Abstract
In recent years, the demand for cytopathological accurate diagnoses has increased as expanding minimally invasive procedures obtain materials from patients with advanced cancer for diagnostic, prognostic, and predictive purposes. However, inadequate knowledge of cytopathological technical procedures and ancillary techniques by clinicians remains the most common reason for the limited availability of cytopathology. The objectives of this review were to understand the technical procedures, ancillary techniques, and application and effectiveness of various types of tests in cytopathology. Each of the many ancillary technologies described in the literature has specific advantages and limitations and laboratories select one or more methods depending on their infrastructure and expertise to achieve the goal from initial screening of the disease to the final diagnosis of the cytopathology. This paper systematically reviews the development of cytopathology, summarizes the existing problems in cytopathology and the new progress of auxiliary examination, to provide a theoretical basis for the advanced development of cytopathological diagnostic technologies and to consolidate the minimally invasive and accurate diagnosis of cytopathologies for clinicians. Cytopathology offers many advantages over other clinical examinations, particularly for minimally invasive and accurate diagnosis.
Keywords: Cytopathology, minimally invasive, accurate diagnosis, technical procedures, ancillary techniques, review
The emergence of cell theory, the discovery of physiology in the 17th century, the use of the microscope, and the establishment of pathological anatomy in the 18th century contributed to the development of cytopathology. Rudolf Virchow, a German scientist published the book Cytopathology, marking the birth of cytopathology in 1858 (1,2). At the beginning of the 20th century, Yang Dawang founded China's first cytology laboratories in the Department of Obstetrics and Gynecology of Peking Union Medical College and Beijing Hospital.
Cytopathology attributed the occurrence of the pathological state to cellular changes, which was an advance in pathology. Cytopathology is based on histology and is used to study the morphology and structure of tissue fragments, groups of cells and individual cells, as well as the relationship between cells and the tissue origin. It includes abscission cytology (AC) and fine needle aspiration cytology (FNA). AC is the examination of sputum, pleural and abdominal fluid, gastric juice, urine, cervical smear, and others. FNA requires a fine needle to absorb a relatively small number of cells from the lesion site, such as the lymph node, thyroid, breast, and lung. Cytopathological examination is simple, minimally invasive, and accurate, providing an important method for early diagnosis of malignant tumors. It is widely used in clinical and tumor investigation.
With the development of minimally invasive techniques, cytology is gradually becoming the main means of tissue diagnosis. Cytological diagnosis refers to the identification of cellular changes under a light microscope. Cytopathological diagnosis is a complex process, influenced by many factors. A large number of well-preserved cells on the smear is required for accurate diagnosis. In contrast, the absence of background information, poor images and blurred staining may lead to a misdiagnosis. Therefore, the methods and techniques of cytopathology are crucial for cytopathological diagnoses. When necessary, immunocytochemistry (ICC), electron microscopy, and molecular biology techniques are used to differentiate between reactive lesions and tumor changes (3).
Advances in cytopathological diagnosis methods and techniques have been reported previously (4-6). In this review, we focus on the technical advances in cytopathology, including AC and FNA. We describe the current state of ICC, flow cytometry (FCM), fluorescence in situ hybridization (FISH), DNA ploidy analysis, gene sequencing, artificial intelligence (AI), remote pathological diagnosis, deep learning, and digital storage for clinicians and cytopathologists.
Cytopathological Specimen Collection
Abscission cytology collection. Cancer cells are characterized by fast metabolism and a depletion in calcium and hyaluronidase, resulting in weakening of cell-cell connections and detachment of cells (7,8). Adequate sample volume of AC, a representative cell collection and time-dependent smear fixation are requirements for a correct diagnosis.
Cervical exfoliation cytology is a method for cervical cancer screening, mainly consisting of cervical smear and thinPrep cytologic test (TCT) examination. The cervical smear contains pathological cells scraped with a specific curette to extract a relatively small number of cells. TCT is recommended to effectively improve the acquisition rate and diagnostic accuracy (9-11). SurePath and ThinPrep 2000 are two testing systems recommended by the FDA for cervical or vaginal usage (12,13). The basic purpose of collecting cells is to increase the sampling of the complete transformation zone (TZ) and squamous columnar epithelial junction (SCJ), which is important for the pathogenesis of cervical cancer (14). Therefore, collecting cells from this region is crucial. Human papillomavirus (HPV) combined with TCT, P16/Ki-67, and E6/E7 provide effective markers for cancer screening (15,16).
Non-gynecological cytology includes the innovation and improvement of various specimen collectors, washing fluid microporous membranes, and sputum releasers (17). Hemolytic substances and anticoagulants are added to thorax, ascites, and other body fluids containing high protein concentrations, followed by the preparation of a centrifugal precipitation smear (18). For urine and other low protein body fluids, sufficient cells can be obtained by centrifugal precipitation collector and microporous filtration membrane technology, increasing the positive rate (19).
Fine needle aspiration cytology specimen collection. FNA is a quick, economical, and minimally invasive method in which cells of suspicious lesions are repeatedly aspirated with a fine needle (the outer diameter of the needle is 0.6-0.9 mm). Then, the cytopathologist observes the samples under a microscope and issues a pathology report (20,21). FNA can be used to puncture tumors and non-tumor lesions in almost all parts of the body, assuring a high positive rate and few false positives (22). Cytopathological diagnosis of superficial tumors, in the thyroid, breast, lymph nodes, subcutaneous masses, and deep tumors such as in the pancreas, retroperitoneum, liver, and kidney, is a very practical and convenient means of assessment (23,24). Metastatic tumor is the most common cause of lymphadenopathy. Lymph node puncture can not only be used for the diagnosis of metastatic tumors, but also for the determination of the histological type and originating organ (25,26).
The advent of cell chips has recently promoted the rapid development of FNA, enabling the use of few remaining cells in the needle after needle aspiration to perform high-throughput examination (27-30) with a wide usage of ultrasound-guided FNA (31-34). Fiber optic instruments and advanced imaging technology enabled FNA to obtain cells from almost all anatomical sites, which poses a challenge to the diagnostic level of cytologists (35,36). Cell blocks (CB) are created by absorbing more specimens (Figure 1) by FNA for ICC and genetic testing (37,38). CB offers versatility for diagnostic, prognostic, and predictive assays (39,40).
Figure 1. Cell block preparation by fine needle aspiration. Fixed with 95% alcohol and paraffin embedded.
Fixation and staining. Cell fixation preserves cells close to their living state by artificial methods. The purpose of fixation is to prevent cell autolysis and conserving the original structure. Methods of cell fixation include wet fixation, dry fixation, liquid-based cytological fixation and other. Wet fixation is based on a fresh specimen smear combined with a fixative solution containing alcohol as the main component in the wet state. Dry fixation method is defined by smear naturally drying in air or by heat. For convenience, cell spray fixator can be used to cover the smear or it could be dropped directly onto the smear. 95% ethanol is the most used cell fixator. The smear is fixed for at least 15 min. Polyethylene glycol can be used as an alternative method for immobilization. Ether ethanol is comparable to 95% ethanol but is rarely used today due to toxic effects. Pure methanol, a fixative containing glacial acetic acid, anhydrous ethanol, acetone, and 4% neutral buffer formaldehyde solution are fixatives that can be used in cytopathology. Rapid alcohol fixation is recommended for Pap stain, which can significantly improve the interpretability of the results (41). Routine cervical cytology and pap smears originated in the 1940s and are accepted as the standard of gynecological care (42,43).
