Abstract
Multidisciplinary treatment for esophageal cancer leads to nutritional and inflammatory changes. Recent studies showed that nutritional and inflammatory changes during multidisciplinary treatment affect both short and long-term oncological outcomes in esophageal cancer treatment. Therefore, evaluation of the nutritional and inflammatory status during treatment is necessary in order to optimize and utilize multidisciplinary therapy for esophageal cancer. If patients with esophageal cancer are able to determine their nutritional and inflammatory status, they will be able to select the optimal esophageal cancer, anti-inflammation, and nutritional treatments. Various types of nutrition and inflammation assessment tools have been developed and reported for esophageal cancer, with each tool having its own clinical characteristics, which must be understood before being applied in clinical practice. This review summarizes the background, current status, and future perspectives on the application of nutrition and inflammation assessment tools in esophageal cancer treatment.
Keywords: Nutritional assessment, inflammation assessment, esophageal cancer, GPS, NLR, CAR, LCR, review
Esophageal cancer is the eighth most common type of cancer and the sixth-leading cause of cancer-related death in the world (1,2). Curative resection and perioperative adjuvant treatment is a standard treatment for resectable esophageal cancer (3-5). Although the survival rate after surgery and adjuvant treatment is gradually increasing, almost half of all patients develop recurrent disease, even after curative treatment (6,7).
Recently, some studies have reported that the perioperative nutritional and inflammatory status affect both the short-term and long-term oncological outcomes in various malignancies (8,9). Therefore, it is necessary to evaluate the nutritional and inflammatory status during the perioperative period in patients with esophageal cancer. If a physician can determine the nutritional and inflammatory status during treatment, they can control and manage the nutritional and inflammatory status to optimize esophageal cancer treatment. Thus far, the application of various nutritional and inflammation assessment tools, such as the Glasgow Prognostic Score (GPS), Prognostic Nutritional Index (PNI), and Controlling Nutritional Status (CONUT) have been reported in esophageal cancer (10-14). To introduce these various nutritional and inflammation assessment tools into daily clinical practice, it is necessary to understand the characteristics of each.
This review summarizes the background, current status, and future perspectives of nutrition and inflammation assessment tools in esophageal cancer treatment.
Search Strategy
The search strategy used in the current study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A literature search was performed on PubMed. The following keywords were used: “Glasgow Prognostic Score (GPS)” and “esophageal cancer (or carcinoma)”; “Prognostic Nutritional Index” and “esophageal cancer (or carcinoma)”; “Controlling Nutritional Status” and “esophageal cancer (or carcinoma)”; “neutrophil-lymphocyte ratio (NLR)” and “esophageal cancer (or carcinoma).” “CRP to albumin ratio (CAR)” and “esophageal cancer (or carcinoma)”; “platelet-to-lymphocyte ratio (PLR)” and “esophageal cancer (or carcinoma)”; “albumin-to-globulin ratio (AGR)” and “esophageal cancer (or carcinoma)”; and “lymphocyte-to-C-reactive-protein ratio (LCR)” and “esophageal cancer (or carcinoma)”. In addition, the references of the cited articles were overlooked. In total, 920 articles were identified. However, 782 were excluded.
Clinical Use of the GPS and Modified GPS (mGPS) in Esophageal Cancer Treatment
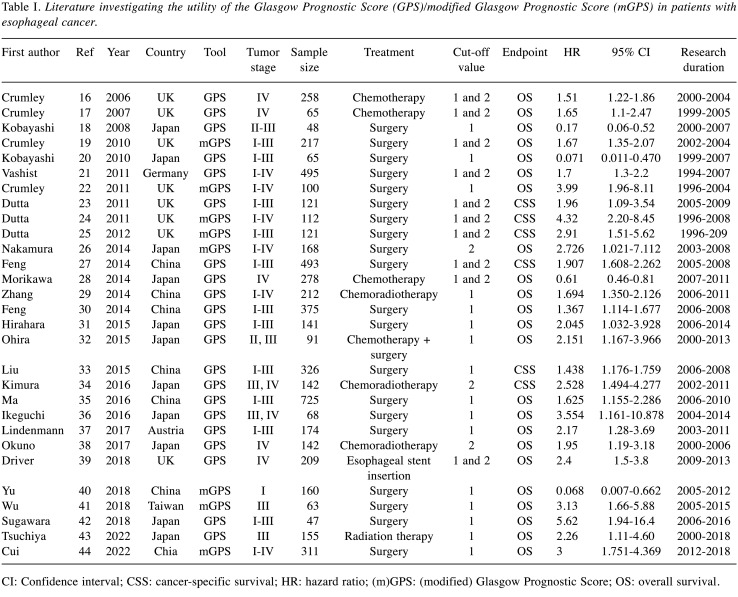
The GPS is calculated using the serum C-reactive protein level (CRP) and serum albumin level (15). Both CRP and albumin are produced by the liver. The serum CRP level reflects the systemic inflammation status, and the serum albumin level reflects the nutritional status. Therefore, the GPS can assess both the inflammatory and nutritional status during treatment. The mGPS has also been investigated and reported in various malignancies. A total of 29 studies have evaluated the clinical impact of the GPS/mGPS in esophageal cancer, using a cut-off value of 1 or 2. Table I summarizes each study (16-44). Among them, 21 studies evaluated patients with esophageal cancer who underwent esophagectomy, four evaluated patients who received chemotherapy, four evaluated patients who received chemoradiation therapy, one evaluated patients who received radiation therapy, and one evaluated patients who underwent esophageal stent insertion. All studies demonstrated that a high GPS/mGPS (GPS/mGPS >1 or 2) was associated with a poor prognosis. The hazard ratio (HR) of a high GPS/mGPS for OS was 1.367-5.62 in the surgery group, 1.51-2.151 in the chemotherapy group, and 1.694-2.528 in the chemoradiation group. Accordingly, the GPS/mGPS had a clinical impact on the oncological outcomes in esophageal cancer, irrespective of the treatment method. Further studies are needed to clarify whether the GPS/mGPS has a clinical impact on short-term oncological outcomes (e.g., occurrence of postoperative surgical complications, continuation of chemotherapy/radiation therapy, and occurrence of chemotherapy/radiation therapy toxicity).
Table I. Literature investigating the utility of the Glasgow Prognostic Score (GPS)/modified Glasgow Prognostic Score (mGPS) in patients with esophageal cancer.
CI: Confidence interval; CSS: cancer-specific survival; HR: hazard ratio; (m)GPS: (modified) Glasgow Prognostic Score; OS: overall survival.
The CRP to Albumin Ratio (CAR) in Esophageal Cancer Treatment
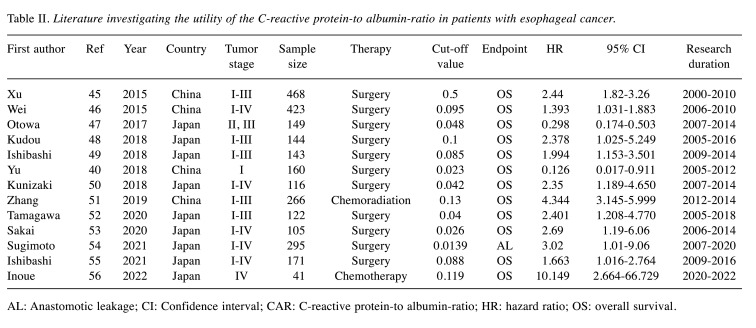
The CAR is derived from laboratory tests and is determined by dividing the serum CRP level by the albumin level. The CAR evaluates both the inflammatory status and the nutritional status. Thirteen studies evaluated the clinical impact of CAR in esophageal cancer. Table II summarizes each study (45-56). Among them, 12 studies evaluated CAR as a prognostic factor and one study evaluated it as a predictive factor for postoperative surgical complications. All studies showed that a high CAR was associated with a poor oncological outcome. In the evaluation as a prognostic factor, 10 studies evaluated patients with esophageal cancer who underwent esophagectomy, one study evaluated patients who received chemotherapy, and one study evaluated patients who received chemoradiation therapy. The reported cut-off values of CAR as a prognostic factor in these studies ranged from 0.0139 to 0.5. Among the patients who underwent esophagectomy, the HR of a high CAR for OS was 1.393-3.02. Further studies are needed to clarify whether the CAR is an optimal tool for unresectable esophageal cancer.
