technology, questions remain on how to analyze and interpret results from the subsequently massive behavioral dataset from HCS. Additionally, while many inducible models of pain have been used in the HCS system, it is unclear from these studies whether this system can resolve behavior changes in preclinical genetic models of pain.
We utilized a genetic rat model of Fabry disease to determine whether HCS could detect behavioral differences that might be related to ongoing pain. We first took a high-level approach to identify broad changes in Fabry rat genotype and sex using two distinct data reduction methods: principal component analysis (PCA) and hierarchical clustering (Fig. 1). This method allowed us to pull out behavioral traits that defined the Fabry genotype including increased sleeping, and decreased grooming and locomotion. Since the PCA and hierarchical clustering indicated a global decrease in movement by the Fabry animals, we categorized all active behaviors together and found decreased activity in Fabry animals (Fig. 2). This decrease in active behaviors is consistent with prior pain-depressed behaviors (drinking, nesting, and locomotion) seen in models of visceral and inflammatory pain (Negus et al., 2015, Negus et al., 2010, Stevenson et al., 2009). Similar observations of depressed locomotion, rearing, and distance traveled with a corresponding increase of immobility were also observed in a model of inflammatory pain (Hasriadi et al., 2021) and vasectomy (Roughan et al., 2009). We previously showed that Fabry rats exhibit long-term chronic evoked withdrawal responses, and this finding aligns with data on the prevalence of life-long pain in patients with FD (Miller et al., 2018). Therefore, using this screening process using, we were able to detect behavioral differences in Fabry rats which may be a result of their chronic pain. However, Further analysis of resting, grooming, rearing, movement, and sniffing behaviors may clarify interpretation of these differences.
Although many previously published HCS analysis papers have only assessed one sex (predominately male), we have conducted HCS analysis on both male and female FD rats and utilized PCA to distinguish sex differences in animal behavior (Fig. 1). We found that locomotion-related behaviors were increased in female rats compared to males, while rearing and sniffing were decreased (Fig. 2). Consistent with this result, previous research has shown that female rats increase movement in novel spaces more than males (Scholl et al., 2019, Seib et al., 2018). Additionally, female mice in the HCS setup spend more time engaging in the active behavior cage lid hanging (Zhang et al., 2021). Although we saw a sex-dependent increase in locomotion/self-care behaviors, female animals had significantly decreased exploration behaviors (Fig. 4). It is possible that the less locomotive males spent more of their time eating, sniffing, and rearing whereas the females moved more and therefore explored less. There is a lack of consideration of both male and female animals in published home cage experiments. We have observed striking differences in how female and male animals behave in their home cage environment. Therefore, home cage allows for objective observation of sex differences and further utilization of this tool to assess sex differences in pain-related behavior will be beneficial in addition to careful assessment of the estrus cycle.
Although broad changes in activity and behaviors are useful for screening, they are also subject to experimenter bias, therefore, the use of an unbiased behavioral clustering method was employed to group behaviors that correlated with each other (Fig. 3). Consistent with a priori approaches, we found behaviors we would predict to group together including eating and chewing and additionally many of the rearing behaviors correlated with sniffing, and horizontal locomotion (Zhang et al., 2021). However, we discovered novel groupings of behaviors that we would not have expected to group together. For instance, we found that not all rearing behaviors grouped together - most types of rearing were more closely associated with eating and exploring but remain reared up was more closely correlated with behavior group that included stretching, drinking, and grooming. Additionally, we expected resting behaviors like sleep, pausing, remaining stationary, twitching, and awakening to all correlate together. However, many of these fell into distinct groups indicating that these inactive behaviors are more closely associated with animals stopping movement prior to grooming or drinking. In a previous study, unbiased grouping of behaviors of immune-deficient male mice showed similarities to our findings as many of the rearing behaviors grouped together as did drinking, walking slow, stretching, and remaining reared up (V Gris et al., 2018). However, their exploratory-like behaviors (e.g., sniffing and rearing) grouped with locomotion behaviors like walking, while ours did not. Additionally, they found that sleeping and drinking had similar correlation patterns, whereas our data suggested that sleep was distinct from all other behaviors. Differences in species and animal sex could account for these differences in behaviors. Notably in our study unbiased clustering could detect associations that may not be readily apparent.
