Abstract
Background:
This systematic review and meta-analysis aimed to overview the observational studies on the association of exposure to air pollution and type 1 diabetes mellitus (T1DM).
Materials and Methods:
Based on PRISMA guidelines, we systematically reviewed the databases of PubMed, Scopus, Embase, and Web of Science databases to determine the association of air pollution exposure and T1DM. Quality assessment of the papers was evaluated using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for observational studies. The odds ratios (OR) and their 95% confidence intervals (CI) were calculated to assess the strength of the associations between air pollutants (gases and particulate matter air pollutants including PM10, PM2.5, NO2, volatile organic compound, SO4, SO2, O3) and T1DM.
Results:
Out of 385 initially identified papers, 6 studies were used for this meta-analysis. Fixed effects meta-analysis showed a significant association between per 10 μg/m3 increase in O3 and PM2.5 exposures with the increased risk of T1DM (3 studies, OR = 1.51, 95% CI: 1.26, 1.80, I2 = 83.5% for O3 and two studies, OR = 1.03, 95% CI: 1.01, 1.05, I2 = 76.3% for PM2.5). There was no evidence of association between increased risk of T1DM and exposure to PM10 (OR = 1.02, 95% CI: 0.99–1.06, I2 = 59.4%), SO4 (OR = 1.16, 95% CI: 0.91–1.49, I2 = 93.8%), SO2 (OR = 0.94, 95% CI: 0.83–1.06, I2 = 85.0%), and NO2 (OR = 0.995,95% CI: 1.05–1.04, I2 = 24.7%).
Conclusion:
Recent publications indicated that exposure to ozone and PM2.5 may be a risk factor for T1DM. However, due to limited available studies, more prospective cohort studies are needed to clarify the role of air pollutants in T1DM occurrence.
Keywords: Adolescents, air pollution, children, meta-analysis, type 1 diabetes mellitus
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is one of the most common chronic diseases in children. It accounts for approximately 10% of the total diabetic cases that stems from the autoimmune destruction of insulin-producing pancreatic beta cells and leads to lack or insufficient insulin secretion.[1,2]
Epidemiologic studies indicated a 2%–5% increasing trend for T1DM worldwide. The causes of this increasing rate have not been determined yet.[3] Numerous prospective studies have been conducted so far to understand the etiology of T1DM, but no definitive answers have been reached. Genetic factors have been demonstrated to play profound roles in T1DM development.[4] However, data from identical twins show that there might be other non-genetic factors (such as environmental triggers) that initiate the disease process and act together with genetic factors in T1DM development and progression.[5] In this regard, more recent studies have highlighted the potential impact of exposure to air pollution on the increased prevalence and mortality of T1DM.[6]
Air pollution has been recognized as the most important environmental health risk factor globally, leads to 4.6 million deaths annually according to the World Health Organization.[7] Based on their state in the atmosphere, air pollutants comprised both gaseous and particulate matters (PMs) which include compounds of ozone, sulfur, nitrogen, carbon oxides, hydrocarbons, halogens, and total suspended PMs, inhalable PM10, fine PM2.5, and ultrafine PM.[8] Several epidemiological studies have demonstrated a link between exposure to one or some of these air pollutants and adverse human health effects including respiratory diseases, cerebrovascular diseases, and cardiovascular diseases.[9] Similarly, in recent years, accumulating evidence suggest that exposure to these air pollutants (ambient and outdoor) increases the risks of several autoimmune disorders, including diabetes[9]. More recent studies also reported some mechanisms of air pollutants in developing immune disorders.[10,11]
Although previous studies have revealed an association between exposure to air pollution and T2DM,[12] no conclusive relationship has been found for air pollution exposure and the development or progression of T1DM. In this regard, some studies have shown that prenatal exposure to air pollution may be associated with a higher risk of offspring T1DM.[13] Moreover, higher risk of T1DM incidence has been reported in countries by higher concentrations of PM10 <10μm, nitrogen oxides, (NOx), and nonmethane volatile organic compounds (VOCs) emissions.[14] Several other investigations have also implicated that cumulative exposure to specific air pollutants may predispose to the development of T1DM in children.[15] Furthermore, in another study conducted in the USA, the authors concluded that exposure to ambient pollutants like ozone may contribute to T1DM development, while exposure to PM[12] might be associated with T1DM before 5 years of age.[16] In contrast to the mentioned researches, there are also other studies showing no association between exposure to air pollution with T1DM development or its metabolic control.[17]
Based on the mentioned notes, it is controversial whether there is any relationship between exposure to air pollution and the risk of T1DM. Therefore, in the present study, we attempted to systematically review the literature to identify any relationship between exposure to a wide array of gaseous and particulate air pollutants with T1DM. To the best of our knowledge, this is the first effort aimed to examine the overall association of air pollution and the risk of T1DM through a meta-analysis and literature review.
