Abstract
Background/Aim: The combination of bevacizumab and atezolizumab (Bev-Ate) has been established as a standard first-line systemic treatment option for unresectable hepatocellular carcinoma (HCC) since 2020. This study examined the outcomes of HCC patients who received the combination in southern Taiwan.
Patients and Methods: All patients were enrolled from four hospitals in Taiwan. They received Bev-Ate therapy for unresectable HCC.
Results: Thirty-five patients were included; 28 (80%) had Barcelona Clinic Liver Cancer stage C disease. Hepatitis etiology was chronic hepatitis B and C in 63% and 17% of patients, respectively. Eleven (31%) patients had received prior systemic treatment for unresectable HCC. The response rate was 51%, and the disease control rate was 72% for all patients. The median progression-free survival (PFS) and overall survival (OS) was 5.2 and 22.2 months, respectively. For patients who received prior systemic treatment, the efficacy of Bev-Ate in terms of response rates was similar compared with those without prior systemic treatment. Patients who received lower doses of bevacizumab (<15 mg/kg per dose) had non-inferior PFS and OS compared with those receiving a standard dose of bevacizumab. The incidence of proteinuria of all grades (15.8%) was less common when lower doses of bevacizumab were used.
Conclusion: Real world data from HCC patients in southern Taiwan disclosed that the efficacy outcomes of Bev-Ate treatment were generally consistent with those of clinical trials in other countries. In patients who were exposed to prior systemic treatment or who received lower doses of bevacizumab, the Bev-Ate regimen retained its clinical efficacy.
Keywords: Antiangiogenic therapy, hepatocellular carcinoma, immune checkpoint inhibitor, real world data
Sorafenib was the first drug approved for unresectable hepatocellular carcinoma (HCC) in 2008 (1). Recent trends in the management of unresectable HCC involve the combination of an immune checkpoint inhibitor and anti-angiogenic agent, since the combination of atezolizumab and bevacizumab significantly prolonged progression-free survival (PFS) and overall survival (OS) in patients with unresectable HCC in the IMbrave 150 phase 3 trial in 2020 (2). All enrolled cases in this trial were systemic treatment naïve, had unresectable HCC, and a liver reserve of Child-Pugh A. The recommended standard dose of atezolizumab was 1,200 mg intravenously and 15 mg/kg for bevacizumab, which was administrated every 3 weeks. HCC patients with liver reserves of Child-Pugh B were excluded from two important phase III clinical trials that supported current standard first treatment with lenvatinib or atezolizumab plus bevacizumab for unresectable HCC (2,3). Only 20 patients with Child-Pugh B, accounting for 3.3% of study subjects, were enrolled in the landmark SHARP study of sorafenib as first line treatment (1). As a result, the poor inclusion of liver reserve led to poor clinical evidence to suggest the standard upfront treatment in HCC patients with Child-Pugh B.
By reviewing the efficacy and safety of the combination of atezolizumab and bevacizumab in treating patients with HCC, Chinese subjects tended to develop more proteinuria in all grades compared with the global population (4). Vascular endothelial growth factor (VEGF) inhibitors, including bevacizumab and ramucirumab are associated with nephrotoxicity, most commonly proteinuria and hypertension. Several studies have demonstrated that VEGF inhibition induces proteinuria in a dose-dependent manner. Higher dose of bevacizumab and longer treatment duration may contribute to higher incidence of proteinuria in Chinese subjects in IMbrave 150 trial (5). The optimal dose of bevacizumab remains a challenge because there is need to balance the efficacy of the atezolizumab/bevacizumab regimen and high incidence of proteinuria. It is important to explore the efficacy of atezolizumab/bevacizumab for patients who receive lower doses of bevacizumab in this combination.
