Abstract
Approximately two-thirds of those suffering with Alzheimer’s disease (AD) are women, however, the biological mechanisms underlying this sex divergence of AD prevalence remain unknown. Previous research has shown sex-specific biochemical differences that bias female mice toward pro-AD signaling on the phosphoproteomic level via corticotropin releasing factor (CRF) receptor 1 activation after CRF overexpression. Here we aimed to determine if chronic stress would induce a similar response in AD mouse models. We stressed 4-month-old APP/PS1 mice using a chronic unpredictable mild stress (CUMS) paradigm for up to 1 month. Following CUMS and behavioral assessments, we quantified whole protein and phosphoprotein levels in the cortex of stressed and non-stressed APP/PS1 mice using mass spectrometry-based proteomics. While there were no statistically significant differences at the total protein and peptide abundance levels, we found 909 and 841 statistically significant phosphopeptides between stressed and unstressed females and males, respectively, using a false discovery rate of 5%. Of these significant phosphopeptides, only 301 were the same in males and females. These results indicate that while both males and females undergo protein phosphorylation changes following stress, the peptides that are phosphorylated differ between sexes. We then used Metacore analysis to determine which biological pathways were affected. We found that several pathways were changed differently between male and female mice including NMDA receptor trafficking, cytoskeleton organization, and tau pathology. The differing biological pathways affected between males and females in response to chronic stress may help us to better understand why women are at a higher risk of AD.
Keywords: Alzheimer’s disease, APP/PS1 mice, chronic stress, phosphoproteomics, sex differences
INTRODUCTION
Alzheimer’s disease (AD) is a common neurodegenerative disorder that currently affects approximately 5.5 million Americans, of which, nearly two-thirds are women [1]. The prevailing theory for the disproportionate number of women affected by AD has been the attribution of increased prevalence to women’s longer average lifespan, but increasing evidence suggests that there remains an intrinsic susceptibility toward AD pathogenesis in women. In fact, studies have shown that at the young age of 45, women already have a 19.5% risk for AD, nearly double that of the 10.3% risk of men of the same age, and that this risk increases after the age of 65 [2]. However, the underlying biological mechanisms that make women more susceptible to AD pathogenesis remain unknown.
One possible mechanism is that women respond differently to and are more negatively affected by stress. Stress and its downstream pathways have long been connected with AD, as studies have shown that those that experience higher stress have an increased risk of AD [3, 4]. Cortisol, or corticosterone in mice, is a stress hormone released following hypothalamic-pituitary-adrenal (HPA) axis pathway activation which releases corticotropin releasing factor (CRF) followed by adrenalcorticotropin hormone (ACTH) release in response to stress. High and sustained levels of cortisol or corticosterone cause many negative effects including influencing the progression and severity of AD pathogenesis along with memory deficits, as shown by both human and animal studies [5–10]. How stress directly or indirectly leads to these results still requires further investigation. Recently in a study led by Dr. Valentino, we found that genetic overexpression of CRF triggered the phosphoproteomic expression of biological pathways connected to specific disease processes, including AD, in a sex-specific manner [11]. We found that male mice overexpressing CRF had a higher activation of Rho/Rac pathways involved with β-arrestin while females instead had activation of amyloid, perhaps through Gs-, PKA pathways [11].
In this study, we aimed to determine if chronic unpredictable mild stress (CUMS) could induce a subsequent increase in CRF-expression via chronic HPA-axis activation and result in sex-divergent responses at total protein and phosphoproteomic levels. CUMS has been used by many previous studies and includes a variety of mild to moderate stressors administered randomly to create an environment of stress meant to mimic the daily life stressors of an unresolvable nature that humans experience as part of their daily lives [12, 13]. We chose this approach to stress APP/PS1 mice starting at 4 months, an age before significant memory deficits onset [14], to see whether CUMS could induce memory and behavioral impairment and whether CUMS affected any biological pathways in a sex-specific manner via proteomic and phosphoproteomic analysis.
MATERIALS AND METHODS
Subjects
A total of 36 APP/PS1 mice (19 females, 17 males) bred from male APP/PS1 and female C57/BL6 (Jackson Laboratory, Bar Harbor, Maine) were used in this study. At weaning age, pups were genotyped and APP/PS1 positive littermates were then group-housed before randomly being divided into stressed or non-stressed conditions (n = 8–10 per group per sex for behavioral testing, and later n = 5–7 per group per sex were used for proteomics analysis). Animals were maintained in a temperature-controlled facility at 60% relative humidity and 20–21°C on a 12-h light/dark cycle, except during stressors that inverted their light cycles. Food and water were available ad libitum at all times except during certain stress conditions. CUMS was started at 4 months of age in the stressed groups. All animals underwent behavioral assessment by 5 months of age, then 13 (7 females, 6 males) CUMS and 10 (5 females, 5 males) non-stressed APP/PS1 were used for corticosterone and proteomic assessment. All procedures were performed in accordance with the NIH guidelines for the treatment of rodents and the current Guide for the Care and Use of Laboratory Animals under a protocol approved by the Northwestern University Animal Studies Committee.
Chronic unpredictable mild stress (CUMS)
Our CUMS protocol included the use of three stressors at variable times of the day that differed between days. After two weeks, the number of stressors per day decreased to two as behavioral assessments began to be conducted and were counted as a stressor. Briefly, the stressors included: no bedding, wet bedding, cage tilt, restraint, cold water swim, food deprivation, water deprivation, isolation, and alternation of light/dark cycles. Detailed information is included in Supplementary Figure 1 and a week’s schedule is presented in Fig. 1A as an example.
Fig. 1.
