Abstract
Experimental infection of beagle dogs with Helicobacter pylori induces recruitment to the gastric mucosae of neutrophils at early stages and later of mononuclear cells that organize into lymphoid follicles. These structures become macroscopically evident and consist of peripheral CD4+ T lymphocytes and central CD21+ B lymphocytes. Furthermore, transient expression of interleukin-8 (IL-8) parallels the presence of neutrophils in the gastric mucosae, whereas expression of IL-6 tends to persist chronically.
Helicobacter pylori is a gram-negative bacterium that chronically colonizes the gastric mucosae of humans, causing chronic gastritis, peptic ulcer, and in some individuals gastric adenocarcinoma and lymphoma (3). Little is known about the natural history of the infection, especially the early events which follow colonization. Since most infections with H. pylori occur during childhood (18) and since most of these infections are usually not diagnosed, most of the early pathological events following H. pylori infection can be better investigated in appropriate animal models.
Various animal models have been proposed for the study of the pathogenesis of H. pylori-induced lesions (3, 6). Recently we showed that in conventional beagle dogs, colonization with H. pylori and disease can be monitored in the same individuals without any need for necropsy (17). In that study, we showed early infiltration with polymorphonuclear cells that were then replaced by mononuclear cells, which later became organized in very well structured lymphoid follicles in the gastric mucosa (17).
As a follow-up of that study, immunohistochemical investigation of the lymphoid cells infiltrating the gastric mucosa of those dogs and of uninfected controls was performed. The characteristics of the dogs, the experimental infection with H. pylori strain SPM326s, and the clinical and pathological events following infection have already been reported (17).
Biopsies were taken under endoscopy from the antrum, corpus, and fundus at 1, 2, 4, 8, 12, 18, and 24 weeks postinfection. Sections (3 μm) were prepared and stained with hematoxylin-eosin. Histological examinations included assessment of the number of inflammatory cells at ×400 magnification using a visual analogue scale modified for canine specimens (10). Gastritis score was defined using the Sydney system (4). For immunohistochemistry, sections were allowed to adhere to pretreated slides. After standard treatments, sections were assayed with the following monoclonal antibodies (MAbs): anti-human interleukin-6 (IL-6) (Genzyme Diagnostics, Cambridge, Mass.); anti-human IL-8 (Genzyme); rat anti-canine monocytes/macrophages, rat anti-canine CD3, rat anti-canine CD4, and rat anti-canine CD8 (all from Serotec Ltd., Oxford, United Kingdom); mouse anti-canine CD21 (VMRD Inc., Pullman, Wash.); and mouse anti-canine neutrophils (VMRD Inc.). Antibody binding was revealed with avidin-biotin complex (ABC)–peroxidase or ABC-alkaline phosphatase techniques using biotin-conjugated horse anti-mouse immunoglobulins or biotin-conjugated rabbit anti-rat immunoglobulins. The enzymatic reaction was developed using the relevant substrates. All histologic examinations were carried out blind and included controls using appropriate isotype-matched antibodies.
Prior to infection with H. pylori, the gastric mucosae of dogs were normal, both endoscopically and histologically, with no evidence of infection with other Helicobacter species. Similarly, uninfected dogs kept as controls never showed signs of gastritis or of cellular infiltrations. One week after infection, marked edema and hyperemia appeared in the lamina propria, especially in the corpus and antrum, with an important infiltration of polymorphonucleates (Fig. 1) in the lamina propria around the glands, the structure of which, in many cases, appeared to be altered. Several neutrophils were observed immunohistochemically. At the same time, epithelial cells of the deeper glandular crypts exhibited a clear cytoplasmic positivity for IL-8 (17).
