Abstract
Background
In clinical studies, the EQ-5D-5L is often employed with disease-specific health-related quality of life instruments. The questions in the former are more general than the latter; however, it is known that responses to general questions can be influenced by preceding specific questions. Thus, the responses to the EQ-5D-5L have the possibility of being influenced by the preceding disease-specific health-related quality of life instruments. This may lead to bias in the cost-effectiveness analysis results. Therefore, this study aimed to evaluate the impact of the preceding cancer-specific health-related quality of life instruments on the EQ-5D-5L responses.
Methods
We prepared questionnaire booklets containing the EQ-5D-5L, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, and the Functional Assessment of Cancer Therapy General with different orders. Using a quasi-randomized design, they were distributed to the patients undergoing drug therapy for advanced cancer, who were classified into three groups: Groups 1, 2, and 3 (the EQ-5D-5L placed first, second, and last, respectively). We compared the EQ-5D-5L index and the missingness of EQ-5D-5L among the groups.
Results
The mean EQ-5D-5L index was 0.796, 0.760, and 0.789 for groups 1 (n = 300), 2 (n = 306), and 3 (n = 331), respectively. The difference between Groups 2 and 1 was − 0.036 (95% CI − 0.065 to − 0.007; p = 0.015). The proportion of patients with an incomplete EQ-5D-5L was 0.11, 0.11, and 0.05 for Groups 1, 2, and 3, respectively. The difference of the proportions between group 3 and 1 and between 3 and 2 was − 0.06 (95% CI − 0.10 to − 0.02; p = 0.003) and − 0.06 (95% CI − 0.10 to − 0.02; p = 0.003), respectively.
Conclusions
Although the EQ-5D-5L index differed according to the instrument orders, the difference size would not be considerably larger than the minimally important difference. The patients tended to complete the EQ-5D-5L when they were placed at the end of the questionnaire.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12955-022-02085-8.
Keywords: Cost-effectiveness analysis, EORTC QLQ-C30, EQ-5D-5L, FACT-G, Health-related quality of life, Order effect
Introduction
Since the results of a cost-effectiveness analysis help to properly allocate limited medical resources, many countries utilize it for decision-making regarding healthcare systems [1, 2]. Thus, conducting it using reliable methods is important, for which several guidelines are available [3–9]. The quality-adjusted life year (QALY), weighting lifetime with health state utilities, is usually recommended as an outcome measure [3, 4, 8]. Furthermore, the EQ-5D index is often used as a health state utility [10]; it is a multi-attribute preference-based measure consisting of five dimensions [11]. Although previously only the three-level version of the EQ-5D was available, the one with five levels, the EQ-5D-5L, was developed for increasing sensitivity and decreasing the ceiling effect [12, 13].
The EQ-5D is frequently employed with disease-specific health-related quality of life (HRQOL) instruments in clinical studies, such as randomized controlled trials that often provide health utility information for health economic evaluation. The EQ-5D and the disease-specific HRQOL instruments are used simultaneously; this is because the former’s five items incompletely describe patients’ health status for clinical assessment due to the smallness of the number of items, while the latter can detect disease-specific symptoms and treatment impacts with high sensitivity. Additionally, the two are utilized concurrently in research developing mapping algorithms from the disease-specific HRQOL instruments onto the EQ-5D indexes [14].
However, theory of order effects and previous studies evaluating the order effects on the responses of the HRQOL instruments indicate the EQ-5D’s possible susceptibility to it. In this paper, order effects refer to the phenomenon that the different orders in which instruments or questions presented influence the responses to them. On the one hand, the order effects on HRQOL instruments consisting of many questions has been unobserved in three studies [15–18], and small differences in few subscales have been observed in another two [19, 20]. On the other hand, for those comprising one question, although a large sample size may be a cause, four studies have reported order effects [21–24]. Thus, the less questions the HRQOL instruments contain, the more susceptible their responses may be to the preceding instruments. This tendency is consistent with the theory of order effects; if the HRQOL instruments contain less questions the questions would be more general, and it is known that responses to general questions can be influenced by preceding specific questions [25]. Additionally, the responses to the EQ-5D correlate with the responses to the disease-specific HRQOL instruments [26–30]. Therefore, the EQ-5D, consisting of one item for each dimension, may be influenced by the HRQOL instrument orders, though direction of the effects was unpredictable. Although a study examining the order effects on the EQ-5D-3L index did not find a difference, this could be because it was continually preceded by the MOS 36-Item Short-Form Health Survey, which is an HRQOL measure consisting of 36 detailed questions [31]. Furthermore, thus far, no study has assessed the order effects on the EQ-5D-5L responses.
If instrument orders impact the EQ-5D-5L responses, the order effects could bias the cost-effectiveness analysis results. For example, when health state utilities are extracted from multiple studies in which different orders of the disease-specific HRQOL instruments and the EQ-5D-5L are adopted, the aforementioned analysis’ results may be influenced due to the order effects. In mapping research, the latter may or may not be preceded by disease-specific measures that may also bias mapping algorithms, resulting in biased results of the cost-effectiveness analysis.
This research investigated the impact of the preceding disease-specific HRQOL instruments on responses to the EQ-5D-5L in the field of oncology. Specifically, we focused on two common cancer-specific HRQOL instruments, namely, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and the Functional Assessment of Cancer Therapy General (FACT-G) and evaluated the differences in the responses to the EQ-5D-5L’s five questions, its mean index, its correlations with the cancer-specific HRQOL instruments’ subscales, and the missingness of its index among the instrument orders.
