Abstract
Background/Aim: Sinonasal metastases arising from renal cell cancer are rare and usually managed with surgery. Few studies describe the use of radiotherapy in this specific setting, while the use of stereotactic body radiotherapy (SBRT) has been rarely reported as well.
Case Report: We present the case of a solitary left sinonasal metastasis in a 65-year-old man with clear cell renal cancer who also received bilateral nephrectomy and subsequent kidney transplantation. The patient received subtotal surgery and subsequently he was candidate to SBRT to avoid systemic treatment, due to renal comorbidities.
Conclusion: The patient was treated with SBRT for a total dose of 35 Gy in 5 fractions and after 24 months of follow-up there is no evidence of local relapse. No major side-effects were reported. Our experience supports SBRT as a safe and feasible treatment option in the case of sinonasal metastases from RCC.
Keywords: Renal cell cancer, solitary metastasis, stereotactic body radiotherapy
Renal cell carcinoma (RCC) is a tumor usually observed in the male population with a major incidence between 30 and 60 years of age (1). Approximately 80% of RCCs are clear cell renal carcinomas (2). The most frequent sites of metastatic spread of RCC are the regional lymph nodes, bones, lungs, and liver. In a small proportion of cases, the head and neck area may be a site of recurrent disease, accounting for about 15-30% of cases, mainly involving the parotid glands, larynx, or thyroid (3,4). Late recurrences from RCC occur in almost 10% of patients, and sinonasal metastases have been reported in less than 100 cases in the literature (5,6).
In the vast majority of available literature, the preferred management of sinonasal metastases is surgery. Regarding radiotherapy, there is a substantial lack of evidence, with few studies reporting its use in the post-operative setting (7). RCCs are generally considered radioresistant tumors. Recent in vitro studies have hypothesized a low α/β ratio (a radiobiological measure of the fractionation sensitivity of the cells) for RCC, estimated at about α/β=3 Gy, thus leading clinicians to consider the use of higher doses per fraction as a means to improve the outcomes in terms of local control (8).
In the primary disease setting, stereotactic body radiotherapy (SBRT) represents a non-invasive alternative in case of non-feasibility for a surgical approach, with growing evidence in support of the role of SBRT for this specifical histological subtype. The main advantage of SBRT is its ability to deliver high doses with ablative intent to small volumes, with minimal exposure to nearby healthy structures (9).
On the other hand, response assessment after SBRT for renal lesions is a matter of debate, given the low specificity and sensitivity of metabolic imaging for this cancer subtype. Moreover, conventional computed tomography (CT) imaging assessment after radiotherapy is challenging due to the slow response and changes induced by SBRT.
Herein, we report a case of a 65-year-old man who received SBRT treatment for a RCC maxillary sinus metastasis. A review of the currently available literature on this topic is also included.
Case Report
In September 2020, a 65-year-old man was referred at our Institution with a contrast-enhanced total body CT-scan reporting an isolated recurrence of RCC in the left maxillary sinus. In 2015, the patient had received complete bilateral nephrectomy due to a clear cell carcinoma of the right kidney, with a personal history of polycystic renal disease. Subsequently, he received kidney transplantation and started an immunosuppressive regimen with basiliximab, mycophenolate mofetil, tacrolimus and steroids. In November 2017, the patient received a left pneumonectomy due to evidence of multiple ipsilateral lung metastases.
In March 2020, a contrast-enhanced total body CT-scan revealed a left maxillary sinus lesion of 4 cm. Radiologically, the lesion presented as an osteolytic mass located in the anterior side of the right maxillary sinus, extended to the hard palate and the ipsilateral nasal fossa down to the alveolar process. After contrast-enhancement, the lesion was hypodense with a polypoid behavior in the context of the maxillary sinus. The first biopsy was compatible with a giant cellular inflammatory tissue. Afterwards, the otolaryngologist (ear, nose, throat - ENT) surgeons planned a maximal safe resection of the lesion, which was performed in July 2020, with pathological confirmation of clear cell carcinoma metastasis.
The post-operative CT-scan revealed the persistence of a 2×2 cm lesion located in the right maxillary sinus extended to the ipsilateral nasal fossa and the hard palate. At physical examination, in the right maxillary region, we observed a large tumefaction with minimal erythema. The patient suffered from right nasal obstruction as a consequence of the surgical treatment, with no evidence of nasal bleeding. Also, minimal paresthesia in the right maxillary region was reported.
Given the presence of this lesion as the only active site of disease, and the difficulty to perform further surgical treatment, the patient was a candidate for radiotherapy treatment. In light of the radiobiological features of the disease, SBRT was proposed for the site of recurrence.