MATERIALS AND METHODS
Search strategy
In this systematic review study, the literature was searched in PubMed, Scopus, Embase, and Web of Science databases until May 2020. All cross-sectional, case–control, and cohort studies were considered. The search strategy used in the databases was as follows: (“Air Pollutions” OR “Pollution, Air” OR “Air Quality” OR “air contamination” OR “air pollution” OR “air pollutioning” OR “atmosphere pollution” OR “atmospheric pollution” OR “polluted air” OR “polluted atmosphere”) in combination with (“Type 1 Diabetes Mellitus” OR “Diabetes Mellitus, Type I” OR “Type 1 Diabetes” OR “Diabetes, Type 1” OR “Diabetes Mellitus, Ketosis-Prone” OR “Diabetes Mellitus, Ketosis Prone” OR “Ketosis-Prone Diabetes Mellitus” “Diabetes, Autoimmune” OR “Autoimmune Diabetes” OR “Diabetes Mellitus, Juvenile-Onset” OR “Diabetes Mellitus, Juvenile Onset” OR “Juvenile-Onset Diabetes Mellitus” OR “Juvenile-Onset Diabetes” OR “Diabetes, Juvenile-Onset” OR “Juvenile Onset Diabetes” OR “Diabetes Mellitus, Insulin-Dependent” OR “Diabetes Mellitus, Insulin Dependent” OR “Insulin-Dependent Diabetes Mellitus” OR “IDDM” OR “Diabetes Mellitus, Insulin-Dependent, 1” OR “Insulin-Dependent Diabetes Mellitus 1”OR “Insulin-Dependent Diabetes Mellitus 1” OR “Diabetes Mellitus, Brittle” OR “Brittle Diabetes Mellitus” OR “Diabetes Mellitus, Sudden-Onset” OR “Diabetes Mellitus, Sudden Onset” OR “Sudden-Onset Diabetes Mellitus” OR “ketoacidotic diabetes” OR “labile diabetes mellitus” OR “mckusick 22210”OR “T1DM”) AND (child OR adolescent OR school-aged OR youth OR teenager OR boy OR girl OR student OR pediatrics). Relevant articles were obtained without any language restriction. In addition, references of the relevant papers were screened to further identify eligible papers manually. All the retrieved publications were entered into reference-manager software (EndNote X7, Thomson Scientific, Stamford, CT, USA).
Study selection
Studies included if they met the following criteria: (i) observational study (ii) participants: children and adolescents; (iii) main outcome was T1DM; (iv) reporting the odds ratio (OR), hazard ratio (HR), relative risk (RR) or β-coefficient of air pollutions with T1DM. In this study, we focused on gaseous pollutants PM10, PM2.5, O3, SO2, NO2, and VOCs. Studies reporting duplicated results and studies with low quality were excluded.
Data extraction and quality assessment
Two reviewers screened the retrieved literatures by title and abstracts and then, full texts were assessed independently based on inclusion criteria. Information was extracted from the studies included the first author's name, year, country, number of participants and cases of diabetes and study design, follow-up period (for cohort studies), participants’ characteristics (age and gender), exposure measurement, covariates, and study results.
Two investigators (NM and MY) independently assessed the quality of eligible studies according to the checklist from STROBE in which the studies were divided into three groups of high, medium, and low quality.
Different results of the quality assessment were resolved by consultation and consensus.
Statistical analysis
As this meta-analysis aimed to investigate the relationship between air pollution and T1DM in children. The effect sizes of the RR, OR, and HR. RR and HR were considered as OR because incidence and prevalence of T1DM is low in the children.[18] The units of measurement of air pollution in the individual studies were μg/m3 or ppb. To coordinate the air pollution unit, we converted ppb to μg/m3. The OR of T1DM was calculated for a 10-μg/m3 increase in air pollutant concentration for PM2.5, PM10, NO2, and O3, and for a 1-μg/m3 increase in SO4 and SO2.[15,16]
A pooled effect size was estimated if there were two or more studies that adopted the same exposure. Heterogeneity across the enrolled studies was evaluated by Cochran's Q-statistic and I2 statistic was used to estimate the heterogeneity of the studies in the meta-analysis.[19] Standard random-effects meta-analysis methods perform poorly when applied to few numbers studies.[20] Therefore, for synthesizing specific ORs for each pollutant we used fixed-effect method. Funnel plot asymmetry tests were performed to assess the potential publication bias.[21] Two-tailed P < 0.05 was considered statistically significant. All analyses were performed using STATA version 14.0 ((StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.).
