Abstract
Background:
To investigage the thiol and disulphide levels in Helicobacter pylori-positive patients with non-ulcer dyspepsia and investigate the change in these levels with eradication therapy.
Methods:
This is a prospective observational study. A total of 320 patients diagnosed with dyspepsia according to Rome IV criteria were included in the study. First, blood samples were drawn from patients to determine their serum thiol and disulphide levels. Endoscopic biopsy was performed on all patients and the biopsy specimens obtained were examined pathologically. Patients positive for H. pylori were administered eradication therapy. Blood samples were drawn from these patients for the second time, and their serum thiol and disulphide levels were measured. The thiol–disulfide levels of the patients who were successful in H. pylori eradication treatment, with those who were not, were compared before and after the treatment.
Results:
The mean plasma disulphide level decreased significantly from 14.0 ± 6.6 to 10.9 ± 5.9 μmol/L in H. pylori-positive patients that responded to the H. pylori eradication treatment (P = 0.033). On the other hand, there was an insignificant increase in the mean serum thiol level (341.4 ± 30.5 vs. 342.6 ± 29.8 μmol/L; P = 0.273) and an insignificant decrease in the mean serum disulphide level (15.2 ± 2.5 vs. 14.8 ± 2.3 μmol/L; P = 0.163) in H. pylori-positive patients that did not respond to the H. pylori eradication treatment.
Conclusion:
The inflammation caused by H. pylori shifted the thiol–disulphide equilibrium in the cell redox system towards the direction of disulphide. The study findings suggest that the restoration of the said hemostatic balance with eradication therapy relieved the organism from oxidative stress.
Keywords: Eradication, helicobacter pylori, oxidative stress, thiol-disulphide hemostasis
INTRODUCTıON
Helicobacter pylori, which was discovered in 1982, is the most common cause of chronic inflammation in the stomach. This bacterium has colonized the stomachs of about half of the world's population.[1] Chronic inflammation caused by H. pylori remains an important etiological cause of chronic gastritis, atrophic gastritis, gastric ulcer, duodenal ulcer, malt lymphoma, and gastric cancer.[2]
H. pylori is asymptomatic in most people but may cause serious diseases such as ulcers and cancer in some cases. The factors that increase the risk for these diseases in the presence of H. pylori are not clear. Studies on the relevant immune and inflammatory mechanisms are expected to shed light on this subject.[3]
Oxygenated aerobic reactions that take place in cell mitochondria are essential for life. However, these reactions give rise to the release of reactive oxygen radicals (ROS) that are very harmful to the cell membrane and nucleus. ROS are neutralized by the antioxidant mechanisms of the cells. Hence, the decrease in the antioxidant capacity can cause irreversible damage to both cell membrane and cell DNA.[4]
Thiol groups (mercaptans) are powerful antioxidant molecules. They contain hydrogen and sulfhydryl groups that play an important role in neutralizing ROS by enzymatic or non-enzymatic means. Thiol–disulphide homeostasis (TDH) is the reversal of thiol oxidation in proteins. Disulphides (DS) are dynamic and redox-sensitive compounds. The covalent bonds formed between two thiol groups result in the formation of DS. As stress or inflammation increases in the cell, the bonds between these antioxidant thiol molecules are broken down and DSs are released.[5,6] In physiological conditions, the DS bonds are reduced back to thiol groups using two-way reactions, and hence, TDH is preserved. Thiol and dynamic DSs have critical roles in many mechanisms in the body, such as stabilization of protein structures, regulation of proteins and enzyme functions, Na–K channel, detoxification, antioxidant defence, apoptosis, and cellular signal transduction.[7]
The results of many studies carried out in recent years revealed that TDH is affected by many diseases that progress with acute and chronic inflammation.[8]
The presence of H. pylori causes oxidative stress in the body. ROS is produced in the gastric mucosa, especially with the effect of epithelial cells and neutrophils.[9] ROS production is catalyzed particularly by nicotinamide adenine dinucleotide phosphate (NADPH oxidase) in the cell membrane to eliminate H. pylori.[10] However, H. pylori become difficult to kill because of the separation between the gastric mucosa and the lumen. Oxidative stress caused by continued ROS production results in a harmful microenvironment for the host. Chronic active inflammation primarily damages the gastric mucosa, and in the long term, the DNA.[11] In this study, we aimed to investigate the effect of H.pylori eradication on the thiol–DS balance in patients with dyspeptic complaints, based on the hypothesis that H. pylori infection harms antioxidant balance.
