Abstract
Fibronectin-binding protein I (SfbI) of Streptococcus pyogenes binds to mouse immunoglobulin G (IgG) but not to IgA or IgM in a nonimmune fashion. The fibronectin-binding domains of SfbI were responsible for this activity, which was targeted to the IgG F(ab′)2 fragment. SfbI also binds to B cells but not to CD4+ or CD8+ lymphocytes.
The expression of bacterial surface proteins that interact nonspecifically with immunoglobulins (Igs) from different mammalian species has been described for many microorganisms (1, 6, 7, 12, 13, 15). Nonimmune binding to Igs is generally mediated by the Fc fragment of the Ig molecules (7, 12, 13, 15). However, few of these proteins can also bind the F(ab′)2 fragment through an alternative binding pathway (2–5). The consistent presence of these Ig-binding proteins in many pathogenic bacteria suggests that these molecules might be required for bacterial survival during the infection process. We have recently shown that fibronectin-binding protein I (SfbI) of Streptococcus pyogenes, a main adhesin and invasin, also binds to human IgG in a nonimmune fashion through the Fc component (8). This binding affected Fc-mediated phagocytosis by macrophages and antibody-dependent cell-mediated cytotoxicity. SfbI was also reactive with mouse, rabbit, pig, and horse IgG (8). In an attempt to further characterize the wide range of immunomodulatory activities exerted by SfbI in the murine system (8–10), which constitutes the model for S. pyogenes in vivo studies, we characterized the interactions between SfbI and mouse Igs.
SfbI binds to mouse IgG.
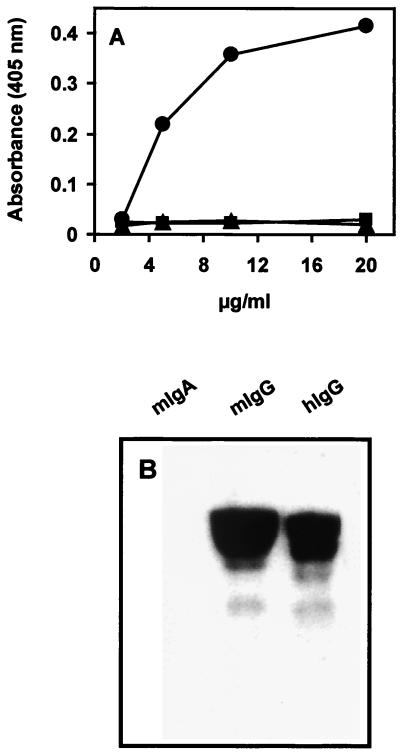
Microtiter plates coated with purified mouse IgA, IgG, or IgM (Dianova, Hamburg, Germany) were incubated with different concentrations of SfbI to test by enzyme-linked immunosorbent assay (ELISA) the ability of SfbI to bind mouse Ig. The SfbI-IgG complexes were detected using rabbit polyclonal anti-SfbI antibodies and a peroxidase-conjugated goat anti-rabbit antibody as a secondary reagent. The results (Fig. 1A) show that SfbI binds to immobilized IgG but not to IgA or IgM. The binding of SfbI to mouse IgG was further confirmed by Western blot analysis under denaturing conditions. Mouse IgA and IgG and human IgG were immobilized onto nitrocellulose and incubated with the SfbI protein. Blots were then exposed to an SfbI-specific rabbit antiserum, which was detected using a peroxidase-conjugated goat anti-rabbit antibody. Appropriate controls were used to exclude possible cross-reactions with secondary reagents. The results that we obtained confirmed that SfbI bound to mouse IgG (Fig. 1B).
FIG. 1.
Binding of SfbI protein to mouse Ig. (A) Binding of SfbI to immobilized mouse IgA (■), IgG (●), and IgM (▴) as determined by ELISA. The reported data are representative of three independent experiments. Results are the averages of triplicate samples. Standard deviations were lower than 10%. (B) Western blot analysis of SfbI binding to mouse (m) and human (h) Igs.
SfbI interacts with mouse IgG through the F(ab′)2 component of the Ig molecule.
