Abstract
Introduction
The COVID-19 pandemic strongly affected every aspect of the modern society, from health to socioeconomics, leading people to experience high levels of stress.
Methods
A double-blind, cross-over, placebo-controlled clinical study was performed to investigate the ability of a food supplement containing two probiotic strains, Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077, in supporting 33 healthy adults, working at a university, in stress management. The efficacy of the tested strains in influencing the stress response, in terms of mood and sleep behavior, was assessed using the following validated questionnaires: Profile of Mood State (POMS) and Pittsburgh Sleep Quality Index (PSQI).
Results
Outcomes of the POMS and the PSQI demonstrated a significant reduction of the questionnaire's scores both versus baseline and placebo after 30 days of probiotic intake.
Conclusions
According to the results, the probiotic food supplement investigated showed a remarkable effect on stress management by improving the quality of sleep and the mood.
Keywords: Gut-brain axis, Probiotics, Mood, Sleep, COVID-19
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused a global, unprecedented crisis that involved public health, economic system, and society in general, affecting populations all over the world [1]. As a consequence, people had to face not only the health emergency and the economic crisis but also the insecurity of the future and the fear for their health and employment [2, 3]. Furthermore, restrictions like social distancing and lockdowns led to a worsening of the quality of life, both from a physical and a psychological point of view [4, 5]. In Italy, the ongoing uncertainty due to the oscillation from strict to less strict restrictions (and vice versa) produced an increasingly unstable psychological condition, leading to a situation of high anxiety and stress level in the population [6, 7]. Moreover, the impact of introducing changes in work habits (i.e., smart working, layoffs, children at home, and distance learning) to manage the so-called “new normal” further exacerbated this mental state [8, 9, 10]. In this context, the need of new strategies for managing stressful conditions has become warranted.
Scientific evidence pointed out that stress induces several physiological alterations such as cortisol increases and sleep disturbances. Indeed, long-duration stress stimuli result in a response that involves the hypothalamic-pituitary-adrenal axis, leading to an impaired production of cortisol [11]. A compromised communication along this axis may cause structural changes in the brain with long-term effects on the nervous and immune system [12]. Furthermore, stress hormone levels correlate positively with reduced sleep duration, which in turn is associated with a higher incidence of metabolic disorders such as obesity and type 2 diabetes [13]. Stress can also affect the gastrointestinal functions, increasing mucosal permeability that causes an enhanced uptake of potentially harmful molecules such as lipopolysaccharide and pro-inflammatory agents (TNF-α, IL-1b, IL-6) [14]. These compounds can overstimulate the immune system, triggering autoimmune and inflammatory responses [15].
Microbiota plays a pivotal role in maintaining gut microbiota integrity, also known as eubiosis status, a condition of dynamic equilibrium of resident gut microorganisms [16]. Besides intestinal functionality, gut microbial communities are also actively involved in immune system regulation through the production of bioactive molecules that act as signals at immunological and neurological level [17, 18, 19]. Indeed, in the past decades, the link between the intestinal microbiota and the brain, along the so-called gut-brain axis, has been confirmed by several studies indicating an intimate connection between gut health and brain functions, including behavior and sleep [20, 21, 22]. A gut microbiota imbalance, as a consequence of stress, may lead to gut-brain miscommunication. Thus, maintaining a microbiota in eubiosis could be a valid approach to cope with such alteration.
In the last decades, clinical evidence indicates that probiotics can influence the gut-brain axis and modulate mood and stress responses [23, 24]. Indeed, probiotics are able to maintain mucosal barrier functions, mitigate stress-induced glucocorticoids and inflammatory cytokine responses, and increase neurochemicals such as tryptophan, dopamine, serotonin, gamma-aminobutyric acid known to modulate host neural activities and functions such as stress management, emotions, and cognition [25, 26, 27]. Moreover, there is increasing evidence that probiotics can restore the gut microbiota balance through several mechanisms [28, 29, 30]. For these reasons, probiotic intake to modulate microbiota could contribute to mental health in stressful conditions.
In this double-blind, cross-over, placebo-controlled clinical study, we investigated the ability of a food supplement containing two probiotic strains, Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077, in supporting healthy adults, working alternately from home/university, in stress management, monitoring their mood state and sleep quality. These two probiotic strains have been selected among different strains for their in vitro performance and further tested in a proof of concept clinical study reporting an improvement of cognitive functions and sleep quality in stressed students [31].
Materials and Methods
A randomized, double-blind, placebo-controlled, cross-over clinical study was carried out at the Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Italy. The study was performed in accordance with the principles of the Declaration of Helsinki, good clinical practice, and applicable national regulatory requirements. All procedures involving human subjects were approved by the Ethics Committee of the University of Calabria. The study was registered at ISRCTN registry with the following registration number: ISRCTN15033071.
Study Participants
Subjects participating in this study were healthy men and women, 25–60 years old, working at the Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, with an initial Pittsburgh Sleep Quality Index (PSQI) score in the range 4–6 [32] and Profile of Mood State (POMS) in the range 10–30. To the best of our knowledge, to discriminate between good and bad mood, no Total Mood Disturbance (TMD) score guidelines are available. Thus, considering that the TMD score can run from 0 to 200, the range 10–30 has been chosen to enroll only subjects with non-pathological mood disturbance.
