Abstract
Background:
Inferior vena cava tumor thrombus (IVC-TT) is a rare yet deadly sequel of renal cell carcinoma (RCC) with limited treatment options. The standard treatment is extirpative surgery, which has high rates of morbidity and mortality. As a result, many patients are unfit or unwilling to undergo surgery and face poor prognosis. This stresses the need for alternative options for local disease control. Our study aims to assess the feasibility and oncological outcomes of stereotactic ablative radiation (SAbR) for IVC-TT.
Methods:
A retrospective study reviewing six leading international institutions’ experience in treating RCC with IVC-TT with SAbR. Primary end point was overall survival using Kaplan-Meier.
Results:
Fifteen patients were included in the cohort. Over 50% of patients had high level IVC-TT (level III or IV), 66.7% had metastatic disease. Most eschewed surgery due to high surgical risk (7/15) or recurrent thrombus (3/15). All patients received SAbR to the IVC-TT with a median biologically equivalent dose (BED10) of 72 Gy (range: 37.5–100.8) delivered in a median of 5 fractions (range 1–5).
Median overall survival was 34 months. Radiographic response was observed in 58% of patients. Symptom palliation was recorded in all patients receiving SAbR for this indication. Only grade 1–2 adverse events were noted.
Conclusions:
SAbR for IVC-TT appears feasible and safe. In patients who are not candidates for surgery, SAbR may palliate symptoms and improve outcomes. SAbR may be considered as part of a multimodal treatment approach for patients with RCC IVC-TT.
Keywords: inferior vena cava thrombus, renal cell carcinoma, Radiation, SBRT, T3b
Introduction
Despite a recent trend towards earlier diagnosis of renal cell carcinoma (RCC), 4–10% of patients are still diagnosed with advanced disease and tumor invasion into the inferior vena cava (IVC-TT) (1).
The only therapy with curative potential for these patients is extirpative surgery, but this is a highly complex procedure which may require cardio-pulmonary bypass, and is associated with rates of perioperative high-grade complications (≥ Clavien 3A) as high as 35% and post-operative mortality ranging from 5 to 13% (2). Furthermore, many of these patients are high-risk surgical candidates due to age and/or comorbidities and are unwilling or unable to undergo such procedures.
Untreated IVC-TT carries a dismal prognosis with a median disease specific survival (DSS) ranging from 3–4 months to 8–9 months for those with or without metastatic disease, respectively (3). IVC-TT may cause further complications including venous congestion, Budd-Chiari syndrome, pulmonary emboli, and even contribute to metastatic progression, thus emphasizing the unmet need for alternative measures for local disease control in those unfit or unwilling to undergo surgery.
Contemporary data suggest that stereotactic ablative radiation therapy (SAbR) is effective for both primary and metastatic RCC (4, 5). Reports on the use of SAbR for the treatment of hepatocellular carcinoma related IVC-TT showed favorable results with response rates ranging from 36% to 70%(6, 7). We previously reported on our initial experience with SAbR for IVC TT in two patients with unresectable RCC, which showed good local control and prolonged overall survival of 18 and 34 months without additional systemic therapy (8, 9).
Since IVC-TT for RCC is relatively rare and experience treating it with SAbR is scarce, we endeavored to combine the experiences from leading institutions across the world as reflected in this manuscript.
Methods and patients
We reviewed patients’ records from 6 participating institutions with experience in treating RCC patients with SAbR. Institutional review board approval was obtained, as needed, by participating institutions.
Patients were included if they received SAbR directed at the RCC IVC-TT. Patients with metastatic disease were included, and systemic treatments before, during or after SAbR were allowed. Radiation to additional sites including the primary tumor was also allowed.
Patient, tumor and treatment characteristics, toxicity and oncological outcomes were retrospectively reviewed.
The primary endpoint was defined as overall survival from time of SAbR to the IVC-TT. Kaplan-Meier curves were used to evaluate median overall survival.
