Abstract
Abstract
Endophytic fungi have proved to be a major source of secondary metabolites, wherein the genus Chaetomium has emerged as a source of multifarious bioactive natural compounds belonging to diverse classes such as chaetoglobosins, epipolythiodioxopiperazines, azaphilones, xanthones, anthraquinone, chromones, depsidones, terpenoids, and steroids. The objective of this review is to encapsulate recent findings on various Chaetomium strains, such as C. globosum, C. cupreum, C. elatum, C. subspirale, C. olivaceum, C. indicum, and C. nigricolor known for production of beneficial secondary metabolites, with an insight into their origin and function. A thorough literature survey was conducted for obtaining Chaetomium-derived secondary metabolites, with a scope of future application into drug development efforts. More than 100 secondary metabolites, with various beneficial properties such as antitumor, cytotoxic, antimalarial, and enzyme inhibitory activities, were enlisted. We believe this review will enhance the understanding of beneficial effects conferred by various Chaetomium-derived secondary metabolites and emphasize their potential in serving novel drug development efforts.
Key points
• Identified Chaetomium-derived metabolites with potential for drug development.
• More than 100 beneficial metabolites are enlisted.
• Benefits include anti-cancerous, antimalarial, and anti-enzymatic properties.
Graphical Abstract
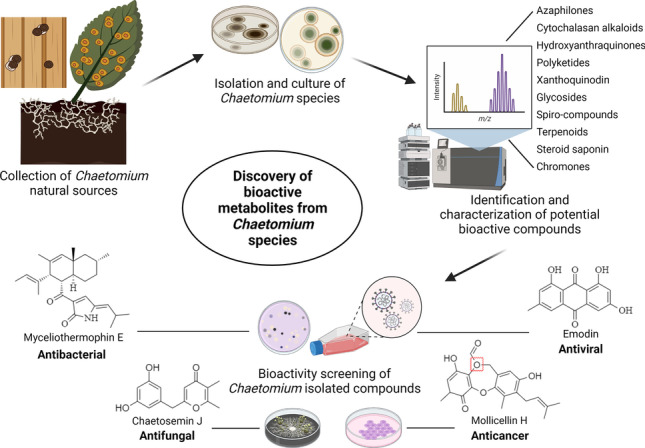
Keywords: Chaetomium, Endophytes, Drug discovery, Natural products, Bioactivities
Introduction
Natural compounds offer a great alternative in the human quest for disease curing medicines. In drug development research, secondary metabolites and their derivatives obtained from plants, bacteria, marine, and animal sources serve as attractive components of new drugs in the fields of microbial chemistry, biology, and medicine. As compared to synthetic drugs, these naturally derived drugs have better structural diversity, complexity, persistent nature and exhibit a wide range of biological activities (Atanasov et al. 2021; Dwibedi et al. 2022).
Endophytic fungi have a huge potential as a source of bioactive compounds (Dwibedi and Saxena 2018; Keller 2019). They have adapted to life inside the tissues of other eukaryotes, which might trigger biosynthesis of novel secondary metabolites with potent bioactivity (Dwibedi and Saxena 2020; Rokas et al. 2020). A myriad of endophyte-derived, secondary metabolites belonging to diverse chemical classes like phenolic acids, phenylpropanoids, cytochalasins, alkaloids, isocoumarins, steroids, furandiones, terpenoid derivatives, flavonoids, quinones, lignans, peptides, and aliphatic compounds have been reported (Ancheeva et al. 2020; Dwibedi and Saxena 2019; Torres-Mendoza et al. 2020). These metabolites are well known for their antioxidant, antibiotic, antiviral, anti-diabetic, immunosuppressive, anticancer, and insecticidal properties (Dwibedi and Saxena 2019; Torres-Mendoza et al. 2020). Recently, several novel bioactive compounds have been isolated from fungal endophytes, including xylarosides, penicidones, xylariol, and benzoquinone derivatives (Poveda et al. 2022).
Various reports enlist the secondary metabolites, isolated from different endophytic fungi and their biological applications. Rustamova et al. (2020) summarized nearly 220 biologically relevant metabolites, including meroterpenoids, sesquiterpenoids, polyketides, and steroids, isolated from various endophytic fungi genus, such as Alternaria, Ascomycota, Cerrena, Cytospora, Fusarium, Mucor, Preussia, Trichoderma, and Xylaria (Rustamova et al. 2020). In 2021, Cao et al. reviewed nine strains of various endophytic fungi (Penicillium sp. LDL4.4, Colletotrichum gloeosporioides ESO26 and Cg01, Alternaria brassicae AGF041, Penicillium polonicum hy4, Shiraia sp. Slf14, Paecilomyces tenuis YS-13, Trichoderma harzianum L44, Colletotrichum cladosporioides LF70, and Ceriporia lacerate HS-ZJUT-C13A), isolated from Huperzia serrata, that can produce Huperzine A, a known acetylcholinesterase inhibitor (Cao et al. 2021). In an interesting review from University of Panama and Institute for Scientific Research and Technology Services, Panama, the authors enlisted endophytic fungi-derived secondary metabolites with a novel, uncommon structure (Ortega et al. 2021). These compounds, including cytochalasans, indole alkaloids, pyridine derivatives, and peptides, were known for various biological activities, such as antitumor, antiviral, and anti-inflammatory activities (Ortega et al. 2021).
In 1817, Gustav Kunze discovered the genus Chaetomium, an endophytic fungal genus in the family Chaetomiaceae (Abdel-Azeem 2019). It is a dark-walled mold normally found in soil, air, plant, and cellulose debris. Chaetomium occurs in a wide variety of substrates that have gained attention for their ability to generate a spectrum of biologically active compounds (Salo et al. 2020). According to the Fungi dictionary, the widespread genus includes about 95 species. Chaetomium is characterized by ostiolate ascomata, covered with hair, or setae and clavate, fusiform or cylindrical, fascicular, evanescent asci, and brown to gray-colored, single-celled ascospores with one or two germ pores. Chaetomium has been related to many anamorphic genera such as Acremonium, Botryotrichum, Chrysosporium, Histoplasma, Humicola, Phialophora, Scopulariopsis, and Scytalidium. More than 400 species have been described since the creation of the genus, many of which have been synonymized/excluded, and only 273 Chaetomium species have been recognized under the Index Fungorum Partnership (Abdel-Azeem 2020; Calaça et al. 2020). Chaetomium sp., due to the variety of species and habitats, can activate different clusters of biosynthetic genes, thus expressing different bioactive compounds to adjust to various ecosystems. Over 200 compounds with a broad range of bioactive effects have been isolated from Chaetomium sp. so far (Table 1) (Elkhateeb et al. 2021), yet more bioactive secondary metabolites can potentially be found.
Table 1.
A list of Chaetomium-derived bioactive compounds, along with their known bioactivity against various diseases/pathogens
| Species | Metabolite | Metabolite class | Target | Tested system | Biological activity | Reference |
|---|---|---|---|---|---|---|
| Chaetomium globosum | Chaetone C | Dibenzoxepine | Various cancer | A549, Raji, HepG2, MCF-7, and HL-60 cell lines | IC50 values of 1.2, 1.8, 1.9, 2.3, and 1.6 µg/mL, respectively | (Shen et al. 2012) |
| Chrysophanol | Trihydroxyanthraquinone | Cerebral ischemia | CD1 mice | Suppressed activation of NALP3 inflammasome | (Zhang et al. 2014) | |
| Chaetoglobosin A | Cytochalasan alkaloid | Colon cancer | HCT116 cell line | IC50 value of 3.15 μM | (Li et al. 2014) | |
| Chaetoglobosin Fa | Cytochalasan alkaloid | Colon cancer | HCT116 cell line | IC50 value of 5.85 μM | (Li et al. 2014) | |
| Chaetoglobosin A | Cytochalasan alkaloid | Myelogenous leukemia | K562 cells | IC50 value of 60 pM | (Ko et al. 1998) | |
| Armochaetoglosin C | Cytochalasan alkaloid | Bacterial activity |
Klebsiella pneumonia and Escherichia coli ATCC 35,218 |
MIC = 4.0 μg/mL and 16.0 μg/mL, respectively | (Gao et al. 2019) | |
| Chaetomugilide A | Azaphilone alkaloid | Hepatocellular carcinoma | HePG2 cell line | IC50 value of 1.7 Μm | (Li et al. 2013) | |
| Chaetomugilide B | Azaphilone alkaloid | Hepatocellular carcinoma | HePG2 cell line | IC50 value of 19.8 μM | (Li et al. 2013) | |
| Chaetomugilide C | Azaphilone alkaloid | Hepatocellular carcinoma | HePG2 cell line | IC50 value of 53.4 μM | (Li et al. 2013) | |
| Chaetoviridin E | Azaphilone | Bacterial activity |
Staphylococcus aureus and Enterococcus faecalis |
MIC > 100 μg/mL | (Kingsland and Barrow 2009) | |
| Chaetoviridin B | Azaphilone | Bacterial activity |
Staphylococcus aureus and Enterococcus faecalis |
MIC > 100 μg/mL | (Kingsland and Barrow 2009) | |
| Chaetomugilin I | Azaphilone | Cancer | RAW 264.7 cells | IC50 value of 0.9 μM | (Youn et al. 2015) | |
| Chaetomugilin N | Azaphilone | Cancer | RAW 264.7 cells | IC50 value of 0.9 μM | (Youn et al. 2015) | |
| Chaetomugilin J | Azaphilone | Cancer | RAW 264.7 cells | IC50 value of 7.6 μM | (Youn et al. 2015) | |
| Chaetomugilin E | Azaphilone | Cancer | RAW 264.7 cells | IC50 value of 11.6 μM | (Youn et al. 2015) | |
| Chaetomugilin F | Azaphilone | Cancer | RAW 264.7 cells | IC50 value of 5.1 μM | (Youn et al. 2015) | |
| Chaetomugilin G | Azaphilone | Leukemia | P388 and HL-60 cell lines | IC50 value of 24.1 and 19.8 μM, respectively | (Yamada et al. 2009) | |
| Chaetomugilin H | Azaphilone | Leukemia and epithelial carcinoma | P388, HL-60 and KB cell lines | IC50 value of 4.1 and 19.8 μM, respectively | (Yamada et al. 2009) | |
| Chaetomugilin D | Azaphilone | Phytotoxic activity | Lactuca sativa | IC50 value of 24.2 ppm | (Piyasena et al. 2015) | |
| Chaetomugilin J | Azaphilone | Phytotoxic activity | Lactuca sativa | IC50 value of 22.6 ppm | (Piyasena et al. 2015) | |
| Chaetomugilin I | Azaphilone | Various cancer | 39 human cancer cell lines | Potent selective cytotoxic activity | (Muroga et al. 2009) | |
