Abstract
Pharmacological reperfusion remains the primary strategy for ST-elevation myocardial infarction (STEMI) in low- and medium-income countries. Literature has reported inconsistent incidences and outcomes of failed thrombolysis (FT). This study aimed to identify the incidence, mortality outcomes and predictors of FT in STEMI pharmacological reperfusion. This single-centre retrospective cohort study analyzed data on consecutive STEMI patients who received thrombolytic therapy from 2016 to 2020 in a public tertiary hospital. Total population sampling was used in this study. Logistic regression analyses were used to assess independent predictors of the mortality outcomes and FT. We analyzed 941 patients with a mean age of 53.0 ± 12.2 years who were predominantly male (n = 846, 89.9%). The in-hospital mortality was 10.3% (n = 97). FT occurred in 86 (9.1%) patients and was one of the predictors of mortality (aOR 3.847, p < 0.001). Overall, tenecteplase use (aOR 1.749, p = 0.021), pre-existing hypertension (aOR 1.730, p = 0.024), history of stroke (aOR 4.176, p = 0.004), and heart rate ≥ 100 bpm at presentation (aOR 2.333, p < 0.001) were the general predictors of FT. The predictors of FT with streptokinase were Killip class ≥ II (aOR 3.197, p = 0.004) and heart rate ≥ 100 bpm at presentation (aOR 3.536, p = 0.001). History of stroke (aOR 6.144, p = 0.004) and heart rate ≥ 100 bpm at presentation (aOR 2.216, p = 0.015) were the predictors of FT in STEMI patients who received tenecteplase. Mortality following STEMI thrombolysis remained high in our population and was attributed to FT. Identified predictors of FT enable early risk stratification to evaluate the patients’ prognosis to manage them better.
Keywords: STEMI, Streptokinase, Tenecteplase, Failed thrombolysis, Emergency Department, Asian
Introduction
Globally, ischemic heart disease (IHD) remains the leading cause of mortality despite the evolution and advancement of coronary reperfusion strategies over 40 years [1, 2]. The well-established superiority of primary percutaneous coronary intervention (PCI) over thrombolysis in STEMI has made PCI-capable facilities highly accessible in developed countries [3–5]. However, pharmacological reperfusion using the thrombolytic agent remains the primary strategy in low- to medium-income countries due to the lack of PCI-capable facilities [6–8].
Failed thrombolysis (FT) is a concern in STEMI pharmacological reperfusion strategy as it is associated with early mortality, ventricular dysfunction, and arrhythmias [6, 9, 10]. Studies worldwide have reported inconsistent rates of FT in STEMI, ranging from 10 to 56.8%, for both fibrin-specific (alteplase, tenecteplase) and non-fibrin-specific (streptokinase) thrombolytic agents [11–26]. The predictors of FT (mainly on streptokinase) were identified in some studies: anterior location STEMI, higher Killip class, longer door-to-needle time, pre-existing diabetes and hypertension, higher total white cells count, longer symptoms-onset-to-thrombolysis time, hyperglycaemia, and higher thrombolysis in myocardial infarction (TIMI) score [18, 19, 21]. However, the small sample size in most studies is a major limitation. The studies conducted before the combination therapy of dual-antiplatelet (DAPT) plus antithrombotic along with STEMI thrombolysis also limit their applicability in contemporary practice. Additionally, predictors of FT on streptokinase cannot be generalized to STEMI patients who received fibrin-specific agents such as tenecteplase.
Streptokinase and tenecteplase are the only two thrombolytic agents commonly used for STEMI. Prior studies have shown that tenecteplase achieved a higher patency rate (90 min TIMI 2 or 3 flow) than streptokinase (85% versus 60–68%) %) [26]. However, the current evidence on FT between streptokinase and tenecteplase was inconsistent but favouring tenecteplase [12, 14, 25].
The evidence on mortality outcomes of FT and its predictive factors can allow early risk stratification in the Emergency Department (ED) to accurately evaluate the patient's prognosis and guide in triaging and managing the patients. Thus, this study aimed to assess the incidence, mortality outcomes and predictors of FT in STEMI reperfusion.
Methods
Design, setting, and population
This single-centre retrospective observational study was conducted at Hospital Kuala Lumpur, the largest tertiary care hospital with 2300 beds under the Ministry of Health Malaysia and one of the biggest hospitals in Asia. Hospital Kuala Lumpur is a non-PCI-capable hospital. The dose of streptokinase was 1.5 million units, given over 1 h. Tenecteplase was dosed according to the patient’s body weight as per the Metalyse® product insert and given as a bolus. All STEMI patients who received the thrombolytic agent were given DAPT (clopidogrel plus acetylsalicylic acid) and fondaparinux (majority) as part of acute STEMI management. Enoxaparin was used only in patients with creatinine clearance of below 30 ml/min. This study was approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-20-2630-57346). Informed consent was waived due to the retrospective nature of the study. The study conformed to the principles outlined in the Declaration of Helsinki.
Inclusion and exclusion criteria
This study included adult patients aged ≥ 18 years diagnosed with STEMI upon admission to the ED from 01 January 2016 to 31 December 2020 and who received intravenous streptokinase or tenecteplase. The exclusion criteria for this study were: incomplete data/missing records, received thrombolytic agent in the presence of absolute contraindication, incomplete administration of the thrombolytic agent, received lower than recommended dose, did not receive the standard concurrent antiplatelet and anticoagulant therapy for STEMI, discharged against medical advice and diagnosis revised to other than STEMI by the Cardiology physician.
Data collection
Total population sampling was used in this study. Medical records of consecutive STEMI patients who received thrombolytic therapy were traced from the Records Office and were screened based on eligibility criteria.
Pertinent data obtained from the patients’ medical records include (A) patients’ socio-demographic, (B) comorbidities, (C) STEMI diagnosis, (D) thrombolytic therapy, (E) efficacy outcomes (all-cause mortality, successful or failed thrombolysis), and (F) in-hospital safety outcomes, including stroke, bleeding, cardiac events and other complications.
Definitions
The diagnosis of STEMI followed both international and local guidelines, i.e., the 2017 European Society of Cardiology (ESC) STEMI guidelines and the Malaysian Clinical Practice Guidelines on STEMI management [4, 6]. In-hospital all-cause mortality is defined as death associated with any cause during the index admission date. Thrombolysis outcomes (successful or failed) were based on the Cardiology physician’s documentation in the patient’s medical record. Our local Malaysia STEMI guideline defined FT as continuing chest pain, persistent ST-segment elevation and haemodynamic instability [6]. However, no established guidelines stated the definite time frame to repeat electrocardiograms post-thrombolysis to define FT. Thus, this study used the repeated electrocardiogram criteria of < 50% ST-segment resolution at 90–180 min post-thrombolysis to define FT based on all literature reported on FT [9, 11–25, 27]. Lastly, bleeding events were categorized using the Thrombolysis in Myocardial Infarction (TIMI) bleeding criteria [28].