Results
Our cohort included fifteen patients from six institutions. Table 1 summarizes patient and tumor characteristics. Thrombus level (Mayo classification(10)) was I in 13.3% (2/15), II in 33.3% (5/15), III in 26.7% (4/15) and IV in 26.7% (4/15) of patients. Median thrombus diameter was 3.0 cm (range: 1.0–4.1). 46.6% (7/15) did not undergo surgery due to high surgical risk; 20% (3/15) had recurrent thrombus (following previous nephrectomy and IVC-TT thrombectomy). Other reasons for not undergoing surgery included inoperable tumor (n=2), disseminated metastatic disease (n=1) and patient refusal (n=1).
Table 1.
Patient and tumor characteristics
| Median age, years (Range) | 72 | (38–91) | |
| Thrombus level | I | 2 | (13.3%) |
| II | 5 | (33.3%) | |
| III | 4 | (26.7%) | |
| IV | 4 | (26.7%) | |
| Median thrombus diameter in cm (range) | 3 | (1–4.1) | |
| Metastatic disease | Yes | 10 | (66.7%) |
| No | 5 | (33.3%) | |
| IMDC risk group (for metastatic patients) | Good | 1 | (10.0%) |
| Intermediate | 8 | (80.0%) | |
| Poor | 1 | (10.0%) | |
| Tumor type | Clear cell | 12 | (80.0%) |
| Papillary | 1 | (6.66%) | |
| Other | 1 | (6.66%) | |
| N/A | 1 | (6.66%) | |
| Previous nephrectomy | Yes | 4 | (26.6%) |
| No | 11 | (73.3%) | |
| Systemic treatment before SAbR | Yes | 11 | (73.3%) |
| No | 4 | (26.6%) | |
| Reason for SAbR* | Progressive disease | 8 | (53.3%) |
| Palliation of symptoms | 5 | (33.3%) | |
| Other | 3 | (20.0%) | |
| Median SAbR dose in Gy (range) / No. of Fractions (range) | 40 (25–50) / 5 (1–5) | ||
| Median radio-biological equivalent dose (BED10) in Gy (range) | 72 (37.5 – 100.8) | ||
| Number of systemic treatments after SAbR | 0 | 7 | (46.6%) |
| 1 | 4 | (26.6%) | |
| 2+ | 4 | (26.6%) | |
IMDC - International Metastatic RCC Database Consortium, SAbR – Stereotactic Ablative Radiation, Gy - Gray
Patients may have had more than one indication
Median radiobiological equivalent dose (BED10), was 72 Gy (range: 37.5–100.8). Median SAbR dose was 40 Gy (range: 25–50) delivered in a median of 5 (range: 1–5) fractions (individual SAbR data is detailed in table 2). 33% (5/15) of patients also received SAbR to the primary tumor with a median (range) – dose of 30 Gy (25–42) delivered in 5 (1–5) fractions and a BED10 of 48 (37.5–100.8) Gy. Three patients had additional radiation to metastatic sites.
Table 2.
Individual patient SAbR data
| Patient number | Duration (days) | Total dose (Gy) | Number of fractions (Gy) | Dose per fraction (Gy) | BED-10 (Gy) |
|---|---|---|---|---|---|
| 1 | 14 | 50 | 5 | 10 | 100 |
| 2 | 5 | 36 | 4 | 9 | 68.4 |
| 3 | 12 | 40 | 5 | 8 | 72 |
| 4 | 9 | 40 | 5 | 8 | 72 |
| 5 | 14 | 40 | 5 | 8 | 72 |
| 6 | 11 | 40 | 5 | 8 | 72 |
| 7 | 10 | 30 | 5 | 6 | 48 |
| 8 | 17 | 35 | 5 | 7 | 59.5 |
| 9 | 5 | 50 | 5 | 10 | 100 |
| 10 | 9 | 42 | 3 | 14 | 100.8 |
| 11 | 1 | 26 | 1 | 26 | 93.6 |
| 12 | 7 | 30 | 5 | 6 | 48 |
| 13 | 6 | 25 | 5 | 5 | 37.5 |
| 14 | 9 | 40 | 5 | 8 | 72 |
| 15 | 9 | 31.25 | 5 | 6.25 | 50.78 |
| Median | 9 | 40 | 5 | 8.5 | 72 |
SAbR – Stereotactic ablative radiation; Gy – Grey, BED - Biological equivalent dose
Median follow-up after SAbR was 19 months (range: 1–43). Median overall survival was 34 months (95% CI 6.9–61.0). Six patients did not receive any additional systemic treatment following SAbR, in this subgroup median survival was 18 months (range 1–34). Twelve patients had available post-SAbR imaging of the tumor thrombus, which showed regression in 58% (7/12) within a median of 478 days from SAbR (range: 69–668), stable size in the remaining 25% (3/12) and enlargement in 16% (2/12) (18 and 740 days). Five patients who did not receive any systemic therapy following SAbR still showed either stable or reduced IVC-TT size.
