Abstract
Background
The ratio of gamma-glutamyltransferase to high-density lipoprotein cholesterol (GGT/HDL-C) has been highlighted in nonalcoholic fatty liver disease (NAFLD) by previous studies. However, there have been fewer investigations into the correlation between the GGT/HDL-C ratio and type 2 diabetes mellitus (T2DM) incidence. Our secondary analysis used published data from a Japanese population and aimed to investigate the role of the GGT/HDL-C ratio in the incidence of T2DM.
Methods
The research was a longitudinal cohort study completed by Okamura, Takuro et al. We obtained the data from the DATADRYAD website and used it for secondary analysis only. The participants recruited from a medical program called the NAGALA database received regular medical examinations and standardized questionnaires to obtain the baseline variables. Abdominal ultrasound was used to diagnose fatty liver disease. The participants were followed up, and the duration and occurrence of T2DM were documented. The GGT/HDL-C ratio evaluated at baseline served as the independent variable, while the occurrence of diabetes served as the dependent variable.
Results
A total of 15,453 cases (8,419 men and 7,034 women) were included in our study. After adjusting for age, sex, BMI, DBP, SBP, ALT, AST, TG, TC, HbA1C, FPG, drinking status, smoking status, exercise status, and fatty liver, we observed that the GGT/HDL-C ratio was positively associated with the incidence of T2DM (hazard ratio = 1.005, 95% confidence interval: 1.000 to 1.010, P = 0.0667). The results were consistent when the GGT/HDL-C quartile was used as a categorical variable (P for trend < 0.00396). A curvilinear relationship with a threshold effect was identified between the GGT/HDL-C ratio and the risk of incident T2DM. On the left of the point, a one-unit increase in the GGT/HDL-C ratio was associated with a 1.5-fold increase in the risk of incident T2DM (hazard ratio 2.57, 95% confidence interval 1.20 to 5.49). On the right of the point, when GGT/HDL-C was greater than 6.53, their relationship became saturated.
Conclusion
The GGT/HDL-C ratio correlated with the incidence of T2DM in a curvilinear form with a threshold effect. Their positive relationship could be observed when GGT/HDL-C was less than 6.53.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-023-01772-9.
Keywords: Ratio of gamma-glutamyltransferase to high-density lipoprotein cholesterol, Incidence of T2DM, Nonlinear relationship, Threshold effect
Background
Type 2 diabetes mellitus (T2DM) is an epidemic metabolic disorder with a rapidly increasing incidence worldwide. The disease can lead to subsequent metabolic disorders and other complications that are hazardous to health and potentially lethal, increasing mortality.
Abnormal glucose and lipid metabolism often accompany the onset and development of T2DM [1]. The liver is the leading site in charge of lipid metabolism, glucose metabolism, and homeostasis. In clinical practice, we often use lipid indicators such as triglyceride (TG), total cholesterol (TC), HDL-C, and files of liver enzyme indicators such as ALT (alanine aminotransferase), AST (aspartate aminotransferase) and GGT (gamma-glutamyltransferase) to reflect lipid metabolism and liver function. Single and complex liver enzymes, as well as the lipid index, have been explored in research on risk factors for diabetes [2–5]. Previous studies have reported that TG/HDL-C, ALT/AST, and GGT all have associations with the incidence of T2DM, and some of them have predictive value for the incidence of T2DM [6]. Recently, the role of the GGT/HDL-C ratio in NAFLD patients has been discussed. The authors suggested that this GGT/HDL-C ratio might predict the risk of NAFLD and metabolic diseases such as diabetes [7]. To date, very little attention has been given to the role of GGT/HDL-C in patients with T2DM. To determine whether there is a link between GGT/HDL-C and the incidence of T2DM or whether GGT/HDL-C is a more comprehensive indicator for assessing the risk of T2DM, we used published data on the Japanese population to conduct secondary data analysis. This secondary analysis used the GGT/HDL-C ratio as the independent variable and the incidence of T2DM as the dependent variable to probe into the independent association.
Methods
Data source
We downloaded the data for this study from www.Datadryad.org, the ‘DATADRYAD’ website. According to the website's request for data usage, the Dryad data package ([8] Dryad data package: Okamura, Takuro et al. (2019), Data from Ectopic fat obesity presents the greatest risk for incident T2DM: a population-based longitudinal study, Dryad, Dataset, https://doi.org/10.5061/ dryad. 8q0p192) need to be cited when using these data.
Study population
The longitudinal study was based on the medical examination program NAGALA (NAfld in the Gifu Area, Longitudinal Analysis). The database comes from the medical examination program at Murakami Memorial Hospital (Gifu, Japan), founded in 1994. The objective of this project is to investigate risk factors for chronic diseases. Takuro Okamura et al. [8] extracted cases from 2004 to 2015 who participated in the medical examination program at Murakami Memorial Hospital in their analysis.
The study initially enrolled 20,944 participants (12,498 male and 8,446 female), of which 863 participants (504 male and 359 female) were excluded because of missing data. Another 416 participants (278 male and 138 female) were excluded due to known liver disease; 739 participants (635 male and 104 female) were excluded due to ethanol consumption over the level regarded as toxic to the liver (420 g/w for male, 280 g/w for female) [9]; 2,321 participants (1,709 male and 612 female) were also excluded due to any medication usage at baseline; 322 patients (265 male and 58 female) and 128 patients (677 male and 131 female) were excluded due to diabetes at the baseline examination or FPG ≥ 6.1 mmol/L [10], respectively. Another 11 participants (11 male) were excluded due to having an invalid GGT/HDL-C ratio. Thus, 15,453 subjects (7,034 female and 8,419 male) remained to be part of this study.