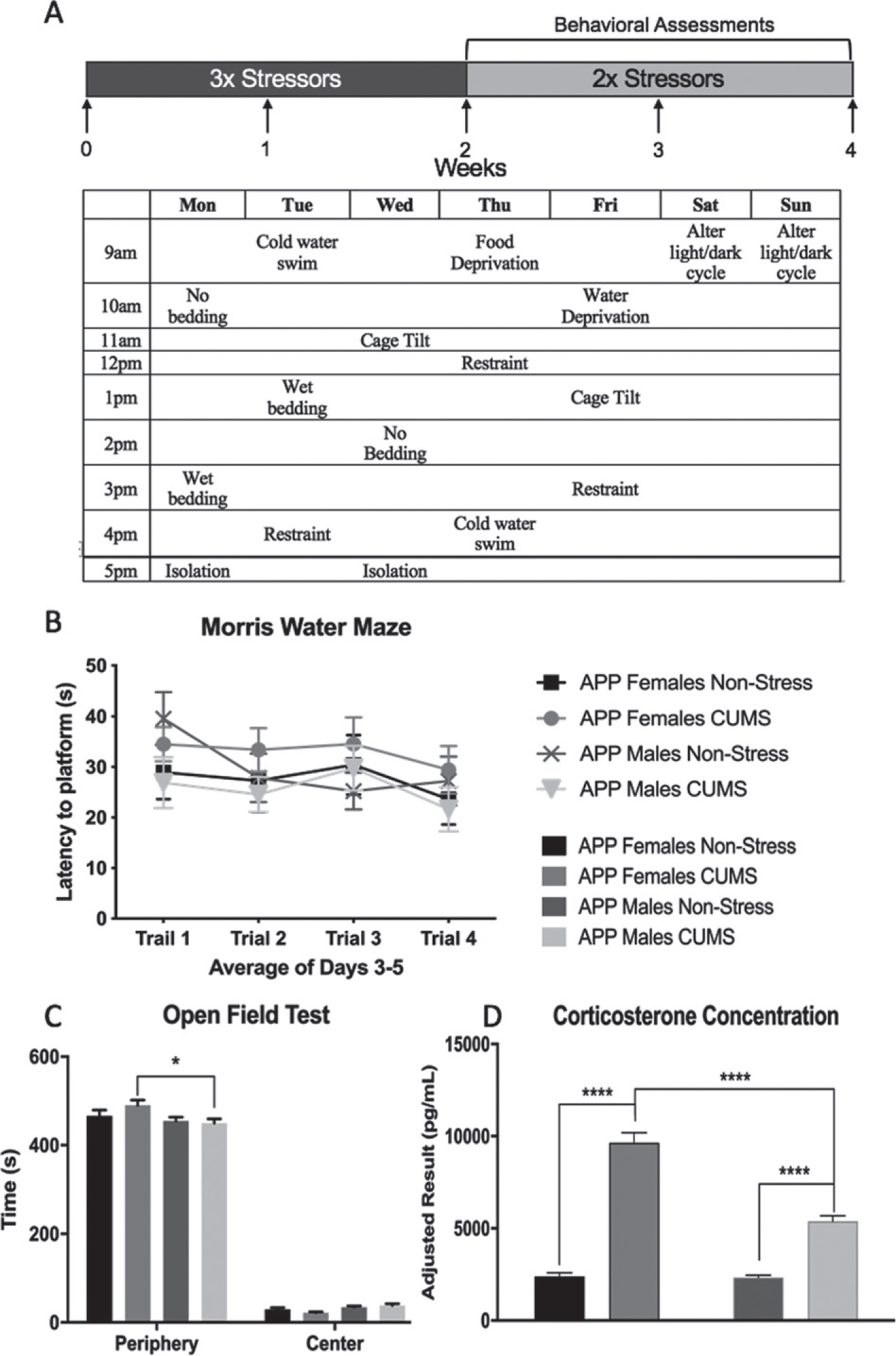
Chronic Unpredictable Mild Stress and Biochemical Assessments. A) The schematic for CUMS in which mice underwent 1-month of chronic stress. Behavioral tests were completed the last 2 weeks of stress and included both memory and anxiety-like assessments. B) Morris Water Maze showed no significant differences between non-stressed and CUMS conditions with no significant changes between sexes. C) Open Field test showed that both conditions, both sexes overwhelming preferred the periphery over the center area. CUMS females significantly preferred the periphery more than CUMS males. D) Corticosterone concentration in non-stressed and CUMS APP mice divided by sex in pg/mL. Stress increased corticosterone expression and females of both conditions had higher overall expression than their male counterparts. All data is presented as Mean ± SEM, n = 8–10 per group in B and C, n = 5–7 in D, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Behavioral tests
A series of behavioral tests were conducted starting after two weeks of CUMS. These included:
Locomotor activity (LMA) and open field (OF)
An evenly illuminated plexiglass box (25 cm×25 cm×25 cm) was placed on a stable table with overhead video recording (Canon, Melville, IL). Locomotion was defined as the distance traveled (mm) during a 10 min trial. Animal activity was recorded using an automated tracking system (Any-Maze, Shoelting, Wood Dale, IL). This recorded data was further used to determine the time the mice spent in the periphery (outer 2/3rds) or center (inner third) of the arena.
Morris water maze (MWM)
In this study, we used a modified MWM task to test working memory (also called Delayed Matching to Place procedure) [15]. Mice were acclimated to the room for 30 min on each test day prior to testing. The task was conducted in a tank 1.8 m in diameter and 0.9 m deep, filled with tap water (23°C) made opaque with non-toxic tempera paint over 5 consecutive days with four trials per day. At the beginning of each trial, the mouse was placed in a starting position and had a maximum of 60 s to reach a hidden platform. The mouse was removed once it reached the platform or if 60 s had elapsed, then placed into a drying cage. The location of the platform and the starting position of the mouse changed each day but remained constant for all the trials for that day. Trials 1 and 2 were conducted between 9 and 11 am, with an inter-trial interval of 25 s. Animals were then placed in the home cage and remained in the testing room for 3 h prior to trial 3. Trials 3 and 4 were conducted between 12–2 pm, with an inter-trial interval of 25 s. Days 1 and 2 were considered habituation days and data from these is not reported. Data from days 3–5 was recorded and the latency to find the platform was averaged across days for each trial.
Sucrose preference test
Sucrose preference test is often used to measure stress-induced anhedonia particularly in stress-based models of depression [16]. The mice are presented with a choice between two bottles, one with water and the other with 1% sucrose. Mice are habituated to both bottles in their home cage for 24 h then transferred to a single housing cage for 48 h beginning at approximately 10 am. Afterwards, preference is measured as a ratio of amount of sucrose water consumed daily divided by the total liquid consumed (water+sucrose).