Sniffing behavior in rodents is complex; it can be a way to explore the environment, but it can also accompany different social interactions (Deschênes et al., 2012, Pitcher et al., 2019, Wesson, 2013). It is possible that sniffing could be more reflective of exploration of the home cage environment (Fig. 5). Since we have seen elevated levels of lyso-Gb3 in the brain, it is possible that buildup of glycosphingolipids could cause damage to the olfactory sensory system leading to decreased sniffing (Miller et al., 2018). Additionally, a case study of a patient with Fabry disease found accumulations of Gb3 in the cortical accessory (olfactory) nucleus in the amygdala (Del Tredici et al., 2020). However, we found that gabapentin administration reverses this decrease in sniffing only in Fabry rats, suggesting that the decreased sniffing may be pain-related in the context of the Fabry rats. In the patient population, no significant smelling deficits have been noted to date, but there is evidence that pain in Fabry disease impacts daily activities (Arends et al., 2015, Gold et al., 2002, Morand et al., 2019). Therefore, although the Fabry rat and patient affected activities are different, Fabry disease impacts the rats’ daily function which may also be true in other preclinical genetic models.
One limitation of this study is that Fabry disease is a complex multi-system disease and other non-pain symptoms can potentially influence animal behavior. Patients with Fabry disease can have cardiovascular complications, kidney problems, decreased vision, tinnitus, digestive issues, angiokeratomas, fatigue, depression, and anxiety (Miller et al., 2020). All of these symptoms can contribute to the dramatic decrease in activity and quality of life in patients (Morand et al., 2019). Patients report pain significantly impacting their daily activity (Morand et al., 2019), but it is unclear how much other non-pain co-morbidities may influence changes in daily behavior or even pain perception. In rodents we are limited to behavior observation which can be substantially impacted by the aforementioned systemic complications. Therefore, in this study we used an analgesic in order to determine whether some of these behavioral changes in the Fabry rat could be pain-related versus inherent differences due to non-pain complications. As observed in this study there is only a partial reversal of the sniffing phenotype and therefore, it is possible that other non-pain complications in the Fabry rat may also contribute to the deceased sniffing observed. Although sniffing was altered in both male and female Fabry rats and was the behavior that was most greatly affected by gabapentin administration, there were other behaviors that were altered in both male and female rats that could be validated and assessed further. Additionally, behaviors that were changed in one sex but not in another provide other interesting avenues to pursue in future studies. While we have tested the effectiveness of gabapentin in male rats, studies have shown that there can be sex differences in analgesic effectiveness. Therefore, sex differences in analgesic performance can be an additional method utilized in HCS. Another limitation is that we assume that gabapentin is causing pain relief and that pain relief is resulting in a change in rat behavior. Although gabapentin can reduce pain, it also has off-target effects which may affect behavior. Although not addressed in this study, administration of multiple analgesics may give greater confidence to behavior changes being pain-related.
HCS is a powerful tool to study ongoing behavior in rats, however, there are limitations to the technique. One such limitation is that animals often are singly housed in the home cage. Rats and mice are social animals and therefore, isolating them has been shown to cause memory deficits, increased anxiety-like, and increased depressive-like behaviors over the course of weeks (Liu et al., 2020, Manouze et al., 2019). To avoid this effect, we limited the separation of the rats from the cage mates to less than a week. However, for longer-term studies this could have a significant impact on the behaviors measured. An important caution in interpreting HCS data is avoiding over ascribing rodent behaviors to patient experience. As shown in this study, there are some behaviors such as sleep that are increased in the Fabry rat population. Patients with severe Fabry pain are more physically inactive and report disruption of their sleep. However, rats sleep more than humans and their sleep is non-continuous compared to humans (Campbell and Tobler, 1984, Clancy et al., 1978, Stenvers et al., 2016, Zhang et al., 2021). Therefore, it is possible that the sleep behavior measured by HCS is more akin to human inactivity rather than what is typically thought of as human sleep. It is important to note that the HomeCageScan software determines if an animal is sleeping by looking for longer periods of inactivity. However, an actual sleep state needs to be confirmed using electroencephalogram (EEG) monitoring. Despite these limitations, HCS is a powerful tool to investigate non-evoked behavioral differences in Fabry rats that resemble lifestyle changes in humans.