MATERıALS AND METHODS
This research was designed as a prospective observational study. A total of 320 patients who applied to the gastroenterology outpatient clinic, where this study was conducted, with dyspeptic complaints, between March 2019 and January 2021, were included in this study.
Ethical approvals
Before starting the research, a study protocol was prepared. In this protocol, the importance of H. pylori infection and the benefits of eradication therapy were explained to the patients. The purpose of the study was informed and they were asked to come at regular intervals for collecting blood and stool samples. Patients who accepted eradication treatment and signed the study protocol were included in the study. This study was approved by the Dicle University Faculty of Medicine Non-Invasive Clinical Research Ethics Committee with Decision Number 127 dated April 18, 2019.
Study Inclusion Criteria:
Having been diagnosed with dyspepsia according to Rome IV[12] criteria; that is, having symptoms, such as postprandial fullness, bloating, early satiety, epigastric pain, and burning sensation for at least 6 months;
Being more than 16 years old;
Absence of diseases, such as peptic ulcer, erosive gastritis, and esophagitis, that would explain the symptoms;
Having white blood cell (WBC) and C-reactive protein (CRP) levels within normal range; and
Having albumin (which may affect the thiol–DS equilibrium[13]) level within the normal range.
Study Exclusion Criteria:
Being under the age of 16;
Having lesions revealed by upper gastrointestinal tract endoscopy suggesting the presence of ulcers, polyps, esophagitis, metaplasia, dysplasia, or malignancy;
Having a history of gastrointestinal malignancy;
Having undergone an operation on the gastrointestinal tract;
Having chronic systemic diseases, such as hypertension, diabetes mellitus, heart failure, chronic kidney disease, chronic lung disease, and chronic liver disease;
Patients with any active infection
Having a disease that may cause low blood albumin levels[13] ;
Having celiac disease;
Smoking or using alcohol[14] ;
Being pregnant;
Using vitamins and/or herbal products for antioxidant purposes; and
Not having signed the informed consent form.
Method of H. pylori detection
An invasive method, endoscopic biopsy, was used to detect H. pylori infection.[15] To increase the probability of detecting H. pylori, at least four biopsies were taken from the antrum and corpus. Gastric biopsies were taken from the following sites according to the recommendations of the updated Sydney system: lesser curvature of the antrum, greater curvature of the antrum, lesser curvature of the corpus, greater curvature of the corpus, and incisura angularis.[16] Mucosal samples taken with biopsy forceps were placed in different tubes containing formaldehyde solution and conveyed to the pathology laboratory.
Endoscopy
It was ensured that the patients undergoing the endoscopy procedure fasted the previous 12 h. Signed endoscopic consent was obtained from all patients before the procedure. The esophagus, stomach, and duodenum mucosa were evaluated by endoscopy using Olympus CV-260 and Olympus GIF CV-70 video-endoscope devices. During the endoscopic examination, two biopsy specimens were taken from the antrum and corpus mucosa. The biopsy specimens were fixed in formaldehyde solution and sent to the pathology clinic.
Biopsy
After the completion of the routine pathological tissue follow-up, paraffin blocks were prepared from the samples fixed in formaldehyde solution for 24 h, and 4-μm sections were prepared using a standard microtome. These sections were stained with Hemotoxylin–Eosin and Giemsa and examined under a light microscope (Nikon ECLIPSE 80i) at 200 magnification. Sydney classification[16] was determined by pathologists and the results were reported.