| Chaetomugilin J | Azaphilone | Leukemia and epithelial carcinoma | P388, HL-60, L1210 and KB cell lines | IC50 value of 12.6, 12.6, 2.8, and 8.5 μM, respectively | (Muroga et al. 2009) | |
| Chaetomugilin K | Azaphilone | Leukemia and epithelial carcinoma | P388, HL-60, L1210 and KB cell lines | IC50 value of 8.2, 14.1, 11.2, and 18.7 μM, respectively | (Muroga et al. 2009) | |
| Chaetomugilin L | Azaphilone | Leukemia and epithelial carcinoma | P388, HL-60, L1210 and KB cell lines | IC50 value of 10.9, 13.1, 15.6, and 20.1 μM, respectively | (Muroga et al. 2009) | |
| Chaetomugilin N | Azaphilone | Leukemia and epithelial carcinoma | P388, HL-60, L1210 and KB cell lines | IC50 value of 2.3, 2.3, 10.6, and 10.6 μM, respectively | (Muroga et al. 2009) | |
| Chaetomugilin O | Azaphilone | Leukemia and epithelial carcinoma | P388, HL-60, L1210 and KB cell lines | IC50 value of 11.1, 11.1, 10.1, and 7.2 μM, respectively | (Muroga et al. 2009) | |
| Chaetomium cupreum | Rotiorinol | Azaphilone | White root disease | Rigidoporus microporus | ED50 value of 26 µg/l | (Kaewchai and Soytong 2010) |
| Rotiorinol A | Azaphilone | Fungal activity | Candida albicans | IC50 value of 10.5 µg/mL | (Kanokmedhakul et al. 2006) | |
| Rotiorinol C | Azaphilone | Fungal activity | Candida albicans | IC50 value of 16.7 µg/mL | (Kanokmedhakul et al. 2006) | |
| (-)-Rotiorin | Azaphilone | Fungal activity | Candida albicans | IC50 value of 24.3 µg/mL | (Kanokmedhakul et al. 2006) | |
| Rubrorotiorin | Azaphilone | Fungal activity | Candida albicans | IC50 value of 0.6 µg/mL | (Kanokmedhakul et al. 2006) | |
| Isochromophilonol | Azaphilone | Epithelial carcinoma | KB cell line | IC50 value of 9.63 µg/mL | (Panthama et al. 2015) | |
| Isochromophilonol | Azaphilone | Epithelial carcinoma | NCI-H187 cell line | IC50 value of 27.18 µg/mL | (Panthama et al. 2015) | |
| Ochrephilonol | Azaphilone | Epithelial carcinoma | KB cell line | IC50 value of 30.2 µg/mL | (Panthama et al. 2015) | |
| Clearanol B | α-Pyrone derivative | Epithelial carcinoma | KB cell line | IC50 value of 32.42 µg/mL | (Panthama et al. 2015) | |
| Clearanol B | α-Pyrone derivative | Breast cancer | MCF-7 cell line | IC50 value of 13.01 µg/mL | (Panthama et al. 2015) | |
| Chaetomium elatum | Xanthoquinodin A4 | Xanthoquinodin | Various cancer | SMMC-7721, A-549 and SW480 cell lines | IC50 value of 19.18, 25.47, and 18.85 μM, respectively | (Chen et al. 2013) |
| Xanthoquinodin A5 | Xanthoquinodin | Various cancer | HL-60, SMMC-7721, A-549, MCF-7, and SW480 cell lines | IC50 value of 26.62, 16.81, 18.60, 23.96, and 16.19 μM, respectively | (Chen et al. 2013) | |
| Xanthoquinodin A6 | Xanthoquinodin | Various cancer | HL-60, SMMC-7721, A-549, MCF-7, and SW480 cell lines | IC50 value of 3.75, 2.87, 2.04, 5.64, and 6.44 μM, respectively | (Chen et al. 2013) | |
| Chaetomugilin S | Azaphilone | Apoptosis | Caspase-3 | IC50 value of 20.6 μM | (Chen et al. 2012) | |
| 7,5′-Bis-epi-chaetoviridin A | Azaphilone | Apoptosis | Caspase-3 | IC50 value of 10.9 μM | (Chen et al. 2012) | |
| 7-Epi-chaetoviridin E | Azaphilone | Apoptosis | Caspase-3 | IC50 value of 7.9 μM | (Chen et al. 2012) | |
| Xanthoquinodin B4 | Xanthoquinodin | Various cancer | HL-60, SMMC-7721, and A-549 cell lines | IC50 value of 3.01, 27.70, and 25.13 μM, respectively | (Chen et al. 2013) | |
| Xanthoquinodin B5 | Xanthoquinodin | Various cancer | HL-60, SMMC-7721, and A-549 cell lines | IC50 value of 4.74, 14.99, and 11.38 μM, respectively | (Chen et al. 2013) | |
| Chaetomium subspirale | Oxaspirodion | Spiro compound | Inflammatory activity | Jurkat T cells | IC50 value of 2.5 μg/ml | (Rether et al. 2004) |
| Chaetomium olivaceum | Myceliothermophin E | Polyketide | Microbial activity | Methicillin-resistant Staphylococcus aureus (MRSA) | MIC value of 15.8 μM | (Wang et al. 2020b) |
| Chaetolivacine B | Polyketide | Microbial activity | Methicillin-resistant Staphylococcus aureus (MRSA) | MIC value of 27.1 μM | (Wang et al. 2020b) | |
| Chaetolivacine B | Polyketide | Microbial activity | Staphylococcus aureus | MIC value of 10.8 μM | (Wang et al. 2020b) | |
| Miliacin | Tritepenoid | Cytotoxic activity | Mouse erythrocytes | HC50 value of 2 × 10−4 mol/l | (Smetanina et al. 2001) | |
| Pseudoprotodioscin | Steroidal saponin | Cardiac disease | H9c2 cells | Increased the viability of H9c2, induced by H2O2, in a dose-dependent manner | (Dong et al. 2016) | |
|
5-ene-3β, 20β-diol-22, 16-lactone-3-O-α-L-rhamnopyranosyl- (1 → 4)-β-D-glucopyranoside |
Steroidal saponin | Cardiac disease | H9c2 cells | Increased the viability of H9c2, induced by H2O2, in a dose-dependent manner | (Dong et al. 2016) | |
|
26-O-β-D-glucopyranosyl- 23(S)-methoxyl-(25R)-furosta-5,20(22)-diene-3β,26-diol − 3-O-α- L-rhamnopyranosyl-(1 → 4)-β-D-glucopyranoside |
Steroidal saponin | Cardiac disease | H9c2 cells | Increased the viability of H9c2, induced by H2O2, in a dose-dependent manner | (Dong et al. 2016) | |
| Chaetomium indicum | Chaetochromone A | Polyketide | Fungal activity | Poria placenta (Fr.) Cooke | > 60% inhibitory activity | (Lu et al. 2013) |
| Chaetomium nigricolor | (aS)-asperpyrone A | bis-naphtho-γ-pyrone | Inflammation | RAW 264.7 cells | Inhibited NO production | (Kim et al. 2020) |
| (aS)-fonsecinone A | bis-naphtho-γ-pyrone | Inflammation | RAW 264.7 cells | Inhibited NO production | (Kim et al. 2020) | |
| Chamiside A | Cytochalasan | Bacterial activity | Staphylococcus aureus | MIC value of 25 μg/ml | (Wang et al. 2019) | |
| Other Chaetomium sp. | Gliocladinin C | p-terphenyl glycoside | Larynx and hepatocellular carcinoma | Hep-2 and HepG-2 cell lines | IC50 value of 0.18 mM and 0.12 μM, respectively | (Han et al. 2019) |
| Chaetominin A | Furano-polyene derivative | Larynx and hepatocellular carcinoma | Hep-2 and HepG-2 cell lines | IC50 value of 0.32 mM and 0.38 μM, respectively | (Han et al. 2019) | |
| Chaetocochin A | Epipolythiodioxopiperazine | Various cancer | Bre-04, Lu-04, and N-04 cell lines | GI50 value of 4.1, 3.4, and 7.0 μg/ml, respectively | (Li et al. 2006b) | |
| Chaetocochin C | Epipolythiodioxopiperazine | Various cancer | Bre-04, Lu-04, and N-04 cell lines | GI50 value of 0.4, 1.9, and 0.4 μg/ml, respectively | (Li et al. 2006b) | |
| Dethio-tetra (methylthio) chetomin | Thiodiketopiperazine alkaloid | Various cancer | Bre-04, Lu-04, and N-04 cell lines | GI50 value of 0.06, 0.05, and 0.2 μg/ml, respectively | (Li et al. 2006b) | |
| Mollicellin J | Depsidone | Various cancer | Bre-04, Lu-04, and N-04 cell lines | GI50 value of 5.9, 8.6, and 3.8 μg/ml, respectively | (Li et al. 2008) | |
| Mollicellin H | Depsidone | Various cancer | Bre-04, Lu-04, and N-04 cell lines | GI50 value of 5.1, 6.5, and 2.5 μg/ml, respectively | (Li et al. 2008) | |
| Mollicellin K | Depsidone | Malaria | Plasmodium falciparum | IC50 value of 1.2 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin L | Depsidone | Malaria | Plasmodium falciparum | IC50 value of 3.4 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin M | Depsidone | Malaria | Plasmodium falciparum | IC50 value of 2.9 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin B | Depsidone | Malaria | Plasmodium falciparum | IC50 value of 4.7 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin C | Depsidone | Malaria | Plasmodium falciparum | IC50 value of 9.1 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin E | Depsidone | Malaria | Plasmodium falciparum | IC50 value of 3.2 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin J | Depsidone | Malaria | Plasmodium falciparum | IC50 value of 4.9 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin K | Depsidone | Tuberculosis | Mycobacterium tuberculosis | MIC value of 12.5 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin K | Depsidone | Fungal activity | Candida albicans | IC50 value of 1.2 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin K | Depsidone | Epithelial carcinoma, breast cancer and small cell lung cancer | KB, BCI and NCI-H187 cell lines | IC50 value of 1.9, 6.8, and 0.35 μg/ml, respectively | (Khumkomkhet et al. 2009) | |
| Mollicellin L | Depsidone | Small cell lung cancer | NCI-H187 cell line | IC50 value of 9.5 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin M | Depsidone | Small cell lung cancer | NCI-H187 cell line | IC50 value of 0.68 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin N | Depsidone | Epithelial carcinoma and small cell lung cancer | KB and NCI-H187 cell lines | IC50 value of 25.9 and 13.5 μg/ml, respectively | (Khumkomkhet et al. 2009) | |
| Mollicellin B | Depsidone | Small cell lung cancer | NCI-H187 cell line | IC50 value of 14.7 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin C | Depsidone | Small cell lung cancer | NCI-H187 cell line | IC50 value of 3.1 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin E | Depsidone | Small cell lung cancer | NCI-H187 cell line | IC50 value of 1.0 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin F | Depsidone | Small cell lung cancer | NCI-H187 cell line | IC50 value of 13.1 μg/ml | (Khumkomkhet et al. 2009) | |