Staining allows to highlight one part of the tissue and cells with different color intensities or alternatively use different colors for different parts, leading to different refractive indices and subsequently the display of various fine structures in the nucleus and cytoplasm more clearly. Cytological staining can be classified into three categories: conventional cytological staining, special cytological staining, and immunohistochemical staining. Common staining methods include HE, Pap, Diff-Quick, and Swiss staining. The main advantage of Pap staining is that it clearly shows the fine structure of the nucleus. The cytoplasm is bright and transparent, reflecting the change in the differentiation of the cytoplasm (44,45). Diff-Quick staining is a fast staining method modified on the basis of Swiss staining. CellDetect® staining encompasses a dual color discrimination and a morphological analysis that has the potential to be one of the most effective methods for cervical cancer screening and early diagnosis (46). Quality control should be performed during routine dyeing (47). Many factors affect the accuracy of cytopathological examination in clinical work (Table I). Auxiliary examination or auxiliary combined examination is necessary.
Table I. Influencing factors of cytopathological examination.
Auxiliary Diagnostic
Immunocytochemistry. The recognition of benign and malignant cells in cytopathology is influenced by experience (Table II) and subjective deviations should be avoided. Molecular targeted therapy has been increasing in cancer therapy, especially with recurring and metastasizing tumors, and is considered the first choice of tumor treatment. For investigating multiple drug resistance, ICC is required and provides a way to investigate related gene and protein expression levels.
Table II. Morphological differences between benign and malignant cells.
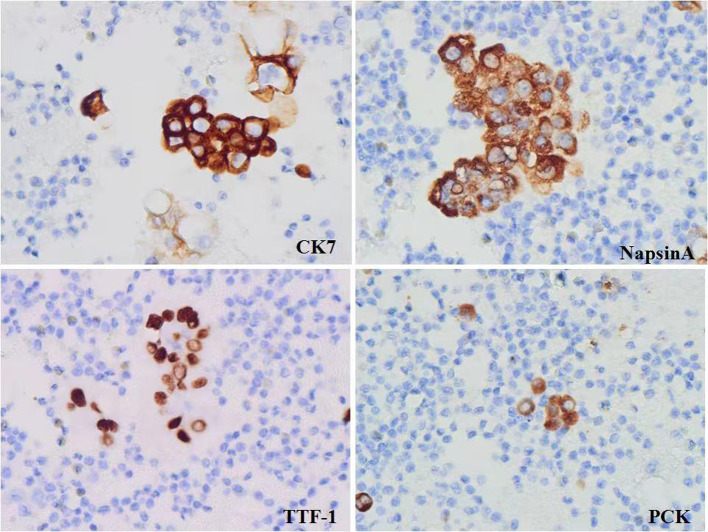
ICC is a useful auxiliary diagnostic in cytopathology (48-50) and plays a key role in diagnosis, prognosis and in the identification of predictive markers (51-53). There are four methods of specimen preparation commonly used in ICC: direct smear, cytospin, paraffin embedded CB, and liquid-based thin layer preparation method. CB method is widely established (54-56). CB is essential for the future of cytology; more output could be achieved with less material and with a low degree of invasiveness. Strong evidence indicates that the combination of conventional cytology and CB improves the diagnostic accuracy of endosonography with FNA (EUS-FNA) (57). This approach should be implemented in general practice, especially where rapid onsite evaluation (ROSE) is not available. ICC staining is easy to operate, independent of cell morphology, uncomplicated to read, and can assist the identification of benign and malignant diseases (Figure 2). CB in combination with ICC is an effective supplement to conventional FNA smears, which can further improve the accuracy of cytopathological diagnosis and facilitate the selection of preoperative chemotherapy (58).
Figure 2. Immunocytochemistry of cell blocks.
ICC also has its own limitations: 1) No absolute specific antibody is available. 2) A considerable number of tumors lack specific antigen expression. 3) The same antigen can be expressed in multiple tumors. 4) Related antigens are absent in some tumors due to low differentiation. 5) In some cases, different antibody titers have different positive results. 6) Endogenous biotin can lead to false positives. 7) The results may be inconsistent between different laboratories. There are still many problems to be solved in quantitative analysis. Standard and correct technical operation in the immunization laboratory is the most important premise to ensure the correct application of ICC. Cytological specimens are mainly suitable for ICC, but proper optimization and strict quality control of high-quality stainings are fundamental (59).
Flow cytometry. With the rapid development of modern medicine, the proportion of diagnostics in clinical medicine is gradually increasing. The number of new diagnostic tools is also rising (60). To comprehensively analyze all kinds of independent diagnostic data to obtain useful information for clinical treatment is a major challenge to modern medicine. FCM is a technology used to conduct qualitative and quantitative analysis and sorting of single cells or biological particles one by one by multiple parameters in a state of rapid linear flow. As a technology platform, modern FCM was developed in the 1960s and 1970s. After about 40 years of development and improvement, FCM matured. It has been widely used in all aspects from basic research to clinical practice, covering the fields of cell biology, immunology, hematology, oncology, pharmacology, genetics, and clinical laboratory, playing a major part in various disciplines (61-64).
When carcinogenesis and precancerous lesions with malignant potential occur in the body, abnormal changes in DNA content may precede the current state of the tissue. FCM can accurately detect changes in DNA content by quantifying the DNA content of precancerous cells and determining the distribution of cell cycle according to the DNA distribution square diagram (65,66). FCM has been successfully used to detect tumor cells in malignant effusions (67,68). FCM combined with morphological analysis and ICC can overcome the individual limitations of each method and provide reliable results in a faster and more efficient manner, thereby improving the diagnosis and prognosis of breast cancer (69,70). Intraoperative FCM can evaluate the margins of pancreatic cancer (71,72). FCM detection of circulating tumor cells in portal venous blood is valuable in predicting postoperative metastasis of pancreatic or periampullary tumors (73). FCM can be used to diagnose lymphoma subtypes (74-76). Cerebrospinal fluid FCM can be used to screen for primary CNS lymphoma (77). FCM is developing towards high sensitivity, high speed, multiparameter measurement, and morphological information acquisition (78).
The limitations of FCM are the following: 1) The tissue structure cannot be addressed and some rare large cells are difficult to analyze. 2) Some cells are highly adhered and are difficult to extract by bone marrow puncture. 3) The prognostic value is lower than that of chromosome and gene analysis. 4) The equipment is expensive and requires professional and technical personnel to operate.
Fluorescence in situ hybridization. FISH is a technique that uses fluorescence-labeled specific nucleic acid probes to hybridize with corresponding target DNA or RNA molecules in cells. By observing fluorescence signals under a fluorescence microscope or confocal laser scanner, the morphology and distribution of cells or organelles that are stained after hybridization with specific probes, or the location of DNA regions or RNA molecules bound to fluorescent probes in chromosomes or other organelles could be resolved (79). CellDetect and FISH show equal value in diagnosing urothelial carcinoma, both are superior to conventional urine cytology (80). With the rapid scientific and technological development, more and more FISH probe markers have been identified, laying a solid foundation for the clinical application of FISH technology (81). Single fluorescence to multi-color fluorescence detection is possible, enabling the observation of cells in mitosis and also in interphase states.
The limitations of FISH are as follows: 1) The numerous steps are easy to cause signal loss and false negative results. 2) Only qualitative detection is possible, not quantitative. 3) RNA detection is only an indirect way to infer gene expression, inconsistent with protein level. 4) Hybridization cannot be achieved in 100% of cases. Shorter cDNA probes will result in a significant reduction in efficiency. 5) FISH is circumstantial and expensive.