Data analysis and sample size
Using the rule of thumb of an event of variable (EPV) of 50, the minimum sample size for multiple logistic regression was calculated using the formula n = 100 + 50i, where i refers to the number of independent variables in the final model. The calculated minimum sample sizes for multiple logistic regression models were: 500 (in-hospital all-cause mortality), 350 (failed thrombolysis), and 200 (failed thrombolysis of individual thrombolytic agent). Data analysis was performed using the statistical package for social sciences (SPSS) for Windows version 26 (IBM Corp., Armonk, N.Y., USA). Descriptive analysis used to describe continuous data was expressed as mean and standard deviations (SD) or median and interquartile range (IQR) depending on normality distribution, whereas categorical data were reported as counts and percentages. Post hoc power analyses were obtained using the online Open Source Epidemiologic Statistics for Public Health (OpenEpi)Version 3.01.
Logistic regression (LR) models were used to analyze risk factors significantly associated with in-hospital mortality and failed thrombolysis. We included variables with a p-value < 0.25 from the simple LR analysis in the multiple LR model to assess the independent predictors for the outcomes of interest. LR results were presented as odds ratios with a confidence interval of 95%. Variance Inflation Factors (VIF) was used to detect multicollinearity in the multivariable analysis, with an acceptable VIF cut-off value of ≤ 10. All statistical tests with a p-value of < 0.05 denote statistical significance.
Results
Subjects’ demographics
A total of 1043 STEMI patients received thrombolytic therapy from 2016 to 2020. Streptokinase and tenecteplase were given to 528 and 515 patients, respectively, over the period of 5 years. After screening, 941 patients were included in the final analyses (Fig. 1). The post hoc power analysis with a population size of 1043, an expected frequency of 9.1%, and a margin of error of 5%, our sample size of 941 patients would give a confidence level above 99.99%.
Fig. 1.
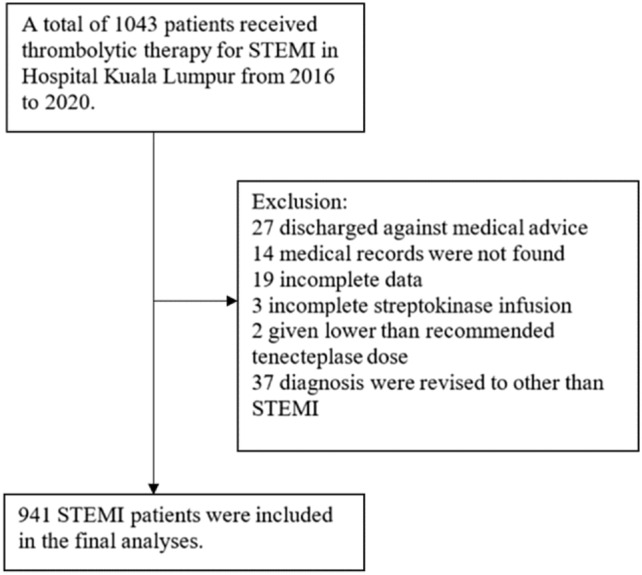
Study profile
The majority of the patients were male (n = 846, 89.9%) and current smoker (n = 642, 68.2%) with a mean age of 53.0 (± 12.2) years. Hypertension (n = 432, 45.9%) and diabetes (n = 349, 37.1%) were the most common pre-existing comorbid. Most STEMI involved the anterior location (n = 540, 57.4%) and almost half of the patients were presented with Killip II and above (n = 463, 49.2%) (Table 1). The majority of the baseline and clinical characteristics of STEMI patients who received streptokinase and tenecteplase were similar except for mean age, race, pre-existing hypertension, anterior involvement STEMI, Killip class, systolic pressure on presentation and door-to-needle time (DNT) (Table 1).
Table 1.
Baseline demographics and clinical characteristics of STEMI patients
| Parameters (n = 941) | Counts and percentage, n (%) | p-value, ()* | ||
|---|---|---|---|---|
| Total (n = 941) | SK (n = 481) | TNK (n = 460) | ||
| Age | ||||
| Mean (± SD), in years | 53.0 (12.2) | 51.48 (12.8) | 54.58 (11.3) | < 0.001a |
| Range | 20–92 | 22–91 | 20–92 | - |
| ≥ 75 years old | 39 (4.1) | 22 (4.6) | 17 (3.7) | 0.499 |
| Race | ||||
| Malay | 330 (35.1) | 129 (26.8) | 201 (43.7) | < 0.001 |
| Chinese | 86 (9.1) | 41 (8.5) | 45 (9.8) | 0.503 |
| Indian | 193 (20.5) | 78 (16.2) | 115 (25.0) | 0.001 |
| Other Malaysians | 13 (1.4) | 5 (1.0) | 8 (1.7) | 0.358 |
| Permanent residents | 93 (9.9) | 49 (10.2) | 44 (9.6) | 0.749 |
| Foreigners | 226 (24.0) | 179 (37.2) | 47 (10.2) | < 0.001 |
| Male Gender | 846 (89.9) | 433 (90.0) | 413 (89.8) | 0.904 |
| Current smoker | 642 (68.2) | 326 (67.8) | 316 (68.7) | 0.762 |
| Has family history of IHD | 188 (20.0) | 92 (19.1) | 96 (20.9) | 0.504 |
| Comorbidities | ||||
| Hypertension | 432 (45.9) | 200 (41.6) | 232 (50.4) | 0.006 |
| Diabetes | 349 (37.1) | 181 (37.6) | 168 (36.5) | 0.725 |
| IHD | 142 (15.1) | 65 (13.5) | 77 (16.7) | 0.167 |
| History of PCI | 16 (1.7) | 8 (1.7) | 8 (1.7) | 0.928 |
| History of CABG | 5 (0.5) | 2 (0.4) | 3 (0.7) | 0.680b |
| Heart failure | 22 (2.3) | 7 (1.5) | 15 (3.3) | 0.067 |
| Ischemic stroke | 21 (2.2) | 9 (1.9) | 12 (2.6) | 0.444 |
| Dyslipidaemia | 206 (21.9) | 100 (20.8) | 106 (23.0) | 0.403 |