Proteomic assessment
Sample preparation: Protein digestion and peptides desalting
Sample preparation was done as previously described [17–19]. Briefly, tissue from one hemisphere of the entire cortex of 23 mouse brains, approximately 80 mg wet weight per sample, were homogenized in 2 × 1 mL cold fresh urea buffer. After homogenization, brain lysates were centrifuged for 10 min at 20,000 g at 4°C. The protein concentration of the lysates was determined via MicroBCA assay (Thermo Scientific, San Jose, CA). 6 mg protein from each brain sample was spiked in with 2 μg chicken ovalbumin (1 : 3000, Sigma, St. Louis, MO) which served as a phosphoprotein internal standard. The proteins from all 23 samples were reduced, then diluted with 50 mM Tris HCl pH 8.0 to reduce urea concentration to 0.8 M before trypsin digestion. Following Trypsin hydrolysis and desalting, peptides were eluted from the desalting columns with 3 × 0.5 mL 80% acetonitrile/0.1% trifluoroacetic acid, lyophilized and stored at −80°C.
Separation of peptides for proteomic and phosphoproteomic work
Basic reversed phase-high performance liquid chromatographic (RP-HPLC) was used to separate peptides. Peptide concentrations were determined spectrophotometrically by monitoring the absorbance @ 280 nm (NanoDrop ND-1000) before loading 3 mg for first dimension high pH reversed phase chromatography (Acquity H-Class UPLC, Waters, Milford, MA). RP-HPLC was conducted on a Zorbax 300 Å Extend-C18 4.6 mm×250 mm column (3.5 μm bead size, Agilent, Santa Clara, CA). Solvent A (2% acetonitrile, 5 mM ammonium formate, pH 10), and solvent B (90% acetonitrile, 5 mM ammonium formate, pH 10) at a flow rate of 1 mL/min was used and increased the percentage of solvent B in a nonlinear gradient as previously described [17–19]. Peptide fractions were then recombined into 12 fractions to be used for proteome analysis. About 5% of each sub-fraction was reserved for whole proteome analysis and the remaining 95% of each 12 sub-fractions were further combined into 6 sub-fractions before enrichment for PTM workflow [18].
Immobilized metal affinity chromatography (IMAC) for phosphopeptide enrichment
Peptides from each of the 6 sub-fractions were dissolved in 50% acetonitrile/0.1% water, then diluted 1 : 1 with 100% acetonitrile/0.1% TFA as described before [17–19]. The final concentration of peptides was 0.5 μg/μL. Phos-select iron affinity beads (Sigma, St. Louis, MO) were washed twice with 30% acetonitrile/250 mM acetic acid (W/E) at 8000 rcf for 1 min [18]. Peptide mixtures were incubated with 20 μL of IMAC beads for 1 h at 1000 rpm at room temperature. The enriched phosphopeptides on the IMAC beads were loaded onto the wells of a pre-washed 96 well glass fiber filter plate (Phenomenex, Torrance, CA) then washed 2x with 400 μL W/E buffer for 20 s under shaking with a DPC MicroMix 5 Plate Shaker where the beads were kept on the filters while the wells were emptied under vacuum with a vacuum manifold (Waters, Milford, MA). The IMAC beads were then washed sequentially 2 × 400 μL with 80% acetonitrile/0.1% TFA, 2 × 400 μL 30% acetonitrile/water. Phosphorylated peptides were eluted twice from the IMAC beads with 400 μL of 400 mM ammonium hydroxide/30% acetonitrile for 1 min under shaking and collected with a new LoBind Plate 96 well collection plate (Eppendorf, Hauppauge, NY). The eluted peptides were lyophilized and dissolved in 11 μL water/0.1% TFA including the HRM peptides for QC/QA measures and system suitability.
LC-MS/MS analysis
Reconstituted peptides for phosphoproteome analysis were analyzed by LC-MS/MS on a QExactive HF mass spectrometer (Thermofisher Scientific, San Jose, CA) coupled with an Ultimate 3000. Peptides were separated by reverse phase (RP)-HPLC on a nanocapillary column, 75 μm ID×50 cm 2 μm PepMap Acclaim column. The mass spectrometer was set to repetitively scan m/z from 300 to 1400 (R = 240,000) followed by data-dependent MS/MS scans on the twenty most abundant ions, minimum AGC 1e4, dynamic exclusion with a repeat count of 1, repeat duration of 30 s, (R = 15000) FTMS full scan AGC target value was 3e6, while MSn AGC was 1e5, respectively. MSn injection time was 160 ms; microscans were set at one. Rejection of unassigned and 1+,6–8 charge states was set. As a measure for QC/QA, we injected standard E. coli protein digest in between samples (one for every 6 phosphoproteomics injections) and analyzed the data to track and maintain the quality of the instrumentation. For online monitoring of the instrument, PRM analysis for the HRM peptides was performed though Skyline AutoQC and the data was uploaded and accessed in Skyline Panorama.
Data processing and bioinformatics analysis
Protein and phosphopeptide identification/quantification was performed with MaxQuant (1.6.1.0) using a mouse reference database from Uniprot (reviewed canonical and isoforms; downloaded on January 4, 2018). For phosphoproteome samples the phosphorylation of Ser, Thr, and Tyr were also set as variable modifications. The False Discovery Rate (FDR) for peptides and proteins were set at 1%. Fragment ion tolerance was set to 0.5 Da. The MS/MS tolerance was set at 20 ppm. The minimum peptide length was set at 7 amino acids. The match-between-runs was turned on for the biological replicates. The rest of the parameters were kept as default. The quality of the generated results by MaxQuant was further verified by PTXQC [20]. Perseus (1.6.1.3) was used for downstream proteomics data processing and statistical analysis. The MaxLFQ intensity values were used to analyze the whole cell proteome data. Metacore was used to identify enriched canonical pathways for the phosphoproteins in each dataset. Additional information for annotating the phosphoproteome, such as linear motifs, known sites, and kinase-substrate relations was obtained from PhosphoSitePlus (Cell Signaling Technology, Danvers, MA) and accessed through Perseus. Motif-x was used to do motif analysis of the identified phosphopeptides [21].
