Abstract
Introduction
3M syndrome is an autosomal recessive disorder characterized by characteristic facial features, severe pre- and postnatal growth restriction (<–4 SDS), and normal mental development. 3M syndrome is genetically heterogeneous. Up to date, causative mutations have been demonstrated in 3 genes, cullin-7 (CUL7), obscurin-like 1 (OBSL1), and coiled coil domain containing protein 8 (CCDC8).
Case presentation
Here, we report a patient who was referred to our clinic due to short stature and developmental delay. Physical examination revealed prenatal onset short stature, low birth weight, and normal head circumference. She displayed several dysmorphic facial features in addition to developmental delay and bilateral sensorineural hearing loss. The physical findings were suggestive of 3M syndrome. Genetic assessment revealed a novel homozygous frameshift c.418_419delAC (p.Thr140Cysfs*11) variant in the CUL7 gene and a previously reported pathogenic nonsense homozygous c.942C>A (p.Cys314Ter) variant in the ILDR1 gene. The parents were heterozygous for the same variant.
Discussion
3M syndrome should be considered in the differential diagnosis of patients with short stature and typical facial features even if in the presence of other inconsistent features such as developmental delay. In addition, it is important to take into account the co-occurrence of rare autosomal recessive genetic disorders especially in countries with a high consanguineous marriage rate.
Keywords: 3M syndrome, CUL7 gene, Short stature, Developmental delay, Sensorineural hearing loss
Established Facts
3M syndrome is characterized by severe pre- and postnatal growth deficiency, characteristic facial features, and normal intelligence.
Novel Insights
3M syndrome should be considered in the differential diagnosis of patients with short stature and typical facial features even in the presence of other inconsistent features such as developmental delay and hearing loss.
Introduction
The Miller-McKusick-Malvaux (3M) syndrome is a rare autosomal recessive disorder characterized by severe intrauterine and postnatal growth retardation with dysmorphic facial features as gloomy face and skeletal abnormalities. Microcephaly, intellectual disability (ID), and organ malformations are not observed [Van der Wal et al., 2001]. 3M syndrome is grouped under “Slender Bone Dysplasia Group” according to Nosology and Classification of Genetic Skeletal Disorders [Mortier et al., 2019]. Slender appearance of long tubular bones, tall vertebrae with shortness in the anteroposterior diameter, and narrow pelvis are among the radiographic features [Spranger et al., 2018]. The genetic aetiology is heterogeneous, CUL7 (MIM 609577), OBSL1 (MIM 610991), and CCDC8 (MIM 614145) variants have been identified in the aetiology so far, with CUL7 mutations representing the major gene defect [Holder-Espinasse, 2012]. Nevertheless, it is suggested that some other genes, probably in the same growth regulatory pathway, might be involved in the aetiology [Clayton et al., 2012].
Case Presentation
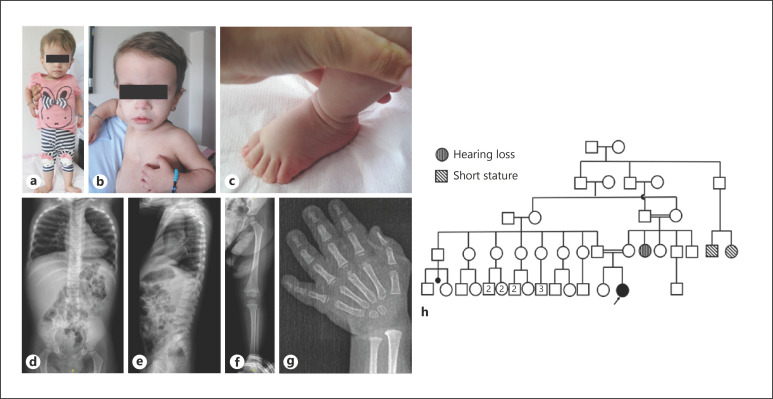
A 26-month-old girl was referred with short stature, global developmental delay (GDD), and dysmorphic facial features. The patient was the second live-born child of first cousin parents following an uneventful term pregnancy with a birth weight of 2,000 g (−3.02 SDS) and a birth length of 38 cm (−4.58 SDS). Her head circumference was not noted. After birth, she had respiratory distress and required neonatal intensive care unit support for one day. In the postnatal period, Bera (brainstem evoked response audiometry) test was performed owing to bilateral hearing loss. Auditory brainstem response trace showing no V wave at 100 dB nHL, indicating at least bilateral profound hearing loss. Her family history was positive for deafness since her aunt had bilateral hearing loss as well. Before admission to our center, she was evaluated for growth retardation and recurrent bronchiolitis. Karyotype analysis was consistent with 46,XX, and FGFR3 sequence analysis was found to be normal. Growth hormone