Methods
Data collection
This research used data from Quality of Life Mapping Algorithm for Cancer (QOL-MAC) study’s data that mainly purported to develop mapping algorithms for the EORTC QLQ-C30 and the FACT-G on the EQ-5D-5L index. Its details have been published elsewhere [32]. The QOL-MAC study was conducted in accordance with the Declaration of Helsinki; the research protocol was approved by each participating hospital.
In the QOL-MAC research, patients with unresectable, locally advanced, recurrent, or metastatic cancers were recruited from 14 hospitals in Japan from November 2018 to March 2019. The eligible patients had lung, stomach, colorectal, breast cancer, or other solid tumors; they were aged 20 years or above and were undergoing drug therapy, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–3. Those who were receiving treatment for multiple primary tumors or were unable to respond to the questionnaires were excluded. All enrolled patients provided written informed consent before participating in the study.
We utilized those questionnaire booklets that contained the EQ-5D-5L, FACT-G, and EORTC QLQ-C30 in different orders; let us denote them as E, F, and Q, respectively. We created six types of questionnaire booklets, each of which contained the HRQOL instruments in any of the following orders: EFQ, EQF, FEQ, QEF, FQE, or QFE. For example, EFQ represents questionnaire booklets comprising the HRQOL instruments in the following order: EQ-5D-5L > FACT-G > EORTC QLQ-C30. We designed the booklets in such a manner that the medical staff could not identify these measures’ order by observing their covers.
We distributed the questionnaire booklets to the patients by a quasi-randomization design using the order in which patients enrolled; therefore, each patient was assigned to any of the six groups defined by its types. Firstly, we repeatedly lined the six questionnaire booklet types in a fixed order (specifically, EFQ > EQF > FQE > FEQ > QFE > QEF) and sent them to all hospitals. Subsequently, each hospital’s medical staff recruited the patients and distributed the questionnaire booklets in the aforementioned order. Finally, the patients answered the questionnaire booklets, which were principally collected in the hospital. When this was unfeasible, the patients returned them directly to the data center.
The HRQOL instruments
The EQ-5D-5L is a multi-attribute preference-based measure comprising five questions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [12]. Each item is rated on a five-point scale: no, slight, moderate, severe, and extreme problems (wording differs according to the items). We calculated the EQ-5D-5L index using the value sets for Japan, England, and the United States [33–35]; one and zero represented full health and death, respectively. The three value sets were used to serve to future studies using some of them.
The 30-item EORTC QLQ-C30 (version 3) is a cancer-specific HRQOL instrument [36]. It includes five functional subscales (physical, role, cognitive, emotional, and social functioning), a global health and quality-of-life subscale, three symptom subscales calculated using several questions (fatigue, nausea and vomiting, and pain), and six symptom items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Each subscale score ranged from 0 and 100; it was calculated when half or more subscale questions were answered. High scores on the functional and global health and quality-of-life subscales denoted better health status, while those on the symptom subscales or items signified severe symptoms.
The FACT-G (version 4) is a cancer-specific HRQOL instrument containing 27 questions [37]. It comprises four subscales regarding well-being: physical, social/family, emotional, and functional. Each subscale’s score was computed when over half of its questions were answered. The FACT-G total score was calculated when more than 80% of all its questions were answered and all subscale scores were obtained. High subscale and FACT-G total scores represented better health status.
Statistical analysis
The QOL-MAC study’s sample size was determined based on the main purpose (i.e., the mapping algorithms’ development) and feasibility. Hence, no sample size calculation for the order effects’ evaluation was conducted.
We employed two analysis populations: the eligible and the completed EQ-5D-5L. The former included all enrolled and suitable patients; it was used for the statistical analysis of the missing EQ-5D-5L indexes. The latter comprised all participating and eligible patients whose EQ-5D-5L indexes were calculated; it was utilized for the statistical analysis of the EQ-5D-5L items and index.
The patients were classified into three groups based on the EQ-5D-5L’s placement in the questionnaire booklets: Groups 1, 2, and 3 consisted of the two booklet types that had the EQ-5D-5L in the first, second, and last places, respectively. The differences in the responses of this scale’s each item between the groups were examined using the Wilcoxon rank sum test. Furthermore, the differences in the mean EQ-5D-5L indexes between the groups were estimated using analysis of variance with the EQ-5D-5L’s order as the only explanatory variable. Additionally, we conducted multivariable analysis and analysis with inverse probability weighting (IPW) (see Additional file 1 for details) [38]. Spearman rank correlation analysis between the EQ-5D-5L index and the subscales of EORTC QLQ-C30 and FACT-G were conducted (see Additional file 1).
Using a linear binomial regression (also called linear probability model) with the EQ-5D-5L’s order as the only explanatory variable, we compared the proportions of those patients whose EQ-5D-5L were incomplete due to some reason; who failed to return the questionnaire booklets to the data center; who submitted the questionnaire booklets unanswered; and who returned these booklets with some incomplete responses. Additional details regarding the statistical analysis including multivariable analysis and analysis of proportions of the FACT-G and the EORTC QLQ-C30 with missing subscales are provided in Additional file 1.
Each p-value was two-tailed, and p < 0.05 was considered to be nominally statistically significant. All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute).