| Mollicellin H | Depsidone | Epithelial carcinoma and small cell lung cancer | KB and NCI-H187 cell lines | IC50 value of 16.6 and 3.9 μg/ml, respectively | (Khumkomkhet et al. 2009) | |
| Mollicellin J | Depsidone | Epithelial carcinoma and small cell lung cancer | KB and NCI-H187 cell lines | IC50 value of 29.1 and 23.3 μg/ml, respectively | (Khumkomkhet et al. 2009) | |
| Chaetosemin B | Chromone | Fungal activity |
Magnaporthe oryzae and Gibberella saubinettii |
MIC value of 6.25 and 12.5 μM, respectively | (Li et al. 2015) | |
| Chaetosemin C | Chromone | Oxidants | DPPH free radical scavenging activity | 50.7% activity at 50 μM | (Li et al. 2015) | |
| Chaetoquadrin J | Chromone | Hypertension and Inflammation | Soluble epoxide hydrolase | IC50 value of 63.0 μM | (Li et al. 2015) | |
| Chaetocin | Piperazine | Bacterial activity | Staphylococcus aureus | MIC value of 0.1 μg/ml | (SAITO et al. 1988) | |
| Chaetocin B | Piperazine | Bacterial activity | Staphylococcus aureus | MIC value of 0.05 μg/ml | (SAITO et al. 1988) | |
| Chaetocin C | Piperazine | Bacterial activity | Staphylococcus aureus | MIC value of 0.025 μg/ml | (SAITO et al. 1988) | |
| Chetracin A | Epipolythiodioxopiperazine | Bacterial activity | Staphylococcus aureus | MIC value of 0.39 μg/ml | (SAITO et al. 1988) | |
| 11α,11′α-Dihydroxychaetocin | Epipolythiodioxopiperazine | Bacterial activity | Staphylococcus aureus | MIC value of 0.2 μg/ml | (SAITO et al. 1988) | |
| Chaetocin | Piperazine | Cervical cancer | HeLa cells | IC50 value of 0.04 μg/ml | (SAITO et al. 1988) | |
| Chaetocin B | Piperazine | Cervical cancer | HeLa cells | IC50 value of 0.03 μg/ml | (SAITO et al. 1988) | |
| Chaetocin C | Piperazine | Cervical cancer | HeLa cells | IC50 value of 0.02 μg/ml | (SAITO et al. 1988) | |
| Chetracin A | Epipolythiodioxopiperazine | Cervical cancer | HeLa cells | IC50 value of 0.02 μg/ml | (SAITO et al. 1988) | |
| 11α,11′α-Dihydroxychaetocin | Epipolythiodioxopiperazine | Cervical cancer | HeLa cells | IC50 value of 0.04 μg/ml | (SAITO et al. 1988) | |
| Radicicol | Macrolide | Breast cancer | MCF-7 cell line | IC50 value of 0.03 μM | (Turbyville et al. 2006) | |
| Chaetoquadrin G | Spiro compound | Depression | Monoamine oxidase inhibition in mouse liver | IC50 value of 0.045 μM | (Fujimoto et al. 2003) | |
| Chaetoquadrin H | Spiro compound | Depression | Monoamine oxidase inhibition in mouse liver | IC50 value of 0.023 μM | (Fujimoto et al. 2003) | |
| Mollipilin A | Polyketide | Colon cancer | HCT-116 cells | GI50 value of 1.8 μM | (Asai et al. 2012) | |
| Mollipilin B | Polyketide | Colon cancer | HCT-116 cells | GI50 value of 3.7 μM | (Asai et al. 2012) | |
| Sclerotiorin | Azaphilone | Hsp90 chaperoning activity | PR reconstitution assay | Inhibitory activity at par with known compounds | (Kabbaj et al. 2015) | |
| Chaetoatrosin A | Naphthalene skeleton | Fungal activity | Rhizoctonia solani | MIC value of 50 μg/ml | (Hwang et al. 2000) |
Many works summarize the specific beneficial effects of various Chaetomium species. Moya et al. (2020) highlighted the role of Chaetomium globosum in the agricultural industry as a plant growth promoter and a biocontrol agent, specifically in Argentina (Moya et al. 2020). A recent comprehensive study conducted in Saudi Arabia and Egypt focused on enlisting the Chaetomium-derived enzymes, such as L-methioninase, β-1,3-glucanase, laccase, dextranase, amylolytic, chitinolytic, and proteolytic enzymes, and their applications (Ibrahim et al. 2021). This study focused on the studies reported between 2016 and 2021 (Ibrahim et al. 2021). In another study, Tian et al. focused on bioactive compounds derived from marine Chaetomium species (Tian and Li 2022). Interestingly, they related the structure of the metabolites to their bioactivities, thus providing an unique insight into their biological functioning (Tian and Li 2022). Further, in mid-2022, a study on soil-derived Chaetomium madrasense 375 discussed various secondary metabolites, including chaetoviridins, chaetomugilins, and chaetoglobosin, isolated from the species (Guo et al. 2022).
This has encouraged us to prepare a comprehensive review on the bioactive compounds derived from various Chaetomium species. Various studies have tried to provide details on the Chaetomium-derived secondary metabolites and their multifarious application. However, this is perhaps one of the first reviews wherein an exhaustive compilation of data on the secondary metabolites from various Chaetomium strains, such as C. globosum, C. cupreum, C. elatum, C. subspirale, C. olivaceum, C. indicum, and C. nigricolor, with an insight into their origin and function, has been provided. A thorough literature survey (2000–2022) was conducted for obtaining Chaetomium-derived secondary metabolites, resulting in identification of more than 100 secondary metabolites with various beneficial properties, such as antitumor, cytotoxic, antimalarial, and enzyme inhibitory activities. The review spotlights the benefits of natural compounds and should supplement various drug development efforts.
Chaetomium-derived secondary metabolites
Secondary metabolites from Chaetomium globosum
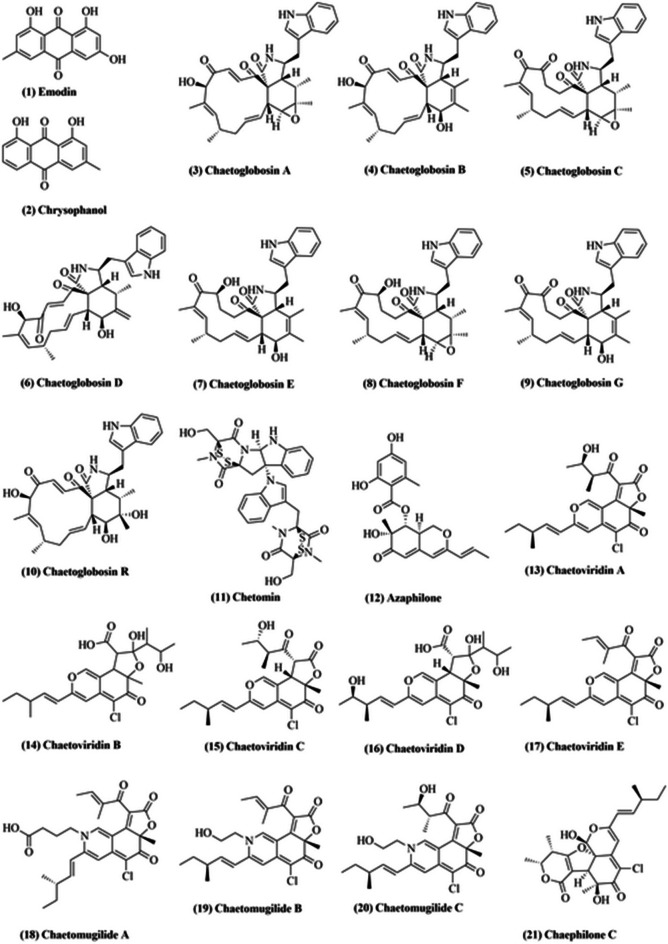
Chaetomium globosum is a common mesophilic member of the Chaetomiaceae family of molds. It is a saprophytic fungus, which lives primarily on seeds, dirt, grass, and dung, and is also reported to live inside the plant tissue as an endophyte (Vivi et al. 2019). Their asymptomatic colonization confers tolerance to plants against toxicity from copper-like heavy metals, which inhibit plant production and disrupt metabolic processes such as photosynthesis. Abou Alhamed et al. 2012 reported that maize crop, upon administration with Chaetomium globosum, demonstrated increased biomass and lesser growth inhibition against metal toxicity (Abou Alhamed and Shebany 2012). Chaetomium globosum produces an array of biologically active compounds (Fig. 1) such as emodins (1), chrysophanols (2), chaetoglobosins A-G (3–9), isochaetoglobosin, chetomin (11), azaphilones (12), and chaetoviridins (13–17) (Madbouly and Abdel-Wareth 2020). Emodin (1) is an active component of many plants such as Rheum palmatum, Polygonum cuspidatum, and Polygonum multiflorum, which are used in traditional Chinese medicine. Emodin (1) reportedly exhibits laxative, antibacterial and anti-inflammatory effects (Akkol et al. 2021). Additionally, it might exhibit potential antiviral activity against SARS-CoV-2 (Horvat et al. 2021), being one of the most active components of Lianhua Qingwen, a traditional antiviral Chinese medicine formulation (Runfeng et al. 2020).
Fig. 1.
Some of the compounds isolated from Chaetomium globosum
Chrysophanol (2) is an environmentally significant anthraquinone with wide-spectrum medicinal properties. Traditional Chinese and Korean medicinal systems provide evidence that chrysophanol (2) has important health benefits (Kikiowo et al. 2020). It is the first polyketide reported to be biosynthesized in an organism-specific way. Chrysophanol (2) exerts a therapeutic effect on cerebral ischemia – reperfusion (I / R) via its anti-inflammatory action. Zhang et al. (2014) reported that chrysophanol suppressed NALP3 inflammasome activation (which consists of NALP3, ASC, and caspase‐1) for occlusion of the median cerebral artery (MCAO) and reperfusion (Zhang et al. 2014). Analysis of proteomic iTRAQ highlighted decorin (DCN) as another target of chrysophanol, while DCN knockdown considerably flouted chrysophanol-induced apoptosis in colorectal cancer (CRC) cells (Zhang et al. 2014). Taken together, chrysophanol exerts an anti-neoplastic effect under in vitro and in vivo conditions in CRC cells by modulating DCN, indicating its therapeutic potential in CRC cells (Zhang et al. 2014).
Chaetoglobosins (3–9) belong to cytochalasan alkaloids and constitute a large range of secondary fungal metabolites (Perlatti et al. 2020). Till date, more than 100 types of chaetoglobosins and their analogs have been isolated and characterized from a wide range of fungi, including Chaetomium elatum (Soytong et al. 2021), Chaetomium globosum (Darwish et al. 2020), Phomopsis sp. (Zhu et al. 2021), Botryosphaeria dothidea (Carvalho et al. 2019), and Chaetomium subaffine (Liu et al. 2021). Chaetoglobosins reportedly exhibit wide spectrum antitumor activity. They reportedly inhibited cell lines L929 (murine fibroblast cell line), KB3.1 (human epidermoid carcinoma cell line), PC-3 (human prostatic carcinoma cell line), and HUVEC (human umbilical vein endothelial cell line) with IC50 values of 1.6, 0.15, 0.42, and 0.78 μg/mL, respectively (Flewelling et al. 2015). Li et al. (2014) reported cytotoxic effect of chaetoglobosins towards HL60 (human promyelocytic cell line), A549 (hypotriploid human cell line), SMMC7721 (human hepatocarcinoma cell line), MCF-7 (human breast cancer cell line), and SW480 (human colon cancer cell line) cell lines with an inhibitory activity ratio range of 51–96% at 40 μmol/L (Li et al. 2014). In addition, MDA-MB-435 (human breast cancer cell line), SGC-7901 (human gastric cancer cell line), and A549 cell lines were found to be inhibited with IC50 values of 4.65, 5.32, and 8.73 μmol/L, respectively (Tikoo et al. 1999).