Table II. Literature investigating the utility of the C-reactive protein-to albumin-ratio in patients with esophageal cancer.
AL: Anastomotic leakage; CI: Confidence interval; CAR: C-reactive protein-to albumin-ratio; HR: hazard ratio; OS: overall survival.
The Neutrophil-to-Lymphocyte Ratio (NLR) in Esophageal Cancer Treatment
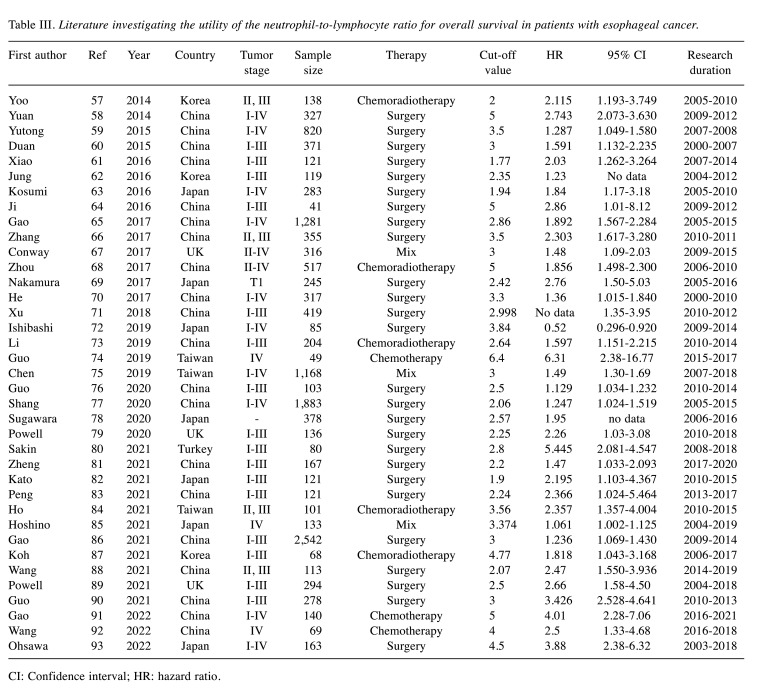
The NLR is determined by dividing the absolute neutrophil count by the absolute lymphocyte count. Recent studies demonstrated that a high NLR is associated with a poor prognosis in various malignancies. Thus far, 63 studies have evaluated the clinical impact of the NLR in esophageal cancer. Table III summarizes each study (57-93). Thirty-eight studies evaluated the NLR as a prognostic factor. Among them, 26 evaluated patients with esophageal cancer who underwent esophagectomy, five evaluated patients who received chemoradiation therapy, three evaluated patients who underwent chemotherapy, and three evaluated patients who received combined treatment. All studies demonstrated that a high NLR was associated with a poor prognosis. The reported cut-off values of the NLR as a prognostic factor ranged from 1.77 to 6.4 in these studies. The HR of the NLR for OS was 1.129-5.445 in the surgery group, 2.5-6.31 in the chemotherapy group, and 1.597-2.357 in the chemoradiation group. Accordingly, the NLR has a clinical impact on the oncological outcomes in esophageal cancer, irrespective of the treatment method.
Table III. Literature investigating the utility of the neutrophil-to-lymphocyte ratio for overall survival in patients with esophageal cancer.
CI: Confidence interval; HR: hazard ratio.
The Platelet-to-Lymphocyte Ratio (PLR) in Esophageal Cancer Treatment
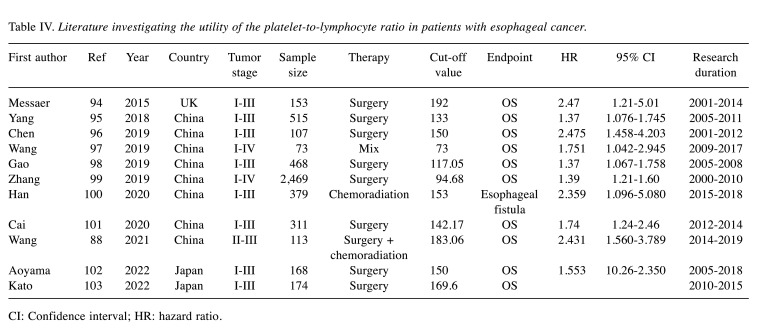
Recently, the PLR was reported as a promising prognostic factor for gastrointestinal malignancies. The PLR is calculated by dividing the platelet count by the lymphocyte count. Eleven studies evaluated the clinical impact of the PLR in esophageal cancer. Table IV summarizes each study (94-103). Among them, 10 evaluated the PLR as a prognostic factor, while one study evaluated the PLR as a predictive factor for postoperative surgical complications. The cut-off value of the PLR as a prognostic factor was reported to be 73-192 in these studies. In studies that evaluated the PLR as a prognostic factor, a high PLR was associated with a poor prognosis. The HR of the PLR for OS was 1.37-2.475. Although the PLR showed prognostic value in esophageal cancer treatment, most studies assessed patients who underwent esophagectomy. Therefore, further studies are needed to clarify the clinical impact of the PLR in patients who receive chemotherapy or chemoradiation therapy.
Table IV. Literature investigating the utility of the platelet-to-lymphocyte ratio in patients with esophageal cancer.
CI: Confidence interval; HR: hazard ratio.
The PNI in Esophageal Cancer Treatment
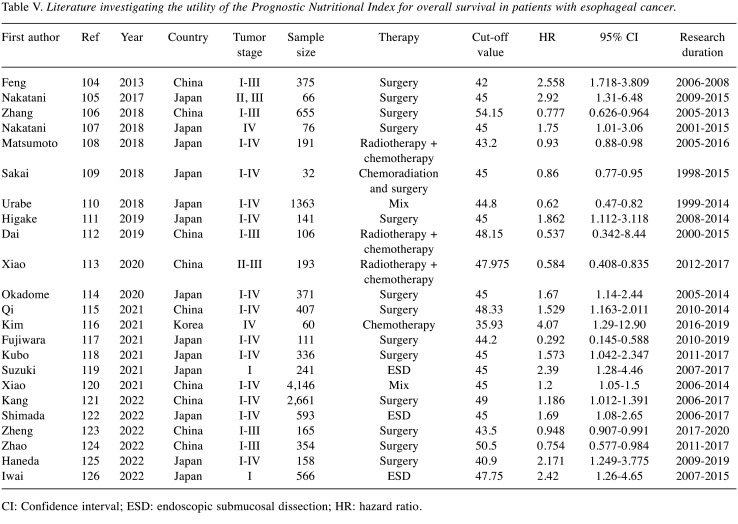
The PNI is a novel index that is used to assess the immune and nutritional status based on the serum lymphocyte count and albumin level. Twenty-six studies evaluated the clinical impact of the PNI in esophageal cancer. Table V summarizes each study (104-126). Among the 23 studies that evaluated the PNI as a prognostic factor, 13 evaluated patients with esophageal cancer who underwent esophagectomy, three evaluated patients who underwent endoscopic submucosal dissection, one evaluated patients who received chemotherapy, and six studies evaluated patients who received combined treatment. The cut-off value of the PNI as a prognostic factor in these studies was reported to range from 35.93 to 54.15. All studies demonstrated that a low PNI was associated with a poor prognosis. The HR of the PNI for OS was 1.186-2.92 in the surgery group and 1.69-2.42 in the endoscopic submucosal dissection group. Accordingly, the PNI had a clinical impact on the oncological outcomes in esophageal cancer, irrespective of the treatment method.
Table V. Literature investigating the utility of the Prognostic Nutritional Index for overall survival in patients with esophageal cancer.
CI: Confidence interval; ESD: endoscopic submucosal dissection; HR: hazard ratio.