RESULTS
Search results and quality assessment
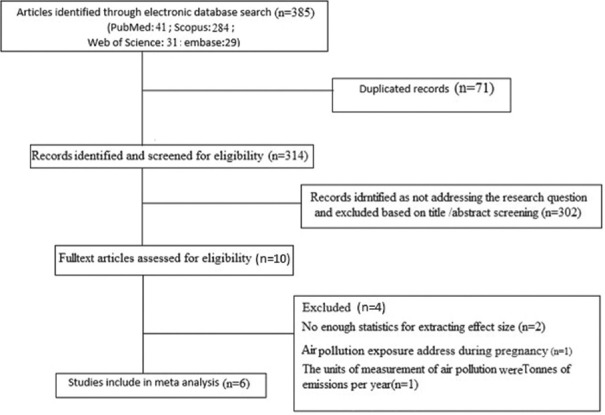
In the primary search based on the terms, 385 literatures were identified from electronic databases.
(PubMed, Embase, Web of Science, Cochrane Library and Scopus) and manual search. Subsequently, 12 studies were remained after the removal of duplicates and irrelevant titles and/or abstracts (13-18, 22-27). By reviewing the full text of articles, a total of six studies met the inclusion criteria in the current meta-analysis (15, 16,24-27), and others were removed for the following reasons: studies with insufficient data, reviews, non-human studies, and studies unrelated to this meta-analysis [Figure 1]. We excluded one case-control study because it evaluated prenatal exposures to air pollutants and the later development of T1DM in children.[13] We also excluded one study because it did not report the measure of association between air pollution and the risk of T1DM in children and adolescents.[22] A population-based study also was excluded because the air pollution was measured as Tonnes of emissions per year.[14] Additional two studies were excluded due to insufficient data to extract the effect size.[22,23]
Figure 1.
Flow diagram of study selection process
Studies were conducted from European and American countries. The characteristics of the included studies are summarized in Table 1 (14-16, 24-27). Overall, the studies were published between 2002 and 2020. We selected six studies for extracting data and quality assessment.
Table 1.
Summaries of studies included in the systematic review
| Author/date | Type of study (year) | Location | Number of participants | Gender, age (years) | Matching and adjustment factors | Exposure | Effect size (95% CI) |
|---|---|---|---|---|---|---|---|
| Di Ciaula[24] | Population-based study (2016) | Apulia, Italy | 631,275 (1501 T1DM case) | Male/female, 0-14 | Aggregate data over 13 years 2001-2013, adjusted for age group, gender, year of diagnosis and other pollutants (NOx, CO, ozone) | PM10 (μg/m3) | OR: Tertile 1: Ref Tertile 2: 1.018(0.986-1.051) Tertile 3: 1.037(1.0021.07) |
| Gonzalez et al.[25] | Population-based study (2013) | Santiago, Chile | Very large, (total number of T1DM cases not provided) | Male/female, 0-15 | Aggregate weekly data over 7 years (period 2000-2007), not adjusted for individual patient characteristics RR: per 1 - μg/m3 increase Coefficient of β |
PM2.5 | RR=1.003 (1.001-1.005) β=0.003116 SE=0.00104 |
| Di Ciaula[14] | Population-based study (2014) | 16, European countries | Very large, (total number of T1DM cases not provided) | Male/female, 0-15 | Aggregate nation-wide data over 20 years (1990-2010); not adjusted for individual patient characteristics | PM10 | OR: Tertile 1: Ref Tertile 2: 1.069(0.921-1.23) Tertile 3: 1.24(1.051.46) |
| NOx | OR: Tertile 1: Ref Tertile 2: 1.08(0.941.24) Tertile 3: 1.20(1.03-1.39) |
||||||
| VOCs | OR: Tertile 1: Ref Tertile 2: 1.033 (0.91-1.183) Tertile 3: 1.20 (1.031.38) |