The most important exclusion criteria were the use of oral antibiotics within 30 days before the screening visit and use of drugs, food, or herbal supplements for the mood or sleep. A complete list of the inclusion and exclusion criteria is reported in Table 1. A total of 33 subjects were considered eligible to participate in the study.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Good general health |
| Female or male subjects |
| Aged between 25 and 60 years |
| Subjects who have not been recently involved in any other similar study |
| Commitment to complete questionnaire |
| Commitment to use during the entire study period only the products to be tested |
| Commitment to not use products likely to interfere with the product to be tested |
| Commitment to not vary the normal daily routine (i.e. lifestyle, physical activity, etc.) |
| Subjects aware of the study procedures and having signed an informed consent form |
| Profile of Mood State score between 10 and 30 at the baseline |
| Pittsburgh Sleep Quality Index score between 4 and 6 at the baseline |
| Exclusion criteria |
| Current antibiotic administration |
| Treatment with probiotics in the 6 months preceding enrolment |
| Subjects with known or suspected sensitization to one or more test formulation ingredients |
| Subjects who do not meet the inclusion criteria |
| Women who are pregnant/breast feeding or not using adequate contraceptive methods |
| Any condition that the principal investigator deems inappropriate for participation |
| Adult protected by the law (under guardianship, or hospitalized in a public or private institution, for a reason other than the research, or incarcerated) |
| Volunteer unable to communicate or cooperate with the Investigator due to language problems, poor mental development, or impaired cerebral function |
| Suffering from other psychiatric disorders such as schizophrenia, other psychotic disorders, bipolar disorder or substance use disorder |
| Herbal remedies or psychotropic drugs that are intended for depression taken within the last 2 weeks prior to baseline or during the study |
Study Design
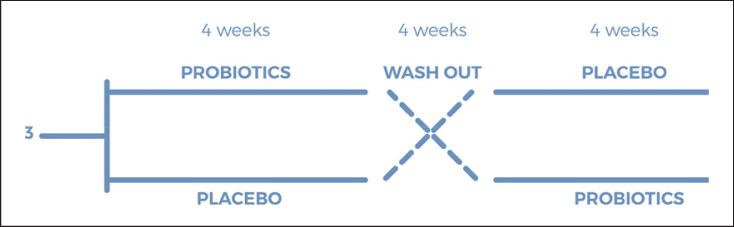
The study was conducted with a cross-over design with the primary aim to reduce perceived stress during the pandemic period in terms of improvement of mood and sleep quality. At the screening visit, eligible subjects were enrolled in a two-treatment period (probiotic and placebo or vice versa) of 30 days each, alternated with a washout period of 30 days. The total duration of the study was 90 days. Outcome measures were evaluated at each of the four visits: visit 1 (beginning of the study), visit 2 (end of the first intervention), visit 3 (start of the second intervention, after a washout period of 30 days), and visit 4 (end of the second intervention). The flowchart of the study is reported in Figure 1.
Fig. 1.
Flowchart of the study.
Study Product
Subjects were randomly assigned to receive one capsule a day of the active or the placebo product, away from meals and stored at room temperature. The active capsules contained two probiotic strains, Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077, for a final dose of 4×109 CFU/day (2 × 109 CFU for each strain) and maltodextrin, while the placebo capsules were composed only of maltodextrin. The active and placebo products presented a similar appearance and taste. The products were manufactured by ROELMI HPC, Origgio (VA), Italy. Compliance was evaluated at the end of each treatment period, when the subjects were asked to return all treatment capsules, and the number of unused capsules was counted.
Outcome Measures
The endpoint was to assess the ability of the probiotic formulation in enhancing mood and sleep behavior. All participants completed a battery of validated psychological questionnaires, the Profile of Mood State (POMS) and the Pittsburgh Sleep Quality Index (PSQI), at the beginning and at the end of each treatment, for a total of four time points. Sessions were carried out half in person and half online. Each session lasted about 25 min.
Profile of Mood State
The Profile of Mood State (POMS) is a self-report questionnaire that assesses mood. The version used in the present study is the Italian adaptation of the original text [33]. It consists of 58 items (words or sentences) that describe feelings that people usually have. For each item, participants rate on a 5-point scale from 0 (not at all) to 4 (extremely) how they have been feeling in the past week including the current day. The items are grouped into 6 categories: tension (9 items), depression (15 items), anger (12 items), fatigue (7 items), confusion (7 items), and vigor (8 items). The Vigor scale is in inverted relationship with the other scales since it assesses positive feelings (e.g., to be lively, active, energetic). The total score, defined as the Total Mood Disturbance (TMD), can be calculated by adding the scores for tension, depression, anger, fatigue, and confusion and then subtracting the score for vigor as explained by the following equation: TMD = (Tension + Depression + Anger + Fatigue + Confusion) − Vigor (1)
The TMD global score ranges from 0 to 200: the reduction of TMD score over time is expected as a reflection of an improvement of general mood. A subgroup analysis of each category has been assessed to evaluate a possible modification toward a specific feeling category.
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) is a self-report questionnaire that assesses sleep quality for the majority of days and nights in the past month. The test used was the validated Italian version [34]. It consists of 19 questions. The first four investigate usual bedtime, the number of minutes needed to fall asleep, the usual getting up time, and the number of hours of sleep per night. The other questions are related to other aspects of sleep quality rated on scales appropriate for the specific question. The items are weighted on a 0–3 interval scale and grouped in seven component scores (i.e., subjective sleep quality, sleep latency, sleep duration, use of sleeping medication, daytime dysfunction, habitual sleep efficiency). A global PSQI score (range 0–21) was obtained by adding up the component scores, wherein the lower the PSQI global score, the better the sleep quality. A subgroup analysis of the different categories was assessed to determine if probiotic intake specifically modulates a certain sleep condition.