Collection and storage of venous blood samples
Venous blood samples were taken twice from the patients who were positive for H. pylori, once before the start of the eradication treatment and once 3 months after the end of the treatment. On the other hand, venous blood samples from the patients who were negative for H. pylori were taken only once. The blood samples taken were centrifuged at 1000 rpm for 10 min and their serum was separated. The samples were stored at –80°C until the time they were studied. Then, these serums were studied with the technique described in the Material–Methods Department of Yıldırım Bayezid University Biochemistry Laboratory (Ankara), and the levels of native thiol (NT), total thiol (TT), and disulfide were measured.
The Eradication Protocol
The patients positive for H. pylori were treated with a four-fold treatment regimen containing bismuth.[17] Accordingly, they were given 4 × 1 500 mg tetracycline + 3 × 1 500 mg metronidazole + 2 × 1 a proton pump inhibitor (PPI), and 4 × 1 262 mg bismuth subsalicylate for 14 days.
Evaluation of H. pylori eradication
Patients were checked for eradication with stool Hp antigen test[18] after at least 15 days after eradication without any PPI and antibiotic use, and again 3 months later.
Thiol–DS measurement
NT and TT concentrations were measured synchronously using a paired test. In the first container, the amount of NT groups was measured with a modified Ellman's reagent. Synchronously, in another container, first, dynamic DS bonds were reduced to free thiol groups with sodium borohydride. Second, sodium borohydride (NaBH4) was removed with formaldehyde to prevent reduction of unused reduced sodium borohydride to dithionite-2-nitrobenzoic acid (DTNB). Third, after the reaction with DTNB, NT and TT levels were measured. The amount of NT was subtracted from the TTcontent and half of the difference is deemed to indicate the DS level.[19]
The specified reference range for NT (–SH), TT ((S–S) + (–SH)), and DS (S–S) were taken as 278–826 μmol/L, 441–740 μmol/L, and 2–52 μmol/L, respectively.[20]
Ratios of DS/NT, DS/TT, and NT/TT ratios were obtained by dividing the DS, NT, and TT levels to one another. DS/NT, DS/TT, and NT/TT ratios were expressed as percentages.
The statistical comparisons between the groups of patients positive and negative for H. pylori, and between the groups of patients positive for H. pylori and in whom eradication therapy was and was not successful were made, based on the said ratios.
Statistical analysis
The Kolmogorov–Smirnov test, the Shapiro–Wilk test, coefficient of variation, skewness, and kurtosis methods were used to determine whether the patient data conforms to the normal distribution or not. Continuous variables were expressed as mean and standard deviation whereas categorical variables were expressed as percentage values. Independent samples t test was used to determine the difference between H. pylori-positive and negative patient groups in age, gender, and laboratory parameters. Independent samples t test or Mann–Whitney U test was used to determine the difference between the H. pylori-positive and negative patient groups in native and TT and DS levels. Paired samples t test was used in the case of parameters that were determined to conform to normal distribution and Wilcoxon test was used in the case of parameters that were determined not to conform to normal distribution in the determination of the differences in native and TT and DS levels, before and after treatments and the ratios thereof between the groups of patients positive for H. pylori and in whom eradication therapy was and was not successful. All tests were bilateral and probability values of <0.05 indicated statistical significance. Statistical analyses were performed using the SPSS24.0 (Statistical Package for Social Sciences for Windows, Version 24.0, IBM Corp., Armonk, NY, 2016) software package.
RESULTS
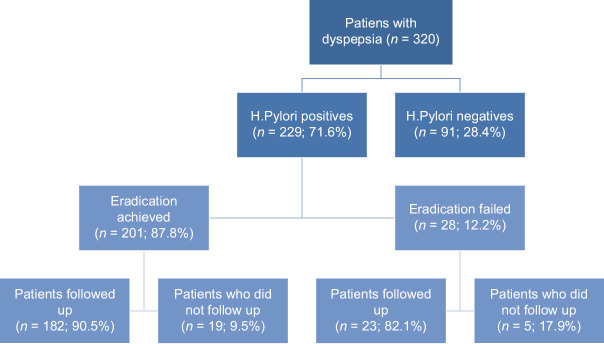
Of the 320 patients included in the study, 229 (71.6%) were H. pylori positive and 91 (28.4%) were H. pylori negative. H. pylori eradication treatment was successful in 201 (87.8%) patients. H. pylori could not be eradicated in 28 (12.2%) patients. Of the 229 patients positive for H. pylori, 205 came to the clinic within the scope of follow up visits. Serum samples of the patients who showed up for follow up visits were taken [Figure 1].