Statistical analysis
Statistical analyses for behavioral experiments were conducted using GraphPad Prism software (San Diego, CA). Data are expressed as mean ± standard error of the mean (S.E.M.) (n = 8–10/group behavioral assessment, n = 5–7/group corticosterone and proteomic assessment). LMA data, sucrose preference, and corticosterone levels were analyzed using Two-way analysis of variance (ANOVA). MWM and OF were analyzed using a Three-way repeated measures ANOVA. Post-hoc Tukey’s HSD test was used to test for between group differences. To identify differentially expressed proteins following proteomic analysis we employed student’s t-test then generated volcano plots to visualize the affected proteins while comparing different groups of samples. We used Benjamini-Hochberg FDR of 0.05 as false discovery control (i.e., significant hits should have an FDR of less than 0.05). To analyze proteomics and phosphoproteomics data, protein groups containing matches to decoy database or contaminants were discarded. The data were log2 transformed and normalized by subtracting the median for each sample. For phosphoproteomics data, we filtered out the phosphopeptides with localization probability of less than 75%. After log2 transformation, the data was normalized by median subtraction. Subsequently, student t-test was used for comparing different cohorts. Benjamini-Hochberg FDR of 0.05 was set to identify significantly affected phosphopeptides. Lists of differentially abundant phosphopeptides (with an FDR < 0.05) were selected and used for bioinformatics analysis. For proteins with multiple phosphopeptides, the median intensity values for the phosphopeptides was calculated and used for further analysis. All behavioral and neurobiological assessments were used on the same group of animals.
RESULTS
Memory and anxiety behavior after CUMS
For APP/PS1 mice at five months of age, we did not expect to see robust behavioral changes in terms of memory impairment [14]. However, we wanted to determine if CUMS could induce memory and behavioral changes as compared to unstressed mice at this age.
Motor function
The effect of 2 weeks of CUMS, the amount of stress given at the point of this behavioral test, on locomotor activity during a 10-min session was assessed using the LMA test described in the methods. Analysis of distance traveled using a Two-way ANOVA indicated no significant effect of stress (F1,32 = 0.6220, p = 0.4361), sex (F1,32 = 2.854, p = 0.1009), or an interaction between stress × sex (Supplementary Figure 2A, F1,32 = 0.01297, p = 0.9100).
Working memory
For memory assessment, we conducted a modified MWM meant to determine working memory in which the latency to reach a hidden platform was measured. Analysis with a Three-way repeated measures ANOVA of the latency during the trials showed a main effect for trials (F2.610,83.50 = 4.871, p = 0.0053) but not for sex (F1,32 = 0.3285, p = 0.5706) or stress (F1,32 = 0.01868, p = 0.8921). Nor were there any significant interactions between trial × stress (F3,96 = 1.507, p = 0.2177), trial × sex (F3,96 = 1.177, p = 0.3228), sex × stress (F1,32 = 1.299, p = 0.2629), or sex × stress × trial (Fig. 1B, F396 = 2.077, p = 0.1083). The lack of memory deficits is to be expected at this age in APP/PS1 mice as, despite there likely being plaque formation, memory deficits occur at a later stage [22]. Our results indicate that two to three weeks of CUMS could not induce memory deficits in APP/PS1 mice at this age.
Anxiety-like behaviors
In this study, we selected two commonly used behavioral tests, sucrose preference and OF, to measure potential anxiety-like behaviors in our cohort. For sucrose preference, Two-way ANOVA showed there was no main effect of sex (F1,32 = 0.4402, p = 0.5118), stress (F1,32 = 0.06651, p = 0.7981), or an interaction between sex × stress (Supplementary Figure 2B, F1,32 = 0.7911, p = 0.3804). We further analyzed LMA data to determine where the mice spent the most time during their 10-min trial while in the OF apparatus. Previous studies have shown that increased time spent in the periphery as compared to the center of the apparatus can indicated increased anxiety [23]; however, we saw that all mice spent a substantial amount of time in the periphery as compared with the center (F1,32 = 3973, p < 0.0001). We also found that there was no effect of sex (F1,32 = 2.843, p = 0.1015) or stress (F1,32 = 0.7517, p = 0.3924). Furthermore, there were no interactions between the area in which mice spent their time × stress (F1,32 = 0.6841, p = 0.4143) or sex × stress (F1,32 = 0.9185, p = 0.3451). There was however a significant interaction of area × stress (F1,32 = 7.068, p = 0.0121) with a significant difference between time spent in the periphery between stressed females and stressed males (p = 0.0149), but with all animals having spent the majority of the time in the periphery, this suggests that all animals approached the assessment with high levels of anxiety. Finally, there was no interaction between sex × stress × area (Fig. 1C, F1,32 = 2.161, p = 0.1513).
Corticosterone expression
Immediately prior to sacrifice and brain collection, blood serum was collected and then analyzed for corticosterone levels. Using Two-Way ANOVA, we found both a stress (F1,19 = 198.2, p < 0.0001) and sex effect (F1,19 = 34.87, p < 0.0001) and an interaction of stress × sex (Fig. 1D, F1,19 = 32.93, p < 0.0001). Post-hoc analysis showed that female mice that had undergone CUMS had significantly higher corticosterone expression than their male counterparts, signifying either a higher activation of the HPA-axis or a down-regulation in negative feedback mechanisms resulting in a higher level of circulating glucocorticoids. Thus we were then able to confirm that CUMS did in fact activate innate stress-pathways creating a difference in expression of stress hormones between non-stressed and stressed conditions, and our evidence showed that the extent of response was sex-specific.
Total protein and phosphoprotein expression following proteomic analysis
When comparing the total proteome of female unstressed to stressed mice (Fig. 2A) and the total proteome of male unstressed to stressed mice (Fig. 2B), there were no statistically significance changes in protein expression due to stress. However, when we looked at the expression levels of different phosphopeptides, we found that there were 909 statistically significant phosphopeptides between stressed and unstressed females (Fig. 2C) and 841 statistically significant phosphopeptides between stressed and unstressed males (Fig. 2D) using an FDR of 5% as a cutoff for significance. Furthermore, when these identified phosphopeptides and their direction of change were allowed to cluster via unsupervised hierarchy, we see that they form clusters based on stress condition for both females (Fig. 2E) and males (Fig. 2F), thus confirming that these changes in phosphopeptide expression is directly connected to exposure of chronic stress.
