Abstract
Background/Aim: Radiotherapy regimens for brain or bone metastases vary substantially. This study compared regimens utilized in Northern Germany and Denmark.
Patients and Methods: Three centers participating in the Interreg-Project TreaT completed questionnaires regarding preferred radiotherapy regimens for brain or bone metastases.
Results: Brain metastases: In poor-prognosis patients, all centers prefer short-course whole-brain irradiation (WBI) for multiple metastases. For oligometastatic disease, two centers prefer WBI, one center fractionated stereotactic radiotherapy (FSRT). For single lesions, all centers use FSRT. In intermediate- or favorable-prognosis patients, longer-course WBI is preferred for multiple lesions, sometimes with simultaneous-integrated boost. For oligo-metastasis, regimens vary. FSRT is preferred for single lesions. Bone metastases: For poor-prognosis patients, single-fraction radiotherapy is used for uncomplicated metastases and short-course radiotherapy for (impending) fractures, large soft-tissue components, and spinal cord compression. Multi-fraction regimens are preferred for intermediate-prognosis and longer-course regimens for favorable-prognosis patients.
Conclusion: Regimens are relatively similar for bone metastases, single and multiple brain lesions, but vary considerably for few brain metastases. Further cross-border collaboration is required to provide more uniform and optimized treatment standards.
Keywords: Radiotherapy, brain metastases, bone metastases, treatment concepts, cross-border activities
In cancer patients, brain and bone metastases occur in up to 70% and 40%, respectively. The frequency depends on survival time and primary tumor type (1-5). Many of these patients are assigned to radiotherapy, either alone or following surgery. Different radiotherapy techniques and dose-fractionation schedules are available. Techniques include 3-dimensional conformal radiotherapy (3D-CRT), intensity-modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT), single-fraction stereotactic radiosurgery (SRS), and fractionated stereotactic radiotherapy (FSRT) (2-5). Dose-fractionation schedules include single-fraction treatments and several different multi-fraction radiotherapy regimens mainly administered over one to four weeks.
Worldwide, the preferred techniques and dose-fractionation schedules for brain and bone metastases vary considerably, despite existing international guidelines (3-7). This may hold true also for neighboring countries or even centers in one country. A higher degree of standardization could lead to better research and outcomes. The first step to achieve these goals would be a survey to get an impression, for which situations preferred treatment concepts are the same, similar, or different between radiotherapy centers. Therefore, the present study was performed as part of the German-Danish Interreg-Project TreaT. This project allowed us to compare the preferred concepts of three participating centers located in Northern Germany and Southern Denmark.
Patients and Methods
Experienced radiation oncologists of the three centers participating in the German-Danish Interreg-Project TreaT (University of Lübeck; Medical Practice for Radiotherapy and Radiation Oncology, Hannover; Vejle Hospital, University Hospital of Southern Denmark) completed questionnaires regarding their current radiotherapy opinions for different situations of brain or bone metastases. These include single brain metastasis, oligometastatic situation (2-4 lesions), 5-10 lesions and >10 lesions, as well as painful bone metastases and bone metastases complicated by (impending) pathological fractures, large soft-tissue components, or metastatic spinal cord compression (MSCC). Radiation regimens preferred by each institution for the situations described above were specified for patients with poor, intermediate or favorable survival prognoses. In addition, the radiation oncologists also stated whether the preferred regimens were different for metastases from less radiosensitive tumors such as malignant melanoma and renal cell carcinoma.
Results
Brain metastases.