Figure 1.
Number and distribution of patients included in the study and followed up
The mean ages of dyspeptic patients positive and negative for H. pylori were 36.3 (min. 17–max. 56) years and 38.5 (min. 20–max. 61) years, respectively (P = 0.421). The distribution of the patient groups positive and negative for H. pylori by gender, revealed that 110 (48%) were female and 119 (52%) were male in the patient group who were positive for H. pylori, and 47 (51.6%) were female and 44 (48.4%) were male in the patient group who were negative for H. pylori (P = 0.353). Accordingly, there was no statistically significant difference between the patient groups positive and negative for H. pylori in terms of demographic characteristics.
The mean WBC counts of patients positive and negative for H. pylori were 6810 ± 1880 and 7000 ± 1920 per mm3, respectively, and within the normal range (P = 0.510). The mean CRP levels of patients who were positive and negative for H.pylori were 0.43 and 0.40mg/L, respectively, and within the normal range (P = 0.310). The mean albumin levels, which may affect the serum thiol level, were 4.8 ± 0.3 and 4.9 ± 0.3g/dL in the patient groups positive and negative for H.pylori, respectively (P = 0.808) [Table 1]. The mean NT levels of patients positive and negative for H. pylori were 329.0 ± 53.7 and 319.1 ± 73.6 mmol/L, respectively (P = 0.396). The mean TT levels of patients who were positive and negative for H.pylori were 356.1 ± 58.0 and 344.1 ± 77.0 mmol/L (P = 0.328). The mean DS levels of patients who were positive and negative for H. pylori were 13.5 ± 6.4 and 12.4 ± 5.5 μmol/L, respectively (P = 0.317). Accordingly, there was no statistically significant difference between the patient groups positive and negative for H. pylori in terms of laboratory parameters. Based on these results, serum DS/NT, DS/TT, and NT/TT ratios were also found to be similar between the two patient groups [Table 2].
Table 1.
Demographic data and laboratory characteristics of patients with dyspeptic complaints included in the study
| H. pylori (+) (n=229) | H. pylori (–) (n=91) | P | |
|---|---|---|---|
| Age | 36.3 (17–56) | 38.5 (20–61) | 0.421 |
| Gender F/M (n/%) | 110/119 (%48.0/%52.0) | 47/44 (%51.6/%48.4) | 0.353 |
| WBC | 6810±1880 | 7000±1920 | 0.510 |
| CRP | 0.43±0.76 | 0.40±0.36 | 0.309 |
| Albumin | 4.8±0.3 | 4.9±0.3 | 0.808 |
WBC=White Blood Cell, CRP=C reactive protein
Table 2.