CONUT in Esophageal Cancer Treatment
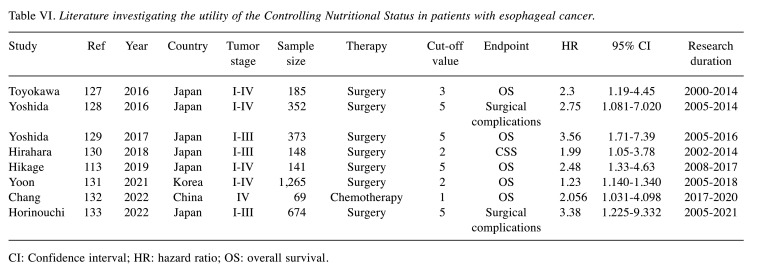
The CONUT score was developed as an accessible nutritional screening tool for evaluating patients’ nutritional status. The CONUT score is calculated from the serum albumin level, the total cholesterol level, and the total lymphocyte count. The clinical impact of the CONUT score on the outcomes of esophageal cancer was first reported in 2016. Eight studies evaluated the clinical impact of the CONUT in esophageal cancer. Table VI summarizes each study (127-133). Among them, six studies evaluated the CONUT score as a prognostic factor and two evaluated it as a predictive factor for postoperative surgical complications. Six studies showed that a high CONUT score was associated with a poor oncological outcome. In the studies that evaluated the CONUT score as a prognostic factor, five studies evaluated esophageal cancer patients who received esophagectomy, and one study evaluated patients who received chemotherapy. The reported cut-off values of the CONUT score as a prognostic factor ranged from 1 to 5 in these studies. Among the patients who underwent esophagectomy, the HR of a high CONUT score for OS was 1.23-3.56.
Table VI. Literature investigating the utility of the Controlling Nutritional Status in patients with esophageal cancer.
CI: Confidence interval; HR: hazard ratio; OS: overall survival.
The Albumin-to-Globulin Ratio (AGR) and the Lymphocyte-to-C-reactive Protein Ratio (LCR) in Esophageal Cancer Treatment
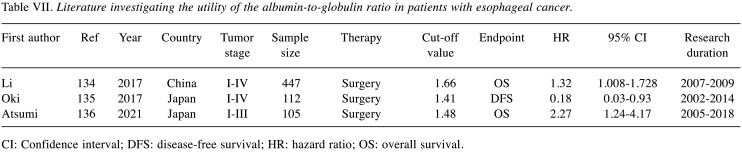
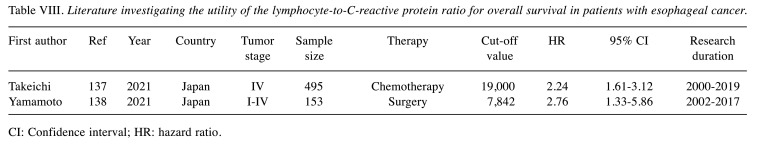
Recently, the clinical utility of albumin and globulin as tumor prognostic markers have aroused great interest, due to the close relationship with the nutritional status and the inflammatory responses of cancer patients. The AGR, which is calculated as AGR = albumin/(total protein–albumin) has been considered a possible effective combination of the two individual prognostic indicators. In addition, the lymphocyte-to-C-reactive-protein ratio (LCR) is a particularly promising marker of systemic inflammation in the perioperative period. Table VII and Table VIII showed the clinical impact of the AGR and LCR in esophageal cancer treatment (134-138). However, limited studies have shown its significance as a prognostic factor in esophageal cancer treatment. Additional studies are needed to clarify the clinical impact of the AGR and LCR in esophageal cancer treatment.
Table VII. Literature investigating the utility of the albumin-to-globulin ratio in patients with esophageal cancer.
CI: Confidence interval; DFS: disease-free survival; HR: hazard ratio; OS: overall survival.
Table VIII. Literature investigating the utility of the lymphocyte-to-C-reactive protein ratio for overall survival in patients with esophageal cancer.
CI: Confidence interval; HR: hazard ratio.
Future Prospects for Nutrition and Inflammation Assessment Tools in Esophageal Cancer Treatment
Thus far, various nutrition and inflammation assessment tools have been applied in esophageal cancer treatment. To utilize the nutrition and inflammation assessment tools in esophageal cancer treatment, the following points should be clarified. Firstly, setting the optimal cut-off value of each tool is an issue. In the previous studies, patient background factors and treatment methods were heterogeneous. In addition, the sample sizes of the previous studies were relatively small, and the studies were retrospective in nature. Therefore, these differences may have affected the cut-off values of each tool. In addition, the timing at which each tool should be applied is also unclear. It remains necessary to establish the optimal timing for assessment by these tools. Secondly, the mechanisms through which nutrition and inflammation affect the prognosis of esophageal cancer are unclear. Recently, the nutritional and inflammatory status was reported to affect postoperative surgical complications, the introduction of chemotherapy, and adverse events of chemotherapy. However, the precise mechanism through which the nutritional and inflammatory status, as assessed by these tools, influence the prognosis of esophageal cancer is unclear. Thirdly, it is unclear whether these nutrition and inflammation assessment tools will become promising indicators for treatment approaches targeting nutrition/inflammation in esophageal cancer. The clinical relationship between changes in the nutritional and inflammatory status and perioperative oral nutritional treatment need to be clarified.
Conclusion
The nutritional and inflammatory status, as assessed by nutrition and inflammation assessment tools, may have some clinical influence on both the short-term and long-term oncological outcomes of patients with esophageal cancer. However, the optimal cut-off values for each tool are unclear, as are the mechanisms through which these parameters influence prognosis. To optimize the nutrition and inflammation assessment tools for patients with esophageal cancer, it is necessary to clarify these points in further studies.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
TA and KK made substantial contributions to the concept and design. TA, YM, KH, and KK made substantial contributions to the acquisition of data and the analysis and interpretation of the data. TA and KK were involved in drafting the article or revising it critically for important intellectual content. TA, YM, and KH gave their final approval of the version to be published.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, Kato K, Li J, Ryu MH, Zamaniah WIW, Yong WP, Yeh KH, Nakajima TE, Shitara K, Kawakami H, Narita Y, Yoshino T, Van Cutsem E, Martinelli E, Smyth EC, Arnold D, Minami H, Tabernero J, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):34–43. doi: 10.1093/annonc/mdy498. [DOI] [PubMed] [Google Scholar]
- 4.Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D, ESMO Guidelines Committee Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 5.Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, Willett C, Stiles B, Sharma P, Tang L, Wijnhoven BPL, Hofstetter WL. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol. 2020;38(23):2677–2694. doi: 10.1200/JCO.20.00866. [DOI] [PubMed] [Google Scholar]
- 6.Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y, CheckMate 648 Trial Investigators Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 7.Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 8.Aoyama T. Perioperative body composition changes in the multimodal treatment of gastrointestinal cancer. Surg Today. 2020;50(3):217–222. doi: 10.1007/s00595-019-01815-8. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SL, Grundmann O, Williams JJ, Gordan L, George TJ Jr. Body composition changes differ by gender in stomach, colorectal, and biliary cancer patients with cachexia: Results from a pilot study. Cancer Med. 2018;7(8):3695–3703. doi: 10.1002/cam4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Xu D, Song H, Qiu B, Tian D, Li Z, Ji Y, Wang J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ Open. 2021;11(9):e048324. doi: 10.1136/bmjopen-2020-048324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao J, Chen C, Wan F, Zhu Y, Jin H, Zhou J, Chen N, Yang J, Pu Q. Prognostic value of pre-treatment prognostic nutritional index in esophageal cancer: a systematic review and meta-analysis. Front Oncol. 2020;10:797. doi: 10.3389/fonc.2020.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takagi K, Buettner S, Ijzermans JNM, Wijnhoven BPL. Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res. 2020;40(10):5343–5349. doi: 10.21873/anticanres.14541. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Shen A, Chen X, Guo L, Peng H, Gao M. Clinical significance of nutrition and inflammation in esophageal cancer patients with surgery: a meta-analysis. Nutr Cancer. 2022;74(9):3128–3139. doi: 10.1080/01635581.2022.2056620. [DOI] [PubMed] [Google Scholar]