| Anterior involvement STEMI | 540 (57.4) | 235 (48.9) | 305 (66.3) | < 0.001 |
| Killip class | ||||
| I | 478 (50.8) | 264 (54.9) | 214 (46.5) | 0.010 |
| II | 227 (24.1) | 111 (23.1) | 116 (25.2) | 0.443 |
| III | 59 (6.3) | 29 (6.0) | 30 (6.5) | 0.755 |
| IV | 177 (18.8) | 77 (16.0) | 100 (21.7) | 0.025 |
| Cardiac arrest before thrombolysis | 12 (1.3) | 5 (1.0) | 7 (1.5) | 0.510 |
| Door-to-needle time | ||||
| Median (IQR), minutes | 20.0 (15.0–35.0) | 20.0 (15.0–30.0) | 20.0 (14.0–40.0) | 0.359c |
| < 30 min | 701 (74.5) | 378 (78.6) | 323 (70.2) | 0.003 |
| Symptoms-onset-to-thrombolysis time | ||||
| Median (IQR), minutes | 180.0 (120.0–287.5) | 180.0 (125.0–282.5) | 180.0 (120.0–280.0) | 0.431c |
| > 4 h | 318 (33.8) | 165 (34.3) | 153 (33.3) | 0.735 |
| Systolic blood pressure on presentation | ||||
| Mean ± SD, mmHg | 129.9 (23.3) | 130.0 (20.8) | 129.7 (25.8) | 0.789a |
| < 100 mmHg | 80 (8.5) | 29 (6.0) | 50 (10.9) | 0.007 |
| ≥ 160 mmHg | 108 (11.5) | 47 (9.8) | 61 (13.3) | 0.093 |
| Heart rate on presentation | ||||
| Mean ± SD, beats per minute | 84.5 (21.2) | 83.9 (20.2) | 85.1 (25.8) | 0.396 |
| < 60 beats per minute | 102 (10.8) | 49 (10.2) | 53 (11.5) | 0.510 |
| ≥ 100 beats per minute | 211 (22.4) | 99 (20.6) | 112 (24.3) | 0.166 |
bpm beats per minute, CABG coronary artery bypass graph, IHD ischemic heart disease, IQR interquartile range, SD standard deviation, SK streptokinase, TNK tenecteplase, PCI percutaneous coronary intervention, chi-square test
*SK vs TNK
aIndependent t-test
bFisher exact
cMann–Whitney U test
Outcomes following thrombolytic therapy
Ninety seven (10.3%) patients died during hospitalization. Failed thrombolysis occurred in 86 (9.1%) patients. There were 7 (0.8%) and 17 (1.8%) major and minor bleeding cases, respectively. The median number of overnight hospital stays was 4 nights.
In comparison to streptokinase, tenecteplase arm has significantly higher numbers of failed thrombolysis (p = 0.003), in-hospital all-cause mortality (p = 0.040) and stent placement (p < 0.001), but a significantly lower incidence of minor bleeding (p = 0.009), hypotension (p < 0.001), bradycardia (p = 0.005), and allergic reaction (p = 0.003) (Table 2).
Table 2.
Outcomes following thrombolysis
| Outcomes | Counts and Percentage, n (%) | p-value, ()* | ||
|---|---|---|---|---|
| Total (N = 941) | SK (n = 481) | TNK (n = 460) | ||
| Failed thrombolysis | 86 (9.1) | 31 (6.4) | 55 (12.0) | 0.003 |
| All-cause mortalityA | ||||
| In-hospital | 97 (10.3) | 40 (8.3) | 57 (12.4) | 0.040 |
| In-hospital strokes | ||||
| Ischemic | 5 (0.5) | 1 (0.2) | 4 (0.9) | 0.208a |
| Haemorrhagic | 4 (0.4) | 3 (0.6) | 1 (0.2) | 0.625a |
| TIMI Bleeding | ||||
| Major | 7 (0.8) | 4 (0.8) | 3 (0.7) | 1.000a |
| Minor | 17 (1.8) | 14 (2.9) | 3 (0.7) | 0.009 |
| Minimal | 132 (14.0) | 71 (14.8) | 61 (13.3) | 0.508 |
| Number of overnights stays, days | ||||
| Median (IQR) | 4 (4–5) | 4 (4–5) | 4 (4–5) | 0.573b |
| Cardiac events | ||||
| Reinfarction | 17 (1.8) | 6 (1.2) | 11 (2.4) | 0.188 |
| Hypotension | 233 (24.8) | 171 (35.6) | 62 (13.5) | < 0.001 |
| Bradycardia | 82 (8.7) | 54 (11.2) | 28 (6.1) | 0.005 |
| Second- and third-degree AV block | 10 (1.1) | 3 (0.6) | 7 (1.5) | 0.215a |
| Congestive heart failure | 18 (1.9) | 11 (2.3) | 7 (1.5) | 0.392 |
| Cardiogenic shock | 22 (2.3) | 13 (2.7) | 9 (2.0) | 0.449 |
| VF/VT | 148 (16.0) | 66 (14.3) | 82 (17.8) | 0.139 |
| SVT | 4 (0.4) | 3 (0.6) | 1 (0.2) | 0.625a |
| AF | 33 (3.5) | 13 (2.7) | 20 (4.3) | 0.170 |
| Stent placement | 84 (8.9) | 21 (4.4) | 63 (13.7) | < 0.001 |
| Other events | ||||
| Anaphylaxis | 5 (0.5) | 5 (1.0) | 0 (0.0) | 0.062a |
| Allergic reaction | 15 (1.6) | 13 (2.7) | 2 (0.4) | 0.005 |
AF atrial fibrillation, AV atrioventricular, IQR interquartile range, SK streptokinase, SVT supraventricular tachycardia, TIMI thrombolysis in myocardial infarction, TNK tenecteplase, VF ventricular fibrillation, VT ventricular tachycardia, chi-square test
*SK vs TNK; a Fisher exact; b Mann–Whitney U test
A3 cases of in-hospital mortality with the length of stay of more than 30 days
Mortality and failed thrombolysis
Failed thrombolysis is one of the predictors of in-hospital all-cause mortality (aOR 3.847, 95% CI 2.058–7.191, p < 0.001) in STEMI patients who received pharmacological reperfusion therapy (Table 3).
Table 3.