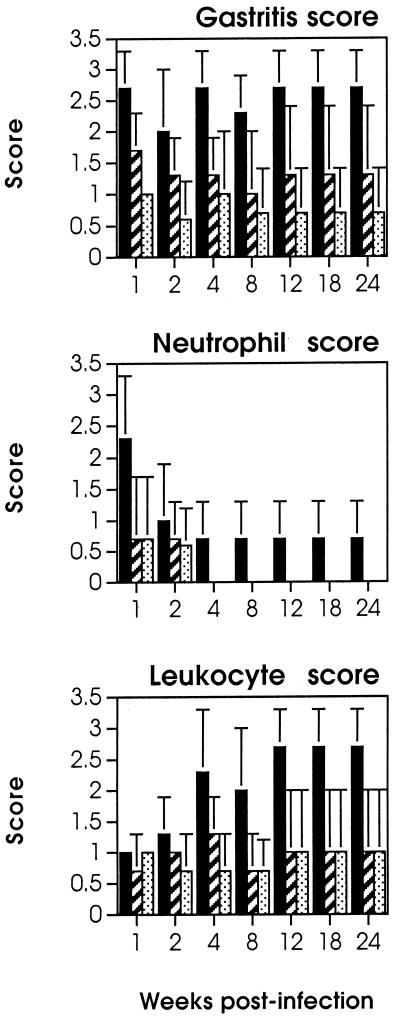
FIG. 1.
Gastritis, neutrophil, and leukocyte scores in beagle dogs experimentally infected with H. pylori. Dogs were infected orally at time zero as described in detail previously (17). Biopsies were taken during endoscopy at the indicated time points (weeks). Scores were calculated as reported earlier (4, 10). Solid bars, antrum; hatched bars, corpus; dotted bars, fundus. Each bar represents the average (plus 1 standard deviation) for three dogs infected in one experiment. Similar results were obtained in a second experiment. Uninfected dogs did not show any sign of gastritis or of neutrophil or leukocyte infiltrations.
At 2 weeks postinfection, we observed an important drop in the number of neutrophils and an increase in that of mononuclear cells (Fig. 1), consisting mostly of CD3+ T lymphocytes. At this stage, IL-8-positive epithelial cells were observed in the glandular structures and in antral mucosa, where a moderate infiltration of neutrophils still persisted (Fig. 2a). Scattered infiltrates of CD3+ T lymphocytes appeared in a juxtaglandular position, organized in small aggregates or dispersed in the corpus and antrum (Fig. 2b). A strong expression of IL-6 was observed in epithelial cells and in mononuclear cells in subepithelial areas, mainly in the antrum. Unlike IL-8, the expression of IL-6 remained unaltered during the entire study, and at later stages its expression could also be seen in the corpus (Fig. 2c). Four weeks after infection with H. pylori, CD3+ (CD4+) lymphocytes appeared more abundantly in the interglandular corion of the corpus and even more of the antrum (Fig. 2d). In some antral areas, the lymphoid infiltrate contained some CD8+ cells grouped in a periglandular position immediately beneath the basal lamina of the epithelium (Fig. 2e). The appearance of these infiltrates was accompanied by erosion of the epithelial surface and by the presence of cytoplasmic vacuoles (17).
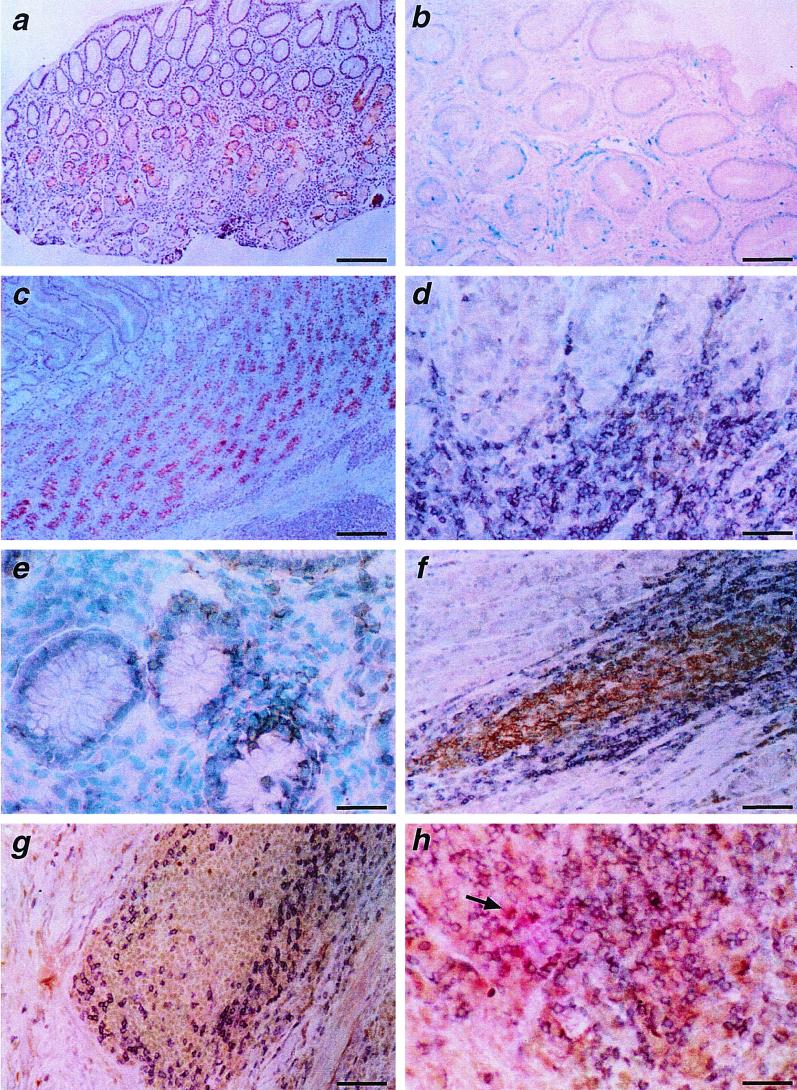
FIG. 2.
