Abstract
Background/Aim: Anti-CD20-depleting monoclonal antibodies predispose patients to the development of severe disease of SARS-CoV-2 infection. These antibodies are given as backbone or maintenance therapy in patients with hematological malignancies and rheumatology diseases, inducing effective B-cell depletion along with antibody-dependent cell-mediated cytotoxicity (ADCC) and disrupting infection-protective antibody responses.
Case Report: We describe two cases of prolonged SARS-CoV-2 infection with common features, in two patients receiving anti-CD20 therapies, the first for chronic lymphocytic leukemia (CLL) and the second for rheumatoid arthritis (RA). For CLL patient, despite administration of antiviral therapy, signs and symptoms of SARS-CoV-2 infection persisted for 43 days, with resolution and lymphocyte recovery from day 33. For RA patient, despite administration of two courses of antiviral therapy, signs and symptoms of SARS-CoV-2 infection persisted for 47 days, without resolution and lymphocyte recovery, leading to a fatal outcome due to acute respiratory distress syndrome (ARDS) and unspecified sepsis.
Conclusion: These two cases highlight the risk for persistent SARS-CoV-2 infection in patients treated with anti-CD20 monoclonal antibodies and support a role for cellular immunity recovery for disease control.
Keywords: COVID-19, cellular immunity, rituximab, obinutuzumab, SARS-CoV-2, chronic lymphocytic leukemia, rheumatoid arthritis
For most immunocompetent patients, infection from SARS-CoV-2 is resolved within 1-3 weeks after the onset of symptoms and virus replication is cleared (1). Pre-existing immunocompromise is significantly associated with increased risk of severe disease (2). Also, variable time to recovery and infectious viral shedding for >2 months has been reported in immunocompromised patients (3,4). The role of ongoing viral infection and evolution versus a secondary heightened inflammation after viral replication for the development of severe COVID-19 is under investigation in patients with severe immunosuppression (5,6). Cases of prolonged and severe SARS-CoV-2 infection with humoral immune deficiencies related to chronic lymphocytic leukemia (CLL) have been described (7). Also, patients with immune-mediated inflammatory diseases like rheumatoid arthritis (RA) are also more vulnerable to represent severe SARS-CoV-2 infection (8).
Case Report
The first case is a 55-year-old female with CLL on obinutuzumab, presented to the emergency department with an approximate 7-day history of fever up to 40˚C and myalgias. On day 1 of illness, she was diagnosed with COVID-19, after positive SARS-CoV-2 nasopharyngeal swab antigen immunoassay. The clinical course is outlined in Figure 1. On day 7 vital signs demonstrated an oxygen saturation of 91% on room air, heart rate of 102, temperature of 38.2˚C, and blood pressure of 135/89. Her medical history included CLL diagnosed 5 years ago, enterectomy of small intestine due to bowel obstruction, perforation, and peritonitis 4 years ago, and anxiety disorder. She had no history of underlying lung disease. The patient’s medication list included obinutuzumab and citalopram, and she was vaccinated with 3 doses of Pfizer-BioNTech coronavirus mRNA vaccine, with the last dose 3 months before COVID-19 infection. She had a known allergy to cefuroxime and was an ex-smoker. On examination, she was dyspneic with fine crackles during aspiration. She had normal abdominal, neurologic, musculoskeletal, and skin exams. A chest radiograph revealed bilateral patchy opacities (Figure 2a). A computed tomography pulmonary angiogram (CTPA) identified multifocal patchy ground glass opacities (GGO) in both lungs without evidence of focal pneumonia, pulmonary abscess, or embolism (Figure 2b). Pneumonia was diagnosed based on clinical symptoms and radiographic findings on admission, and the patient was hospitalized with low supplemental oxygen needs (5 l/min).