Fig. 2.
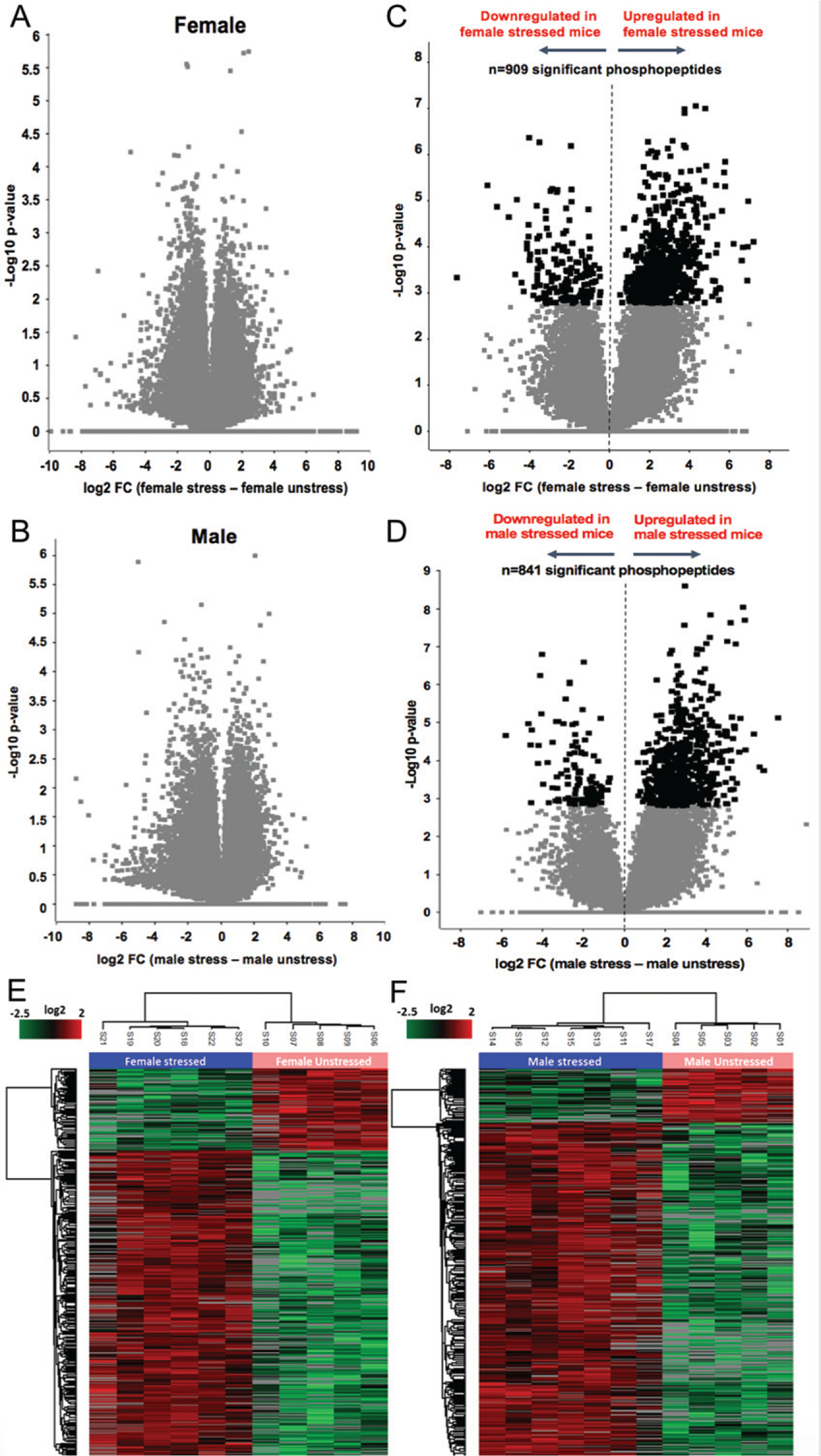
Whole Protein and Phosphopeptide Profiles following Proteomic Analysis. Volcano plots, A and B, showing that there were no statistically significant changes in peptide expression in female and male APP mice between CUMS and non-stress, respectively. C and D show the statistically significant changes of phosphopeptides expression in females and males, respectively, along with their direction of change. Here darker boxes indicate significance at an FDR of 5%. Finally, E and F show heatmaps where phosphopeptides were allowed to cluster via unsupervised hierarchy. Phosphopeptides and their changes grouped stressed and unstressed separately for both females and males. N = 5–7 per group.
Pathway analysis using phosphopeptide expression changes and maintained motifs
Following identification of these significant phosphopeptides, the next step was to analyze how these phosphopeptides may differ by sex and the pathways to which they belonged. Of these two groups, males and females only shared 301 phosphopeptides. Thus there were 540 phosphopeptides that resulted after stress in males alone and 608 phosphopeptides unique to females following stress (Fig. 3A). This indicates that while both males and females undergo changes in phosphorylation and by extension different conditions for the activation of biological pathways following stress, the exact phosphorylation events that occur differ in males versus females. We then analyzed all significant phosphopeptides within each sex, including common peptides, to determine the significant pathways that underwent changes in phosphorylation following stress. The top twenty pathways (Fig. 3B), all well above the 5% FDR cutoff, depict some interesting similarities and differences. As part of the study, we also determined distinct motifs within our phosphopeptide sequences that were specific to either females or males (Supplementary Table 1). We identified 10 different kinase and binding motifs for males and 8 for females using an FDR limit of < 5%. While the majority of these motifs are repeated between genders, males showed an overrepresentation of amino acid sequence patterns to which PKC bind while females showed enriched MAP kinase activated kinases motifs following stress. Again, these indicate that while there are many similar changes in terms of potential phosphorylation sites, there are unique patterns of enrichment in either gender following stress.
Fig. 3.