The ethics committee of Murakami Memorial Hospital approved this study. Takuro Okamura et al. [8] obtained written informed consent for data usage from each participant.
The data collection and measurement procedure
All the baseline variables included in the database were collected at baseline by a regular medical exam, including basic information such as age, sex, BMI (body mass index), DBP (diastolic blood pressure), SBP (systolic blood pressure), and biochemical indicators ALT, AST, GGT, TG, TC, HDL-C, glycosylated hemoglobin (HbA1C), and fasting plasma glucose (FPG). Other covariates related to lifestyle habits, such as smoking and drinking status, exercise status, and ethanol consumption, were obtained by questionnaire. B-ultrasonography was used to diagnose fatty liver disease by trained technicians. A follow-up study was performed on participants’ incidence of T2DM [11].
BMI is obtained by dividing weight (in kg) by the square of height (in meters). Okamura et al. [8] applied a standardized self-administered questionnaire to ascertain the participants’ information on medication usage, drinking, smoking, and exercise habits. We estimated average ethanol intake per week by the type and amount of alcohol consumed weekly during the prior month, and we divided ethanol consumption into four groups: 1) no or minimal, 2) light, 3) moderate, and 4) heavy; the corresponding ethanol consumption was < 40 g/week, 40 to 140 g/week, 140 to 280 g/week, and > 280 g/week [12]. Smoking status was divided into three categories: never, ex-smoker, or current smoker. Regular exercise habits were defined as participating in any sport regularly, more than once a week [13, 14]. Trained technicians diagnosed fatty liver disease by the criteria of the scoring system of abdominal ultrasonography [15]. The technicians were not informed of other personal information of the specific participants. Finally, T2DM was diagnosed by FPG ≥ 7 mmol/L, HbA1c ≥ 6.5% [16] or by self-report, and the date of diagnosis was recorded.
Statistical analyses
Data management and analysis were performed using the statistical software package R (http://www. R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). P < 0.05 (two-sided) was considered statistically significant.
We carried out the data analysis according to the following procedures: For the data of baseline characteristics of participants (the GGT/HDL-C was grouped in quartiles), data were presented as the means ± standard deviations for normal distribution, medians (quartiles) for skewed distribution, frequency or percentage for categorical variables. The significant differences among the GGT/HDL-C quartiles were tested with one-way ANOVA for continuous variables with a normal distribution, the Kruskal‒Wallis H test for continuous variables with a skewed distribution, and the chi-square test for categorical variables. We used Cox proportional hazard regression models to explore the predictive effect of GGT/HDL-C on the risk of T2DM. The hazard ratio (HR) and 95% confidence interval (CI) were calculated. To satisfy the recommendations comprising the STROBE statement [17], if the covariance added to the model altered the matched effect size by at least ten percent, they were adjusted [18], and we presented the results of the unadjusted model and models with different covariates that were adjusted simultaneously. For trend testing, we converted the GGT/HDL-C continuous variables into categorical variables, and the P for trend was calculated. Next, considering possible nonlinear relationships that might exist between dependent and independent variables, a generalized additive model (GAM) was applied. In terms of the smoothing plot, we used a two-piecewise linear regression model to calculate the inflection point of GGT/HDL-C on the incidence of T2DM. The recursive method automatically calculated the inflection point by using the maximum model likelihood when the inflection point appeared in the curve. In the next step, subgroup analysis was also explored by stratified Cox regression models, and we used the likelihood ratio test to test for interactions across subgroups. We used the Kaplan–Meier method to compare cumulative diabetes event rates within specific time intervals. The log-rank test compared HR and corresponding 95% CI for the events of T2DM in each GGT/HDL-C quartile.
Results
Baseline characteristics of participants
The baseline characteristics of the patients divided by GGT/HDL-C quartiles are shown in Table 1. The average age of the participants was 43.71 ± 8.90 years. The average GGT/HDL-C was 10.78 (1.57–342.82). The factors of age, sex, BMI, ALT, AST, TC, TG, SBP, DBP, FPG, HbA1C, and ethanol consumption were compared across the GGT/HDL-C quartiles. In the higher GGT/HDL-C group (Q3, Q4), males accounted for the majority, and relatively more participants in Q3 and Q4 were diagnosed with fatty liver disease and were current smokers compared with the lower GGT/HDL-C groups (Q1, Q2). The participants of the Q3 and Q4 groups also had a relatively higher ethanol consumption compared with the lower GGT/HDL-C group (Q1 and Q2).
Table 1.
