Abstract
Background
Ultrathin bronchoscopy (external diameter, ≤3.5 mm) is useful for the diagnosis of peripheral pulmonary lesions because of its good accessibility.
Objectives
We performed a meta-analysis to investigate the diagnostic yield of ultrathin bronchoscopy for peripheral pulmonary lesions.
Methods
We performed a systematic search of MEDLINE and EMBASE (from inception to May 2021), and meta-analysis was performed using R software. The diagnostic yield was evaluated by dividing the number of successful diagnoses by the total number of lesions, and subgroup analysis was performed to identify related factors.
Results
Nineteen studies with a total of 1,977 peripheral pulmonary lesions were included. The pooled diagnostic yield of ultrathin bronchoscopy was 0.65 (95% confidence interval, 0.60–0.70). Significant heterogeneity was observed among studies (χ<sup>2</sup>, 87.75; p < 0.01; I<sup>2</sup>, 79.5%). In a subgroup analysis, ultrathin bronchoscopy with 1.2 mm channel size showed a diagnostic yield of 0.61 (95% confidence interval, 0.53–0.68), whereas ultrathin bronchoscopy with 1.7 mm channel size showed 0.70 (95% confidence interval, 0.66–0.74) (χ<sup>2</sup>, 5.35; p = 0.02). In addition, there was a significant difference in diagnostic yield based on lesion size, histologic diagnosis (malignant vs. benign), bronchus sign, and lesion location from the hilum, whereas no significant difference was found based on lobar location. The overall complication rate of ultrathin bronchoscopy was 2.7% (pneumothorax, 1.1%).
Conclusions
Ultrathin bronchoscopy is an excellent tool for peripheral pulmonary lesion diagnosis with a low complication rate. The diagnostic yield of ultrathin bronchoscopy was significantly higher with larger channel size, which might be attributed to the availability of radial endobronchial ultrasound.
Keywords: Bronchoscopy, Endobronchial ultrasound, Lung cancer, Solitary pulmonary nodule, Meta-analysis
Introduction
Lung cancer is reportedly one of the most common cancers worldwide (2.09 million cases, 11.6% of all cancers) and is the main cause of cancer-related mortality (1.76 million deaths, 18.4%) [1]. A recent meta-analysis by Huang et al. [2] evaluated the effect of low-dose computed tomography on lung cancer screening compared with no screening or chest X-ray, and low-dose computed tomography proved to be a sensitive tool for the detection of stage I lung cancer (risk ratio [RR], 2.08). As an increase in the number of peripheral pulmonary lesions (PPLs) is anticipated, the importance of their accurate pathological diagnosis increases.
Transbronchial biopsy (TBB) and transthoracic needle biopsy (TNB) are generally recommended as non-surgical approaches for diagnosing PPLs [3]. Conventional TBB with a standard bronchoscope (external diameter [ED], 6.0 mm) and fluoroscopy showed a median sensitivity of 31% for diagnosis of malignant PPLs <20 mm, whereas TNB showed a sensitivity of ≥90% (70–82% for PPLs ≤15 mm) [3]. However, in the US population-based studies, TNB was associated with a higher risk of complications such as haemorrhage (1.0%), pneumothorax (15%), and pneumothorax requiring chest tube insertion (6.6%) [4], whereas TBB was associated with a lower risk of haemorrhage (0.58%), pneumothorax (0.97%), and pneumothorax requiring chest tube insertion (0.55%) [5].
To increase the diagnostic yield of TBB, new adjunct modalities for bronchoscopy such as radial endobronchial ultrasonography (r-EBUS) [6], guide sheath (GS) [7], computed tomography (CT)-guided [8], virtual bronchoscopic navigation (VBN) [9], and electromagnetic navigation bronchoscopy (ENB) [10] have been introduced. In a recent meta-analysis by McGuire et al. [11], TBB with either r-EBUS or ENB showed overall sensitivity of 70.7% for detecting PPLs. Although safe, TBB still needs further improvement in sensitivity compared with TNB.
Recently, the ultrathin bronchoscope (UTB) (ED, ≤3.5 mm) has been developed for diagnosis of PPLs [12], which is smaller in diameter than the thin bronchoscope (ED, 4.0 or 4.2 mm) commonly used for small PPLs in previous studies [11]. Therefore, UTB showed greater accessibility to peripheral bronchi with mobility compared with thin bronchoscope (median 5th vs. median 4th bronchial generation; p < 0.001) [13]. Although UTB seems promising for PPLs, systematic research on this modality is currently lacking. Thus, we performed a meta-analysis on the diagnostic yield of UTB for PPLs. In addition, we also evaluated the factors affecting its performance and associated complications.
Material and Methods
Literature Search
We performed a meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines [14]. We searched MEDLINE and EMBASE (from their inception until May 2021) to identify all studies that employed UTB for evaluation of PPLs using a predetermined protocol (Table 1). A manual search of references cited in original and review papers was also done for relevant studies, which might have been missed by electronic search.
Table 1.
Search strategy for meta-analysis
| Search strategy | |
|---|---|
| 1 | “Bronchoscope and lung nodule(s)” |
| 2 | “Bronchoscope and lung lesion(s)” |
| 3 | “Bronchoscope and pulmonary nodule(s)” |
| 4 | “Bronchoscope and pulmonary lesion(s)” |
| 5 | “Bronchoscope and peripheral nodule(s)” |
| 6 | “Bronchoscope and peripheral lesion(s)” |
| 7 | “Bronchoscopic and lung nodule(s)” |
| 8 | “Bronchoscopic and lung lesion(s)” |
| 9 | “Bronchoscopic and pulmonary nodule(s)” |
| 10 | “Bronchoscopic and pulmonary lesion(s)” |
| 11 | “Bronchoscopic and peripheral nodule(s)” |
| 12 | “Bronchoscopic and peripheral lesion(s)” |
| 13 | “Bronchoscopy and lung nodule(s)” |
| 14 | “Bronchoscopy and lung lesion(s)” |
| 15 | “Bronchoscopy and pulmonary nodule(s)” |
| 16 | “Bronchoscopy and pulmonary lesion(s)” |
| 17 | “Bronchoscopy and peripheral nodule(s)” |
| 18 | “Bronchoscopy and peripheral lesion(s)” |
Selection of Studies
All articles identified by the search strategy were independently assessed by two authors (S.H.K. and J.S.E.). Discordance was resolved by consensus. Abstracts were initially examined, and studies were selected for inclusion only after both reviewers assessed the full-text articles. Criteria for inclusion were: (1) UTB used for diagnosis of PPL and diagnostic yield was provided, (2) diagnosis confirmed histologically or by close clinical follow-up, and (3) studies enrolled at least 20 patients. We excluded review articles, letters, papers not available in English, or case reports with fewer than 20 patients. When two or more studies were published by the same author(s), the methods sections were reviewed to check if study periods were overlapping. If so, we included only one publication with the greatest number of patients to prevent duplication of the study cohorts.