levels were normal as well. Due to GDD, the Denver Developmental Screening Test (DDST) II was performed to screen the patient's development at the age of 3 years 7 months. In this study, the Turkish version of DDST was used [Durmazlar et al., 1998]. The development of fine motor, gross motor, personal-social, and language was compatible with the age of 21–22 months, 20–21 months, 21–22 months, and 19–20 months, respectively. Due to a moderate-to-severe speech delay, the patient was referred to speech-language therapy, and physical therapy was offered for mildly delayed gross motor developmental milestones. Physical examination at her admission revealed a head circumference of 47 cm (−0.54 SDS), height of 69 cm (−5.03 SDS), weight of 6.9 kg (−6.93 SDS), and arm span of 64 cm. She had a triangular face with pointy chin, frontal bossing, depressed nasal bridge, pectus excavatum, and prominent heels (Fig. 1a–c). Cardiac evaluation with echocardiography and abdomen ultrasonography were all normal. Radiographic evaluation revealed slender long bones, tall vertebral bodies, and delayed bone age (Fig. 1d–g). After informed consent was taken from the parents, molecular tests were planned.
Fig. 1.
a–c Clinical pictures at 26 months of age. Note the triangular face with pointy chin, gloomy face, relative macrocephaly, frontal bossing, depressed nasal bridge, pectus excavatum, and prominent heels. d, e Radiographic evaluation revealed wide and broad chest, tall vertebral bodies, and shortening of posteroanterior diameter of lumbar vertebral bodies. f, g Mild slender appearance in short and long tubular bones. h Pedigree of the proband. The other family members with short stature and hearing loss are indicated in the pedigree.
Materials and Methods
Genomic DNA was extracted from peripheral blood using standardized protocols for salt precipitation.
Sanger Sequencing
Exon-specific primers for CUL7 and GJB2 were designed using PerlPrimer (http://perlprimer.sourceforge.net/) and used to amplify the coding exons 1–26 of CUL7 and exon 2 of GJB2 and their flanking regions (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/524703). Amplification was done with 1 U of Taq polymerase (Qiagen), 0.2 mM of each of the dNTPs, 1× PCR reaction buffer, 1.5 mM MgCl2, 5 pmol of each forward and reverse primers, and 50 ng of genomic DNA template in 25 μL reactions.
PCR was performed by an initial 5 min denaturation at 95°C followed by 40 cycles of 95°C for 30 s, 58–62°C for 35 s, and 72°C for 45 s with a final elongation at 72°C for 10 min.
Sanger sequencing was performed using the BigDye Terminator v.3.1 Cycle Sequencing Kit, and sequencing products were applied onto ABI 3500 genetic analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The Sanger sequencing was also performed to evaluate cosegregation within family members. Variants are designated on the coding CUL7 and GJB2 sequence with reference to Genbank accession number NM_014780.5 and NM_004004.6, respectively, with the A of the initiation codon ATG as number +1.
Chromosomal Microarray Analysis
Microarray procedure was performed using the GeneChip® CytoScan Optima Array (Affymetrix, Santa Clara, CA, USA) according to the manufacturer's instructions. The data aligned to the human genome build 37 (GRCh37/hg19) reference genome. Chromosome Analysis Suite 3.1 (ChAS®3.1) software (Affymetrix) was utilized for the analysis. The CNVs (copy number variations) found in the patient were analyzed with in-house data and public databases, such as Database of Chromosomal Imbalance and Phenotype in Humans using Ensemble Resources (DECIPHER), ClinVar Variants, and Database of Genomic Variants (DGV).
Exome Sequencing (ES)
Ion AmpliSeqTM Exome RDY Kit (Thermo Fisher Scientific) was used for exome library preparation. Emulsion PCR was performed on Ion OneTouchTM 2 instrument using the Ion PI Hi-Q OT2 200 Kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Enrichment of Ion Sphere Particles (ISPs) was performed using the One TouchTM ES module. ISPs were loaded on Ion PI chips and sequenced with Ion ProtonTM platform (Thermo Fisher Scientific).