Statistical Analysis
Statistical analysis was performed using NCSS 8 (version 8.0.4 for Windows; NCSS, Kaysville, UT, USA) running on Windows Server 2008 R2 Standard SP1 64-bit edition (Microsoft, USA). Data normality was checked using the Shapiro-Wilk W normality test and data shape. Intragroup (vs. baseline) statistical analysis was carried out using the Wilcoxon signed-rank test, while intergroup statistical analysis was carried out using the Mann-Whitney U test. A p < 0.05 was considered statistically significant. Statistical analysis output was reported as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Subjects of the Study
The cross-over study was conducted between March 2021 and June 2021. Recruitment was carried out via email invitation among subjects working at the Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Quattromiglia, Cosenza. A total of 33 male and female subjects, employees of university (researchers, professors, administrative employees) working alternately from home/university were successfully enrolled. At the end of the study, a total of 24 subjects completed the treatment. Nine subjects discontinued the study, five withdrew because of their inability to collect the last questionnaires of the second treatment period, and four had to take antibiotics during the washout period. The population was composed of 15 women and 9 men with an average age of 36 ± 9 (±SD) years. In general, both products used in this clinical study were well tolerated, and the level of compliance to the treatment was high.
Mood-Related Aspects
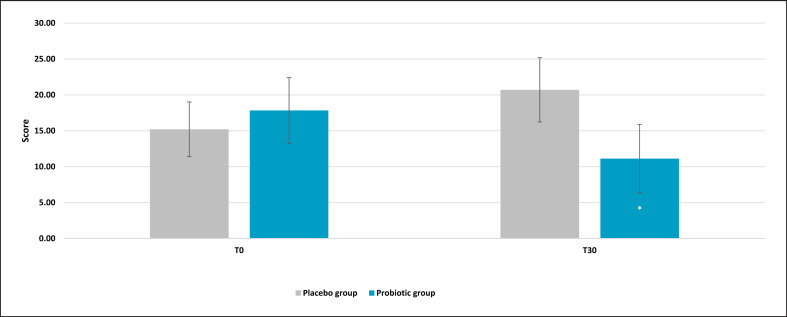
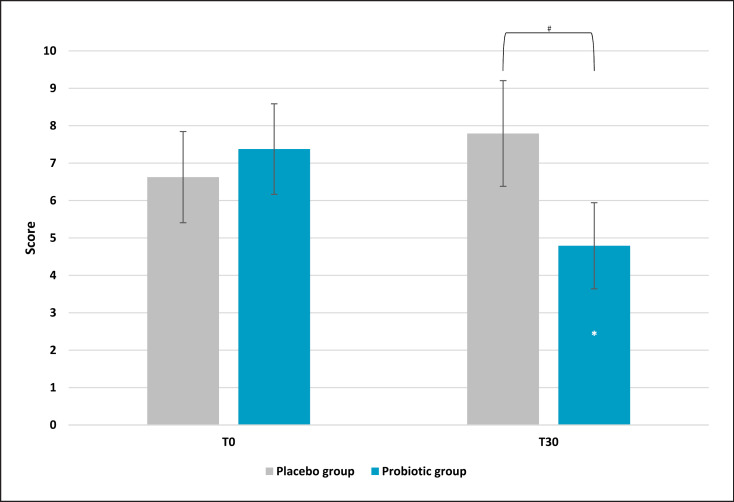
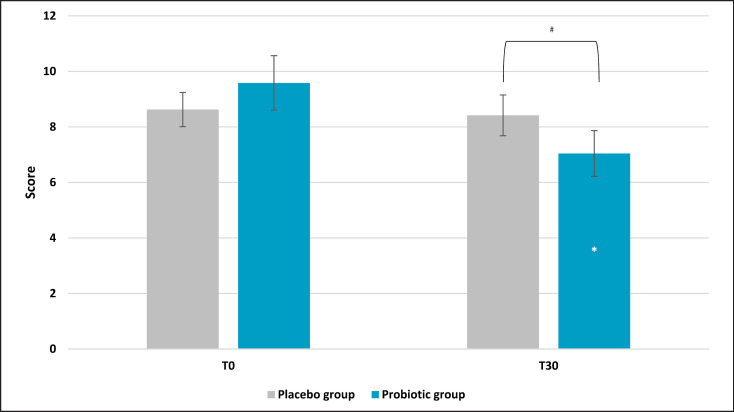
Results obtained from the global score, the TMD, showed a strong reduction of the overall mood score. Indeed, after 30 days of treatment, the active group showed a significant reduction of 44% (p = 0.025 vs. T0), while the placebo reported a higher score (+27%), reflecting a worsening of the general mood state (Fig. 2). The comparison between active and placebo results reported a significance of p = 0.0572, meaning that there was a borderline statistical significance versus placebo. In addition, a sub-analysis of each questionnaire category was assessed (see M&M section for categories' description). Anger evaluation showed a significant effect on the scores for the experimental group (Fig. 3). Results showed a lower score in the active group after probiotic intervention (mean ± SE, 4.79 ± 1.14; p = 0.028 vs. T0) compared to baseline (T0) and placebo (p = 0.032). Also, in this case, the placebo group showed a higher value compared to the active treatment. No significant results on the anger category for the placebo group were recorded. A significant effect was also found on the tension subscale scores for the experimental group (Fig. 4). Post hoc comparisons showed that this effect was lower after 30 days of probiotic intake (T30) (mean ± SE, 7.04 ± 0.82; p = 0.011 vs. T0) compared to baseline (T0) and placebo (p = 0.046). The placebo group reported a light but no statistical reduction (mean ± SE, 8.42 ± 0.73; p = 0.72 vs. T0) of tension score with respect to the active group. For the other categories, results showed no significant variation (data not shown).