Data Extraction
All data were independently extracted by S.H.K. and J.S.E and compared. Disagreements were resolved by further discussion with third investigator (K.P.). The following were retrieved: author, year of publication, study design (randomized controlled trial, prospective, retrospective, or unknown), total number of lesions, number of successful diagnoses, UTB channel size, use of guidance modalities, lesion size, prevalence of malignancy, histologic diagnosis, presence of bronchus sign, distance from the hilum, lobar location of the lesion, complications, and reference standard.
Quality of selected studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 tool [15]. This validated tool contains 14 signalling questions to evaluate four main components (patient selection, index test, reference standard, and flow and timing) in two categories (risk of bias and applicability concerns).
Statistical Analysis
Meta-analysis was performed using meta package of R statistical software (version 4.0.5, http://www.R-project.org). A p value of <0.05 was considered statistically significant. The primary outcome was the diagnostic yield with 95% confidence interval (CI), which was calculated by dividing the number of successful diagnoses by the total number of lesions. Inverse variance weighting across selected studies was applied to evaluate the pooled diagnostic yield, where the weight of each study was based on the number of lesions.
Subgroup analysis was performed to identify the factors associated with diagnostic yield. Stratified analysis on diagnostic yield was based on channel size (1.2 mm vs. 1.7 mm), lesion size (≤20 mm vs. >20 mm), histologic diagnosis (malignant vs. benign), bronchus sign (present vs. absent), distance from the hilum (peripheral vs. non-peripheral [central or intermediate]), and location of lesion (upper lobe vs. other lobes). For each study, the diagnostic yield was determined for a single-channel size (1.2 mm or 1.7 mm). The other variables, on the other hand, were dichotomous, and the diagnostic yield was determined for each of their two possible values. Dichotomous variables were analysed using Mantel Haenszel RR, which was calculated by dividing the diagnostic yield of the former variable by that of the latter variable. RR >1 was in favour of the former variable for the diagnostic yield, while RR <1 of the latter variable.
Study heterogeneity was assessed using the Cochran Q test (χ2 test) and quantified by the I2 index [16]. Statistical heterogeneity was indicated in cases of p < 0.01 in χ2 test [16], and I2 index value of >50% was considered as significant heterogeneity [17]. Random-effect models with the inverse variance method were applied to reflect the variability of effect sizes among included studies with diversity in adjunctive modalities [18]. Publication bias was evaluated using funnel plot asymmetry [19] based on both the Egger's and Begg tests [20, 21]. We used funnel plots of standard error or diagnostic yield (logit transformed).
Results
Literature Search and Study Selection
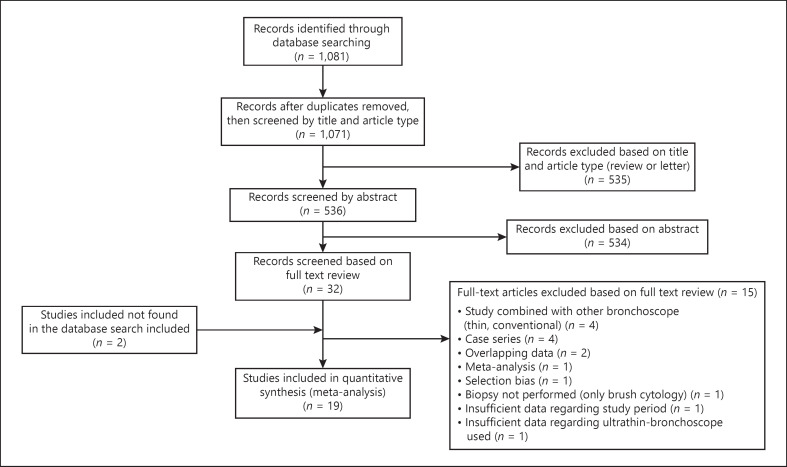
The search algorithm revealed 1,071 potentially relevant papers after removal of duplicates (Fig. 1). Following abstract review, 32 articles were selected for full-text review. Of these, 15 articles were excluded according to the protocol, and two studies missed in the database search were added [22, 23]. Therefore, 19 studies formed the basis of our systematic review [13, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39].
Fig. 1.
Flow diagram of search and study selection.
Study Description
A total of 1,977 PPLs were included. Table 2 lists the study characteristics and summarizes their features [13, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]. Six studies were randomized controlled trials, six were prospective, and seven were retrospective studies. UTB with 1.2 mm channel size was used in 11 studies; the others employed UTB with 1.7 mm channel size. The prevalence of malignancy was reported in 14 studies (median, 72%; interquartile range, 62–82). Among them, eight studies showed prevalence of malignancy ≤75%, whereas 6 studies showed >75%. There was a variation in additional guidance devices used among included studies, such as fluoroscopy (14 studies), VBN (11 studies), r-EBUS (eight studies), CT-fluoroscopy (six studies), and cone-beam CT (two studies).
Table 2.