Bioinformatic Analysis
ES was performed for index case. Variant filtering steps were performed as outlined previously [Utine et al., 2017]. All variants passed the quality score filters and were annotated with the Ion ReporterTM 5.10 software. Exonic and non-synonymous rare variants (minimum allele frequency [MAF] < 0.01) were selected for in-house filtering. After that, the alterations which were in the boundaries of runs of homozygosity (ROH) regions ≥1 Mb within all chromosomes were extracted. We used HomSI, a software that performs mapping of disease loci by SNP genotyping using VCF file obtained from exome sequence data, to identify the ROH in index cases. As a final step, variants which were observed in ExAC Browser (in homozygous state) were ruled out. After selection and filtering steps, we identified candidate variants. A novel homozygous frameshift CUL7 variant and a homozygous previously reported pathogenic nonsense ILDR1 variant were detected among these candidates, which was compatible with the patient's phenotype.
Results
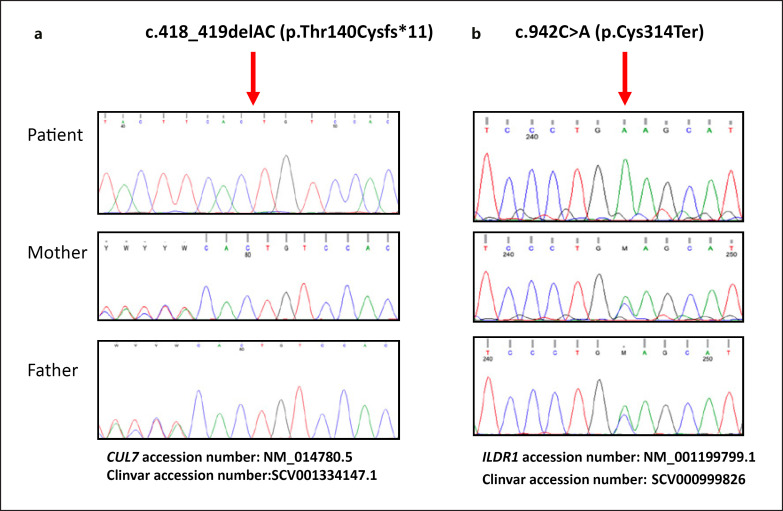
DNA sequence analysis revealed a novel homozygous CUL7 (NM_014780.5) variant; c.418_419delAC (p.Thr140Cysfs*11) in the patient. The length of the ROH including CUL7 is nearly 20 Mb (chr6: 37,446,163–57,244,893). The parents were heterozygous for the same variant (Fig. 2a). The detected variant is submitted to ClinVar with accession number SCV001334147.1. The chromosomal microarray and GJB2 sequence analysis were all normal. ES was performed in the patient in order to identify the genetic aetiology of intellectual disability/global developmental delay (ID/GDD) and sensorineural hearing loss, however, except for a homozygous nonsense ILDR1 variant no other pathogenic variants were detected. Variants that remained after filtering in the exome sequenced patient which might contribute ID/GDD are shown in online suppl. Table 2.
Fig. 2.
a Sequence analysis of CUL7 showing the c.418_419delAC (p.Thr140Cysfs*11) variant in exon 2. b Sequence analysis of ILDR1 showing the c.942C>A (p.Cys314Ter) variant in exon 7. Sanger sequence analysis confirmed that both parents were heterozygous for the 2 variants.
Discussion
In this study, we further expanded the mutational spectrum of 3M syndrome in a patient presenting with short stature, sensorineural hearing loss, and developmental delay, and also highlighted the co-occurrence of rare autosomal recessive conditions that lead to the patient's phenotype.