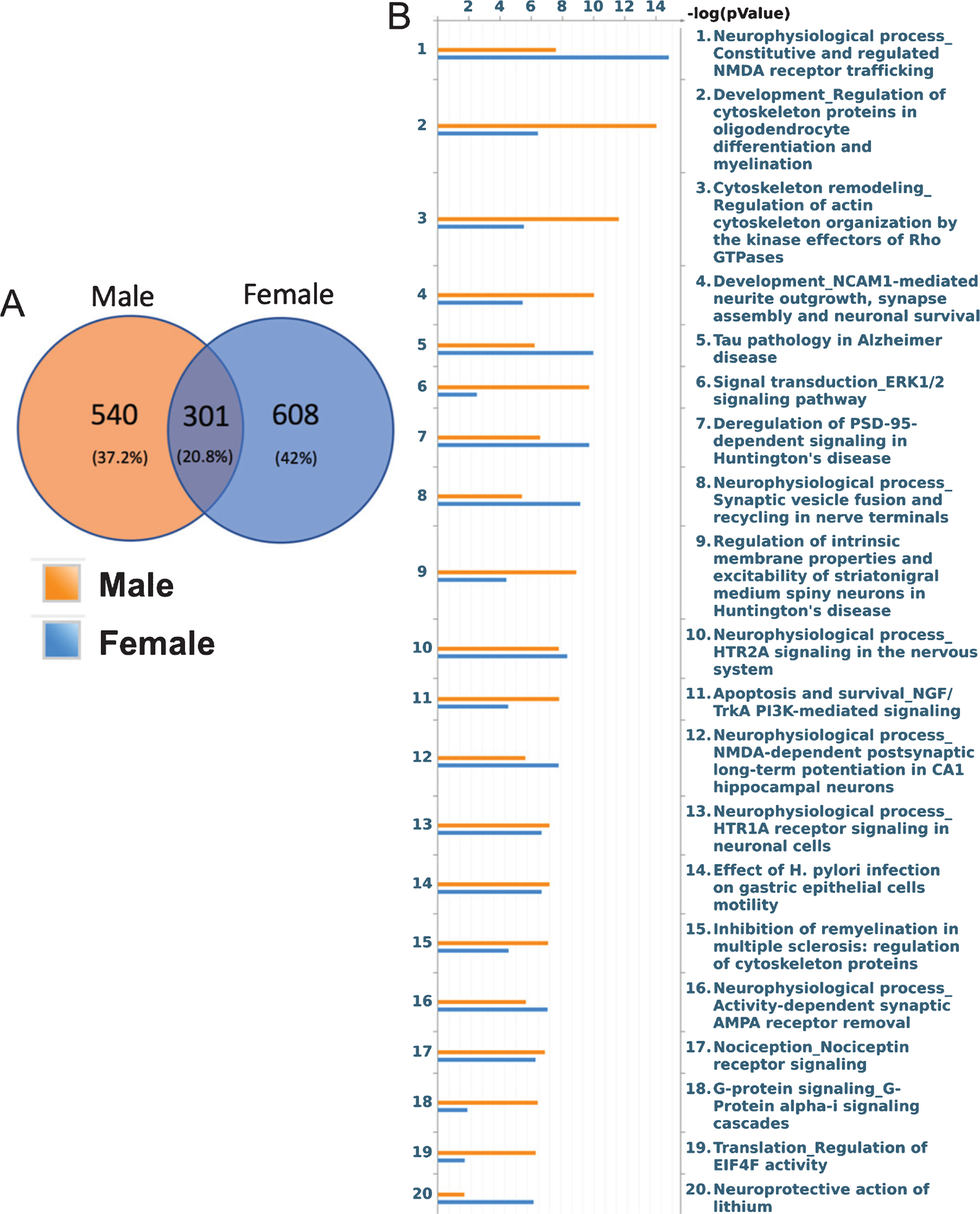
Sex-Divergent Signaling Pathways after CUMS. A) Phosphopeptides were separated into groups whether they were unique to either females, males, or common between the two. B) These phosphopeptides and their fold change were then inputted into Metacore for pathway identification and the top 20 pathways, each with an FDR greater than 5%, are shown for either sex.
DISCUSSION
This study aimed to determine how CUMS, a model meant to mimic the unpredictable life stressors of humans, affected behavior, proteomic, and phosphoproteomic landscapes and whether these changes were sex-specific and associated with AD neuropathology. We found that 2 to 4 weeks of CUMS did not cause significant memory or behavioral impairments but did change the corticosterone expression and phosphoproteomic profiles of both males and females. The downstream signaling pathways linked to these phosphoproteomic alterations may be associated with neuronal function and AD pathogenesis in a sex-specific manner in APP/PS1 mice.
In this study, we chose to use APP/PS1 mice at a stage before neuropathological phenotype presentation to confirm if chronic stress could accelerate disease onset. Despite AD being a disease that increases in severity with age, disease pathogenesis begins in affected individuals well before symptom presentation [24]. This is true in animal models as well. Specifically, in APP/PS1 mice, Aβ plaques have been seen as early as 6 weeks of age with mature plaques at 3–4 months, yet behavioral changes are often not reported until approximately 8 months or later [14, 25, 26]. We supposed that stress might be able to initiate memory impairment earlier as it is well established that chronic stress can impede learning and memory consolidation [27, 28]. However, in our study we did not find memory changes at this time; perhaps our CUMS paradigm of 3 weeks, the time at which we started the memory assessment, was not intense enough to induce working memory impairment.
Following general locomotion and memory tests, we also conducted behavioral assessments of anxiety and depressive-like symptoms such as anhedonia. Since the CUMS model is often used to induce anxiety and as a model of depression, we expected to see significant changes in our assessments. However, our sucrose preference assay showed no significant differences between stress and non-stressed APP/PS1 mice. The OF assay showed high levels of anxiety in all mice as they all spent a majority of their time in the periphery of the novel arena. There was, however, a significant difference between the time spent in the periphery where stressed females spent more time in this area than stressed males. Although CUMS protocols are well-documented and often used in preclinical studies, results are inconsistent in terms of output, mostly depending on the intensity and duration of stressors [29–33]. Nevertheless, we found a significant difference in corticosterone levels between male and female unstressed and stressed mice. In addition, we also saw a sex-difference within the stress condition with females having a significantly higher level of corticosterone expression than their male counterparts. Plasma corticosterone level, while dynamic, is the most commonly used marker for the evaluation of stress levels, although it has been shown that increased corticosterone levels do not always coincide with changes in the behavioral assays we used [34–36]. Additionally, other studies have shown that higher corticosterone levels are correlated with the affective behaviors tested by tail suspension and light-dark box, which we did not use in this study [34, 37]. Regardless, our data suggests that females are indeed more susceptible to chronic stress, even using a milder than expected CUMS, as evidenced by higher corticosterone levels compared to males.
