Abstract
Background/Aim: In clinical practice, platinum-based systemic chemotherapy works to shrink pelvic lymph nodes. Intra-arterial (IA) bolus infusion may result in more favorable results than systemic chemotherapy. In the present study, we investigated the distribution of cisplatin administrated by IA infusion in varying organs, specifically focusing on the node tissue, in comparison with the intra-venous (IV) route.
Materials and Methods: Under anesthesia, cisplatin 0.42 mg/body was administrated by IA or IV infusion in rats to mimic a balloon-occluded arterial infusion model used in clinical practice. The kidney, bladder, lymphatic tissue, and peripheral blood were extracted to analyze the amount of cisplatin by inductively coupled plasma-mass spectrometry.
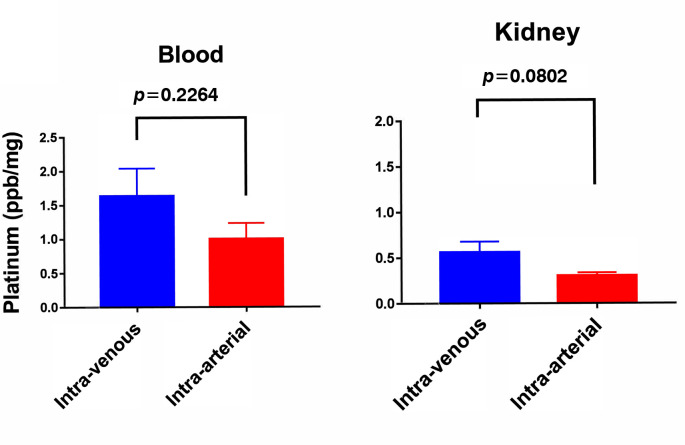
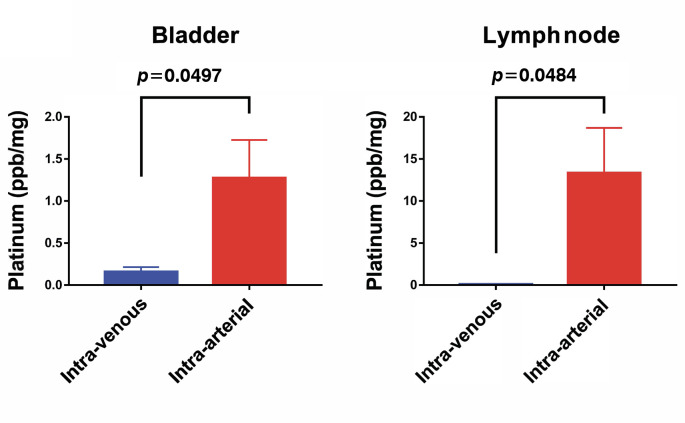
Results: Concertation of cisplatin by IA infusion was higher than that by the IV route in the peripheral blood and kidney. IA infusion led to a significantly high concentration of cisplatin in the bladder compared to IV infusion (1.3±0.452 vs. 0.2 ppb/mg ± 0.055, p=0.050). Furthermore, the IA method led to an extremely high concentration of cisplatin in the lymphatic tissue compared to the IV method (0.1±0.036 vs. 13.3±5.36, p=0.048).
Conclusion: High cisplatin accumulation in the lymphatic tissue and bladder by IA administration may have a potential role for treating patients with node-positive bladder cancer.
Keywords: Urothelial carcinoma, rat model
The last few years have seen significant advancements in the treatment of patients with non-metastatic urothelial bladder carcinoma (nmUBC), given the use of immunological checkpoint inhibitors in the adjuvant setting (1). However, an unmet need still remains for patients who are ineligible for cystectomy. There is currently a lack of novel therapies in these settings, and these patients receive trans-urethral resection of the bladder tumor (TURBT) alone or in combination with chemo-radiation therapy.
All current guidelines for the treatment of nmUBC suggest that radical cystectomy (RC) should be considered as the standard of care in patients who are eligible for surgery. Although surgery remains the standard treatment for nmUBC, bladder-preserving trimodal treatment, including TURBT, radiation, and chemotherapy, offer an alternative conservative option for selected patients who are potential candidates for cystectomy with similar survival rates and high rates of local control (2-7). Lymph node involvement is a poor prognostic factor (8,9). Only 30% of node-positive nmUBC patients survive 3 years following RC and bilateral pelvic lymph node dissection (8,9). Adding chemotherapy to RC has demonstrated improved survival outcomes for node-positive bladder cancer, with pathologic remission to pT0N0 after NAC associated with a 5-year overall survival rate of 85% (10).