Patients and Methods
This study retrospectively reviewed 35 patients with unresectable HCC who were treated with atezolizumab and bevacizumab between June 2019 and November 2021 in four independent institutions (Tainan Medical Oncology Group) in Taiwan. The inclusion criteria included: 1) HCC diagnosed by pathologic diagnosis or radiological evaluation using enhanced computed tomography (CT) or magnetic resonance imaging (MRI); 2) complete follow-up from the initial treatment until death or the study censor time (21 January 2022). Patients with liver reserve of Child-Pugh C were excluded. Clinical characteristics, the information of patients, therapeutic responses including PFS, OS, radiological findings, and simplified adverse events (AE) were recorded for analysis. Patient information from the medical records included sex, age, performance, Child-Pugh class, alpha-fetoprotein (AFP), HCC etiology, and prior systemic therapy for HCC. HCC was classified using the Barcelona Clinic Liver Cancer staging system. Macrovascular invasion (MVI) and extrahepatic spread (EHS) were determined by radiographic information. This study included patients who had MVI of the main portal trunk, bile duct invasion, or at least 50% hepatic involvement - any of these three features was defined as high risk.
The combination therapy of atezolizumab and bevacizumab was performed according to the pharmaceutical recommendations. Patients received 1,200 mg of atezolizumab plus bevacizumab intravenously every 3 weeks. Standard dosage (SD) of bevacizumab was 15 mg/kg and lower dose (LD) of bevacizumab was allowed in this real-world study.
Tumors were assessed by CT or MRI after initiation of treatment. The therapeutic response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (6). Response rate was defined as the percentage of patients who had a confirmed complete or partial response. complete response. Disease control rate (DCR) refers to the percentage of patients whose therapeutic intervention has led to a complete response, partial response, or stable disease
Adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Hypertension and proteinuria were monitored regularly. Other AEs were not required to be reported.
Statistical analysis. All statistical analyses were performed using SigmaPlot (9.0 version, CA, USA). PFS and OS were calculated using the Kaplan-Meier method and statistically analyzed using the log-rank test. p<0.05 was considered statistically significant.
Results
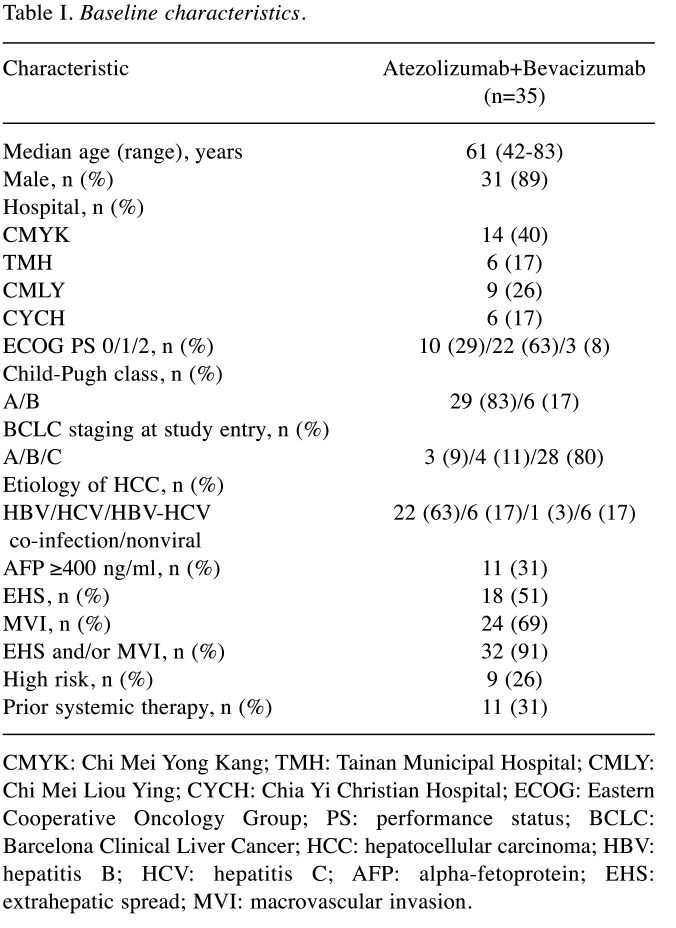
A total of 35 patients from four institutes (Chi Mei Medical Center, Chi Mei Medical Center Liouying, Tainan Municipal Hospital and Chiayi Christian Hospital) were treated with atezolizumab plus bevacizumab during the study period. The characteristics of the patients are summarized in Table I. The median age was 61 years (range=42-83 years) and only 4 patients were female. Three patients with Eastern Cooperative Oncology Group (ECOG) performance status of 2 were enrolled in this study. Child-Pugh class A and B was observed in 29 and 6 patients, respectively. BCLC stage A, B, and C at study entry were 3, 4, and 28 patients, respectively. The most common etiology of liver disease was viral hepatitis, including 22 with hepatitis B, 6 with hepatitis C, and 1 with both hepatitis B and C. Eleven patients had AFP levels more than 400 ng/ml. Thirty-two patients had EHS and/or MVI. Nine patients presented with high-risk features and 11 patients had experienced prior systemic therapy.