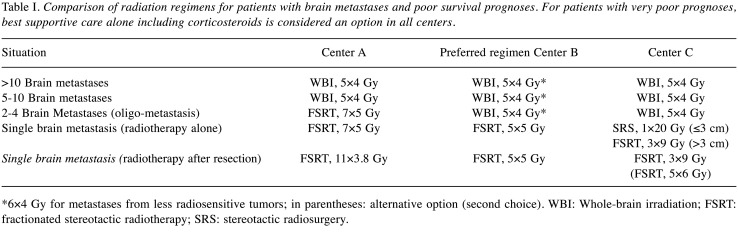
Poor survival prognosis. In patients with poor estimated survival, the preferred radiation concept is the same for >10 lesions and 5-10 lesions in all centers, namely whole-brain irradiation (WBI) with 5×4 Gy over one week (Table I). For patients with oligometastatic disease (2-4 lesions), two centers prefer WBI with 5×4 Gy, whereas one center favors 7×5 Gy of FSRT. In case of irradiation alone for a single brain metastasis, all three centers use local radiotherapy (FSRT or SRS). However, the preferred dose-fractionation regimens vary between the centers, and are 5×5 Gy of FSRT, 7×5 Gy of FSRT, and SRS with 1×20 Gy (lesions ≤3 cm) or 3×9 Gy of FSRT (lesions >3 cm), respectively. Following neurosurgical resection of a single lesion, preferred local treatments include FSRT with 5×4 Gy, 11×3.8 Gy or 3×9 Gy, respectively. One center uses 6×4 Gy of WBI instead of 5×4 Gy for metastases from less radiosensitive tumors. Best supportive care alone including corticosteroids is considered an option in all centers for patients with very poor survival prognoses (8).
Table I. Comparison of radiation regimens for patients with brain metastases and poor survival prognoses. For patients with very poor prognoses, best supportive care alone including corticosteroids is considered an option in all centers.
*6×4 Gy for metastases from less radiosensitive tumors; in parentheses: alternative option (second choice). WBI: Whole-brain irradiation; FSRT: fractionated stereotactic radiotherapy; SRS: stereotactic radiosurgery.
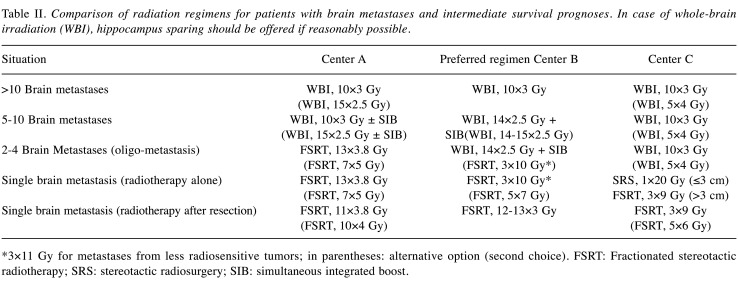
Intermediate survival prognosis. In patients with intermediate prognoses, all three centers prefer WBI with 10×3 Gy over two weeks for >10 lesions (Table II). For 5-10 lesions, WBI with 10×3 Gy is favored in two centers, and WBI with 14-15×2.5 Gy in one center. Two centers use a simultaneous integrated boost (SIB) to metastatic lesions for selected patients. For patients with 2-4 lesions, the radiation concepts vary considerably. One center prefers 13×3.8 Gy or 7×5 Gy of FSRT, one center 18×2 Gy of WBI over 3.5 weeks plus a SIB (18×0.5 Gy) or FSRT with 3×10 Gy, and one center WBI with 10×3 Gy or 5×4 Gy. In case of WBI for a limited number of lesions, hippocampus sparing should be offered and discussed with the patients (9,10). For a single lesion, all centers prefer local irradiation. Dose-fractionation regimens are different and include 3×10 Gy (or 5×7 Gy) of FSRT, 13×3.8 Gy (or 7×5 Gy) of FSRT, and 1×20 Gy of SRS (or 3×9 Gy of FSRT for lesions >3 cm), respectively. Following resection of a single lesion, all centers prefer FSRT with 12-13×3 Gy, 11×3.8 Gy (or 10×4 Gy), and 3×9 Gy (or 5×6 Gy), respectively. One center uses 3×11 Gy of FSRT instead of 3×10 Gy for selected patients with metastases from less radiosensitive tumors.
Table II. Comparison of radiation regimens for patients with brain metastases and intermediate survival prognoses. In case of whole-brain irradiation (WBI), hippocampus sparing should be offered if reasonably possible.