Simple and multiple logistic regression (LR) analyses of the possible predictor of in-hospital mortality on outcomes following thrombolysis
| Predictor variables | In-hospital mortality (N = 941) | |||
|---|---|---|---|---|
| Simple LR | Multiple LR | |||
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Failed thrombolysis | 3.312 (1.919–5.714) | < 0.001 | 3.847 (2.058–7.191) | < 0.001 |
| Hypotension | 1.938 (1.246–3.015) | 0.003 | 2.059 (1.241–3.417) | 0.005 |
| Bradycardia | 1.080 (0.522–2.234) | 0.835 | ||
| Congestive heart failure | 5.890 (2.228–15.572) | < 0.001 | 5.159 (1.509–17.638) | 0.009 |
| Cardiogenic shock | 0.867 (0.200–3.769) | 0.849 | ||
| VF/ VT | 6.915 (4.413–10.836) | < 0.001 | 7.316 (4.475–11.961) | < 0.001 |
| Atrial fibrillation/flutter | 4.779 (2.242–10.189) | < 0.001 | 2.687 (1.074–6.723) | 0.035 |
| TIMI bleeding events | ||||
| No bleeding (ref.) | – | < 0.001 | – | < 0.001 |
| Minimal | 0.788 (0.396–1.567) | 0.496 | 0.621 (0.295–1.307) | 0.210 |
| Minor | 8.541 (3.199–22.801) | < 0.001 | 7.110 (2.116–23.887) | 0.002 |
| Major | 24.020 (4.580–125.978) | < 0.001 | 51.047 (9.052–287.861) | < 0.001 |
| Allergic reaction | 2.213 (0.613–7.982) | 0.225 | ||
aOR adjusted odds ratio, LR logistic regression, OR odds ratio, ROC receiver operating characteristic, TIMI thrombolysis in myocardial infarction, VF ventricular fibrillation, VT ventricular tachycardia
Backward LR multiple logistic method applied in all analyses; no interactions and multicollinearity detected; Hosmer–Lemeshow Test χ2 = 5.003, df = 5, p = 0.416; Overall classification table = 90.5%; Area under ROC curve = 80.9%
Predictors of failed thrombolysis
The final multiple LR model revealed four significant pre-thrombolysis predictors of failed thrombolysis: tenecteplase use (streptokinase as reference) (aOR 1.749, 95% CI 1.087–2.184, p = 0.021), pre-existing hypertension (aOR 1.730, 95% CI 1.074–2.786, p = 0.024), history of stroke (aOR 4.176, 95% CI 1.572–11.091, p = 0.004), and HR ≥ 100 bpm at presentation (aOR 2.333, 95% CI 1.452–3.750, p < 0.001) (Table 4).
Table 4.
Simple and multiple logistic regression (LR) analyses of the possible general predictor of failed thrombolysis among STEMI patients
| Predictor variables | Failed thrombolysis (N = 941) | |||
|---|---|---|---|---|
| Simple LR | Multiple LR | |||
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Tenecteplase use | 1.971 (1.244–3.123) | 0.004 | 1.749 (1.087–2.814) | 0.021 |
| Age ≥ 75 | 1.143 (0.396–3.296) | 0.805 | ||
| Male gender | 0.663 (0.346–1.271) | 0.216 | ||
| Race | ||||
| Malay (ref) | – | 0.534 | ||
| Chinese | 1.106 (0.485–2.522) | 0.810 | ||
| Indian | 1.532 (0.860–2.727) | 0.147 | ||
| Others-Malaysian | 0.000 (0.000) | 0.999 | ||
| Others-PR | 0.613 (0.230–1.634) | 0.328 | ||
| Foreigners | 1.105 (0.611–1.999) | 0.742 | ||
| Current smoker | 0.857 (0.538–1.366) | 0.516 | ||
| Family history of IHD | 1.527 (0.920–2.534) | 0.102 | ||
| IHD | 1.439 (0.819–2.528) | 0.206 | ||
| Heart failure | 0.994 (0.228–4.327) | 0.994 | ||
| Hypertension | 2.129 (1.348–3.365) | 0.001 | 1.730 (1.074–2.786) | 0.024 |
| Diabetes | 1.794 (1.149–2.800) | 0.010 | ||
| Stroke | 5.323 (2.087–13.574) | < 0.001 | 4.176 (1.572–11.091) | 0.004 |
| Anterior MI | 1.803 (1.116–2.912) | 0.016 | 1.524 (0.926–2.510) | 0.097 |
| Killip | ||||
| II–IV | 1.840 (1.165–2.907) | 0.009 | ||
| Symptoms-onset-to- thrombolysis > 4 h | 1.387 (0.882–2.182) | 0.157 | ||
| SBP < 100 mmHg | 1.169 (0.859–3.345) | 0.128 | ||
| SBP ≥ 160 mmHg | 1.146 (0.588–2.233) | 0.689 | ||
| HR ≥ 100 bpm | 2.648 (1.670–4.198) | < 0.001 | 2.333 (1.452–3.750) | < 0.001 |
aOR adjusted odds ratio, HR heart rate, IHD ischemic heart disease, LR logistic regression, MI myocardial infarction, OR odds ratio, ROC receiver operating characteristic, SBP systolic blood pressure
Backward LR multiple logistic method applied in all analyses; no interactions and multicollinearity detected; Hosmer–Lemeshow Test χ2 = 6.625, df = 8, p = 0.578; Overall classification table = 91.9%; Area under ROC curve = 71.9%
Further LR analyses were conducted on individual thrombolytic agents to determine their predictors of failed thrombolysis. The predictors of failed thrombolysis with streptokinase are Killip II–IV (aOR 2.676, 95% CI 1.185–6.043, p = 0.018) and HR ≥ 100 bpm at presentation (aOR 2.936, 95% CI 1.370–6.292, p = 0.006). Conversely, history of stroke (aOR 6.144, 95% CI 1.811–20.843, p = 0.004) and HR ≥ 100 bpm at presentation (aOR 2.216, 95% CI 1.158–3.903, p = 0.015) were the predictors of failed thrombolysis in STEMI patients who received tenecteplase (Table 5).
Table 5.