Table I. Baseline characteristics.
CMYK: Chi Mei Yong Kang; TMH: Tainan Municipal Hospital; CMLY: Chi Mei Liou Ying; CYCH: Chia Yi Christian Hospital; ECOG: Eastern Cooperative Oncology Group; PS: performance status; BCLC: Barcelona Clinical Liver Cancer; HCC: hepatocellular carcinoma; HBV: hepatitis B; HCV: hepatitis C; AFP: alpha-fetoprotein; EHS: extrahepatic spread; MVI: macrovascular invasion.
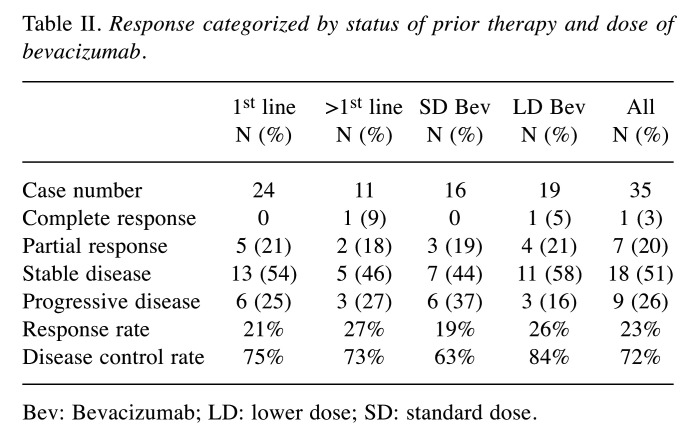
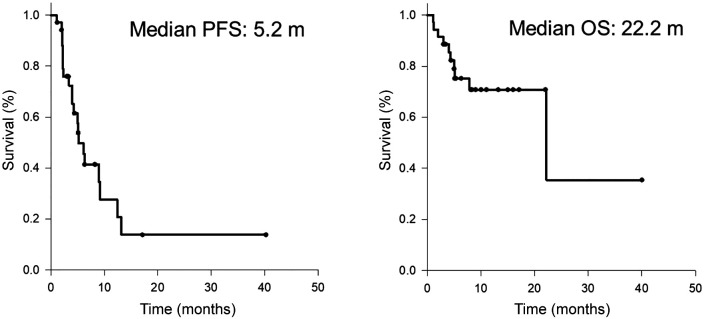
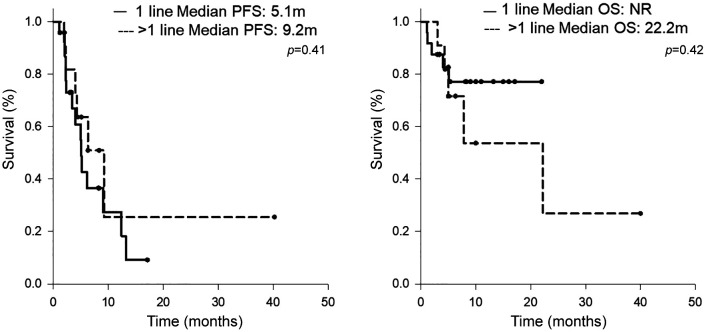
Efficacy analysis. The radiological therapeutic response to atezolizumab plus bevacizumab is shown in Table II. Using RECIST 1.1. to assess the response rate in all patients, complete response (CR) and partial response (PR) were observed in 1 patient (3%) and 7 patients (20%), respectively. Stable disease was observed in 18 patients (51%) and progressive disease (PD) in 9 patients (26%). The disease control rate was 72%. The median PFS was 5.2 months, and the median OS was 22.2 months, as shown in Figure 1. The differences in the therapeutic effects in prior systemic therapy naïve patients and those with prior systemic therapy are demonstrated in Table II and Figure 2. The response rate in prior systemic therapy naïve cases was 21% and 27% in those with prior systemic therapy. The median PFS for prior systemic therapy naïve cases was 5.1 months and the patients did not reach the median OS time during the observation period. In patients who had undergone prior systemic therapy, the median PFS and median OS were 9.2 months and 22.2 months, respectively. There were no significant differences in the response rate and survival outcome between these two groups.