Quantitative analysis of cell DNA ploidy. Automatic cell DNA quantitative analysis system allows the detection of DNA ploidy of genetic material in the nucleus, the investigation of the physiological state and pathological cell changes and allows the evaluation of cancer and precancerous lesions. The system can be used to analyze a variety of gynecological and non-gynecological clinical cytological specimens, including cervical exfoliation specimens, and can also assist the assessment of healing after tumor treatment (82).
At present, staining and image analysis techniques are used in the clinical context to measure the DNA content of solid tumors or puncture specimens. The techniques are applied on gastroscopy, esophagoscopy, tumor flushing fluid, urine and a small amount of tissue, to analyze the cell cycle state and tumor cell alloploidy, one of the most important indicators for differentiating benign and malignant tumors and determining early diagnosis of tumors (83). In parallel, DNA ploidy of tumor cells is analyzed, and the detection rate of DNA ploidy of tumor cells is closely related to clinical biological behaviors such as tumor diffusion and metastasis (84). However, a study has suggested that DNA ploidy alone is nonspecific and may not be a good tool for assessing oral cancer prognosis or metastatic progression (85). The DNA quantitative analysis system can detect cancer and precancerous lesions earlier than traditional cytology (86) and predict malignant transformation well. In addition, when it was combined with dysplasia grading, it gave the highest predictive value (87). In conclusion, the clinical application of tumor cell cycle and DNA ploidy analysis can be used as important indicators for early diagnosis of tumors.
Sequencing. Since Sanger (88) invented the first generation of gene sequencing technology, the technology has gradually become an effective method to analyze gene sequences and was used in the human Genome Project, to map the human genome (89). Fields of applications include disease diagnosis and drug development. Characterized by high throughput, next-generation sequencing (NGS) significantly reduces the cost of sequencing and advances gene sequencing from a single locus to the whole genome (90,91). NGS can be used in cytopathology, including CBs, direct smears, liquid-based cytology and supernatants (91,92). NGS is widely used in cancers (93). The third generation of sequencing technology, while maintaining the flux and speed of the second generation of sequencing, makes up for the deficiency of the second generation of sequencing regarding the read length.
Artificial intelligence. A new science named AI, researches and develops theories, methods, technologies, and application systems for simulating, extending, and expanding human intelligence (94). AI has been used as an important ancillary technique in the diagnosis of lung, breast, colon, and prostate cancers and has achieved good results (95-97). Advances in AI, image analysis, and deep learning are expanding the ways in which computational pathology can be applied to cytopathology (98). AI algorithms have a long history in the field of urine cytology and some of them have proven superior to human review results (99). Trained, tested, and validated models established in other areas of medicine are mature and can assist the diagnosis of cervical cell images (100). AI has its unique advantages in assisting pathological diagnosis of cervical cytology images. AI can significantly reduce the rate of misdiagnosis and missed diagnosis, classify cells more accurately, eliminate interference factors in a more robust manner, and reduce the single slide diagnosis time (101). The practical application of medical AI has been emphasized but the interpretability and the methods of operation have not been addressed. Helping cytopathologists to understand the benefits of the AI's support and its limitations will contribute to its use in diagnostics and research (102).
Remote cytopathological diagnosis. Remote cytopathological diagnosis is a method of using telecommunication technology to transmit cytological images for tele-diagnosis, teaching, and research (103). Remote cytopathological diagnosis involves viewing cytological images on a computer screen rather than in a traditional bright field microscope. The requirements are an optical microscope, a high-resolution digital image acquisition instrument, a computer working platform, and a remote communication link. The accuracy of remote diagnosis is also related to the sampling area, image quality, diagnostic experience of the personnel, and expertise in viewing images on screens. Using the internet to transmit static digital images and selecting representative diagnostic areas from specimens is crucial (104). Currently, remote cytopathological diagnosis has started to be applied in routine diagnosis and education in Europe, the United States, and Japan (105). Camera improvements, software development and software operation on smart phones make smart phones effective tools for tele-pathology and tele-cytology (106). The digital slides generated by full slide scanners are sufficient to make cytological diagnoses and are comparable with physical slides observed by clinical pathologists at different professional levels (107). In cytopathological screening of cervical cancer, high-resolution digital cytopathological sections are very important for the interpretation of pathological cells. The study is expected to help to increase cytopathological screening in remote and poor areas where high-end imaging equipment is not available (108).
Deep learning in cytopathology. In recent decades, the great advances in image processing and speech recognition based on deep learning have greatly promoted the development of quantitative analysis and automatic detection of pathological images (109). Deep learning is especially useful for very large and unstructured data sets and is excellent at handling complex problems such as image classification. Furthermore, the applications in cytopathology are increasing. Zhang et al. (110) developed a deep convolutional neural network (DCNN) system to segment cell clusters and to recognize the cytopathological sections of cancer cell clusters on images. The DCNN system is feasible and robust in identifying pancreatic cancer cell clusters. Deep learning system can screen urothelial carcinoma cells more accurately than traditional cytology approaches and includes an evaluation of the malignant potential of tumors (111). Deep learning systems in cytopathological screening support urologists to develop treatment strategies to benefit the patients. Deep learning models are resistant to changes in the aspect ratio of cells in cervical cytopathological images (112).
Digital storage. Recent advances in digital pathology have decreased the effort and increased the availability of pathology in disease diagnosis, especially in tumor diagnosis. However, despite the potential to include low-cost diagnostics and viable telemedicine, digital pathology is not yet available due to expensive storage, data security requirements, and network bandwidth constraints for streaming high-resolution images and related metadata (113).
Conclusion
In conclusion, cytopathology has developed rapidly in recent years and achieved significant results. Moreover, many research methods using new technologies have emerged, providing more methods for the development of cytopathology and the diagnosis and treatment of clinical diseases. The quality control of cytopathology is an important mechanism to improve the diagnosis of cytopathology and the development of a quality control system will further promote the development of cytopathology. Cytopathology technology is becoming more and more automated and standardized. Although ancillary techniques are fundamental for improving the accuracy and census rate, their high cost is still a major issue. How to better learn and use these technologies is key for us.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
Conggai Huang reviewed and synthesized the relevant information and was the primary author of the review article. Xing Luo and Shaohua Wang reviewed and synthesized the relevant information. Yu Wan and Jieqiong Wang collected the data. Xiaoqin Tang and Huiling Zhang contributed figures and discussion of the review article. Johannes Haybaeck and Zhihui Yang made substantial contributions to the conception, drafting, and revision of the article. All Authors contributed to the article and approved the submitted version.
Acknowledgements
This work was financially supported by the project of the department of science and Technology, Sichuan province (No.22ZDYF3780).