Simple and multiple logistic regression (LR) analyses of the possible predictor of failed thrombolysis among STEMI patients who received streptokinase (N = 481) and tenecteplase (N = 460)
| Predictor variables | Failed thrombolysis | |||||||
|---|---|---|---|---|---|---|---|---|
| aStreptokinase (N = 481) | bTenecteplase (N = 460) | |||||||
| Simple LR | Multiple LR | Simple LR | Multiple LR | |||||
| OR (95% CI) | p-value | aOR (95% CI) | p-value | OR (95% CI) | p-value | aOR | p-value | |
| Age ≥ 75 | 1.483 (0.330–6.655) | 0.607 | 0.981 (0.218–4.411) | 0.980 | ||||
| Male Gender | 1.037 (0.303–3.547) | 0.954 | 0.529 (0.241–1.164) | 0.114 | ||||
| Current smoker | 0.638 (0.304–1.339) | 0.235 | 1.021 (0.555–1.878) | 0.946 | ||||
| Family history of IHD | 1.156 (0.655–3.506) | 0.331 | 1.500 (0.790–2.849) | 0.215 | ||||
| IHD | 0.945 (0.319–2.794) | 0.918 | 1.649 (0.838–3.245) | 0.147 | ||||
| Heart failure | 2.467 (0.288–21.156) | 0.410 | 0.517 (0.067–4.012) | 0.528 | ||||
| Hypertension | 2.353 (1.115–4.966) | 0.025 | 1.848 (1.032–3.310) | 0.039 | ||||
| Diabetes | 1.842 (0.888–3.823) | 0.101 | 1.805 (1.024–3.182) | 0.041 | ||||
| Stroke | 4.365 (0.867–21.961) | 0.074 | 5.686 (1.739–18.591) | 0.004 | 6.144 (1.811–20.843) | 0.004 | ||
| Anterior MI | 1.292 (0.622–2.685) | 0.492 | 1.956 (0.999–3.828) | 0.050 | ||||
| Killip | ||||||||
| II–IV | 3.197 (1.440–7.097) | 0.004 | 2.676 (1.185–6.043) | 0.018 | 1.242 (0.702–2.196) | 0.457 | ||
| Symptoms-onset-to-thrombolysis > 4 h | 0.906 (0.416–1.973) | 0.804 | 1.803 (1.019–3.191) | 0.043 | 1.654 (0.921–2.969) | 0.092 | ||
| SBP < 100 mmHg | 2.519 (0.818–7.757) | 0.108 | 1.224 (0.521–2.2.875) | 0.642 | ||||
| HR ≥ 100 bpm | 3.536 (1.678–7.454) | 0.001 | 2.936 (1.370–6.292) | 0.006 | 2.131 (1.179–3.852) | 0.012 | 2.216 (1.158–3.903) | 0.015 |
aOR adjusted odds ratio, HR heart rate, IHD ischemic heart disease, LR logistic regression, MI myocardial infarction, OR odds ratio, ROC receiver operating characteristic, SBP systolic blood pressure
aBackward LR multiple logistic method applied in all analyses; no interactions and multicollinearity detected; Hosmer–Lemeshow Test χ2 = 1.071, df = 2, p = 0.585; Overall classification table = 93.6%; Area under ROC curve = 70.6%
bBackward LR multiple logistic method applied in all analyses; no interactions and multicollinearity detected; Hosmer–Lemeshow Test χ2 = 0.102, df = 2, p = 0.950; Overall classification table = 88.5%; Area under ROC curve = 63.4%
Discussion
Generally, in the absence of contraindication, thrombolytic therapy is recommended within 12 h of ischemic symptom onset if primary PCI cannot be performed within 120 min of STEMI diagnosis [4]. This is the first Asian study with a relatively large sample size reporting the outcomes of FT and identifying its predictors in STEMI pharmacological reperfusion. Our findings further strengthen the evidence on STEMI thrombolysis in the Asian population, which mainly relies on pharmacological reperfusion [6, 7]. In addition, this study included a substantial amount (almost 30%) of permanent residents and foreign workers from other Asian countries, mainly Indonesia, Bangladesh, India, and Myanmar. Thus, the study’s findings are applicable to the other Asian population.
In this study, 10.3% of in-hospital all-cause mortality in STEMI patients receiving thrombolytic therapy was higher than 9.2% as reported by the Malaysian National Cardiovascular Disease (NCVD) registry [29]. Missing data on patients who died in ED probably contributed to the lower mortality in the registry. Our mortality rate was worrisome as it was comparable to 6.2–10.7% as reported in the major clinical trials on thrombolytic agents before the era of DAPT plus anticoagulant, probably due to more ill patients with higher mortality risk in this study [30–33]. The higher STEMI in-hospital mortality in our non-PCI-capable centre, compared to less than 6% reported by the Malaysia NCVD and international studies, strongly suggests the need to establish more PCI-capable facilities in the country [29, 34].
The incidence of FT in our setting was far lower than up to 56.8% as reported in the literature [11–25, 27]. Nevertheless, STEMI patients given thrombolytic therapy should be considered for a pharmacoinvasive approach whenever possible [6]. The shorter duration to repeat electrocardiogram for ST-segment resolution at 90 min post-thrombolysis may contribute to the higher rate of FT in some studies. It is reasonable to monitor ST-segment resolution for up to 180 min, especially in those given streptokinases, due to its pharmacokinetics and longer administration duration.
Our finding of FT as one of the predictors for in-hospital all-cause mortality agrees with a small-scale Indian study that reported non-ST-segment resolution post-thrombolysis was associated with more adverse events and higher mortality [10]. The infarct-related artery in FT usually has persistent occlusion, where TIMI 3 flow (normal flow that fills the distal coronary bed) is not achieved. In one study, only 83.2% and 6.4% of STEMI patients with successful thrombolysis and FT, respectively, achieved the TIMI 3 flow [19]. In a meta-analysis, only TIMI 3 flow post thrombolysis is associated with a mortality benefit, with short-term mortality (in-hospital or 30-day mortality) of 3.7%, 7.0% and 8.8% among STEMI patients who achieved TIMI flow grade 3, 2, and 0/1, respectively [27].
We identified the predictors of FT of the overall and individual thrombolytic agents. In our study, tenecteplase, surprisingly, is one of the predictors of FT, with an almost two times higher chance of FT than streptokinase. Similarly, the Malaysian National Heart Centre, a PCI-capable centre, also reported a high rate of FT (19%) with tenecteplase in STEMI reperfusion [35]. A network meta-analysis of 40 randomized trials comparing thrombolytic agents in STEMI patients has shown the superiority of tenecteplase over streptokinase [36]. However, the results must be interpreted cautiously as the thrombolytic agents were not compared directly. As mentioned in the study’s limitation, some relevant data was not obtained for adjustment in the final analysis [36]. Also, the differences in standard treatment and changes in practice over time could potentially affect the outcomes of the studies included in the analysis. Five small-scale (≤ 100 patients) studies comparing tenecteplase and streptokinase had reported different findings in FT. Three studies done in India and Malaysia showed that the achievement of > 50% ST-resolution at 90 min was similar between the tenecteplase and streptokinase [14, 25, 37]. On the contrary, another two Indian studies showed that the tenecteplase had a significantly higher achievement of ST-segment resolution than the streptokinase [12, 13]. Our finding indicates that streptokinase is still an effective thrombolytic agent for STEMI reperfusion in our Asian population. Besides, patients who received tenecteplase have a significantly higher incidence of stent placement at other PCI centres due to the higher rate of FT in this group, which requires further investigation.
Hypertension increases the risk of IHD and affects its prognosis. Hypertension is the most common comorbid in STEMI patients and is a risk factor for higher mortality following STEMI [38]. Our finding of hypertension as a predictor of FT was consistent with other studies [10, 18, 23]. A study has postulated that poorly-controlled hypertension, accelerated atherosclerosis, and endothelial dysfunction in hypertensive patients might contribute to thrombolytic failure in STEMI [18].