Upon confirming increased corticosterone levels following chronic stress, we then completed whole proteome and phosphoproteomic analysis, specifically looking at what changes occurred within each sex following chronic stress. Ultimately, we found no significant changes in total protein expression in either males or females following stress, but we did find 909 statistically significant phosphopeptides between stressed and unstressed females and 841 statistically significant phosphopeptides between stressed and unstressed males (FDR < 0.05). Of these, there were 540 phosphopeptides unique to males and 608 phosphopeptides unique to females that were changed following CUMS. This indicates that male and female AD mice, in response to chronic stress, express different phosphopeptide levels, which may trigger different signaling pathways, some of which may be linked to AD pathogenesis. Further pathway analysis indeed showed a multitude of pathways affected by phosphorylation changes and that some pathways were more indicated in either sex.
Among some of the pathways that were similar in both males and females, both sexes showed an activation of the “Tau pathology of AD pathway” after stress, which is to be expected as previous literature has stated that stress works via tau protein and tau pathways to cause neurological deficits [38, 39]. Moreover, it is of interest to note that females still seem to have a higher activation of this pathway than males, perhaps suggesting further susceptibility or an increased speed of disease progression following stress. Among the pathways higher specifically in females are those involved in NMDA receptor trafficking, synaptic vesicle fusion and recycling in nerve terminals, and NMDA-dependent post-synaptic long-term potential in CA1 hippocampal neurons. Meanwhile, pathways higher in males include oligodendrocyte differentiation and myelination, regulation of actin cytoskeletal organization, and NCAM1-mediated outgrowth, synapse assembly and neuronal survival. A recent paper that looked at the whole transcriptome of AD patients to see gene-trait correlations with AD pathology in a cell-specific manner found sex differences in the responses of particular cells types and, of interest to this study, found that global transcriptional activation of oligodendrocytes positively correlated to increased pathology only in males while females have positive disease correlations when there was a global downregulation of excitatory and inhibitory cells [40]. The fact that these cell-specific changes correlate with what pathways we see activated in a sex-specific manner further supports the belief that there are underlying biological mechanisms at play which affect AD pathogenesis differently in men and women.
This data in conjunction with the building library of other scientific studies further proves that stress affects men and women differently, specifically in the context of AD pathogenesis. While our findings did not identify the same pathways as we did in our collaboration with the Valentino laboratory [11], the results are unsurprising due to key differences in the animal model and stress paradigm between these two studies. In this study, many of the significant pathways we found directly connect to proteins involved in AD along with memory and learning functions. Thus, despite there being some differences in phosphoproteomic changes between biological expression of CRF following stress and genetic overexpression of CRF in transgenic mice, this evidence supports that there are underlying sex-specific differences in pathway activation and that females are more inclined toward disease pathways. Future studies are required to: explore these pathways at the biochemical level for further validation of these sex-specific pathway changes, reproduce these findings, investigate the effects of different stress paradigms, and to determine if there are long-term effects of chronic stress on the phosphoproteomic landscape. Likewise, it would be in our interest to conduct a similar study using the same model and stress paradigm at a later time point where disease is well established and has caused behavioral deficits to see the dynamic changes on the proteomic/phosphoproteomic landscape to determine the affect of time on stress vulnerability and if certain pathways are only activated earlier or later in disease pathogenesis. A limitation of this study is the lack of biochemical validation of our phosphoproteomic data or measurement of pathological changes including amyloid levels which were not done due to limited tissue following proteomic assessment. In one of our previous studies, we found sex differences in plaque burden, with females displaying higher plaque count [11]. However, since these mice are not from the same cohort, we are unable to correlate these findings with this work. Future studies are necessary to fill the gaps as to whether proteomic alterations, especially phosphoproteomic alteration, affects amyloid production and if this occurs differently at different time points after CUMS.
In summary, 2–4 weeks of CUMS did not induce memory impairment or anxiety like behavior in both male and females. Four weeks of CUMS did, however, result in sex-specific increases in corticosterone, changes in phosphopeptide expression, and pathway activation. We found that females had higher levels of corticosterone following chronic stress and more unique phosphopeptide expression changes than their male counterparts. Furthermore, the pathways linked to these phosphopeptide changes suggest higher involvement of AD-neuropathogenesis, including pathways such as tau and learning and memory function pathways in females than males, although further validation of these pathways is necessary. The results presented in this paper are intended to aid in the further exploration of sex-specific downstream pathway activation and can hopefully direct future research as to which pathways might be involved in AD. Now that we are further aware of these sex-specific changes in pathway activation, we can better design studies that include both sexes to improve our understanding of AD pathogenesis as a whole. Understanding how AD pathogenesis differs in either gender is imperative in our creation of treatments for patients of either gender as we as a field continue to strive for better, personalized medicine.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Aging grants R56AG053491 and RF1AG057884.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1009r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191009.