References
- 1.de Gouveia RH, Gulczynski J, Canzonieri V, Nesi G, History of Pathology Working Group of the European Society of Pathology Rudolf Virchow: 200th birth anniversary. Virchows Arch. 2021;479(6):1063–1065. doi: 10.1007/s00428-021-03252-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goschler C. [Pathology and modernity: a look back at Rudolf Virchow on the occasion of his 200th birthday] Pathologe. 2021;42(6):617–619. doi: 10.1007/s00292-021-01005-9. [DOI] [PubMed] [Google Scholar]
- 3.Bishop JA. Immunohistochemistry surrogates for molecular alterations: A new paradigm in salivary gland tumor cytopathology. Cancer Cytopathol. 2021;129(2):102–103. doi: 10.1002/cncy.22337. [DOI] [PubMed] [Google Scholar]
- 4.Tobias AHG, Vitalino AC, Rezende MT, Oliveira RRR, Coura-Vital W, Amaral RG, Carneiro CM. Performance of rapid prescreening and 100% rapid review as internal quality control methods for cervical cytopathology. Cytopathology. 2018;29(5):428–435. doi: 10.1111/cyt.12599. [DOI] [PubMed] [Google Scholar]
- 5.Vaickus LJ, Suriawinata AA, Wei JW, Liu X. Automating the Paris System for urine cytopathology-A hybrid deep-learning and morphometric approach. Cancer Cytopathol. 2019;127(2):98–115. doi: 10.1002/cncy.22099. [DOI] [PubMed] [Google Scholar]
- 6.Srebotnik Kirbiš I, Rodrigues Roque R, Bongiovanni M, Strojan Fležar M, Cochand-Priollet B. Immunocytochemistry practices in European cytopathology laboratories-Review of European Federation of Cytology Societies (EFCS) online survey results with best practice recommendations. Cancer Cytopathol. 2020;128(10):757–766. doi: 10.1002/cncy.22311. [DOI] [PubMed] [Google Scholar]
- 7.Fatima K, Masood N, Ahmad Wani Z, Meena A, Luqman S. Neomenthol prevents the proliferation of skin cancer cells by restraining tubulin polymerization and hyaluronidase activity. J Adv Res. 2021;34:93–107. doi: 10.1016/j.jare.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim K, Choi H, Choi ES, Park MH, Ryu JH. Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics. 2019;11(7):301. doi: 10.3390/pharmaceutics11070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan X, Li K, Zhang J, Wang W, Wu B, Wu J, Li X, Huang X. Automatic model for cervical cancer screening based on convolutional neural network: a retrospective, multicohort, multicenter study. Cancer Cell Int. 2021;21(1):35. doi: 10.1186/s12935-020-01742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naizhaer G, Yuan J, Mijiti P, Aierken K, Abulizi G, Qiao Y. Evaluation of multiple screening methods for cervical cancers in rural areas of Xinjiang, China. Medicine (Baltimore) 2020;99(6):e19135. doi: 10.1097/MD.0000000000019135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husaiyin S, Jiao Z, Yimamu K, Maisaidi R, Han L, Niyazi M. ThinPrep cytology combined with HPV detection in the diagnosis of cervical lesions in 1622 patients. PLoS One. 2021;16(12):e0260915. doi: 10.1371/journal.pone.0260915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozemeijer K, Penning C, Siebers AG, Naber SK, Matthijsse SM, van Ballegooijen M, van Kemenade FJ, de Kok IM. Comparing SurePath, ThinPrep, and conventional cytology as primary test method: SurePath is associated with increased CIN II+ detection rates. Cancer Causes Control. 2016;27(1):15–25. doi: 10.1007/s10552-015-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozemeijer K, Naber SK, Penning C, Overbeek LI, Looman CW, de Kok IM, Matthijsse SM, Rebolj M, van Kemenade FJ, van Ballegooijen M. Cervical cancer incidence after normal cytological sample in routine screening using SurePath, ThinPrep, and conventional cytology: population based study. BMJ. 2017;356:j504. doi: 10.1136/bmj.j504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamal M. Pap smear collection and preparation: key points. Cytojournal. 2022;19:24. doi: 10.25259/CMAS_03_05_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benevolo M, Mancuso P, Allia E, Gustinucci D, Bulletti S, Cesarini E, Carozzi FM, Confortini M, Bisanzi S, Rubino T, Rollo F, Marchi N, Farruggio A, Pusiol T, Venturelli F, Giorgi Rossi P, New Technologies for Cervical Cancer 2 (NTCC2) Working Group Determinants of p16/Ki-67 adequacy and positivity in HPV-positive women from a screening population. Cancer Cytopathol. 2021;129(5):383–393. doi: 10.1002/cncy.22385. [DOI] [PubMed] [Google Scholar]
- 16.Giorgi Rossi P, Carozzi F, Ronco G, Allia E, Bisanzi S, Gillio-Tos A, De Marco L, Rizzolo R, Gustinucci D, Del Mistro A, Frayle H, Confortini M, Iossa A, Cesarini E, Bulletti S, Passamonti B, Gori S, Toniolo L, Barca A, Bonvicini L, Mancuso P, Venturelli F, Benevolo M, the New Technology for Cervical Cancer 2 Working Group p16/ki67 and E6/E7 mRNA accuracy and prognostic value in triaging HPV DNA-positive women. J Natl Cancer Inst. 2021;113(3):292–300. doi: 10.1093/jnci/djaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda K, Sato S, Chigira H, Shibuki Y, Hiraoka N. Characterizing the effect of automated cell sorting solutions on cytomorphological changes. Acta Cytol. 2020;64(3):232–240. doi: 10.1159/000500769. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, Oboshi W, Hashimoto Y, Komene T, Yamaguchi Y, Sato S, Maruyama S, Furukawa N, Sakabe N, Nagata K. Characterizing the effect of processing technique and solution type on cytomorphology using liquid-based cytology. Acta Cytol. 2022;66(1):55–60. doi: 10.1159/000519335. [DOI] [PubMed] [Google Scholar]
- 19.Munch MM, Chambers LC, Manhart LE, Domogala D, Lopez A, Fredricks DN, Srinivasan S. Optimizing bacterial DNA extraction in urine. PLoS One. 2019;14(9):e0222962. doi: 10.1371/journal.pone.0222962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Renberg S, Papakonstantinou A, Haglund de Flon F. Diagnosing gastrointestinal stromal tumors: The utility of fine-needle aspiration cytology versus biopsy. Cancer Med. 2022;11(14):2729–2734. doi: 10.1002/cam4.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Chamieh C, Vielh P, Chevret S. Statistical methods for evaluating the fine needle aspiration cytology procedure in breast cancer diagnosis. BMC Med Res Methodol. 2022;22(1):40. doi: 10.1186/s12874-022-01506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdogan-Durmus S. Diagnostic value of liquid-based cytology test in intrathoracic lymph nodes and lung lesions sampled by endobronchial ultrasonography-transbronchial needle aspiration. Diagn Cytopathol. 2021;49(12):1251–1256. doi: 10.1002/dc.24898. [DOI] [PubMed] [Google Scholar]