Usually, chemotherapy is administrated intra-venously. Although not widely adopted in routine clinical practice, the administration route paradigm remains critical for the advancement of nmUBC therapy. In our clinical center experience, intra-arterial (IA) infusion of combination chemotherapy regimen has been found to be effective for treating patients with node-positive muscle-invasive bladder cancer (11). It is an attractive setting to test novel and effective administration routes for patients with positive nodes, because it provides pharmacodynamic endpoints in the subsequent cystectomy specimen as well as a rapid readout with respect to treatment response. In addition, the adoption of IA chemotherapy could be favored if we were able to predict which patients with lymph node metastases are likely to respond to the therapy. Currently, there is a hesitancy to treat patients via the IA route because uncommon adverse events could occur. The identification of active drug distribution by IA route that predicts organ response to chemotherapy may increase the use of IA aiming for bladder preservation or total cystectomy plus lymphadenopathy with curative intent. However, controversy persists about whether cisplatin could reach lymph-nodes or lymphatic tissue by IA infusion. In this study, we compared cisplatin concentration in relation to these two administration routes in an animal model.
Materials and Methods
Rat model for intra-arterial infusion. Wistar rats (body weight 160-300 g) were used. All animal maintenance and experiments were performed according to institutional guidelines established by the Animal Core Facility at OMPU. The rats were anesthetized with intraperitoneal (i.p.) pentobarbital. After exposure of the descending abdominal artery through a lower abdominal incision, the animals were infused with cisplatin by the IA route (12 rats) or IV route (12 rats). At the end of the drug infusion, the animals were sacrificed by physical euthanasia by applying pressure to the neck and dislocating the spinal column from the skull. The kidney, lymphatic tissue, bladder, and circulating blood sampled by cardiac left ventricle puncture of each rat were collected. The drug accumulated in the lumen of the kidney vessels was cleared out by infusing a saline solution both via the renal artery and renal vein for 60 seconds and parenchymal organs were minced. Tissue samples were frozen at –70˚C and kept on dry-ice until use. Tissue levels of cisplatin and its metabolites were measured using inductively coupled plasma mass spectrometry (ICP-MS), which is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected.
Clinical data of patients with bladder cancer treated by intra-arterial infusion. The dataset includes survival data, clinic-pathological records of patients diagnosed with bladder cancer with lymph node metastases treated by bladder preservation regimens, which include IA cisplatin transfusion. The patients included had one or more radiologically measurable lymph node metastatic sites according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guidelines (defined as a measurable lesion of ≥10 mm on spiral computed tomography) (12).
Statistical analyses. Statistical analysis was done using GraphPad Prism Software (Graphpad, San Diego, CA, USA). For in vivo xenograft data, one-way ANOVA was performed, and unpaired t tests with the two-tailed p-value were used to evaluate the differences between each drug concentration and control groups. Statistical significance was set at p<0.05.
Results
Drug accumulation in each sample organ. When the IV route of administration was used, the uptake of cisplatin in the bladder and lymphatic tissue was very low (0.2 ppb/mg±0.055 and 0.1±0.036, respectively), whereas the accumulation of cisplatin was significantly more substantial with 1.3±0.452 (p=0.050) and 13.3±5.36 (p=0.048), respectively. Cisplatin was also distributed preferentially in the circulating blood and kidney by the IV route (Figure 1 and Figure 2). Administration via the IA route largely increased the concentration of drugs in the pelvic organs. Accumulation of cisplatin by the IA route was increased by 6.5 times in the bladder and 133 times in the lymphatic tissue compared to the levels obtained with the IV route. It is noteworthy that although cisplatin administrated by the IA route exhibited higher level concentration in the blood and kidney than that measured in pelvic organs, drug accumulation was still at lower levels than those after IV injection.
Figure 1. Uptake of cisplatin in the circulating blood and kidney, immediately after administration via the intraarterial or intravenous route.
Figure 2. Uptake of cisplatin in the bladder and lymphatic tissue, immediately after administration via the intra-arterial or intra-venous route.