Study characteristics
| No. | Author | Year | Study design | Channel size, mm | Additional guidance | Lesion size, mm | Prevalence of malignancy, % | Complications | Reference/comparison test |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Shinagawa et al. [24] | 2004 | Prospective | 1.2 | CT-Flu, VBN | 13.2 | 69.2 | No | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 2 | Yamamoto et al. [25] | 2004 | Prospective | 1.2 | Flu | NE | 76.1 | NE | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 3 | Shinagawa et al. [26] | 2007 | Prospective | 1.2 | CT-Flu, VBN | 13.7 | 71.8 | 1 Bleed, 1 PNX | NE |
|
| |||||||||
| 4 | Tachihara et al. [27] | 2007 | Retrospective | 1.2 | Flu, VBN | 15.9 | NE | No | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 5 | Oki et al. [28] | 2009 | Prospective | 1.7 | Flu, R-EBUS | 31.2 | 62.0 | No | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 6 | Matsuno et al. [22] | 2011 | Retrospective | 1.2 | CT-Flu, Flu, VBN | NE | 60.8 | 1 PNX | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 7 | Oki et al. [29] | 2012 | RCT | 1.7 | Flu, R-EBUS | 30.6 | 75.2 | 1 Bleed, 3 PNX, 1 PNA | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 8 | Asano et al. [30] | 2013 | RCT | 1.2 | Flu, VBN | 17.3 | 88.0 | 2 Bleed, 2 PNX 1 PNA 2 Others (1 transient bradycardia, 1 xylocaine intoxication) |
Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 9 | Oki et al. [31] | 2015 | RCT | 1.7 | Flu, R-EBUS, VBN | 19.0 | 82.0 | 3 PNX, 1 PNA 1 Others (1 chest pain) |
Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 10 | Franzen et al. [32] | 2016 | RCT | 1.2 | Flu | 27.0 | 40.0 | 6 Others (2 coughing, 3 blocked working channel or weak suction, 1 hypertensive urgency requiring intervention) | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 11 | Tokoro et al. [33] | 2016 | Retrospective | 1.2 | CT-Flu | NE | NE | NE | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 12 | Ali et al. [34] | 2019 | Prospective | 1.2 | CBCT, VBN | 20.0 | 62.5 | 1 PNX (requiring chest tube), 1 PNA | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 13 | Diez-Ferrer et al. [35] | 2019 | Prospective | 1.2 | Flu, VBN | 24.3 | 72.1 | NE | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 14 | Oki et al. [13] | 2019 | RCT | 1.7 | Flu, R-EBUS, VBN | 18.9 | 81.4 | 1 Bleed, 2 PNX (1 requiring chest tube), 2 PNA | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 15 | Sehgal et al. [36] | 2019 | Retrospective | 1.7 | R-EBUS | 16.4 | NE | 1 Bleed, 1 PNX | Clinical/radiologic surveillance |
|
| |||||||||
| 16 | Sumi et al. [37] | 2020 | Retrospective | 1.7 | Flu, R-EBUS | 19.5 | NE | 1 Bleed, 1 PNX (requiring chest tube), 2 PNA | NE |
|
| |||||||||
| 17 | Kawakita et al. [38] | 2021 | Retrospective | 1.2 | CBCT, CT-Flu, Flu, VBN | 19.9 | 68.6 | 3 PNX, 1 others (respiratory failure) | Histology by alternative means or clinical/radiologic surveillance |
|
| |||||||||
| 18 | Sumi et al. [39] | 2021 | Retrospective | 1.7 | Flu, R-EBUS | 20.0 | NE | 2 Bleed, 1 PNA | NE |
|
| |||||||||
| 19 | Zheng et al. [23] | 2021 | RCT | 1.7 | Flu, R-EBUS, VBN | 27.7 | 83.3 | No | Histology by alternative means or clinical/radiologic surveillance |
CBCT, cone-beam computed tomography; CT-Flu, computed tomography-fluoroscopy; Flu, fluoroscopy; NE, not evaluable; No., number; PNA, pneumonia; PNX, pneumothorax; R-EBUS, radial-endobronchial ultrasound; RCT, randomized controlled trial; VBN, virtual bronchoscopic navigation.
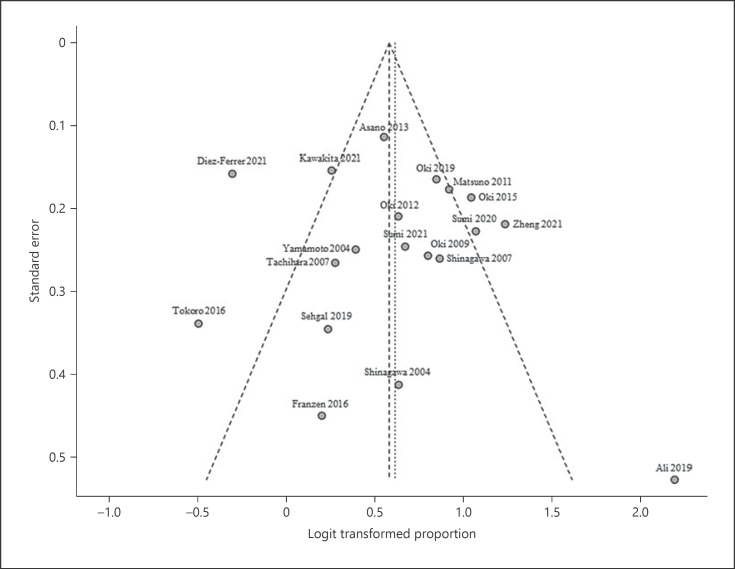
Online supplementary Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000527362) provides a quality assessment of all included studies based on Quality Assessment of Diagnostic Accuracy Studies-2. The overall analysis showed good performance in the patient selection and index test criteria. However, it showed poor performance in the reference standard in addition to flow and timing criteria, which indicates potential for significant bias. Funnel plot (Fig. 2) was not asymmetric, with both Egger's (p = 0.585) and Begg tests (p = 0.944) showing insignificant p values, indicating the absence of publication bias.
Fig. 2.
Funnel plot of publication bias.