Five patients received SAbR for symptoms palliation (Budd-Chiari (n=1), edema (n=2) pain (n=1) and hematuria (n=1)) which were eventually resolved in all cases.
Only minor (grade 1–2) short term adverse events were reported. These included fatigue, nausea and dermatitis.
Discussion
To our knowledge, this is the largest multicenter case series of patients treated with SAbR for RCC IVC-TT. Our median overall survival of 34 months compares favorably with historical data. A large retrospective study reported a median survival of five months in 390 patients with untreated IVC-TT (3). Despite the numerous shortcomings of a small retrospective series, including selection bias and use of systemic therapy, SAbR tumor killing and local control effects give cautious optimism that this could be contributing to the observed survival in this series. We observed both radiographic and symptomatic response in most patients, suggesting a potential rationale for exploring SAbR as a strategy to help render borderline tumors resectable which may answer this critical unmet need. Indeed, in one patient with Budd-Chiari syndrome, thrombus regression and symptom resolution, possibly due to the combined effects of systemic therapy(11) and SAbR, eventually rendered the tumor resectable (Figure 1). In addition, SAbR’s immunomodulatory and antigen presenting properties (12, 13) may have been contributory since the IVC-TT is continuously exposed to dendritic cells. Regardless of survival benefit, palliating local complication-related symptoms may justify using SAbR in the setting of symptomatic RCC IVC-TT.
Figure 1:
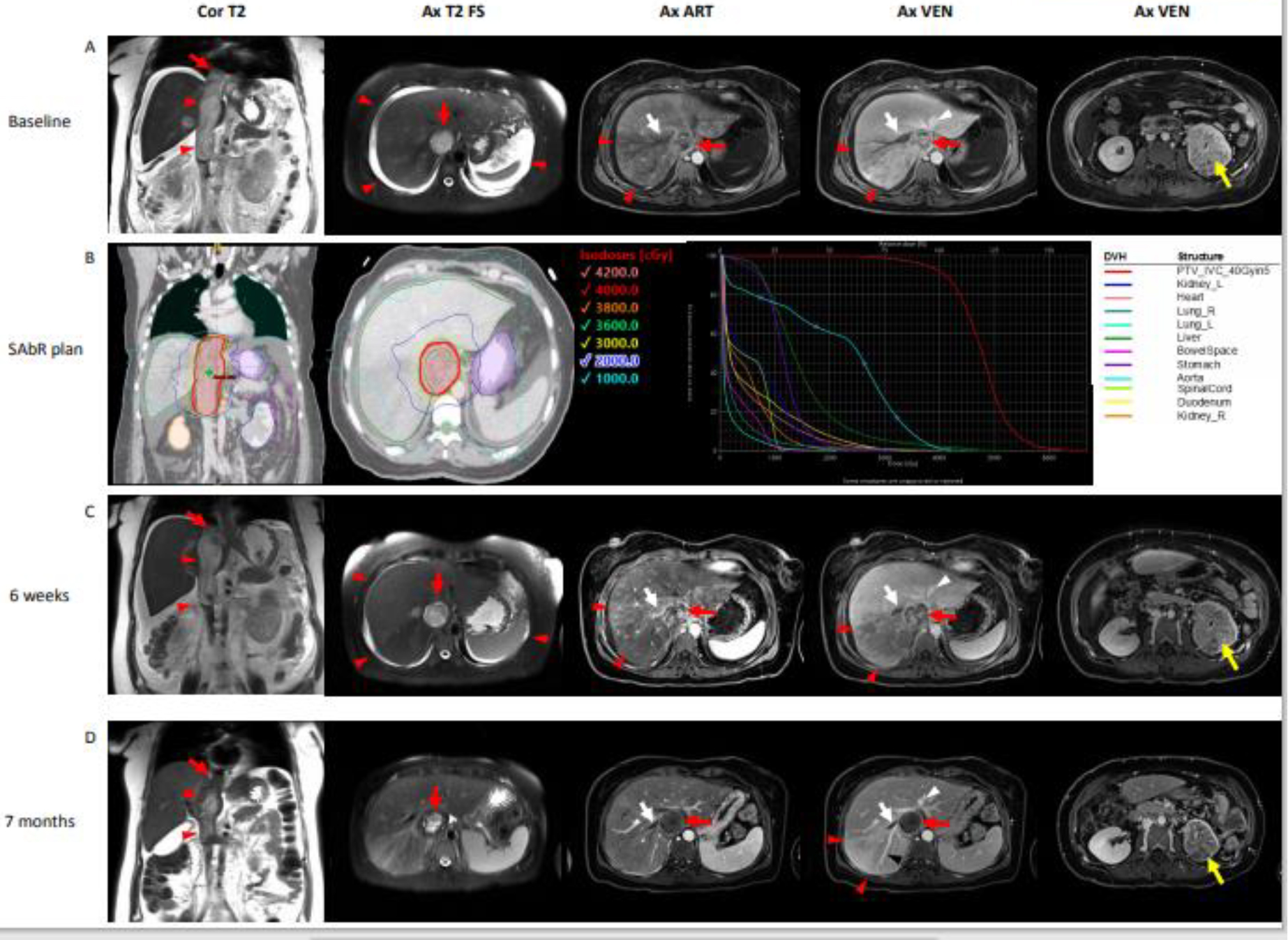
SAbR treatment plan and assessment of treatment response with multi-parametric MRI in a 38 year-old female with renal cell carcinoma, tumor thrombus in the inferior vena cava (IVC-TT) and Budd-Chiari syndrome. (A) Staging MRI at diagnosis. Coronal T2-weighted image demonstrates IVC-TT (arrowheads) with distal tip extending beyond the diaphragm (arrow). Axial T2-weighted image with fat suppression (Ax T2 FS) demonstrates expansion and complete filling of the IVC lumen and ascites (arrowheads). Contrast-enhanced T1-weighted gradient echo images during the arterial (art) and venous (venous) phases after administration of a bolus of gadobutrol (0.1. mmol/kg body weight) demonstrates heterogeneous enhancement of the IVC-TT (red arrow). Note decreased heterogeneous enhancement of the posterior right lobe of the liver (arrowheads) due to congestion caused by bland (i.e., non-enhancing) thrombus in the right hepatic vein (white arrow). The left and middle hepatic veins (white arrowhead) are patent. Axial image acquired at a lower level shows heterogeneous infiltrating primary tumor in the left kidney (yellow arrow). LFT’s at presentation were ALT 241U/L, AST 165 U/L ALK Phos 186 U/L. SAbR of 40 Gy (B) aimed at the IVC-TT was delivered in 5 fractions. Follow-up imaging at 6 weeks (C) and 7 months (D) shows a clear reduction in both the diameter and the length of the IVC-TT. Absence of enhancement in the IVC-TT at 7 months suggests a predominantly non-viable tumor thrombus. Note also progressive decrease in ascites and recanalization of a posterior branch of the right hepatic vein (black arrowhead) with persistent bland thrombus in an anterior branch of the hepatic vein (white arrow). LFT’s improved and normalized within 6 weeks of SAbR.
RCC- Renal cell carcinoma, IVC-TT – Inferior Vena Cava tumor thrombus, Ax- axial, Cor- coronal, T2 – T2-weighted single shot fast spin echo imaging, T2 FS – T2-weighted single shot fast spin echo imaging with fat suppression, ART – T1-weighted gradient echo imaging during the arterial phase, VEN – T1-weighted gradient echo imaging during the venous phase, LFT – Liver Function Tests, ALT - Alanine Transaminase, AST - Aspartate Aminotransferase, ALK Phos – Alkaline Phosphatase, SAbR – Stereotactic Ablative Radiation, DVH – Dose Volume Histogram, PTV – Planning Target Volume.