In 2013, Zhang et al. reported that chaetoglobosin A (3), C (5), D (6), E (7), G (9) and R (10) can inhibit the growth of two phytopathogenic fungi, Rhizopus stolonifer and Coniella diplodiella (Zhang et al. 2013). Multiple studies have shown that there is significant antibacterial potential of chaetoglobosins towards agricultural pathogens. Zhu et al. (2017) displayed that penochalasin K, a novel chaetoglobosin isolated from Penicillium chrysogenum V11, had an effective antimicrobial activity towards Colletotrichum gloeosporioides, with an IC50 value of 6.13 μmol/L (Zhu et al. 2017). Gao et al. (2019) reported a stronger antimicrobial activity of armochaetoglobosin C, a 1′-N-methyl-chaetoglobosin, towards Klebsiella pneumoniae (MIC = 4.0 μg/mL) as compared to commonly used antibiotic meropenem (MIC = 8 μg/mL) (Gao et al. 2019). All these reports point at potential application of these compounds in agricultural and clinical aspects.
Chetomin (11), a metabolite compound produced by genus Chaetomium, was reported to inhibit tumor growth via blockade of hypoxia-inducible transcription (Telarovic et al. 2021). Chetomin (11) changes the confirmation of CH1 domain of p300, a transcriptional coactivator, thereby reducing the communication between p300 and HIF-1α (Telarovic et al. 2021). Li et al. (2013) isolated three novel endophytic Chaetomium globosum TY1 azaphilone alkaloids, namely chaetomugilides A–C (18–20), that reported high cytotoxic activity towards HePG2 (human cancer cell line) with IC50 values ranging from 1.7 to 53.4 μM (Li et al. 2013). Two phytotoxic azaphilone derivatives, chaetomugiline D and chaetomugiline J, isolated from Amaranthus viridis derived C. globosum exhibited phytotoxic activity in seed germination bioassay of Lactuca sativa (Piyasena et al. 2015). The root growth inhibition IC50 values of chaetomugiline D and chaetomugiline J were 24.2 and 22.6 ppm, respectively, whereas the shoot growth inhibition IC50 values were 27.8 and 21.9 ppm, respectively (Piyasena et al. 2015). Recently, a novel azaphilone, chaephilone C (21), isolated from Polygonatum sibiricum derived C. globosum displayed cytotoxicity against HepG-2 cells with IC50 values of 38.6 µM (Song et al. 2020).
In 1990, Takahashi et al. extracted four novel azaphilones, namely chaetoviridins A–D (13–16), from C. globosum culture and reported their structure (TAKAHASHI et al. 1990). In 2009, a revised structure of chaetoviridin B and D was proposed, along with the structure of chaetoviridin E (17) (Kingsland and Barrow 2009). Youn et al. (2015) isolated two novel azaphilones, chaetoviridins J and K, from Wikstroemia uva-ursi derived C. globosum (Youn et al. 2015). Chetoviridin J presented encouraging results when assessed for cancer chemo-preventive-potential based on the capacity to prevent TNF-α-induced nuclear factor-kappa B (NF-κB) (Youn et al. 2015).
Secondary metabolites from Chaetomium cupreum
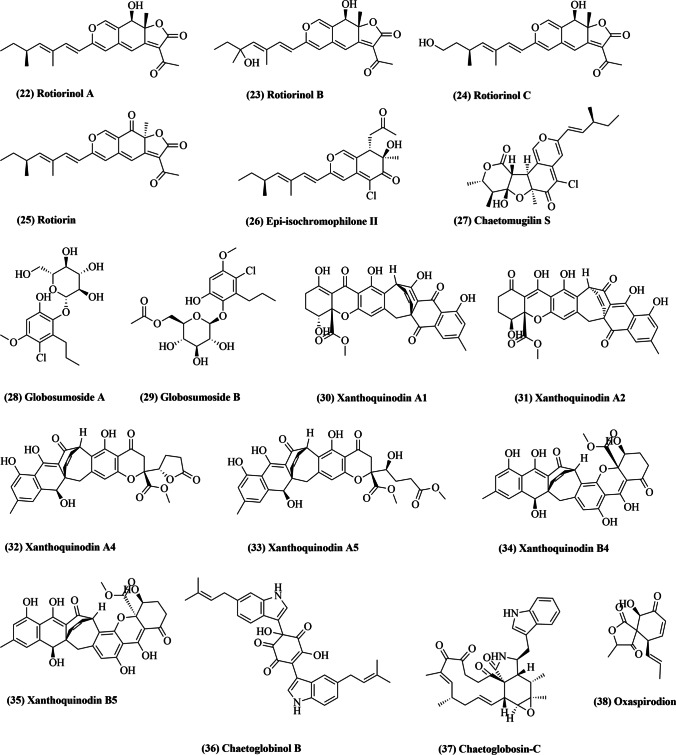
Chaetomium cupreum, a member the family, Chaetomiaceae, can grow on synthetic cellulosic materials and is known to infect a broad variety of soil microorganisms. C. cupreum is mesophilic and rapidly colonizes organic substances in soil (Dionizio et al. 2022). Broad spectrum fungicides obtained from several C. cupreum and C. globosum strains reportedly induce tolerance against Phytophthora palmivora, Phytophthora parasitica, Fusarium oxysporum, and Sclerotium rolfsii (Soytong et al. 2021). Also, C. cupreum RY202 crude extracts in hexane, ethyl acetate, and methanol with ED50 values of 170, 402, and 1220 μg/L, respectively, prevent the growth of Rigidoporus microporus, the causative agent of white root disease in rubber trees (Kaewchai and Soytong 2010). Moreover, rotiorinol, a bioactive compound formed by C. cupreum, inhibits the growth of R. microporus with ED50 value of 26 μg/L (Kaewchai and Soytong 2010).
In 2006, three novel azaphilones, rotiorinols A-C (22–24; Fig. 2), and two additional stereoisomers, (-)-rotiorin (25) and epi-isochromophilone II (26), were isolated from C. cupreum (Kanokmedhakul et al. 2006). Rotiorinol A (22), C (24), and (-)-rotiorin (25) displayed strong antifungal activity against Candida albicans with IC50 values of 10.5, 16.7, and 24.3 µg/mL, respectively (Kanokmedhakul et al. 2006). In 2015, two novel angular azaphilone forms, isochromophilonol and ochrephilonol, were isolated from C. cupreum (Panthama et al. 2015). Both novel compounds displayed mild cytotoxicity against KB (epidermoid carcinoma) and NCI-H187 (lung cancer) cell lines, with IC50 value ranging between 9.63 and 32.42 μg/mL (Panthama et al. 2015).
Fig. 2.
Some of the compounds isolated from Chaetomium cupreum (22–26), Chaetomium elatum (27–37), and Chaetomium subspirale (38)
In vitro analysis suggested that C. cupreum, C. globosum and C. lucknowense induce an inhibitory effect on growth parameters of Phytophthora palmivora, the causative organism of root rot in Citrus maxima (Hung et al. 2015). In another experiment, C. cupreum extracts in hexane, ethyl acetate and methanol demonstrated substantial inhibition of Colletotrichum gloeosporioides (an anthracnose pathogen), with ED50 values of 13, 11, and 28 ppm, respectively (Vilavong and Soytong 2017). In addition, use of powdered, nano-rotiorinol, nano-trichotoxin, and a spore suspension of C. cupreum reduced anthracnose by 54.77%, 46.23%, 42.71%, and 18.59%, respectively (Vilavong and Soytong 2017). An analysis of antioxidant properties of different organic solvent extracts of C. cupreum was conducted by Wani and Tirumale (2018) by employing multiple antioxidant assays (Wani and Tirumale 2018). The results indicated at significant antioxidant activity of C. cupreum and warranted further experimental evaluation (Wani and Tirumale 2018).
Secondary metabolites from Chaetomium elatum
Chaetomium elatum is a significant saprotrophic mold-fungus of the Chaetomiaceae family, believed to grow on many different substances worldwide (Moya et al. 2020). It was discovered by Gustav Kunze on dead leaves. The distinguishing characteristic of this fungus is its extremely coarse coating.
C. elatum has been isolated from various products, and many studies have investigated its biochemical properties for possible biotechnological applications. Thohinung et al. (2010) extracted anticancer fungal 10-(Indol-3-yl)-[13] cytochalasans from endophytic C. elatum, reporting possible cytotoxicity against human breast cancer (IC50 2.54–21.29 μM) and cholangiocarcinoma (IC50 3.41–86.95 μM) cell lines (Thohinung et al. 2010). In 2012, three new azaphilones, chaetomugilin S (27; Fig. 2), 7,5′-bis-epi-chaetoviridine A and 7-epi-chaetoviridine E were isolated from C. elatum raw extract (Chen et al. 2012). These three compounds were the first examples of 7R-configured azaphilones with a chlorinated isochromen, found in Chaetomium species. They displayed inhibitory activity in the caspase-3 enzymatic assay, with IC50 values of 20.6, 10.9, and 7.9 μM, respectively (Chen et al. 2012). Additionally, two new chlorinated phenolic glycosides, globosumoside A (28) and globosumoside B (29), were also isolated (Chen et al. 2012). In yet another study, five new xanthoquinodins, A4-A6 (32, 33), B4 (34), and B5 (35), along with three existing xanthoquinodins, A1-A3 (30, 31), were isolated from methanolic extracts of the endolichenic fungal strain C. elatum (Chen et al. 2013). The cytotoxic activity of all compounds was tested against various human cancer cell lines (HL-60, SMMC7721, A-549, MCF-7, and SW480), and xanthoquinodin A6 displayed significant activity with IC50 values of 3.75, 2.87, 2.04, 5.64, and 6.44 μM, respectively (Chen et al. 2013).
In 2019, Yao et al. isolated a new compound, chaetoglobinol B (36), from C. elatum solid-state fermented rice culture (Yao et al. 2019). It reportedly inhibited α-glycosidase at a 2.5 mg/mL concentration (Yao et al. 2019). A highly virulent isolate from Fusarium oxysporum f. sp. Lycopersici, responsible for causing wilt of tomato, was reported to be effectively inhibited by C. elatum, C. lucknowense, and Emericella rugulosa strains (Sibounnavong et al. 2011). Chaetoglobosin-C (37) was identified as the compound responsible for antifungal activity, with ED50 of 5.98 μg/ml (Sibounnavong et al. 2011). The study further indicated that antibiosis was involved in the process of disease prevention by these antagonistic fungi.
Secondary metabolites from Chaetomium subspirale
Chaetomium subspirale fungi belongs to phylum Ascomycota (Netz 2019). It was discovered by A.H. Chivers in 1912, America. The organism has sexual fruiting bodies decorated with signature, coiled hairs, which give it a puffy look. C. subspirale has brown colored colonies and is usually found in various types of soil and dung (Attia and Abdel-Azeem 2020).
C. subspirale generates mycotoxin oxaspirodion (38; Fig. 2) that inhibits TNF expression and stimulation of the NF-κB transcription factor (Abdel-Azeem et al. 2019). Oxaspirodion (38) inhibits the expression of the TNF-α-driven luciferase reporter gene with an IC50 value of 2.5 μg/ml in TPA-/ionomycin-stimulated Jurkat T-cells by intervening in phosphorylation of the ERK1/2 kinases (Abdel-Azeem et al. 2019). In addition, oxaspirodion (38) also inactivated the transcription factor NF-κB, which is implicated in inducible expression of several pro-inflammatory genes (Abdel-Azeem et al. 2019).