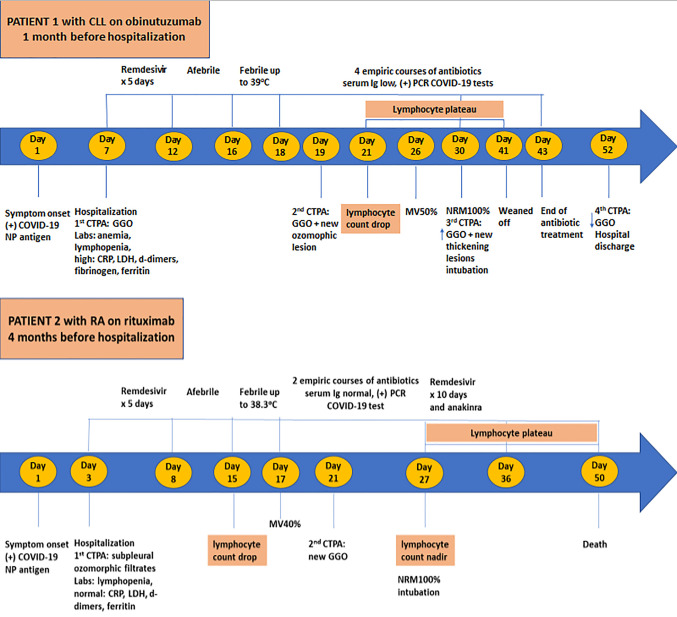
Figure 1. Timeline of COVID-19 illness from symptom onset, disease progression and treatment course for both patients. CLL, Chronic lymphocytic leukemia; RA, rheumatoid arthritis; CTPA, computed tomography of the chest; GGO, ground glass opacities; COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; NP, nasopharyngeal; PCR, polymerase chain reaction; MV, mask venturi; NRM, non-rebreathing mask.
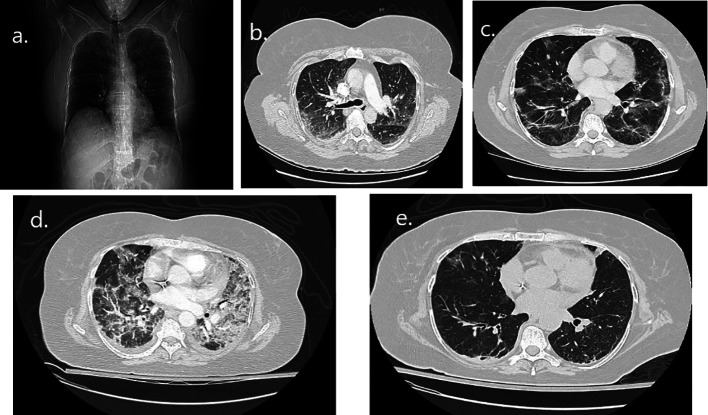
Figure 2. Radiographic imaging of the first patient during COVID-19 illness. (a) Chest radiograph on day 2 of illness with bilateral hazy opacities. (b) Computed tomography pulmonary angiogram (CTPA) on day 2 of illness with bilateral ground glass opacities (GGO) infiltrates. (c) CTPA on day 12 of illness with GGO infiltrates and stripped atelectasis with a new ozomophic lesion in the periphery of right upper lobe (RUL). (d) CTPA on day 23 of illness with old GGO infiltrates increased in both lungs and new condenser hobs with peripheral location. (e) Chest CT on day 43 of illness with old GGO infiltrates diminished and a residual ozomophic lesion still depicted in anterior segment of RUL.