In conclusion, we used PCA and unbiased behavioral groupings in conjunction with the HCS system to demonstrate that rats with Fabry disease exhibit decreases in active behaviors including self-care, locomotion, and exploration while increasing the amount of time they slept. These changes in activity are consistent with reductions in movement and daily activity that patients with Fabry disease report. We also found sniffing was depressed in rats with Fabry disease, but that could be reversed with the analgesic gabapentin. Therefore, HCS measurement of daily behaviors in Fabry disease rats may also provide insight into ongoing pain-related behaviors in a way that evoked tests cannot. This platform can be used for subsequent unbiased screening for pain-relieving drugs in Fabry rats and other genetic models of pain.
CRediT authorship contribution statement
Anthony J. Burand Jr.: Writing – original draft. Tyler B. Waltz: Writing – review & editing. Anna D. Manis: Writing – review & editing. Matthew R. Hodges: Writing – review & editing. Cheryl L. Stucky: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Vanessa Ehlers for her proof reading and editing expertise on this manuscript. This research was supported by the National Institute of Health (NIH) R37 NS108278-02 (CLS), NIH RO1 NS070711-11 (CLS), NIH HL122358 (MRH), and NIH DK122647 (AM).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynpai.2022.100113.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Allchorne, A.J., Broom, D.C., Woolf, C.J., 2005. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol. Pain 1, 1744-8069-1–36. https://doi.org/10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed]
- Arends M., Hollak C.E.M., Biegstraaten M. Quality of life in patients with Fabry disease: A systematic review of the literature. Orphanet J. Rare Dis. 2015;10:1–10. doi: 10.1186/s13023-015-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegstraaten M., Hollak C.E.M., Bakkers M., Faber C.G., Aerts J.M.F.G., van Schaik I.N. Small fiber neuropathy in Fabry disease. Mol. Genet. Metab. 2012;106:135–141. doi: 10.1016/j.ymgme.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Brenner D.S., Golden J.P., Gereau R.W., Sakakibara M. A novel behavioral assay for measuring cold sensation in mice. PLoS One. 2012;7(6):e39765. doi: 10.1371/journal.pone.0039765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S.S., Tobler I. Animal sleep: A review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-X. [DOI] [PubMed] [Google Scholar]
- Carnevali L., Sgoifo A., Trombini M., Landgraf R., Neumann I.D., Nalivaiko E., Schmidt M.V. Different patterns of respiration in rat lines selectively bred for high or low anxiety. PLoS One. 2013;8(5):e64519. doi: 10.1371/journal.pone.0064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H., Jang, Y., Lee, B., Chun, H., Jung, J., Kim, S.M., Hwang, S.W., Oh, U., 2013. Voluntary movements as a possible non-reflexive pain assay. Mol. Pain 9, 1744-8069-9–25. https://doi.org/10.1186/1744-8069-9-25. [DOI] [PMC free article] [PubMed]
- Clancy J., Caldwell D., Villeneuve M., Sangiah S. Daytime sleep-wake cycle in the rat. Physiol. Behav. 1978;21:457–459. doi: 10.1016/0031-9384(78)90109-9. [DOI] [PubMed] [Google Scholar]