Results
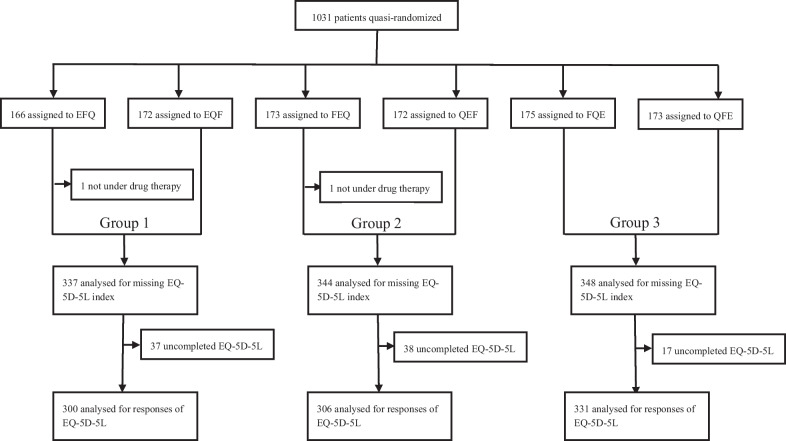
Overall, 1,031 patients were enrolled in the QOL-MAC study (Fig. 1), of which 2 were excluded due to eligibility criteria violations (not receiving drug therapy); thus, the eligible population comprised 1,029 patients. The completed EQ-5D-5L population included 937 patients, after excluding 92 patients. Their demographic and clinical characteristics were similar among the three groups (Table 1). The median ages of Groups 1, 2, and 3 were 68, 67, and 68, respectively; 54% were male. Furthermore, lung cancer was the most common (35%), followed by colorectal cancer (25%); 50% and 41% had an ECOG performance status of 0 and 1, respectively. The patient characteristics between those with complete and incomplete EQ-5D-5Ls have been shown in Additional file 1: Table S1.
Fig. 1.
Patient flow chart. EFQ, EQF, FEQ, QEF, FQE and QFE respectively stand for the questionnaire type containing HRQOL instruments in the order of EQ-5D-5L > FACT-G > EORTC QLQ-C30, EQ-5D-5L > EORTC QLQ-C30 > FACT-G, FACT-G > EQ-5D-5L > EORTC QLQ-C30, EORTC QLQ-C30 > EQ-5D-5L > FACT-G, FACT-G > EORTC QLQ-C30 > EQ-5D-5L and EORTC QLQ-C30 > FACT-G > EQ-5D-5L. Groups 1, 2, and 3 consisted of the two questionnaire types that had EQ-5D-5L in the first, second, and last places, respectively. EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; FACT-G, Functional Assessment of Cancer Therapy General
Table 1.
Demographic and clinical characteristics of the eligible patients with a completed EQ-5D-5L
| Characteristic | Group 1 (N = 300) | Group 2 (N = 306) | Group 3 (N = 331) |
|---|---|---|---|
| Age (in years) | 68 (58–75) | 67 (58–73) | 68 (59–74) |
| Sex | |||
| Male | 157 (52%) | 163 (53%) | 189 (57%) |
| Female | 143 (48%) | 143 (47%) | 142 (43%) |
| Tumor type | |||
| Lung cancer | 108 (36%) | 108 (35%) | 113 (34%) |
| Stomach cancer | 21 (7%) | 22 (7%) | 23 (7%) |
| Colorectal cancer | 79 (26%) | 73 (24%) | 80 (24%) |
| Breast cancer | 37 (12%) | 35 (11%) | 44 (13%) |
| Other solid tumors | 55 (18%) | 68 (22%) | 71 (21%) |
| Stage at diagnosis | |||
| I | 12 (4%) | 19 (6%) | 18 (5%) |
| II | 23 (8%) | 17 (6%) | 34 (10%) |
| III | 63 (21%) | 64 (21%) | 65 (20%) |
| IV | 200 (67%) | 204 (67%) | 206 (62%) |
| Unknown | 2 (1%) | 2 (1%) | 8 (2%) |
| Site of metastasis or recurrencea | |||
| None | 26 (9%) | 22 (7%) | 35 (11%) |
| Liver | 60 (20%) | 72 (24%) | 79 (24%) |
| Lung | 110 (37%) | 95 (31%) | 91 (27%) |
| Bone | 48 (16%) | 54 (18%) | 67 (20%) |
| Brain | 34 (11%) | 35 (11%) | 31 (9%) |
| Lymph nodes | 124 (41%) | 129 (42%) | 120 (36%) |
| Others | 69 (23%) | 73 (24%) | 90 (27%) |
| History of surgery | |||
| Yes | 159 (53%) | 152 (50%) | 186 (56%) |
| No | 141 (47%) | 154 (50%) | 144 (44%) |
| Unknown | 0 (0%) | 0 (0%) | 1 (0%) |
| Hospitalization | |||
| Yes | 60 (20%) | 71 (23%) | 64 (19%) |
| No | 240 (80%) | 235 (77%) | 267 (81%) |
| ECOG performance status | |||
| 0 | 156 (52%) | 148 (48%) | 160 (48%) |
| 1 | 115 (38%) | 127 (42%) | 143 (43%) |
| 2 | 21 (7%) | 21 (7%) | 25 (8%) |
| 3 | 7 (2%) | 10 (3%) | 3 (1%) |
| Unknown (0, 1, 2, or 3) | 1 (0%) | 0 (0%) | 0 (0%) |
| Type of treatmenta | |||
| Chemotherapy | 186 (62%) | 195 (64%) | 225 (68%) |
| Endocrine therapy | 20 (7%) | 22 (7%) | 25 (8%) |
| Molecular targeted therapy | 56 (19%) | 49 (16%) | 58 (18%) |
| Immunotherapy | 38 (13%) | 43 (14%) | 31 (9%) |
| Palliative therapy | 17 (6%) | 10 (3%) | 10 (3%) |
| Others | 3 (1%) | 2 (1%) | 3 (1%) |
| Adverse event at enrollment | |||
| Yes | 186 (62%) | 200 (65%) | 220 (66%) |
| No | 113 (38%) | 106 (35%) | 110 (33%) |
| Unknown | 1 (0%) | 0 (0%) | 1 (0%) |
The median (IQR) and the number (%) were reported for age and other characteristics, respectively. Groups 1, 2, and 3 consisted of the two questionnaire types that had the EQ-5D-5L in the first, second, and last places, respectively
ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range
aMultiple choices were allowed
Regarding mobility, the responses between Groups 1 and 2 and between 1 and 3 were significantly different (Table 2). Specifically, the proportion of the patients who reported “no problems” with mobility was smaller in Groups 2 (48%) and 3 (50%) than in Group 1 (65%), whereas the proportion of those with “slight problems” was larger in Groups 2 (29%) and 3 (30%) as compared to Group 1 (17%). Moreover, the responses to usual activities between Groups 1 and 2 and between 2 and 3 varied significantly. Specifically, the proportion of the patients who did not indicate problems with usual activities was smaller in Group 2 (40%) than in Groups 1 (50%) and 3 (50%), while that of those who reported slight difficulties was larger in Group 2 (40%), as compared to Groups 1 (31%) and 3 (29%). The results distinguishing FEQ and QEF in the questionnaire booklets that had the EQ-5D-5L in the second place are displayed in Additional file 1: Table S2.
Table 2.
Responses to the EQ-5D-5L’s five items in the three groups
| Response level | P-value | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | vs Group 1 | vs Group 2 | |
| Mobility | |||||||
| Group 1 | 194 (65%) | 51 (17%) | 26 (9%) | 23 (8%) | 6 (2%) | – | – |
| Group 2 | 146 (48%) | 88 (29%) | 41 (13%) | 21 (7%) | 10 (3%) | < 0.001 | – |
| Group 3 | 165 (50%) | 100 (30%) | 47 (14%) | 17 (5%) | 2 (1%) | 0.004 | 0.310 |
| Self-care | |||||||
| Group 1 | 243 (81%) | 28 (9%) | 16 (5%) | 8 (3%) | 5 (2%) | – | – |
| Group 2 | 242 (79%) | 40 (13%) | 12 (4%) | 5 (2%) | 7 (2%) | 0.657 | – |
| Group 3 | 271 (82%) | 42 (13%) | 10 (3%) | 6 (2%) | 2 (1%) | 0.606 | 0.322 |
| Usual activities | |||||||
| Group 1 | 150 (50%) | 92 (31%) | 31 (10%) | 22 (7%) | 5 (2%) | – | – |
| Group 2 | 122 (40%) | 123 (40%) | 32 (10%) | 16 (5%) | 13 (4%) | 0.045 | – |
| Group 3 | 167 (50%) | 95 (29%) | 43 (13%) | 22 (7%) | 4 (1%) | 0.998 | 0.045 |
| Pain/discomfort | |||||||
| Group 1 | 122 (41%) | 126 (42%) | 39 (13%) | 10 (3%) | 3 (1%) | – | – |
| Group 2 | 118 (39%) | 140 (46%) | 31 (10%) | 12 (4%) | 5 (2%) | 0.807 | – |
| Group 3 | 136 (41%) | 137 (41%) | 40 (12%) | 15 (5%) | 3 (1%) | 0.988 | 0.784 |
| Anxiety/depression | |||||||
| Group 1 | 188 (63%) | 85 (28%) | 16 (5%) | 7 (2%) | 4 (1%) | – | – |
| Group 2 | 169 (55%) | 100 (33%) | 24 (8%) | 10 (3%) | 3 (1%) | 0.057 | – |
| Group 3 | 206 (62%) | 93 (28%) | 22 (7%) | 10 (3%) | 0 (0%) | 0.896 | 0.068 |
The number (%) was reported for each response level. Responses 1 to 5 correspond to no problems, slight problems, moderate problems, severe problems, and extreme problems, respectively (wording differs according to the items). Groups 1, 2, and 3 consisted of the two questionnaire types that had the EQ-5D-5L in the first, second, and last places, respectively
The mean EQ-5D-5L indexes based on the value set for Japan in Groups 1, 2, and 3 were 0.796 (95% CI 0.776–0.817), 0.760 (0.740–0.781), and 0.789 (0.769–0.808), respectively (Table 3); it was the lowest in Group 2. The difference between Groups 2 and 1 was − 0.036 (95% CI − 0.065, − 0.007; p = 0.015), and that between Groups 3 and 2 was 0.029 (0.000, 0.057; 0.049). The discrepancies based on the three value sets in the EQ-5D-5L index’s mean differences between the groups were less than 0.01 for all pairwise comparisons. The analysis adjusted for covariates and the analysis adjusted for the missing EQ-5D-5L indexes through the IPW showed similar results (Additional file 1: Tables S3 and S4). A forest plot for the subgroup analysis is shown in Additional file 1: Fig. S1. Additional file 1: Table S5 contains the results discriminating between FEQ and QEF.