Test Performance: Meta-analysis
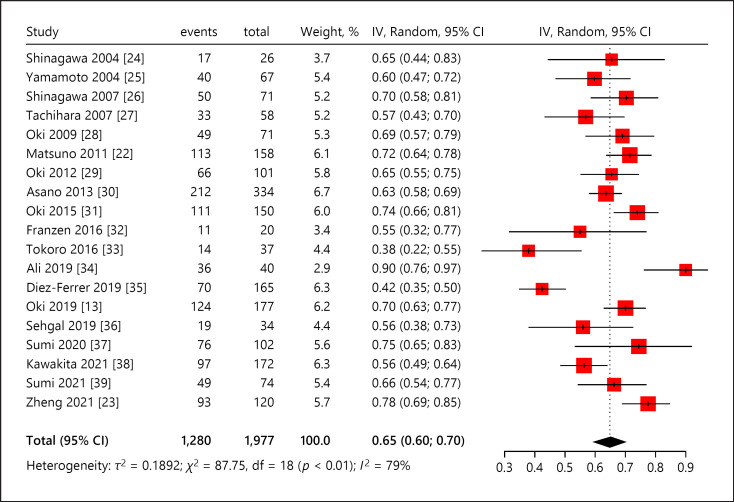
The inverse variance weighted overall diagnostic yield was 0.65 (95% CI, 0.60–0.70) (Fig. 3) [13, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]. The diagnostic yield among studies ranged from 0.38 to 0.90. χ2 value of 87.75 (p < 0.01) and I2 index of 79.5% (95% CI, 68.7–86.6) indicated substantial heterogeneity across studies.
Fig. 3.
Overall diagnostic yield of ultrathin bronchoscope. CI, confidence interval; IV, inverse variance.
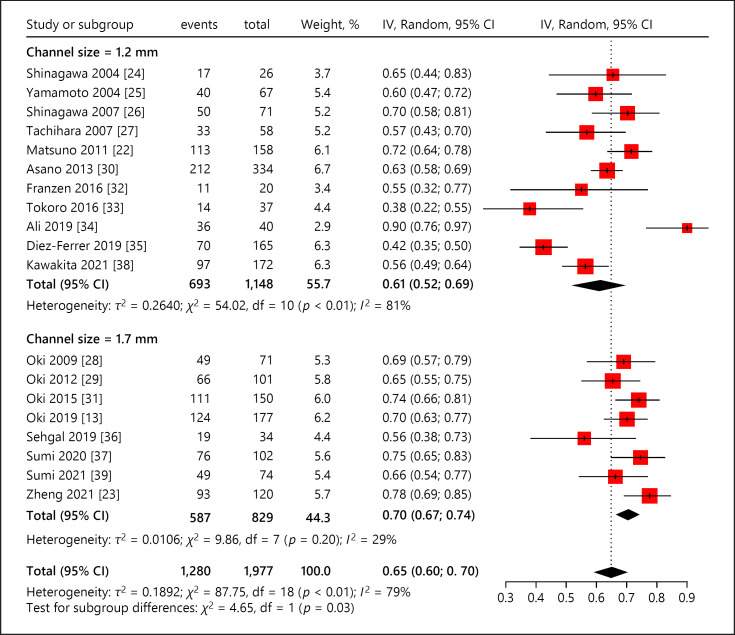
The factors underlying diagnostic yield were further evaluated by subgroup meta-analysis. As for independent variables, the weighted diagnostic yield of UTB with 1.2 mm channel size (1,148 lesions) was 0.61 (95% CI, 0.53–0.68) [22, 24, 25, 26, 27, 30, 32, 33, 34, 35, 38], whereas that with 1.7 mm channel size (829 lesions) was 0.70 (95% CI, 0.66–0.74) (Fig. 4) [13, 23, 28, 29, 31, 36, 37, 39]. The difference of pooled diagnostic yield between these two subgroups was statistically significant (χ2 value, 5.35; p = 0.02).
Fig. 4.
Diagnostic yield of ultrathin bronchoscope based on channel size (1.2 mm vs. 1.7 mm). CI, confidence interval; IV, inverse variance.
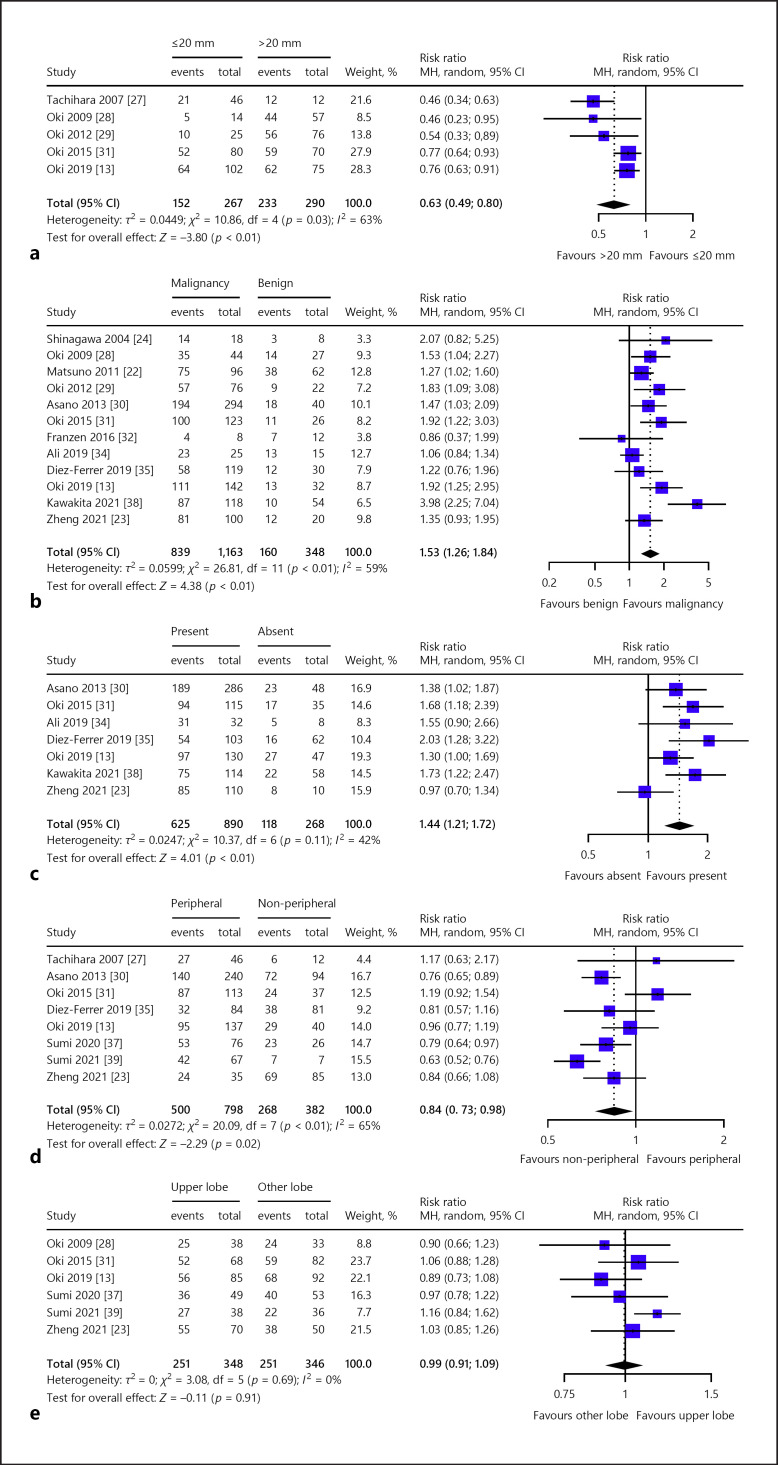
Regarding dichotomous variables (Table 3), the pooled diagnostic yield was significantly different based on lesion size in five studies (≤20 mm vs. >20 mm; RR, 0.63; 95% CI, 0.49–0.80) (Fig. 5a) [13, 27, 28, 29, 31], histologic diagnosis in 12 studies (malignancy vs. benign; RR, 1.53; 95% CI, 1.26–1.84) (Fig. 5b) [13, 22, 23, 24, 28, 29, 30, 31, 32, 34, 35, 38], presence of bronchus sign in seven studies (present vs. absent; RR, 1.44; 95% CI, 1.21–1.72) (Fig. 5c) [13, 23, 30, 31, 34, 35, 38], and distance from the hilum in eight studies (peripheral vs. non-peripheral; RR, 0.84; 95% CI, 0.73–0.98) (Fig. 5d) [13, 23, 27, 30, 31, 35, 37, 39]. However, there was no significant difference in the pooled diagnostic yield based on lobar location of lesion in six studies (upper vs. other lobes; RR, 0.99; 95% CI, 0.91–1.09) (Fig. 5e) [13, 23, 28, 31, 37, 39].
Table 3.
Stratified meta-analysis for dichotomous variables
| Variable | Studies, n | Observations, n | Events, n | Pooled sensitivity (95% CI) | Risk ratio (95% CI) | Heterogeneity |
Overall effect |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| x2 | I2 (%) | p value | z | p value | ||||||
| Lesion size | ||||||||||