3M syndrome was first clinically described by Miller et al. [1975]. To date, approximately 100 patients with 3M syndrome have been reported in the literature [Holder-Espinasse, 2012]. Many newborns with 3M syndrome are initially diagnosed with achondroplasia as was the case in the present patient. However, the clinical and radiographic findings are quite distinct. Achondroplasia can be easily excluded with the absence of typical radiographic findings and the presence of less severe short stature compared to that of 3M syndrome. In addition, children with 3M syndrome are significantly shorter at birth than those with achondroplasia. Another differential diagnosis of 3M syndrome is Russel Silver syndrome (RSS). The characteristic limb asymmetry of RSS is absent in 3M syndrome and the facial features are different as well. In Mulibrey nanism, which is another diagnosis to consider, J-shaped sella turcica and hepatomegaly may be distinctive. The other disorder to consider in the differential diagnosis of 3M syndrome is Dubowitz syndrome which can be distinguished by microcephaly, skin lesions such as eczema, characteristic facial features (small face, sloping forehead, broad nasal bridge, road nasal tip, short palpebral fissures, telecanthus, ptosis, dysplastic ears), and ID [Huber et al., 2011]. Due to coarse facial features and small stature, most patients with 3M syndrome have been suspected of having storage disorders such as mucopolysaccharidoses in the first year of life. Patients with mucopolysaccharidoses display hepatomegaly, hearing loss, dysostosis multiplex, and ID in addition to coarse face and short stature, which are absent in 3M syndrome [Clarke, 2016]. ID/GDD is not among the expected findings in 3M syndrome. However, 3M syndrome patients with mild motor developmental delay and normal intelligence have previously been reported [Hu et al., 2017; Jacob and Girisha, 2021]. Interestingly, only a 3-year-old patient with mild developmental delay particularly in speech was reported. This patient had fetal distress after birth which required resuscitation and admission to the neonatal intensive care unit for 3 weeks. He also had seizures and feeding problems. During the investigation of developmental delay, cranial MRI revealed cerebellar atrophy with brain stem and supratentorial volume loss [HabibUllah et al., 2019]. Interestingly, the present patient had GDD, and the diagnosis of 3M syndrome was not considered as a differential diagnosis in her previous admissions before her referral to our center. According to DDST, while her gross motor skills were mildly delayed, she had a significant delay in language and social developmental fields. In addition, she had bilateral sensorineural hearing loss which was detected in the early postnatal period. GJB2, mutations of which represent the most common cause of non-syndromic hearing loss, was found to be normal. Chromosomal microarray analysis was also performed due to ID/GDD, however, no pathogenic copy number variations were detected. Finally, we performed ES to elucidate the molecular aetiology of ID/GDD and bilateral sensorineural hearing loss, however, no pathogenic variants were found except for the variant in CUL7. In order to explain the patient's phenotype, we further expanded the filtering, which can lead to ID/GDD and bilateral hearing loss, and we finally identified a previously reported pathogenic homozygous nonsense variant in the ILDR1 gene. The length of the ROH including ILDR1 is nearly 1.5 Mb (chr3:121,527,902–122,647,891). Sanger sequencing confirmed that the parents were heterozygous for the same variant (Fig. 2b). The patient's aunt, who had bilateral sensorineural hearing loss, was born to first cousin parents. Molecular test could not be performed, however, due to consanguinity, the aetiology of hearing loss was considered to be the same as in the present patient. ID/GDD has many causes with a contribution of both genetic and environmental factors. Since some of the genetic aetiologies of ID/GDD remain still unclear, it is possible that other genetic causes yet undefined and various environmental factors might have contributed to the patient's phenotypic features.
Radiographic findings in 3M syndrome may not be detected in the first 2 years of life [Huber et al., 2011; Clayton et al., 2012]. Typical radiological findings include slender long bones, shortening of the posteroanterior diameter of vertebral bodies, delayed bone age, and narrow pelvis. Distal shortening of the ulna and kyphoscoliosis are rarely reported in these patients [Hennekam et al., 1987]. It has been suggested that bone abnormalities are less common in patients with CUL7 mutations [Maksimova et al., 2007]. The present patient had radiological findings suggestive of 3M syndrome.
The genetic aetiology of 3M syndrome is heterogeneous. Homozygous or compound heterozygous pathogenic variants in CUL7 (MIM 609577), OBSL1 (MIM 610991), and CCDC8 (MIM 614145) have previously been revealed in the aetiology. Nevertheless, the presence of 3M syndrome patients with no CUL7, OBSL1, and CCDC8 pathogenic variants suggests that other gene(s) yet unknown might be involved [Dias et al., 2002]. Huber et al. described CUL7 pathogenic variants as the first gene variants associated with 3M syndrome. More than 40 different CUL7 pathogenic variants have been reported in 3M syndrome patients so far, including frameshift, nonsense, and missense mutations [Huber et al., 2011; Holder-Espinasse, 2012; Simsek-Kiper et al., 2019; Isik et al., 2021].