- Clark J.D. Preclinical pain research: Can we do Better? Anesthesiology. 2016;125:846–849. doi: 10.1097/ALN.0000000000001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K., Ludolph A.C., Feldengut S., Jacob C., Reichmann H., Bohl J.R., Braak H. Fabry disease with concomitant Lewy body disease. J. Neuropathol. Exp. Neurol. 2020;79:378–392. doi: 10.1093/jnen/nlz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M., Moore J., Kleinfeld D. Sniffing and whisking in rodents. Curr. Opin. Neurobiol. 2012;22:243–250. doi: 10.1016/j.conb.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuis J.R., Dvorakova L.S., Vetter I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017;10:1–17. doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A.S., Lanigan M.T., Upton N., Lione L.A. Preclinical neuropathic pain assessment; the importance of translatability and bidirectional research. Front. Pharmacol. 2021;11:1–21. doi: 10.3389/fphar.2020.614990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstenpointner J., Ruscheweyh R., Attal N., Baron R., Bouhassira D., Enax-Krumova E.K., Finnerup N.B., Freynhagen R., Gierthmühlen J., Hansson P., Jensen T.S., Maier C., Rice A.S.C., Segerdahl M., Tölle T., Treede R.-D., Vollert J. No pain, still gain (of function): the relation between sensory profiles and the presence or absence of self-reported pain in a large multicenter cohort of patients with neuropathy. Pain. 2021;162:718–727. doi: 10.1097/j.pain.0000000000002058. [DOI] [PubMed] [Google Scholar]
- Fried N.T., Chamessian A., Zylka M.J., Abdus-Saboor I. Improving pain assessment in mice and rats with advanced videography and computational approaches. Pain. 2020;161:1420–1424. doi: 10.1097/j.pain.0000000000001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold K.F., Pastores G.M., Botteman M.F., Yeh J.M., Sweeney S., Aliski W., Pashos C.L. Quality of life of patients with Fabry disease. Qual. Life Res. 2002;11:317–327. doi: 10.1023/a:1015511908710. [DOI] [PubMed] [Google Scholar]
- Gris V., K., Yamamoto, K., Gharagozloo, M., Mahmoud, S., Simard, C., Gris, P., Gris, D. Exhaustive behavioral profile assay to detect genotype differences between wild-type, inflammasome-deficient, and Nlrp12 knock-out mice. AIMS Med. Sci. 2018;5:238–251. doi: 10.3934/medsci.2018.3.238. [DOI] [Google Scholar]
- Hargreaves K., Dubner R., Brown F., Flores C., Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hasriadi, Wasana P.W.D., Vajragupta O., Rojsitthisak P., Towiwat P. Automated home-cage for the evaluation of innate non-reflexive pain behaviors in a mouse model of inflammatory pain. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-91444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkin R.J., Bissler J., Banikazemi M., Clarke L., Eng C.M., Germain D.P., Lemay R., Tylki-Szymanska A., Wilcox W.R. Characterization of Fabry Disease in 352 Pediatric Patients in the Fabry Registry. Pediatr. Res. 2008;64:550–555. doi: 10.1203/PDR.0b013e318183f132. [DOI] [PubMed] [Google Scholar]
- Lehmann M.L., Mustafa T., Eiden A.M., Herkenham M., Eiden L.E. PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology. 2013;38:702–715. doi: 10.1016/j.psyneuen.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Wang Y., An A.Y., Banker C., Qian Y., O’Donnell J.M. Single housing-induced effects on cognitive impairment and depression-like behavior in male and female mice involve neuroplasticity-related signaling. Eur. J. Neurosci. 2020;52:2694–2704. doi: 10.1111/ejn.14565. [DOI] [PubMed] [Google Scholar]
- Loeser J.D. Chronic Pain Is More Than a Peripheral Event. J. Pain. 2012;13:930–931. doi: 10.1016/j.jpain.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Manis A.D., Palygin O., Isaeva E., Levchenko V., LaViolette P.S., Pavlov T.S., Hodges M.R., Staruschenko A. Kcnj16 knockout produces audiogenic seizures in the Dahl salt-sensitive rat. JCI Insight. 2021;6:1–14. doi: 10.1172/jci.insight.143251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manouze, H., Ghestem, A., Poillerat, V., Bennis, M., Ba-M’hamed, S., Benoliel, J.J., Becker, C., Bernard, C., 2019. Effects of Single Cage Housing on Stress, Cognitive, and Seizure Parameters in the Rat and Mouse Pilocarpine Models of Epilepsy. eneuro 6, ENEURO.0179-18.2019. https://doi.org/10.1523/ENEURO.0179-18.2019. [DOI] [PMC free article] [PubMed]