- 14.Pirozzolo G, Gisbertz SS, Castoro C, van Berge Henegouwen MI, Scarpa M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis. 2019;11(7):3136–3145. doi: 10.21037/jtd.2019.07.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87(3):264–267. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer. 2006;94(5):637–641. doi: 10.1038/sj.bjc.6602998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crumley AB, Stuart RC, McKernan M, McDonald AC, McMillan DC. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J Gastroenterol Hepatol. 2008;23(8 Pt 2):e325–e329. doi: 10.1111/j.1440-1746.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Teruya M, Kishiki T, Endo D, Takenaka Y, Tanaka H, Miki K, Kobayashi K, Morita K. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144(5):729–735. doi: 10.1016/j.surg.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Crumley AB, Stuart RC, McKernan M, Going JJ, Shearer CJ, McMillan DC. Comparison of pre-treatment clinical prognostic factors in patients with gastro-oesophageal cancer and proposal of a new staging system. J Gastrointest Surg. 2010;14(5):781–787. doi: 10.1007/s11605-010-1162-6. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi T, Teruya M, Kishiki T, Kaneko S, Endo D, Takenaka Y, Miki K, Kobayashi K, Morita K. Inflammation-based prognostic score and number of lymph node metastases are independent prognostic factors in esophageal squamous cell carcinoma. Dig Surg. 2010;27(3):232–237. doi: 10.1159/000276910. [DOI] [PubMed] [Google Scholar]
- 21.Vashist YK, Loos J, Dedow J, Tachezy M, Uzunoglu G, Kutup A, Yekebas EF, Izbicki JR. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011;18(4):1130–1138. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 22.Crumley AB, Going JJ, Hilmy M, Dutta S, Tannahill C, McKernan M, Edwards J, Stuart RC, McMillan DC. Interrelationships between tumor proliferative activity, leucocyte and macrophage infiltration, systemic inflammatory response, and survival in patients selected for potentially curative resection for gastroesophageal cancer. Ann Surg Oncol. 2011;18(9):2604–2612. doi: 10.1245/s10434-011-1658-7. [DOI] [PubMed] [Google Scholar]
- 23.Dutta S, Al-Mrabt NM, Fullarton GM, Horgan PG, McMillan DC. A comparison of POSSUM and GPS models in the prediction of post-operative outcome in patients undergoing oesophago-gastric cancer resection. Ann Surg Oncol. 2011;18(10):2808–2817. doi: 10.1245/s10434-011-1676-5. [DOI] [PubMed] [Google Scholar]
- 24.Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg. 2011;35(8):1861–1866. doi: 10.1007/s00268-011-1130-7. [DOI] [PubMed] [Google Scholar]
- 25.Dutta S, Going JJ, Crumley AB, Mohammed Z, Orange C, Edwards J, Fullarton GM, Horgan PG, McMillan DC. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer. 2012;106(4):702–710. doi: 10.1038/bjc.2011.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura M, Iwahashi M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, Kato T, Yamaue H. New prognostic score for the survival of patients with esophageal squamous cell carcinoma. Surg Today. 2014;44(5):875–883. doi: 10.1007/s00595-013-0628-z. [DOI] [PubMed] [Google Scholar]
- 27.Feng JF, Zhao Q, Chen QX. Prognostic significance of Glasgow prognostic score in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Saudi J Gastroenterol. 2014;20(1):48–53. doi: 10.4103/1319-3767.126319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriwaki T, Kajiwara T, Matsumoto T, Suzuki H, Hiroshima Y, Matsuda K, Hirai S, Yamamoto Y, Yamada T, Sugaya A, Kobayashi M, Endo S, Ishige K, Nishina T, Hyodo I. Survival analysis of platinum-refractory patients with advanced esophageal cancer treated with docetaxel or best supportive care alone: a retrospective study. Dis Esophagus. 2014;27(8):737–743. doi: 10.1111/dote.12246. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, Xi M, Li QQ, He LR, Liu SL, Zhao L, Shen JX, Liu MZ. The modified glasgow prognostic score is an independent prognostic factor in patients with inoperable thoracic esophageal squamous cell carcinoma undergoing chemoradiotherapy. J Cancer. 2014;5(8):689–695. doi: 10.7150/jca.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng JF, Huang Y, Chen QX. The combination of platelet count and neutrophil lymphocyte ratio is a predictive factor in patients with esophageal squamous cell carcinoma. Transl Oncol. 2014;7(5):632–637. doi: 10.1016/j.tranon.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirahara N, Matsubara T, Hayashi H, Takai K, Fujii Y, Tajima Y. Impact of inflammation-based prognostic score on survival after curative thoracoscopic esophagectomy for esophageal cancer. Eur J Surg Oncol. 2015;41(10):1308–1315. doi: 10.1016/j.ejso.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Ohira M, Kubo N, Masuda G, Yamashita Y, Sakurai K, Toyokawa T, Tanaka H, Muguruma K, Hirakawa K. Glasgow Prognostic Score as a prognostic clinical marker in T4 esophageal squamous cell carcinoma. Anticancer Res. 2015;35(9):4897–4901. [PubMed] [Google Scholar]
- 33.Liu JS, Huang Y, Yang X, Feng JF. A nomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am J Cancer Res. 2015;5(7):2180–2189. [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura J, Kunisaki C, Makino H, Oshima T, Ota M, Oba M, Takagawa R, Kosaka T, Ono HA, Akiyama H, Endo I. Evaluation of the Glasgow Prognostic Score in patients receiving chemoradiotherapy for stage III and IV esophageal cancer. Dis Esophagus. 2016;29(8):1071–1080. doi: 10.1111/dote.12420. [DOI] [PubMed] [Google Scholar]
- 35.Ma Q, Liu W, Jia R, Jiang F, Duan H, Lin P, Zhang L, Long H, Zhao H, Ma G. Inflammation-based prognostic system predicts postoperative survival of esophageal carcinoma patients with normal preoperative serum carcinoembryonic antigen and squamous cell carcinoma antigen levels. World J Surg Oncol. 2016;14:141. doi: 10.1186/s12957-016-0878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeguchi M. Glasgow prognostic score and neutrophil-lymphocyte ratio are good prognostic indicators after radical neck dissection for advanced squamous cell carcinoma in the hypopharynx. Langenbecks Arch Surg. 2016;401(6):861–866. doi: 10.1007/s00423-016-1453-9. [DOI] [PubMed] [Google Scholar]
- 37.Lindenmann J, Fink-Neuboeck N, Avian A, Pichler M, Habitzruther M, Maier A, Smolle-Juettner FM. Preoperative Glasgow Prognostic Score as additional independent prognostic parameter for patients with esophageal cancer after curative esophagectomy. Eur J Surg Oncol. 2017;43(2):445–453. doi: 10.1016/j.ejso.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Okuno T, Wakabayashi M, Kato K, Shinoda M, Katayama H, Igaki H, Tsubosa Y, Kojima T, Okabe H, Kimura Y, Kawano T, Kosugi S, Toh Y, Kato H, Nakamura K, Fukuda H, Ishikura S, Ando N, Kitagawa Y, Japan Esophageal Oncology Group/Japan Clinical Oncology Group Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303) Int J Clin Oncol. 2017;22(6):1042–1049. doi: 10.1007/s10147-017-1154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driver RJ, Handforth C, Radhakrishna G, Bennett MI, Ford AC, Everett SM. The Glasgow Prognostic Score at the time of palliative esophageal stent insertion is a predictive factor of 30-day mortality and overall survival. J Clin Gastroenterol. 2018;52(3):223–228. doi: 10.1097/MCG.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Wen Y, Lin Y, Zhang X, Chen Y, Wang W, Wang G, Zhang L. The value of preoperative Glasgow Prognostic Score and the C-Reactive Protein to Albumin Ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer. 2018;9(5):807–815. doi: 10.7150/jca.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu CC, Li SH, Lu HI, Lo CM, Wang YM, Chou SY, Chen YH. Inflammation-based prognostic scores predict the prognosis of locally advanced cervical esophageal squamous cell carcinoma patients receiving curative concurrent chemoradiotherapy: a propensity score-matched analysis. PeerJ. 2018;6:e5655. doi: 10.7717/peerj.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugawara K, Mori K, Yagi K, Aikou S, Uemura Y, Yamashita H, Seto Y. Association of preoperative inflammation-based prognostic score with survival in patients undergoing salvage esophagectomy. Dis Esophagus. 2019;32(4):doy066. doi: 10.1093/dote/doy066. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya N, Kunisaki C, Sato S, Tanaka Y, Sato K, Watanabe J, Takeda K, Kosaka T, Akiyama H, Endo I. Chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Langenbecks Arch Surg. 2022;407(5):1911–1921. doi: 10.1007/s00423-022-02445-4. [DOI] [PubMed] [Google Scholar]