Immunohistochemical findings in the gastric mucosae of beagle dogs experimentally infected with H. pylori. (a) Expression of IL-8 2 weeks after H. pylori infection. At this stage, positivity for IL-8 is observed in glandular structures of the antral mucosa. Bar, 100 μm. (b) Scattered infiltrates, 2 weeks after infection, of CD3+ T lymphocytes dispersed among the glands of the antrum. Bar, 40 μm. (c) Strong expression of IL-6 in the epithelial cells of the corpus 12 weeks after infection. The presence of chronic gastritis is evident. Bar, 100 μm. (d) Small aggregates of CD4+ T lymphocytes (black stained) in the interglandular corion of the antrum 4 weeks after infection. Bar, 16.6 μm. (e) CD8+ T lymphocytes (black stained) in a periglandular position immediately beneath the basal lamina propria of the antral epithelium 4 weeks after infection. Bar, 16.6 μm. (f) Well-structured lymphoid follicle 8 weeks after infection. Note the germinal center-like portion consisting of CD21+ lymphocytes (brown stained) and the cortical portion consisting of CD3+ T lymphocytes (violet stained). Bar, 25 μm. (g) Presence of a few scattered CD8+ T lymphocytes (black stained) in the cortical area of a well-structured antral lymphoid follicle 8 weeks after infection. Bar, 25 μm. (h) CD4+ T lymphocytes (brown stained), CD8+ T lymphocytes (violet stained), and some macrophages (red stained, arrow) in the cortical area of a well-structured antral lymphoid follicle 12 weeks after infection. Bar, 25 μm.
From the eighth week, CD3+ cell infiltrates spread in bands or organized in very well structured lymphoid follicles with a germinal center-like portion composed of CD21+ lymphocytes and a peripheral portion consisting predominantly of CD3+ CD4+ lymphocytes (Fig. 2f). A few scattered CD8+ T lymphocytes (Fig. 2g) and macrophages (Fig. 2h) were also seen in the cortical areas. From the 12th week postinfection onwards, the number and size of the follicular structures (mainly in the antrum) significantly increased, causing the appearance of macroscopic nodules easily visible by endoscopy (data not shown). Subsequently we also observed a more diffuse infiltration in the antrum of CD4+ lymphocytes, with rare CD8+ lymphocytes in juxtaglandular or subepithelial areas. At this stage, the presence of neutrophils interspersed within the mononuclear cell population was suggestive of an active chronic gastritis.