Table 3.
Mean EQ-5D-5L indexes in the three groups
| Mean (95% CI) | Difference (95% CI; P-value) | ||
|---|---|---|---|
| vs Group 1 | vs Group 2 | ||
| Japanese value set | |||
| Group 1 | 0.796 (0.776, 0.817) | – | – |
| Group 2 | 0.760 (0.740, 0.781) | − 0.036 (− 0.065, − 0.007; 0.015) | – |
| Group 3 | 0.789 (0.769, 0.808) | − 0.008 (− 0.036, 0.021; 0.597) | 0.029 (0.000, 0.057; 0.049) |
| England value set | |||
| Group 1 | 0.821 (0.797, 0.844) | – | – |
| Group 2 | 0.791 (0.768, 0.814) | − 0.030 (− 0.063, 0.003; 0.071) | – |
| Group 3 | 0.822 (0.800, 0.844) | 0.001 (− 0.031, 0.033; 0.948) | 0.031 (− 0.001, 0.063; 0.056) |
| US value set | |||
| Group 1 | 0.783 (0.754, 0.813) | – | – |
| Group 2 | 0.744 (0.715, 0.773) | − 0.039 (− 0.081, 0.002; 0.061) | – |
| Group 3 | 0.779 (0.751, 0.807) | − 0.005 (− 0.045, 0.036; 0.822) | 0.035 (− 0.006, 0.075; 0.091) |
Groups 1, 2, and 3 comprised the two questionnaire types having the EQ-5D-5L in the first, second, and last places, respectively
CI, confidence interval; US, the United States
The rank correlation only between the EQ-5D-5L index and the EORTC QLQ-C30’s role functioning subscale demonstrated significant differences between the EQF and QEF groups (difference 0.11; Additional file 1: Table S6). The rank correlations between the EQ-5D-5L’s five questions and the EORTC QLQ-C30 and FACT-G subscales have been presented in Additional file 1: Tables S7–S11.
The proportions of the patients with incomplete EQ-5D-5L in Groups 1, 2, and 3 were 0.11 (95% CI 0.08, 0.14), 0.11 (0.08, 0.14), and 0.05 (0.03, 0.07), respectively (Table 4). It was the lowest in Group 3. The difference in the proportions between Groups 3 and 1 was − 0.06 (95% CI − 0.10, − 0.02; p = 0.003), while that between Groups 3 and 2 was − 0.06 (− 0.10, − 0.02; 0.003). The analysis adjusted for covariates showed almost identical results (Additional file 1: Table S12). Both of the proportions of the patients with missing subscales of the FACT-G and those of the EORTC QLQ-C30 were lowest in Group 3 (Additional file 1: Tables S13 and S14).
Table 4.
Proportions of the incomplete EQ-5D-5L in the three groups
| Proportion (95% CI) | Difference (95% CI; P-value) | ||
|---|---|---|---|
| vs Group 1 | vs Group 2 | ||
| Incomplete EQ-5D-5L for any reasons | |||
| Group 1 | 0.11 (0.08, 0.14) | – | – |
| Group 2 | 0.11 (0.08, 0.14) | 0.00 (− 0.05, 0.05; 0.978) | – |
| Group 3 | 0.05 (0.03, 0.07) | − 0.06 (− 0.10, − 0.02; 0.003) | − 0.06 (− 0.10, − 0.02; 0.003) |
| Did not return to the data center | |||
| Group 1 | 0.05 (0.03, 0.08) | – | – |
| Group 2 | 0.06 (0.04, 0.09) | 0.01 (− 0.03, 0.04; 0.668) | – |
| Group 3 | 0.02 (0.01, 0.04) | − 0.03 (− 0.06, 0.00; 0.038) | − 0.04 (− 0.07, − 0.01; 0.012) |
| Returned the questionnaire without any response | |||
| Group 1 | 0.02 (0.00, 0.03) | – | – |
| Group 2 | 0.02 (0.01, 0.04) | 0.01 (− 0.02, 0.03; 0.616) | – |
| Group 3 | 0.01 (0.00, 0.03) | 0.00 (− 0.02, 0.02; 0.721) | − 0.01 (− 0.03, 0.01; 0.390) |
| Incomplete EQ-5D-5L with some responses to the returned questionnaire | |||
| Group 1 | 0.04 (0.02, 0.06) | – | – |
| Group 2 | 0.03 (0.01, 0.04) | − 0.01 (− 0.04, 0.01; 0.360) | – |
| Group 3 | 0.01 (0.00, 0.02) | − 0.03 (− 0.05, 0.00; 0.023) | − 0.01 (− 0.03, 0.01; 0.156) |
Groups 1, 2, and 3 contained the two questionnaire types that had the EQ-5D-5L in the first, second, and last places, respectively
CI, confidence interval
Discussion
This study investigated the impact of the preceding cancer-specific HRQOL instruments on the subsequent EQ-5D-5L’s responses. Regarding the mobility question, the answers differed between the groups with the EQ-5D-5L placed first and second or third. The responses to the usual activities question varied between those with the EQ-5D-5L positioned first and second. As compared to the mean EQ-5D-5L indexes of the former, those of the latter were lower. Few correlation coefficients between the EQ-5D-5L index and the subscales of disease-specific HRQOL instruments differed between the groups with the EQ-5D-5L positioned first and second. The patients in the group with the EQ-5D-5L placed last tended to complete the EQ-5D-5L.