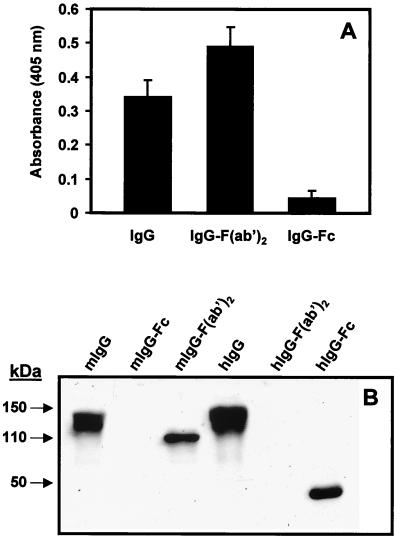
To identify the binding site within the mouse IgG molecule, purified IgG, IgG F(ab′)2, and IgG Fc fragments (Dianova) were tested for their binding to SfbI. The results demonstrate that SfbI interacts with mouse IgG through the F(ab′)2 portion (Fig. 2A). These results were further confirmed by Western blotting (Fig. 2B). The biological significance of a pathogen expressing a single protein with different mammalian Ig-binding patterns is not clear. However, this type of multipattern binding is not unprecedented but rather is common among bacterial Ig-binding proteins (2–5, 11), suggesting that the expression of these proteins may play a role in the adaptive response of the pathogen to an unfavorable host environment.
FIG. 2.
SfbI binds specifically to the F(ab′)2 fragment of mouse IgG. (A) ELISA of SfbI binding to immobilized mouse IgG, IgG F(ab′)2, or IgG Fc fragments. Results are the averages of triplicate samples. Standard deviations are indicated by vertical lines. (B) Western blot analysis of SfbI binding to mouse (m) and human (h) IgG, IgG F(ab′)2, and IgG Fc fragments.
Mouse IgG F(ab′)2 inhibits the binding of SfbI to human IgG Fc.
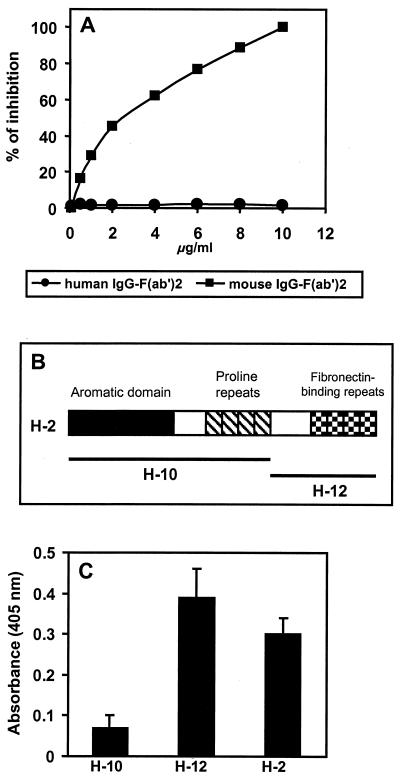
Inhibition experiments were performed to determine whether the binding of SfbI to human IgG Fc and mouse IgG F(ab′)2 was mediated by either a single site or two separate sites. The binding of SfbI to human IgG Fc was tested in the presence of increasing concentrations of mouse IgG F(ab′)2. Figure 3A shows that mouse IgG F(ab′)2 competitively inhibited the binding of SfbI to human IgG Fc in a dose-dependent manner. No effect was observed when human IgG F(ab′)2 fragments were used in the competition test. These results suggest either that the same domain of the SfbI protein is responsible for binding to both human IgG Fc and mouse IgG F(ab′)2 or that the binding sites for both molecules are near each other. Alternatively, the binding of the SfbI domain to one of the moieties may either affect the overall conformation of SfbI or sterically hinder the binding capacities of a putative second domain.
FIG. 3.
(A) Mouse IgG F(ab′)2 fragments inhibit the binding of SfbI to human IgG Fc. The binding of SfbI to human IgG Fc was performed in the presence of increasing concentrations of either mouse or human IgG F(ab′)2 fragments. The values are means of three determinations; one representative out of three independent experiments is shown. Standard deviations were lower than 10%. (B) Schematic representation of the different domains of the SfbI protein. (C) Identification of the SfbI domain able to bind to the F(ab′)2 fragment of mouse IgG. Results are the averages of three independent determinations. Standard deviations are indicated by vertical lines.