- 44.Cui C, Wu X, Deng L, Wang W, Cui W, Wang Y. Modified Glasgow prognostic score predicts the prognosis of patients with advanced esophageal squamous cell carcinoma: A propensity score-matched analysis. Thorac Cancer. 2022;13(14):2041–2049. doi: 10.1111/1759-7714.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10(9):e0138657. doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei XL, Wang FH, Zhang DS, Qiu MZ, Ren C, Jin Y, Zhou YX, Wang DS, He MM, Bai L, Wang F, Luo HY, Li YH, Xu RH. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer. 2015;15:350. doi: 10.1186/s12885-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otowa Y, Nakamura T, Yamamoto M, Kanaji S, Matsuda Y, Matsuda T, Oshikiri T, Sumi Y, Suzuki S, Kakeji Y. C-reactive protein to albumin ratio is a prognostic factor for patients with cStage II/III esophageal squamous cell cancer. Dis Esophagus. 2017;30(12):1–5. doi: 10.1093/dote/dox107. [DOI] [PubMed] [Google Scholar]
- 48.Kudou K, Saeki H, Nakashima Y, Kamori T, Kawazoe T, Haruta Y, Fujimoto Y, Matsuoka H, Sasaki S, Jogo T, Hirose K, Hu Q, Tsuda Y, Kimura K, Ando K, Oki E, Ikeda T, Maehara Y. C-reactive protein/albumin ratio is a poor prognostic factor of esophagogastric junction and upper gastric cancer. J Gastroenterol Hepatol. 2019;34(2):355–363. doi: 10.1111/jgh.14442. [DOI] [PubMed] [Google Scholar]
- 49.Ishibashi Y, Tsujimoto H, Hiraki S, Kumano I, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Aosasa S, Yamamoto J, Ueno H. Prognostic value of preoperative systemic immunoinflammatory measures in patients with esophageal cancer. Ann Surg Oncol. 2018;25(11):3288–3299. doi: 10.1245/s10434-018-6651-y. [DOI] [PubMed] [Google Scholar]
- 50.Kunizaki M, Tominaga T, Wakata K, Miyazaki T, Matsumoto K, Sumida Y, Hidaka S, Yamasaki T, Yasutake T, Sawai T, Hamamoto R, Nanashima A, Nagayasu T. Clinical significance of the C-reactive protein-to-albumin ratio for the prognosis of patients with esophageal squamous cell carcinoma. Mol Clin Oncol. 2018;8(2):370–374. doi: 10.3892/mco.2017.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Guo XW, Yin XX, Liu YC, Ji SJ. Nomogram-integrated C-reactive protein/albumin ratio predicts efficacy and prognosis in patients with thoracic esophageal squamous cell carcinoma receiving chemoradiotherapy. Cancer Manag Res. 2019;11:9459–9468. doi: 10.2147/CMAR.S228113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamagawa H, Aoyama T, Tamagawa A, Komori K, Maezawa Y, Kano K, Murakawa M, Atsumi Y, Hara K, Kazama K, Numata M, Oshima T, Yukawa N, Masuda M, Rino Y. Influence of the preoperative C-reactive protein-to-albumin ratio on survival and recurrence in patients with esophageal cancer. Anticancer Res. 2020;40(4):2365–2371. doi: 10.21873/anticanres.14205. [DOI] [PubMed] [Google Scholar]
- 53.Sakai M, Sohda M, Saito H, Ubukata Y, Nakazawa N, Kuriyama K, Hara K, Sano A, Ogata K, Yokobori T, Shirabe K, Saeki H. Comparative analysis of immunoinflammatory and nutritional measures in surgically resected esophageal cancer: a single-center retrospective study. In Vivo. 2020;34(2):881–887. doi: 10.21873/invivo.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugimoto A, Toyokawa T, Miki Y, Yoshii M, Tamura T, Sakurai K, Kubo N, Tanaka H, Lee S, Muguruma K, Yashiro M, Ohira M. Preoperative C-reactive protein to albumin ratio predicts anastomotic leakage after esophagectomy for thoracic esophageal cancer: a single-center retrospective cohort study. BMC Surg. 2021;21(1):348. doi: 10.1186/s12893-021-01344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishibashi Y, Tsujimoto H, Einama T, Mochizuki S, Kouzu K, Nomura S, Ito N, Harada M, Sugasawa H, Shinto E, Kishi Y, Ueno H. Correlation between immunoinflammatory measures and periostin expression in esophageal squamous cell carcinoma: a single-center, retrospective cohort study. Ann Surg Oncol. 2021;28(2):1228–1237. doi: 10.1245/s10434-020-08765-3. [DOI] [PubMed] [Google Scholar]
- 56.Inoue H, Shiozaki A, Fujiwara H, Konishi H, Kiuchi J, Ohashi T, Shimizu H, Arita T, Yamamoto Y, Morimura R, Kuriu Y, Ikoma H, Kubota T, Okamoto K, Otsuji E. Absolute lymphocyte count and C-reactive protein-albumin ratio can predict prognosis and adverse events in patients with recurrent esophageal cancer treated with nivolumab therapy. Oncol Lett. 2022;24(2):257. doi: 10.3892/ol.2022.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo EJ, Park JC, Kim EH, Park CH, Shim CN, Lee HJ, Chung HS, Lee H, Shin SK, Lee SK, Lee CG, Lee YC. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis. 2014;46(9):846–853. doi: 10.1016/j.dld.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Yuan D, Zhu K, Li K, Yan R, Jia Y, Dang C. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol. 2014;110(3):333–340. doi: 10.1002/jso.23651. [DOI] [PubMed] [Google Scholar]
- 59.Yutong H, Xiaoli X, Shumei L, Shan S, Di L, Baoen S. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with esophageal cancer in a high incidence area in China. Arch Med Res. 2015;46(7):557–563. doi: 10.1016/j.arcmed.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, Ma QL, Lin P. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21(18):5591–5597. doi: 10.3748/wjg.v21.i18.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao Q, Zhang B, Deng X, Wu J, Wang H, Wang Y, Wang W. The preoperative neutrophil-to-lymphocyte ratio is a novel immune parameter for the prognosis of esophageal basaloid squamous cell carcinoma. PLoS One. 2016;11(12):e0168299. doi: 10.1371/journal.pone.0168299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung J, Park SY, Park SJ, Park J. Prognostic value of the neutrophil-to-lymphocyte ratio for overall and disease-free survival in patients with surgically treated esophageal squamous cell carcinoma. Tumour Biol. 2016;37(6):7149–7154. doi: 10.1007/s13277-015-4596-3. [DOI] [PubMed] [Google Scholar]
- 63.Kosumi K, Baba Y, Ishimoto T, Harada K, Nakamura K, Ohuchi M, Kiyozumi Y, Izumi D, Tokunaga R, Taki K, Higashi T, Miyata T, Kurashige J, Hiyoshi Y, Iwagami S, Sakamoto Y, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today. 2016;46(4):405–413. doi: 10.1007/s00595-015-1197-0. [DOI] [PubMed] [Google Scholar]
- 64.Ji WH, Jiang YH, Ji YL, Li B, Mao WM. Prechemotherapy neutrophil : lymphocyte ratio is superior to the platelet : lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus. 2016;29(5):403–411. doi: 10.1111/dote.12322. [DOI] [PubMed] [Google Scholar]