- 23.Budhwar A, Kataria SP, Kumar S, Singh G, Kaushik N, Sen R. Fine needle aspiration cytology of cervical lymph nodes: Comparison of liquid based cytology (SurePath) and conventional preparation. Diagn Cytopathol. 2021;49(1):18–24. doi: 10.1002/dc.24589. [DOI] [PubMed] [Google Scholar]
- 24.Mais DD, Crothers BA, Davey DD, Natale KE, Nayar R, Souers RJ, Blond BJ, Hackman S, Tworek JA. Trends in thyroid fine-needle aspiration cytology practices: Results from a College of American Pathologists 2016 practice survey. Arch Pathol Lab Med. 2019;143(11):1364–1372. doi: 10.5858/arpa.2018-0429-CP. [DOI] [PubMed] [Google Scholar]
- 25.Ronchi A, Caputo A, Pagliuca F, Montella M, Marino FZ, Zeppa P, Franco R, Cozzolino I. Lymph node fine needle aspiration cytology (FNAC) in paediatric patients: Why not? Diagnostic accuracy of FNAC in a series of heterogeneous paediatric lymphadenopathies. Pathol Res Pract. 2021;217:153294. doi: 10.1016/j.prp.2020.153294. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka E, Oda N, Kobayashi S, Ogawa T, Mitani R, Nawa T, Takata I, Ueki T, Okada H. Mediastinal lymph node metastasis of esophageal cancer with esophageal stenosis diagnosed via transesophageal endoscopic ultrasound with bronchoscope-guided fine-needle aspiration. Intern Med. 2022;61(7):1007–1010. doi: 10.2169/internalmedicine.8214-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekita-Hatakeyama Y, Fujii T, Nishikawa T, Mitoro A, Sawai M, Itami H, Morita K, Uchiyama T, Takeda M, Sho M, Yoshiji H, Hatakeyama K, Ohbayashi C. Evaluation and diagnostic value of next-generation sequencing analysis of residual liquid-based cytology specimens of pancreatic masses. Cancer Cytopathol. 2022;130(3):202–214. doi: 10.1002/cncy.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao JJ, Chan HP, Soon YY, Huang Y, Soo RA, Kee ACL. A systematic review and meta-analysis of the adequacy of endobronchial ultrasound transbronchial needle aspiration for next-generation sequencing in patients with non-small cell lung cancer. Lung Cancer. 2022;166:17–26. doi: 10.1016/j.lungcan.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Carrara S, Soldà G, Di Leo M, Rahal D, Peano C, Giunta M, Lamonaca L, Auriemma F, Anderloni A, Fugazza A, Maselli R, Malesci A, Laghi L, Repici A. Side-by-side comparison of next-generation sequencing, cytology, and histology in diagnosing locally advanced pancreatic adenocarcinoma. Gastrointest Endosc. 2021;93(3):597–604.e5. doi: 10.1016/j.gie.2020.06.069. [DOI] [PubMed] [Google Scholar]
- 30.Freiberger SN, Brada M, Fritz C, Höller S, Vogetseder A, Horcic M, Bihl M, Michal M, Lanzer M, Wartenberg M, Borner U, Bode PK, Broglie MA, Rordorf T, Morand GB, Rupp NJ. SalvGlandDx - a comprehensive salivary gland neoplasm specific next generation sequencing panel to facilitate diagnosis and identify therapeutic targets. Neoplasia. 2021;23(5):473–487. doi: 10.1016/j.neo.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sydney GI, Ioakim KJ, Michaelides C, Sepsa A, Sopaki-Valalaki A, Tsiotos GG, Theocharis S, Salla C, Nikas I. EUS-FNA diagnosis of pancreatic serous cystadenoma with the aid of cell blocks and α-inhibin immunochemistry: A case series. Diagn Cytopathol. 2020;48(3):239–243. doi: 10.1002/dc.24348. [DOI] [PubMed] [Google Scholar]
- 32.Nikas IP, Sepsa A, Kleidaradaki E, Salla C. EUS-FNA diagnosis of a metastatic adult granulosa cell tumor in the stomach. Lab Med. 2022;53(5):533–536. doi: 10.1093/labmed/lmac024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho IR, Jeong SH, Kang H, Kim EJ, Kim YS, Cho JH. Comparison of contrast-enhanced versus conventional EUS-guided FNA/fine-needle biopsy in diagnosis of solid pancreatic lesions: a randomized controlled trial. Gastrointest Endosc. 2021;94(2):303–310. doi: 10.1016/j.gie.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 34.van Riet PA, Quispel R, Cahen DL, Erler NS, Snijders-Kruisbergen MC, Van Loenen P, Poley JW, van Driel LMJW, Mulder SA, Veldt BJ, Leeuwenburgh I, Anten MGF, Honkoop P, Thijssen AY, Hol L, Hadithi M, Fitzpatrick CE, Schot I, Bergmann JF, Bhalla A, Bruno MJ, Biermann K. Optimizing cytological specimens of EUS-FNA of solid pancreatic lesions: A pilot study to the effect of a smear preparation training for endoscopy personnel on sample quality and accuracy. Diagn Cytopathol. 2021;49(2):295–302. doi: 10.1002/dc.24645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu H, Jiang F, Zhu J, Du Y, Jin Z, Li Z. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig Endosc. 2017;29(6):667–675. doi: 10.1111/den.12851. [DOI] [PubMed] [Google Scholar]
- 36.Stegehuis PL, Boogerd LS, Inderson A, Veenendaal RA, van Gerven P, Bonsing BA, Sven Mieog J, Amelink A, Veselic M, Morreau H, van de Velde CJ, Lelieveldt BP, Dijkstra J, Robinson DJ, Vahrmeijer AL. Toward optical guidance during endoscopic ultrasound-guided fine needle aspirations of pancreatic masses using single fiber reflectance spectroscopy: a feasibility study. J Biomed Opt. 2017;22(2):24001. doi: 10.1117/1.JBO.22.2.024001. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Jing H, Gong Y, Tam AL, Stewart J, Staerkel G, Guo M. Diagnostic efficacy and molecular testing by combined fine-needle aspiration and core needle biopsy in patients with a lung nodule. Cancer Cytopathol. 2020;128(3):201–206. doi: 10.1002/cncy.22234. [DOI] [PubMed] [Google Scholar]
- 38.Singh P, Kumar P, Rohilla M, Gupta P, Gupta N, Dey P, Srinivasan R, Rajwanshi A, Nada R. Fine needle aspiration cytology with the aid of immunocytochemistry on cell-block confirms the diagnosis ofsolid pseudopapillary neoplasm of the pancreas. Cytopathology. 2021;32(1):57–64. doi: 10.1111/cyt.12838. [DOI] [PubMed] [Google Scholar]
- 39.Sale MS, Kulkarni VV, Kulkarni PV, Patil CA. Efficacy of modified cell block cytology compared to fine needle aspiration cytology for diagnostic oral cytopathology. Biotech Histochem. 2021;96(3):197–201. doi: 10.1080/10520295.2020.1780314. [DOI] [PubMed] [Google Scholar]
- 40.McGoogan E, Colgan TJ, Ramzy I, Cochand-Priollet B, Davey DD, Grohs HK, Gurley AM, Husain OA, Hutchinson ML, Knesel EA Jr, Linder J, Mango LJ, Mitchell H, Peebles A, Reith A, Robinowitz M, Sauer T, Shida S, Solomon D, Topalidis T, Wilbur DC, Yamauchi K. Cell preparation methods and criteria for sample adequacy. International Academy of Cytology Task Force summary. Diagnostic Cytology Towards the 21st Century: An International Expert Conference and Tutorial. Acta Cytol. 1998;42(1):25–32. doi: 10.1159/000331532. [DOI] [PubMed] [Google Scholar]