Secondary metabolites from Chaetomium olivaceum
In 1961, a report stated that after a week at 21 °C, a culture of Chaetomium fruited on Pablum-cereal-agar abundantly, and it was described as being nearest to Chaetomium olivaceum (Macлиeнкo 2019).
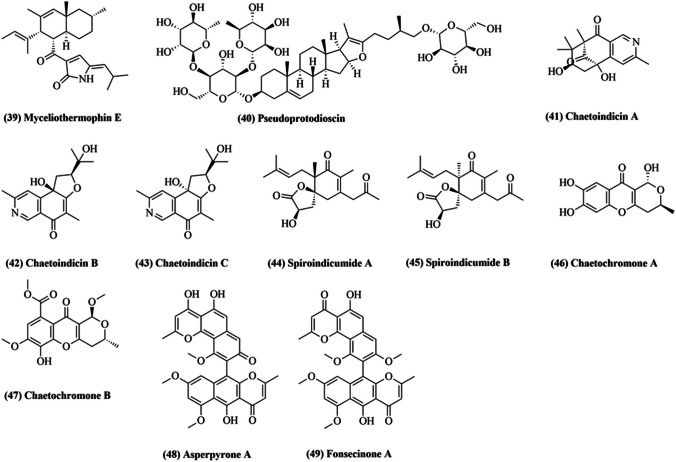
In 2003, El-Gindy and Saad purified enzyme exo-1,4-beta-glucanase from C. olivaceum, achieving 72.8% yield (El-Gindy et al. 2003). The enzyme’s optimum pH was reported to be 5.2, while maximal activity was at 45 °C. Km value was 0.65 mg/mL against alpha-cellulose (El-Gindy et al. 2003). Recently, three new polyketides, Chaetolivacines A–C, and one known compound, myceliothermophin E (39; Fig. 3), were isolated from C. olivaceum (Wang et al. 2020b). Chaetolivacines B and myceliothermophin E (39) displayed moderate activity when tested against Staphylococcus aureus for their antibacterial properties (Wang et al. 2020b).
Fig. 3.
Some of the compounds isolated from Chaetomium olivaceum (39, 40), Chaetomium indicum (41–47), and Chaetomium nigricolor (48, 49)
Five novel pseudoprotodioscin (40) derivatives were obtained via pseudoprotodioscin transformation by C. (Dong et al. 2016). Pseudoprotodioscin and its derivatives were examined for their beneficial role against H2O2-mediated myocardial cell injury. It was confirmed that a few derivatives improved the efficacy of H9c2 mediated by H2O2 in the concentration range from 3125 to 25 g/mL in a dose-dependent way (Dong et al. 2016).
Secondary metabolites from Chaetomium indicum
The Chaetomium indicum group has ascomata hair branched dichotomously (Kedves et al. 2021). The presence of multiple hair variant types has complicated the classification of C. indicum and related organisms. Skolko and Grover (1948) focused on the presence/absence of branched hair characters and unbranched terminal hairs (Wang et al. 2016). Burtseva et al. (2000) successfully isolated beta-1,3-glucanase from marine C. indicum (Burtseva et al. 2000).
Li et al. (2006a, b) isolated Chaetoindicins A − C (41–43; Fig. 3), three isoquinolines with novel skeletons, from a fermented solid-state culture of C. indicum (Li et al. 2006a). Two novel spironolactone polyketides, spiroindicumides A (44) and B (45), were isolated from C. indicum with the help of histone deacetylase inhibitor (Asai et al. 2013). In 2013, two novel polyketides, Chaetochromones A (46) and B (47), were discovered along with three known analogs, PI-3, PI-4, and SB236050, from the crude fungal extract of C. indicum (Lu et al. 2013). The biological activities of all the isolated bioactive compounds was tested against eight plant pathogens, namely Alternaria alternata, Ilyonectria radicicola, Trichoderma viride pers, Aspergillus niger, Fusarium verticillioides, Irpex lacteus (Fr.), Poria placenta (Fr.) Cooke, and Coriolus versicolor (L.) Quél (Lu et al. 2013). Chaetochromone A (46) showed high inhibitory activity (> 60%) against Poria placenta (Fr.) Cooke, a brown rot fungus responsible for wood decay (Lu et al. 2013). However, the cytotoxic activities were also tested against A549, MDA-MB-231, and PANC-1 cancer cell lines, without finding any inhibitory activities (Lu et al. 2013).
Secondary metabolites from Chaetomium nigricolor
Chaetomium nigricolor is similar to Ovatospora members, with respect to ascospore morphology (Abdel-Azeem et al. 2021). C. nigricolor ascopore differs in being attenuated at one end and apiculate at the other end, while Ovatospora ascopores are attenuated at one and round at the other end (Abdel-Azeem et al. 2021).
Chamiside A, a novel cytochalasan with a new 6/6/5-fused tricyclic core skeleton, was isolated from Mahonia fortunei derived C. nigricolor (Wang et al. 2019). Chamiside A was found to display antibacterial activity against Staphylococcus aureus (Wang et al. 2019). In another study, Catharanthus roseus derived C. nigricolor was reported to exhibit potent cytotoxic, apoptotic, and antioxidant properties (Dhayanithy et al. 2019). Recently, twelve secondary metabolites from C. nigricolor were isolated, including a new furan derivative, methyl succinyl-sumiki’s acid, and two novel atropisomers of bis-naphtho-γ-pyrones, (aS)-asperpyrone A (48; Fig. 3) and (aS)-fonsecinone A (49) (Kim et al. 2020). The two atropisomers inhibited nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophages (Kim et al. 2020). Additionally, (aS)-asperpyrone A (48) reportedly inhibited NF-κB, consequently, suppressing pro-inflammatory mediators and cytokine release (Kim et al. 2020).
Secondary metabolites from other Chaetomium sp
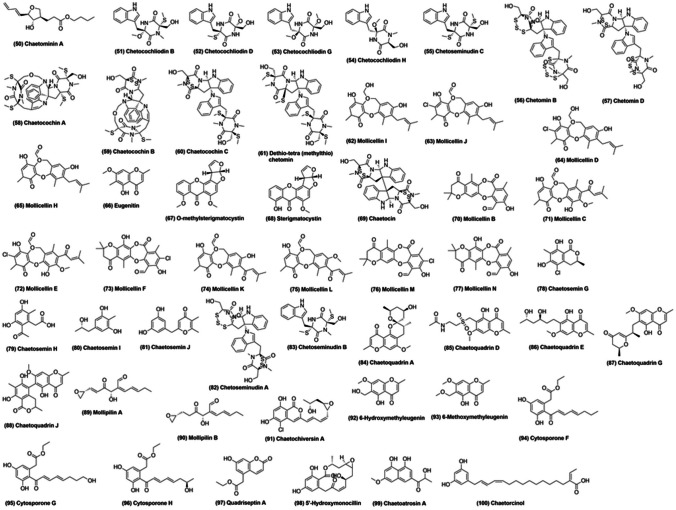
In 1992, precursors of chaetoglobosin A, viz., prochaetoglobosins I, II, III, and IV, were isolated from Chaetomium subaffin, an endophytic potato fungus (Oikawa et al. 1992). In another study, C. subaffin was used to isolate gliocladinin C (a natural p-terphenyl glycoside) and two furano-polyene derivatives, chaetominins A (50; Fig. 4) and B. Gliocladinin C and chaetominin A (50) reportedly inhibited human tumor cell lines, Hep-2 and HepG-2 (Han et al. 2019).
Fig. 4.
Some of the compounds isolated from various Chaetomium species
Recently, Wang et al. (2020a, b) extracted nine novel epipolythiodioxopiperazine analogs, chetocochliodins A-I (51–54), along with two existing ones, chetoseminudins E and C, from Chaetomium cochliodes (Wang et al. 2020a). Chetocochliodin I was shown to inhibit cancerous cell lines (Wang et al. 2020a). In another study, the same group worked on C. cochliodes and reported cytotoxic activity of four novel chetomin analogues, chetomins A–D (56, 57), against HepG2, MCF-7, and HeLa cancer cell lines (Wang et al. 2018). In 2006, three new epipolythiodioxopiperazines, chaetocochins A–C (58–60), along with dethio-tetra (methylthio) chetomin (61) and chetomin, were obtained from C. cochliodes (Li et al. 2006b). Chaetocochin A (58), C (60) and chetomin demonstrated substantial inhibitory activity towards Bre-04 (breast cancer), Lu-04 (lung cancer), and N-04 (neuroma) cell lines (Li et al. 2006b).
An ethyl acetate extract of Chaetomium brasiliense was used to isolate three novel compounds, mollicellin I (62; Fig. 4), mollicellin J (63), and 2-(hydroxymethyl)-6-methylmethyleugenin, along with six known compounds, mollicellin D (64), mollicellin H (65), eugenetin (66), o-methylsterigmatocystin (67), sterigmatocystin (68), and chaetocin (69) (Li et al. 2008). Mollicellins I (62) and H (65) demonstrated inhibitory activity against three human cancer cell lines, Bre-04, Lu-04, and N-04, with GI50 in the range of 2.5–8.6 μg/mL (Li et al. 2008). In another report, four novel compounds, mollicellins K-N (74–77), and six known compounds, mollicellins B (70), C (71), E (72), F (73), H (65), and J (63), were derived from C. brasiliense and exhibited cytotoxic activity against various cancerous cell lines (Khumkomkhet et al. 2009). In addition, mollicellin K (74) also displayed antimycobacterial and antifungal activities against Mycobacterium tuberculosis and Candida albicans, respectively (Khumkomkhet et al. 2009). Interestingly, mollicellins B (70), C (71), E (72), J (63), K (74), L (75), and M (76) were reported to exhibit antimalarial activity against Plasmodium falciparum (Khumkomkhet et al. 2009).
Li et al. (2018) isolated numerous secondary metabolites from Chaetomium seminudum and suggested monaschromone as a potent pesticide based on in vitro analysis (Li et al. 2018). Also, they reported epicoccone B and falvipin as better α-glucosidase inhibitors as compared to the standard drug acarbose (Li et al. 2018). Four novel compounds, chaetosemins G-J (78–81), were also isolated during the same study and chaetosemin J (81) was found to inhibit various plant pathogenic fungi such as Botrytis cinerea, Alternaria solani, Magnaporthe oryzae, and Gibberella saubinettii (Li et al. 2018). In 2004, three novel metabolites, chetoseminudins A (82), B (83), and C (55), were isolated from C. seminudum along with the known epipolythiodioxopiperazine, chetomin (Fujimoto et al. 2004). Chetoseminudin A (82) and chetomin were found to be responsible for the characteristic immunosuppressive features of C. seminudum (Fujimoto et al. 2004).
Eleven chaetoquadrins were derived from an EtOAc extract of ascomycetes Chaetomium quadrangulatum by Fujimoto et al. (2003) (Fujimoto et al. 2003). Off them, five (chaetoquadrins A-E; 84–86) were previously reported by the same group (Fujimoto et al. 2002), while six novel compounds (chaetoquadrins F-K; 87, 88) were found in this study (Fujimoto et al. 2003). Chaetoquadrins A–E, G, and H were found to exhibit monoamine oxidase inhibitory activity (Fujimoto et al. 2002, 2003). In 2012, Chaetomium mollipilium culture, in the presence of nicotinamide, a HDAC inhibitor, led to isolation of five novel C13-polyketides, mollipilin A–E (89, 90) (Asai et al. 2012). Mollipilins A (89) and B (90) were reported to mildly inhibit human colon cancer cell line, HCT-116 (Asai et al. 2012).