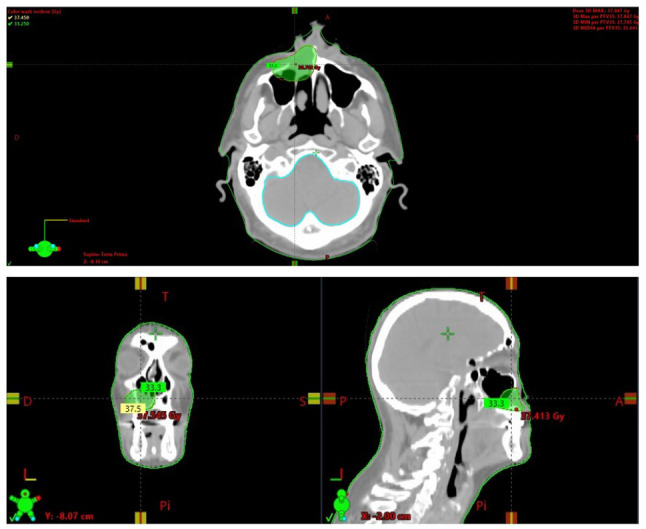
For treatment planning, the immobilization for both simulation and treatment was performed with the aim of a head/shoulders thermoplastic mask (Civco Medical Solutions, Coralville, IA, USA) in a supine position acquired from the vertex to the carina. For target volume delineation, the gross tumor volume (GTV) was identified with the aim of a rigid-fusion of the planning CT with the diagnostic contrast-enhanced CT. Given the critical kidney function of the patient, we decided not to perform further examinations with contrast medium. The planning target volume (PTV) was generated by applying a 3 mm isotropic margin to the GTV. The treatment plan was generated in order to deliver to the PTV a total dose of 35 Gy in 5 fractions using volumetric modulated arc therapy with daily image guidance by means of cone-beam CT (Figure 1).
Figure 1. Axial, coronal, and sagittal views of the stereotactic body radiotherapy (SBRT) treatment plan provided in our case report (35 Gy in 5 daily fractions with the volumetric modulated arc-technique).
Radiotherapy sessions were planned on alternate days and the treatment was well tolerated with no relevant side-effects. The subsequent follow-up exams revealed substantial stability of the treated lesion, with a lower density detected on the last contrast-enhanced CT scan. After 24 months of follow-up, the patient is alive with no evidence of local progression and no relevant side-effects from the radiation treatment.
Discussion
Clear cell carcinoma head and neck metastases are associated with a poor prognosis, since they are mostly followed by concurrent secondary lesions in other organs. On the contrary, isolated head and neck recurrences are rarely reported (10).
A recent review by Bastier et al. reported a total of 53 sinonasal metastases arising from RCC, describing a >10-year latency from the primary kidney cancer diagnosis in 39% of cases. The usual presentation of paranasal sinuses metastases is nasal obstruction and facial pain, similar to the case of our study (7).
Surgical resection is reported as the preferred option, given the possibility to obtain a pathological confirmation of the disease. In agreement, with available literature data, surgery is the most frequently adopted treatment option, alone or in combination with radiotherapy. In fact, the latter option is mainly proposed in the post-operative setting, although the impact in terms of overall survival may be limited; otherwise, radiotherapy alone is reported with palliative intent (11).
To the best of our knowledge, this is among the first experiences reporting the role of SRBT after surgery for a maxillary sinus RCC metastasis. Recently, Marchand Crety et al. reported the first SBRT treatment for a sphenoid metastasis arising from RCC in a patient who received 27 Gy in 3 fractions with a complete response detected after 4 months re-evaluation (12).
In light of several comorbidities of the patient that would not allow for systemic therapy, we aimed to propose a radiotherapy treatment with curative intent, given the subtotal resection of the lesion and the radiobiological features of the primary histology (12). As described for primary kidney cancer, higher doses per fraction are supposed to lead to a major advantage in terms of local control, with several experiences supporting SBRT as a viable option, in the case of the non-feasibility of a surgical approach (13). In the setting of kidney SBRT, a major issue is also represented by response assessment, also due to the absence of a fully reliable metabolic imaging. As hypothesized by some authors, SBRT is supposed to activate an immune reaction within the tumor, which may provide a transient volume increase and a subsequent tumor shrinkage, easily detectable with periodic contrast-enhanced CT scans (14,15). In agreement, in our specific case, we observed at each follow-up CT scan a substantial change in density of the treated lesion, with no relevant changes in terms of volume or dimensions. Interestingly, this evidence has been reported also with initial experiences of carbon ion radiotherapy for primary RCC (16).
Conclusion
We herein reported the use of SBRT for a maxillary sinus metastasis arising from RCC, as a safe and effective treatment option. To our knowledge, this is the first report describing the use of SBRT for sinonasal RCC metastases. This study adds further evidence in support of extreme hypofractionated treatments for renal cancer lesions, with improved local control rates and minimal impact in terms of toxicity.
Conflicts of Interest
The Authors have no competing interests.
Authors’ Contributions
FC, LN, and FR: manuscript drafting and concept. EP, CV, NGL, RM, and VF: literature research. MR, RR, and FA: final revision.