Serum thiol and disulfide levels of dyspeptic patients with H. pylori positive and negative
| H. Pylori positives (n=229) | H.Pylori negatives (n=91) | P | |
|---|---|---|---|
| Native Thiol (µmol/L) | 329.0±53.7 | 319.1±73.6 | 0.396 |
| Total Thiol (µmol/L) | 356.1±58.0 | 344.1±77.0 | 0.328 |
| Disulfide (µmol/L) | 13.5±6.4 | 12.4±5.5 | 0.317 |
| (Disulfide/Native Thiol)*100(%) | 4.1±1.9 | 4.1±2.0 | 0.905 |
| (Disulfide/Total Thiol)*100(%) | 3.7±1.6 | 3.7±1.7 | 0.891 |
| (Native Thiol/Total Thiol)*100(%) | 92.4±3.3 | 92.5±3.4 | 0.891 |
On the other hand, the comparison of the pre-treatment and post-treatment laboratory parameters of the H. pylori-positive patient group in whom eradication therapy was successful, revealed significant differences. Accordingly, the mean pre-treatment and post-treatment NT, TT, and DS levels of the H. pylori-positive patient group were 325.1 ± 49.7 and 343.8 ± 32.0 μmol/L (P < 0.001), 346.3 ± 54.3 and 365.6 ± 35.3 μmol/L (P = 0.002), and 14.0 ± 6.6 and 10.9 ± 5.9 μmol/L (P = 0.033), respectively. In parallel, the mean pre-treatment and post-treatment DS/NT, DS/TT, and NT/TT ratios of the H. pylori-positive patient group were 4.3 ± 2.0 and 3.2 ± 1.8 (P = 0.007), 4.0 ± 1.6 and 2.9 ± 1.6 (P = 0.005), and 90.8 ± 3.7 and 94.0 ± 3.2 (P = 0.005), respectively [Table 3 and Figure 2].
Table 3.
Serum thiol and disulfide levels before and after treatment of H. pylori patients whose eradication treatment was successful
| Before Eradication Therapy (n=182) | After Eradication Therapy (n=182) | P | |
|---|---|---|---|
| Native Thiol (µmol/L) | 325.1±49.7 | 343.8±32.0 | <0.001 |
| Total Thiol (µmol/L) | 346.3±54.3 | 365.6±35.3 | 0.002 |
| Disulfide(µmol/L) | 14.0±6.6 | 10.9±5.9 | 0.033 |
| (Disulfide/Native Thiol)*100 (%) | 4.3±2.0 | 3.2±1.8 | 0.007 |
| (Disulfide/Total Thiol)*100 (%) | 4.0±1.6 | 2.9±1.6 | 0.005 |
| (Native Thiol/Total Thiol)*100(%) | 90.8±3.7 | 94.0±3.2 | 0.005 |
Figure 2.
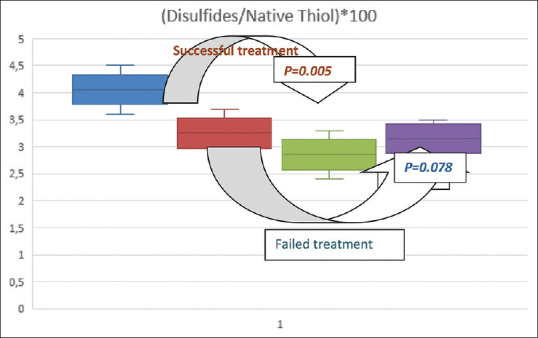
Among the patient groups with and without successful H. pylori eradication treatment; change of disulfide/native thiol ratio before and after treatment
In contrast, the comparison of the pre-treatment and post-treatment laboratory parameters of the H. pylori-positive patient group in whom eradication therapy was unsuccessful did not reveal any significant difference. Accordingly, the mean pre-treatment and post-treatment NT, TT, and DS levels of the H. pylori-positive patient group were 315.2 ± 16.3 and 320.8 ± 19.5 μmol/L (0.201), 341.4 ± 30.5 and 342.6 ± 29.8 μmol/L (P = 0.273), and 15.2 ± 2.5 and 14.8 ± 2.3 μmol/L (P = 0.163), respectively. In parallel, the mean pre-treatment and post-treatment DS/NT, DS/TT, and NT/TT ratios of the H. pylori-positive patient group were 3.3 ± 1.0 and 3.2 ± 0.7 (P = 0.741), 3.8 ± 0.7 and 3.4 ± 0.6 (P = 0.078), and 90.2 ± 2.2 and 90.9 ± 2.5 (P = 0.457), respectively [Table 4 and Figure 2].
Table 4.