On Day 7, initial laboratory testing revealed anemia with hemoglobin of 10.7, leukopenia with a leukocyte count of 3.30 (×103/μl), lymphopenia with a lymphocyte count of 0.57 (×103/μl), a platelet count of 292 (×103/μl), and normal renal and hepatic function. Acute inflammation biomarkers were increased (CRP=11.77 mg/dl, fibrinogen=459 mg/dl, ferritin >2,000 ng/ml), with d-dimers=1.61 μg/ml and LDH=559 IU/l, while procalcitonin was negative. She received antiviral treatment for SARS-CoV-2 with remdesivir for 5 days, immunomodulation treatment with dexamethasone, antimicrobial treatment with ceftriaxone, and prophylactic anticoagulation therapy. The patient was afebrile and hemodynamically stable with low oxygen needs (2 l/min). On day 16 a low-grade fever up to 37.8˚C was recorded, reaching up to 39˚C until day 18 with no respiratory worsening. On day 19 a new CTPA was performed which was negative for pulmonary embolism, where GGO infiltrates and stripped atelectasis were identified again with a new ozomophic lesion in the periphery of right upper lobe (RUL) (Figure 2c). On day 21, the lymphocyte count dropped to 0.60 (×103/μl) forming a plateau until day 41, and oxygen requirements started to increase (MV50%) from day 26. Blood cultures, urine cultures, respiratory viral PCR panel, and serum fungal biomarkers did not reveal any bacterial, fungal, or viral infection. Procalcitonin was still negative while CRP (≥0.7 mg/dl) and d-dimers (≥0.5 μg/ml) were positive. On day 30, a third CTPA was performed but now old diffuse GGO infiltrates were increased bilaterally, and new thickening lesions were identified with peripheral location (Figure 2d). Also, on day 30 serum immunoglobulin A (76.1 mg/dl), G (277 mg/dl), and M (18.3 mg/dl) levels were low and the patient had a positive nasopharyngeal swab for SARS-CoV-2 by nucleic acid amplification at cycle threshold ct22/22. Until day 30, the patient consecutively received 4 empiric courses of antibiotics for presumed superimposed bacterial pneumonia; the first course consisted of piperacillin/tazobactam, the second with meropenem and vancomycin, the third with meropenem together with daptomycin, amikacin and anidulafungin, and the fourth with ceftazidime/avibactam, colistin, vancomycin, sulfamethoxazole/ trimethoprim and isavuconazole. On day 30, the patient was put on a non-rebreathing mask (100%) and was intubated due to worsening respiratory failure. The patient remained in the ICU for 2 weeks where amikacin was added in the existing antibiotic treatment. The patient recovered gradually and was successfully weaned off mechanical ventilation on day 41 and all antibiotics were stopped on day 43. Repeated nasopharyngeal swabs for SARS-CoV-2 by nucleic acid amplification were positive at cycle threshold ct24/22 and 28/27 on day 37 and 46, respectively. The patient exited the ICU on day 43 with low oxygen needs (2 l/min) and was discharged from the hospital on day 52 with an SpO2=96% on room air. On the last day of hospitalization, a chest CT was performed where old GGO lesions were diminished and a residual ozomophic lesion was still depicted in the anterior segment of RUL (Figure 2e). The patient had no other known medical risk factors for prolonged SARS-CoV-2 infection except CLL treated with the anti-CD20 antibody obinutuzumab every month, with the last course performed one month before hospitalization (Figure 1).
The second case is a 73-year-old female with rheumatoid arthritis (RA) on rituximab, presented to the emergency department with an approximate 2-day history of fever up to 38.5˚C, weakness and fatigue. On day 1 of the illness, she was diagnosed with COVID-19 by a positive SARS-CoV-2 nasopharyngeal swab antigen immunoassay. The clinical course is outlined in Figure 1. On day 3 vital signs were notable for an oxygen saturation of 93% on room air, heart rate of 95, temperature of 38˚C, and blood pressure of 145/85. Her medical history included RA, hypothyroidism, arterial hypertension, and dyslipidemia. She had no history of underlying lung disease. The patient’s medication list included rituximab infusion every 6 months, levothyroxine, irbesartan, metoprolol, rosuvastatin, while she was vaccinated with 3 doses of Pfizer-BioNTech coronavirus mRNA vaccine, with the last dose 4 months before COVID-19 infection. She had no known allergies, and no tobacco or alcohol use. On examination, she was dyspneic with a dry cough and diminished bibasilar breath sounds and crackles. She had normal abdominal neurologic, musculoskeletal, and skin exams. A chest radiograph revealed bilateral patchy opacities (Figure 3a). A CTPA identified subpleural ozomorphic infiltrates in the posterior segment of RUL and right lower lobe (RLL) and stripped fibro-atelectatic elements without evidence of focal pneumonia, pulmonary abscess, or embolism (Figure 3b). Pneumonia was diagnosed based on symptoms and radiographic findings on admission and the patient was hospitalized, with low needs of supplemental oxygen (2 l/min).