Table II. Response categorized by status of prior therapy and dose of bevacizumab.
Bev: Bevacizumab; LD: lower dose; SD: standard dose.
Figure 1. Kaplan-Meier curves of (A) progression-free survival and (B) overall survival.
Figure 2. Kaplan-Meier curves of (A) progression-free survival and (B) overall survival, categorized by lines of treatment.
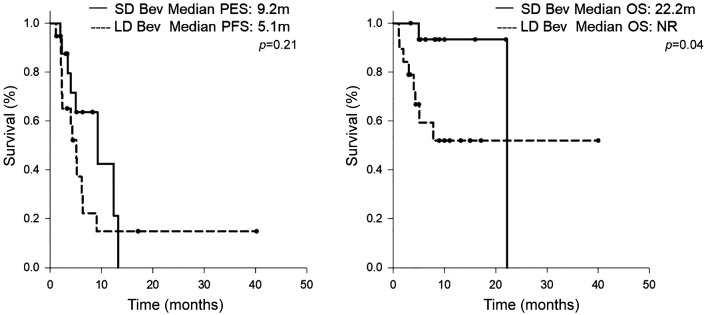
The recommended dose of bevacizumab based on the IMbrave 150 trial was 15 mg/kg. To investigate the standard dose of bevacizumab compared with the lower dose of bevacizumab in the atezolizumab/bevacizumab combination therapy for patients with advanced HCC, we assessed the response rate and survival outcome in the SD bevacizumab group and LD bevacizumab group. The dosage of LD bevacizumab was either 7.5 mg/kg or a fixed dose of 500 mg. The response rate and disease control rate were 19% and 63% for 16 cases of SD bevacizumab. For 19 cases of LD bevacizumab, the response rate was 26% and one case achieved complete response. The median PFS and median OS for the SD bevacizumab group were 9.2 months and 22.2 months, respectively. The patients who received LD bevacizumab in the combination had 5.1 months of median PFS (Figure 3). However, in respect to median OS, the LD bevacizumab group did not reach the median OS time during the observation period.
Figure 3. Kaplan-Meier curves of (A) progression-free survival and (B) overall survival, categorized by the dosage of bevacizumab. LD: Low dose; SD: standard dose.