The course of serum native thiol, total thiol, and disulfide levels before and after treatment in patients with H.pylori (+) dyspepsia who failed eradication therapy
| Before treatment (n=23) | After treatment (n=23) | P | |
|---|---|---|---|
| Native Thiol (µmol/L) | 315.2±16.3 | 320.8±19.5 | 0.201 |
| Total Thiol (µmol/L) | 341.4±30.5 | 342.6±29.8 | 0.273 |
| Disulfide (µmol/L) | 15.2±2.5 | 14.8±2.3 | 0.163 |
| (Disulfide/Native Thiol)*100(%) | 3.3±1.0 | 3.2±0.7 | 0.741 |
| (Disulfide/Total Thiol)*100(%) | 3.8±0.7 | 3.4±0.6 | 0.078 |
| (Native Thiol/Total Thiol)*100(%) | 90.2±2.2 | 90.9±2.5 | 0.457 |
DISCUSSION
H. pylori eradication and acid suppression therapies are the most commonly used management strategies in patients who are determined to be positive for H. pylori, after early endoscopy in patients presenting with dyspeptic complaints.[20]
The results of this study revealed a significant improvement in the dynamic thiol DS equilibrium in H. pylori-positive patients after successful eradication therapy. Increasing thiol and decreasing DS levels are indicators of the increase in the antioxidant levels and resistance of the body. These findings indicate that the mucosal inflammation caused by H. pylori can have systemic effects on the body. The fact that there was no significant difference found between H. pylori-positive and negative patient groups was attributed to the wide reference range of the amount of thiol in the body and the large differences between the TT amount of patients.[21] In this context, DS/NT, DS/TT, and NT/TT ratios were also taken into account in the evaluation of the results of the statistical analyses.
Although there was a partial improvement in the thiol–DS equilibrium of H. pylori-positive patients in whom eradication therapy was unsuccessful, this improvement was not statistically significant. The said improvement maybe attributed to the decreased clearance of H. pylori and its transition to spore form in this patient group. It would not be unreasonable to speculate that this partial recovery will likely be temporary, as H. pylori could not be completely eradicated.
There are methods available to measure ROS both directly and indirectly. Methods such as protein damage, lipid damage, lipid peroxidation markers, and thymidine glycol showing DNA damage, directly provide information about ROS.[22,23,24] 2,2-diphenyl-1-picryl-hydrazil reduction assay,[25] total radical scavenging antioxidant parameter[25] test, and 2,2′-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (developed by Erel) (ABTS) test also provide indirect information about ROS by measuring antioxidant capacity. In this method, ABTS is first oxidized to the radical cation form (ABTS•+), a blue-green chromophore with absorption at 750 nm by metmyoglobin, and hydrogen peroxide. When antioxidants are added, ABTS•+ is reduced to ABTS and becomes decolorized again. Therefore, this method also follows the discoloration of the stable radical spectrophotometrically to measure the relative antioxidant ability of the samples.[26] Thus, measured thiol levels can be considered as an indirect indicator of antioxidant capacity.[23]
However, the fact that the total TT capacity is different for everyone and that these biomarkers have not been compared with methods that directly measure ROS prevents us from making a clear judgment on this issue. For this, a meta-analysis comparing oxidative stress-related tests can give us a better understanding.
A previous study involving patients with H. pylori infection showed a correlation between the severity of endoscopically detected gastritis and the plasma disulfide level.[27]
Studies show that chronic inflammation caused by H. pylori causes lifelong production of ROS.[28] In addition, it was emphasized previously that the maintenance of TDH is an important part of the antioxidant defence system and may prevent the development of many potentially fatal diseases.[29] In another study conducted in this context, it was stated that the loss of physiological balance during redox reactions, which is of vital importance in cell renewal and differentiation, contributed to the pathogenesis of stomach disorders. The disturbances in the oxidant and antioxidant balance in particular have been blamed for a wide range of clinical problems ranging from chronic gastritis, intestinal metaplasia, peptic ulcer, and gastric cancer.[30]
This study could not measure the level of proinflammatory cytokines such as interleukin and interferon, which are important indicators of inflammation. Although there is limited published data, there are studies that determine a correlation between cytokine levels and disulfide levels, especially in chronic inflammatory diseases.[31] However, there is no study showing the relationship between inflammatory cytokines and thiol–disulfide levels in H. pylori. Designing such a study in the future will provide us with much more comprehensive data.