||||||
| SOx | OR=l.16 (1.2-1.3) | ||||||
| Ammonia | OR=1.15 (1.0-1.3) | ||||||
| Hathout et o/.[15] | Casecontrol (2006) | Loma Linda, California |
402 children (102 T1DM case) | Male/female, age 0-17 | Adjusted variables were not reported OR: Per 10- μg/m3 increase NO2, O3, PM10, SO4 and for a 1 - μg/m3 increase in SO2 | Ozone (ppb) | OR=2.89 (1.86-4.58) |
| N02 (ppb) | OR=1.03 (0.71-1.50) | ||||||
| PM10 (ng/m3) | OR=1.08 (0.87-1.34) | ||||||
| S02 (ppb) | OR=1.42 (0.91-2.21) | ||||||
| S04 (ng/m3) | OR=1.65 (1.20-2.28) | ||||||
| Hathout et al.[16] | Case control study (2002) | California, USA | 100 children (61 case) | Male/female, under and over 5 year | Adjusted for age group OR: Per IQR increase Coefficient of β |
Ozone (ppb) | OR=4.22 (1.96-9.10) IQR=10.9 |
| PM10 (ng/m3) | OR=2.37 (1.11-5.03) IQR=22.65 | ||||||
| S02 (ppb) | OR=0.52 (1.31-0.88) IQR=1.235 | ||||||
| N02 (ppb) | OR=0.56 (0.30-1.03) IQR=11.175 | ||||||
| S04 (ng/m3) | OR=0.55 (0.35-0.85) IQR=1.025 | ||||||
| Elten et al.[26] | Retrospective population-based cohort study, (2020) | Ontario, Canada | 754,698 children (1094 case) | Male/female, 0-5 years | Adjusted for exposures to the selected pollutant during pregnancy, maternal age at delivery, infant sex, parity, maternal smoking during pregnancy, gestational age, birth weight, residential greenness exposure during pregnancy, maternal diabetes, maternal preeclampsia, season of conception and family income | PM25 (ng/m3) | HR=0.70 (0.52-1.05) IQR (in ng/m3)=3.80 |
| N02 (ppb) | HR=0.79 (0.38-1.64) IQR (ppb)=6.56 | ||||||
| Ozone (ppb) | HR=0.89 (0.69-1.14) IQR (ppb)=3.31 | ||||||
| Ox (ppb) | HR=0.58 (0.24-1.14) IQR (ppb)=5.00 | ||||||
| Sheehan et al.[27] | An environment wide association study | England | 13,948 cases of type 1 diabetes | Male/female, 0-9 years | Adjusted for age and sex OR: Per 1 - μg/m3 increase NO2 | N02 (ng/m3) | RR=1 (0.995-1.005) |
SE: Standard error, CI: Confidence interval, IQR: Interquartile range, HR: Hazard ratio, OR: Odds ratio, RR: Relative risk, T1DM: Type 1 diabetes mellitus, PM10: Particulate matter <10 μm, NOx: Nitrogen oxides, VOCs: Volatile organic compounds, NO2: Nitrogen dioxide, O3: Ozone, SO4: Sulfate, SO2: Sulfur dioxide, PM2.5: Fine particulate matter
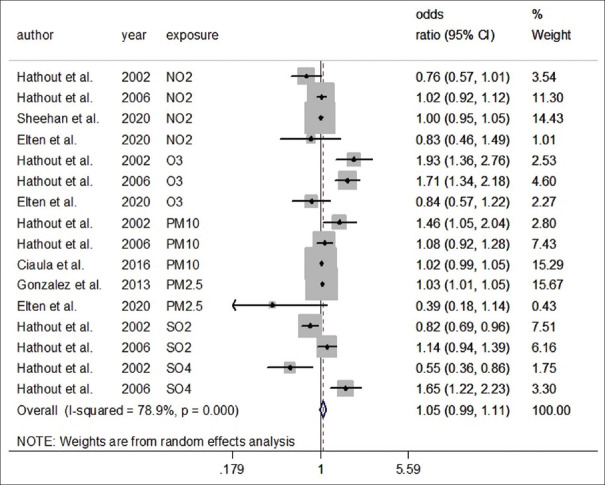
The association between Type 1 Diabetes and PM10 and O3 exposure was assessed in three studies. Two studies reported the effects of SO4, SO2, and PM2.5 exposure, and four studies reported the effects of NO2 exposure on T1DM incidence.
Figure 2 and Table 2 shows the pooled results of association (95% CI) for all 16 effect estimates from 6 studies. It showed that air pollution exposure was not associated with T1DM. Overall effect size was statistically significant (OR = 1.05, 95% confidence interval [CI]: 0.99, 1.11, I2 = 78.9%).
Figure 2.
Forest plot for the association of exposure to air pollution and type 1 diabetes mellitus (random effect model)
Table 2.