The outcomes of STEMI thrombolysis in the population with an ischemic stroke history (above three months) are scarce, as most major trials of thrombolytic agents did not include subjects with a previous history of stroke [32, 33, 39, 40]. Surprisingly, this study found that a history of stroke was the predictor with the highest chance of FT in the overall and tenecteplase arm but not in the streptokinase arm. This finding was not observed or reported in other studies on STEMI-related FT. The population with prior stroke commonly has a higher incidence of metabolic syndrome, which is associated with defective endogenous fibrinolysis and plasminogen activator inhibitor-1 (PAI-1) enhancement [41]. Thrombolysis failure with streptokinase has been reported to be associated with a higher PAI-1 level. However, tenecteplase with a 15-fold higher fibrin specificity and 80-fold reduced binding affinity to the PAI-1 (in comparison with alteplase) should be less affected by the PAI-1 level [42, 43]. Thus, the high STEMI thrombolysis failure with tenecteplase in patients with prior stroke warrants further investigation in our setting.
A heart rate above 100 beats per minute during STEMI presentation is a risk factor of mortality [38]. In this study, STEMI patients with a heart rate above 100 bpm at presentation is one of the predictors of FT in both streptokinase and tenecteplase arms. A similar finding, however, was not reported in other studies.
In STEMI thrombolysis, Killip classification of II–IV during STEMI presentation is another risk factor of all-cause mortality at 30 days [38]. Similarly, significantly higher mortality at 30 days was reported among cardiogenic shock (Killip 4) STEMI patients who had undergone primary PCI [34]. In our study, the proportion of STEMI patients with Killip II-IV was higher than 12.2–29.3% reported in clinical trials related to thrombolytic agents, indicating that STEMI patients in this study were more ill during the presentation in ED [31, 33]. However, it is encouraging to discover a lower incidence of FT despite the higher proportion of more ill patients. We identified that STEMI patients with Killip II-IV at presentation to ED is a predictor of FT in the streptokinase arm but not in the tenecteplase arm, suggesting that tenecteplase is a better option in this population. Conversely, another Indian study has reported Killip II-IV as one of the predictors of FT in STEMI patients receiving either streptokinase or tenecteplase [19].
Total ischemic time can affect STEMI patients’ prognosis as the duration of symptoms onset to reperfusion of more than 4 h is a mortality risk factor [38]. A longer total ischemia time due to reperfusion delay can be patient-related or system-related [44]. Among STEMI patients undergoing primary PCI, an Italian study has shown that patient-related delay has a higher one-year all-cause mortality than system-related delay [44]. However, in this study, the symptoms-onset-to-thrombolysis time was not associated with FT and in-hospital mortality. We postulate that the language barrier due to racially heterogeneous subjects with significant numbers of foreigners (foreign workers) and illiterate elderly patients, who could not communicate in English or Malay, partially contributed to this finding due to the possibility of the inaccurate description of their symptoms onset and misunderstanding of the physician’s questions.
In Malaysia, pharmacological thrombolysis remains the primary reperfusion strategy for STEMI despite the availability of STEMI networks due to the lack of PCI-capable facilities and high patient loads. Similarly, the vast geographical terrain in certain states of Malaysia also presents a challenge for the timely administration of reperfusion strategy in STEMI patients. Besides, Malaysia is a country that provides subsidized healthcare to its citizens. The vast cost difference, with tenecteplase three times more expensive than streptokinase, is a concern which may affect the physician's decision in selecting the thrombolytic agent. Nevertheless, our study findings provide evidence tailored to our population in selecting thrombolytic agents for better outcomes in STEMI patients.
Next, Coronavirus Disease 2019 (COVID-19)—an ongoing public health emergency since 2019, has caused a crisis in the worldwide healthcare system. Several studies have reported the impact of COVID-19 on STEMI, such as the reduction in cardiovascular/STEMI cases, delayed symptoms onset to first medical contact and increased mortality during the early COVID-19 outbreak [45–48]. We did an extended analysis (Appendix 1) to explore the potential impact of COVID-19 on STEMI outcomes; although the COVID-19 cases in Malaysia (less than 120,000 cases) were much lower than in Western and European countries such as Italy (more than 2 million cases), at the end of the year 2020. Our ED utilized a binary system and was categorized into ‘clean’ and ‘dirty’ zones to cover COVID-19 and non-COVID-19 patients, respectively. Each of these ‘clean’ and ‘dirty’ zones has red, yellow, and green zones that cater for patients based on illness severity [49]. Besides, Malaysia's local STEMI guideline has recommended “thrombolysis first” in STEMI during the COVID-19 outbreak. However, we were not affected by the change in guidelines as our centre is not a PCI-capable centre.
Only one STEMI patient was positive for COVID-19 during the study period. Compared to a similar duration before the pandemic (10 months; May 2019 to February 2020), there was a 12.5% reduction in thrombolysis performed during the pandemic (10 months; March 2020–December 2020). The reduction in STEMI thrombolysis cases was similar to an Italian study and likely contributed by the national movement control order [45]. Contrary to another study, the time of symptoms-onset-to-thrombolysis before and during the COVID-19 outbreak were similar in this study [46]. However, the in-hospital mortality among our STEMI patients was significantly higher during the virus outbreak, despite the similar symptoms-onset-to-thrombolysis time, preserved door-to-needle time and similar rates of failed thrombolysis. Conversely, PCI-capable facilities have reported insignificant changes in all-cause mortality for STEMI patients during the COVID-19 outbreak, suggesting that PCI remains a better option for STEMI despite the pandemic [45, 46].
We acknowledged several limitations in this study. The inherited retrospective observational nature of this study may affect the data quality. However, missing data were minimized as the reporting of STEMI cases is the key performance indicator for the ED, and the Cardiology Unit is the source data provider for the Malaysian NCVD Registry. Nevertheless, despite the retrospective design, this study provided “real world” data involving consecutive STEMI patients over 5 years.
The difference in the concurrent anticoagulant used may partially affect the results of this study. Most studies on STEMI FT did not report the anticoagulant used. However, two Indian studies using unfractionated heparin (UFH) have reported a higher FT of 32.7% and 38.7%, respectively [19, 20]. In our setting, fondaparinux was the preferred anticoagulant for STEMI patients receiving thrombolytic therapy; enoxaparin is used only in patients with renal impairment. The subgroup analysis of the OASIS-6 trial reported that fondaparinux significantly improved outcomes in STEMI patients treated with thrombolytic agents (predominantly streptokinase) compared with UFH [50]. Hence, the results of this study may not apply to facilities that use UFH or enoxaparin in the majority of STEMI cases, as the use of fondaparinux may partially contribute to the lower FT in this study. Nevertheless, the outcomes of fondaparinux on different thrombolytic agents used in STEMI patients are scarce and warrant further exploration.
Next, Hospital Kuala Lumpur is the largest tertiary hospital and is one of the largest users of tenecteplase in Malaysia. The involvement of consecutive STEMI patients over five years ensures the robustness of our findings, although this was a single-centre study. Lastly, the incidence result obtained in this study may not represent all hospitals in Malaysia.
Conclusion
Mortality following STEMI thrombolysis remained high in our Asian population and was contributed by FT. Identified predictors of FT enable early risk stratification to accurately evaluate the STEMI patients’ prognosis and serve as a guide in triaging and managing the patients.