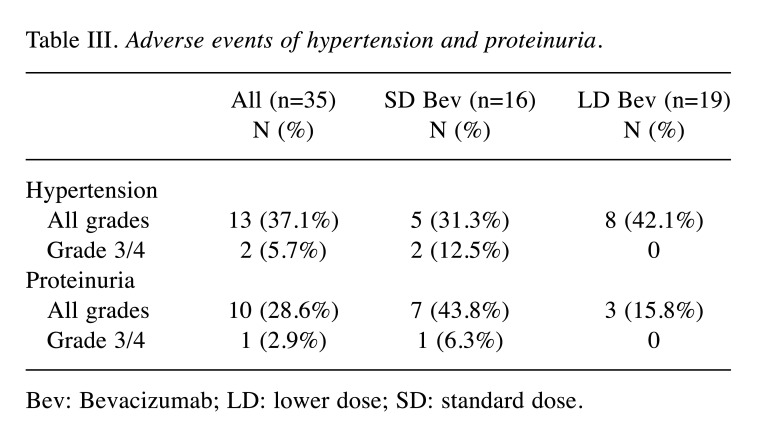
Hypertension and proteinuria. The incidence and grading of hypertension and proteinuria are listed in Table III. Among the 35 study patients, 13 (37.1%) experienced hypertension of any grade and two patients (5.7%) had grade 3 or 4 of hypertension. Ten patients (28.6%) experienced proteinuria of any grade and grade 3 or 4 was noted in one patient. The incidences of hypertension and proteinuria were similar to those of the Chinese extension cohort of IMbrave 150 study (4). However, when compared with the SD bevacizumab group, patients who received LD bevacizumab experienced a lower incidence of proteinuria of all grades (15.8%).
Table III. Adverse events of hypertension and proteinuria.
Bev: Bevacizumab; LD: lower dose; SD: standard dose.
Discussion
Efficacy. Treatment with the combination of atezolizumab and bevacizumab has become the new standard of care for the first line treatment of patients with advanced HCC. Many real-world reports have defined the clinical efficacy and safety of the new standard regimen and also explored the efficacy of the combination for these patients who cannot merit the original eligibility criteria of IMbrave 150 trail. The efficacy and safety based on real world data (RWD) were consistent with those reported from IMbrave 150 trial (7,8). A report of 147 HCC patients treated with atezolizumab/bevacizumab in Germany and Austria demonstrated that prior systemic treatment did not affect OS or PFS, which reflected our data presented here. So far, no phase III randomized trial of immune checkpoint inhibitor combined regimens has defined the clinical efficacy of atezolizumab/bevacizumab compared with current standard therapy of regorafenib or ramucirumab in second line setting (9,10). A phase III study (KEYNOTE-240) of pembrolizumab monotherapy, a PD-1 monoclonal antibody, failed to achieve statistical significance in OS and PFS in previously treated patients with advanced HCC (11). It thus remains controversial to utilize immune checkpoint inhibitor therapy in second line setting or beyond.
Pugh B HCC. Systemic therapies for advanced HCC have only been approved for patients with Child-Pugh class A. Only limited data are available regarding sorafenib in patients with Child-Pugh class B (1,12). Among 35 patients in our study, 6 patients had liver function of Child-Pugh score (CPS) 7 and 8. Stable disease was observed in 5 patients with Child-Pugh class B and one patient had PD. No randomized trial of immune checkpoint inhibitor has been performed in patients with Child-Pugh class B. Only a prospective trial of nivolumab (CheckMate 040), an immune checkpoint inhibitor, evaluated advanced HCC patients with liver dysfunction. An overall response rate of 10.2% was reported in 49 patients with CPS 7 and 8. In addition, the safety profile was comparable with that of cohorts of patients with Child-Pugh class A (13).
In a study based on RWD of 73 patients with impaired liver function and prior systemic therapy, Child-Pugh class A patients reached a median OS of 12.0 months compared to 6.8 months in the Child-Pugh class B group treated with atezolizumab and bevacizumab (5), Although there is no evidence to support the benefit of systemic therapy in HCC patients with Child-Pugh class B, first line treatment with sorafenib or lenvatinib has been applied to treat HCC patients with Child-Pugh class B in the USA (14).