Two new isocoumarins, chaetochiversins A (91) and B, and four known compounds radicicol, 6-hydroxymethyleugenin (92), eugenetin (66), and 6-methoxymethyleugenin (93), were identified from the endophyte Chaetomium chiversii as Hsp90 inhibitors (Turbyville et al. 2006; Wang et al. 2008; Wijeratne et al. 2006). Chaetoatrosin A (99), a novel inhibitor of chitin synthase II (IC50 = 104 μg/ml), was isolated from Chaetomium atrobrunneum and reportedly displayed antifungal activity against Rhizoctonia solani, Pyricularia oryzae, Botrytis cinerea, Cryptococcus neoformans, and Trichophyton mentagrophytes (Hwang et al. 2000). In another interesting study, two novel, thermostable β-glucosidases of the GH3 family, were isolated from C. atrobrunneum and suggested as enzyme mixture components for better cellulose saccharification at high temperatures (Colabardini et al. 2016). Kabbaj et al. (2015) successfully isolated a novel compound, chaetorcinol (100), along with five known compounds, ( +)-sclerotiorin, ( +)-sclerotioramin, ( +)-isochromophilone IV, ( +)-isochromophilone VII, and SB 236,050 (Kabbaj et al. 2015). Sclerotiorin was reported to be an efficient Hsp90 inhibitor, while deacetylated sclerotiorin displayed inhibitory activity towards breast cancer (Hs578T, MDA-MB-231) and prostate cancer (LNCaP) cell lines (Kabbaj et al. 2015).
Conclusion and future perspectives
More than 200 bioactive compounds, belonging to various classes such as azaphilones, cytochalasan alkaloids, hydroxyanthraquinones, polyketides, xanthoquinodin, glycosides, spiro-compounds, terpenoids, steroid saponin, and chromones, have been discovered as secondary metabolites in various Chaetomium species. These compounds are known to exhibit anticancer, antiviral, antibacterial, and antifungal activities, making them potential candidates for therapeutic and drug development efforts. However, there are many challenges in studying Chaetomium species. Firstly, multiple species are known to dwell in different environments, leading to difficulties in procurement and fermentation. Also, large-scale extraction of bioactive compounds is required for validation of their activity in animal models. Moreover, determining and analyzing the stereochemistry of isolated bioactive compounds may prove to be tedious. Additionally, once the structure has been determined, it is a tough ask to extrapolate it to the various known activities of the compound. Also, new strategies need to be developed to analyze and optimally interpret the interaction between Chaetomium and other species.
Despite these hurdles, further research on Chaetomium species might elucidate yet unknown secondary metabolites. A deeper analysis of their biosynthetic pathways, pharmacokinetic properties, structure–activity relationships, mechanism of action, and ecological roles might highlight more candidates against multiple cancers and other disorders.
Acknowledgements
The authors are grateful to the scientific community.
Author contribution
VD: Original draft preparation. SKR: Conceptualization; methodology; supervision. SJ: Review and editing; formal analysis and investigation. NMA: Formal analysis and investigation. RP: Review and editing. SS: Review and editing. LRS: Conceptualization; methodology; supervision.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Santosh Kumar Rath, Email: skrath1985@gmail.com, Email: santoshk.rath@dituniversity.edu.in.
Leonardo Rios-Solis, Email: leo.rios@ncl.ac.uk.
References
- Abdel-Azeem AM (2020) Taxonomy and biodiversity of the genus Chaetomium in different habitats Recent Developments on Genus Chaetomium. Springer, pp 3–77
- Abdel-Azeem AM. Recent developments on genus Chaetomium. Springer; 2019. [Google Scholar]
- Abdel-Azeem AM, Abu-Elsaoud AM, Abo Nahas HH, Abdel-Azeem MA, Balbool BA, Mousa MK, Ali NH, Darwish AM (2021) Biodiversity and industrial applications of genus Chaetomium Industrially Important Fungi for Sustainable Development. Springer, pp 147–206
- Abdel-Azeem AM, Abdel-Azeem MA, Khalil WF (2019) Endophytic fungi as a new source of antirheumatoid metabolites Bioactive Food as dietary interventions for arthritis and related inflammatory diseases. Elsevier, pp 355–384
- Abou Alhamed M, Shebany Y. Endophytic Chaetomium globosum enhances maize seedling copper stress tolerance. Plant Biol. 2012;14(5):859–863. doi: 10.1111/j.1438-8677.2012.00608.x. [DOI] [PubMed] [Google Scholar]
- Akkol EK, Tatlı II, Karatoprak GŞ, Ağar OT, Yücel Ç, Sobarzo-Sánchez E, Capasso R. Is emodin with anticancer effects completely innocent? Two Sides of the Coin Cancers. 2021;13(11):2733. doi: 10.3390/cancers13112733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancheeva E, Daletos G, Proksch P. Bioactive secondary metabolites from endophytic fungi. Curr Med Chem. 2020;27(11):1836–1854. doi: 10.2174/0929867326666190916144709. [DOI] [PubMed] [Google Scholar]
- Asai T, Morita S, Shirata N, Taniguchi T, Monde K, Sakurai H, Ozeki T, Oshima Y. Structural diversity of new C13-polyketides produced by Chaetomium mollipilium cultivated in the presence of a NAD+-dependent histone deacetylase inhibitor. Org Lett. 2012;14(21):5456–5459. doi: 10.1021/ol302539s. [DOI] [PubMed] [Google Scholar]
- Asai T, Taniguchi T, Yamamoto T, Monde K, Oshima Y. Structures of spiroindicumides A and B, unprecedented carbon skeletal spironolactones, and determination of the absolute configuration by vibrational circular dichroism exciton approach. Org Lett. 2013;15(17):4320–4323. doi: 10.1021/ol401741z. [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discovery. 2021;20(3):200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia EA, Abdel-Azeem AM (2020) Chaetomium in indoor environment and medically important species of Chaetomium Recent Developments on Genus Chaetomium. Springer, pp161–173
- Burtseva YV, Sova V, Pivkin M, Zvyagintseva T. Enzymes of carbohydrate metabolism of mycelial fungi from marine environments beta-1, 3-glucanase of the marine fungus Chaetomium indicum. Biochemistry Biokhimiia. 2000;65(10):1175–1183. [PubMed] [Google Scholar]
- Calaça FJS, Xavier-Santos S, Abdel-Azeem AM (2020) Recent advances on occurrence of genus Chaetomium on dung. Recent Developments on Genus Chaetomium, pp 143–159
- Cao D, Sun P, Bhowmick S, Wei Y, Guo B, Wei Y, Mur LAJ, Sun Z (2021) Secondary metabolites of endophytic fungi isolated from Huperzia serrata. Fitoterapia 155:104970. 10.1016/j.fitote.2021.104970 [DOI] [PubMed]
- Carvalho CRd, Ferreira MC, Amorim SS, Silva Florindo RHd, Assis JCSd, Zani CL, Rosa LH (2019) Bioactive compounds of endophytic fungi associated with medicinal plants Recent Advancement in White Biotechnology Through Fungi. Springer, pp 303–361
- Chen G-D, Li Y-J, Gao H, Chen Y, Li X-X, Li J, Guo L-D, Cen Y-Z, Yao X-S. New azaphilones and chlorinated phenolic glycosides from Chaetomium elatum with caspase-3 inhibitory activity. Planta Med. 2012;78(15):1683–1689. doi: 10.1055/s-0032-1315211. [DOI] [PubMed] [Google Scholar]
- Chen G-D, Chen Y, Gao H, Shen L-Q, Wu Y, Li X-X, Li Y, Guo L-D, Cen Y-Z, Yao X-S. Xanthoquinodins from the endolichenic fungal strain Chaetomium elatum. J Nat Prod. 2013;76(4):702–709. doi: 10.1021/np400041y. [DOI] [PubMed] [Google Scholar]
- Colabardini AC, Valkonen M, Huuskonen A, Siika-Aho M, Koivula A, Goldman GH, Saloheimo M. Expression of two novel β-glucosidases from Chaetomium atrobrunneum in Trichoderma reesei and characterization of the heterologous protein products. Mol Biotechnol. 2016;58(12):821–831. doi: 10.1007/s12033-016-9981-7. [DOI] [PubMed] [Google Scholar]
- Darwish AM, Abdelmotilib NM, Abdel-Azeem AM, Abo Nahas HH, Mohesien MT (2020) Applications of Chaetomium functional metabolites with special reference to antioxidants Recent Developments on Genus Chaetomium. Springer, 227–240
- Dhayanithy G, Subban K, Chelliah J. Diversity and biological activities of endophytic fungi associated with Catharanthus roseus. BMC Microbiol. 2019;19(1):1–14. doi: 10.1186/s12866-019-1386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionizio BS, Rabelo CABS, de Jesus HCR, Varesche MBA, de Souza DHF (2022) The deconstruction of the lignocellulolytic structure of sugarcane bagasse by laccases improves the production of H2 and organic acids. Appl Biochem Biotechnol 194(7):3145–3166 [DOI] [PubMed]
- Dong X, Gao Z, Hu H, Gao R, Sun D. Microbial transformation of pseudoprotodioscin by Chaetomium olivaceum. J Mol Catal B Enzym. 2016;130:88–95. doi: 10.1016/j.molcatb.2016.05.001. [DOI] [Google Scholar]
- Dwibedi V, Saxena S. Arcopilus aureus, a resveratrol-producing endophyte from Vitis vinifera. Appl Biochem Biotechnol. 2018;186(2):476–495. doi: 10.1007/s12010-018-2755-x. [DOI] [PubMed] [Google Scholar]
- Dwibedi V, Saxena S. Diversity and phylogeny of resveratrol-producing culturable endophytic fungi from Vitis species in India. 3 Biotech. 2019;9(5):1–8. doi: 10.1007/s13205-019-1712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwibedi V, Saxena S. In vitro anti-oxidant, anti-fungal and anti-staphylococcal activity of resveratrol-producing endophytic fungi. Proceedings of the Nat Acad Sci, India Section b: Biol Sci. 2020;90(1):207–219. doi: 10.1007/s40011-019-01098-6. [DOI] [Google Scholar]
- Dwibedi V, Jain S, Singhal D, Mittal A, Rath SK, Saxena S (2022) Inhibitory activities of grape bioactive compounds against enzymes linked with human diseases. Applied Microbiology and Biotechnology:1–19 [DOI] [PubMed]