Although there is no widespread opinion about the use of antioxidants in the treatment of H. pylori, it was reported in a meta-analysis conducted in 2018 that the addition of antioxidants (especially N-acetyl cysteine) to the H. pylori antibiotic regimen increased the treatment success significantly from 68.6 to 81.3% (P = 0.03), and that there was no significant difference between the two groups in terms of the side effects of the treatments.[32] In another study, it was reported that the addition of vitamins C and E to the eradication protocol increased the treatment success from 44.7 to 66.2% (P < 0.005).[33] However, the net effect of using antioxidants in the treatment of H. pylori on the body's total antioxidant capacity is unknown.
Based on our results, we have shown that H. pylori disrupts the redox balance, however, this balance can be restored with eradication therapy. In this way, it would be possible to avoid many chronic gastric problems that the patients may encounter in the future.
It can be stated that thiol–DS hemostasis in the body shifts towards the DS direction because of the reactive oxygen metabolites formed as a result of inflammation caused by H. pylori. The restoration of this hemostatic balance with successful eradication therapy revealed that the organism was also relieved from oxidative stress. Therefore, H. pylori eradication treatment is highly recommended even in asymptomatic patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We appreciate all the participants who supported this study.
REFERENCES
- 1.Wang C, Yuan Y, Hunt RH. “The association between Helicobacter pylori infection and early gastric cancer: A meta-analysis”. Am J Gastroenterol. 2007;102(8):1789–98. doi: 10.1111/j.1572-0241.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 2.Lehours P, Ferrero RL. Review: Helicobacter: Inflammation, immunology, and vaccines. Helicobacter. 2019;24(Suppl 1):e12644. doi: 10.1111/hel.12644. [DOI] [PubMed] [Google Scholar]
- 3.Robinson K, Kaneko K, Andersen LP. Helicobacter: Inflammation, immunology and vaccines. Helicobacter. 2017;22(Suppl 1):e12406. doi: 10.1111/hel.12406. doi: 10.1111/hel.12406. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1:1396–97. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 5.Yi MC, Khosla C. Thiol-disulfide exchange reactions in the mammalian extracellular environment. Annu Rev Chem Biomol Eng. 2016;7:197–222. doi: 10.1146/annurev-chembioeng-080615-033553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brulisauer L, Gauthier MA, Leroux JC. Disulfide-containing parenteral delivery systems and their redox-biological fate. J Control Release. 2014;195:147–54. doi: 10.1016/j.jconrel.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Hurd TR, Prime TA, Harbour ME, Lilley KS, Murphy MP. Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis: Implications for mitochondrial redox signalling. J Biol Chem. 2007;282:22040–51. doi: 10.1074/jbc.M703591200. [DOI] [PubMed] [Google Scholar]
- 8.Erel Ö, Erdoğan S. Thiol-disulfide homeostasis: An integrated approach with biochemical and clinical aspects. Turk J Med Sci. 2020;50:1728–38. doi: 10.3906/sag-2003-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med. 2002;33:323–36. doi: 10.1016/s0891-5849(02)00868-7. [DOI] [PubMed] [Google Scholar]
- 10.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 11.Butcher LD, den Hartog G, Ernst PB, Crowe SE. Oxidative stress resulting fromhelicobacter pyloriınfectioncontributes to gastric carcinogenesis. Cell Mol Gastroenterol Hepatol. 2017;3:316–22. doi: 10.1016/j.jcmgh.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. 2016;150:1380–92. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Go YM, Jones DP. Thiol/disulfide redox states in signalling and sensing. Crit Rev Biochem Mol Biol. 2013;48:173–81. doi: 10.3109/10409238.2013.764840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solak I, Cetinkaya CD, Gederet YT, Kozanhan B, Erel O, Eryilmaz MA. Effects of smoking on thiol/disulfide homeostasis. Eur Rev Med Pharmacol Sci. 2018;22:2477–82. doi: 10.26355/eurrev_201804_14842. [DOI] [PubMed] [Google Scholar]