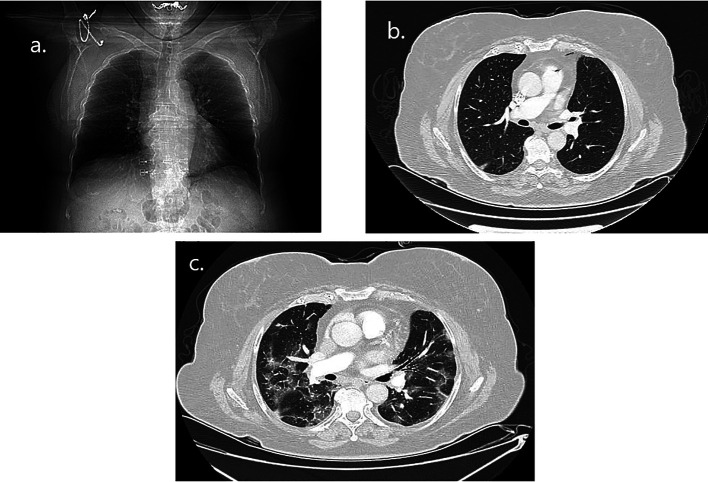
Figure 3. Radiographic imaging of the second patient during COVID-19 illness. (a) Chest radiograph on day 3 of illness with bilateral hazy opacities. (b) Computed tomography pulmonary angiogram (CTPA) on day 3 of illness with bilateral ground glass opacities (GGO) filtrates. (c) CTPA on day 21 of illness with new lesions of multifocal patchy GGO in both lungs.
On day 3, initial laboratory testing revealed hemoglobin levels equal to 12.0, leukopenia with a leukocyte count of 3.87 (×103/μl), lymphopenia with a lymphocyte count of 0.98 (×103/μl), a platelet count of 218 (×103/μl), and normal renal and hepatic function. Acute inflammation biomarkers, d-dimers and LDH were normal and procalcitonin was negative. She received antiviral treatment for SARS-CoV-2 with remdesivir for 5 days, immunomodulation treatment with dexamethasone, antimicrobial treatment with ceftriaxone, and prophylactic anticoagulation therapy. The patient was afebrile and hemodynamically stable with no oxygen need. On day 15, the lymphocyte count dropped further to 0.68 (×103/μl) with the lowest count of 0.13 (×103/μl) on day 27 forming a plateau until day 49, without rebound. On day 16, she had a low-grade fever up to 37.5oC reaching up to 38.3˚C on day 17, with respiratory failure and increased oxygen requirements (MV40%). On day 21, a new CTPA was performed where new lesions were identified with multifocal patchy GGO infiltrates in both lungs (Figure 3c). Blood cultures, urine cultures, respiratory viral PCR panel, and serum fungal biomarkers did not reveal any bacterial, fungal, or viral infection. Procalcitonin was still negative while CRP (≥0.7 mg/dl) and d-dimers (≥0.5 μg/ml) were positive. Until day 26, the patient consecutively received 2 empirical courses of antibiotics for presumed superimposed bacterial pneumonia; the first course consisted of piperacillin/tazobactam and linezolid, and the second with meropenem, amikacin, sulfamethoxazole/trimethoprim and voriconazole. No clinical improvement was observed with any of these interventions. On day 26, serum immunoglobulins were normal, but due to a positive nasopharyngeal swab for SARS-CoV-2 by nucleic acid amplification at cycle threshold ct 29.95/30.9, a new 10-day remdesivir course was decided, with daily anakinra and methylprednisolone as immunomodulation treatment. On day 27, the patient was put on a non-rebreathing mask (100%) and was intubated due to worsening respiratory failure. The patient remained in the ICU for 3 weeks with a fatal outcome on day 50, due to ARDS and unspecified sepsis. The patient had no other known medical risk factors for prolonged SARS-CoV-2 infection except treatment with the anti-CD20 antibody rituximab for RA every six months, with the last course performed four months before hospitalization (Figure 1).
The patient’s written consent was obtained from both patients. The work conforms to standards currently applied in Greece. We have followed the procedures in accordance with the Helsinki Declaration.