- Melo-Carrillo A., Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia. 2013;33:1096–1105. doi: 10.1177/0333102413486320. [DOI] [PubMed] [Google Scholar]
- Miller, J.J., Aoki, K., Moehring, F., Murphy, C.A., O’Hara, C.L., Tiemeyer, M., Stucky, C.L., Dahms, N.M., 2018. Neuropathic pain in a Fabry disease rat model. JCI Insight 3. https://doi.org/10.1172/jci.insight.99171. [DOI] [PMC free article] [PubMed]
- Miller J.J., Kanack A.J., Dahms N.M. Progress in the understanding and treatment of Fabry disease. Biochim. Biophys. Acta Gen. Subj. 2020;1864(1):129437. doi: 10.1016/j.bbagen.2019.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand O., Johnson J., Walter J., Atkinson L., Kline G., Frey A., Politei J., Schiffmann R. Symptoms and Quality of Life in Patients with Fabry Disease: Results from an International Patient Survey. Adv. Ther. 2019;36:2866–2880. doi: 10.1007/s12325-019-01061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus S.S., Bilsky E.J., Carmo G.P.D., Stevenson G.W. In: Emerging Strategies for the Treatment of Neuropathic Pain., Methods in Molecular Biology. Szallasi A., editor. Humana Press; Totowa, NJ: 2010. Rationale and Methods for Assessment of Pain-Depressed Behavior in Preclinical Assays of Pain and Analgesia; pp. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus S.S., Neddenriep B., Altarifi A.A., Carroll F.I., Leitl M.D., Miller L.L. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher M.H., Tarum F., Lehmann M., Bushnell M.C. Persistent inflammatory pain alters sexually-motivated behavior in male rats. Behav. Brain Res. 2019;356:380–389. doi: 10.1016/j.bbr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politei J.M., Bouhassira D., Germain D.P., Goizet C., Guerrero-Sola A., Hilz M.J., Hutton E.J., Karaa A., Liguori R., Üçeyler N., Zeltzer L.K., Burlina A. Pain in Fabry Disease: Practical Recommendations for Diagnosis and Treatment. CNS Neurosci. Ther. 2016;22:568–576. doi: 10.1111/cns.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic L.L., Türck D., von Hodenberg A., Vollmer K.O., McNally W.P., DeHart P.D., Hanson B.J., Bockbrader H.N., Chang T. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab. Dispos. 1995;23:441–448. [PubMed] [Google Scholar]
- Roughan J.V., Wright-Williams S.L., Flecknell P.A. Automated analysis of postoperative behaviour: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab. Anim. 2009;43:17–26. doi: 10.1258/la.2008.007156. [DOI] [PubMed] [Google Scholar]
- Sadler K.E., Mogil J.S., Stucky C.L. Innovations and advances in modelling and measuring pain in animals. Nat. Rev. Neurosci. 2021;23(2):70–85. doi: 10.1038/s41583-021-00536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M. What can we learn from the failure of quantitative sensory testing? Pain. 2021;162:663–664. doi: 10.1097/j.pain.0000000000002059. [DOI] [PubMed] [Google Scholar]
- Scholl J.L., Afzal A., Fox L.C., Watt M.J., Forster G.L. Sex differences in anxiety-like behaviors in rats. Physiol. Behav. 2019;211 doi: 10.1016/j.physbeh.2019.112670. [DOI] [PubMed] [Google Scholar]
- Seib D.R., Chahley E., Princz-Lebel O., Snyder J.S., Christie B.R. Intact memory for local and distal cues in male and female rats that lack adult neurogenesis. PLoS One. 2018;13(5):e0197869. doi: 10.1371/journal.pone.0197869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotin Y.B., Costa M.E., Laplagne D.A. Rodent ultrasonic vocalizations are bound to active sniffing behavior. Front. Behav. Neurosci. 2014;8:1–12. doi: 10.3389/fnbeh.2014.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E., Martin L.J., Isbester K.A., Sotocinal S.G., Rosen S., Tuttle A.H., Wieskopf J.S., Acland E.L., Dokova A., Kadoura B., Leger P., Mapplebeck J.C.S., McPhail M., Delaney A., Wigerblad G., Schumann A.P., Quinn T., Frasnelli J., Svensson C.I., Sternberg W.F., Mogil J.S. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Stenvers D.J., van Dorp R., Foppen E., Mendoza J., Opperhuizen A.-L., Fliers E., Bisschop P.H., Meijer J.H., Kalsbeek A., Deboer T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 2016;6:35662. doi: 10.1038/srep35662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan F.K., Zucker I. Circadian Rhythms in Drinking Behavior and Locomotor Activity of Rats Are Eliminated by Hypothalamic Lesions. Proc. Natl. Acad. Sci. 1972;69:1583–1586. doi: 10.1016/B978-0-12-374984-0.00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G.W., Cormier J., Mercer H., Adams C., Dunbar C., Negus S.S., Bilsky E.J. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: Drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl K.P., Thomas A.J., St. Jean P., Schlenker E.H., Koletsky R.J., Schork N.J. Ventilation and metabolism among rat strains. J. Appl. Physiol. 1997;82(1):317–323. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- Üçeyler N., Ganendiran S., Kramer D., Sommer C. Characterization of Pain in Fabry Disease. Clin. J. Pain. 2014;30:915–920. doi: 10.1097/AJP.0000000000000041. [DOI] [PubMed] [Google Scholar]
- Usui S., Okazaki T., Honda Y. Interruption of the rat circadian clock by short light-dark cycles. Am. J. Physiol. Integr. Comp. Physiol. 2003;284:R1255–R1259. doi: 10.1152/ajpregu.00717.2002. [DOI] [PubMed] [Google Scholar]
- Vierck C.J., Hansson P.T., Yezierski R.P. Clinical and pre-clinical pain assessment: Are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Vollmer K.O., von Hodenberg A., Kölle E.U. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung. 1986;36:830–839. [PubMed] [Google Scholar]
- Waltz T.B., Burand A.J., Sadler K.E., Stucky C.L. Sensory-specific peripheral nerve pathology in a rat model of Fabry disease. Neurobiol. Pain. 2021;10:100074. doi: 10.1016/j.ynpai.2021.100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson D.W. Sniffing behavior communicates social hierarchy. Curr. Biol. 2013;23:575–580. doi: 10.1016/j.cub.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Wesson D.W., Verhagen J.V., Wachowiak M. Why Sniff Fast? The Relationship Between Sniff Frequency, Odor Discrimination, and Receptor Neuron Activation in the Rat. J. Neurophysiol. 2009;101:1089–1102. doi: 10.1152/jn.90981.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright-Williams S., Flecknell P.A., Roughan J.V., Price T.J. Comparative Effects of Vasectomy Surgery and Buprenorphine Treatment on Faecal Corticosterone Concentrations and Behaviour Assessed by Manual and Automated Analysis Methods in C57 and C3H Mice. PLoS One. 2013;8(9):e75948. doi: 10.1371/journal.pone.0075948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Gris K.V., Sotelo Fonseca J.E., Gharagozloo M., Mahmoud S., Simard C., Houle-Martel D., Cloutier T., Gris P., Gris D. Exhaustive Multi-Parametric Assessment of the Behavioral Array of Daily Activities of Mice Using Cluster and Factor Analysis. Front. Behav. Neurosci. 2018;12:1–13. doi: 10.3389/fnbeh.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Lecker I., Collymore C., Dokova A., Pham M.C., Rosen S.F., Crawhall-Duk H., Zain M., Valencia M., Filippini H.F., Li J., D’Souza A.J., Cho C., Michailidis V., Whissell P.D., Patel I., Steenland H.W., Virginia Lee W.-J., Moayedi M., Sterley T.-L., Bains J.S., Stratton J.A., Matyas J.R., Biernaskie J., Dubins D., Vukobradovic I., Bezginov A., Flenniken A.M., Martin L.J., Mogil J.S., Bonin R.P. Cage-lid hanging behavior as a translationally relevant measure of pain in mice. Pain. 2021;162:1416–1425. doi: 10.1097/j.pain.0000000000002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.