References
- 1.Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, Gore JL, Sun M, Wood C, Russo P. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal R, Gersbach E, Yang XJ, Rohan SM. Differential diagnosis of renal tumors with clear cytoplasm: clinical relevance of renal tumor subclassification in the era of targeted therapies and personalized medicine. Arch Pathol Lab Med. 2013;137(4):467–480. doi: 10.5858/arpa.2012-0085-RA. [DOI] [PubMed] [Google Scholar]
- 3.Ziari M, Shen S, Amato RJ, Teh BS. Metastatic renal cell carcinoma to the nose and ethmoid sinus. Urology. 2006;67(1):199. doi: 10.1016/j.urology.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Choong CV, Tang T, Chay WY, Goh C, Tay MH, Zam NA, Tan PH, Tan MH. Nasal metastases from renal cell carcinoma are associated with Memorial Sloan-Kettering Cancer Center poor-prognosis classification. Chin J Cancer. 2011;30(2):144–148. doi: 10.5732/cjc.010.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Múgica M, Bulnes Vázquez V, Jalón Monzón A, Gil A, Rodríguez Robles L, Miranda Aranzubía O. Late recurrence from a renal cell carcinoma: solitary right maxilar mass 17 years after surgery. Arch Esp Urol. 2010;63(2):147–150. [PubMed] [Google Scholar]
- 6.Prescher A, Brors D. Metastases to the paranasal sinuses: case report and review of the literature. Laryngorhinootologie. 2001;80(10):583–594. doi: 10.1055/s-2001-17835. [DOI] [PubMed] [Google Scholar]
- 7.Bastier PL, Dunion D, de Bonnecaze G, Serrano E, de Gabory L. Renal cell carcinoma metastatic to the sinonasal cavity: A review and report of 8 cases. Ear Nose Throat J. 2018;97(9):E6–E12. doi: 10.1177/014556131809700902. [DOI] [PubMed] [Google Scholar]
- 8.Siva S, Pham D, Gill S, Corcoran NM, Foroudi F. A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int. 2012;110(11 Pt B):E737–E743. doi: 10.1111/j.1464-410X.2012.11550.x. [DOI] [PubMed] [Google Scholar]
- 9.Ingrosso G, Becherini C, Francolini G, Lancia A, Alì E, Caini S, Teriaca MA, Marchionni A, Filippi AR, Livi L, Sanguineti G, Aristei C, Detti B. Stereotactic body radiotherapy (SBRT) in combination with drugs in metastatic kidney cancer: A systematic review. Crit Rev Oncol Hematol. 2021;159:103242. doi: 10.1016/j.critrevonc.2021.103242. [DOI] [PubMed] [Google Scholar]
- 10.Ishak AI, Md Pauzi SH, Masir N, Goh BS. Multiple metastatic deposits in the head and neck region from a renal cell carcinoma. Malays J Med Sci. 2010;17(4):71–74. [PMC free article] [PubMed] [Google Scholar]
- 11.Franzen AM, Schneider C, Lebentrau S, Günzel T. [Extranodal metastases of renal cell carcinoma to the head and neck: a diagnostic challenge] Laryngorhinootologie. 2015;94(11):745–748. doi: 10.1055/s-0034-1395571. [DOI] [PubMed] [Google Scholar]
- 12.Marchand Crety C, Vigneau E, Invernizzi C. Stereotactic body radiotherapy of a solitary metachronous sphenoid metastasis from renal cell cancer: a case report. Case Rep Oncol. 2021;14(1):269–273. doi: 10.1159/000513743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzola R, Cuccia F, Bertani A, Tubin S, Conaldi PG, Corradini S, Tolia M, Guba M, Alongi F. The role of radiotherapy in patients with solid tumours after solid organ transplantation: a systematic review. Lancet Oncol. 2021;22(3):e93–e104. doi: 10.1016/S1470-2045(20)30590-8. [DOI] [PubMed] [Google Scholar]
- 14.Franzese C, Francolini G, Nicosia L, Alongi F, Livi L, Scorsetti M. Stereotactic body radiation therapy in the management of oligometastatic and oligoprogressive bladder cancer and other urothelial malignancies. Clin Oncol (R Coll Radiol) 2021;33(1):50–56. doi: 10.1016/j.clon.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Kothari G, Louie AV, Pryor D, Vela I, Lo SS, Teh BS, Siva S. Stereotactic body radiotherapy for primary renal cell carcinoma and adrenal metastases. Chin Clin Oncol. 2017;6(Suppl 2):S17. doi: 10.21037/cco.2017.06.30. [DOI] [PubMed] [Google Scholar]
- 16.Kasuya G, Tsuji H, Nomiya T, Makishima H, Haruyama Y, Kobashi G, Hayashi K, Ebner DK, Omatsu T, Kishimoto R, Yasuda S, Igarashi T, Oya M, Akakura K, Suzuki H, Ichikawa T, Shimazaki J, Kamada T, Working Group for Genitourinary Tumors Prospective clinical trial of 12-fraction carbon-ion radiotherapy for primary renal cell carcinoma. Oncotarget. 2019;10(1):76–81. doi: 10.18632/oncotarget.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]