Fig. 2.
Profile of Mood State: Total Mood Disturbance (TMD). Results are reported as mean values. *p < 0.05 versus baseline.
Fig. 3.
Profile of Mood State: anger subscale score. Results are reported as mean values. *p < 0.05 versus baseline, #p < 0.05 versus placebo.
Fig. 4.
Profile of Mood State: tension subscale score. Results are reported as mean values. **p < 0.01 versus baseline, #p < 0.05 versus placebo.
Sleep Quality
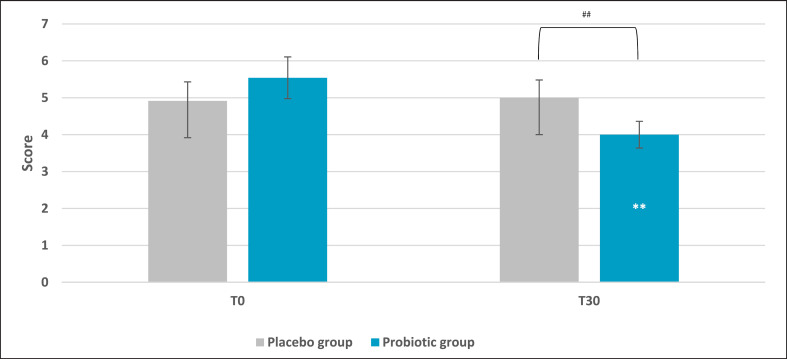
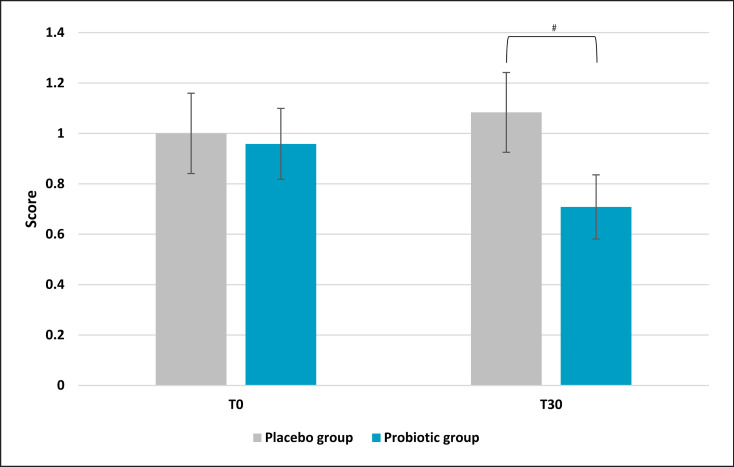
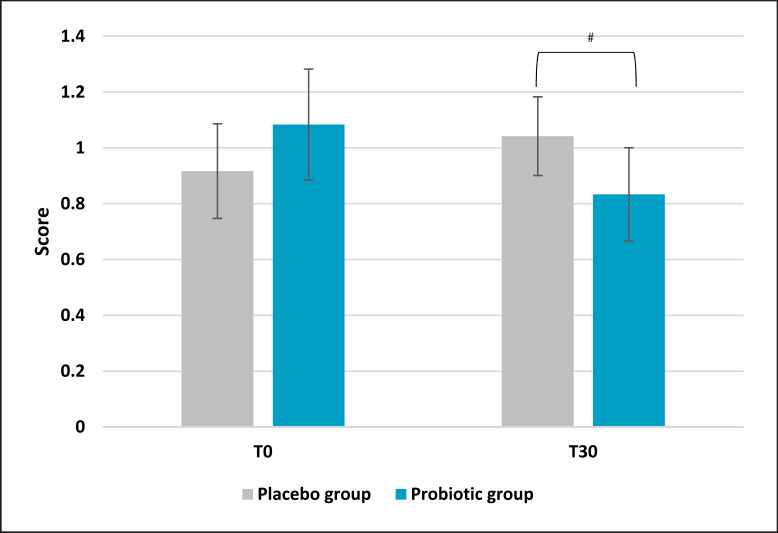
Sleep quality presented a significant improvement in the active group compared to the placebo. Results demonstrated a lower score after 30 days of probiotic intake (mean ± SD, 4.00 ± 1.64) compared to baseline (T0) (mean ± SE, 5.5 ± 0.56; p = 0.002 vs. T0) and placebo (p = 0.009) as reported in Figure 5. On the same trend, the placebo group reported a higher score if compared to the active treatment, which is linked to a worse sleep quality (+20% and −20%, respectively). No significant results were noted for the placebo group. Subscale analysis reported that among all the categories composing the questionnaire, only subjective sleep quality and sleep latency showed statistically significant results. Subjective sleep quality subscale evaluation indicated a slight but significant reduction in the active group (mean ± SE, 0.71 ± 0.13; p = 0.047 vs. placebo) compared to placebo (Fig. 6). Concerning the placebo group, no statistical results were observed at the end of the 30 days of treatment. The initial condition of subjects in the placebo group seemed to worsen, as indicated by an increase in the questionnaire score. The same trend was observed for sleep latency (Fig. 7). The statistical analysis showed a low but significant reduction of the score in the active group compared to the placebo. Comparisons showed that the score was lower after 30 days of probiotic intake (T30) (mean ± SE, 0.83 ± 0.17; p = 0.035 vs. placebo) compared to placebo. The placebo group did not show any improvement and no statistical reduction of sleep latency score.