Baseline characteristics of participants
| GGT/HDL-C ratio quartiles |
Q1 (1.57–7.20) |
Q2 (7.20–10.77) |
Q3 (10.78–17.95) |
Q4 (17.96–342.82) |
P value |
|---|---|---|---|---|---|
| N | 3862 | 3861 | 3866 | 3864 | |
| Sex | < 0.001 | ||||
| Male | 441 (11.42%) | 1576 (40.82%) | 2881 (74.52%) | 3521 (91.12%) | |
| Female | 3421 (88.58%) | 2285 (59.18%) | 985 (25.48%) | 343 (8.88%) | |
| Age (years) | 42.31 ± 8.36 | 43.12 ± 9.05 | 44.31 ± 9.25 | 45.09 ± 8.65 | < 0.001 |
| BMI (kg/m2) | 20.36 ± 2.31 | 21.26 ± 2.64 | 22.56 ± 2.95 | 24.28 ± 3.08 | < 0.001 |
| SBP (mmHg) | 107.48 ± 13.14 | 111.86 ± 13.90 | 116.78 ± 14.32 | 121.85 ± 14.50 | < 0.001 |
| DBP (mmHg) | 66.40 ± 9.16 | 69.54 ± 9.52 | 73.23 ± 10.02 | 77.15 ± 10.09 | < 0.001 |
| ALT (IU/L) | 13.00 (10.00–15.00) | 15.00 (12.00–18.00) | 18.00 (15.00–23.00) | 26.00 (20.00–36.00) | < 0.001 |
| AST (IU/L) | 15.00 (13.00–18.00) | 16.00 (13.00–19.00) | 18.00 (15.00–21.00) | 21.00 (17.00–26.00) | < 0.001 |
| TC (mmol/L) | 5.02 (4.47–5.61) | 4.94 (4.40–5.51) | 5.04 (4.47–5.66) | 5.33 (4.76–5.87) | < 0.001 |
| TG (mmol/L) | 0.51 (0.38–0.69) | 0.61 (0.44–0.86) | 0.84 (0.60–1.20) | 1.23 (0.86–1.80) | < 0.001 |
| HbA1C (mmol/mol) | 32.24 (30.06–35.52) | 32.24 (30.52–35.52) | 33.34 (31.15–35.52) | 33.34 (31.15–36.09) | < 0.001 |
| FPG (mg/dL) | 89.00 (85.00–94.00) | 91.00 (87.00–96.00) | 94.00 (90.00–99.00) | 97.00 (92.00–102.00) | < 0.001 |
| Ethanol consumption (g/week) | 1.00 (0.00–12.00) | 1.00 (0.00–36.00) | 10.27 (0.00–84.00) | 36.00 (1.00–132.00) | < 0.001 |
| Fatty liver | < 0.001 | ||||
| No | 3777 (97.80%) | 3600 (93.24%) | 3123 (80.78%) | 2216 (57.35%) | |
| Yes | 85 (2.20%) | 261 (6.76%) | 743 (19.22%) | 1648 (42.65%) | |
| Habit of exercise | < 0.001 | ||||
| No | 3206 (83.01%) | 3139 (81.30%) | 3123 (80.78%) | 3279 (84.86%) | |
| Yes | 656 (16.99%) | 722 (18.70%) | 743 (19.22%) | 585 (15.14%) | |
| Smoking status | < 0.001 | ||||
| Never | 3204 (82.96%) | 2645 (68.51%) | 1811 (46.84%) | 1367 (35.38%) | |
| Past | 374 (9.68%) | 598 (15.49%) | 929 (24.03%) | 1048 (27.12%) | |
| Current | 284 (7.35%) | 618 (16.01%) | 1126 (29.13%) | 1449 (37.50%) | |
| Incidence of diabetes | < 0.001 | ||||
| No | 3842 (99.48%) | 3819 (98.91%) | 3791 (98.06%) | 3628 (93.89%) | |
| Yes | 20 (0.52%) | 42 (1.09%) | 75 (1.94%) | 236 (6.11%) |
Values are mean ± SD or median (interquartile range) or n (%)
BMI Body mass index, SBP Systolic blood pressure, DBP Diastolic blood pressure, ALT Alanine aminotransferase, AST Aspartate aminotransferase, HbA1C Glycosylated hemoglobin, TG Triglyceride, TC total cholesterol, GGT Gamma-glutamyltransferase, HDL-C High-density lipoprotein cholesterol, FPG Fasting plasma glucose
Univariate analysis
Univariate analysis was performed, and the results are shown in Table 2. In summary, age, BMI, SBP, DBP, ALT, AST, TC, TG, FPG, ethanol consumption, past and current smoking status, and GGT/HDL-C were positively associated with the incidence of T2DM. Habitual exercise had no relationship with the incidence of diabetes mellitus. The risk of developing T2DM in males was higher than that in females, and smokers (current and past) also had a higher risk of developing T2DM than never smokers.