- 41.Swailes AL, Hossler CE, Kesterson JP. Pathway to the Papanicolaou smear: The development of cervical cytology in twentieth-century America and implications in the present day. Gynecol Oncol. 2019;154(1):3–7. doi: 10.1016/j.ygyno.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Kamal M, Topiwala F. Nonneoplastic cervical cytology. Cytojournal. 2022;19:25. doi: 10.25259/CMAS_03_06_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uppada SB, Madduri LSV, Singu S, Lawson B, Bauer L, Freifeld A, Bhatt VR, Byrareddy SN. Modified Papanicolaou staining for oral swab samples stored long term. Biotech Histochem. 2021;96(5):359–363. doi: 10.1080/10520295.2020.1804075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pote A, Boghenco O, Marques-Ramos A. Molecular analysis of H&E- and Papanicolau-stained samples-systematic review. Histochem Cell Biol. 2020;154(1):7–20. doi: 10.1007/s00418-020-01882-w. [DOI] [PubMed] [Google Scholar]
- 45.He S, Wang GL, Zhu YY, Wu MH, Ji ZG, Seng J, Ji Y, Zhou JM, Chen L. Application of the CellDetect® staining technique in diagnosis of human cervical cancer. Gynecol Oncol. 2014;132(2):383–388. doi: 10.1016/j.ygyno.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 46.Chandra S, Chandra H, Kusum A, Singh Gaur D. Study of the pre-analytical phase of an ISO 15189: 2012-certified cytopathology laboratory: a 5-year institutional experience. Acta Cytol. 2019;63(1):56–62. doi: 10.1159/000494567. [DOI] [PubMed] [Google Scholar]
- 47.Kanber Y, Pusztaszeri M, Auger M. Immunocytochemistry for diagnostic cytopathology-A practical guide. Cytopathology. 2021;32(5):562–587. doi: 10.1111/cyt.12993. [DOI] [PubMed] [Google Scholar]
- 48.Kapoor D, Handa U, Kundu R, Das A. Diagnostic utility of p16 immunocytochemistry in metastatic cervical lymph nodes in head and neck cancers. Diagn Cytopathol. 2021;49(4):469–474. doi: 10.1002/dc.24696. [DOI] [PubMed] [Google Scholar]
- 49.Kundu R, Singh B, Dey P. Cytology coupled with immunocytochemistry identifies Merkel cell carcinoma: A rare intruder in the cerebrospinal fluid. Cytopathology. 2022;33(4):530–533. doi: 10.1111/cyt.13127. [DOI] [PubMed] [Google Scholar]
- 50.Metovic J, Righi L, Delsedime L, Volante M, Papotti M. Role of immunocytochemistry in the cytological diagnosis of pulmonary tumors. Acta Cytol. 2020;64(1-2):16–29. doi: 10.1159/000496030. [DOI] [PubMed] [Google Scholar]
- 51.VanderLaan PA. Non-small cell lung cancer predictive biomarker testing via immunocytochemistry: Ways of future past. Cancer Cytopathol. 2019;127(5):278–280. doi: 10.1002/cncy.22138. [DOI] [PubMed] [Google Scholar]
- 52.Iwahashi H, Miyamoto M, Minabe S, Hada T, Sakamoto T, Ishibashi H, Kakimoto S, Matsuura H, Suzuki R, Matsukuma S, Tsuda H, Takano M. Diagnostic efficacy of ascites cell block for ovarian clear cell carcinoma. Diagn Cytopathol. 2021;49(6):735–742. doi: 10.1002/dc.24734. [DOI] [PubMed] [Google Scholar]
- 53.Zaidi A, Srinivasan R, Rajwanshi A, Dey P, Gupta K. Ameloblastoma diagnosis by fine-needle aspiration cytology supplemented by cell block samples. Diagn Cytopathol. 2021;49(3):E93–E98. doi: 10.1002/dc.24600. [DOI] [PubMed] [Google Scholar]
- 54.Saha BK, Bonnier A. Cell block examination of pleural fluid, an underused and overlooked method for evaluation of malignant pleural effusion. Am J Med Sci. 2022;363(6):556–557. doi: 10.1016/j.amjms.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Alrajjal A, Choudhury M, Yang J, Gabali A. Cell-blocks and hematolymphoid lesions. Cytojournal. 2021;18:7. doi: 10.25259/Cytojournal_10_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pausawasdi N, Hongsrisuwan P, Chalermwai WV, Butt AS, Maipang K, Charatchareonwitthaya P. The diagnostic performance of combined conventional cytology with smears and cell block preparation obtained from endoscopic ultrasound-guided fine needle aspiration for intra-abdominal mass lesions. PLoS One. 2022;17(3):e0263982. doi: 10.1371/journal.pone.0263982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanjilal B, Das RN, Chatterjee U, Sengupta M, Sarkar R, Saha K. Utility of cell block preparation in diagnosis of paediatric abdominal neoplasms. Diagn Cytopathol. 2021;49(3):404–411. doi: 10.1002/dc.24670. [DOI] [PubMed] [Google Scholar]
- 58.Jain D, Nambirajan A, Borczuk A, Chen G, Minami Y, Moreira AL, Motoi N, Papotti M, Rekhtman N, Russell PA, Savic Prince S, Yatabe Y, Bubendorf L, IASLC Pathology Committee Immunocytochemistry for predictive biomarker testing in lung cancer cytology. Cancer Cytopathol. 2019;127(5):325–339. doi: 10.1002/cncy.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uno N, Kaku N, Morinaga Y, Hasegawa H, Yanagihara K. Flow cytometry assay for the detection of single-copy DNA in human lymphocytes. Nucleic Acids Res. 2020;48(15):e86. doi: 10.1093/nar/gkaa515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choy B, Venkataraman G, Biernacka A, Lastra RR, Mueller J, Setia N, Reeves W, Antic T. Correlation of cytopathology with flow cytometry and histopathology for the diagnosis of hematologic malignancies in young adults presenting with cervical lymphadenopathy. Diagn Cytopathol. 2019;47(6):579–583. doi: 10.1002/dc.24157. [DOI] [PubMed] [Google Scholar]
- 61.Willner J, Zhou F, Moreira AL. Diagnostic challenges in the cytology of thymic epithelial neoplasms. Cancers (Basel) 2022;14(8):301. doi: 10.3390/cancers14082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lo Gullo R, Cloutier Lambert C, Lin O, Jochelson MS, D’Alessio D. Yield of flow cytometry in addition to cytology for lymph node sampling in patients with incidental axillary adenopathy without a concurrent diagnosis of primary breast malignancy. Breast Cancer Res Treat. 2022;191(3):677–683. doi: 10.1007/s10549-021-06473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cattin S, Fellay B, Calderoni A, Christinat A, Negretti L, Biggiogero M, Badellino A, Schneider AL, Tsoutsou P, Pellanda AF, Rüegg C. Circulating immune cell populations related to primary breast cancer, surgical removal, and radiotherapy revealed by flow cytometry analysis. Breast Cancer Res. 2021;23(1):64. doi: 10.1186/s13058-021-01441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nair A, Manohar SM. A flow cytometric journey into cell cycle analysis. Bioanalysis. 2021;13(21):1627–1644. doi: 10.4155/bio-2021-0071. [DOI] [PubMed] [Google Scholar]