Pooled effect sizes and between study heterogeneity stratified by gaseous and particulate air pollutants
| Exposure | Number of studies | Random effect Effect size | Heterogeneity | Fixed effect Effect size Pooled OR(95% CI) |
||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Pooled OR(95% CI) | I2(%) | Q(Pheterogeneity) | T2 | |||
| Total | 16 | 1.05(0.991.11) | 78.9 | 71.19(<0.001) | 0.006 | 1.03(1.011.04) |
| PM10 | 3 | 1.09(0.951.25) | 59.4 | 4.93(0.08) | 0.009 | 1.02(0.991.06) |
| PM2.5 | 2 | 0.71(0.281.79) | 76.3 | 4.22(0.04) | 0.360 | 1.03(1.011.05) |
| O3 | 3 | 1.42(0.892.25) | 83.5 | 12.09(0.002) | 0.140 | 1.51(1.261.80) |
| SO4 | 2 | 0.97(0.332.83) | 93.8 | 16.17(<0.001) | 0.560 | 1.16(0.911.49) |
| SO2 | 2 | 0.96(0.691.34) | 85.0 | 6.67(0.0.01) | 0.050 | 0.94(0.831.06) |
| NO2 | 4 | 0.99(0.921.06) | 24.7 | 3.98(0.26) | 0.001 | 1.00(0.951.04) |
T2: Estimation of between-studies variance, OR: Odds ratio, CI: Confidence interval, PM10: Particulate matter <10 μm, O3: Ozone, SO4: Sulfate, SO2: Sulfur dioxide, NO2: Nitrogen dioxide, PM2.5: Fine particulate matter
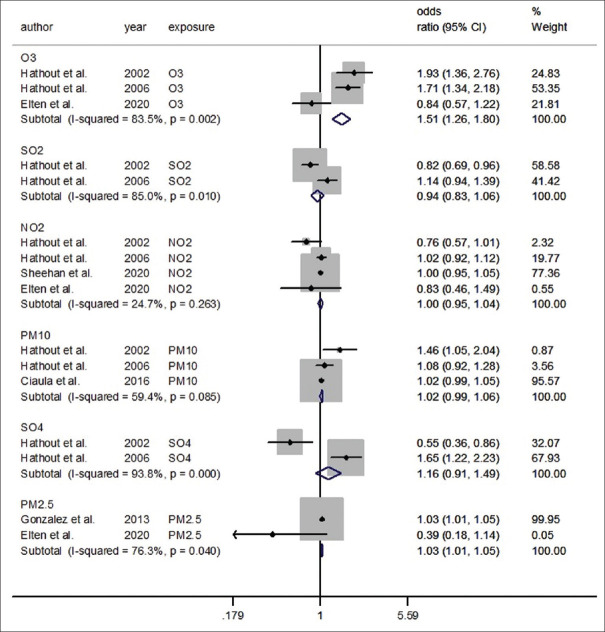
We investigated the association of each particular matter with T1DM risk in separate meta-analyses [Figure 3]. Because of the small number of studies for each pollutant, we used a fixed-effect meta-analysis (<5). The individual study results and the pooled effect sizes of OR (by pollutants) are shown in Figure 3.
Figure 3.
Forest plot of the association between air pollution and type 1 diabetes mellitus regarding each pollutant (fixed effect model)
With regard to exposure to PM10 pollutants of three effect sizes, the pooled results using the fixed-effect model did not show a significant association (OR = 1.02, 95% CI: 0.99, 1.0, I2 = 59.4%).
In an analysis restricted to the O3, two effect sizes found a statistically significant association between O3 and T1DM; the pooled estimated OR using the fixed-effect model was 1.62 (95% CI: 1.24, 2.12) and the test for heterogeneity was statistically significant (I2 = 91.5%; Pheterogeneity≤ 0.001).
In a fixed-effect model meta-analysis restricted to the PM2.5, the pooled estimated OR was 1.03 (95% CI: 1.01, 1.05) and the test for heterogeneity was statistically significant (I2 = 76.3%; Pheterogeneity≤ 0.04). In contrast, random-effect model meta-analysis showed no significant association (OR = 0.71, 95% CI: 0.28, 1.79).
There was no evidence for the association between exposure to air pollution (NO2, SO2, and SO4) and T1DM risk. The pooled effect using the fixed-effect model was 1.00 (95% CI: 0.95–1.04) for NO2, 0.94 (95% CI: 0.83–1.06) for SO2, 1.16 (95% CI: 0.91–1.49) for SO4. The degree of heterogeneity (I2) in the current meta-analysis for NO2, SO2, and SO4, were 24.7%, 85%, and 93.8%, respectively [Figure 3].
Publication bias
Funnel plot showed no publication bias in our meta-analysis [Figure 4]. Begg's adjusted rank correlation (z = −0.27, P = 0.79) and Egger's regression asymmetry test (t = 0.25, P = 0.81) did not inferred to publication bias, too.
Figure 4.
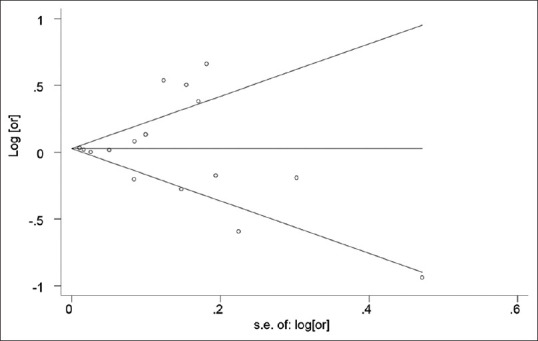
Funnel plot for the effects of air pollution and type 1 diabetes mellitus
DISCUSSION
Studies on the association between air pollution exposure and T1DM are rare and results are inconsistent. This study assessed the link between exposure to air pollutants (O3, PM10, PM2.5, SO4, SO2, and NO2) and the risk of T1DM. This systematic review and meta-analysis showed a positive association between exposure to ozone and PM2.5 and the risk of T1DM in children and adolescents. However, the overall association between T1DM and higher exposures to PM10, SO4, SO2, and NO2 was not statistically significant.