Table 2.
The results of univariate analysis
| Statistics | HR (95% CI) | P value | |
|---|---|---|---|
| Sex | |||
| Female | 7034 (45.52%) | Ref | |
| Male | 8419 (54.48%) | 2.53 (1.99, 3.21) | < 0.0001 |
| Age (years) | 43.71 ± 8.90 | 1.06 (1.04, 1.07) | < 0.0001 |
| BMI (kg/m2) | 22.12 ± 3.13 | 1.24 (1.22, 1.27) | < 0.0001 |
| SBP (mmHg) | 114.49 ± 14.97 | 1.03 (1.03, 1.04) | < 0.0001 |
| DBP (mmHg) | 71.58 ± 10.50 | 1.05 (1.04, 1.06) | < 0.0001 |
| ALT (IU/L) | 19.99 ± 14.35 | 1.006 (1.005, 1.007) | < 0.0001 |
| AST (IU/L) | 18.40 ± 8.64 | 1.008 (1.006, 1.010) | < 0.0001 |
| TC (mmol/L) | 198.22 ± 33.41 | 1.010 (1.008, 1.013) | < 0.0001 |
| TG (mmol/L) | 80.78 ± 58.07 | 1.007 (1.006, 1.007) | < 0.0001 |
| HbA1C (mmol/mol) | 33.03 ± 3.52 | 1.44 (1.40, 1.48) | < 0.0001 |
| FPG (mg/dl) | 92.96 ± 7.44 | 1.20 (1.18, 1.22) | < 0.0001 |
| Ethanol consumption (g/week) | 47.71 ± 82.31 | 1.002 (1.001, 1.003) | 0.0011 |
| Fatty liver | |||
| No | 12,716 (82.288%) | Ref | |
| Yes | 2737 (17.712%) | 7.03 (5.71, 8.64) | < 0.0001 |
| Habit of exercise | |||
| No | 12,747 (82.49%) | Ref | |
| Yes | 2706 (17.51%) | 0.76 (0.56, 1.02) | 0.0652 |
| Smoking status | |||
| Never | 9027 (58.42%) | Ref | |
| Past | 2949 (19.08%) | 1.66 (1.26, 2.19) | 0.0003 |
| Current | 3477 (22.50%) | 2.59 (2.06, 3.25) | < 0.0001 |
| GGT/HDL-C ratio | 15.55 ± 15.73 | 1.02 (1.01, 1.02) | < 0.0001 |
CI Confidence interval, Ref Reference
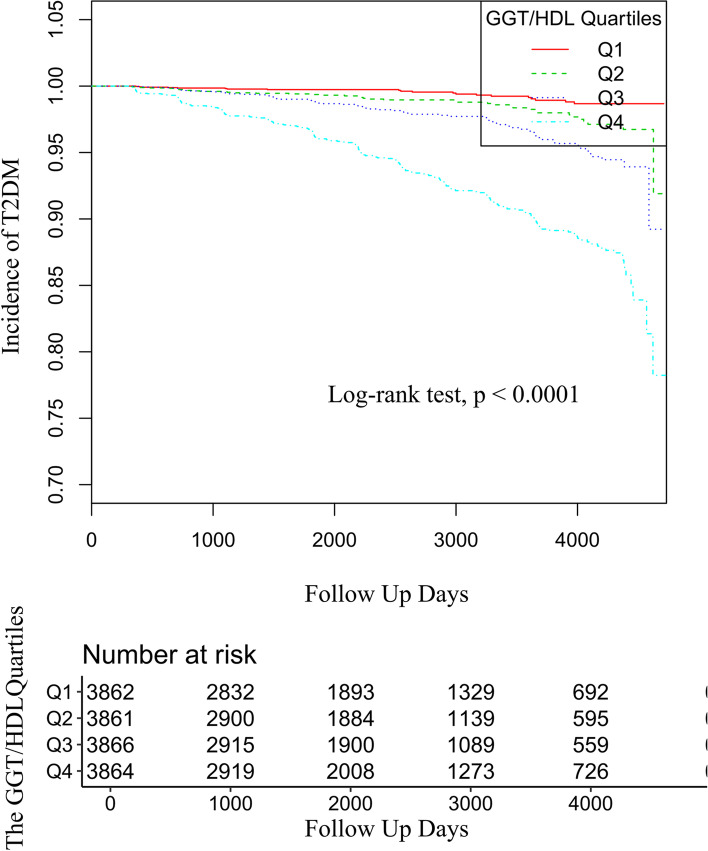
The Kaplan‒Meier curves of the four GGT/HDL-C quartile groups showed that the diabetes incidence risk between each of them was significantly different (the P value of the log-rank test < 0.0001). The increase in cumulative diabetes event rates kept pace with the increased GGT/HDL-C quartiles (Fig. 1).
Fig. 1.
Kaplan–Meier event-free survival curve. Kaplan–Meier event-free survival curve. Kaplan–Meier analysis of the incidence of diabetes based on GGT/HDL-C quartiles (log rank, P < 0.0001)
Relationship between GGT/HDL-C and the incidence of T2DM
In Table 3, we used a Cox proportional hazard regression model to evaluate the associations between GGT/HDL-C and the incidence of T2DM. A nonadjusted model and models with different covariates adjusted are presented. In the crude model and model I (minimally adjusted model), the GGT/HDL-C ratio had a significant positive correlation with the incidence of T2DM: in the crude model, HR = 1.015, 95% CI: 1.013 to 1.017, P < 0.00001; in the adjusted model I (adjusted age, sex, and BMI), HR = 1.010, 95% CI: 1.007 to 1.014, P < 0.00001. In adjusted model II (fully adjusted model), HR = 1.005, 95% CI: 1.000 to 1.010, P = 0.0670; the positive correlation between them was insignificant. The GGT/HDL-C ratio was then handled as a categorical variable (quartile) for the purpose of sensitivity analysis. There was an increased risk for developing T2DM as the quartiles of GGT/HDL-C increased in the crude model and the adjusted model I (both P for trend < 0.00001), and we also observed a significant P for trend (< 0.00396) in the fully adjusted model.
Table 3.