Responses to EQ-5D-5L
The assimilation and contrast effects may explain the order effects on the EQ-5D-5L responses. If respondents answered a specific question before a general one, they may interpret the latter as having a similar meaning to the former, which is called the assimilation effect [25, 39]. However, if they are aware of the preceding specific question while answering the subsequent general one, they may infer the latter excluding the meaning of the former to avoid the redundancy of responses, which is known as the contrast effect [25, 39]. The main differences among the orders were observed in response levels of one (“no problems”) and two (“slight problems”). The patients in the group with the EQ-5D-5L placed second or last would have considered many situations before answering it to respond to the specific questions in the disease-specific HRQOL instruments; moreover, in this process, they may recall any problem relating to the EQ-5D-5L questions. Therefore, the assimilation effect of the specific questions on the EQ-5D-5L responses may have resulted in a small and a large proportion of “no problems” and “slight problems” reported, respectively. However, when two disease-specific HRQOL instruments preceded, the repetition of questions overlapping with each other may have rendered the patients more aware of the specific questions’ content; this may have caused a contrast effect due to the preceding question’s awareness.
For example, regarding usual activities, the specific questions’ contrast effect may help in explaining the difference in the responses between the groups with the EQ-5D-5L placed second and last; this is because most questions that would be related to usual activities overlap between the FACT-G and the EORTC QLQ-C30, such as GF1, GF2, and Q6; GF6 and Q7; and GP3 and Q26 (Table 5). Nevertheless, most specific questions related to mobility were only in the EORTC QLQ-C30, such as Q1, Q2, and Q3. Thus, the patients may have a likelihood of being unaware of these specific questions while answering the EQ-5D-5L’s mobility item; therefore, less contrast effect may occur. This may have resulted in a small proportion of “no problems” reported on the mobility questions in not only the group with the EQ-5D-5L placed second, but also the one where it was positioned last. Regarding anxiety/depression, similar to usual activities, majority of the specific questions would overlap between the FACT-G and the EORTC QLQ-C30, such as GE4, Q21, and Q23; GE5, GE6, and Q22; GE1, GE3, and Q24, although those of the former would be more detailed than those of the latter. Consequently, a small proportion of “no problems” may have been indicated regarding anxiety/depression only in the group with the EQ-5D-5L placed second, though the differences were not significant. Regarding self-care and pain/discomfort, limited specific questions were associated with the self-care or pain/discomfort items. This may explain the absence of discrepancies in these questions’ responses among the instrument orders.
Table 5.
Questions in the FACT-G or the EORTC QLQ-C30 that may be related to the EQ-5D-5L
The question of the FACT-G or the EORTC QLQ-C30 should overlap with those of the other instrument in the same line
EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; FACT-G, Functional Assessment of Cancer Therapy General
One of the previous studies has examined and found assimilation effect of specific health questions on self-rated health question [21]. This would be consistent with the above explanation.
EQ-5D-5L index
We focused on the EQ-5D-5L index based on the Japanese value set, because the mean differences in the EQ-5D-5L’s index were similar among the three value sets. Thus far, no prior research has estimated the minimally important difference (MID) of the EQ-5D-5L index in cancer. Hence, we briefly estimated it for interpretation using the completed EQ-5D-5L population. In the distribution-based approach, 0.3 and 0.5 standard deviations are often used [40], and they were 0.055 (95% CI 0.052, 0.057) and 0.091 (0.087, 0.096), respectively. In the anchor-based approach, the performance status is employed frequently [40], and in cross-sectional studies, the differences between groups having varying anchors are utilized [41]. In our research, the difference between the ECOG performance status of 0 and 1 was 0.082 (95% CI 0.063, 0.102). Thus, the difference in the mean EQ-5D-5L indexes between the instrument orders was smaller than these values; furthermore, its 95% lower confidence limit was located among these values. These results suggest that the order effects on the EQ-5D-5L index would not be considerably larger than MID. However, considering the potential application of the EQ-5D-5L index to a long duration, the degree to which the instrument order affects the cost-effectiveness analysis is uncertain and should be evaluated.
Correlation
Few correlation coefficients between the EQ-5D-5L index and the subscales of disease-specific HRQOL instruments differed among the instrument orders. This finding suggests that only a small assimilation or contrast effect existed; alternatively, both these effects existed in the population, however, mutually nullified the discrepancy in the correlation. In either case, the order effects on the coefficients of the subscales in regression models for mapping algorithms were considered to be limited.
HRQOL instruments with missing values
The reasons why the EQ-5D-5L indexes, subscales of the FACT-G and those of the EORTC QLQ-C30 tended to be missing when the EQ-5D-5L was placed last are not clear. However, the first possible reason is that answering the EQ-5D-5L’s general questions may be easier after responding to the disease-specific HRQOL instruments’ specific ones than before answering them. The second possible reason is that patients may tend to decide to respond to the questionnaire after looking over the questionnaire with the EQ-5D-5L placed last. This is because the last instrument may be the instrument recalled mainly in the decision-making process, since in psychology it is known that, in free recall-task, the last item in a word list is easier to be recalled than other items (called recency effect) [42, 43]. Although the tendency of the first item in the list to be recalled (called primacy effect) is also known, a main cause of the primacy effect would be rehearsal of the item [42]. Contrary to the free-recall task, patients would not rehearse HRQOL instruments to memorize it; thus, primacy effect may not have occurred in the decision-making process. Therefore, the decision may be based largely on the burden of responding to the last HRQOL instrument. It might be recommended that the EQ-5D-5L should be placed at the last of questionnaires.