REFERENCES
- [1].Alzheimer’s Association (2018) 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 14, 367–429. [Google Scholar]
- [2].Alzheimer’s Association (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15, 321–387. [Google Scholar]
- [3].Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, Mills PJ, Khandrika S, Galasko D (2007) The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry 62, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA (2011) Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry 19, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OFX (2007) The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatr 14, 95. [DOI] [PubMed] [Google Scholar]
- [6].Karten YJG, Nair SM, van Essen L, Sibug R, Joëls M (1999) Long-term exposure to high corticosterone levels attenuates serotonin responses in rat hippocampal CA1 neurons. Proc Natl Acad Sci USA 96, 13456–13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VMY, Trojanowski JQ (2011) Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci 31, 14436–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McEwen BS (2017) Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 1, 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cuadrado-Tejedor MRA, Frechilla D, Franco R, Perez-Mediavilla A, Garcia-Osta A (2012) Chronic mild stress accelerates the onset and progression of the Alzheimer’s disease phenotype in Tg2576 mice. J Alzheimers Dis 28, 567–578. [DOI] [PubMed] [Google Scholar]
- [10].Finsterwald C, Alberini CM (2014) Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol Learn Mem 112, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bangasser DA, Dong H, Carroll J, Plona Z, Ding H, Rodriguez L, McKennan C, Csernansky JG, Seeholzer SH, Valentino RJ (2017) Corticotropin-releasing factor overexpression gives rise to sex differences in Alzheimer’s disease-related signaling. Mol Psychiatr 22, 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Frisbee JC, Brooks SD, Stanley SC, d’Audiffret AC (2015) An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes and negative health outcomes in rodents. J Vis Exp, 53109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Boxelaere M, Clements J, Callaerts P, D’Hooge R, Callaerts-Vegh Z (2017) Unpredictable chronic mild stress differentially impairs social and contextual discrimination learning in two inbred mouse strains. PLoS One 12, e0188537–e0188537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Hölscher C, Mathews PM, Jucker M (2006) Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7, 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cho YH, Jaffard R (1994) The entorhinal cortex and a delayed non-matching-to-place task in mice: Emphasis on preoperative training and presentation procedure. Eur J Neurosci 6, 1265–1274. [DOI] [PubMed] [Google Scholar]
- [16].Liu M-Y, Yin C-Y, Zhu L-J, Zhu X-H, Xu C, Luo C-X, Chen H, Zhu D-Y, Zhou Q-G (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13, 1686–1698. [DOI] [PubMed] [Google Scholar]
- [17].Mertins P, Qiao JW, Patel J, Udeshi ND, Clauser KR, Mani DR, Burgess MW, Gillette MA, Jaffe JD, Carr SA (2013) Integrated proteomic analysis of post-translational modifications by serial enrichment. Nat Methods 10, 634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McNulty DE, Annan RS (2008) Hydrophilic interaction chromatography reduces the complexity of the phosphoproteome and improves global phosphopeptide isolation and detection. Mol Cell Proteomics 7, 971–980. [DOI] [PubMed] [Google Scholar]
- [19].Ugras S, Daniels MJ, Fazelinia H, Gould NS, Yocum AK, Luk KC, Luna E, Ding H, McKennan C, Seeholzer S, Martinez D, Evans P, Brown D, Duda JE, Ischiropoulos H (2018) Induction of the immunoproteasome subunit Lmp7 links proteostasis and immunity in α-synuclein aggregation disorders. EBioMedicine 31, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bielow C, Mastrobuoni G, Kempa S (2016) Proteomics quality control: Quality control software for MaxQuant results. J Proteome Res 15, 777–787. [DOI] [PubMed] [Google Scholar]
- [21].Chou MF, Schwartz D (2011) Biological sequence motif discovery using motif-x. Curr Protoc Bioinformatics 35, 13.15.11–13.15.24. [DOI] [PubMed] [Google Scholar]
- [22].Vickers JC, Mitew S, Woodhouse A, Fernandez-Martos CM, Kirkcaldie MT, Canty AJ, McCormack GH, King AE (2016) Defining the earliest pathological changes of Alzheimer’s disease. Curr Alzheimer Res 13, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choleris E, Thomas AW, Kavaliers M, Prato FS (2001) A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev 25, 235–260. [DOI] [PubMed] [Google Scholar]
- [24].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on Aging; Alzheimer’s Association (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maia LF, Kaeser SA, Reichwald J, Hruscha M, Martus P, Staufenbiel M, Jucker M (2013) Changes in amyloid-β and tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med 5, 194re192. [DOI] [PubMed] [Google Scholar]
- [26].Gengler S, Hamilton A, Hölscher C (2010) Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer’s disease is impaired in old but not young mice. PLoS One 5, e9764–e9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Park CR, Campbell AM, Diamond DM (2001) Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychiatry 50, 994–1004. [DOI] [PubMed] [Google Scholar]
- [28].Luethi M, Meier B, Sandi C (2009) Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front Behav Neurosci 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Willner P (2017) The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress 6, 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Willner P (2016) Reliability of the chronic mild stress model of depression: A user survey. Neurobiol Stress 6, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fanceschellli AHS, Thelen C, Papadopoulou-Daifoti Z, Pitychoutis PM (2014) Sex differences in the chronic mild stress model of depression. Behav Pharmcol 25, 372–383. [DOI] [PubMed] [Google Scholar]
- [32].Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019) Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev 99, 101–116. [DOI] [PubMed] [Google Scholar]
- [33].Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z (2005) Chronic mild stress impact: Are females more vulnerable? Neuroscience 135, 703–714. [DOI] [PubMed] [Google Scholar]
- [34].Sturm M, Becker A, Schroeder A, Bilkei-Gorzo A, Zimmer A (2015) Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav 14, 292–300. [DOI] [PubMed] [Google Scholar]
- [35].Gong S, Miao Y-L, Jiao G-Z, Sun M-J, Li H, Lin J, Luo M-J, Tan J-H (2015) Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 10, e0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mora F, Segovia G, del Arco A, de Blas M, Garrido P (2012) Stress, neurotransmitters, corticosterone and body–brain integration. Brain Res 1476, 71–85. [DOI] [PubMed] [Google Scholar]
- [37].Ardayfio P, Kim K-S (2006) Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav Neurosci 120, 249–256. [DOI] [PubMed] [Google Scholar]
- [38].Lopes S, Teplytska L, Vaz-Silva J, Dioli C, Trindade R, Morais M, Webhofer C, Maccarrone G, Almeida OFX, Turck CW, Sousa N, Sotiropoulos I, Filiou MD (2016) Tau deletion prevents stress-induced dendritic atrophy in pre-frontal cortex: Role of synaptic mitochondria. Cereb Cortex 27, 2580–2591. [DOI] [PubMed] [Google Scholar]
- [39].Steinmetz D, Ramos E, Campbell SN, Morales T, Rissman RA (2015) Reproductive stage and modulation of stress-induced tau phosphorylation in female rats. J Neuroendocrinol 27, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai L-H (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