- 65.Gao GD, Sun B, Wang XB, Wang SM. Neutrophil to lymphocyte ratio as prognostic indicator for patients with esophageal squamous cell cancer. Int J Biol Markers. 2017;32(4):e409–e414. doi: 10.5301/ijbm.5000294. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Su Y, Chen Z, Wei Z, Han W, Xu A. The prognostic value of preoperative inflammation-based prognostic scores and nutritional status for overall survival in resected patients with nonmetastatic Siewert type II/III adenocarcinoma of esophagogastric junction. Medicine (Baltimore) 2017;96(30):e7647. doi: 10.1097/MD.0000000000007647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conway AM, Salih Z, Papaxoinis G, Fletcher K, Weaver J, Patrao A, Noble R, Stamatopoulou S, Owen-Holt V, Mansoor W. Significance of blood neutrophil-to-lymphocyte ratio for prognostic stratification of patients with gastroesophageal junction adenocarcinoma in the era of the 8th edition of the American Joint Committee on Cancer (AJCC8) staging. Med Oncol. 2017;34(6):116. doi: 10.1007/s12032-017-0976-4. [DOI] [PubMed] [Google Scholar]
- 68.Zhou XL, Li YQ, Zhu WG, Yu CH, Song YQ, Wang WW, He DC, Tao GZ, Tong YS. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Sci Rep. 2017;7:42581. doi: 10.1038/srep42581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamura K, Yoshida N, Baba Y, Kosumi K, Uchihara T, Kiyozumi Y, Ohuchi M, Ishimoto T, Iwatsuki M, Sakamoto Y, Watanabe M, Baba H. Elevated preoperative neutrophil-to-lymphocytes ratio predicts poor prognosis after esophagectomy in T1 esophageal cancer. Int J Clin Oncol. 2017;22(3):469–475. doi: 10.1007/s10147-017-1090-5. [DOI] [PubMed] [Google Scholar]
- 70.He YF, Luo HQ, Wang W, Chen J, Yao YW, Yan Y, Wu SS, Hu XX, Ke LH, Niu JY, Li HM, Ji CS, Hu B. Preoperative NLR and PLR in the middle or lower ESCC patients with radical operation. Eur J Cancer Care (Engl) 2017;26(2) doi: 10.1111/ecc.12445. [DOI] [PubMed] [Google Scholar]
- 71.Xu GW, Wu HR, Xiong R, Li CW, Liu CQ, Xu MQ, Xie MR. Value of the preoperative neutrophil-to-lymphocyte ratio as a prognostic factor for long-term survival in postoperative esophageal squamous cell carcinoma patients. Thorac Cancer. 2018;9(12):1707–1715. doi: 10.1111/1759-7714.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishibashi Y, Tsujimoto H, Yaguchi Y, Kishi Y, Ueno H. Prognostic significance of systemic inflammatory markers in esophageal cancer: Systematic review and meta-analysis. Ann Gastroenterol Surg. 2019;4(1):56–63. doi: 10.1002/ags3.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li KJ, Xia XF, Su M, Zhang H, Chen WH, Zou CL. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer. 2019;19(1):1004. doi: 10.1186/s12885-019-6157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo JC, Lin CC, Lin CY, Hsieh MS, Kuo HY, Lien MY, Shao YY, Huang TC, Hsu CH. Neutrophil-to-lymphocyte ratio and use of antibiotics associated with prognosis in esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitors. Anticancer Res. 2019;39(10):5675–5682. doi: 10.21873/anticanres.13765. [DOI] [PubMed] [Google Scholar]
- 75.Chen MF, Chen PT, Kuan FC, Chen WC. The predictive value of pretreatment neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma. Ann Surg Oncol. 2019;26(1):190–199. doi: 10.1245/s10434-018-6944-1. [DOI] [PubMed] [Google Scholar]
- 76.Guo Q, Shao Z, Xu D, Fan L, Xiong H, Ding X, You C, Zhang L. Prognostic value of neutrophil-to-lymphocyte ratio in peripheral blood and pathological tissue in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2020;99(29):e21306. doi: 10.1097/MD.0000000000021306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shang QX, Yang YS, Hu WP, Yuan Y, He Y, Zhao JY, Ji AF, Chen LQ. Clinical and prognostic significance of preoperative lymphocyte-monocyte ratio, neutrophil-lymphocyte ratio and neutrophil-monocyte ratio on esophageal squamous cell carcinoma patients. Transl Cancer Res. 2020;9(6):3903–3914. doi: 10.21037/tcr-19-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugawara K, Yagi K, Uemura Y, Okumura Y, Nishida M, Aikou S, Yamashita H, Seto Y. Associations of systemic inflammation and sarcopenia with survival of esophageal carcinoma patients. Ann Thorac Surg. 2020;110(2):374–382. doi: 10.1016/j.athoracsur.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Powell AGMT, Chin C, Coxon AH, Chalishazar A, Christian A, Roberts SA, Lewis WG. Neutrophil to lymphocyte ratio as a predictor of response to neoadjuvant chemotherapy and survival in oesophageal adenocarcinoma. BJS Open. 2020;4(3):416–423. doi: 10.1002/bjs5.50277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakin A, Alay M, Sahin S, Aydemir O, Aldemir MN, Sakin A, Kotan C. Prognostic significance of neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma. North Clin Istanb. 2021;8(5):435–442. doi: 10.14744/nci.2020.63004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Z, Yang C, Cai C, Zhu H. The preoperative neutrophil lymphocyte ratio and platelet lymphocyte ratio predicts disease-free survival in resectable esophageal squamous cell carcinoma. Cancer Manag Res. 2021;13:7511–7516. doi: 10.2147/CMAR.S321326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kato T, Oshikiri T, Goto H, Urakawa N, Hasegawa H, Kanaji S, Yamashita K, Matsuda T, Nakamura T, Suzuki S, Kakeji Y. Preoperative neutrophil-to-lymphocyte ratio predicts the prognosis of esophageal squamous cell cancer patients undergoing minimally invasive esophagectomy after neoadjuvant chemotherapy. J Surg Oncol. 2021;124(7):1022–1030. doi: 10.1002/jso.26611. [DOI] [PubMed] [Google Scholar]
- 83.Peng H, Tan X. The prognostic significance of sarcopenia and the neutrophil-to-lymphocyte ratio in elderly patients with esophageal squamous cell carcinoma. Cancer Manag Res. 2021;13:3209–3218. doi: 10.2147/CMAR.S302274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho YC, Lai YC, Lin HY, Ko MH, Wang SH, Yang SJ, Lin PJ, Chou TW, Hung LC, Huang CC, Chang TH, Lin JB, Lin JC. Low cardiac dose and neutrophil-to-lymphocyte ratio predict overall survival in inoperable esophageal squamous cell cancer patients after chemoradiotherapy. Sci Rep. 2021;11(1):6644. doi: 10.1038/s41598-021-86019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoshino S, Takeuchi M, Kawakubo H, Matsuda S, Mayanagi S, Irino T, Fukuda K, Nakamura R, Wada N, Kitagawa Y. Usefulness of neutrophil to lymphocyte ratio at recurrence for predicting long-term outcomes in patients with recurrent esophageal squamous cell carcinoma. Ann Surg Oncol. 2021;28(6):3001–3008. doi: 10.1245/s10434-021-09637-0. [DOI] [PubMed] [Google Scholar]
- 86.Gao X, Pan Y, Han W, Hu C, Wang C, Chen L, Guo Y, Shi Y, Pan Y, Xie H, Yao L, Yang J, Zheng J, Li X, Liu X, Hong L, Li J, Li M, Ji G, Li Z, Xia J, Zhao Q, Fan D, Wu K, Nie Y. Association of systemic inflammation and body mass index with survival in patients with resectable gastric or gastroesophageal junction adenocarcinomas. Cancer Biol Med. 2021;18(1):283–297. doi: 10.20892/j.issn.2095-3941.2020.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koh HK, Park Y, Koo T, Park HJ, Lee MY, Chang AR, Hong S, Bae H. Neutrophil-to-lymphocyte ratio after definitive concurrent chemoradiotherapy predicts survival in patients with esophageal squamous cell carcinoma. In Vivo. 2021;35(2):1133–1139. doi: 10.21873/invivo.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C, Tong J, Tang M, Lu Y, Liang G, Zhang Z, Chen T. Pretreatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as prognostic factors and reference markers of treatment options for locally advanced squamous cell carcinoma located in the middle and upper esophagus. Cancer Manag Res. 2021;13:1075–1085. doi: 10.2147/CMAR.S294344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Powell AGMT, Eley C, Chin C, Coxon AH, Christian A, Lewis WG, South East Wales Oesophagogastric Cancer Collaborative Prognostic significance of serum inflammatory markers in esophageal cancer. Esophagus. 2021;18(2):267–277. doi: 10.1007/s10388-020-00772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo XW, Zhou JY, Jiang W, Ji L, Liu YC, Yin XX. The combination of preoperative nutritional risk screening-2002 and neutrophil-to-lymphocyte ratio is a useful prognostic marker in patients with esophageal squamous cell carcinoma. Nutr Cancer. 2021;73(4):588–595. doi: 10.1080/01635581.2020.1766090. [DOI] [PubMed] [Google Scholar]