Our results revealed a link between O3 exposure and increased risk of T1DM. The interaction of NOx, hydrocarbons, and ultraviolet energy led to O3 production. Ozone can oxidation or peroxidation of biomolecules directly or indirectly (free radical reactions).[16,28,29] The biological mechanism through which O3 exposure can increase the prevalence of T1DM is complex and not fully understood. O3 exposure creates the free oxygen radicals that can cause damage to βcells or increase the presentation of diabetogenic antigens and resulted in predisposing to T1DM. Ozone can make an internal milieu that is typical of autoimmune diseases such as T1DM with altering T-cell and affecting CD4+ cells.[30] The presence of certain diabetogenic antigens in children that occurred because of the immature pulmonary or ambient air barriers may be elevated due to the synergism effect of ozone in case of simultaneous presence with sulfate in ambient.[31] A case–control study among 402 children aged 0–17 years showed that exposure to O3 and sulfate in ambient air may predispose to the development of T1DM. Hathout et al. found that ozone exposure has a strong positive association withT1DM.[16] However, some recent studies indicated that levels of O3 were not significantly associated with T1DM incidence in children.[24]
Findings of this review indicated that there was no evidence of the association between increased risk of T1DM and exposure to NO2, SO2, and PM10 levels. In 2020, a retrospective cohort study by Elten et al. in Ontario, Canada showed that exposure to air pollution (O3, NO2, PM2.5) during childhood was not associated with pediatric diabetes incidence.[26]
There are also some evidence regarding prenatal exposure to pollutants and the development of T1DM in offsprings. The positive association between prenatal exposures to NOx and O3 with increased risks of developing T1DM was reported by Malmqvist et al.[13]
Few studies have investigated the relation between air pollution and T1DM in children and adolescents. For instance, a population-based study from southern Italy on 0-to 14-year-old children showed a positive relationship between levels of PM10 and T1DM incidence.[24] Similarly, a study from 16 European Countries (1990-2010) among children aged 0–15 years found positive associations between nationwide emissions of PM10, NOx, and VOCs and T1DM incidence.[14] Moreover, González et al. in a population-based study (2013) on children aged less than 15 years in Chile from 2000 to 2007, found that (PM10, PPM2.5, is associated with T1DM incidence.[25] Results of a study among children and adolescents, in Poland by Michalska et al. revealed that mean annual PM10 concentration was positively associated with T1DM incidence in 2016, but not in the year 2015.[22] In 2020, Michalska et al. in Poland showed that high exposure to PM10, SO2, and CO related to the risk of developing T1DM in children, however, NO2 and NO were not found associated with the risk of developing T1DM.[23] Using an ecological regression model, Sheehan et al. showed that nitrogen dioxide was not associated with T1DM risk in children in England.[27] The exact mechanisms linking air pollution to T1DM are not fully understood. Some possible mechanisms have been introduced in this field. Both in vivo and in vitro studies demonstrated that exposure to pollutant particles even for short time could trigger inflammatory reactions.[32,33] Some evidence indicated that exposure to PM results in the formation of reactive oxygen species in pulmonary endothelial cells and circulating monocytes, which consequently result in DNA damage and activation of inflammatory reactions.[23,34,35]
A multicenter observational study conducted by Puett et al., 2019, on 2566 participants showed that PM2.5 exposures were associated with indicators of inflammation, interleukin-6 levels, after adjusting for demographic and lifestyle variables among youth.[36] A recent systematic reviews and meta-analyses showed that elevated fasting blood glucose was associated with exposure to both PM10 and PM2.5.[37] However, a cross-sectional study conducted by Tamayo et al. in Germany showed that exposure to PM10 and NO2 were not associated with glycemic control in children and young adults with T1DM.[17]
The main limitation of this study was the limited number of included studies. Furthermore, most published studies were from European and American countries; limited studies are available for other countries. Given the limited evidence, further prospective studies should be conducted to verify the effect of air pollutants on the risk of T1DM in children, particularly from developing countries. Another limitation was the considerable heterogeneity among the studies. The heterogeneity is likely to be related to different exposure assessment methods, study design, and other uncontrolled confounders in the studies included in the meta-analysis.
Considering the limited number of included studies, we conducted fixed-effect model meta-analysis.