Interestingly, in one of the two patients whose eventual thrombus size was larger compared to pre SAbR imaging, initial imaging within two months showed IVC-TT size increase, yet regression (compared to early post SAbR imaging) was observed on subsequent imaging – this may be due to the interval between pre and post SAbR imaging studies, but may also indicate some initial IVC-TT size increase due to local inflammatory process. In most patients, response was slow and durable, and best response occurred within a median of 478 days. Only two patients had maximal response in <90 days post-SAbR; neither had later imaging available. This suggests overall durable response and may guide clinicians in designing follow-up protocols after SAbR. The fact that IVC-TT regression was also observed in patients not receiving additional systemic therapy further indicate the possible effectiveness of SAbR in this setting.
Our cohort showed favorable safety profile of SAbR for IVC-TT, comparable to preliminary reports of a safety phase of a prospective phase II trial of neoadjuvant SAbR (NCT02473536)(14). Together, these accumulating data indicate that SAbR in this setting is feasible and safe. Nevertheless, late toxicity data from prospective trials are still lacking, and longer follow-up is required.
Our study’s main limitation is that it cannot isolate SAbR’s benefit within multimodality treatments. Nevertheless, favorable survival was noted even in those not receiving additional systemic therapy. Further limitations include the retrospective nature of this study, the heterogeneous patient population and various patient selection criteria employed by each institution, variability in systemic treatments as well as in SAbR protocols.
Although high level evidence is needed to establish the benefit of SAbR for IVC-TT, such evidence based on randomized or prospective trials is unlikely to be available in the near future due to the relative rareness of this condition. Despite it’s limitations, our data suggest that SAbR may play a role in the multimodality management of IVC-TT. We postulate that SAbR for RCC IVC-TT may be considered in the following settings in patients with localized disease: 1) Non-surgical candidates (i.e. due to comorbidities, high surgical risk or patient refusal). 2) Patients with symptomatic disease, or those expected to experience near future complications, specifically Budd-Chiari syndrome, which is associated with a dire prognosis. In this setting, SAbR to IVC-TT, with concurrent systemic therapy if possible, may prove useful as a palliative measure and could even enable later extirpative surgery. 3) Patients with locally recurrent IVC-TT who cannot or chose not to undergo repeat surgery.
In addition, for those with metastatic disease, where the role of cytoreductive surgery is debatable (15) - especially when considering the associated morbidity of extirpative surgery, SAbR may be used in conjunction with systemic therapy for local disease control in case of thrombus progression, for palliation of symptoms and possibly as consolidative debulking treatment while including the primary tumor. In this setting, SAbR’s immunogenic and antigen presentation properties may synergize with immunotherapy (16).
Conclusions
In conclusion, SAbR for RCC-related IVC-TT is feasible and safe. In selected patients who are not candidates for surgery, SAbR may achieve local control and palliate symptoms. Prospective trials investigating the optimal integration of SAbR into multimodality management of RCC IVC-TT are warranted.
Highlights.
Treatment options for renal cell carcinoma with inferior vena cava thrombus (RCC-IVCTT) are limited and carry substantial risks.
This is a retrospective multicenter cohort including fifteen RCC-IVCTT patients treated with stereotactic ablative radiation therapy (SAbR).
Median follow-up after SAbR was 19 months (range: 1–43). Median overall survival was 34 months. No severe adverse events were reported.
Palliation of symptoms was recorded in all patients receiving SAbR for this indication and radiographic tumor response was observed in 58% of patients.
SAbR for RCC-IVCTT seems feasible and safe – further research is needed to establish oncological benefit.
Acknowledgements:
The authors acknowledge Dr. Jonathan Feinberg for the scientific editing of the manuscript
Funding:
Raquibul Hannan is funded by American Cancer Society RSG-16–004-01-CCE and CPRIT MIRA RP180725. James Brugarolas, and Ivan Pedrosa are funded by P50CA196516. Ivan Pedrosa is funded by 5RO1CA154475 and by U01CA207091.