- 91.Gao Y, Zhang Z, Li Y, Chen S, Lu J, Wu L, Ma Z, Hu Y, Zhang G. Pretreatment neutrophil-to-lymphocyte ratio as a prognostic biomarker in unresectable or metastatic esophageal cancer patients with anti-PD-1 therapy. Front Oncol. 2022;12:834564. doi: 10.3389/fonc.2022.834564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L, Zhu Y, Zhang B, Wang X, Mo H, Jiao Y, Xu J, Huang J. Prognostic and predictive impact of neutrophil-to-lymphocyte ratio and HLA-I genotyping in advanced esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitor monotherapy. Thorac Cancer. 2022;13(11):1631–1641. doi: 10.1111/1759-7714.14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohsawa M, Hamai Y, Emi M, Ibuki Y, Kurokawa T, Yoshikawa T, Hirohata R, Kitasaki N, Okada M. Neutrophil-to-lymphocyte ratio as a predictor of postoperative recurrence and prognosis in oesophageal squamous cell carcinoma. Anticancer Res. 2022;42(3):1499–1507. doi: 10.21873/anticanres.15622. [DOI] [PubMed] [Google Scholar]
- 94.Messager M, Neofytou K, Chaudry MA, Allum WH. Prognostic impact of preoperative platelets to lymphocytes ratio (PLR) on survival for oesophageal and junctional carcinoma treated with neoadjuvant chemotherapy: A retrospective monocentric study on 153 patients. Eur J Surg Oncol. 2015;41(10):1316–1323. doi: 10.1016/j.ejso.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 95.Yang Y, Xu H, Zhou L, Deng T, Ning T, Liu R, Zhang L, Wang X, Ge S, Li H, Ba Y. Platelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinoma. Clin Chim Acta. 2018;479:160–165. doi: 10.1016/j.cca.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 96.Chen LC, Li SH, Lo CM, Chen YH, Huang SC, Wang YM, Chou SY, Lu HI. Platelet-to-lymphocyte ratio is an independent prognosticator in patients with esophageal squamous cell carcinoma receiving esophagectomy. J Thorac Dis. 2019;11(11):4583–4590. doi: 10.21037/jtd.2019.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang N, Li X, Luo H, Sun Y, Zheng X, Fan C, Wang H, Ye K, Ge H. Prognostic value of pretreatment inflammatory biomarkers in primary small cell carcinoma of the esophagus. Thorac Cancer. 2019;10(10):1913–1918. doi: 10.1111/1759-7714.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao QF, Qiu JC, Huang XH, Xu YM, Li SQ, Sun F, Zhang J, Yang WM, Min QH, Jiang YH, Chen QG, Zhang L, Wang XZ, Ying HQ. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int. 2018;18:153. doi: 10.1186/s12935-018-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X, Hu D, Lin X, Zhang H, Xia Y, Lin J, Zheng X, Peng F, Jie J, Niu W. Prognostic value of an inflammation-related index in 6,865 Chinese patients with postoperative digestive tract cancers: The FIESTA study. Front Oncol. 2019;9:427. doi: 10.3389/fonc.2019.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han D, Zhang J, Zhao J, Lei T, Chen X, Zhang T, Wei H, Guan Y, Wang J, Zhang W, Zhao L, Wang J, Yuan Z, Song Y, Liu N, Pang Q, Wang P. Platelet-to-lymphocyte ratio is an independent predictor of chemoradiotherapy-related esophageal fistula in esophageal cancer patients. Ann Transl Med. 2020;8(18):1163. doi: 10.21037/atm-20-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cai G, Yu J, Meng X. Predicting prognosis and adverse events by hematologic markers in patients with locally advanced esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Cancer Manag Res. 2020;12:8497–8507. doi: 10.2147/CMAR.S257058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aoyama T, Ju M, Komori K, Tamagawa H, Tamagawa A, Onodera A, Morita J, Hashimoto I, Ishiguro T, Endo K, Cho H, Onuma S, Fukuda M, Oshima T, Yukawa N, Rino Y. The platelet-to-lymphocyte ratio is an independent prognostic factor for patients with esophageal cancer who receive curative treatment. In Vivo. 2022;36(4):1916–1922. doi: 10.21873/invivo.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kato T, Oshikiri T, Goto H, Sawada R, Harada H, Urakawa N, Hasegawa H, Kanaji S, Yamashita K, Matsuda T, Kakeji Y. Impact of the platelet-to-lymphocyte ratio as a biomarker for esophageal squamous cell carcinoma. Anticancer Res. 2022;42(5):2775–2782. doi: 10.21873/anticanres.15757. [DOI] [PubMed] [Google Scholar]
- 104.Feng JF, Chen QX. Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Ther Clin Risk Manag. 2014;10:1–7. doi: 10.2147/TCRM.S56159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakatani M, Migita K, Matsumoto S, Wakatsuki K, Ito M, Nakade H, Kunishige T, Kitano M, Kanehiro H. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus. 2017;30(8):1–7. doi: 10.1093/dote/dox020. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, Ma R, Wu X, Xiao X, Jiang H, Tang P, Yu Z. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234(2):1794–1802. doi: 10.1002/jcp.27052. [DOI] [PubMed] [Google Scholar]
- 107.Nakatani M, Migita K, Matsumoto S, Wakatsuki K, Ito M, Nakade H, Kunishige T, Kitano M, Sho M. Prognostic significance of the Prognostic Nutritional Index in patients with recurrent esophageal squamous cell carcinoma. Nutr Cancer. 2018;70(3):467–473. doi: 10.1080/01635581.2018.1445771. [DOI] [PubMed] [Google Scholar]
- 108.Matsumoto Y, Zhou Q, Kamimura K, Moriyama M, Saijo Y. The Prognostic Nutrition Index predicts the development of hematological toxicities in and the prognosis of esophageal cancer patients treated with cisplatin plus 5-fluorouracil chemotherapy. Nutr Cancer. 2018;70(3):447–452. doi: 10.1080/01635581.2018.1445765. [DOI] [PubMed] [Google Scholar]
- 109.Sakai M, Sohda M, Miyazaki T, Yoshida T, Kumakura Y, Honjo H, Hara K, Ozawa D, Suzuki S, Tanaka N, Yokobori T, Kuwano H. Association of preoperative nutritional status with prognosis in patients with esophageal cancer undergoing salvage esophagectomy. Anticancer Res. 2018;38(2):933–938. doi: 10.21873/anticanres.12306. [DOI] [PubMed] [Google Scholar]
- 110.Urabe M, Yamashita H, Watanabe T, Seto Y. Comparison of prognostic abilities among preoperative laboratory data indices in patients with resectable gastric and esophagogastric junction adenocarcinoma. World J Surg. 2018;42(1):185–194. doi: 10.1007/s00268-017-4146-9. [DOI] [PubMed] [Google Scholar]
- 111.Hikage M, Taniyama Y, Sakurai T, Sato C, Takaya K, Okamoto H, Konno T, Ujiie N, Naitoh T, Unno M, Kamei T. The influence of the perioperative nutritional status on the survival outcomes for esophageal cancer patients with neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(13):4744–4753. doi: 10.1245/s10434-019-07742-9. [DOI] [PubMed] [Google Scholar]
- 112.Dai Y, Fu X, Li T, Yao Q, Su L, Su H, Li J. Long-term impact of prognostic nutritional index in cervical esophageal squamous cell carcinoma patients undergoing definitive radiotherapy. Ann Transl Med. 2019;7(8):175. doi: 10.21037/atm.2019.03.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao L, Lyu J, Liu X, Li K, Wang Y, Zhang R, Chen T, Li T. Clinical application value of the Prognostic Nutritional Index for predicting survival in patients with esophageal squamous cell carcinoma undergoing chemoradiotherapy or radiotherapy. Nutr Cancer. 2021;73(10):1933–1940. doi: 10.1080/01635581.2020.1817511. [DOI] [PubMed] [Google Scholar]
- 114.Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic Nutritional Index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700. doi: 10.1097/SLA.0000000000002985. [DOI] [PubMed] [Google Scholar]