Most studies aimed at characterizing the lymphocytic infiltration of gastric mucosae in natural or experimental infection with H. pylori have been performed by cell sorting techniques or reverse transcription-PCR (1, 5, 19, 22, 23). These approaches do not allow determination of the location and organization of lymphoid cell populations or the nature and location of cytokine-producing cells, which can be better determined by immunohistochemistry (2, 12). In this study, the use of immunohistochemistry has allowed the identification and localization over time of the inflammatory cells infiltrating the gastric mucosa of experimentally infected dogs and of the gastric cells expressing some cytokines which may be of importance in H. pylori-induced pathology. Of course, the availability of specific antibodies to certain dog cytokines may represent a limit of the histopathological approach.
The important infiltration of neutrophils at initial stages of infection in beagle dogs has also been reported upon accidental or voluntary infections in humans (13, 15, 20). Furthermore, the presence of neutrophils among the mononuclear cells at later stages is the hallmark of chronic active gastritis.
It is noteworthy that at early stages of infection both IL-8 and IL-6 were detectable. The anti-human cytokine MAbs used in this study clearly gave specific stainings because of the consistently negative results in uninfected controls and because of the high degree of homology shared by human and canine IL-8 and IL-6 (11, 14). IL-8 tended to disappear at later stages together with the neutrophilic infiltration, whereas the expression of IL-6 remained unchanged over time. This suggests that IL-8 production is regulated by H. pylori products, e.g., pathogenicity island-encoded antigens (3), more than IL-6 production, which may be sustained by the inflammatory phenomena induced by H. pylori. In previous studies in humans (1), increased IL-6 levels were observed in chronic H. pylori infection, together with a concomitant increase in CD8+ lymphocytes and natural killer cells. In the present study, the strong positivity of epithelial cells for IL-6 in biopsies from the antrum and corpus suggests that gastric epithelial cells may contribute to the proinflammatory response to H. pylori infection, either by cytokine production or by uptake of IL-6 produced by the lamina propria or intraepithelial leukocytes, as observed in humans (12).
The lymphocyte infiltrate, initially diffuse and later organized in follicular structures, represents a consequence of H. pylori infection in both natural (7) and experimental (6, 8, 9) infections. In the present study, the recruitment of lymphocytes to the gastric mucosa represents an early event. Later, classical follicular structures appear in the gastric mucosa, with germinal centers containing mostly CD21+ B lymphocytes and CD3+ CD4+ lymphocytes in the cortical areas. This suggests that in our experimentally infected dogs, as in patients with chronic gastritis, chronic H. pylori infection may favor the development and persistence of lymphoid follicles in which continuous helper T-cell activation may eventually lead to uncontrolled follicular B-cell proliferation (21). It would be interesting to investigate over time the clonality of the B lymphocytes present in these gastric follicular structures and compare it with that found in chronically infected individuals and in patients with mucosa-associated lymphoid tissue lymphoma of the stomach (16, 24).
The appearance of CD3+ CD8+ cells dispersed around the lymphoid follicles and at the base of the antral glands has also been observed in naturally infected patients (1). It is not clear yet what role these CD8+ cells may play in the immune response to H. pylori. Given the strictly extracellular localization of H. pylori, it is unlikely that these CD8+ cells have cytolytic activity. However, they may contribute to the inflammatory events occurring at both early and chronic stages of infection by producing gamma interferon or other proinflammatory cytokines and chemokines (2, 12). It would be interesting to test this hypothesis in our beagle model of infection.