Footnotes
Declaration of interests:
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim HL, Zisman A, Han K-R, Figlin RA, Belldegrun AS. Prognostic Significance of Venous Thrombus in Renal Cell Carcinoma. Are Renal Vein and Inferior Vena Cava Involvement Different? The Journal of Urology. 2004;171(2, Part 1):588–91. [DOI] [PubMed] [Google Scholar]
- 2.Haddad AQ, Leibovich BC, Abel EJ, Luo J-H, Krabbe L-M, Thompson RH, et al. Preoperative multivariable prognostic models for prediction of survival and major complications following surgical resection of renal cell carcinoma with suprahepatic caval tumor thrombus. Urologic Oncology: Seminars and Original Investigations. 2015;33(9):388.e1–e9. [DOI] [PubMed] [Google Scholar]
- 3.Reese AC, Whitson JM, Meng MV. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urologic Oncology: Seminars and Original Investigations. 2013;31(7):1305–9. [DOI] [PubMed] [Google Scholar]
- 4.Zaorsky NG, Lehrer EJ, Kothari G, Louie AV, Siva S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): a meta-analysis of 28 studies. European Urology Oncology. 2019;2(5):515–23. [DOI] [PubMed] [Google Scholar]
- 5.Correa RJM, Louie AV, Zaorsky NG, Lehrer EJ, Ellis R, Ponsky L, et al. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. European Urology Focus. 2019;5(6):958–69. [DOI] [PubMed] [Google Scholar]
- 6.Xi M, Zhang L, Zhao L, Li Q-Q, Guo S-P, Feng Z-Z, et al. Effectiveness of Stereotactic Body Radiotherapy for Hepatocellular Carcinoma with Portal Vein and/or Inferior Vena Cava Tumor Thrombosis. PLoS ONE. 2013;8(5):e63864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuo Y, Yoshida K, Nishimura H, Ejima Y, Miyawaki D, Uezono H, et al. Efficacy of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis/inferior vena cava tumor thrombosis: evaluation by comparison with conventional three-dimensional conformal radiotherapy. Journal of Radiation Research. 2016;57(5):512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freifeld Y, Margulis V, Woldu SL, Timmerman R, Brugarolas J, Hannan R. Stereotactic Body Radiation Therapy for Renal Cell Carcinoma with Inferior Vena Cava Thrombus – Initial Experience Report and Literature Review. Kidney Cancer. 2019;3:71–7. [Google Scholar]
- 9.Hannan R, Margulis V, Chun SG, Cannon N, Kim DW, Abdulrahman RE, et al. Stereotactic radiation therapy of renal cancer inferior vena cava tumor thrombus. Cancer Biol Ther. 2015;16(5):657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neves RJ, Zincke H. Surgical Treatment of Renal Cancer with Vena Cava Extension. British Journal of Urology. 1987;59(5):390–5. [DOI] [PubMed] [Google Scholar]
- 11.Vergho DC, Loeser A, Kocot A, Spahn M, Riedmiller H. Tumor thrombus of inferior vena cava in patients with renal cell carcinoma - clinical and oncological outcome of 50 patients after surgery. BMC Res Notes. 2012;5:5-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nature Medicine. 2018;24(12):1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. New England Journal of Medicine. 2012;366(10):925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margulis V, Freifeld Y, Pop LM, Manna S, Kapur P, Pedrosa I, et al. Neoadjuvant SABR for Renal Cell Carcinoma Inferior Vena Cava Tumor Thrombus; Safety Lead-in Results of a Phase 2 Trial. International Journal of Radiation Oncology, Biology, Physics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Méjean A, Ravaud A, Thezenas S, Colas S, Beauval J-B, Bensalah K, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine. 2018;379(5):417–27. [DOI] [PubMed] [Google Scholar]
- 16.Hammers HJ, Vonmerveldt D, Ahn C, Nadal RM, Drake CG, Folkert MR, et al. Combination of dual immune checkpoint inhibition (ICI) with stereotactic radiation (SBRT) in metastatic renal cell carcinoma (mRCC) (RADVAX RCC). Journal of Clinical Oncology. 2020;38(6_suppl):614-. [Google Scholar]