| ≤20 mm | 5 | 267 | 152 | 0.53 (0.41–0.64) | 0.63 (0.49–0.80) | 10.86 | 63 | 0.0281 | −3.80 | 0.0001 |
| >20 mm | 5 | 290 | 233 | 0.80 (0.74–0.84) | ||||||
| Histologic diagnosis | ||||||||||
| Malignancy | 12 | 1,163 | 839 | 0.74 (0.68–0.80) | 1.53 (1.26–1.84) | 26.81 | 59 | 0.0049 | 4.38 | <0.0001 |
| Benign | 12 | 348 | 160 | 0.47 (0.38–0.56) | ||||||
| Bronchus sign | ||||||||||
| Present | 7 | 890 | 625 | 0.72 (0.63–0.80) | 1.44 (1.21–1.72) | 10.37 | 42 | 0.1099 | 4.01 | <0.0001 |
| Absent | 7 | 268 | 118 | 0.47 (0.36–0.59) | ||||||
| Distance from hilum | ||||||||||
| Peripheral | 8 | 798 | 500 | 0.63 (0.54–0.71) | 0.84 (0.73–0.98) | 20.09 | 65 | 0.0054 | −2.29 | 0.0220 |
| Central or intermediate | 8 | 382 | 268 | 0.71 (0.59–0.81) | ||||||
| Location of lesion | ||||||||||
| Upper lobe | 6 | 348 | 251 | 0.72 (0.67–0.77) | 0.99 (0.91–1.09) | 3.08 | 0 | 0.6873 | −0.11 | 0.9087 |
| Other lobe | 6 | 346 | 251 | 0.72 (0.67–0.77) | ||||||
CI, confidence interval; n, number.
Fig. 5.
Diagnostic yield of ultrathin bronchoscope based on a lesion size (≤20 mm vs. > 20 mm), b histologic diagnosis (malignant vs. benign), c bronchus sign (present vs. absent), d distance from the hilum (peripheral vs. non-peripheral), and e location of lesion (upper lobe vs. other lobes). CI, confidence interval; MH, Mantel Haenszel.
Complication Rates
Complication rates were 2.7% (46 out of 1,708 lesions) across 16 studies [13, 22, 23, 24, 26, 27, 28, 29, 30, 31, 32, 34, 36, 37, 38, 39], whereas three studies did not report complication rates (Table 2) [25, 33, 35]. Among 16 studies, no complication was noted in four studies. The most common complication was pneumothorax with a pooled rate of 1.1% (18 out of 1,708) (range, 0.6–3.0%), whereas that of chest tube insertion was 0.2% (three out of 1,708). The pooled rate of bleeding was 0.5% (nine out of 1,708) (range, 0.6–2.9%). The pooled rate of pneumonia was 0.5% (nine out of 1,708) (range, 0.3–2.5%). No deaths were reported in any study.
Discussion
To our knowledge, this is the first systematic meta-analysis investigating the diagnostic yield of UTB for diagnosing PPLs. Subgroup analysis showed significantly higher diagnostic yield of UTB with 1.7 mm channel size compared to 1.2 mm channel size. In addition, the funnel plot of included studies was symmetrical, implying a low risk of publication bias. Lastly, our study reported an excellent safety profile of UTB, with 2.7% overall complication rate.