*3×11 Gy for metastases from less radiosensitive tumors; in parentheses: alternative option (second choice). FSRT: Fractionated stereotactic radiotherapy; SRS: stereotactic radiosurgery; SIB: simultaneous integrated boost.
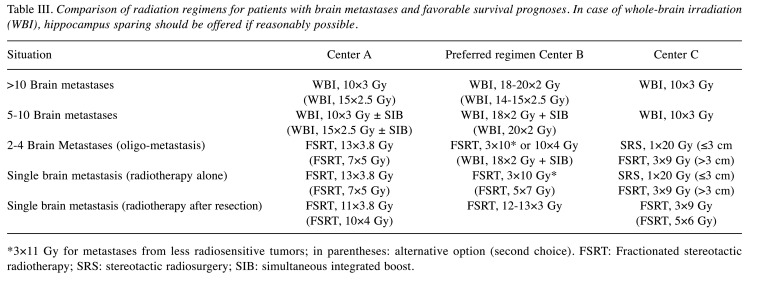
Favorable survival prognosis. In patients with favorable prognoses and >10 lesions, two centers consider WBI with 10×3 Gy the first choice of radiotherapy, and one center WBI with 18-20×2 Gy (Table III). For 5-10 lesions, the same regimens are preferred as for >10 lesions. Two centers administer a SIB for selected patients with 5-10 lesions. For patients with 2-4 lesions, primary choices of treatment vary and include 10×4 Gy of FSRT, 13×3.8 Gy of FSRT and 1×20 Gy of SRS (or 3×9 Gy of FSRT for lesions >3 cm), respectively. For radiotherapy alone of a single lesion, preferred regimens are the same as for patients with intermediate survival prognoses, i.e., local irradiation with 3×10 Gy (or 5×7 Gy) of FSRT, 13×3.8 Gy (or 7×5 Gy) of FSRT, and 1×20 Gy of SRS (or 3×9 Gy of FSRT for lesions >3 cm), respectively. Like for patients with intermediate prognoses, one center uses 3×11 Gy of FSRT for metastases from less radiosensitive tumors in selected patients. Following resection, favored FSRT regimens are almost the same as for intermediate prognoses, i.e., 12-13×3 Gy, 11×3.8 Gy (or 10×4 Gy) and 3×9 Gy (or 5×6 Gy), respectively.
Table III. Comparison of radiation regimens for patients with brain metastases and favorable survival prognoses. In case of whole-brain irradiation (WBI), hippocampus sparing should be offered if reasonably possible.
*3×11 Gy for metastases from less radiosensitive tumors; in parentheses: alternative option (second choice). FSRT: Fractionated stereotactic radiotherapy; SRS: stereotactic radiosurgery; SIB: simultaneous integrated boost.
Bone metastases.
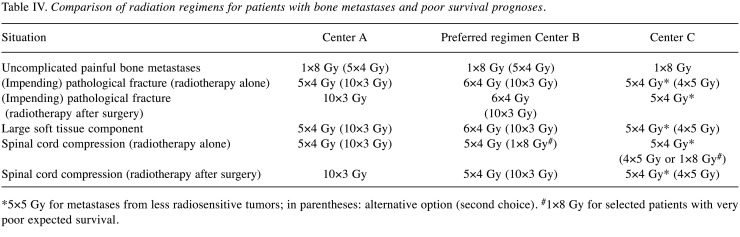
Poor survival prognosis. In patients with poor survival prognoses and uncomplicated bone metastases, all three centers prefer 1×8 Gy, two centers use also 5×4 Gy for selected patients (Table IV). In case of radiotherapy alone for (impending) pathological fractures, short-course regimens are preferred (5×4 Gy in two centers, 6×4 Gy in one center). In addition, two centers use 10×3 Gy and one center 4×5 Gy for selected patients. Moreover, one center uses 5×5 Gy instead of 5×4 Gy for metastases from less radiosensitive tumors. Following surgical stabilization, two centers prefer the same short-course regimens as without surgery, one center prefers 10×3 Gy over two weeks instead of 5×4 Gy. For bone metastases with a large soft tissue component, two centers prefer the same regimens as for pathological fractures, namely 6×4 Gy, 5×4, and 5×4 (or 4×5) Gy, respectively. For radiotherapy alone of MSCC, all centers consider short-course treatment with 5×4 Gy the first choice. In addition, two centers use 1×8 Gy for selected patients with very poor prognoses. Following surgery of MSCC, two centers prefer 5×4 Gy and one center 10×3 Gy.