- El-Gindy A, Saad R, Fawzi E. Purification and some properties of exo-1, 4-beta-glucanase from Chaetomium olivaceum. Acta Microbiol Pol. 2003;52(1):35–44. [PubMed] [Google Scholar]
- Elkhateeb WA, Kolaibe A, Elnahas MO, Daba GM (2021) Highlights on Chaetomium morphology, secondary metabolites and biological activates. J Pharm Pharmacol Res. Available online. 10.31579/2693-7247/030
- Flewelling AJ, Bishop AL, Johnson JA, Gray CA (2015) Polyketides from an endophytic Aspergillus fumigatus isolate inhibit the growth of Mycobacterium tuberculosis and MRSA. Natural product communications 10(10):1934578X1501001009 [PubMed]
- Fujimoto H, Nozawa M, Okuyama E, Ishibashi M. Five new chromones possessing monoamine oxidase inhibitory activity from an ascomycete. Chaetomium Quadrangulatum Chem Pharmaceut Bull. 2002;50(3):330–336. doi: 10.1248/cpb.50.330. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Nozawa M, Okuyama E, Ishibashi M. Six new constituents from an ascomycete, Chaetomium quadrangulatum, found in a screening study focused on monoamine oxidase inhibitory activity. Chem Pharm Bull. 2003;51(3):247–251. doi: 10.1248/cpb.51.247. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Sumino M, Okuyama E, Ishibashi M. Immunomodulatory Constituents from an Ascomycete, Chaetomium s eminudum. J Nat Prod. 2004;67(1):98–102. doi: 10.1021/np0302201. [DOI] [PubMed] [Google Scholar]
- Gao W, He Y, Li F, Chai C, Zhang J, Guo J, Chen C, Wang J, Zhu H, Hu Z. Antibacterial activity against drug-resistant microbial pathogens of cytochalasan alkaloids from the arthropod-associated fungus Chaetomium globosum TW1-1. Bioorg Chem. 2019;83:98–104. doi: 10.1016/j.bioorg.2018.10.020. [DOI] [PubMed] [Google Scholar]
- Guo Q-f, Chen J-H, Yin Z-h, Zhang J-j, Zeng J, Liu Q-g, Yang B-c, Chen L. Secondary metabolites of a desert soil-derived fungus Chaetomium madrasense 375. Chem Nat Compd. 2022;58(3):534–537. doi: 10.1007/s10600-022-03727-5. [DOI] [Google Scholar]
- Han XY, Xie YX, Wu CQ, Ai HL, Lei XX, Wang XJ. Novel metabolites from the endophytic fungus Chaetomium subaffine L01. Chem Biodivers. 2019;16(12):e1900471. doi: 10.1002/cbdv.201900471. [DOI] [PubMed] [Google Scholar]
- Horvat M, Avbelj M, Durán-Alonso MB, Banjanac M, Petković H, Iskra J. Antiviral activities of halogenated emodin derivatives against human coronavirus NL63. Molecules. 2021;26(22):6825. doi: 10.3390/molecules26226825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung PM, Wattanachai P, Kasem S, Poaim S. Biological control of Phytophthora palmivora causing root rot of pomelo using Chaetomium spp. Mycobiology. 2015;43(1):63–70. doi: 10.5941/MYCO.2015.43.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E-I, Yun B-S, Kim Y-K, Kwon B-M, Kim H-G, Lee H-B, Bae K-S, Kim S-U. Chaetoatrosin A, a novel chitin synthase II inhibitor produced by Chaetomium atrobrunneum F449. J Antibiot. 2000;53(3):248–255. doi: 10.7164/antibiotics.53.248. [DOI] [PubMed] [Google Scholar]
- Ibrahim SRM, Mohamed SGA, Sindi IA, Mohamed GA. Biologically active secondary metabolites and biotechnological applications of species of the family Chaetomiaceae (Sordariales): an updated review from 2016 to 2021. Mycol Prog. 2021;20(5):595–639. doi: 10.1007/s11557-021-01704-w. [DOI] [Google Scholar]
- Kabbaj FZ, Lu S, Faouzi MEA, Meddah B, Proksch P, Cherrah Y, Altenbach H-J, Aly AH, Chadli A, Debbab A. Bioactive metabolites from Chaetomium aureum: structure elucidation and inhibition of the Hsp90 machine chaperoning activity. Bioorg Med Chem. 2015;23(1):126–131. doi: 10.1016/j.bmc.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewchai S, Soytong K. Application of biofungicides against Rigidoporus microporus causing white root disease of rubber trees. J Agricul Technol. 2010;6(2):349–363. [Google Scholar]
- Kanokmedhakul S, Kanokmedhakul K, Nasomjai P, Louangsysouphanh S, Soytong K, Isobe M, Kongsaeree P, Prabpai S, Suksamrarn A. Antifungal azaphilones from the fungus Chaetomium c upreum CC3003. J Nat Prod. 2006;69(6):891–895. doi: 10.1021/np060051v. [DOI] [PubMed] [Google Scholar]
- Kedves O, Kocsubé S, Bata T, Andersson MA, Salo JM, Mikkola R, Salonen H, Szűcs A, Kedves A, Kónya Z. Chaetomium and chaetomium-like species from European indoor environments include Dichotomopilus finlandicus sp. nov. Pathogens. 2021;10(9):1133. doi: 10.3390/pathogens10091133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol. 2019;17(3):167–180. doi: 10.1038/s41579-018-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khumkomkhet P, Kanokmedhakul S, Kanokmedhakul K, Hahnvajanawong C, Soytong K. Antimalarial and cytotoxic depsidones from the fungus Chaetomium brasiliense. J Nat Prod. 2009;72(8):1487–1491. doi: 10.1021/np9003189. [DOI] [PubMed] [Google Scholar]
- Kikiowo B, Ogunleye JA, Metibemu DS, Omotuyi I, Adelakun NS. Virtual screening and pharmacokinetic studies of potential MAO-B inhibitors from traditional Chinese medicine. J Biol Engin Res Rev. 2020;7(1):08–15. [Google Scholar]
- Kim MJ, Kim D-C, Kwon J, Ryu SM, Kwon H, Guo Y, Hong S-B, Kim Y-C, Oh H, Lee D. Anti-inflammatory metabolites from Chaetomium nigricolor. J Nat Prod. 2020;83(4):881–887. doi: 10.1021/acs.jnatprod.9b00560. [DOI] [PubMed] [Google Scholar]
- Kingsland SR, Barrow RA. Identification of chaetoviridin E from a cultured microfungus, Chaetomium sp and structural reassignment of chaetoviridins B and D. Aus J Chem. 2009;62(3):269–274. doi: 10.1071/CH08259. [DOI] [Google Scholar]
- Ko H-R, Kim B-Y, Ahn S-C, Oh W-K, Kim J-H, Lee H-S, Kim H-M, Han S-B, Mheen T-I, Ahn J-S. Chaetoglobosin A, an inhibitor of bleb formation on K562 cells induced by phorbol 12, 13-dibutyrate. J Microbiol Biotechnol. 1998;8(6):705–709. [Google Scholar]
- Li G-Y, Li B-G, Yang T, Liu G-Y, Zhang G-L. Chaetoindicins A− C, three isoquinoline alkaloids from the fungus Chaetomium indicum. Org Lett. 2006;8(16):3613–3615. doi: 10.1021/ol061525k. [DOI] [PubMed] [Google Scholar]
- Li G-Y, Li B-G, Yang T, Yan J-F, Liu G-Y, Zhang G-L. Chaetocochins A− C, epipolythiodioxopiperazines from Chaetomium cochliode s. J Nat Prod. 2006;69(9):1374–1376. doi: 10.1021/np0602970. [DOI] [PubMed] [Google Scholar]
- Li GY, Li BG, Yang T, Liu GY, Zhang GL. Secondary metabolites from the fungus Chaetomium brasiliense. Helv Chim Acta. 2008;91(1):124–129. doi: 10.1002/hlca.200890002. [DOI] [Google Scholar]
- Li X, Tian Y, Yang S-x, Zhang Y-m, Qin J-c. Cytotoxic azaphilone alkaloids from Chaetomium globosum TY1. Bioorg Med Chem Lett. 2013;23(10):2945–2947. doi: 10.1016/j.bmcl.2013.03.044. [DOI] [PubMed] [Google Scholar]
- Li H, Xiao J, Gao Y-Q, Tang JJ, Zhang A-L, Gao J-M. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in Ginkgo biloba, and their phytotoxic and cytotoxic activities. J Agric Food Chem. 2014;62(17):3734–3741. doi: 10.1021/jf500390h. [DOI] [PubMed] [Google Scholar]
- Li H, Tian J-M, Tang H-Y, Pan S-Y, Zhang A-L, Gao J-M. Chaetosemins A-E, new chromones isolated from an Ascomycete Chaetomium seminudum and their biological activities. RSC Adv. 2015;5(37):29185–29192. doi: 10.1039/C5RA00525F. [DOI] [Google Scholar]
- Li H, Liao Z-B, Tang D, Han W-B, Zhang Q, Gao J-M. Polyketides from two Chaetomium species and their biological functions. J Antibiot. 2018;71(7):677–681. doi: 10.1038/s41429-018-0047-x. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao J, Cao H. Study on the antifungal effect and mycolytic activity of the biocontrol agent Chaetomium subaffine LB-1. Plant Prot Sci. 2021;57(4):289–296. doi: 10.17221/65/2020-PPS. [DOI] [Google Scholar]
- Lu K, Zhang Y, Li L, Wang X, Ding G. Chaetochromones A and B, two new polyketides from the fungus Chaetomium indicum (CBS 860.68) Molecules. 2013;18(9):10944–10952. doi: 10.3390/molecules180910944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macлиeнкo Л (2019) Биoлoгичecкaя эффeктивнocть лaбopaтopныx oбpaзцoв микpoбиoпpeпapaтoв нa ocнoвe пepcпeктивныx штaммoв-пpoдyцeнтoв из poдoв Chaetomium и Bacillus пpoтив вoзбyдитeля лoжнoй мyчниcтoй pocы нa пoдcoлнeчникe. Macличныe кyльтypы(1 (177)):85–91
- Madbouly AK, Abdel-Wareth MT (2020) The use of Chaetomium taxa as biocontrol agents Recent Developments on Genus Chaetomium. Springer 251–266
- Moya P, Cipollone J, Sisterna M (2020) The fungal genus Chaetomium and its agricultural applications. Plant Defence: Bioll Control 289–308
- Muroga Y, Yamada T, Numata A, Tanaka R. Chaetomugilins I-O, new potent cytotoxic metabolites from a marine-fish-derived Chaetomium species. Stereochem Biol Act Tetrahed. 2009;65(36):7580–7586. doi: 10.1016/j.tet.2009.06.125. [DOI] [Google Scholar]
- Netz I (2019) Studien zur Totalsynthese von Oxaspirodion. Universitätsbibliothek Mainz. 10.25358/openscience-2352
- Oikawa H, Murakami Y, Ichihara A. Useful approach to find the plausible biosynthetic precursors of secondary metabolites using P-450 inhibitors: postulated intermediates of chaetoglobosin A. J Chem Soc, Perkin Transact. 1992;1(21):2949–2953. doi: 10.1039/p19920002949. [DOI] [Google Scholar]
- Ortega HE, Torres-Mendoza D, Caballero EZ, Cubilla-Rios L. Structurally uncommon secondary metabolites derived from endophytic fungi. Journal of Fungi. 2021;7(7):570. doi: 10.3390/jof7070570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthama N, Kanokmedhakul S, Kanokmedhakul K, Soytong K. Chemical constituents from the fungus Chaetomium cupreum RY202. Arch Pharmacal Res. 2015;38(5):585–590. doi: 10.1007/s12272-014-0418-1. [DOI] [PubMed] [Google Scholar]