Limitation
This study has certain limitations. First, the questionnaire booklets’ assignment to the patients was quasi-randomized and not strictly randomized with random numbers generated. Additionally, the medical staff could identify the questionnaire booklet types if they looked inside them. Thus, we cannot dismiss the possibility that they distributed the questionnaire booklets in an order different from instruction. However, they had no motivation to allocate these questionnaire booklets selectively to the patients with specific characteristics. Indeed, the patients’ observed characteristics were not unbalanced. Second, the patients might not have answered from the questionnaire booklet’s start, thus resulting in the order effects’ underestimation. Third, the order effects’ impact on the EQ-5D-5L may be different from this study when more severe patients or other disease-specific instruments are examined.
Conclusions
In the patients undergoing drug therapy for advanced cancer, the preceding cancer-specific HRQOL instruments were found to impact the mobility and usual activities questions in the EQ-5D-5L. This resulted in a difference in the EQ-5D-5L index; however, our findings indicated that the difference size would not be considerably larger than the MID. Few correlation coefficients between the EQ-5D-5L index and the subscales of the disease-specific HRQOL instruments varied among the instrument orders. The patients tended to complete the EQ-5D-5L when it was placed at the end of the questionnaire.
Supplementary Information
Additional file 1. Supplementary methods and tables.
Acknowledgements
We thank the patients and the researchers who participated in the QOL-MAC study that was supported by the of the Public Health Research Foundation. We are grateful to Associate Prof. Koji Oba and Assistant Prof. Yoshinori Takeuchi at the University of Tokyo for their comments on the manuscript’s earlier version. We would like to thank Editage (www.editage.com) for English language editing. This research was also supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP22lk0201701.
Abbreviations
- ECOG
Eastern Cooperative Oncology Group
- EORTC QLQ-C30
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30
- ePRO
Electric patient-reported outcome
- FACT-G
Functional Assessment of Cancer Therapy General
- HRQOL
Health-related quality of life
- IPW
Inverse probability weighting
- MID
Minimally important difference
- QALY
Quality-adjusted life year
- QOL-MAC
Quality of Life Mapping Algorithm for Cancer
Author contributions
YH, TS, NT, TK, KK, SN, TF, and KS contributed to the study’s conception and design. NT contributed to the data acquisition. SI, YH, and YM contributed to the data analysis. All authors interpreted the data. SI and YH composed the manuscript’s first draft; all authors revised it critically. All authors read and approved the final manuscript.
Funding
The QOL-MAC research was sponsored by the Public Health Research Foundation that received the research fund by the Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health, under the study contract. This research was also supported by AMED under Grant Number JP22lk0201701.
Availability of data and materials
The data that support this study’s findings are available from the Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health; however, restrictions apply to their availability, which were used under license for the current study. Hence, they are not publicly available.
Declarations
Ethics approval and consent to participate
The study protocol was approved by each participating hospital. All patients provided written informed consent before their enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao Y, Feng H, Qu J, Luo X, Ma W, Tian J. A systematic review of pharmacoeconomic guidelines. J Med Econ. 2018 doi: 10.1080/13696998.2017.1387118. [DOI] [PubMed] [Google Scholar]
- 2.Sharma D, Aggarwal AK, Downey LE, Prinja S. National Healthcare Economic Evaluation Guidelines: a cross-country comparison. PharmacoEconomics Open. 2021 doi: 10.1007/s41669-020-00250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Outcomes Research and Economic Evaluation for Health National Institute of Public Health (C2h). Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council. version 2.0. 2019. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed 8 Aug 2021.