Fig. 5.
Pittsburgh Sleep Quality Index score. Results are reported as mean values. **p < 0.01 versus baseline, ##p < 0.01 versus placebo.
Fig. 6.
Pittsburgh Sleep Quality Index: subjective sleep quality subscale score. Results are reported as mean values. #p < 0.05 versus placebo.
Fig. 7.
Pittsburgh Sleep Quality Index: sleep latency subscale score. Results are reported as mean values. #p < 0.05 versus placebo.
Discussion
The recent pandemic caused by the severe acute respiratory syndrome coronavirus 2 has had a dramatic impact on modern global society [35]. The panic caused by threats to health and the future uncertainty related to socioeconomic crisis had a devastating effect on many individuals and many aspects of everyday life [36]. Furthermore, the necessary restriction measures contributed to the worsening of the emotional state of many, leading to increased stress and anxiety [37, 38]. Several scientific papers highlighted how COVID-19 raised the level of perceived anxiety and tension all over the world [39, 40, 41, 42, 43]. In Italy, the first western country to experience lockdown, effects were overwhelming [44, 45, 46]. Even before this pandemic, stress represented an enormous burden for many people, being defined as the “Health Epidemic of the 21st Century” by the World Health Organization [47]. For these reasons, the need of a novel approach to influence mood and well-being is even more urgent. In recent years, it has become more and more evident that, through the gut-brain axis, gut microbiota can influence response to stress and that probiotics are useful to affect in a positive manner these commensal bacteria [48]. During the pandemic, probiotics were largely suggested as a possible tool to manage respiratory tract infection, gastrointestinal symptoms, and inflammation caused by severe acute respiratory syndrome coronavirus 2 [49, 50, 51, 52, 53, 54]. However, to the best of our knowledge, no evidence of actual usage for stress management was proposed.
Results obtained from this cross-over, double-blind clinical study demonstrated how probiotic intake could contribute to the modulation of mood and sleep during a stressful period. Profile of Mood State questionnaire showed a decrease of the Total Mood Disturbance score, underlining a significant reduction compared to T0 of the active group. It is worth noting that the result was close to the significance (p = 0.0572) when compared to the placebo. Therefore, we can speculate that, with a larger cohort of subjects, these results may reach a p value <0.05. The TMD is used as a marker of negative feelings: a higher TMD score is indicative of a greater mood disturbance (i.e., greater negative mood) [55, 56]. To the best of our knowledge, no other clinical studies with probiotics have focused their attention on TMD but rather on the improvement of the single category [57, 58]. The anger category showed a significant reduction in score in the active group compared to the placebo. The same trend was observed for the tension category: in the active group, there was improvement of this negative feeling with respect to both T0 and placebo. In this context, it is difficult to make a comparison with other clinical studies since there are only few using POMS with probiotics, and they show conflicting results [59, 60]. The current circumstances caused by the pandemic mean that people returned to their workplaces after months of lockdown. They have done so with paradoxical emotions; thus, it is without question that working on negative feelings is now becoming a prerogative to support worker's mental health [60, 61, 62].
Sleep quality was assessed through the validated questionnaire Pittsburgh Sleep Quality Index. Also, in this case, a reduction of the total score indicated an improvement compared to the initial condition. Several clinical trials with probiotics have focused their attention on this topic using the same questionnaire; however, no significant effect has been found [63]. According to the meta-analysis performed by Irwin and colleagues, only subgroup analysis suggested a greater but no significant effect in healthy subjects with an average treatment period of 8 weeks. On the contrary, in this cross-over clinical study, the active group experienced a significant reduction of the global PSQI score, denoting a general improvement of the sleep condition in a shorter period of time (4 weeks of probiotic intake). In addition, subscale analysis of the single category evidenced a significant reduction of perceived sleep quality as well as sleep latency score, which corresponds to an improvement of the abovementioned category. Furthermore, it is worth noting that, although we enrolled healthy subjects, the current living situation has further affected the normal life routine of many people, which is fundamental for circadian rhythms. Indeed, the study was carried out during a period when the government decided to alternate stricter to softer restrictions due to the fluctuations of contagions. It is worth noting that, even though we performed a cross-over study, we did not observe significant variations between the two groups, according to the period they took the probiotic supplement. Furthermore, as reported in several probiotic cross-over clinical trials, 4 weeks of washout were sufficient to avoid any putative bias linked to the design of the study [64, 65, 66].
In the last decade, several clinical trials have been carried out to evaluate the role of probiotics in the gut-brain axis, pointing out their ability to improve cognitive functions, ameliorate stress management, as well as sleep quality, and underlining their positive effect toward negative feelings such as anxiety [67]. Other studies demonstrated that probiotics can improve psychological and physiological markers of stress and anxiety. However, it is interesting to note that, in those studies, probiotic concentrations were dramatically higher compared to the one presented in this clinical study [68, 69, 70, 71]. Furthermore, the same concentration of the product was already tested in a proof of concept study to evaluate stress-related parameters in a group of stressed students, showing significant positive effect on mood and cognitive functions [31].