Relationship between GGT/HDL-C and incident of DM2 in different models
| Exposure | Crude model (HR, 95% CI, P) | Model I (HR, 95% CI, P) | Model II (HR, 95% CI, P) |
|---|---|---|---|
| GGT/HDL-C | 1.015 (1.013, 1.017) < 0.00001 | 1.010 (1.007, 1.014) < 0.00001 | 1.005 (1.000, 1.010) 0.0670 |
| GGT/HDL-C quartiles | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 2.22 (1.30, 3.78) 0.0033 | 1.87 (1.09, 3.22) 0.0228 | 1.28 (0.74, 2.20) 0.3790 |
| Q3 | 4.04 (2.47, 6.62) < 0.0001 | 2.75 (1.62, 4.68) 0.0002 | 1.25 (0.73, 2.16) 0.4171 |
| Q4 | 11.59 (7.34, 18.30) < 0.0001 | 6.08 (3.59, 10.29) < 0.0001 | 1.84 (1.04, 3.24) 0.0361 |
| P for trend | < 0.0001 | < 0.0001 | 0.00396 |
Crude model did not adjust for other covariants
Model I adjusted for age, sex, BMI
Model II adjusted for age, sex, BMI, SBP, DBP, ALT, AST, TC, TG, HbA1C, FPG, fatty liver, smoking and exercise status and ethanol consumption
CI Confidence interval, Ref Reference
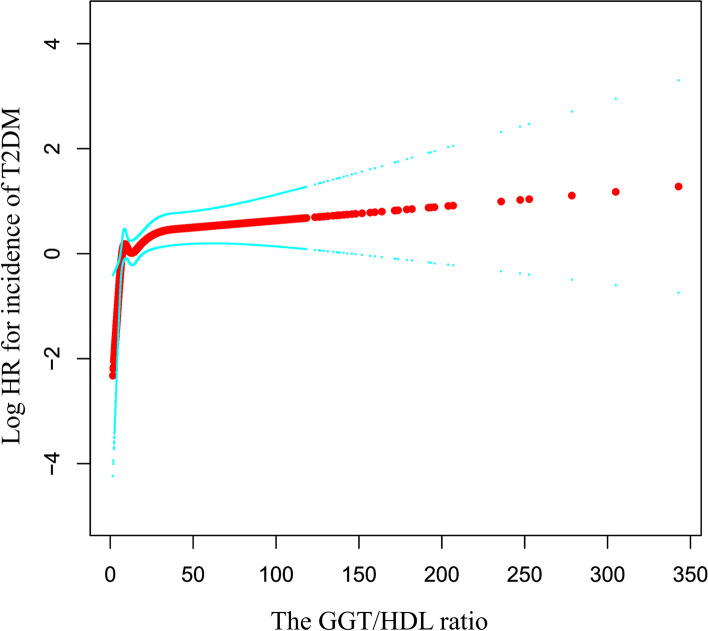
Nonlinear relationship between GGT/HDL-C and T2DM
Next, we used GAM to explore whether there was a curvilinear relationship between the independent and dependent variables and showed the results in Fig. 2. We observed a curvilinear relationship after covariates (sex, age, BMI, ALT, AST, TC, TG, HbA1C, FPG, SBP, DBP, smoking, exercise status, ethanol consumption, and fatty liver disease) were adjusted. Subsequent threshold effect analysis found an inflection point in their curvilinear relationship at GGT/HDL equal to 6.35 (log-likelihood ratio test, P = 0.001). When GGT/HDL was less than 6.53, the risk of incident T2DM increased with increasing GGT/HDL-C (HR: 2.57, 95% CI: 1.20 to 5.49, P = 0.0151). When the GGT/HDL-C ratio was greater than 6.53, the incidence of T2DM no longer increased (HR: 1.00, 95% CI: 1.00 to 1.01, P = 0.0803), and their relationship tended to saturate (Table 4).
Fig. 2.
The nonlinear relationship between GGT/HDL-C and the incidence of diabetes
Table 4.
The results of the two-piecewise linear regression model
| Incident of diabetes (HR, 95%CI) | P value | |
|---|---|---|
| Fitting model by standard linear regression | 1.01 (1.00, 1.01) | 0.0670 |
| Fitting model by two-piecewise linear regression | ||
| Inflection point of GGT/HDL-C | 6.53 | |
| ≤ 6.53 | 2.57 (1.20, 5.49) | 0.0151 |
| ≥ 6.53 | 1.00 (1.00, 1.01) | 0.0803 |
| P for log likelihood ratio test | 0.001 | |
Adjusted age, sex, BMI, SBP, DBP, FPG, ALT, AST, HbA1C, TC, TG, fatty liver, smoking and drinking status, exercise status
A nonlinear relationship between them was detected after adjusting for age, sex, BMI, SBP, DBP, ALT, AST, TC, TGs, HBA1C, FPG, fatty liver, smoking and exercise status and ethanol consumption.
Subgroup analysis
We used age, sex, BMI, SBP, DBP, smoking, drinking, exercise status, and fatty liver disease as categorical variables to evaluate the effect sizes in different subgroups and to explore potential interactions in Table 5. Tests for interactions were not statistically significant across all the subgroups listed above (all P values for interactions were > 0.05). Considering that the nonlinear relationship between the independent variable and dependent variable might also exist in other subgroups, we supplemented the smoothing plots in stratified age, sex, BMI, smoking, drinking, exercise status, SBP, DBP, and fatty liver disease in Supplemental Fig. 1 (Figure S 1). We discovered that a curvilinear relationship existed in each of the above subgroups. Therefore, the test for interaction needed to consider the existence of the curvilinear relationship and required further in-depth research.
Table 5.