- Perlatti B, Nichols CB, Lan N, Wiemann P, Harvey CJ, Alspaugh JA, Bills GF. Identification of the antifungal metabolite chaetoglobosin P from Discosia rubi using a Cryptococcus neoformans inhibition assay: Insights into mode of action and biosynthesis. Front Microbiol. 2020;11:1766. doi: 10.3389/fmicb.2020.01766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piyasena KNP, Wickramarachchi W, Kumar NS, Jayasinghe L, Fujimoto Y. Two phytotoxic azaphilone derivatives from Chaetomium globosum, a fungal endophyte isolated from Amaranthus viridis leaves. Mycology. 2015;6(3–4):158–160. doi: 10.1080/21501203.2015.1089332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda J, Díaz-González S, Díaz-Urbano M, Velasco P, Sacristán S. Fungal endophytes of Brassicaceae: molecular interactions and crop benefits. Front Plant Sci. 2022;13:932288. doi: 10.3389/fpls.2022.932288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rether J, Erkel G, Anke T, Sterner O (2004) Inhibition of inducible TNF-α expression by oxaspirodion, a novel spiro-compound from the ascomycete Chaetomium subspirale. Biol Chem 385(9):829–34 [DOI] [PubMed]
- Rokas A, Mead ME, Steenwyk JL, Raja HA, Oberlies NH. Biosynthetic gene clusters and the evolution of fungal chemodiversity. Nat Prod Rep. 2020;37(7):868–878. doi: 10.1039/C9NP00045C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, Chufang L, Jin Z, Zhenhua J, Haiming J. Lianhua Qingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustamova N, Bozorov K, Efferth T, Yili A. Novel secondary metabolites from endophytic fungi: synthesis and biological properties. Phytochem Rev. 2020;19(2):425–448. doi: 10.1007/s11101-020-09672-x. [DOI] [Google Scholar]
- Saito T, Suzuki Y, Koyama K, Natori S, Iitaka Y, KINOSITA T. Chetracin A and Chaetocins B and C, Three new epipolythiodioxo-piperazines from Chaetomium spp. Chem Pharm Bull. 1988;36(6):1942–1956. doi: 10.1248/cpb.36.1942. [DOI] [Google Scholar]
- Salo JM, Kedves O, Mikkola R, Kredics L, Andersson MA, Kurnitski J, Salonen H. Detection of Chaetomium globosum, Ch. cochliodes and Ch. rectangulare during the diversity tracking of mycotoxin-producing Chaetomium-like isolates obtained in buildings in Finland. Toxins. 2020;12(7):443. doi: 10.3390/toxins12070443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Gao S, Gao YX, Wang AR, Xu YB, Sun R, Hu PG, Yang GF, Li AJ, Zhong D, Liu HY, Dong JY. Novel dibenzo[b, e]oxepinones from the freshwater-derived fungus Chaetomium sp YMF 1.02105. Planta Med. 2012;78(17):1837–43. doi: 10.1055/s-0032-1327828. [DOI] [PubMed] [Google Scholar]
- Sibounnavong P, Charoenporn C, Kanokmedhakul S, Soytong K. Antifungal metabolites from antagonistic fungi used to control tomato wilt fungus Fusarium oxysporum f sp. lycopersici. African J Biotechnol. 2011;10(85):19714–19722. doi: 10.5897/AJB11.3343. [DOI] [Google Scholar]
- Smetanina O, Kuznetzova T, Denisenko V, Pivkin M, Khudyakova YV, Gerasimenko A, Popov DY, Elyakov G. 3β-Methoxyolean-18-ene (miliacin) from the marine fungus Chaetomium olivaceum. Russ Chem Bull. 2001;50(12):2463–2465. doi: 10.1023/A:1015068520694. [DOI] [Google Scholar]
- Song C, Ding G, Wu G, Yang J, Zhang M, Wang H, Wei D, Qin J, Guo L. Identification of a unique azaphilone produced by Chaetomium globosum isolated from Polygonatum sibiricum. Chem Biodivers. 2020;17(3):e1900744. doi: 10.1002/cbdv.201900744. [DOI] [PubMed] [Google Scholar]
- Soytong K, Kahonokmedhakul S, Song J, Tongon R (2021) Chaetomium Application in Agriculture. Technology in Agriculture:229
- Takahashi M, Koyama K, NATORI S, Four new azaphilones from Chaetomium globosum var flavo-viridae. Chem Pharmac Bulletin. 1990;38(3):625–628. doi: 10.1248/cpb.38.625. [DOI] [Google Scholar]
- Telarovic I, Wenger RH, Pruschy M. Interfering with tumor hypoxia for radiotherapy optimization. J Exp Clin Cancer Res. 2021;40(1):1–26. doi: 10.1186/s13046-021-02000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thohinung S, Kanokmedhakul S, Kanokmedhakul K, Kukongviriyapan V, Tusskorn O, Soytong K. Cytotoxic 10-(indol-3-yl)-[13] cytochalasans from the fungus Chaetomium elatum ChE01. Arch Pharmacal Res. 2010;33(8):1135–1141. doi: 10.1007/s12272-010-0801-5. [DOI] [PubMed] [Google Scholar]
- Tian Y, Li Y. A review on bioactive compounds from marine-derived Chaetomium species. J Microbiol Biotechnol. 2022;32(5):541–550. doi: 10.4014/jmb.2201.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikoo A, Cutler H, Lo SH, Chen LB, Maruta H. Treatment of Ras-induced cancers by the F-actin cappers tensin and chaetoglobosin K, in combination with the caspase-1 inhibitor N1445. Cancer J Sci Am. 1999;5(5):293–300. [PubMed] [Google Scholar]
- Torres-Mendoza D, Ortega HE, Cubilla-Rios L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications. Journal of Fungi. 2020;6(2):58. doi: 10.3390/jof6020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbyville TJ, Wijeratne EK, Liu MX, Burns AM, Seliga CJ, Luevano LA, David CL, Faeth SH, Whitesell L, Gunatilaka AL. Search for Hsp90 inhibitors with potential anticancer activity: isolation and SAR studies of radicicol and monocillin I from two plant-associated fungi of the Sonoran desert. J Nat Prod. 2006;69(2):178–184. doi: 10.1021/np058095b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilavong S, Soytong K. Application of a new bio-formulation of Chaetomium cupreum for biocontrol of Colletotrichum gloeosporioides causing coffee anthracnose on Arabica variety in Laos. AGRIVITA, J Agricul Sci. 2017;39(3):303–310. doi: 10.17503/agrivita.v39i3.1070. [DOI] [Google Scholar]
- Vivi VK, Martins-Franchetti SM, Attili-Angelis D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2019;64(1):1–7. doi: 10.1007/s12223-018-0621-4. [DOI] [PubMed] [Google Scholar]
- Wang S, Xu Y, Maine EA, Wijeratne EK, Espinosa-Artiles P, Gunatilaka AL, Molnár I. Functional characterization of the biosynthesis of radicicol, an Hsp90 inhibitor resorcylic acid lactone from Chaetomium chiversii. Chem Biol. 2008;15(12):1328–1338. doi: 10.1016/j.chembiol.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Wang XW, Houbraken J, Groenewald JZ, Meijer M, Andersen B, Nielsen KF, Crous PW, Samson RA. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud Mycol. 2016;84:145–224. doi: 10.1016/j.simyco.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M-H, Hu Y-C, Sun B-D, Yu M, Niu S-B, Guo Z, Zhang X-Y, Zhang T, Ding G, Zou Z-M. Highly photosensitive poly-sulfur-bridged chetomin analogues from Chaetomium cochliodes. Org Lett. 2018;20(7):1806–1809. doi: 10.1021/acs.orglett.8b00304. [DOI] [PubMed] [Google Scholar]
- Wang H-H, Li G, Qiao Y-N, Sun Y, Peng X-P, Lou H-X. Chamiside A, a cytochalasan with a tricyclic core skeleton from the endophytic fungus Chaetomium nigricolor F5. Org Lett. 2019;21(9):3319–3322. doi: 10.1021/acs.orglett.9b01065. [DOI] [PubMed] [Google Scholar]
- Wang M-H, Zhang X-Y, Tan X-M, Niu S-B, Sun B-D, Yu M, Ding G, Zou Z-M. Chetocochliodins AI, Epipoly (thiodioxopiperazines) from Chaetomium cochliodes. J Nat Prod. 2020;83(4):805–813. doi: 10.1021/acs.jnatprod.9b00239. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao L, Liu C, Qi J, Zhao P, Liu Z, Li C, Hu Y, Yin X, Liu X. New tetramic acids comprising of decalin and pyridones from Chaetomium olivaceum SD-80A with antimicrobial activity. Front Microbiol. 2020;10:2958. doi: 10.3389/fmicb.2019.02958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani NA, Tirumale S. Evaluation of antioxidant properties of different extracts of Chaetomium cupreum SS02. Bull Faculty Pharmacy, Cairo Univ. 2018;56(2):191–198. doi: 10.1016/j.bfopcu.2018.08.001. [DOI] [Google Scholar]
- Wijeratne EK, Paranagama PA, Gunatilaka AL. Five new isocoumarins from Sonoran desert plant-associated fungal strains Paraphaeosphaeria quadriseptata and Chaetomium chiversii. Tetrahedron. 2006;62(36):8439–8446. doi: 10.1016/j.tet.2006.06.089. [DOI] [Google Scholar]
- Yamada T, Yasuhide M, Shigeta H, Numata A, Tanaka R. Absolute stereostructures of chaetomugilins G and H produced by a marine-fish-derived Chaetomium species. J Antibiot. 2009;62(7):353–357. doi: 10.1038/ja.2009.39. [DOI] [PubMed] [Google Scholar]
- Yao C, Bai H-H, Zhang Q, Qian X-Q, Zhang X, Wu L-W, Yang T, Li G-Y. Secondary metabolites from the fungus Chaetomium elatum CIB-412. Chem Nat Compd. 2019;55(5):899–901. doi: 10.1007/s10600-019-02841-1. [DOI] [Google Scholar]
- Youn UJ, Sripisut T, Park E-J, Kondratyuk TP, Fatima N, Simmons CJ, Wall MM, Sun D, Pezzuto JM, Chang LC. Determination of the absolute configuration of chaetoviridins and other bioactive azaphilones from the endophytic fungus Chaetomium globosum. Bioorg Med Chem Lett. 2015;25(21):4719–4723. doi: 10.1016/j.bmcl.2015.08.063. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhang X, Liu X, Wang H, Xue J, Yu J, Kang N, Wang X (2014) Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediators of Inflammation 2014:370530 [DOI] [PMC free article] [PubMed]
- Zhang G, Zhang Y, Qin J, Qu X, Liu J, Li X, Pan H. Antifungal metabolites produced by Chaetomium globosum No 04, an endophytic fungus isolated from Ginkgo biloba. Ind J Microbiol. 2013;53(2):175–180. doi: 10.1007/s12088-013-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhou D, Liang F, Wu Z, She Z, Li C. Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia. 2017;123:23–28. doi: 10.1016/j.fitote.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Zhu H, Chen C, Tong Q, Zhou Y, Ye Y, Gu L, Zhang Y. Progress in the chemistry of cytochalasans. Prog Chem Org Nat Prod. 2021;114:1–134. doi: 10.1007/978-3-030-59444-2_1. [DOI] [PubMed] [Google Scholar]