Table IV. Comparison of radiation regimens for patients with bone metastases and poor survival prognoses.
*5×5 Gy for metastases from less radiosensitive tumors; in parentheses: alternative option (second choice). #1×8 Gy for selected patients with very poor expected survival.
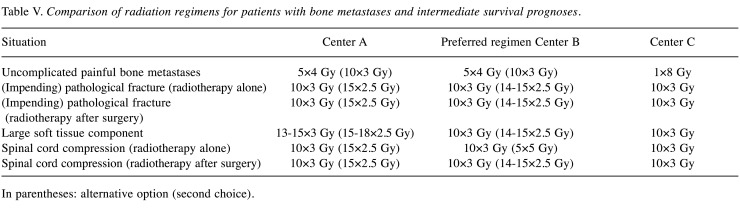
Intermediate survival prognosis. For uncomplicated painful bone metastases in patients with intermediate survival prognoses, two centers prefer 5×4 Gy, one center 1×8 Gy (Table V). In case of impending or existing pathological fractures, 10×3 Gy is the first choice of all centers for both, radiotherapy alone and postoperative radiotherapy. In case of a large soft tissue component, two centers prefer 10×3 Gy and one center 13-15×3 Gy (15-18×2.5 Gy). For radiotherapy alone and postoperative radiotherapy of MSCC, all centers prefer 10×3 Gy. One center uses also 15×2.5 Gy for selected patients, and one center 5×5 Gy for radiotherapy alone and 14-15×2.5 Gy following decompressive surgery.
Table V. Comparison of radiation regimens for patients with bone metastases and intermediate survival prognoses.
In parentheses: alternative option (second choice).
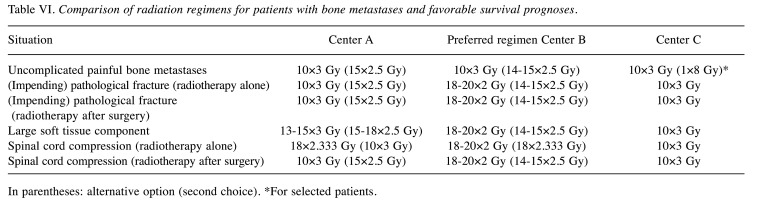
Favorable survival prognosis. In patients with favorable survival prognoses and uncomplicated painful bone metastases, all centers prefer longer-course radiotherapy with 10×3 Gy (Table VI). One center uses 1×8 Gy in selected patients. In two centers, alternative options include 15×2.5 Gy and 14-15×2.5 Gy, respectively. For radiotherapy alone or following surgery in case of (impending) pathological fractures, all centers prefer longer-course programs with 10×3 Gy (two centers) or 18-20×2 Gy (one center). The same regimens are used for postoperative radiotherapy in patients with MSCC. In case of a large soft tissue component, preferred longer-course regimens are 10×3 Gy, 13-15×3 Gy (or 15-18×2.5 Gy), and 18-20×2 Gy (or 14-15×2.5 Gy), respectively. In patients with MSCC, preferred regimens for radiotherapy alone include longer-course radiotherapy with 10×3 Gy, 18×2.333 Gy (or 10×3 Gy), and 18-20×2 Gy (or 18×2.333 Gy), respectively.
Table VI. Comparison of radiation regimens for patients with bone metastases and favorable survival prognoses.
In parentheses: alternative option (second choice). *For selected patients.
Discussion
Brain and bone metastases are common situations facing radiation oncologists (11-15). For both situations, different techniques and dose-fractionation regimens are available. Preferred treatment regimens vary, even between neighboring countries.