Effect size of GGT/HDL-C on incident DM2 in prespecified and exploratory subgroups
| Character | Number of participant | Effect size (95%CI) | P value | P for interaction |
|---|---|---|---|---|
| Age (years) | 0.7182 | |||
| < 60 | 14,741 | 1.00 (1.00, 1.01) | 0.0903 | |
| > = 60 | 712 | 1.00 (0.98, 1.02) | 0.9106 | |
| Sex | 0.9279 | |||
| Male | 8419 | 1.005 (0.983, 1.027) | 0.6853 | |
| Female | 7034 | 1.006 (1.000, 1.011) | 0.0455* | |
| BMI (kg/m2) | 0.6066 | |||
| < 23 | 10,059 | 1.004 (0.996, 1.012) | 0.3575 | |
| > = 23 | 5394 | 1.006 (1.000, 1.012) | 0.0375* | |
| SBP (mmHg) | 0.4241 | |||
| < 140 | 14,668 | 1.00 (1.00, 1.01) | 0.1285 | |
| > = 140 | 785 | 1.01 (1.00, 1.03) | 0.1511 | |
| DBP (mmHg) | 0.3525 | |||
| < 90 | 14,690 | 1.00 (1.00, 1.01) | 0.1642 | |
| > = 90 | 763 | 1.01 (1.00, 1.02) | 0.0866 | |
| Current smoker | 0.7553 | |||
| Never | 9027 | 1.00 (0.99, 1.02) | 0.3915 | |
| Past | 2949 | 1.01 (1.00, 1.02) | 0.1355 | |
| Current | 3477 | 1.00 (0.99, 1.01) | 0.4486 | |
| Drinking status | 0.5413 | |||
| Non | 11,802 | 1.00 (1.00, 1.01) | 0.2545 | |
| Light | 1754 | 1.00 (0.99, 1.02) | 0.5992 | |
| Moderate | 1357 | 1.00 (0.98, 1.01) | 0.6772 | |
| Heavy | 540 | 1.02 (1.00, 1.03) | 0.0150* | |
| Fatty liver | 0.4586 | |||
| No | 12,716 | 1.00 (1.00, 1.01) | 0.4310 | |
| Yes | 2737 | 1.007 (1.001, 1.014) | 0.0290* | |
| Exercise | 0.3589 | |||
| No | 12,747 | 1.004 (0.998, 1.010) | 0.1552 | |
| Yes | 2706 | 1.012 (0.997, 1.027) | 0.1164 |
Note 1: Above model adjusted for age, sex, BMI, SBP, DBP, FPG, ALT, AST, HBA1C, TC, TG, fatty liver, smoking and drinking status, exercise status
Note 2: In each case, the model is not adjusted for the stratification variable
Discussion
The role of liver enzymes and lipid profiles in the pathogenesis and progression of T2DM has long been discussed in the literature [4, 19–21]. To the best of our knowledge, this is the first study focusing on the independent relationship between the GGT/HDL-C ratio and the incidence of T2DM. We found that in the Japanese population, GGT/HDL-C was positively associated with the incidence of diabetes, independent of age, sex, BMI, SBP, DBP, FPG, ALT, AST, HbA1C, TC, TGs, fatty liver, smoking status, drinking status, and exercise status. A further nonlinear relationship was explored, and a threshold or a saturation effect was found. The relationship could be characterized as follows: When GGT/HDL-C was less than 6.53, the risk of diabetes increased 1.56 times as the GGT/HDL-C ratio increased by 1 unit, and the risk leveled off when the ratio was greater than 6.53. Further subgroup analysis was conducted, but no interaction was found in the subgroups investigated.
The enzyme GGT is a sensitive index of liver enzymes. Serum GGT can sensitively reflect liver function, and obvious and rapid elevation of the GGT level can be observed in fatty liver (both nonalcoholic and alcoholic), hepatic inflammation, and hepatitis [22]. In addition, elevated serum GGT levels can also be related to various biochemical reactions and various systemic diseases, such as systemic inflammation, oxidative stress, atherosclerosis, metabolic syndrome, and an increased risk of T2DM [23–25]. Since GGT activity is related to oxidative stress, aggravated inflammation, and potential NAFLD, which are major pathological processes involved in T2DM, we speculate that GGT may be involved in the pathogenesis of T2DM [26, 27]. Previous research has already provided considerable evidence of GGT and the incidence of T2DM [6, 28–30].
Several human studies have provided evidence to support that plasma HDL-C levels are inversely correlated with the risk of T2DM [31–33]. Furthermore, rising serum HDL-C levels through extra interventions can be related to remarkable improvement of glycemic regulation in T2DM patients [34]. Dyslipidemia is a known risk factor for T2DM. As one of the major blood lipid components, HDL-C not only undertakes the function of cholesterol retro transportation but also modulates glycometabolism through the improvement of insulin sensitivity and elevation of insulin secretion [34]. Moreover, HDL-C could inhibit oxidative stress and enhance insulin secretion efficiently, which may exert protective effects on the beta cells of the pancreas [35]. In other tissues and organs involved in glucose metabolism, such as skeletal muscle, adipose tissue, and liver tissue, HDL-C has also been found to have the ability to enhance glucose uptake in both insulin-dependent and noninsulin-dependent ways [36]. These findings suggest that abnormal HDL-C levels and dysfunctionality of HDL-C in the human body may give rise to the risk of developing diabetes [37]. Both elevated GGT levels and decreased HDL-C levels can be related to an increased risk of developing T2DM. Therefore, the GGT/HDL-C ratio is posited to have predictive value for T2DM. In a cross-sectional study conducted on Chinese populations, Guofang Feng et al. investigated the independent association between the GGT/HDL-C ratio and NAFLD. They suggested that the function of the ratio was the combination of the two indicators and might exert a predictive value on the prevalence of NAFLD or other related metabolic disorders [7]. A recent cross-sectional study in a Chinese population of T2DM patients also found that in the overweight (BMI greater than 23 kg/m2) subgroup, GGT/HDL-C has a positive relationship with NAFLD incidence [38]. Our study is the first to highlight the role of the GGT/HDL-C ratio in T2DM and further confirm a nonlinear relationship. Further and more profound research is needed to reveal the intrinsic mechanism.
Study strengths and limitations
The following are strong points in our study. First, we use the GAM to clarify that the relationship between the independent and dependent variables is nonlinear, which assists in better understanding the actual correlation between exposures and outcomes. Second, we used rigorous statistical strategies to reduce the impact of a set of confounders on the results, thus minimizing residual confounders. Third, we obtained the positive finding that when the GGT/HDL-C ratio was less than 6.53, the risk of diabetes increased 1.56 times as the GGT/HDL-C ratio increased by 1 unit. The clinical value of this finding is that the positive correlation between GGT/HDL-C and the risk of T2DM could only be observed when GGT/HDL-C is under a certain threshold.