Acknowledgements
We would like to thank the Director-General of Health, Ministry of Health Malaysia, for his permission to publish this article.
Appendix 1. Comparison of STEMI presentations and outcomes before and during the early COVID-19 outbreak in Hospital Kuala Lumpur
| Variable | Pre-COVID-19 outbreak (May 2019–February 2020) | During the COVID-19 outbreak (March 2020–December 2020) | P-value, ()* |
|---|---|---|---|
| Numbers of STEMI thrombolysis cases | 168 | 147 | – |
| COVID-19 cases | 0 | 1 | – |
| Time symptoms onset to fibrinolysis | |||
| Median (IQR), mins | 196.00 (129.5–285.0) | 195.0 (130.0–300.0) | 0.892a |
| > 4 h | 60 (37.0) | 50 (35.0) | 0.707 |
| Door-to-needle time | |||
| Median (IQR), mins | 20.0 (15.0–30.0) | 25.0 (15.0–40.0) | 0.151a |
| ≤ 30 min | 128 (76.2) | 102 (69.4) | 0.175 |
| Failed thrombolysis | 18 (10.7) | 15 (10.2) | 0.883 |
| In-hospital all-cause mortality | 16 (9.5) | 25 (17.0) | 0.049 |
COVID-19 Coronavirus disease 2019, IQR interquartile range, STEMI ST-segment elevated myocardial infarction
aMann–Whitney U test; chi-square test
Funding
This study was a self-funded work.
Data availability
Raw data are not attached. The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-20–2630-57346).
Footnotes
Hock Peng Koh: takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2018) The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 12 Nov 2021
- 2.Van de Werf F. The history of coronary reperfusion. Eur Heart J. 2014;35:2510–2515. doi: 10.1093/eurheartj/ehu268. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi S, Garratt KN, Li S, et al. Ten-year trends in patient characteristics, treatments, and outcomes in myocardial infarction from National Cardiovascular Data Registry Chest Pain-MI Registry. Circ Cardiovasc Qual Outcomes. 2022;15:e008112. doi: 10.1161/CIRCOUTCOMES.121.008112. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, James S, Agewall S, et al. (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.Zeymer U, Ludman P, Danchin N, et al. Reperfusion therapies and in-hospital outcomes for ST-elevation myocardial infarction in Europe: the ACVC-EAPCI EORP STEMI Registry of the European Society of Cardiology. Eur Heart J. 2021;42:4536–4549. doi: 10.1093/eurheartj/ehab342. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health Malaysia (2019) Clinical Practice Guidelines: Management of Acute ST-Segment Elevation Myocardial Infarction (STEMI) 2019. Putrajaya
- 7.Tern PJW, Ho AKH, Sultana R, et al. Comparative overview of ST-elevation myocardial infarction epidemiology, demographics, management, and outcomes in five Asia-Pacific countries: a meta-analysis. Eur Heart J Qual Care Clin outcomes. 2021;7:6–17. doi: 10.1093/ehjqcco/qcaa057. [DOI] [PubMed] [Google Scholar]
- 8.Chandrashekhar Y, Alexander T, Mullasari A, et al. Resource and infrastructure-appropriate management of ST-segment elevation myocardial infarction in low- and middle-income countries. Circulation. 2020;141:2004–2025. doi: 10.1161/CIRCULATIONAHA.119.041297. [DOI] [PubMed] [Google Scholar]
- 9.De Belder MA. Acute myocardial infarction: failed thrombolysis. Heart. 2001;85:104–112. doi: 10.1136/heart.85.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandal R, Yadav K. Assessment of ST segment resolution as a predictor of outcome in acute myocardial infarction after thrombolysis. Int J Contemp Med Res. 2019 doi: 10.21276/ijcmr.2019.6.8.10. [DOI] [Google Scholar]
- 11.Bhatia L, Clesham GJ, Turner DR. Clinical implications of ST-segment non-resolution after thrombolysis for myocardial infarction. J R Soc Med. 2004;97:566–570. doi: 10.1258/jrsm.97.12.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ChandraBabu S, Chakka G, Dornadula GRS, et al (2019) Comparison of safety and efficacy of streptokinase and tenecteplase in patients of myocardial infarction. IOSR J Dent Med Sci 18:14–18
- 13.Neela B, Gunreddy VR, Chandupatla MR, et al. Safety and efficacy of streptokinase, tenecteplase, and reteplase in patients diagnosed with ST-elevation myocardial infarction: a comparative study. J Indian Coll Cardiol. 2020;10:138. doi: 10.4103/jicc.jicc_62_20. [DOI] [Google Scholar]
- 14.Ng SS, Lim TH, Tan SP, et al (2016) Comparison of efficacy and safety of streptokinase and tenecteplase in patients with ST-segment elevated acute myocardial infarction (STEMI) in Melaka Hospital. In: Proceedings of the 9th National Pharmacy R&D Conference, 2016. Malaysian J Pharm 2:26-undefined
- 15.Araiza- Garaygordobil D, Gopar-Nieto R, Cabello-López A, et al. Pharmacoinvasive strategy vs primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction: results from a study in Mexico city. CJC Open. 2021;3:409–418. doi: 10.1016/j.cjco.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam SA, Faruque M, Rahman F, et al (2019) Clinical impacts of ST-segment non-resolution after thrombolysis for myocardial infarction. Univ Hear J 15:3–7
- 17.Nik Muhamad NA, Azizan MS, Masani NA, et al. Association of risk factors and its bleeding complication for tenecteplase administered in acute myocardial infarction (AMI) Med J Malaysia. 2013;68:381–383. [PubMed] [Google Scholar]
- 18.Lee YY, Tee MH, Zurkurnai Y, et al. Thrombolytic failure with streptokinase in acute myocardial infarction using electrocardiogram criteria. Singap Med J. 2008;49:310. [PubMed] [Google Scholar]
- 19.Mishra A, Prajapati J, Dubey G, et al. Characteristics of ST-elevation myocardial infarction with failed thrombolysis. Asian Cardiovasc Thorac Ann. 2020;28:266–272. doi: 10.1177/0218492320932074. [DOI] [PubMed] [Google Scholar]
- 20.Girdhar R, Kothari Y, Anil Raj R, et al. Successful or unsuccessful thrombolysis with streptokinase in acute myocardial infarction: a descriptive study. J Med Sci Clin Res. 2018;6:731–735. doi: 10.18535/jmscr/v6i3.122. [DOI] [Google Scholar]
- 21.Mahendra S, Setianto BY, Hariawan H. Prediction of failed fibrinolytic using scoring system in ST elevation myocardial infarction patients. Acta Cardiol Indones. 2017;2:39–46. [Google Scholar]
- 22.Palmieri EA, Migliaresi P, Palmieri V, et al. Lytic failure in the current pharmacointensive ST-elevated acute myocardial infarction care: insights from a pilot real-world study. J Cardiovasc Med. 2013;14:35–42. doi: 10.2459/jcm.0b013e328356a2be. [DOI] [PubMed] [Google Scholar]