Patients would likely benefit from standardized therapies for brain and bone metastases, since treatment standards are generally created from evidence-based data and knowledge. For treating physicians, such standardized treatments would facilitate selection of a suitable regimen for specific situations, and can be especially helpful for less experienced physicians. When considering the benefits of standardized radiotherapy for brain and bone metastases, it appears likely to improve patient outcomes. Before treatments can be standardized, it is important to compare the preferred treatment regimens from different centers, in order to identify similarities and differences. The second step would be a consensus conference to harmonize the favored treatment concepts.
In the present study, preferred radiation regimens for different situations of brain and bone metastases of three centers from Germany and Denmark participating in the Interreg-Project TreaT were recorded and compared. The radiation regimens preferred in the participating centers are relatively similar for bone metastases, single brain metastasis, and multiple brain lesions. For example, in all centers, the favored regimens for uncomplicated bone metastases are 1×8 Gy for patients with poor, 5×4 Gy for patients with intermediate, and 10×3 Gy for patients with favorable survival prognoses. Several meta-analyses have shown that 1×8 Gy is as effective as multi-fraction regimens with respect to pain relief (16,17). However, re-irradiation of the same regions for recurrent pain is required significantly more often after 1×8 Gy than after multi-fraction treatment. Moreover, multi-fraction treatment was demonstrated to result in more pronounced re-calcification of osteolytic bone in a randomized trial (18). Indications for re-irradiation and significant re-calcification usually occur only after several months and are, therefore, more important for patients with intermediate or favorable survival prognoses. This is reflected by the preferred concepts of the centers participating in the present study. When bone metastases are complicated by (impending) pathological fractures, large soft-tissue components or MSCC, multi-fraction regimens are generally recommended. For MSCC, 5×4 Gy and longer-course radiotherapy programs are similarly effective with respect to post-treatment motor function and ambulatory status (19,20). Therefore, patients with poor expected survival should receive 5×4 Gy to keep the overall treatment time short. In contrast, patients with intermediate and favorable prognoses can benefit from longer-course programs in terms of better local control of MSCC (20,21). This aspect is reflected by the preferred concepts of the centers participating in this study, namely longer-course programs. According to a retrospective matched-pair study, patients with expected longer-term survival can achieve better local control of MSCC with doses >30 Gy compared to 10×3 Gy (22). These results are considered by two centers of the present study.
Preferred regimens are relatively similar for single brain metastasis. All three centers use local radiotherapy (FSRT or SRS) alone in accordance with international guidelines (4-7). For multiple brain metastases, WBI with 5×4 Gy is favored by all centers for patients with poor survival prognoses, who are not candidates for best supportive care alone including corticosteroids. These preferences agree with the results of a previous study that compared 5×4 Gy and 10×3 Gy of WBI in patients with multiple brain metastases (23). Since patients with intermediate or favorable prognoses may benefit from longer-course WBI in terms of intracerebral control and survival, all three centers favor longer-course WBI for these patients (24). Moreover, lower doses per fraction are associated with a reduced risk of neuro-cognitive decline (25). Patients with favorable prognoses assigned to WBI may even benefit from 20×2 Gy when compared to 10×3 Gy (24).
In contrast to the situations described above, preferred radiation regimens vary considerably between the three centers for the treatment of very few brain metastases (oligo-metastatic situation). Two centers prefer WBI with 5×4 Gy for poor-prognosis patients, one center FSRT with 7×5 Gy. The preferred treatments are even more heterogeneous for intermediate-prognosis patients. One center favors WBI with 10×3 Gy or 5×4 Gy, one center WBI with 14×2.5 Gy plus SIB (14×0.5 Gy) or FSRT (3×10-11 Gy), and one center FSRT with 13×3.8 Gy or 7×5 Gy. For favorable-prognosis patients, treatments are less heterogeneous, since all centers prefer local radiotherapy with FSRT or SRS. However, the preferred dose-fractionation regimens are quite different (Table III). The differences regarding the favored regimens for oligo-metastatic situations may be explained to a certain extent by the facts that the definition of the maximum number of lesions in an oligo-metastatic situation varies between 2-3 and 2-4 in previous studies, that the available guidelines do not explicitly consider the patient’s survival prognosis, that the use of FSRT or SRS alone (without WBI) has become increasingly popular only during the last decade, and that only a few studies investigated the role of a SIB for oligo-metastatic brain disease (4-7,26,27). The heterogeneity of the radiotherapy concepts preferred by the centers of this study demonstrates that it is important to intensify cross-border collaboration in order to establish and optimize common treatment standards.