The methods of measuring air pollution concentration were not uniform. In some studies, the concentrations of air pollutants were estimated from fixed-site monitoring stations to zip code centroids which are more accurate scale,[15,16] but some studies estimated air pollution exposure through citywide air pollution exposure levels.[24]
Given that, T1DM in the pediatric age group is affected by multiple factors. Some studies did not adjust for individual patient characteristics, which may affect the study results.
Together, they illustrate that the role of chemicals in T1DM may be complex and may depend on a variety of factors, such as exposure level, the timing of exposure, nutritional status, and chemical metabolism.[38]
CONCLUSIONS
This systematic review and meta-analysis indicated a positive association between PM2.5 and O3 exposure with the increased risk of T1DM in children and adolescents. While the evidence that these exposures may increase the risk of T1DM is still preliminary, it is critical to investigate this possibility further as a means of preventing T1DM.
Interventions and prevention programs should be considered to reduce exposure to air pollution and its adverse health effects in children and adolescents by health policymakers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–62. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redondo MJ, Concannon P. Genetics of type 1 diabetes comes of age. Diabetes Care. 2020;43:16–8. doi: 10.2337/dci19-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao CN, Xu Z, Wu GC, Mao YM, Liu LN, Qian-Wu, et al. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev. 2019;18:607–14. doi: 10.1016/j.autrev.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Giwa AM, Ahmed R, Omidian Z, Majety N, Karakus KE, Omer SM, et al. Current understandings of the pathogenesis of type 1 diabetes: Genetics to environment. World J Diabetes. 2020;11:13–25. doi: 10.4239/wjd.v11.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet. 2016;388:1939–51. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Xu L, Shan Z, Teng W, Han C. Association between air pollution and type 2 diabetes: An updated review of the literature. Ther Adv Endocrinol Metab. 2019;10:2042018819897046. doi: 10.1177/2042018819897046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiering E, Heinrich J. Epidemiology of air pollution and diabetes. Trends Endocrinol Metab. 2015;26:384–94. doi: 10.1016/j.tem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Hidaka T, Kumagai Y, Yamamoto M. Environmental pollutants and the immune response. Nat Immunol. 2020;21:1486–95. doi: 10.1038/s41590-020-0802-6. [DOI] [PubMed] [Google Scholar]
- 11.Liston A, Humblet-Baron S, Duffy D, Goris A. Human immune diversity: From evolution to modernity. Nat Immunol. 2021;22:1479–89. doi: 10.1038/s41590-021-01058-1. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Zheng S, Nie Y, Weng J, Cheng N, Hu X, et al. Association between short-term exposure to air pollution and dyslipidemias among type 2 diabetic patients in northwest china: A population-based study. Int J Environ Res Public Health. 2018;15:631. doi: 10.3390/ijerph15040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malmqvist E, Larsson HE, Jönsson I, Rignell-Hydbom A, Ivarsson SA, Tinnerberg H, et al. Maternal exposure to air pollution and type 1 diabetes – Accounting for genetic factors. Environ Res. 2015;140:268–74. doi: 10.1016/j.envres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Di Ciaula A. Association between air pollutant emissions and type 1 diabetes incidence in European countries. Adv Res. 2014;2:409–425. [Google Scholar]
- 15.Hathout EH, Beeson WL, Ischander M, Rao R, Mace JW. Air pollution and type 1 diabetes in children. Pediatr Diabetes. 2006;7:81–7. doi: 10.1111/j.1399-543X.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 16.Hathout EH, Beeson WL, Nahab F, Rabadi A, Thomas W, Mace JW. Role of exposure to air pollutants in the development of type 1 diabetes before and after 5 yr of age. Pediatr Diabetes. 2002;3:184–8. doi: 10.1034/j.1399-5448.2002.30403.x. [DOI] [PubMed] [Google Scholar]
- 17.Tamayo T, Rathmann W, Stahl-Pehe A, Landwehr S, Sugiri D, Krämer U, et al. No adverse effect of outdoor air pollution on HbA1c in children and young adults with type 1 diabetes. Int Jo Hyg Environ Health. 2016;219:349–55. doi: 10.1016/j.ijheh.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007;4:e78. doi: 10.1371/journal.pmed.0040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16:e1002742. doi: 10.1371/journal.pmed.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolakopoulou A, Mavridis D, Salanti G. How to interpret meta-analysis models: Fixed effect and random effects meta-analyses. Evid Based Ment Health. 2014;17:64. doi: 10.1136/eb-2014-101794. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, Chu H, Murad MH, Hong C, Qu Z, Cole SR, et al. Empirical comparison of publication bias tests in meta-analysis. J Gen Intern Med. 2018;33:1260–7. doi: 10.1007/s11606-018-4425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalska M, Bartoszewicz M, Wąż P, Kozaczuk S, Beń-Skowronek I, Zorena K. PM10 concentration and microbiological assessment of air in relation to the number of acute cases of type 1 diabetes mellitus in the Lubelskie Voivodeship. Preliminary report. Pediatr Endocrinol Diabetes Metab. 2017;23:70–6. doi: 10.18544/PEDM-23.02.0076. [DOI] [PubMed] [Google Scholar]