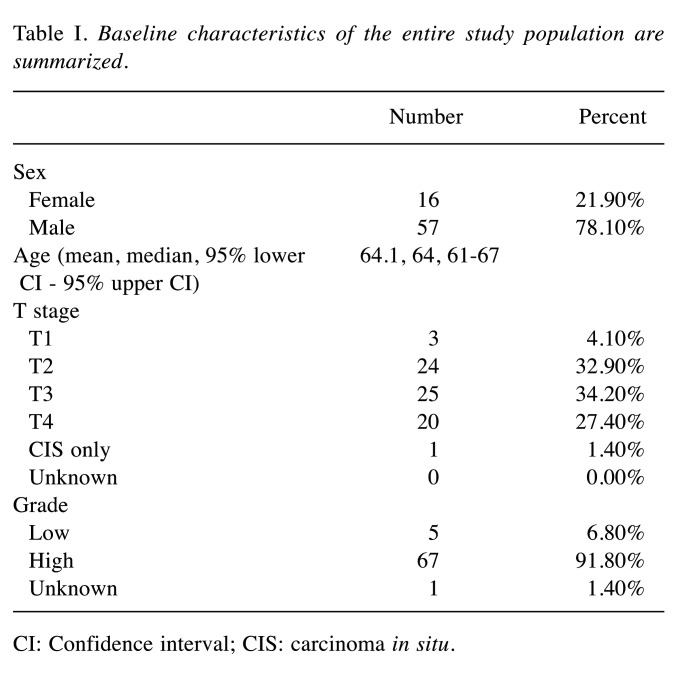
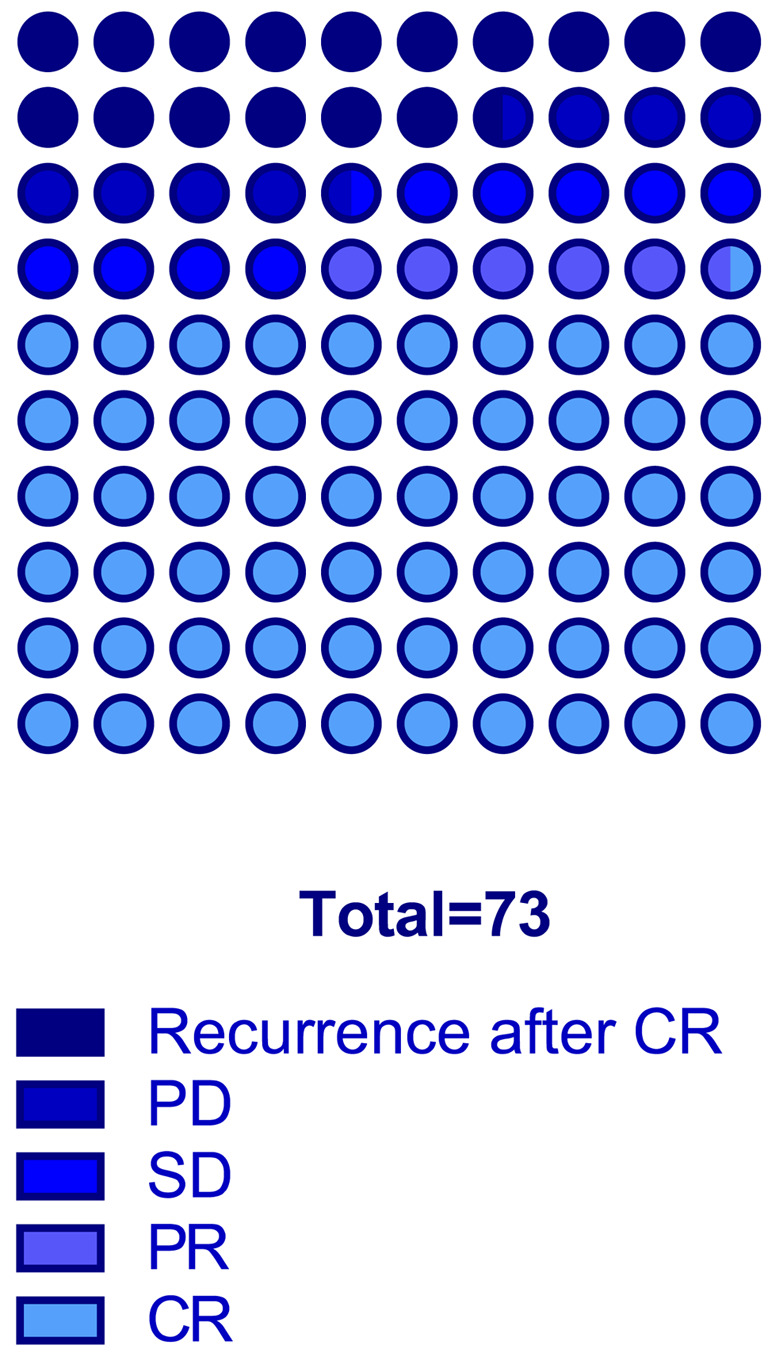
Clinical results of patients with node metastases treated with cisplatin via the IA route. A total of 73 patients were enrolled in the analysis. The median age of all patients was 64 years (95%CI=61-67 years). Baseline characteristics of the entire study population are summarized in Table I. All patients had pure urothelial transitional carcinoma. Eight patents with N1, 19 with N2, and 46 with N3 were included (Figure 3). Forty-four patients achieved CR, 4 had PR, 7 had SD, 6 had PD, and the remaining 12 experienced recurrence after CR. The total objective response rate (ORR; defined as the proportion of patients with CR and PR) was 65.8% (Figure 4).