The inverse variance-weighted pooled diagnostic yield was 0.65, which was slightly inferior to previous studies on other bronchoscopes. In a meta-analysis by Steinfort et al. [40], the pooled diagnostic yield of standard or thin bronchoscope was 0.73 (95% CI, 0.70–0.76) when combined with r-EBUS. This difference might be attributed to the median lesion size of the included studies, which is a major determinant of the diagnostic yield [41]. Steinfort et al. [40] included seven studies (580 lesions) with lesion size <25 mm and 6 studies (510 lesions) ≥25 mm, whereas our study included 12 studies (1,403 lesions) <25 mm and four studies (312 lesions) ≥25 mm. Furthermore, there is a possibility of selection bias in our study, since PPLs not observable on fluoroscopy, undiagnosed by previous conventional bronchoscopy under fluoroscopy, or difficult to access were included in some studies [22, 25, 33]. In addition, the smaller working channel of UTB prohibits the suction capability compared to thin bronchoscopy [12, 29, 31]. However, due to a small ED, UTB can pass through small airways with less resistance, thereby increasing performance and ease for operators. Furthermore, UTB provides good accessibility with visibility for PPL [13] and can play a role as a thick GS [23]. Lastly, UTB combined with fluoroscopy, r-EBUS, and VBN showed a higher diagnostic yield of 0.70 (95% CI, 0.63–0.77) than that of thin bronchoscopy (0.59; 95% CI, 0.51–0.66; p = 0.027) for PPL less than ≤30 mm [13].
In our study, UTB with 1.7 mm channel size showed a significantly higher diagnostic yield compared to that with 1.2 mm channel size. Conventional UTB mostly consisted of 1.2 mm channel size, which allowed the passage of mini-forceps and cytology brush <1.2 mm in diameter [42]. This small channel size limits adequate specimen collection, suction capability, and the use of r-EBUS [9, 28, 42], which might have led to the lower diagnostic yield observed with 1.2 mm channel size compared to that of thin bronchoscope. The recent development of 1.7 mm channel size allows the passage of r-EBUS [28], which provides a 360° view of the surrounding lesion by the ultrasound transducer [43] and shows a pooled diagnostic yield of 0.73 (95% CI, 0.70–0.76) [40]. The r-EBUS probe position relative to the lesion was known to be associated with the diagnostic yield of TBB, where the diagnostic yield of within, adjacent to, and outside the lesion was 0.83, 0.61, and 0.04, respectively (p < 0.001) [44]. In line with this, Nishi et al. [45] reported that UTB showed improved r-EBUS probe position for PPL after substituting thin bronchoscope (adjacent, 12; not visible, 32) by UTB (within, 26; adjacent, 11; not visible, 7) combined with fluoroscopy and VBN. This implies better applicability of r-EBUS in UTB compared with thin bronchoscope, which might be attributed to UTB's greater accessibility. Lastly, UTB with 1.7 mm channel size allows the passage of 1.5 mm biopsy forceps for adequate specimens and enhances the suction capability, which improves visualization around obstacles such as secretions and bleeding during the procedure [31]. These findings support the use of UTB with 1.7 mm over 1.2 mm channel size, as well as thin bronchoscope, emphasizing the importance of r-EBUS for the diagnosis of PPL.
Our subgroup analysis showed that UTB provided a significantly lower diagnostic yield of lesions located within the outer third of the lung field. On the contrary, Oki et al. [13] reported no significant difference in the diagnostic yield of UTB with multimodalities between the lesion in intermediate and peripheral location from the hilum (0.73 vs. 0.69; p = 0.701). In the same study, UTB showed significantly higher diagnostic yield than that of thin bronchoscope in the peripheral (0.69 vs. 0.56; p = 0.019) but not in the intermediate location (0.73 vs. 0.70; p > 0.999) [13]. This might be attributed to the good accessibility, bronchial selectivity, and ability of the thick GS of UTB in peripheral bronchi [12, 23, 30]. The discrepancy between these findings with our study might be attributed to inclusion of studies using 1.2 mm channel size (three out of eight) and the variability of adjunct modalities among included studies. Thus, UTB might be a good option during TBB for peripheral as well as central or intermediate lesions.
Furthermore, we found no difference in the diagnostic yield of UTB based on the upper lobe position of PPL, contrary to previous study findings on other bronchoscope [46]. When combined with fluoroscopy, GS, and r-EBUS, Kurimoto et al. [46] reported the lowest diagnostic yield of standard bronchoscope in the apico-posterior segment of left upper lobe compared with all other locations (0.40 vs. 0.76, p = 0.003). This might be attributed to the limitation of previous bronchoscope insertion in the upper lobes due to their acute angle, especially in the apical segment. On the contrary, UTB permits relatively easy access to upper lobes because of its wide angulation range (210° up and 130° down in BF-MP190F; Olympus Medical Systems, Tokyo, Japan). Thus, UTB might be the best option for TBB for lesions in the upper lobes. In addition, lesions in the superior segment of lower lobes with their acute angles might also be a good candidate for UTB, which warrants further investigation.
However, our study has some limitations. First, there was substantial heterogeneity in diagnostic yield among the included studies, as their quality was not uniform. To overcome this issue, we performed subgroup analysis to explore the factors regarding heterogeneity. However, we could not evaluate the diagnostic yield according to each guidance system due to different combinations of guidance use and study design among included studies, which might limit interpretation of our study results. Second, information on the other predictive factors for successful diagnosis was lacking due to variability among included study designs, such as r-EBUS findings, experience of operators, radiologic appearance on CT, distance from the pleura, and so on. Third, the reference standard of diagnostic yield was inconsistent among included studies, perhaps leading to different interpretations of successful diagnosis, which might have impacted the overall diagnostic yield of UTB. Fourth, the number of studies in subgroup analysis on PPL size was relatively small. This is attributed to the different method of measurement on lesion size (longest or mean) and lack of diagnostic yield based on lesion size (20 mm) among included studies. Fifth, our study excluded systematic reviews and conference abstracts, which are likely to show a low study quality. However, this may predispose to publication bias. Finally, our study was not registered prior to the review, but we followed the suggested PRISMA protocol.