- 115.Qi Q, Song Q, Cheng Y, Wang N. Prognostic significance of preoperative Prognostic Nutritional Index for overall survival and postoperative complications in esophageal cancer patients. Cancer Manag Res. 2021;13:8585–8597. doi: 10.2147/CMAR.S333190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim JH, Ahn B, Hong SM, Jung HY, Kim DH, Choi KD, Ahn JY, Lee JH, Na HK, Kim JH, Kim YH, Kim HR, Lee HJ, Kim SB, Park SR. Real-world efficacy data and predictive clinical parameters for treatment outcomes in advanced esophageal squamous cell carcinoma treated with immune checkpoint inhibitors. Cancer Res Treat. 2022;54(2):505–516. doi: 10.4143/crt.2020.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujiwara Y, Higashida M, Kubota H, Okamoto Y, Mineta S, Endo S, Ueno T. Perioperative predictive markers for recurrence of esophageal cancer after esophagectomy. Gastrointest Tumors. 2021;8(2):87–95. doi: 10.1159/000513961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kubo Y, Tanaka K, Yamasaki M, Yamashita K, Makino T, Saito T, Yamamoto K, Takahashi T, Kurokawa Y, Motoori M, Kimura Y, Nakajima K, Eguchi H, Doki Y. Influences of the Charlson Comorbidity Index and nutrition status on prognosis after esophageal cancer surgery. Ann Surg Oncol. 2021;28(12):7173–7182. doi: 10.1245/s10434-021-09779-1. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki T, Furukawa K, Funasaka K, Ishikawa E, Sawada T, Maeda K, Yamamura T, Ishikawa T, Ohno E, Nakamura M, Kawashima H, Miyahara R, Fujishiro M. Long-term prognostic predictors of esophageal squamous cell carcinoma potentially indicated for endoscopic submucosal dissection. Digestion. 2021;102(4):563–571. doi: 10.1159/000510091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiao FK, Wang L, Zhang WC, Wang LD, Zhao LS. Preoperative Prognostic Nutritional Index is a significant predictor of survival in esophageal squamous cell carcinoma patients. Nutr Cancer. 2021;73(2):215–220. doi: 10.1080/01635581.2020.1757129. [DOI] [PubMed] [Google Scholar]
- 121.Kang J, Yang G, Wang D, Lin Y, Wang Q, Luo H. The clinical application value of the Prognostic Nutritional Index for the overall survival prognosis of patients with esophageal cancer: a robust real-world observational study in China. Comput Math Methods Med. 2022;2022:3889588. doi: 10.1155/2022/3889588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimada T, Hatta W, Takahashi S, Koike T, Ohira T, Hikichi T, Toya Y, Tanaka I, Onozato Y, Hamada K, Fukushi D, Watanabe K, Kayaba S, Ito H, Mikami T, Oikawa T, Takahashi Y, Kondo Y, Yoshimura T, Shiroki T, Nagino K, Hanabata N, Funakubo A, Nakamura J, Matsumoto T, Iijima K, Fukuda S, Masamune A, Ito K, Tohoku GI Endoscopy Group Combined assessment of clinical and pathological prognostic factors for deciding treatment strategies for esophageal squamous cell carcinoma invading into the muscularis mucosa or submucosa after endoscopic submucosal dissection. Dig Endosc. 2022;34(7):1382–1391. doi: 10.1111/den.14378. [DOI] [PubMed] [Google Scholar]
- 123.Zheng Z, Zhu H, Cai H. Preoperative Prognostic Nutritional Index predict survival in patients with resectable esophageal squamous cell carcinoma. Front Nutr. 2022;9:824839. doi: 10.3389/fnut.2022.824839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao Y, Shen W, Song C, Su J, Wu P, Wang X, Yan K, Xu J, Zhu S. Prognostic significance of Prognostic Nutritional Index in esophageal squamous cell carcinoma patients undergoing radical radiotherapy: a propensity score matching analysis. Nutr Cancer. 2022;74(6):2095–2104. doi: 10.1080/01635581.2021.1982997. [DOI] [PubMed] [Google Scholar]
- 125.Haneda R, Hiramatsu Y, Kawata S, Honke J, Soneda W, Matsumoto T, Morita Y, Kikuchi H, Kamiya K, Takeuchi H. Survival impact of perioperative changes in prognostic nutritional index levels after esophagectomy. Esophagus. 2022;19(2):250–259. doi: 10.1007/s10388-021-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iwai N, Dohi O, Yamada S, Harusato A, Horie R, Yasuda T, Yamada N, Horii Y, Majima A, Zen K, Kimura H, Yagi N, Naito Y, Itoh Y. Prognostic risk factors associated with esophageal squamous cell carcinoma patients undergoing endoscopic submucosal dissection: a multi-center cohort study. Surg Endosc. 2022;36(4):2279–2289. doi: 10.1007/s00464-021-08502-1. [DOI] [PubMed] [Google Scholar]
- 127.Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16(1):722. doi: 10.1186/s12885-016-2696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshida N, Baba Y, Shigaki H, Harada K, Iwatsuki M, Kurashige J, Sakamoto Y, Miyamoto Y, Ishimoto T, Kosumi K, Tokunaga R, Imamura Y, Ida S, Hiyoshi Y, Watanabe M, Baba H. Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg. 2016;40(8):1910–1917. doi: 10.1007/s00268-016-3549-3. [DOI] [PubMed] [Google Scholar]
- 129.Yoshida N, Harada K, Baba Y, Kosumi K, Iwatsuki M, Kinoshita K, Nakamura K, Sakamoto Y, Miyamoto Y, Karashima R, Mima K, Sawayama H, Ohuchi M, Chikamoto A, Imamura Y, Watanabe M, Baba H. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg. 2017;402(2):333–341. doi: 10.1007/s00423-017-1553-1. [DOI] [PubMed] [Google Scholar]
- 130.Hirahara N, Matsubara T, Hayashi H, Takai K, Nakada S, Tajima Y. Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther. 2018;25(5):e524–e532. doi: 10.1097/MJT.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoon JP, Nam JS, Abidin MFBZ, Kim SO, Lee EH, Choi IC, Chin JH. Comparison of preoperative nutritional indexes for outcomes after primary esophageal surgery for esophageal squamous cell carcinoma. Nutrients. 2021;13(11):4086. doi: 10.3390/nu13114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang L, Cheng Q, Ma Y, Wu C, Zhang X, Ma Q, He L, Li Q, Tao J. Prognostic effect of the controlling nutritional status score in patients with esophageal cancer treated with immune checkpoint inhibitor. J Immunother. 2022;45(9):415–422. doi: 10.1097/CJI.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horinouchi T, Yoshida N, Harada K, Eto K, Sawayama H, Iwatsuki M, Iwagami S, Baba Y, Miyamoto Y, Baba H. A retrospective study of preoperative malnutrition based on the Controlling Nutritional Status score as an associated marker for short-term outcomes after open and minimally invasive esophagectomy for esophageal cancer. Langenbecks Arch Surg. 2022 doi: 10.1007/s00423-022-02655-w. [DOI] [PubMed] [Google Scholar]
- 134.Li XH, Gu WS, Wang XP, Lin JH, Zheng X, Zhang L, Kang T, Zhang ZX, Liu WL. Low preoperative albumin-to-globulin ratio predict poor survival and negatively correlated with fibrinogen in resectable esophageal squamous cell carcinoma. J Cancer. 2017;8(10):1833–1842. doi: 10.7150/jca.19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oki S, Toiyama Y, Okugawa Y, Shimura T, Okigami M, Yasuda H, Fujikawa H, Okita Y, Yoshiyama S, Hiro J, Kobayashi M, Ohi M, Araki T, Inoue Y, Mohri Y, Kusunoki M. Clinical burden of preoperative albumin-globulin ratio in esophageal cancer patients. Am J Surg. 2017;214(5):891–898. doi: 10.1016/j.amjsurg.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 136.Atsumi Y, Kawahara S, Kakuta S, Onodera A, Hara K, Kazama K, Numata M, Aoyama T, Tamagawa A, Tamagawa H, Oshima T, Yukawa N, Rino Y. Low preoperative albumin-to-globulin ratio is a marker of poor prognosis in patients with esophageal cancer. In Vivo. 2021;35(6):3555–3561. doi: 10.21873/invivo.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takeuchi M, Kawakubo H, Hoshino S, Matsuda S, Mayanagi S, Irino T, Fukuda K, Nakamura R, Wada N, Kitagawa Y. Lymphocyte-to-C-reactive protein ratio as a novel marker for predicting oncological outcomes in patients with esophageal cancer. World J Surg. 2021;45(11):3370–3377. doi: 10.1007/s00268-021-06269-z. [DOI] [PubMed] [Google Scholar]
- 138.Yamamoto A, Toiyama Y, Okugawa Y, Ichikawa T, Imaoka H, Yasuda H, Fujikawa H, Okita Y, Yokoe T, Ohi M. Clinical implications of the preoperative lymphocyte C-reactive protein ratio in esophageal cancer patients. Surg Today. 2021;51(5):745–755. doi: 10.1007/s00595-020-02166-5. [DOI] [PubMed] [Google Scholar]