Table I. Baseline characteristics of the entire study population are summarized.
CI: Confidence interval; CIS: carcinoma in situ.
Figure 3. Distribution of patients stratified by node metastases.
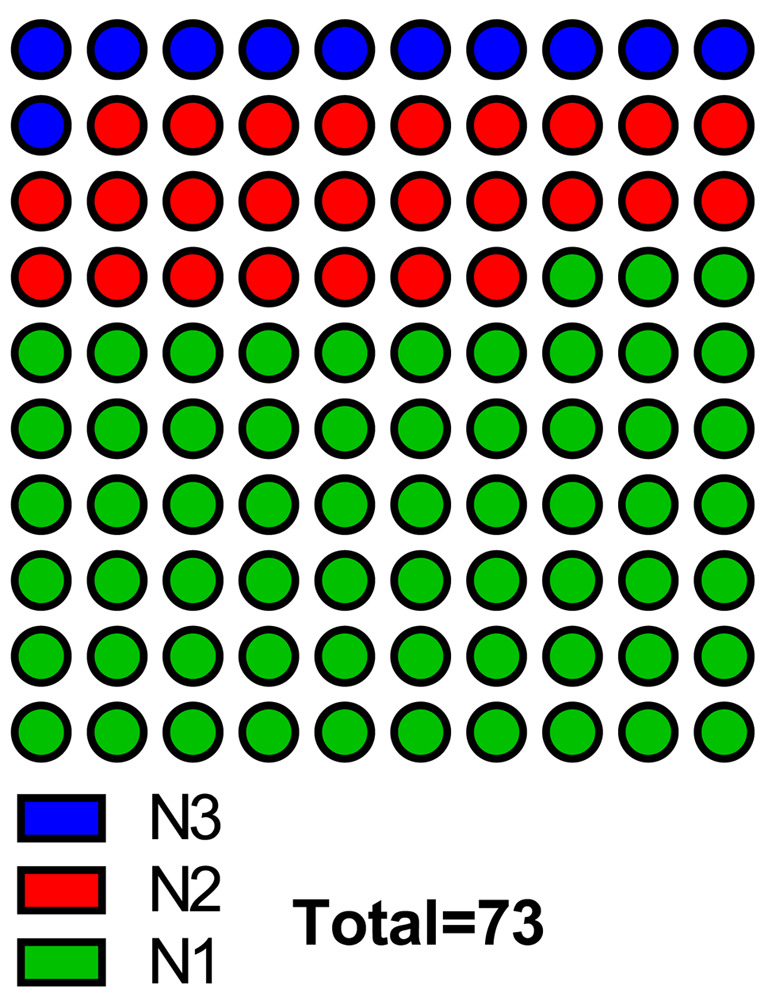
Figure 4. Objective response rates were evaluated using standard bidimensional measurements. CR: Complete response; PD: progressive disease; SD: stable disease; PR: partial response.
Discussion
We developed a new rabbit cisplatin IA administration model using a catheter infusion to investigate the pharmacokinetics of drug delivery to the extremity. The drug uptake in pelvic organs was higher after IA administration than after IV infusion, but not in the circulating blood and kidney. It is widely known that the main organ of excretion of platinum is the kidney and drug-induced renal toxicity is the limiting factor for dosage. The IA administration of cisplatin could be considered less toxic compared with IV infusion considering the lower renal tissue concentration after the former compared with the latter. The amount of cisplatin in the circulating blood showed no difference between IV and IA administration. This could be explained by the time-dependent distribution of cisplatin.