- 23.Michalska M, Zorena K, Wąż P, Bartoszewicz M, Brandt-Varma A, Ślęzak D, et al. Gaseous pollutants and particulate matter (PM) in ambient air and the number of new cases of type 1 diabetes in children and adolescents in the pomeranian voivodeship, Poland. Biomed Res Int 2020. 2020 doi: 10.1155/2020/1648264. 1648264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Ciaula A. Type I diabetes in paediatric age in Apulia (Italy): Incidence and associations with outdoor air pollutants. Diabetes Res Clin Pract. 2016;111:36–43. doi: 10.1016/j.diabres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 25.González RN, Torres-Avilés F, Carrasco PE, Salas PF, Pérez BF. Association of the incidence of type 1 diabetes mellitus with environmental factors in Chile during the period 2000-2007. Rev Med Chil. 2013;141:595–601. doi: 10.4067/S0034-98872013000500007. [DOI] [PubMed] [Google Scholar]
- 26.Elten M, Donelle J, Lima I, Burnett RT, Weichenthal S, Stieb DM, et al. Ambient air pollution and incidence of early-onset paediatric type 1 diabetes: A retrospective population-based cohort study. Environ Res. 2020;184:109291. doi: 10.1016/j.envres.2020.109291. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan A, Freni Sterrantino A, Fecht D, Elliott P, Hodgson S. Childhood type 1 diabetes: An environment-wide association study across England. Diabetologia. 2020;63:964–76. doi: 10.1007/s00125-020-05087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gangwar RS, Bevan GH, Palanivel R, Das L, Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020;34:101545. doi: 10.1016/j.redox.2020.101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burg AR, Tse HM. Redox-sensitive innate immune pathways during macrophage activation in type 1 diabetes. Antioxid Redox Signal. 2018;29:1373–98. doi: 10.1089/ars.2017.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirowsky JE, Dailey LA, Devlin RB. Differential expression of pro-inflammatory and oxidative stress mediators induced by nitrogen dioxide and ozone in primary human bronchial epithelial cells. Inhal Toxicol. 2016;28:374–82. doi: 10.1080/08958378.2016.1185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner MC, Krewski D, Diver WR, Pope CA, 3rd, Burnett RT, Jerrett M, et al. Ambient air pollution and cancer mortality in the cancer prevention study II. Environ Health Perspect. 2017;125:087013. doi: 10.1289/EHP1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu BY, Li WK, Li JS, Hong QH, Khodahemmati S, Gao JF, et al. Effects of DNA damage and oxidative stress in human bronchial epithelial cells exposed to PM2.5 from Beijing, China, in winter. Int J Environ Res Public Health. 2020;17:4874. doi: 10.3390/ijerph17134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ljubimova JY, Braubach O, Patil R, Chiechi A, Tang J, Galstyan A, et al. Coarse particulate matter (PM2.5-10) in Los Angeles Basin air induces expression of inflammation and cancer biomarkers in rat brains. Sci Rep. 2018;8:5708. doi: 10.1038/s41598-018-23885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bengalli R, Molteni E, Longhin E, Refsnes M, Camatini M, Gualtieri M. Release of IL-1β triggered by milan summer PM10: Molecular pathways involved in the cytokine release. BioMed Res Int 2013. 2013 doi: 10.1155/2013/158093. 158093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilz V, Wolf K, Breitner S, Rückerl R, Koenig W, Rathmann W, et al. C-reactive protein (CRP) and long-term air pollution with a focus on ultrafine particles. Int J Hyg Environ Health. 2018;221:510–8. doi: 10.1016/j.ijheh.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Puett RC, Yanosky JD, Mittleman MA, Montresor-Lopez J, Bell RA, Crume TL, et al. Inflammation and acute traffic-related air pollution exposures among a cohort of youth with type 1 diabetes. Environ Int. 2019;132:105064. doi: 10.1016/j.envint.2019.105064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma R, Zhang Y, Sun Z, Xu D, Li T. Effects of ambient particulate matter on fasting blood glucose: A systematic review and meta-analysis. Environ Pollut. 2020;258:113589. doi: 10.1016/j.envpol.2019.113589. [DOI] [PubMed] [Google Scholar]
- 38.Howard SG. Exposure to environmental chemicals and type 1 diabetes: An update. J Epidemiol Community Health. 2019;73:483–8. doi: 10.1136/jech-2018-210627. [DOI] [PubMed] [Google Scholar]