Conclusion
This study revealed that the radiation regimens preferred in the three participating centers are relatively similar for bone metastases, single brain metastasis, and multiple brain metastases. However, radiation regimens vary considerably for the treatment of very few brain metastases (oligo-metastatic situation). Thus, further cross-border collaboration including consensus development is required for standardization of radiation regimens, particularly for patients with oligo-metastatic brain disease. Additionally, future research in radiotherapy should focus on optimizing outcome in patients with a few brain metastases.
Conflicts of Interest
On behalf of all Authors, the corresponding Author states that there are no conflicts of interest related to this study.
Authors’ Contributions
D.R., C.K., D.K., S.E.S. and S.J. participated in the design of the study. D.R., C.K. and S.J. provided the data and completed the questionnaire. The article was drafted by D.R. and S.E.S., reviewed by all Authors, and approved by all Authors.
Acknowledgements
The study was funded by the European Regional Development Fund through the Interreg Deutschland-Danmark program as part of the project TreaT (148-1.1-21).
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Rades D, Schild SE, Abrahm JL. Treatment of painful bone metastases. Nat Rev Clin Oncol. 2010;7(4):220–229. doi: 10.1038/nrclinonc.2010.17. [DOI] [PubMed] [Google Scholar]
- 3.Lutz S, Balboni T, Jones J, Lo S, Petit J, Rich SE, Wong R, Hahn C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(1):4–12. doi: 10.1016/j.prro.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondi V, Bauman G, Bradfield L, Burri SH, Cabrera AR, Cunningham DA, Eaton BR, Hattangadi-Gluth JA, Kim MM, Kotecha R, Kraemer L, Li J, Nagpal S, Rusthoven CG, Suh JH, Tomé WA, Wang TJC, Zimmer AS, Ziu M, Brown PD. Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12(4):265–282. doi: 10.1016/j.prro.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, Weinberg JS, Zadeh G, Schiff D. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492–516. doi: 10.1200/JCO.21.02314. [DOI] [PubMed] [Google Scholar]
- 7.Schiff D, Messersmith H, Brastianos PK, Brown PD, Burri S, Dunn IF, Gaspar LE, Gondi V, Jordan JT, Maues J, Mohile N, Redjal N, Stevens GHJ, Sulman EP, van den Bent M, Wallace HJ, Zadeh G, Vogelbaum MA. Radiation therapy for brain metastases: ASCO guideline endorsement of ASTRO guideline. J Clin Oncol. 2022;40(20):2271–2276. doi: 10.1200/JCO.22.00333. [DOI] [PubMed] [Google Scholar]
- 8.Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D, Holt T, Morgan S, Lee C, Waite K, Bayman N, Pugh C, Sydes B, Stephens R, Parmar MK, Langley RE. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, Bovi JA, Robinson C, Konski A, Khuntia D, Grosshans D, Benzinger TLS, Bruner D, Gilbert MR, Roberge D, Kundapur V, Devisetty K, Shah S, Usuki K, Anderson BM, Stea B, Yoon H, Li J, Laack NN, Kruser TJ, Chmura SJ, Shi W, Deshmukh S, Mehta MP, Kachnic LA, for NRG Oncology Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: Phase III trial NRG oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. doi: 10.1200/JCO.19.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rades D, Delikanli C, Blanck O, Schild SE, Janssen S. A survival score for very elderly patients with brain metastases assigned to stereotactic radiosurgery or fractionated stereotactic radiotherapy. Anticancer Res. 2022;42(11):5629–5634. doi: 10.21873/anticanres.16071. [DOI] [PubMed] [Google Scholar]