- 65.Kusama H, Shimoda M, Miyake T, Tanei T, Kagara N, Naoi Y, Shimazu K, Kim SJ, Noguchi S. Prognostic value of tumor cell DNA content determined by flow cytometry using formalin-fixed paraffin-embedded breast cancer tissues. Breast Cancer Res Treat. 2019;176(1):75–85. doi: 10.1007/s10549-019-05222-y. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi K, Kurashina K, Saito S, Kanamaru R, Ohzawa H, Yamaguchi H, Miyato H, Hosoya Y, Lefor AK, Sata N, Kitayama J. Flow cytometry-based analysis of tumor-leukocyte ratios in peritoneal fluid from patients with advanced gastric cancer. Cytometry B Clin Cytom. 2021;100(6):666–675. doi: 10.1002/cyto.b.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rikkert LG, de Rond L, van Dam A, van Leeuwen TG, Coumans FAW, de Reijke TM, Terstappen LWMM, Nieuwland R. Detection of extracellular vesicles in plasma and urine of prostate cancer patients by flow cytometry and surface plasmon resonance imaging. PLoS One. 2020;15(6):e0233443. doi: 10.1371/journal.pone.0233443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wopereis S, Walter LO, Vieira DSC, Ribeiro AAB, Fernandes BL, Wilkens RS, Santos-Silva MC. Evaluation of ER, PR and HER2 markers by flow cytometry for breast cancer diagnosis and prognosis. Clin Chim Acta. 2021;523:504–512. doi: 10.1016/j.cca.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Markopoulos GS, Harissis H, Andreou M, Alexiou GΑ, Vartholomatos G. Intraoperative flow cytometry for invasive breast cancer conserving surgery: A new alternative or adjunct to cavity shaving technique. Surg Oncol. 2022;42:101712. doi: 10.1016/j.suronc.2022.101712. [DOI] [PubMed] [Google Scholar]
- 70.Markopoulos GS, Goussia A, Bali CD, Messinis T, Alexiou GΑ, Vartholomatos G. Resection margins assessment by intraoperative flow cytometry in pancreatic cancer. Ann Surg Oncol. 2022 doi: 10.1245/s10434-022-11645-7. [DOI] [PubMed] [Google Scholar]
- 71.Shah MM, Kooby DA. Landmark Series: Importance of pancreatic resection margins response to comments to the editorresection margins assessment by intraoperative flow cytometry in pancreatic cancer. Ann Surg Oncol. 2022 doi: 10.1245/s10434-022-11648-4. [DOI] [PubMed] [Google Scholar]
- 72.Tao L, Su L, Yuan C, Ma Z, Zhang L, Bo S, Niu Y, Lu S, Xiu D. Postoperative metastasis prediction based on portal vein circulating tumor cells detected by flow cytometry in periampullary or pancreatic cancer. Cancer Manag Res. 2019;11:7405–7425. doi: 10.2147/CMAR.S210332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grewal RK, Chetty M, Abayomi EA, Tomuleasa C, Fromm JR. Use of flow cytometry in the phenotypic diagnosis of Hodgkin’s lymphoma. Cytometry B Clin Cytom. 2019;96(2):116–127. doi: 10.1002/cyto.b.21724. [DOI] [PubMed] [Google Scholar]
- 74.Cherian S, Soma LA. How I diagnose minimal/measurable residual disease in B lymphoblastic leukemia/lymphoma by flow cytometry. Am J Clin Pathol. 2021;155(1):38–54. doi: 10.1093/ajcp/aqaa242. [DOI] [PubMed] [Google Scholar]
- 75.Wang JC, Deng XQ, Liu WP, Gao LM, Zhang WY, Yan JQ, Ye YX, Liu F, Zhao S. Comprehensive flow-cytometry-based immunophenotyping analysis for accurate diagnosis and management of extranodal NK/T cell lymphoma, nasal type. Cytometry B Clin Cytom. 2020;98(1):28–35. doi: 10.1002/cyto.b.21838. [DOI] [PubMed] [Google Scholar]
- 76.Au KLK, Latonas S, Shameli A, Auer I, Hahn C. Cerebrospinal fluid flow cytometry: Utility in central nervous system lymphoma diagnosis. Can J Neurol Sci. 2020;47(3):382–388. doi: 10.1017/cjn.2020.22. [DOI] [PubMed] [Google Scholar]
- 77.Huang S, Jin L, Yang J, Duan Y, Zhang M, Zhou C, Zhang YH. Characteristics of central nervous system (CNS) involvement in children with non-Hodgkin’s lymphoma (NHL) and the diagnostic value of CSF flow cytometry in CNS positive disease. Technol Cancer Res Treat. 2021;20:15330338211016372. doi: 10.1177/15330338211016372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z, Deng X, Ye Y, Zhang W, Liu W, Zhao S. Flow cytometry-assessed PD1/PDL1 status in tumor-infiltrating lymphocytes: a link with the prognosis of diffuse large B-cell lymphoma. Front Oncol. 2021;11:687911. doi: 10.3389/fonc.2021.687911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marchetti A, Barberis M, Papotti M, Rossi G, Franco R, Malatesta S, Buttitta F, Ardizzoni A, Crinò L, Gridelli C, Taddei GL, Clemente C, Scagliotti G, Normanno N, Pinto C. ALK rearrangement testing by FISH analysis in non-small-cell lung cancer patients: results of the first italian external quality assurance scheme. J Thorac Oncol. 2014;9(10):1470–1476. doi: 10.1097/JTO.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 80.Shang D, Liu Y, Xu X, Chen Z, Wang D. Diagnostic value comparison of CellDetect, fluorescent in situ hybridization (FISH), and cytology in urothelial carcinoma. Cancer Cell Int. 2021;21(1):465. doi: 10.1186/s12935-021-02169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borak S, Siegal GP, Reddy V, Jhala N, Jhala D. Metastatic inflammatory myofibroblastic tumor identified by EUS-FNA in mediastinal lymph nodes with ancillary FISH studies for ALK rearrangement. Diagn Cytopathol. 2012;40 Suppl 2:E118–E125. doi: 10.1002/dc.21663. [DOI] [PubMed] [Google Scholar]
- 82.Guo Y, Peng Q, Wang Y, Li L, Yi X, Yan B, Zou M, Dai G, Guo P, Ma Q, Wu X. The application of DNA ploidy analysis in large-scale population screening for cervical cancer. Acta Cytol. 2021;65(5):385–392. doi: 10.1159/000518052. [DOI] [PubMed] [Google Scholar]
- 83.El-Gamal EM, Gouida MS. Flow cytometric study of cell cycle and DNA ploidy in bilharzial bladder cancer. Clin Lab. 2015;61(3-4):211–218. doi: 10.7754/clin.lab.2014.140609. [DOI] [PubMed] [Google Scholar]
- 84.Carloni S, Gallerani G, Tesei A, Scarpi E, Verdecchia GM, Virzì S, Fabbri F, Arienti C. DNA ploidy and S-phase fraction analysis in peritoneal carcinomatosis from ovarian cancer: correlation with clinical pathological factors and response to chemotherapy. Onco Targets Ther. 2017;10:4657–4664. doi: 10.2147/OTT.S141117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zargoun IM, Bingle L, Speight PM. DNA ploidy and cell cycle protein expression in oral squamous cell carcinomas with and without lymph node metastases. J Oral Pathol Med. 2017;46(9):738–743. doi: 10.1111/jop.12554. [DOI] [PubMed] [Google Scholar]
- 86.Ghizoni JS, Sperandio M, Lock C, Odell EW. Image cytometry DNA ploidy analysis: Correlation between two semi-automated methods. Oral Dis. 2018;24(7):1204–1208. doi: 10.1111/odi.12888. [DOI] [PubMed] [Google Scholar]