REFERENCES
- 1.Agnihotri N, Bhasin D K, Vohra H, Ray P, Singh K, Ganguly N K. Characterization of lymphocytic subsets and cytokine production in gastric biopsy samples from Helicobacter pylori patients. Scand J Gastroenterol. 1998;33:704–709. doi: 10.1080/00365529850171639. [DOI] [PubMed] [Google Scholar]
- 2.Ahlstedt I, Lindholm C, Lonroth H, Hamlet A, Svennerholm A M, Quiding-Jarbrink M. Role of local cytokines in increased gastric expression of the secretory component in Helicobacter pylori infection. Infect Immun. 1999;67:4921–4925. doi: 10.1128/iai.67.9.4921-4925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 4.Dixon M F, Genta R M, Yardley J F, Correa P. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Dogan A, Du M, Koulis A, Briskin M J, Isaacson P G. Expression of lymphocyte homing receptors and vascular addressins in low-grade gastric B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol. 1997;151:1361–1369. [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton K A. Animal models of Helicobacter gastritis. Curr Top Microbiol Immunol. 1999;241:123–154. doi: 10.1007/978-3-642-60013-5_8. [DOI] [PubMed] [Google Scholar]
- 7.Genta R M, Hammer H W, Graham D Y. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993;24:577–583. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- 8.Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, Covacci A, Telford J L, De Magistris M T, Pizza M, Rappuoli R, Del Giudice G. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handt L K, Fox J G, Stalis I H, Rufo R, Lee G, Linn J, Li X T, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995;33:2280–2289. doi: 10.1128/jcm.33.9.2280-2289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happonen I, Linden J, Saari S, Karjalainen M, Hanninen M L, Jalava K, Westermark E. Detection and effects of helicobacters in healthy dogs and in dogs with signs of gastritis. J Am Vet Med Assoc. 1998;213:1767–1774. [PubMed] [Google Scholar]
- 11.Kukielka G L, Smith C W, Manning A M, Youker K A, Michael L H, Entman M L. Induction of interleukin-6 synthesis in the myocardium: potential role in postreperfusion inflammatory injury. Circulation. 1995;92:1866–1875. doi: 10.1161/01.cir.92.7.1866. [DOI] [PubMed] [Google Scholar]
- 12.Lindholm C, Quiding-Jarbrink M, Lonroth H, Hamlet A, Svennerholm A M. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall B J, Armstrong J A, McGechie D B, Glancy R J. Attempt to fulfill Koch's postulate for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto Y, Mohamed A, Onodera T, Kato H, Ohashi T, Goitsuka R, Tsujimoto H, Hasegawa A, Furusawa S, Yoshihara K. Molecular cloning and expression of canine interleukin-8 cDNA. Cytokine. 1994;6:455–461. doi: 10.1016/1043-4666(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 15.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raises gastric pH. Am J Gastroenterol. 1987;82:192–199. [PubMed] [Google Scholar]
- 16.Nakamura S, Aoyagi K, Furuse M, Suekane H, Matsumoto T, Yao T, Sakai Y, Fuchigami T, Yamamoto I, Tsuneyoshi M, Fujishima M. B-cell monoclonality precedes the development of gastric MALT lymphomas in Helicobacter pylori-associated chronic gastritis. Am J Pathol. 1998;152:1271–1279. [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi G, Rossi M, Vitali C G, Fortuna D, Burroni D, Pancotto L, Capecchi S, Sozzi S, Renzoni G, Braca G, Del Giudice G, Rappuoli R, Ghiara P, Taccini E. A conventional beagle dog model for acute and chronic infection with Helicobacter pylori. Infect Immun. 1999;67:3112–3120. doi: 10.1128/iai.67.6.3112-3120.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothenbacher D, Bode G, Berg G, Knayer U, Gonser T, Adler G, Brenner H. Helicobacter pylori among preschool children and their parents: evidence of parent-child transmission. J Infect Dis. 1999;179:398–402. doi: 10.1086/314595. [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama T, Everett S M, Dixon M F, Axon A T, Crabtree J E. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol. 1998;51:765–770. doi: 10.1136/jcp.51.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobala G M, Crabtree J E, Dixon M F, Schorah C J, Taylor J D, Rathbone B J, Heatley R V, Axon A T. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991;32:1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terres A M, Pajares J M. An increased number of follicles containing activated CD69+ helper T cells and proliferating CD71+ B cells are found in Helicobacter pylori infected gastric mucosa. Am J Gastroenterol. 1998;93:579–583. doi: 10.1111/j.1572-0241.1998.168_b.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cag gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 23.Ye G, Barrera C, Fan X, Gourley W K, Crowe S E, Ernst P B, Reyes V E. Expression of B7-1 and B7-2 costimulatory molecules by human gastric epithelial cells: potential role in CD4+ T cell activation during Helicobacter pylori infection. J Clin Investig. 1997;99:1628–1636. doi: 10.1172/JCI119325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli F. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid tissue lymphoma of the stomach. N Engl J Med. 1998;338:804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]