The present patient has a homozygous novel frameshift variant in CUL7 considered to be pathogenic according to the ACMG 2015 criteria [Li and Wang, 2017]. The protein alteration caused by the CUL7 c.418_419delAC p.(Thr140Cysfs*11) results in a premature termination codon at position 140 of the CUL7 mRNA. The variant has not previously been reported in neither gnomAD nor HGMD public databases. The parents were heterozygous for the same variant, supporting the autosomal recessive inheritance.
CUL7 belongs to the cullin family, which is composed of 7 cullins (CUL1, 2, 3, 4A, 4B, 5, and 7) playing a scaffold role in assembling a multisubunit ubiquitin ligase (E3s). CUL7 interacts with other cellular proteins (SKP1, FBXW8, and ROC1) to form an E3 ubiquitin ligase complex that promotes ubiquitination [Dias et al., 2002; Maksimova et al., 2007]. It is important for normal functioning of a number of vital biological processes such as cell cycle progression, cell proliferation, apoptosis, and signal transduction pathways. CUL7 plays a fundamental role in in utero growth and development including lung development, endochondral ossification, and chondrogenesis. Variants in CUL7 may contribute to disruption of the GH and IGF-I signaling pathways through IRS-1 deposition; this accumulation results in increased activation of ACT and MAPK, and overstimulation may lead to cellular aging. Recently, variants in OBSL1 and CCDC8 were identified in 3M patients that do not harbor CUL7 variants. Importantly, the variants in these 3 genes occurred in a mutually exclusively manner, strongly suggesting that OBSL1, CCDC8, and CUL7 work in the same pathway and this is called the 3M complex. The dysfunction in this complex leads to serious defects in microtubule dynamics, chromosome separation, cell survival, and organism growth [Xu et al., 2008].
In conclusion, 3M syndrome should be considered in the differential diagnosis of patients with short stature and typical facial features even in the presence of other inconsistent findings. Moreover, co-occurrence of rare autosomal recessive genetic disorders should be kept in mind especially in countries with a high consanguineous marriage rate.
Statement of Ethics
This study was performed in accordance with the Declaration of Helsinki Principles.
The study protocol was approved by Hacettepe University Ethics Committee (GO 13/165-25) and was supported by Hacettepe University (Project ID: 633 and 14392).
Written informed consent was obtained from the parent/legal guardian of the patient for publication of the details of her medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
All the authors have contributed significantly and approved the final content of the manuscript.
A.A. contributed to the literature search and clinical evaluation, collected references, prepared the final manuscript, and is corresponding author.
P.Ö.Ş.-K. contributed to the clinical evaluation and manuscript writing.
E.U. contributed to the clinical evaluation.
E.T. performed molecular testing and analysis.
K.B., the senior professor, revised the clinical and molecular data.
Data Availability Statement
All data generated or analyzed during this study are included. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Clarke LA. GeneReviews® [Internet] Seattle: University of Washington, Seattle; 2016. Mucopolysaccharidosis type, I. [Google Scholar]
- 2.Clayton PE, Hanson D, Magee L, Murray PG, Saunders E, Abu‐Amero SN, et al. Exploring the spectrum of 3‐M syndrome, a primordial short stature disorder of disrupted ubiquitination. Clin Endocrinol (Oxf) 2012;77((3)):335–342. doi: 10.1111/j.1365-2265.2012.04428.x. [DOI] [PubMed] [Google Scholar]
- 3.Dias DC, Dolios G, Wang R, Pan Z-Q. CUL7: a DOC domain-containing cullin selectively binds Skp1⋅Fbx29 to form an SCF-like complex. Proc Natl Acad Sci U S A. 2002;99((26)):16601–6. doi: 10.1073/pnas.252646399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durmazlar N, Ozturk Ç, Ural B, Karaagaoglu E, Anlar B. Turkish children's performance on Denver II: effect of sex and mother's education. Dev Med Child Neurol. 1998;40((6)):411–416. doi: 10.1111/j.1469-8749.1998.tb08217.x. [DOI] [PubMed] [Google Scholar]