To determine the SfbI domain responsible for binding to the IgG F(ab′)2 fragment, full-length SfbI protein and two recombinant polypeptides spanning different domains of SfbI (Fig. 3B) were tested by ELISA for their capacity to bind mouse IgG F(ab′)2. The results demonstrated that the polypeptide encompassing the fibronectin-binding domains of SfbI (H12), but not the one containing the aromatic domain and the proline repeats (H10), was able to bind to mouse IgG F(ab′)2 (Fig. 3C).
It has been observed that bacterial Ig-binding proteins can differ markedly in their levels of reactivity with Ig Fc or Ig F(ab′)2 portions belonging to different mammalian species (5, 14). SfbI was able to bind the Fc fragment of human IgG but failed to bind the F(ab′)2 fragment; conversely, it was able to bind the F(ab′)2 but not the Fc fragment of mouse IgG. This may reflect differences in the avidity of SfbI protein for the different portions of mouse and human IgG. Therefore, the possibility that SfbI has less affinity for interacting with human IgG F(ab′)2 than with IgG Fc cannot be excluded. Weak SfbI-F(ab′)2 binding activity might thus be undetectable during the binding assays.
The stimulatory capacity of SfbI for mouse and human cells.
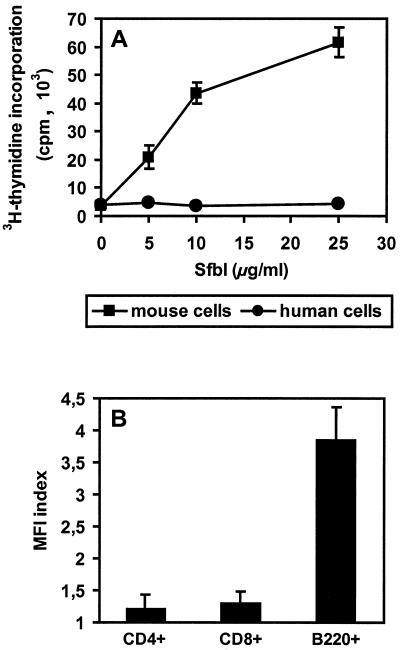
The SfbI protein induces polyclonal activation of murine B cells (9). The observation of different SfbI binding patterns for human and mouse IgG prompted us to further analyze whether human and mouse cells were stimulated by SfbI in a similar manner. Peripheral blood lymphocytes (PBLs) isolated from heparinized venous blood from healthy adult volunteers and cells isolated from mouse spleen were incubated at 37°C in the presence of different concentrations of SfbI for 3 days. Proliferation was determined by [3H]thymidine incorporation after 3 days in culture with SfbI (25 μg/ml). The results presented in Fig. 4A indicate that while mouse spleen cells were stimulated in a dose-dependent manner by SfbI, human PBLs showed no response.
FIG. 4.
(A) Stimulation of human PBLs and mouse spleen cells by SfbI. The values are calculated as follows: mean counts per minute of SfbI-stimulated cells − mean counts per minute in the absence of SfbI. Shown are the means ± standard errors of the means of triplicate samples. (B) Binding of SfbI to mouse spleen cell subsets. Splenocytes were incubated with SfbI, double stained using antibodies specific for SfbI and surface cellular markers, and analyzed by flow cytometry. The mean fluorescence intensity (MFI) index was calculated as follows: MFI value in the presence of SfbI/MFI value in the absence of SfbI.
Binding of SfbI to mouse spleen cells.
Since SfbI binds mouse IgG molecules and stimulates B cells, it might execute in part its biological activities by binding the surface Ig from B cells. Therefore, the binding of SfbI to different populations of mouse spleen cells was further investigated by indirect immunofluorescence using rabbit anti-SfbI and goat anti-rabbit-phycoerythrin antibodies (Dianova). Cells were analyzed on a FACScan (Becton Dickinson, Heidelberg, Germany) gating specific lymphocyte subpopulations stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4, FITC-conjugated anti-CD8, and biotin-conjugated anti-B220 developed with FITC-conjugated streptavidin (Pharmingen, Hamburg, Germany). The results presented in Fig. 4B show that SfbI specifically binds to B220+ B cells but not to CD4+ or CD8+ T-cell subsets. SfbI also did not bind to human PBLs (data not shown).
Our data show that SfbI interacts in a nonimmune fashion with the F(ab′)2 fragment of mouse IgG and binds to mouse B cells. Previous studies demonstrated that SfbI activates B cells, also stimulating an upregulation of major histocompatibility complex class II molecules (9). This suggests that the cross-linking of surface IgG F(ab′)2 on B cells might trigger this process. A better understanding of the underlying molecular events leading to the immunomodulatory activities of this important virulence factor may facilitate the design of new strategies to prevent or treat S. pyogenes infections.