Discussion
We encountered a case of a CLL patient with acquired hypogammaglobulinemia, treated with one course of remdesivir who was recovered from COVID-19 infection, and a second case with RA, with normal serum immunoglobulins treated consecutively with two courses of remdesivir with no disease resolution. Comparing the kinetic of lymphocyte counts between the two patients, along with radiographic findings during the SARS-CoV-2 infection, an interpretation for the disease progression could be applied (Figure 1). In the first patient, lymphocyte drop was observed 5 days after the onset of fever and lasted for 20 days. In this period, the patient was intubated due to respiratory failure but after four empiric antibiotic treatments, she was weaned off on day 41 and the lymphocyte count started to recover. In the second patient after the initial remdesivir course and an afebrile period of 12 days, lymphocyte drop was observed along with fever on day 15. Antibiotic treatment was initiated but lymphocytes continued to drop until day 27, where a nadir count was noticed, and patient required mechanical ventilation due to severe respiratory failure. Then a second remdesivir course was given to the patient, but lymphocyte count formed a plateau until day 50, without any recovery and with fatal disease outcome.
Chronic lymphocytic leukemia (CLL) is characterized by a multifactorial disease-inherent immunodeficiency (9). This immune dysregulation drives CLL patients to develop more severe courses of COVID-19 infections especially when under additional immunosuppression by chemoimmunotherapy (10). Differential risk of adverse clinical outcomes has been reported depending on the type of CLL treatment especially with anti-CD20 monoclonal antibodies, while other targeted therapies might have protective effects against COVID-19 by attenuating hyperinflammatory responses (11,12). Also, differential risk of adverse clinical outcomes has been described among patients with systemic rheumatic disease based on the type of biological agents received (13). Particularly, for anti-CD20 monoclonal antibodies like rituximab, which have been associated with dampened humoral response and infectious complications, lethal COVID-19 cases have been described in patients with RA (14,15).
The impact of prior rituximab therapy, given in adults for any indication, has shown that the median time between rituximab administration and COVID-19 diagnosis was not significantly different between those who developed antibodies and those who did not (16). In cancer patients, significantly lower seroconversion was observed in patients with hematologic malignancies, including those who received anti-CD20 antibody treatment and stem cell transplants (17). Also, seroconversion in patients with RA is influenced by the background therapy, particularly for patients being treated with rituximab (18).
The potential importance of humoral responses for SARS-CoV-2 clearance and illness resolution is supported by several clinical reports (19,20), while others indicate that development of neutralizing antibodies may not be necessary for recovery from COVID-19 infection (16). Also, in patients with inherited agammaglobulinaemia, the COVID-19 infection has shown a mild clinical course, implying that protection from severe COVID-19 may be rather independent of serum IgG (21).
Inside a complex inflammatory milieu, persistent adaptive immune activation has been described in cases with SARS-CoV-2 infection leading to lymphocyte exhaustion (22). This T-cell functional exhaustion has been correlated to viral disease progression, rapid decompensation, and life-threatening disease (23). On the contrary, less immunocompromised and non-lymphopenic cancer patients may response adequately, with cytotoxic T lymphocytes and natural killer cells being crucial for the control of viral infection (24). Recently, it was reported that in severe COVID-19 patients the reduction of T-cell count, shifting from a status of hyperactivation to one of exhaustion by the unleashed inflammasome activation, rapidly increased levels of PD-1 (25-27). The potential role of PD-1/PD-L1 axis in COVID-19 infection suggests a prognostic role of PD-L1 and provides a rationale to implement novel clinical therapeutic approaches in COVID-19 patients, with targeted therapies that could manipulate T cells responses (27-29).
Additional research is needed to further investigate the role of adaptive immunity, cellular and humoral, in controlling SARS-CoV-2 infection and establish optimal treatment strategies especially for patients treated with B-cell-depleting therapies.
Conflicts of Interest
All Authors declare no conflicts of interest.
Authors’ Contributions
A.P. conceptualized the study and wrote the manuscript; V.S. collected the data; E.A., V.R., K.S., G.K., S.N., G.K. and E.P. treated the patients; V.S., E.K., T.M. and G.P. drafted the initial manuscript and revised the manuscript; KNS supervised the study. All Authors approved the final manuscript.
Acknowledgements
The Authors would like to thank all staff who worked for the patients’ care at the Infectious Diseases Clinic of the Third Department of Internal Medicine at Sotiria General Hospital. The opinions presented in this article are those of the authors, and do not necessarily represent those of their institutions.