For patients with lymph node metastases, lymph node dissection is an essential component of RC for bladder cancer. Lymph node positive status usually makes the survival data heterogenous, because the extent of node dissection is not defined. A retrospective study showed that approximately 25-30% of nmUBC patients undergoing RC presented with lymph node positive after pathologic examination (13). In a European large cohort of 1,381 patients with muscle invasive bladder cancer with 1 or 2 positive nodes, 5-year OS was 74% and 38% for pT stage ≤2 and pT stage ≥3 (14). Patients with at least 3 positive nodes show a dismal prognosis of 13% regardless of the pT stage, underlining the importance of node number for patient survival (14). A trend towards a larger benefit from adjuvant or neoadjuvant chemotherapy in node-positive bladder cancer has been observed in randomized controlled trials; however, which patients benefit from this approach remains unknown. Therefore, focusing on the distribution of chemotherapy via a different administration route is reasonable. Almost all chemotherapy regimens are administrated via IV infusion to treat node metastases and has been shown to be effective. In the latest propensity matched cohort analysis including 281 patients, to test the value of adjuvant chemotherapy in node-positive bladder cancer, patients with bladder cancer with pT any and 3 or greater positive nodes were found to have a significant benefit from chemotherapy in terms of OS (HR=0.51) (14). In the present study, we investigated whether neoadjuvant chemoradiation therapy via the IA route could reduce the viable cells in metastatic nodes. To this end, the rate of CR was analyzed in relation to clinical node-positive status in advanced bladder cancer patients. For our patients treated with IA chemotherapy, CR was achieved in 60.3% of the cases. This rate is much larger than that reported by other papers using the IV route (15). Ghodoussipour et al. Investigated the effect of neoadjuvant chemotherapy in clinically node positive muscle invasive bladder cancer, along with the radiologic response of the nodes (15). Complete nodal response by pathological review occurred in 40.8% of patients, post-chemotherapy, whose median OS and recurrence-free survival were significantly longer than those with viable cells in the nodes (1.9 years vs. 12.8 years, p=0.016 and 1.2 years vs. 4.3 years, p=0.013, respectively) (15). They found that each 1 mm decrease in the size of the largest node on a post-chemotherapy computed tomography scan, decreased the chance of having viable cells by 1.57 times (15).
IA administration of active drugs is more advantageous for patients even with clinical lymph-node metastases in terms of achieving high drug concentration and minor systemic toxicity than the IV route. The present study suggests examining the pharmacological advantages of bladder arterial infusion of more recently developed drugs aiming to more qualified bladder preservation and improved patients’ survival.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
Atsushi Ichihashi and Teruo Inamoto substantially contributed to the study conceptualization and the manuscript drafting. Taizo Uchimoto, Ko Nakamura, Kazumasa Komura, Yusuke Yano, Kazuki Nishimura, Shoko Kinoshita, Kyosuke Nishio, Tatsuo Fukushima, Keita Nakamori, Tomohisa Matsunaga, Takeshi Tsutsumi, Takuya Tsujino, Hirofumi Uehara, Kiyoshi Takahara, Kazuhiro Yamamoto, Ryuji Kato, Yoshio Ijiri and Tetsuya Hayashi significantly contributed to data analysis and interpretation. Haruhito Azuma supervised the conduct of this study. All Authors critically reviewed and revised the manuscript draft and approved the final version for submission.
References