Conclusion
In conclusion, our study showed that UTB is an excellent tool for PPL diagnosis with a low complication rate. The diagnostic yield of UTB with 1.7 mm channel size was significantly higher compared to that with 1.2 mm, which might be attributed to availability of r-EBUS in 1.7 mm channel size.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on the published literature.
Conflict of Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Sources
This work was supported by a clinical research grant from Pusan National University Hospital in 2022, and also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2022R1F1A1074117).
Author Contribution
Soo Han Kim contributed to conceptualization, methodology, study search, study selection, data acquisition, data curation, formal analysis, validation, and writing (original draft). Jinmi Kim contributed to formal analysis, validation, and writing (review and editing). Kyoungjune Pak contributed to conceptualization, methodology, data curation, validation, and writing (review and editing). Jung Seop Eom contributed to conceptualization, methodology, study search, study selection, data acquisition, data curation, writing (review and editing), and supervised the project. All authors read and approved the final manuscript.
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material. Further enquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgments
We thank the Department of Biostatistics, the Clinical Trial Center, and the Biomedical Research Institute of Pusan National University Hospital.
Funding Statement
This work was supported by a clinical research grant from Pusan National University Hospital in 2022, and also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2022R1F1A1074117).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Huang KL, Wang SY, Lu WC, Chang YH, Su J, Lu YT. Effects of low-dose computed tomography on lung cancer screening a systematic review, meta-analysis, and trial sequential analysis. BMC Pulm Med. 2019 Jul 11;19((1)):126. doi: 10.1186/s12890-019-0883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules when is it lung cancer? Diagnosis and management of lung Cancer. American college of chest physicians evidence-based clinical practice guidelines chest. (3rd ed) 2013;Vol. 143((5 Suppl)):p. e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule an analysis of discharge records. Ann Intern Med. 2011 Aug 2;155((3)):137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tukey MH, Wiener RS. Population-based estimates of transbronchial lung biopsy utilization and complications. Respir Med. 2012 Nov;106((11)):1559–1565. doi: 10.1016/j.rmed.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002 Oct;20((4)):972–974. doi: 10.1183/09031936.02.00032001. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi E, Yamazaki K, Sukoh N, Kikuchi J, Asahina H, Imura M, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J. 2004 Oct;24((4)):533–537. doi: 10.1183/09031936.04.00138603. [DOI] [PubMed] [Google Scholar]
- 8.Tsushima K, Sone S, Hanaoka T, Takayama F, Honda T, Kubo K. Comparison of bronchoscopic diagnosis for peripheral pulmonary nodule under fluoroscopic guidance with CT guidance. Respir Med. 2006;100((4)):737–745. doi: 10.1016/j.rmed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Asano F, Matsuno Y, Shinagawa N, Yamazaki K, Suzuki T, Ishida T, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest. 2006;130((2)):559–566. doi: 10.1378/chest.130.2.559. [DOI] [PubMed] [Google Scholar]
- 10.Eberhardt R, Anantham D, Herth F, Feller-Kopman D, Ernst A. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest. 2007 Jun;131((6)):1800–1805. doi: 10.1378/chest.06-3016. [DOI] [PubMed] [Google Scholar]
- 11.McGuire AL, Myers R, Grant K, Lam S, Yee J. The diagnostic accuracy and sensitivity for malignancy of radial-endobronchial ultrasound and electromagnetic navigation bronchoscopy for sampling of peripheral pulmonary lesions systematic review and meta-analysis. J bronchol Interv pulmonol. 2020 Apr;27((2)):106–121. doi: 10.1097/LBR.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 12.Oki M, Saka H. Diagnostic value of ultrathin bronchoscopy in peripheral pulmonary lesions a narrative review. J Thorac Dis. 2020 Dec;12((12)):7675–7682. doi: 10.21037/jtd-2020-abpd-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oki M, Saka H, Asano F, Kitagawa C, Kogure Y, Tsuzuku A, et al. Use of an ultrathin vs thin bronchoscope for peripheral pulmonary lesions a randomized trial. Chest. 2019 Nov;156((5)):954–964. doi: 10.1016/j.chest.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 14.McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting Items for a systematic review and meta-analysis of diagnostic test accuracy studies the PRISMA-DTA statement. JAMA. 2018 Jan 23;319((4)):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 15.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct 18;155((8)):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327((7414)):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinnes J, Deeks J, Kirby J, Roderick P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess. 2005;9((12)):1–113. doi: 10.3310/hta9120. iii. [DOI] [PubMed] [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010 Apr;1((2)):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 19.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014 Aug;67((8)):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997 Sep 13;315((7109)):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994 Dec;50((4)):1088–1101. [PubMed] [Google Scholar]
- 22.Matsuno Y, Asano F, Shindoh J, Abe T, Shiraki A, Ando M, et al. CT-guided ultrathin bronchoscopy bioptic approach and factors in predicting diagnosis. Intern Med Open Access. 2011;50((19)):2143–2148. doi: 10.2169/internalmedicine.50.5666. [DOI] [PubMed] [Google Scholar]