The results obtained in this study support the clinical evidence of a beneficial effect on mood and sleep quality of the tested strains, L. reuteri PBS072 and B. breve BB077, in stressed subjects. However, a core limitation of the study was related to the uncertainty of the moment that made it difficult to track fluctuations of life habits (diets, physical activity, etc.) due to frequent ministerial decree modifications according to the pandemic evolution. Furthermore, during the design of the protocol, no validated questionnaire linked to COVID-19 was available. In addition, even though everyday chronic stresses can be in part responsible for general mood and sleep alteration, our results are mainly related to COVID-19 and to the uncertainty of that period. Restrictions like social distancing and lockdowns were responsible for an increased state of anxiety and fear for the future, affecting the quality of life physically and psychologically. Any volunteer was infected during the study period.
Even though the trial was carried out on a small cohort of volunteers, data resulted sufficient to obtain significant results. For the reasons mentioned above, the choice of a cross-over clinical trial was the optimal solution to overcome the enrollment of a high number of people during pandemic and also to minimize the potential emotional bias intrinsic to each volunteer.
Another limitation observed was related to the correlation between physical and psychological parameters and the gut microbiota composition. Actually, the main outcome of the study was to investigate an improvement of mood and sleep through the oral administration of specific multi-strain probiotic formulation. The mutual connection between gut microbiota and psychophysiological aspects will be for sure investigated; further studies are needed to more deeply explore the effect and the mechanism of action of the tested probiotics in this field of research.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. This study protocol was reviewed and approved by the Ethics Committee of the University of Calabria (Italy), approval number 0007582. Written informed consent was obtained from all subjects before enrollment.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
Conceptualization: Francesco Puoci and Vincenzo Nobile; methodology, investigation, resources, writing − original draft preparation, supervision, project administration, and funding acquisition: Francesco Puoci; formal analysis, data curation, and writing − review and editing: Vincenzo Nobile. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data supporting reported results are stored at the Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, and subjected to regular backup. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank ROELMI HPC for providing the products.
Funding Statement
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Allain-Dupré D, Chatry I, Michalun V, Moisio A. The territorial impact of COVID-19 managing the crisis across levels of government. OECD. 2020:70. [Google Scholar]
- 2.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17((5)):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahorsu DK, Lin CY, Imani V, Saffari M, Griffiths MD, Pakpour AH. The fear of COVID-19 scale development and initial validation. Int J Ment Health Addict. 2022;20((3)):1537–1545. doi: 10.1007/s11469-020-00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raza SH, Haq W, Sajjad M. COVID-19 a psychosocial perspective. Front Psychol. 2020;11:554624. doi: 10.3389/fpsyg.2020.554624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuntella O, Hyde K, Saccardo S, Sadoff S. Lifestyle and mental health disruptions during COVID-19. Proc Natl Acad Sci U S A. 2021;118((9)):e2016632118. doi: 10.1073/pnas.2016632118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmastro M, Zamariola G. Depressive symptoms in response to COVID-19 and lockdown a cross-sectional study on the Italian population. Sci Rep. 2020;10((1)):22457. doi: 10.1038/s41598-020-79850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amit Aharon A, Dubovi I, Ruban A. Differences in mental health and health-related quality of life between the Israeli and Italian population during a COVID-19 quarantine. Qual Life Res. 2021;30((6)):1675–1684. doi: 10.1007/s11136-020-02746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonacini L, Gallo G, Scicchitano S. Working from home and income inequality risks of a 'new normal' with COVID-19. J Popul Econ. 2020;34:303–360. doi: 10.1007/s00148-020-00800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auriemma V, Iannaccone C. COVID-19 pandemic socio-economic consequences of social distancing measures in Italy. Front Sociol. 2020;5:575791. doi: 10.3389/fsoc.2020.575791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trougakos JP, Chawla N, McCarthy JM. Working in a pandemic exploring the impact of COVID-19 health anxiety on work, family, and health outcomes. J Appl Psychol. 2020;105((11)):1234–1245. doi: 10.1037/apl0000739. [DOI] [PubMed] [Google Scholar]
- 11.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6((2)):603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function a review. EXCLI J. 2017;16:1057–1072. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirotsu C, Tufik S, Andersen ML. Interactions between sleep and metabolism from physiological to pathological conditions. Sleep Sci. 2015;8((3)):143–152. doi: 10.1016/j.slsci.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes M. The cytokine hypothesis of depression inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008;29((3)):287–291. [PubMed] [Google Scholar]