- 23.Lester G, Eather S, Law D, et al. Clinical factors associated with failed thrombolysis in ST-segment myocardial infarction (STEMI) in a regional setting. Hear Lung Circ. 2021;30:S217. doi: 10.1016/J.HLC.2021.06.268. [DOI] [Google Scholar]
- 24.Hamid S, Kundal V, Mahajan N, Singh P. Failure of thrombolysis with streptokinase in acute myocardial infarction using ECG criteria: an observational study. J Med Educ Res. 2015;17:123–126. [Google Scholar]
- 25.Deshani VR, Mehta MN, Rathod NR (2016) A comparative study of streptokinase v/s tenecteplase in hyper acute stage of myocardial infarction. Int J Sci Res 5:496–497
- 26.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JL, Karagounis LA, Califf RM. Metaanalysis of five reported studies on the relation of early coronary patency grades with mortality and outcomes after acute myocardial infarction. Am J Cardiol. 1996;78:1–8. doi: 10.1016/S0002-9149(96)00217-2. [DOI] [PubMed] [Google Scholar]
- 28.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/circulationaha.110.009449. [DOI] [PubMed] [Google Scholar]
- 29.Wan Ahmad WA (2022) Annual report of the NCVD-ACS registry year 2018–2019. Kuala Lumpur
- 30.Wilcox RG. Randomised, double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. International Joint Efficacy Comparison of Thrombolytics. Lancet. 1995;346:329–336. doi: 10.1016/S0140-6736(95)92224-5. [DOI] [PubMed] [Google Scholar]
- 31.GISSI Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet. 1986;327:397–402. doi: 10.1016/S0140-6736(86)92368-8. [DOI] [PubMed] [Google Scholar]
- 32.The Gusto III Investigators A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med. 1997;337:1118–1123. doi: 10.1056/nejm199710163371603. [DOI] [PubMed] [Google Scholar]
- 33.Van de Werf F, Adgey J, Ardissino D, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–722. doi: 10.1016/S0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 34.Falco L, Fabris E, Gregorio C, et al. Early prognostic stratification and identification of irreversibly shocked patients despite primary percutaneous coronary intervention. J Cardiovasc Med. 2022;23:247–253. doi: 10.2459/JCM.0000000000001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohd Hamdan SNA (2021) A 4 year study on utilization of tenecteplase and outcomes for ST-elevation myocardial infarction in a primary percutaneous coronary intervention capable hospital. Malaysian J Emerg Med 5:77
- 36.Jinatongthai P, Kongwatcharapong J, Foo CY, et al. Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patients with ST-segment elevation myocardial infarction: a systematic review and network meta-analysis. Lancet. 2017;390:747–759. doi: 10.1016/S0140-6736(17)31441-1. [DOI] [PubMed] [Google Scholar]
- 37.Aherrao N, Chopda M, Gulecha V, et al (2018) A randomized, parallel study to compare efficacy & safety of streptokinase vs tenecteplase when given in correct timelines in patients of ST-elevation myocardial infarction (STEMI). Ann Pharmacol Pharm 3:1159
- 38.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for Treatment of Infarcting Myocardium Early II trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.CIR.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 39.Van De Werf F, Cannon CP, Luyten A, et al. Safety assessment of single-bolus administration of TNK tissue-plasminogen activator in acute myocardial infarction: the ASSENT-1 trial. The ASSENT-1 Investigators. Am Heart J. 1999;137:786–791. doi: 10.1016/S0002-8703(99)70400-X. [DOI] [PubMed] [Google Scholar]
- 40.Van de Werf F, Armstrong PW, Granger C, et al. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605–613. doi: 10.1016/S0140-6736(01)05775-0. [DOI] [PubMed] [Google Scholar]
- 41.Anand SS, Yi Q, Gerstein H, et al. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation. 2003;108:420–425. doi: 10.1161/01.cir.0000080884.27358.49. [DOI] [PubMed] [Google Scholar]
- 42.Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41:1229–1245. doi: 10.2165/00003088-200241150-00001. [DOI] [PubMed] [Google Scholar]
- 43.Sinkovic A. Pretreatment plasminogen activator inhibitor-1 (PAI-1) levels and the outcome of thrombolysis with streptokinase in patients with acute myocardial infarction. Am Heart J. 1998;136:406–411. doi: 10.1016/S0002-8703(98)70213-3. [DOI] [PubMed] [Google Scholar]
- 44.Rubartelli P, Bartolini D, Bellotti S, et al. Reasons for reperfusion delay in ST-elevation myocardial infarction and their impact on mortality. J Cardiovasc Med (Hagerstown) 2022;23:157–164. doi: 10.2459/JCM.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 45.Fabris E, Bessi R, De Bellis A, et al. COVID-19 impact on ST-elevation myocardial infarction incidence rate in a Italian STEMI network: a U-shaped curve phenomenon. J Cardiovasc Med (Hagerstown) 2021;22:344–349. doi: 10.2459/JCM.0000000000001153. [DOI] [PubMed] [Google Scholar]
- 46.Zorzi A, Vio R, Rivezzi F, et al. Characteristics and hospital course of patients admitted for acute cardiovascular diseases during the coronavirus disease-19 outbreak. J Cardiovasc Med (Hagerstown) 2021;22:29–35. doi: 10.2459/JCM.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 47.Flori M, Marinucci L, Gabrielli G, et al. Reduction in acute coronary syndromes during coronavirus disease 2019 global pandemic: data from the Marche region of Italy. J Cardiovasc Med (Hagerstown) 2021;22:350–356. doi: 10.2459/JCM.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 48.Morello F, Bima P, Ferreri E, et al. After the first wave and beyond lockdown: long-lasting changes in emergency department visit number, characteristics, diagnoses, and hospital admissions. Intern Emerg Med. 2021;16:1683. doi: 10.1007/S11739-021-02667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abd. Wahab M, Safaai S, Mohd Saiboon I. Impact of a binary triage system and structural reorganization of emergency department on health care workers exposed to suspected COVID-19 patients-a single-centre analysis. Int J Emerg Med. 2021 doi: 10.1186/S12245-021-00384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters RJG, Joyner C, Bassand JP, et al. The role of fondaparinux as an adjunct to thrombolytic therapy in acute myocardial infarction: a subgroup analysis of the OASIS-6 trial. Eur Heart J. 2008;29:324–331. doi: 10.1093/eurheartj/ehm616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are not attached. The data underlying this article will be shared on reasonable request to the corresponding author.