References
- 1.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corse T, Dayan L, Kersten S, Battaglia F, Terlecky SR, Han Z. Clinical outcomes of COVID-19 patients with pre-existing, compromised immune systems: a review of case reports. Int J Med Sci. 2020;17(18):2974–2986. doi: 10.7150/ijms.50537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, Dutta J, van Bakel H, Aberg J, García-Sastre A, Shah G, Hohl T, Papanicolaou G, Perales MA, Sepkowitz K, Babady NE, Kamboj M. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, Adhikari UD, Winkler ML, Mueller AA, Hsu TY, Desjardins M, Baden LR, Chan BT, Walker BD, Lichterfeld M, Brigl M, Kwon DS, Kanjilal S, Richardson ET, Jonsson AH, Alter G, Barczak AK, Hanage WP, Yu XG, Gaiha GD, Seaman MS, Cernadas M, Li JZ. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383(23):2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarhini H, Recoing A, Bridier-Nahmias A, Rahi M, Lambert C, Martres P, Lucet JC, Rioux C, Bouzid D, Lebourgeois S, Descamps D, Yazdanpanah Y, Le Hingrat Q, Lescure FX, Visseaux B. Long-term severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021;223(9):1522–1527. doi: 10.1093/infdis/jiab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, Patel K, Osterborg A, Wojenski D, Kamdar M, Huntington SF, Davids MS, Brown JR, Antic D, Jacobs R, Ahn IE, Pu J, Isaac KM, Barr PM, Ujjani CS, Geyer MB, Berman E, Zelenetz AD, Malakhov N, Furman RR, Koropsak M, Bailey N, Hanson L, Perini GF, Ma S, Ryan CE, Wiestner A, Portell CA, Shadman M, Chong EA, Brander DM, Sundaram S, Seddon AN, Seymour E, Patel M, Martinez-Calle N, Munir T, Walewska R, Broom A, Walter H, El-Sharkawi D, Parry H, Wilson MR, Patten PEM, Hernández-Rivas JÁ, Miras F, Fernández Escalada N, Ghione P, Nabhan C, Lebowitz S, Bhavsar E, López-Jiménez J, Naya D, Garcia-Marco JA, Skånland SS, Cordoba R, Eyre TA. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saadoun D, Vieira M, Vautier M, Baraliakos X, Andreica I, da Silva JAP, Sousa M, Luis M, Khmelinskii N, Gracía JMA, Castrejon I, Gonzalez JCN, Scirè CA, Silvagni E, Bortoluzzi A, Penn H, Hamdulay S, Machado PM, Fautrel B, Cacoub P, Resche-Rigon M, Gossec L. SARS-CoV-2 outbreak in immune-mediated inflammatory diseases: the Euro-COVIMID multicentre cross-sectional study. Lancet Rheumatol. 2021;3(7):e481–e488. doi: 10.1016/S2665-9913(21)00112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. doi: 10.1182/blood-2015-03-567388. [DOI] [PubMed] [Google Scholar]
- 10.Arellano-Llamas AA, Vela-Ojeda J, Hernandez-Caballero A. Chronic lymphocytic leukemia in the SARS-CoV-2 pandemic. Curr Oncol Rep. 2022;24(2):209–213. doi: 10.1007/s11912-022-01198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafat T, Grupel D, Porges T, Levi I, Yagel Y, Nesher L. Treatment with obinutuzumab leads to worse outcomes in haematological patients diagnosed with Omicron variant COVID-19. Br J Haematol. 2022;198(5):826–829. doi: 10.1111/bjh.18315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fürstenau M, Langerbeins P, De Silva N, Fink AM, Robrecht S, von Tresckow J, Simon F, Hohloch K, Droogendijk J, van der Klift M, van der Spek E, Illmer T, Schöttker B, Fischer K, Wendtner CM, Tausch E, Stilgenbauer S, Niemann CU, Gregor M, Kater AP, Hallek M, Eichhorst B. COVID-19 among fit patients with CLL treated with venetoclax-based combinations. Leukemia. 2020;34(8):2225–2229. doi: 10.1038/s41375-020-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, García-García V, Calvo-Sanz L, Del Bosque-Granero I, Terán-Tinedo MA, Boteanu A, Bachiller-Corral J, Vázquez-Díaz M. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40(12):2015–2021. doi: 10.1007/s00296-020-04699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kow CS, Hasan SS. Use of rituximab and the risk of adverse clinical outcomes in COVID-19 patients with systemic rheumatic disease. Rheumatol Int. 2020;40(12):2117–2118. doi: 10.1007/s00296-020-04715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2021;80(5):e67. doi: 10.1136/annrheumdis-2020-218075. [DOI] [PubMed] [Google Scholar]