- 15.Ilchmann-Diounou H, Menard S. Psychological stress intestinal barrier dysfunctions and autoimmune disorders an overview. Front Immunol. 2020;11:1823. doi: 10.3389/fimmu.2020.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooks KB, O'Malley MA. Dysbiosis and its discontents. mBio. 2017;8((5)):e01492–e01517. doi: 10.1128/mBio.01492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30((6)):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonini M, Lo Conte M, Sorini C, Falcone M. How the interplay between the commensal microbiota, gut barrier integrity, and mucosal immunity regulates brain autoimmunity. Front Immunol. 2019;10:1937. doi: 10.3389/fimmu.2019.01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions metabolism of nutrients and other food components. Eur J Nutr. 2018;57((1)):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167((4)):915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19((4)):241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 22.Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340. doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 23.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina-Torres G, Rodriguez-Arrastia M, Roman P, Sanchez-Labraca N, Cardona D. Stress and the gut microbiota-brain axis. Behav Pharmacol. 2019;30((2 and 33-Spec Issue)):187–200. doi: 10.1097/FBP.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 25.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117((1)):93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid G. Disentangling what we know about microbes and mental health. Front Endocrinol. 2019;10:81. doi: 10.3389/fendo.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Niu Z, Zou M, Liu S, Wang M, Gu X, et al. Probiotics and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J Dairy Sci. 2020;103((7)):5816–5829. doi: 10.3168/jds.2019-18003. [DOI] [PubMed] [Google Scholar]
- 29.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6((1)):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieërs G, Belkhir L, Enaud R, Leclercq S, Philippart de Foy JM, Dequenne I, et al. How probiotics affect the microbiota. Front Cell Infect Microbiol. 2019;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nobile V, Giardina S, Puoci F. The effect of a probiotic complex on the gut-brain Axis a translational study. Neuropsychobiology. 2021;81((2)):116–126. doi: 10.1159/000518385. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, et al. Relationships between the Pittsburgh sleep quality index (PSQI) epworth sleepiness scale (ESS) and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;04((06)):563–571. [PMC free article] [PubMed] [Google Scholar]
- 33.Farnè M, Sebellico A, Gnugnoli D, Corallo A. POMS profile of mood states: manuale adattamento italiano. Firenze: GiuntiOS. 1991 [Google Scholar]
- 34.Curcio G, Tempesta D, Scarlata S, Marzano C, Moroni F, Rossini PM, et al. Validity of the Italian version of the Pittsburgh sleep quality index (PSQI) Neurol Sci. 2013;34((4)):511–519. doi: 10.1007/s10072-012-1085-y. [DOI] [PubMed] [Google Scholar]
- 35.Zoumpourlis V, Goulielmaki M, Rizos E, Baliou S, Spandidos DA. (Comment) the COVID-19 pandemic as a scientific and social challenge in the 21st century. Mol Med Rep. 2020;22((4)):3035–3048. doi: 10.3892/mmr.2020.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhtar S. Psychological health during the coronavirus disease 2019 pandemic outbreak. Int J Soc Psychiatry. 2020;66((5)):512–516. doi: 10.1177/0020764020925835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ploumpidis D. Living with covid-19. Psychiatriki. 2020;31((3)):197–200. doi: 10.22365/jpsych.2020.313.197. [DOI] [PubMed] [Google Scholar]
- 38.French MT, Mortensen K, Timming AR. Psychological distress and coronavirus fears during the initial phase of the COVID-19 pandemic in the United States. J Ment Health Policy Econ. 2020;23((3)):93–100. [PubMed] [Google Scholar]
- 39.Droit-Volet S, Gil S, Martinelli N, Andant N, Clinchamps M, Parreira L, et al. Time and Covid-19 stress in the lockdown situation time free, «Dying» of boredom and sadness. PLoS One. 2020;15((8)):e0236465. doi: 10.1371/journal.pone.0236465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torales J, O'Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020;66((4)):317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 41.Khademian F, Delavari S, Koohjani Z, Khademian Z. An investigation of depression and stress and its relating factors during COVID-19 pandemic in Iran. BMC Public Health. 2021;21((1)):275. doi: 10.1186/s12889-021-10329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehman U, Shahnawaz MG, Khan NH, Kharshiing KD, Khursheed M, Gupta K, et al. Depression anxiety and stress among Indians in times of covid-19 lockdown. Community Ment Health J. 2021;57((1)):42–48. doi: 10.1007/s10597-020-00664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdulah DM, Mohammed AA. The consequences of the COVID-19 pandemic on perceived stress in clinical practice experience of doctors in Iraqi Kurdistan. Rom J Intern Med. 2020;58((4)):219–227. doi: 10.2478/rjim-2020-0020. [DOI] [PubMed] [Google Scholar]
- 44.Rossi R, Socci V, Talevi D, Mensi S, Niolu C, Pacitti F, et al. COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatry. 2020;11:790. doi: 10.3389/fpsyt.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simione L, Gnagnarella C. Differences between health workers and general population in risk perception, behaviors, and psychological distress related to COVID-19 spread in Italy. Front Psychol. 2020;11:2166. doi: 10.3389/fpsyg.2020.02166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spinelli M, Lionetti F, Pastore M, Fasolo M. Parents' stress and children's psychological problems in families facing the COVID-19 outbreak in Italy. Front Psychol. 2020;11:1713. doi: 10.3389/fpsyg.2020.01713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fink G. Reference module in neuroscience and biobehavioral psychology. Amsterdam: Elsevier; 2016. Stress concepts, definition and history; pp. p. 549–555. [Google Scholar]