- 5.HabibUllah H, Al-Baradie R, Bashir S. 3-M syndrome: a local case report. Am J Case Rep. 2019;20:36. doi: 10.12659/AJCR.912736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennekam RC, Bijlsma JB, Spranger J, Neri G. Further delineation of the 3-M syndrome with review of the literature. Am J Med Genet. 1987;28((1)):195–209. doi: 10.1002/ajmg.1320280127. [DOI] [PubMed] [Google Scholar]
- 7.Holder-Espinasse M. GeneReviews® [Internet] Seattle: University of Washington, Seattle; 2012. 3-M syndrome. [Google Scholar]
- 8.Hu X, Li H, Gui B, Xu Y, Wang J, Li N, et al. Prenatal and early diagnosis of Chinese 3-M syndrome patients with novel pathogenic variants. Clin Chim Acta. 2017;474:159–164. doi: 10.1016/j.cca.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Huber C, Munnich A, Cormier-Daire V. The 3M syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25((1)):143–151. doi: 10.1016/j.beem.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Huber RS, Houlihan D, Filter K, Dubowitz Syndrome: A Review and Implications for Cognitive Behavioral, and Psychological. J Clin Med Res. 2011;3((4)):147–155. doi: 10.4021/jocmr581w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isik E, Arican D, Atik T, Ooi JE, Darcan S, Ozen S, et al. A rare cause of syndromic short stature: 3M syndrome in three families. Am J Med Genet A. 2021;185((2)):461–468. doi: 10.1002/ajmg.a.61989. [DOI] [PubMed] [Google Scholar]
- 12.Jacob P, Girisha KM. Three M syndrome 2 in two Indian patients. Am J Med Genet A. 2021;185((2)):614–616. doi: 10.1002/ajmg.a.61949. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Wang K. InterVar: clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am J Hum Genet. 2017;100((2)):267–280. doi: 10.1016/j.ajhg.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maksimova N, Hara K, Miyashia A, Nikolaeva I, Shiga A, Nogovicina A, et al. Clinical, molecular and histopathological features of short stature syndrome with novel CUL7 mutation in Yakuts: new population isolate in Asia. J Med Genet. 2007;44((12)):772–778. doi: 10.1136/jmg.2007.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JD, McKusick VA, Malvaux P, Temtamy S, Salinas C. The 3-M syndrome: a heritable low birthweight dwarfism. Birth Defects Orig Artic Ser. 1975;11((5)):39–47. [PubMed] [Google Scholar]
- 16.Mortier GR, Cohn DH, Cormier‐Daire V, Hall C, Krakow D, Mundlos S, et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am J Med Genet A. 2019;179((12)):2393–2419. doi: 10.1002/ajmg.a.61366. [DOI] [PubMed] [Google Scholar]
- 17.Simsek‐Kiper PO, Taskiran E, Kosukcu C, Arslan UE, Cormier‐Daire V, Gonc N, et al. Further expanding the mutational spectrum and investigation of genotype–phenotype correlation in 3M syndrome. Am J Med Genet A. 2019;179((7)):1157–1172. doi: 10.1002/ajmg.a.61154. [DOI] [PubMed] [Google Scholar]
- 18.Spranger JW, Brill PW, Hall C, Superti-Furga A, Unger S. Oxford: Oxford University Press; 2018. Bone Dysplasias: An Atlas of Genetic Disorders of Skeletal Development. [Google Scholar]
- 19.Utine GE, Taşkıran EZ, Koşukcu C, Karaosmanoğlu B, Güleray N, Doğan ÖA, et al. HERC1 mutations in idiopathic intellectual disability. Eur J Med Genet. 2017;60((5)):279–283. doi: 10.1016/j.ejmg.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Van der Wal G, Otten BJ, Brunner HG, Van der Burgt I. 3-M syndrome: description of six new patients with review of the literature. Clin Dysmorphol. 2001;10((4)):241–252. doi: 10.1097/00019605-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Sarikas A, Dias-Santagata DC, Dolios G, Lafontant PJ, Tsai S-C, et al. The CUL7 E3 ubiquitin ligase targets insulin receptor substrate 1 for ubiquitin-dependent degradation. Mol Cell. 2008;30((4)):403–414. doi: 10.1016/j.molcel.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included. Further inquiries can be directed to the corresponding author.