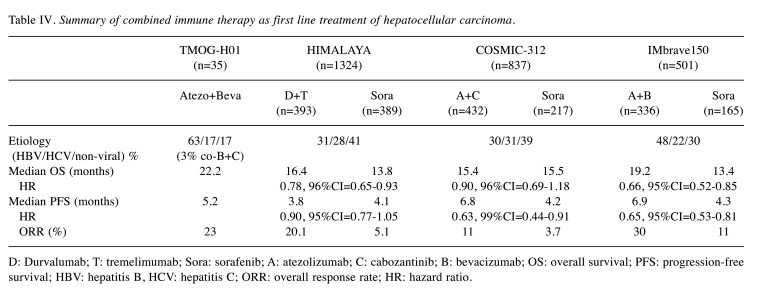
LD Bevacizumab. Hypertension, fatigue, and proteinuria are common adverse effects of atezolizumab-bevacizumab in patients with advanced HCC (2). In 194 Chinese subjects of the IMbrave150 trial, proteinuria of any grade was the most common adverse event in up to 35.6% of the cases treated with the atezolizumab-bevacizumab combination (4). The reason of the high incidence of proteinuria in the Chinese population is unclear. It cannot be merely explained by the exposure duration of bevacizumab, because the median PFS of atezolizumab-bevacizumab group was 6.8 months in the global population and 5.7 months in the Chinese population. A retrospective study of 154 patients with gynecological malignancies demonstrated that the cumulative doses of bevacizumab were associated with proteinuria toxicity. The cumulative incidence of all grades and grade 3/4 proteinuria plateaued at around 35% for all grades and 3% for grade 3 and 4, and occurred at bevacizumab doses above 11,190 mg and 4,530 mg, respectively (15). It is of importance to explore the treatment efficacy and incidence of proteinuria when patients are treated with LD bevacizumab. Nineteen of 35 patients in our study were treated with LD bevacizumab, but complete response was assessed in one patient (5%) and 4 patients had PR (21%). The response rate was not inferior compared to patients who received SD bevacizumab. The impact of different doses of bevacizumab on the safety and treatment outcome in patients with malignancies remains controversial. Half-dose (7.5 mg/kg) of bevacizumab in relapsed ovarian cancer had similar effectiveness compared to a 15 mg/kg dose in a retrospective study in Turkey. No new safety information was reported but a lower rate of grade 3 or above hypertension was observed (16). In a retrospective analysis of 118 patients with progressive glioblastoma, the OS of patients treated with reduced dose (5 mg/kg 2-weekly) of bevacizumab was not inferior to that in those treated with standard dose (10 mg/kg 2-weekly) of bevacizumab monotherapy in Ireland (17). Our study demonstrated that patients treated with LD bevacizumab had less incidence of all grades of proteinuria compared with those treated with SD bevacizumab (15.8% vs. 43.8%). The survival outcomes from another two phase III randomized trials, HIMALAYA trial and COSMIC-312 trial, are summarized in addition to those of the IMbrave150 trial in Table IV (18,19). Compared with these combinational regimens of immune checkpoint inhibitor from the three randomized clinical trials, the response rate, PFS and OS of these 35 cases in this study were comparable with these referenced regimens. Although this conclusion requires further investigation, it provides a potential solution to reduce proteinuria at safety levels, without compromising the efficacy of the combination of atezolizumab and bevacizumab.
Table IV. Summary of combined immune therapy as first line treatment of hepatocellular carcinoma.
D: Durvalumab; T: tremelimumab; Sora: sorafenib; A: atezolizumab; C: cabozantinib; B: bevacizumab; OS: overall survival; PFS: progression-free survival; HBV: hepatitis B, HCV: hepatitis C; ORR: overall response rate; HR: hazard ratio.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Yang-Cheng Lee, Wen-Tsung Huang and Ming-Yang Lee: Study design and data analysis; Chao-Jung Tsao: data analysis; Yin-Hsun Feng: Study design, data analysis, and manuscript preparation.