- 48.Ma T, Jin H, Kwok LY, Sun Z, Liong MT, Zhang H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol Stress. 2021;14:100294. doi: 10.1016/j.ynstr.2021.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darbandi A, Asadi A, Ghanavati R, Afifirad R, Darb Emamie A, Kakanj M, et al. The effect of probiotics on respiratory tract infection with special emphasis on COVID-19 systemic review 2010-20. Int J Infect Dis. 2021;105:91–104. doi: 10.1016/j.ijid.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conte L, Toraldo DM. Targeting the gut-lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Ther Adv Respir Dis. 2020;14:175346662093717. doi: 10.1177/1753466620937170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akour A. Probiotics and COVID-19 is there any link? Lett Appl Microbiol. 2020;71((3)):229–234. doi: 10.1111/lam.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bottari B, Castellone V, Neviani E. Probiotics and covid-19. Int J Food Sci Nutr. 2021;72((3)):293–299. doi: 10.1080/09637486.2020.1807475. [DOI] [PubMed] [Google Scholar]
- 53.Santacroce L, Inchingolo F, Topi S, Del Prete R, Di Cosola M, Charitos IA, et al. Potential beneficial role of probiotics on the outcome of COVID-19 patients an evolving perspective. Diabetes Metab Syndr Clin Res Rev. 2021;15((1)):295–301. doi: 10.1016/j.dsx.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anwar F, Altayb HN, Al-Abbasi FA, Al-Malki AL, Kamal MA, Kumar V. Antiviral effects of probiotic metabolites on COVID-19. J Biomol Struct Dyn. 2021;39((11)):4175–4184. doi: 10.1080/07391102.2020.1775123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breymeyer KL, Lampe JW, McGregor BA, Neuhouser ML. Subjective mood and energy levels of healthy weight and overweight/obese healthy adults on high-and low-glycemic load experimental diets. Appetite. 2016;107:253–259. doi: 10.1016/j.appet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Box AG, Feito Y, Petruzzello SJ, Mangine GT. Mood state changes accompanying the crossfit Open™ competition in healthy adults. Sports. 2018;6((3)):67. doi: 10.3390/sports6030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marotta A, Sarno E, Del Casale A, Pane M, Mogna L, Amoruso A, et al. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front Psychiatry. 2019;10:164. doi: 10.3389/fpsyt.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michalickova D, Minic R, Dikic N, Andjelkovic M, Kostic-Vucicevic M, Stojmenovic T, et al. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2016;41((7)):782–789. doi: 10.1139/apnm-2015-0541. [DOI] [PubMed] [Google Scholar]
- 59.Malinen SK, Wong JHK, Näswall K. Effective workplace strategies to support employee wellbeing during a pandemic. NZJER. 2020;45((2)):17–32. [Google Scholar]
- 60.Manucci M. How People Come Back to Workplaces during the Pandemic three dimensions of intervention for new emotional performance conditions. Hum Resource Development Int. 2021;24((4)):446–453. [Google Scholar]
- 61.Trougakos JP, Chawla N, McCarthy JM. Working in a pandemic exploring the impact of COVID-19 health anxiety on work, family, and health outcomes. J Appl Psychol. 2020;105((11)):1234–1245. doi: 10.1037/apl0000739. [DOI] [PubMed] [Google Scholar]
- 62.Irwin C, McCartney D, Desbrow B, Khalesi S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74((11)):1536–1549. doi: 10.1038/s41430-020-0656-x. [DOI] [PubMed] [Google Scholar]
- 63.Solito A, Bozzi Cionci N, Calgaro M, Caputo M, Vannini L, Hasballa I, et al. Supplementation with Bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross-over randomized double-blind placebo-controlled trial. Clin Nutr. 2021;40((7)):4585–4594. doi: 10.1016/j.clnu.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Mohd Redzwan S, Abd Mutalib MS, Wang JS, Ahmad Z, Kang MS, Abdul Rahman N'A, et al. Effect of supplementation of fermented milk drink containing probiotic Lactobacillus casei Shirota on the concentrations of aflatoxin biomarkers among employees of Universiti Putra Malaysia a randomised, double-blind, cross-over, placebo-controlled study. Br J Nutr. 2016;115((1)):39–54. doi: 10.1017/S0007114515004109. [DOI] [PubMed] [Google Scholar]
- 65.Maneerat S, Lehtinen MJ, Childs CE, Forssten SD, Alhoniemi E, Tiphaine M, et al. Consumption of Bifidobacterium lactis Bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes. J Nutr Sci. 2014;2:e44. doi: 10.1017/jns.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang N, Zhang Y, Li M, Wang W, Liu Z, Xi C, et al. Efficacy of probiotics on stress in healthy volunteers a systematic review and meta-analysis based on randomized controlled trials. Brain Behav. 2020;10((9)):e01699. doi: 10.1002/brb3.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patterson E, Griffin SM, Ibarra A, Ellsiepen E, Hellhammer J. Lacticaseibacillus paracasei Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults a randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study) Neurobiol Stress. 2020;13:100277. doi: 10.1016/j.ynstr.2020.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Önning G, Hillman M, Hedin M, Montelius C, Eriksson J, Ahrné S, et al. Intake of Lactiplantibacillus plantarum HEAL9 reduces the inflammatory markers soluble fractalkine and CD163 during acute stress a randomized, double blind, placebo-controlled study. Physiol Behav. 2020;225:113083. doi: 10.1016/j.physbeh.2020.113083. [DOI] [PubMed] [Google Scholar]
- 69.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder a randomized clinical trial. Clin Nutr. 2019;38((2)):522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Wallace CJK, Foster JA, Soares CN, Milev RV. The effects of probiotics on symptoms of depression protocol for a double-blind randomized placebo-controlled trial. Neuropsychobiology. 2020;79((1)):108–116. doi: 10.1159/000496406. [DOI] [PubMed] [Google Scholar]
- 71.Rudzki L, Ostrowska L, Pawlak D, Małus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting reported results are stored at the Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, and subjected to regular backup. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.