- 12.Takayama Y, Yano J, Seike R, Mishima S, Shoda H. Efficacy of chemoimmunotherapy in NSCLC patients with brain metastasis with or without prior brain radiotherapy. Anticancer Res. 2022;42(10):4805–4812. doi: 10.21873/anticanres.15985. [DOI] [PubMed] [Google Scholar]
- 13.Noyama T, Katano A, Shinya Y, Kawashima M, Shin M, Saito N, Yamashita H. Prognostic factors for patients with brain metastases treated with single-fraction gamma knife radiosurgery. Anticancer Res. 2021;41(6):3179–3185. doi: 10.21873/anticanres.15104. [DOI] [PubMed] [Google Scholar]
- 14.Sturgis R, Mack A, Kim S, Maier J, Heath EI. Symptom outcomes of cancer patients with clival metastases treated with radiotherapy: a study of 44 patients. Anticancer Res. 2021;41(10):5001–5006. doi: 10.21873/anticanres.15314. [DOI] [PubMed] [Google Scholar]
- 15.Nieder C, Mannsåker B, Yobuta R. Independent validation of a comprehensive machine learning approach predicting survival after radiotherapy for bone metastases. Anticancer Res. 2021;41(3):1471–1474. doi: 10.21873/anticanres.14905. [DOI] [PubMed] [Google Scholar]
- 16.Rich SE, Chow R, Raman S, Liang Zeng K, Lutz S, Lam H, Silva MF, Chow E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol. 2018;126(3):547–557. doi: 10.1016/j.radonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24(2):112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Koswig S, Budach V. [Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study] Strahlenther Onkol. 1999;175(10):500–508. doi: 10.1007/s000660050061. [DOI] [PubMed] [Google Scholar]
- 19.Rades D, Šegedin B, Conde-Moreno AJ, Garcia R, Perpar A, Metz M, Badakhshi H, Schreiber A, Nitsche M, Hipp P, Schulze W, Adamietz IA, Norkus D, Rudat V, Cacicedo J, Schild SE. Radiotherapy with 4 Gy×5 versus 3 Gy×10 for metastatic epidural spinal cord compression: Final results of the SCORE-2 trial (ARO 2009/01) J Clin Oncol. 2016;34(6):597–602. doi: 10.1200/JCO.2015.64.0862. [DOI] [PubMed] [Google Scholar]
- 20.Rades D, Lange M, Veninga T, Stalpers LJ, Bajrovic A, Adamietz IA, Rudat V, Schild SE. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79(2):524–530. doi: 10.1016/j.ijrobp.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 21.Lawton AJ, Lee KA, Cheville AL, Ferrone ML, Rades D, Balboni TA, Abrahm JL. Assessment and management of patients with metastatic spinal cord compression: a multidisciplinary review. J Clin Oncol. 2019;37(1):61–71. doi: 10.1200/JCO.2018.78.1211. [DOI] [PubMed] [Google Scholar]
- 22.Rades D, Panzner A, Rudat V, Karstens JH, Schild SE. Dose escalation of radiotherapy for metastatic spinal cord compression (MSCC) in patients with relatively favorable survival prognosis. Strahlenther Onkol. 2011;187(11):729–735. doi: 10.1007/s00066-011-2266-y. [DOI] [PubMed] [Google Scholar]
- 23.Rades D, Kieckebusch S, Lohynska R, Veninga T, Stalpers LJ, Dunst J, Schild SE. Reduction of overall treatment time in patients irradiated for more than three brain metastases. Int J Radiat Oncol Biol Phys. 2007;69(5):1509–1513. doi: 10.1016/j.ijrobp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Rades D, Panzner A, Dziggel L, Haatanen T, Lohynska R, Schild SE. Dose-escalation of whole-brain radiotherapy for brain metastasis in patients with a favorable survival prognosis. Cancer. 2012;118(15):3852–3859. doi: 10.1002/cncr.26680. [DOI] [PubMed] [Google Scholar]
- 25.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 26.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 27.Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]