- 4.Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada (4th ed.). 2017. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed 8 Aug 2021
- 5.Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II—an ISPOR good research practices task force report. Value Health. 2015 doi: 10.1016/j.jval.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ. 2011 doi: 10.1136/bmj.d1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016 doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Developing NICE guidelines: the manual. http://www.nice.org.uk/article/pmg20. Accessed 8 Aug 2021 [PubMed]
- 9.Caro JJ, Briggs AH, Siebert U, Kuntz KM, Modeling Good Research Practices Task Force Overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012 doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Richardson J, McKie J, Bariola E. Multiattribute utility instruments and their use. In: Culyer AJ, editor. Encyclopedia of health economics. Amsterdam: Elsevier; 2014. pp. 341–357. [Google Scholar]
- 11.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990 doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 12.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011 doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013 doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wailoo AJ, Hernandez-Alava M, Manca A, Mejia A, Ray J, Crawford B, et al. Mapping to estimate health-state utility from non–preference-based outcome measures: an ISPOR good practices for outcomes research task force report. Value Health. 2017 doi: 10.1016/j.jval.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Rat AC, Baumann C, Klein S, Loeuille D, Guillemin F. Effect of order of presentation of a generic and a specific health-related quality of life instrument in knee and hip osteoarthritis: a randomized study. Osteoarthr Cartil. 2008 doi: 10.1016/j.joca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Barry MJ, Walker-Corkery E, Chang Y, Tyll LT, Cherkin DC, Fowler FJ. Measurement of overall and disease-specific health status: does the order of questionnaires make a difference? J Health Serv Res Policy. 1996 doi: 10.1177/135581969600100105. [DOI] [PubMed] [Google Scholar]
- 17.Cheung YB, Lim C, Goh C, Thumboo J, Wee J. Order effects: a randomised study of three major cancer-specific quality of life instruments. Health Qual Life Outcomes. 2005 doi: 10.1186/1477-7525-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung YB, Wong LC, Tay MH, Toh CK, Koo WH, Epstein R, et al. Order effects in the assessment of quality of life in cancer patients. Qual Life Res. 2004 doi: 10.1023/B:QURE.0000037499.80080.07. [DOI] [PubMed] [Google Scholar]
- 19.Childs AL, Submacular Surgery Trials Research Group Effect of order of administration of health-related quality of life interview instruments on responses. Qual Life Res. 2005 doi: 10.1007/s11136-004-0727-9. [DOI] [PubMed] [Google Scholar]
- 20.Kieffer JM, Verrips GHW, Hoogstraten J. Instrument-order effects: using the Oral Health Impact Profile 49 and the Short Form 12. Eur J Oral Sci. 2011 doi: 10.1111/j.1600-0722.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 21.Garbarski D, Schaeffer NC, Dykema J. The effects of response option order and question order on self-rated health. Qual Life Res. 2015 doi: 10.1007/s11136-014-0861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Grant D. The effect of question order on self-rated general health status in a multilingual survey context. Am J Epidemiol. 2009 doi: 10.1093/aje/kwp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowling A, Windsor J. The effects of question order and response-choice on self-rated health status in the English Longitudinal Study of Ageing (ELSA) J Epidemiol Community Health. 2008 doi: 10.1136/jech.2006.058214. [DOI] [PubMed] [Google Scholar]
- 24.Crossley TF, Kennedy S. The reliability of self-assessed health status. J Health Econ. 2002 doi: 10.1016/S0167-6296(02)00007-3. [DOI] [PubMed] [Google Scholar]
- 25.Strack F. “Order effects” in survey research: activation and information functions of preceding questions. In: Schwarz N, Sudman S, editors. Context effects in social and psychological research. New York: Springer; 1992. pp. 23–34. [Google Scholar]
- 26.Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021 doi: 10.1007/s11136-020-02688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng X, Sui M, Liu B, Yang H, Liu R, Tan RLY, et al. Measurement properties of the EQ-5D-5L and EQ-5D-3L in six commonly diagnosed cancers. Patient. 2021 doi: 10.1007/s40271-020-00466-z. [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Kim HJ, Lee S, Jo MW. Comparing the psychometric properties of the EQ-5D-3L and EQ-5D-5L in cancer patients in Korea. Qual Life Res. 2012 doi: 10.1007/s11136-011-0018-1. [DOI] [PubMed] [Google Scholar]
- 29.Yang Q, Yu X, Zhang W. Health variations among breast-cancer patients from different disease states: evidence from China. BMC Health Serv Res. 2020 doi: 10.1186/s12913-020-05872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouwenberg CAE, Kranenburg LW, Visser MS, Busschbach JJ, Mureau MAM. The validity of the EQ-5D-5L in measuring quality of life benefits of breast reconstruction. J Plast Reconstr Aesthet Surg. 2019 doi: 10.1016/j.bjps.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 31.McColl E, Eccles MP, Rousseau NS, Nicholas I. From the generic to the condition-specific? Instrument order effects in quality of life assessment. Med Care. 2003 doi: 10.1097/00005650-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hagiwara Y, Shiroiwa T, Taira N, Kawahara T, Konomura K, Noto S, et al. Mapping EORTC QLQ-C30 and FACT-G onto EQ-5D-5L index for patients with cancer. Health Qual Life Outcomes. 2020 doi: 10.1186/s12955-020-01611-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda S, Shiroiwa T, Igarashi A, Noto S, Fukuda T, Saito S, et al. Developing a Japanese version of the EQ-5D-5L value set. J Natl Inst Public Heal. 2015;64:47–55. [Google Scholar]
- 34.Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Heal Econ. 2018 doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019 doi: 10.1016/j.jval.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 37.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 38.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013 doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 39.Bradburn NM, Sudman S, Wansink B. Asking questions: the definitive guide to questionnaire design: For market research, political polls, and social and health questionnaires. 2. California: Jossey-Bass; 2004. [Google Scholar]
- 40.Ousmen A, Touraine C, Deliu N, et al. Distribution- and anchor-based methods to determine the minimally important difference on patient-reported outcome questionnaires in oncology: a structured review. Health Qual Life Outcomes. 2018 doi: 10.1186/s12955-018-1055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrasco-Labra A, Devji T, Qasim A, et al. Minimal important difference estimates for patient-reported outcomes: a systematic survey. J Clin Epidemiol. 2020 doi: 10.1016/j.jclinepi.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Tan L, Ward G. A recency-based account of the primacy effect in free recall. J Exp Psychol Learn Mem Cogn. 2000 doi: 10.1037/0278-7393.26.6.1589. [DOI] [PubMed] [Google Scholar]
- 43.Lohnas LJ, Polyn SM, Kahana MJ. Expanding the scope of memory search: modeling intralist and interlist effects in free recall. Psychol Rev. 2015 doi: 10.1037/a0039036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary methods and tables.
Data Availability Statement
The data that support this study’s findings are available from the Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health; however, restrictions apply to their availability, which were used under license for the current study. Hence, they are not publicly available.