On the other hand, the following limitations must be acknowledged. First, the cohort was conducted by the medical examination program NAGALA in Japan. The data source was the Japanese population, so we could not extrapolate our findings to different ethnic groups or special groups, such as adolescents. Additionally, we could only use available variables in published data and were unable to encompass all variables that may be related to both GGT/HDL-C and T2DM, such as low-density lipoprotein and inflammatory cytokines. In addition, we could only use HDL-C to represent the function of HDLs in our research. Second, T2DM in this cohort study was not diagnosed with a 2-h oral glucose tolerance test, which might lead to some missing cases, and the incidence of diabetes might be lower than they actually were. Third, GGT/HDL-C was only measured at baseline. Its changes over time could not be observed during follow-up days. Finally, residual confounders caused by measurement error in assessing confounders and unmeasured factors could not be completely ruled out. Further research in diverse populations, with more extensive data collection and meticulous research design, is needed.
Conclusion
The GGT/HDL-C ratio correlated with the incidence of T2DM in a curvilinear form with a threshold effect. Their positive relationship could be observed when GGT/HDL-C was less than 6.53. That is, a one-unit increase in the GGT/HDL-C ratio was associated with a 1.5-fold increase in the risk of incident T2DM. Therefore, the possible curvilinear relationship should be taken into account in the future risk studies of T2DM, and the positive relationship between GGT/HDL and T2DM can only be observed in a specific range of GGT/HDL.
Supplementary Information
Additional file 1: Supplemental Figure 1. The smoothing plots between GGT/HDL-C ratio and T2DM in subgroups. Each plot was adjusted for age, sex, BMI, SBP, DBP, FPG, ALT, AST, HBA1C, TC, TGs, fatty liver, smoking and drinking status, and exercise status, except for the stratification variable.
Acknowledgements
We greatly appreciate Takuro Okamura, Yoshitaka Hashimoto, Masahide Hamaguchi, Akihiro Obora, Takao Kojima, and Michiaki Fukui. They provide the data that enable us to conduct a second analysis. We thank Chang-zhong Chen and Xin-lin Chen of Empower U for their assistance with data analysis.
Abbreviations
- T2DM
Type 2 diabetes mellitus
- NAFLD
Nonalcoholic fatty liver disease
- BMI
Body mass index
- DBP
Diastolic blood pressure
- SBP
Systolic blood pressure
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GGT
Gamma-glutamyltransferase
- HDL
High-density lipoprotein cholesterol
- TG
Triglyceride
- TC
Total cholesterol
- HbA1C
Glycosylated hemoglobin
- FPG
Fasting plasma glucose
- HR
Hazard ratio
- CI
Confidence interval
- GAM
General addictive model
Authors’ contributions
Yue Zhao contributed to the manuscript’s data analysis and interpretation and drafting. Xing Xin contributed to data analysis and interpretation. Xiaoping Luo contributed to the manuscript’s conception and critical revision of the manuscript, analysis and interpretation of the data and approved the final version of the submitted manuscript. All authors critically revised or reviewed the manuscript and approved the final version.
Funding
Not applicable.
Availability of data and materials
The dataset used in this study can be downloaded from the ‘DATADRYAD’ database (www.Datadryad.org).
Declarations
Ethics approval and consent to participate
The ethics committee of Murakami Memorial Hospital approved the study.
The committee’s reference number (not applicable).
Consent for publication
Our study is a secondary analysis based on public databases. All data involved are anonymous, and the data provider stated that written informed consent for data usage was obtained from each participant.
Competing interests
All the authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yue Zhao, Email: zhaoyue18@qq.com.
Xiao-ping Luo, Email: xpluo@tjh.tjmu.edu.cn.
References
- 1.Janghorbani M, Amini M. Utility of serum lipid ratios for predicting incident type 2 diabetes: the Isfahan Diabetes Prevention Study. Diabetes Metab Res Rev. 2016;32:572–580. doi: 10.1002/dmrr.2770. [DOI] [PubMed] [Google Scholar]
- 2.Teshome G, Ambachew S, Fasil A, Abebe M: Prevalence of Liver Function Test Abnormality and Associated Factors in Type 2 Diabetes Mellitus: A Comparative Cross-Sectional Study. EJIFCC 2019; 30:303–316.doi. [PMC free article] [PubMed]
- 3.Giandalia A, Romeo EL, Ruffo MC, Muscianisi M, Giorgianni L, Forte F, et al. Clinical correlates of persistently elevated liver enzymes in type 2 diabetic outpatients. Prim Care Diabetes. 2017;11:226–232. doi: 10.1016/j.pcd.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 4.De Silva NMG, Borges MC, Hingorani AD, Engmann J, Shah T, Zhang X, et al. Liver Function and Risk of Type 2 Diabetes: Bidirectional Mendelian Randomization Study. Diabetes. 2019;68:1681–1691. doi: 10.2337/db18-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YL, Koh WP, Yuan JM, Pan A. Association between liver enzymes and incident type 2 diabetes in Singapore Chinese men and women. BMJ Open Diabetes Res Care. 2016;4:e000296. doi: 10.1136/bmjdrc-2016-000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao W, Tong J, Liu J, Liu J, Li J, Cao Y. The Dose-Response Relationship between Gamma-Glutamyl Transferase and Risk of Diabetes Mellitus Using Publicly Available Data: A Longitudinal Study in Japan. Int J Endocrinol. 2020;2020:5356498. doi: 10.1155/2020/5356498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng G, Feng L, Zhao Y. Association between ratio of γ-glutamyl transpeptidase to high-density lipoprotein cholesterol and prevalence of nonalcoholic fatty liver disease and metabolic syndrome: a cross-sectional study. Ann Transl Med. 2020;8:634. doi: 10.21037/atm-19-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond) 2019;43:139–148. doi: 10.1038/s41366-018-0076-3. [DOI] [PubMed] [Google Scholar]