- 1.Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat SF, Park SH, Ye D, Agerbaek M, Enting D, McDermott R, Gajate P, Peer A, Milowsky MI, Nosov A, Neif Antonio J Jr, Tupikowski K, Toms L, Fischer BS, Qureshi A, Collette S, Unsal-Kacmaz K, Broughton E, Zardavas D, Koon HB, Galsky MD. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcangeli G, Strigari L, Arcangeli S. Radical cystectomy versus organ-sparing trimodality treatment in muscle-invasive bladder cancer: A systematic review of clinical trials. Crit Rev Oncol Hematol. 2015;95(3):387–396. doi: 10.1016/j.critrevonc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Giacalone NJ, Shipley WU, Clayman RH, Niemierko A, Drumm M, Heney NM, Michaelson MD, Lee RJ, Saylor PJ, Wszolek MF, Feldman AS, Dahl DM, Zietman AL, Efstathiou JA. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the massachusetts general hospital experience. Eur Urol. 2017;71(6):952–960. doi: 10.1016/j.eururo.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, Bostrom PJ, Kuk C, Li K, Templeton AJ, Sridhar SS, van der Kwast TH, Chung P, Bristow RG, Milosevic M, Warde P, Fleshner NE, Jewett MAS, Bashir S, Zlotta AR. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35(20):2299–2305. doi: 10.1200/JCO.2016.69.2327. [DOI] [PubMed] [Google Scholar]
- 5.Turgeon GA, Souhami L, Cury FL, Faria SL, Duclos M, Sturgeon J, Kassouf W. Hypofractionated intensity modulated radiation therapy in combined modality treatment for bladder preservation in elderly patients with invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2014;88(2):326–331. doi: 10.1016/j.ijrobp.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Krasnow RE, Drumm M, Roberts HJ, Niemierko A, Wu CL, Wu S, Zhang J, Heney NM, Wszolek MF, Blute ML, Feldman AS, Lee RJ, Zietman AL, Shipley WU, Efstathiou JA. Clinical outcomes of patients with histologic variants of urothelial cancer treated with trimodality bladder-sparing therapy. Eur Urol. 2017;72(1):54–60. doi: 10.1016/j.eururo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 7.García-Perdomo HA, Montes-Cardona CE, Guacheta M, Castillo DF, Reis LO. Muscle-invasive bladder cancer organ-preserving therapy: systematic review and meta-analysis. World J Urol. 2018;36(12):1997–2008. doi: 10.1007/s00345-018-2384-6. [DOI] [PubMed] [Google Scholar]
- 8.Fajkovic H, Cha EK, Jeldres C, Robinson BD, Rink M, Xylinas E, Chromecki TF, Breinl E, Svatek RS, Donner G, Tagawa ST, Tilki D, Bastian PJ, Karakiewicz PI, Volkmer BG, Novara G, Joual A, Faison T, Sonpavde G, Daneshmand S, Lotan Y, Scherr DS, Shariat SF. Extranodal extension is a powerful prognostic factor in bladder cancer patients with lymph node metastasis. Eur Urol. 2013;64(5):837–845. doi: 10.1016/j.eururo.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 10.Darwish C, Sparks A, Amdur R, Reddy A, Whalen M. Trends in treatment strategies and comparison of outcomes in lymph node positive bladder cancer: an analysis of the national cancer database. Urology. 2020;146:168–176. doi: 10.1016/j.urology.2020.06.091. [DOI] [PubMed] [Google Scholar]
- 11.Azuma H, Inamoto T, Takahara K, Nomi H, Hirano H, Ibuki N, Uehara H, Komura K, Minami K, Uchimoto T, Saito K, Takai T, Tanda N, Yamamoto K, Narumi Y, Kiyama S. The novel bladder preservation therapy BOAI-CDDP-radiation (OMC-regimen): a new treatment option for invasive bladder cancer patients with lymph node metastasis. Int J Oncol. 2014;44(6):1895–1903. doi: 10.3892/ijo.2014.2378. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Pedrosa JA, Koch MO, Cheng L. Lymph node-positive bladder cancer: surgical, pathologic, molecular and prognostic aspects. Expert Rev Anticancer Ther. 2013;13(11):1281–1295. doi: 10.1586/14737140.2013.850847. [DOI] [PubMed] [Google Scholar]
- 14.Afferi L, Lonati C, Montorsi F, Briganti A, Necchi A, Mari A, Minervini A, Tellini R, Campi R, Schulz GB, Black PC, di Trapani E, de Cobelli O, Karnes RJ, Ahmed M, Mir MC, Algarra MA, Rink M, Zamboni S, Mondini F, Simeone C, Antonelli A, Tafuri A, Krajewski W, Małkiewicz B, Xylinas E, Soria F, Sanchez Salas R, Arora A, Cathelineau X, Hendricksen K, Ammiwala M, Borghesi M, Chierigo F, Teoh JY, Mattei A, Albisinni S, Roghmann F, Roumiguié M, Bajeot AS, Maier E, Aziz A, Hurle R, Contieri R, Pradere B, Carando R, Poyet C, Alvarez-Maestro M, D’Andrea D, Shariat SF, Moschini M, European Association of Urology Young Academic Urologists Urothelial Carcinoma Working Group Selecting the best candidates for cisplatin-based adjuvant chemotherapy after radical cystectomy among patients with pN+ bladder cancer. Eur Urol Oncol. 2022 doi: 10.1016/j.euo.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Ghodoussipour S, Xu W, Tran K, Atkinson R, Cho D, Miranda G, Cai J, Bhanvadia S, Schuckman A, Daneshmand S, Djaladat H. Preoperative chemotherapy in clinically node positive muscle invasive bladder cancer: Radiologic variables can predict response. Urol Oncol. 2021;39(2):133.e1–133.e8. doi: 10.1016/j.urolonc.2020.08.020. [DOI] [PubMed] [Google Scholar]