- 15.Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, Babazadeh A, Koppolu V, Vasigala VR, et al. Diagnostic methods for Helicobacter pylori infection: Ideals, options, and limitations. Eur J Clin Microbiol Infect Dis. 2019;38:55–66. doi: 10.1007/s10096-018-3414-4. [DOI] [PubMed] [Google Scholar]
- 16.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis The updated Sydney System International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Liang X, Zheng Q, Liu W, Xiao S, Gu W, et al. High efficacy of 14-day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter. 2010;15:233–8. doi: 10.1111/j.1523-5378.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 18.Perri F, Quitadamo M, Ricciardi R, Piepoli A, Cotugno R, Gentile A, et al. Comparison of a monoclonal antigen stool test (Hp StAR) with the 13C-urea breath test in monitoring Helicobacter pylori eradication therapy. World J Gastroenterol. 2005;11:5878–81. doi: 10.3748/wjg.v11.i37.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326–32. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Duggan AE, Elliott CA, Miller P, Hawkey CJ, Logan RF. Clinical trial: Arandomized trial of early endoscopy, Helicobacter pylori testing and empirical therapy for the management of dyspepsia in primary care. Aliment Pharmacol Ther. 2009;29:55–68. doi: 10.1111/j.1365-2036.2008.03852.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–38. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito K, Yano T, Morodomi Y, Yoshida T, Kohno M, Haro A, et al. Serum antioxidant capacity and oxidative injury to pulmonary DNA in never-smokers with primary lung cancer. Anticancer Res. 2012;32:1063–67. [PubMed] [Google Scholar]
- 23.Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. OxidMedCell Longev. 2019;2019:1279250. doi: 10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–81. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- 25.Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–52. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Baykan AR, Biçer C, Gerçeker E, Erel E, Cerrah S, Albayrak B, et al. Thiol/disulphide homeostasis in H.pylori infected patients. The European Research Journal. 2019;5:948–56. [Google Scholar]
- 28.Kaakoush NO, Kovach Z, Mendz GL. Potential role of thiol: disulfide oxidoreductases in the pathogenesis of Helicobacter pylori. FEMS Immunol Med Microbiol. 2007;50:177–83. doi: 10.1111/j.1574-695X.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 29.Erenler AK, Yardan T. Clinical utility of thiol/disulfide homeostasis. Clin Lab. 2017;63:867–70. doi: 10.7754/Clin.Lab.2017.161117. [DOI] [PubMed] [Google Scholar]
- 30.Perez S, Talens-Visconti R, Rius-Perez S, Finamor I, Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med. 2017;104:75e103. doi: 10.1016/j.freeradbiomed.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 31.Coppo L, Ghezzi P. Thiol regulation of pro-inflammatory cytokines and innate immunity: Protein S-thiolation as a novel molecular mechanism. Biochem Soc Trans. 2011;39:1268–72. doi: 10.1042/BST0391268. [DOI] [PubMed] [Google Scholar]
- 32.Yang-Ou YB, Hu Y, Zhu Y, Lu NH. The effect of antioxidants on Helicobacter pylori eradication: A systematic review with meta-analysis. Helicobacter. 2018;23:e12535. doi: 10.1111/hel.12535. [DOI] [PubMed] [Google Scholar]
- 33.Sezikli M, Cetinkaya ZA, Güzelbulut F, Sezikli H, Özkara S, Coşgun S, et al. Efficacy of vitamin supplementation to therapy on Helicobacter pylori eradication in patients with low antioxidant capacity. Clin Res Hepatol Gastroenterol. 2011;35:745–9. doi: 10.1016/j.clinre.2011.07.001. [DOI] [PubMed] [Google Scholar]