- 23.Zheng X, Xie F, Li Y, Chen J, Jiang Y, Sun J. Ultrathin bronchoscope combined with virtual bronchoscopic navigation and endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions with or without fluoroscopy a randomized trial. Thorac Cancer. 2021 May 6;12((12)):1864–1872. doi: 10.1111/1759-7714.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinagawa N, Yamazaki K, Onodera Y, Miyasaka K, Kikuchi E, Dosaka-Akita H, et al. CT-guided transbronchial biopsy using an ultrathin bronchoscope with virtual bronchoscopic navigation. Chest. 2004 Mar;125((3)):1138–1143. doi: 10.1378/chest.125.3.1138. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto S, Ueno K, Imamura F, Matsuoka H, Nagatomo I, Omiya Y, et al. Usefulness of ultrathin bronchoscopy in diagnosis of lung cancer. Lung Cancer. 2004;46((1)):43–48. doi: 10.1016/j.lungcan.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Shinagawa N, Yamazaki K, Onodera Y, Asano F, Ishida T, Moriya H, et al. Virtual bronchoscopic navigation system shortens the examination time--feasibility study of virtual bronchoscopic navigation system. Lung Cancer. 2007 May;56((2)):201–206. doi: 10.1016/j.lungcan.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Tachihara M, Ishida T, Kanazawa K, Sugawara A, Watanabe K, Uekita K, et al. A virtual bronchoscopic navigation system under X-ray fluoroscopy for transbronchial diagnosis of small peripheral pulmonary lesions. Lung Cancer. 2007 Sep;57((3)):322–327. doi: 10.1016/j.lungcan.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Oki M, Saka H, Kitagawa C, Kogure Y, Mori K, Kajikawa S. Endobronchial ultrasound-guided transbronchial biopsy using novel thin bronchoscope for diagnosis of peripheral pulmonary lesions. J Thorac Oncol. 2009 Oct;4((10)):1274–1277. doi: 10.1097/JTO.0b013e3181b623e1. [DOI] [PubMed] [Google Scholar]
- 29.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, et al. Randomized study of endobronchial ultrasound-guided transbronchial biopsy thin bronchoscopic method versus guide sheath method. J Thorac Oncol. 2012 Mar;7((3)):535–541. doi: 10.1097/JTO.0b013e3182417e60. [DOI] [PubMed] [Google Scholar]
- 30.Asano F, Shinagawa N, Ishida T, Shindoh J, Anzai M, Tsuzuku A, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med. 2013 Aug 1;188((3)):327–333. doi: 10.1164/rccm.201211-2104OC. [DOI] [PubMed] [Google Scholar]
- 31.Oki M, Saka H, Ando M, Asano F, Kurimoto N, Morita K, et al. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions a randomized trial. Am J Respir Crit Care Med. 2015;192((4)):468–476. doi: 10.1164/rccm.201502-0205OC. [DOI] [PubMed] [Google Scholar]
- 32.Franzen D, Diacon AH, Freitag L, Schubert PT, Wright CA, Schuurmans MM. Ultrathin bronchoscopy for solitary pulmonary lesions in a region endemic for tuberculosis a randomised pilot trial. BMC Pulm Med. 2016 Apr 27;16((1)):62. doi: 10.1186/s12890-016-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokoro Y, Yasuo M, Kobayashi T, Hama M, Ichiyama T, Horiuchi T. Computed tomography-guided bronchoscopy in the diagnosis of small peripheral pulmonary lesions a retrospective study of 240 examinations in a single academic center. Respir Investig. 2016 Sep;54((5)):347–354. doi: 10.1016/j.resinv.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Ali EAA, Takizawa H, Kawakita N, Sawada T, Tsuboi M, Toba H, et al. Transbronchial biopsy using an ultrathin bronchoscope guided by cone-beam computed tomography and virtual bronchoscopic navigation in the diagnosis of pulmonary nodules. Respiration. 2019;98((4)):321–328. doi: 10.1159/000500228. [DOI] [PubMed] [Google Scholar]
- 35.Diez-Ferrer M, Morales A, Tebé C, Cubero N, López-Lisbona R, Padrones S. Ultrathin bronchoscopy with and without virtual bronchoscopic navigation influence of segmentation on diagnostic yield. Respiration. 2019;97((3)):252–258. doi: 10.1159/000493270. [DOI] [PubMed] [Google Scholar]
- 36.Sehgal IS, Dhooria S, Bal A, Gupta N, Ram B, Aggarwal AN, et al. A retrospective study comparing the ultrathin versus conventional bronchoscope for performing radial endobronchial ultrasound in the evaluation of peripheral pulmonary lesions. Lung India. 2019 Mar-Apr;36((2)):102–107. doi: 10.4103/lungindia.lungindia_115_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumi T, Ikeda T, Sawai T, Shijubou N, Kure K, Yamada Y, et al. Comparison of ultrathin bronchoscopy with conventional bronchoscopy for the diagnosis of peripheral lung lesions without virtual bronchial navigation. Respir Invest. 2020;58((5)):376–380. doi: 10.1016/j.resinv.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Kawakita N, Takizawa H, Toba H, Sakamoto S, Miyamoto N, Matsumoto D. Cone-beam computed tomography versus computed tomography-guided ultrathin bronchoscopic diagnosis for peripheral pulmonary lesions a propensity score-matched analysis. Respirology. 2021 May;26((5)):477–484. doi: 10.1111/resp.14016. [DOI] [PubMed] [Google Scholar]
- 39.Sumi T, Kamada K, Sawai T, Shijubou N, Yamada Y, Nakata H, et al. Sedation with fentanyl and midazolam without oropharyngeal anesthesia compared with sedation with pethidine and midazolam with oropharyngeal anesthesia in ultrathin bronchoscopy for peripheral lung lesions. Respir Invest. 2021;59((2)):228–234. doi: 10.1016/j.resinv.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer systematic review and meta-analysis. Eur Respir J. 2011 Apr;37((4)):902–910. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 41.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000 Apr;117((4)):1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 42.Rooney CP, Wolf K, McLennan G. Ultrathin bronchoscopy as an adjunct to standard bronchoscopy in the diagnosis of peripheral lung lesions a preliminary report. Respiration. 2002;69((1)):63–68. doi: 10.1159/000049372. [DOI] [PubMed] [Google Scholar]
- 43.Anantham D, Siyue Koh M, Ernst A. Endobronchial ultrasound. Respir Med. 2009 Oct;103((10)):1406–1414. doi: 10.1016/j.rmed.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, Shinagawa N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007 Aug;132((2)):603–608. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 45.Nishii Y, Nakamura Y, Fujiwara K, Ito K, Sakaguchi T, Suzuki Y, et al. Use of ultrathin bronchoscope on a need basis improves diagnostic yield of difficult-to-approach pulmonary lesions. Front Med. 2020;7:588048. doi: 10.3389/fmed.2020.588048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004 Sep;126((3)):959–965. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material. Further enquiries can be directed to the corresponding author.