- 16.Levavi H, Lancman G, Gabrilove J. Impact of rituximab on COVID-19 outcomes. Ann Hematol. 2021;100(11):2805–2812. doi: 10.1007/s00277-021-04662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakkar A, Pradhan K, Jindal S, Cui Z, Rockwell B, Shah AP, Packer S, Sica RA, Sparano J, Goldstein DY, Verma A, Goel S, Halmos B. Patterns of seroconversion for SARS-CoV2-IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2(4):392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benucci M, Damiani A, Gobbi FL, Lari B, Grossi V, Infantino M, Manfredi M. Role of booster with BNT162b2 mRNA in SARS-CoV-2 vaccination in patients with rheumatoid arthritis. Immunol Res. 2022;70(4):493–500. doi: 10.1007/s12026-022-09283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baang JH, Smith C, Mirabelli C, Valesano AL, Manthei DM, Bachman MA, Wobus CE, Adams M, Washer L, Martin ET, Lauring AS. Prolonged severe acute respiratory syndrome Coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2021;223(1):23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, Barbian K, Judson SD, Fischer ER, Martens C, Bowden TA, de Wit E, Riedo FX, Munster VJ. Case study: Prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183(7):1901–1912.e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinti I, Lougaris V, Milito C, Cinetto F, Pecoraro A, Mezzaroma I, Mastroianni CM, Turriziani O, Bondioni MP, Filippini M, Soresina A, Spadaro G, Agostini C, Carsetti R, Plebani A. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):211–213.e4. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitmire JK. Induction and function of virus-specific CD4+ T cell responses. Virology. 2011;411(2):216–228. doi: 10.1016/j.virol.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep. 2020;22(5):53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and functional exhaustion of T cells in patients with Coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellesi S, Metafuni E, Hohaus S, Maiolo E, Marchionni F, D’Innocenzo S, La Sorda M, Ferraironi M, Ramundo F, Fantoni M, Murri R, Cingolani A, Sica S, Gasbarrini A, Sanguinetti M, Chiusolo P, De Stefano V. Increased CD95 (Fas) and PD-1 expression in peripheral blood T lymphocytes in COVID-19 patients. Br J Haematol. 2020;191(2):207–211. doi: 10.1111/bjh.17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duhalde Vega M, Olivera D, Gastão Davanzo G, Bertullo M, Noya V, Fabiano de Souza G, Primon Muraro S, Castro I, Arévalo AP, Crispo M, Galliussi G, Russo S, Charbonnier D, Rammauro F, Jeldres M, Alamón C, Varela V, Batthyany C, Bollati-Fogolín M, Oppezzo P, Pritsch O, Proença-Módena JL, Nakaya HI, Trias E, Barbeito L, Anegon I, Cuturi MC, Moraes-Vieira P, Segovia M, Hill M. PD-1/PD-L1 blockade abrogates a dysfunctional innate-adaptive immune axis in critical β-coronavirus disease. Sci Adv. 2022;8(38):eabn6545. doi: 10.1126/sciadv.abn6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabbatino F, Conti V, Franci G, Sellitto C, Manzo V, Pagliano P, De Bellis E, Masullo A, Salzano FA, Caputo A, Peluso I, Zeppa P, Scognamiglio G, Greco G, Zannella C, Ciccarelli M, Cicala C, Vecchione C, Filippelli A, Pepe S. PD-L1 dysregulation in COVID-19 patients. Front Immunol. 2021;12:695242. doi: 10.3389/fimmu.2021.695242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Vitetta L. Increased PD-L1 expression may be associated with the cytokine storm and CD8+ T-cell exhaustion in severe COVID-19. J Infect Dis. 2021;223(9):1659–1660. doi: 10.1093/infdis/jiab061. [DOI] [PMC free article] [PubMed] [Google Scholar]