REFERENCES
- 1.Biguzzi S. Fcγ-like determinants on immunoglobulin variable regions: identification by staphylococcal protein A. Scand J Immunol. 1982;15:605–618. doi: 10.1111/j.1365-3083.1982.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 2.Erntell M, Myhre E B, Kronvall G. Alternative non-immune F(ab′)2-mediated immunoglobulin binding to group C and G streptococci. Scand J Immunol. 1983;17:201–209. doi: 10.1111/j.1365-3083.1983.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 3.Erntell M, Myhre E B, Kronvall G. Non-immune F(ab′)2- and Fc-mediated interactions of mammalian immunoglobulins with Staphylococcus aureus and group C and G streptococci. Acta Pathol Microbiol Immunol Scand Sect B. 1986;94:377–385. doi: 10.1111/j.1699-0463.1986.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 4.Erntell M, Myhre E B, Sjobring U, Bjorck L. Streptococcal protein G has affinity for both Fab- and Fc-fragments of human IgG. Mol Immunol. 1988;25:121–126. doi: 10.1016/0161-5890(88)90059-4. [DOI] [PubMed] [Google Scholar]
- 5.Inganäs M. Comparison of mechanisms of interaction between protein A from Staphylococcus aureus and human monoclonal IgG, IgA, and IgM in relation to the classical Fcγ and the alternative F(ab′)2ɛ protein A interactions. Scand J Immunol. 1981;13:343–352. doi: 10.1111/j.1365-3083.1981.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 6.Kornvall G. A surface component in group A, C, and G streptococci with nonimmune reactivity for IgG. J Immunol. 1973;111:1401–1406. [PubMed] [Google Scholar]
- 7.Labbé S, Grenier D. Characterization of the human immunoglobulin G Fc-binding activity of Prevotella intermedia. Infect Immun. 1995;63:2785–2789. doi: 10.1128/iai.63.7.2785-2789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina E, Molinari G, Rohde M, Haase B, Chhatwal G S, Guzmán C A. Fc-mediated non-specific binding between the fibronectin-binding protein I of Streptococcus pyogenes and human immunoglobulins. J Immunol. 1999;163:3396–3402. [PubMed] [Google Scholar]
- 9.Medina E, Talay S R, Chhatwal G S, Guzmán C A. Fibronectin-binding protein I of Streptococcus pyogenes promotes T cell-independent proliferation of murine B lymphocytes and enhances the expression of MHC class II molecules on antigen-presenting cells. Int Immunol. 1998;28:1657–1664. doi: 10.1093/intimm/10.11.1657. [DOI] [PubMed] [Google Scholar]
- 10.Medina E, Talay S R, Chhatwal G S, Guzmán C A. Fibronectin-binding protein I of Streptococcus pyogenes is a promising adjuvant for antigens delivered by mucosal route. Eur J Immunol. 1998;28:1069–1077. doi: 10.1002/(SICI)1521-4141(199803)28:03<1069::AID-IMMU1069>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Raeder R, Faulmann E L, Boyle M D. Evidence for functional heterogeneity in IgG Fc-binding proteins associated with group A streptococci. J Immunol. 1991;146:1247–1253. [PubMed] [Google Scholar]
- 12.Reis K J, Ayoub E M, Boyle M D P. Streptococcal Fc receptor. I. Isolation and partial characterization of the receptors from group C streptococcus. J Immunol. 1984;132:3091–3097. [PubMed] [Google Scholar]
- 13.Schalen C, Christensen P. IgG Fc receptors on group A streptococci. In: Boyle M D P, editor. Bacterial immunoglobulin binding proteins. I. San Diego, Calif: Academic Press; 1990. pp. 57–68. [Google Scholar]
- 14.Schuurman R K B, Gelfand E W, Dosch M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T-cell independent B-cell mitogen. J Immunol. 1980;125:820–826. [PubMed] [Google Scholar]
- 15.Yarnall M, Boyle M D P. Isolation and partial characterization of a type II Fc receptor from a group A streptococcus. Mol Cell Biochem. 1986;70:57–66. doi: 10.1007/BF00233803. [DOI] [PubMed] [Google Scholar]