- 87.Zaini ZM, McParland H, Møller H, Husband K, Odell EW. Predicting malignant progression in clinically high-risk lesions by DNA ploidy analysis and dysplasia grading. Sci Rep. 2018;8(1):15874. doi: 10.1038/s41598-018-34165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pai JA, Satpathy AT. High-throughput and single-cell T cell receptor sequencing technologies. Nat Methods. 2021;18(8):881–892. doi: 10.1038/s41592-021-01201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lopez-Beltran A, Cimadamore A, Montironi R, Cheng L. Molecular pathology of urothelial carcinoma. Hum Pathol. 2021;113:67–83. doi: 10.1016/j.humpath.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 91.Pisapia P, Pepe F, Iaccarino A, Sgariglia R, Nacchio M, Conticelli F, Salatiello M, Tufano R, Russo G, Gragnano G, Girolami I, Eccher A, Malapelle U, Troncone G. Next generation sequencing in cytopathology: focus on non-small cell lung cancer. Front Med (Lausanne) 2021;8:633923. doi: 10.3389/fmed.2021.633923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roy-Chowdhuri S, Pisapia P, Salto-Tellez M, Savic S, Nacchio M, de Biase D, Tallini G, Troncone G, Schmitt F. Invited review-next-generation sequencing: a modern tool in cytopathology. Virchows Arch. 2019;475(1):3–11. doi: 10.1007/s00428-019-02559-z. [DOI] [PubMed] [Google Scholar]
- 93.Conde E, Rojo F, Gómez J, Enguita AB, Abdulkader I, González A, Lozano D, Mancheño N, Salas C, Salido M, Salido-Ruiz E, de Álava E. Molecular diagnosis in non-small-cell lung cancer: expert opinion on ALK and ROS1 testing. J Clin Pathol. 2022;75(3):145–153. doi: 10.1136/jclinpath-2021-207490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology – new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019;16(11):703–715. doi: 10.1038/s41571-019-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiu HY, Chao HS, Chen YM. Application of artificial intelligence in lung cancer. Cancers (Basel) 2022;14(6):1370. doi: 10.3390/cancers14061370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao M, Yao S, Li Z, Wu L, Xu Z, Pan X, Lin H, Xu Y, Yang S, Zhang S, Li Y, Zhao K, Liang C, Liu Z. The Crohn’s-like lymphoid reaction density: a new artificial intelligence quantified prognostic immune index in colon cancer. Cancer Immunol Immunother. 2022;71(5):1221–1231. doi: 10.1007/s00262-021-03079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fitzgerald J, Higgins D, Mazo Vargas C, Watson W, Mooney C, Rahman A, Aspell N, Connolly A, Aura Gonzalez C, Gallagher W. Future of biomarker evaluation in the realm of artificial intelligence algorithms: application in improved therapeutic stratification of patients with breast and prostate cancer. J Clin Pathol. 2021;74(7):429–434. doi: 10.1136/jclinpath-2020-207351. [DOI] [PubMed] [Google Scholar]
- 98.van der Velden BHM, Kuijf HJ, Gilhuijs KGA, Viergever MA. Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med Image Anal. 2022;79:102470. doi: 10.1016/j.media.2022.102470. [DOI] [PubMed] [Google Scholar]
- 99.Sanghvi AB, Allen EZ, Callenberg KM, Pantanowitz L. Performance of an artificial intelligence algorithm for reporting urine cytopathology. Cancer Cytopathol. 2019;127(10):658–666. doi: 10.1002/cncy.22176. [DOI] [PubMed] [Google Scholar]
- 100.Wang CW, Liou YA, Lin YJ, Chang CC, Chu PH, Lee YC, Wang CH, Chao TK. Artificial intelligence-assisted fast screening cervical high grade squamous intraepithelial lesion and squamous cell carcinoma diagnosis and treatment planning. Sci Rep. 2021;11(1):16244. doi: 10.1038/s41598-021-95545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang HP, Cai D, Kong YQ, Ye H, Ma ZX, Lv HS, Tuo LR, Pan QJ, Liu ZH, Han X. Cervical cytology screening facilitated by an artificial intelligence microscope: A preliminary study. Cancer Cytopathol. 2021;129(9):693–700. doi: 10.1002/cncy.22425. [DOI] [PubMed] [Google Scholar]
- 102.McAlpine ED, Michelow P. The cytopathologist’s role in developing and evaluating artificial intelligence in cytopathology practice. Cytopathology. 2020;31(5):385–392. doi: 10.1111/cyt.12799. [DOI] [PubMed] [Google Scholar]
- 103.Della Mea V. Prerecorded telemedicine. J Telemed Telecare. 2005;11(6):276–284. doi: 10.1258/1357633054893382. [DOI] [PubMed] [Google Scholar]
- 104.Lee ES, Kim IS, Choi JS, Yeom BW, Kim HK, Han JH, Lee MS, Leong AS. Accuracy and reproducibility of telecytology diagnosis of cervical smears. A tool for quality assurance programs. Am J Clin Pathol. 2003;119(3):356–360. doi: 10.1309/7ytvag4xnr48t75h. [DOI] [PubMed] [Google Scholar]
- 105.Dennis T, Start RD, Cross SS. The use of digital imaging, video conferencing, and telepathology in histopathology: a national survey. J Clin Pathol. 2005;58(3):254–258. doi: 10.1136/jcp.2004.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sahin D, Hacisalihoglu UP, Kirimlioglu SH. Telecytology: Is it possible with smartphone images. Diagn Cytopathol. 2018;46(1):40–46. doi: 10.1002/dc.23851. [DOI] [PubMed] [Google Scholar]
- 107.Bonsembiante F, Bonfanti U, Cian F, Cavicchioli L, Zattoni B, Gelain ME. Diagnostic validation of a whole-slide imaging scanner in cytological samples: Diagnostic accuracy and comparison with light microscopy. Vet Pathol. 2019;56(3):429–434. doi: 10.1177/0300985818825128. [DOI] [PubMed] [Google Scholar]
- 108.Ma J, Yu J, Liu S, Chen L, Li X, Feng J, Chen Z, Zeng S, Liu X, Cheng S. PathSRGAN: Multi-supervised super-resolution for cytopathological images using generative adversarial network. IEEE Trans Med Imaging. 2020;39(9):2920–2930. doi: 10.1109/TMI.2020.2980839. [DOI] [PubMed] [Google Scholar]
- 109.Xu J, Xiang L, Liu Q, Gilmore H, Wu J, Tang J, Madabhushi A. Stacked sparse autoencoder (SSAE) for nuclei detection on breast cancer histopathology images. IEEE Trans Med Imaging. 2016;35(1):119–130. doi: 10.1109/TMI.2015.2458702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang S, Zhou Y, Tang D, Ni M, Zheng J, Xu G, Peng C, Shen S, Zhan Q, Wang X, Hu D, Li WJ, Wang L, Lv Y, Zou X. A deep learning-based segmentation system for rapid onsite cytologic pathology evaluation of pancreatic masses: A retrospective, multicenter, diagnostic study. EBioMedicine. 2022;80:104022. doi: 10.1016/j.ebiom.2022.104022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nojima S, Terayama K, Shimoura S, Hijiki S, Nonomura N, Morii E, Okuno Y, Fujita K. A deep learning system to diagnose the malignant potential of urothelial carcinoma cells in cytology specimens. Cancer Cytopathol. 2021;129(12):984–995. doi: 10.1002/cncy.22443. [DOI] [PubMed] [Google Scholar]
- 112.Liu W, Li C, Rahaman MM, Jiang T, Sun H, Wu X, Hu W, Chen H, Sun C, Yao Y, Grzegorzek M. Is the aspect ratio of cells important in deep learning? A robust comparison of deep learning methods for multi-scale cytopathology cell image classification: From convolutional neural networks to visual transformers. Comput Biol Med. 2022;141:105026. doi: 10.1016/j.compbiomed.2021.105026. [DOI] [PubMed] [Google Scholar]
- 113.Subramanian H, Subramanian S. Improving diagnosis through digital pathology: Proof-of-concept implementation using smart contracts and decentralized file storage. J Med Internet Res. 2022;24(3):e34207. doi: 10.2196/34207. [DOI] [PMC free article] [PubMed] [Google Scholar]