- 9.Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778–787. doi: 10.1111/j.1440-1746.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 10.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 11.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–8. 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed]
- 12.Hashimoto Y, Hamaguchi M, Kojima T, Ohshima Y, Ohbora A, Kato T, et al. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J Gastroenterol Hepatol. 2015;30:546–552. doi: 10.1111/jgh.12786. [DOI] [PubMed] [Google Scholar]
- 13.Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 14.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71–77. doi: 10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 15.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes A. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama M, Watanabe T, Otaki Y, Takahashi H, Arimoto T, Shishido T, et al. Association of the Aspartate Aminotransferase to Alanine Aminotransferase Ratio with BNP Level and Cardiovascular Mortality in the General Population: The Yamagata Study 10-Year Follow-Up. Dis Markers. 2016;2016:4857917. doi: 10.1155/2016/4857917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KRAUSS R M: Lipids and Lipoproteins in Patients With Type 2 Diabetes. Diabetes Care 2004; 27:1496–1504.doi. [DOI] [PubMed]
- 20.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 2009;32:741–50. 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed]
- 22.Wu L, Zhang M, Hu H, Wan Q. Elevated gamma-glutamyl transferase has a non-linear association with incident non-alcoholic fatty liver disease in the non-obese Chinese population: a secondary retrospective study. Lipids Health Dis. 2021;20:142. doi: 10.1186/s12944-021-01577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndrepepa G, Colleran R, Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin Chim Acta. 2018;476:130–138. doi: 10.1016/j.cca.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Tao L, Li X, Zhu H, Gao Y, Luo Y, Wang W, et al. Association between gamma-glutamyl transferase and metabolic syndrome: a cross-sectional study of an adult population in Beijing. Int J Environ Res Public Health. 2013;10:5523–5540. doi: 10.3390/ijerph10115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DH, Blomhoff R, Jacobs DR, Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535–539. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 26.Kunutsor SK. Gamma-glutamyltransferase-friend or foe within? Liver Int. 2016;36:1723–1734. doi: 10.1111/liv.13221. [DOI] [PubMed] [Google Scholar]
- 27.Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al.: Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016; 31:936–944.doi:10.1111/jgh.13264. [DOI] [PubMed]
- 28.Kim CH, Park JY, Lee KU, Kim JH, Kim HK. Association of serum gamma-glutamyltransferase and alanine aminotransferase activities with risk of type 2 diabetes mellitus independent of fatty liver. Diabetes Metab Res Rev. 2009;25:64–69. doi: 10.1002/dmrr.890. [DOI] [PubMed] [Google Scholar]
- 29.Fujita M, Ueno K, Hata A. Association of gamma-glutamyltransferase with incidence of type 2 diabetes in Japan. Exp Biol Med (Maywood) 2010;235:335–341. doi: 10.1258/ebm.2009.009232. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko K, Yatsuya H, Li Y, Uemura M, Chiang C, Hirakawa Y, et al. Association of gamma-glutamyl transferase and alanine aminotransferase with type 2 diabetes mellitus incidence in middle-aged Japanese men: 12-year follow up. J Diabetes Investig. 2019;10:837–845. doi: 10.1111/jdi.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang YC, Ahn HY, Park SW, Park CY. Association of HDL-C and apolipoprotein A-I with the risk of type 2 diabetes in subjects with impaired fasting glucose. Eur J Endocrinol. 2014;171:137–142. doi: 10.1530/EJE-14-0195. [DOI] [PubMed] [Google Scholar]
- 32.Tabara Y, Arai H, Hirao Y, Takahashi Y, Setoh K, Kawaguchi T, et al. Different inverse association of large high-density lipoprotein subclasses with exacerbation of insulin resistance and incidence of type 2 diabetes: The Nagahama study. Diabetes Res Clin Pract. 2017;127:123–131. doi: 10.1016/j.diabres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Waldman B, Jenkins AJ, Davis TM, Taskinen MR, Scott R, O’Connell RL, et al. HDL-C and HDL-C/ApoA-I predict long-term progression of glycemia in established type 2 diabetes. Diabetes Care. 2014;37:2351–8. 10.2337/dc13-2738. [DOI] [PubMed]
- 34.Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 35.Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8:237–245. doi: 10.1038/nrendo.2011.235. [DOI] [PubMed] [Google Scholar]
- 36.de Haan W, Bhattacharjee A, Ruddle P, Kang MH, Hayden MR. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J Lipid Res. 2014;55:516–523. doi: 10.1194/jlr.M045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold von Eckardstein C W: HDL, beta cells and diabetes. Cardiovascular research 2014; 103:384–394.doi:10.1093/cvr/cvu143. [DOI] [PubMed]
- 38.Xing Y, Chen J, Liu J, Ma H. Associations Between GGT/HDL and MAFLD: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2022;15:383–394. doi: 10.2147/DMSO.S342505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Figure 1. The smoothing plots between GGT/HDL-C ratio and T2DM in subgroups. Each plot was adjusted for age, sex, BMI, SBP, DBP, FPG, ALT, AST, HBA1C, TC, TGs, fatty liver, smoking and drinking status, and exercise status, except for the stratification variable.
Data Availability Statement
The dataset used in this study can be downloaded from the ‘DATADRYAD’ database (www.Datadryad.org).