Acknowledgements
The Authors appreciate the staff from the cancer centers of the four participating hospitals. The Authors also thank the funds from Roche Taipei, Taiwan, R.O.C. and Chi Mei Medical Center, Tainan, Taiwan, R.O.C.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, IMbrave150 Investigators Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4.Qin S, Ren Z, Feng YH, Yau T, Wang B, Zhao H, Bai Y, Gu S, Li L, Hernandez S, Xu DZ, Mulla S, Wang Y, Shao H, Cheng AL. Atezolizumab plus bevacizumab versus sorafenib in the chinese subpopulation with unresectable hepatocellular carcinoma: phase 3 randomized, open-label IMbrave150 study. Liver Cancer. 2021;10(4):296–308. doi: 10.1159/000513486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu C, Rimassa L, Sun HC, Vogel A, Kaseb AO. Immunotherapy in hepatocellular carcinoma: evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther Adv Med Oncol. 2021;13:17588359211031141. doi: 10.1177/17588359211031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, de Vries E. RECIST 1.1 - Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur J Cancer. 2016;62:138–145. doi: 10.1016/j.ejca.2016.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Castro T, Jochheim LS, Bathon M, Welland S, Scheiner B, Shmanko K, Roessler D, Ben Khaled N, Jeschke M, Ludwig JM, Marquardt JU, Weinmann A, Pinter M, Lange CM, Vogel A, Saborowski A. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: a real-world experience. Ther Adv Med Oncol. 2022;14:17588359221080298. doi: 10.1177/17588359221080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwamoto H, Shimose S, Noda Y, Shirono T, Niizeki T, Nakano M, Okamura S, Kamachi N, Suzuki H, Sakai M, Kajiwara A, Itano S, Tanaka M, Yamaguchi T, Kuromatsu R, Koga H, Torimura T, on behalf of The Kurume Liver Cancer Study Group of Japan Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers (Basel) 2021;13(11):2786. doi: 10.3390/cancers13112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G, RESORCE Investigators Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M, REACH Trial Investigators Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL, KEYNOTE-240 investigators Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 12.Abou-Alfa GK, Amadori D, Santoro A, Figer A, De Greve J, Lathia C, Voliotis D, Anderson S, Moscovici M, Ricci S. Safety and efficacy of sorafenib in patients with hepatocellular carcinoma (HCC) and Child-Pugh A versus B cirrhosis. Gastrointest Cancer Res. 2011;4(2):40–44. [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo M, Matilla A, Santoro A, Melero I, Gracián AC, Acosta-Rivera M, Choo SP, El-Khoueiry AB, Kuromatsu R, El-Rayes B, Numata K, Itoh Y, Di Costanzo F, Crysler O, Reig M, Shen Y, Neely J, Tschaika M, Wisniewski T, Sangro B. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75(3):600–609. doi: 10.1016/j.jhep.2021.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Klink AJ, Marshall LZ, Aly A, Seal B, Healey MJ, Feinberg B. Real-world treatment patterns and reasons for therapy selection in patients with advanced hepatocellular carcinoma in US oncology practices. Oncologist. 2022;27(3):e265–e272. doi: 10.1093/oncolo/oyab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SP, Hsu HC, Tai YJ, Chen YL, Chiang YC, Chen CA, Cheng WF. Bevacizumab dose affects the severity of adverse events in gynecologic malignancies. Front Pharmacol. 2019;10:426. doi: 10.3389/fphar.2019.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kose F, Alemdaroglu S, Mertsoylu H, Besen AA, Guler OC, Simsek SY, Erbay G, Onal C, Celik H. Half-dose bevacizumab experience in relapsed ovarian cancer patients in Turkey due to formal regulations: similar effectiveness with lower rate of hypertension. J BUON. 2020;25(4):1928–1934. [PubMed] [Google Scholar]
- 17.Gleeson JP, Keane F, Keegan NM, Mammadov E, Harrold E, Alhusaini A, Harte J, Eakin-Love A, O’Halloran PJ, MacNally S, Hennessy BT, Breathnach OS, Grogan L, Morris PG. Similar overall survival with reduced vs. standard dose bevacizumab monotherapy in progressive glioblastoma. Cancer Med. 2020;9(2):469–475. doi: 10.1002/cam4.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M. Durvalumab Plus Tremelimumab: A novel combination immunotherapy for unresectable hepatocellular carcinoma. Liver Cancer. 2022;11(2):87–93. doi: 10.1159/000523702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]