Abstract
Strategies proficient for relieving cognitive impairments in aging and Alzheimer’s disease (AD) have an enormous impact. Regular physical exercise (PE) can prevent age-related dementia and slow down AD progression. However, such a lifestyle change is likely not achievable for individuals displaying age-related frailty. Hence, drugs or biologics that could simulate the benefits of PE have received much attention. Previous studies suggested that the fibronectin-domain III containing 5 (FNDC5) underlies the PE-mediated improved cognitive function. A recent study reports that PE-related cognitive benefits in aging and AD are mediated by irisin, the cleaved form of FNDC5 released into the blood after PE. Such a conclusion was apparent from the deletion of irisin through a global knockout of FNDC5, leading to the loss of PE-induced cognitive benefits or inducing memory impairments in adult or aged models. Furthermore, in AD models, peripherally administered irisin mimicked the cognitive benefits of PE by modulating neuroinflammation. This short review discusses the promise of irisin to simulate the cognitive benefits of PE in age- and AD-related dementia. In addition, critical issues such as how blood-borne irisin acts on neural cells, the role of the brain-derived neurotrophic factor in irisin-mediated cognitive benefits, and irisin’s ability to inhibit neuroinflammatory cascades in aging and AD are discussed.
Keywords: Brain derived neurotrophic factor, Dementia, Irisin, Physical exercise, Neuroinflammation, Neurogenesis
1. Introduction
Increased life expectancy in the past century has substantially expanded the population of people > 65 years of age globally and is expected to grow further in the coming decades. However, increased lifespan has also contributed to a greater prevalence of age-related conditions, including neurodegenerative diseases (Gurău et al., 2018; Shetty et al., 2018), which are among the leading causes of morbidity worldwide (de Magalhães et al., 2017). Aging is associated with cognitive impairment in a substantial percentage of people, and age-related cognitive decline is associated with various alterations in the brain, particularly the hippocampus (Bettio et al., 2017). These include moderate but chronic neuroinflammation (Barrientos et al., 2015; Kodali et al., 2015), increased oxidative stress and mitochondrial dysfunction (Li et al., 2020), diminished autophagy (Glatigny et al., 2019; Kodali et al., 2021a), waned neurogenesis (van Praag et al., 2005; Rao et al., 2006, 2008; Hattiangady and Shetty, 2008), and synapse loss (Xu et al., 2019).
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, accounts for ~80% of dementia and affects ~47 million people worldwide (AD association, 2019). The adverse changes in the AD brain include lasting neuroinflammation, extracellular deposition of β-amyloid (Aβ), neuronal dysfunction, hyperphosphorylated tau, intraneuronal neurofibrillary tangles, loss of synapses, and neurodegeneration (Wang and Colonna, 2019; Joe and Ringman, 2019; van der Kant et al., 2020; Duyckaerts et al., 2019). Deposition of Aβ plaques likely contributes to the accumulation of neurofibrillary tangles and the progression of activated microglia-mediated neuroinflammation, which leads to the loss of synapses and neurodegeneration (Long and Holtzman, 2019). Many factors, including genetic susceptibility, histone modifications, DNA methylations, altered RNA expression, cellular level changes in the brain, and systemic inflammation, interplay in the progression of aging and AD (Xia et al., 2018). No therapy can reverse cognitive and mood impairments observed in aging or AD. Hence, discovering strategies that promote successful aging, a state where older individuals maintain better cognitive function, actively participate in life, and develop no significant disease (Bettio et al., 2017; Fan et al., 2017), and approaches that restrain the AD progression and improve brain function have immense value.
One of the well-known lifestyle changes to maintain better brain function in aging is physical exercise (PE), which also has the potential to prevent age-related dementia, including AD, as well as slow down disease progression in individuals diagnosed with the early stage of AD (Jia et al., 2019; Morris et al., 2017; Hoffmann et al., 2016). Previous studies have shown that exercise induces hippocampal expression of a glycosylated type 1 membrane protein, the fibronectin-domain III containing 5 (FNDC5), which can activate hippocampal neuroprotective genes (Wrann et al., 2013). Consequently, it is believed that FNDC5 underlies the PE-mediated cognitive benefits. In line with this concept, a recent study by Islam and associates provides novel insights on the role of FNDC5 in cognitive function in aging and AD (Islam et al., 2021). Notably, the authors demonstrated that irisin, the cleaved and N-terminal portion of FNDC5 released into the circulation after PE, is the active moiety mediating the exercise-related cognitive benefits, and peripherally administered irisin can mimic the benefits of PE in alleviating cognitive impairments in mouse models of AD. The purpose of this short review is to discuss the significance and implications of these findings for treating aging and AD-related dementia and point out critical issues that require further investigation.
2. Potential mechanisms by which PE enhances cognitive function and modulates inflammation
While many studies have implied that PE promotes better brain function, prevents cognitive impairments in aging, and slows down the progression of AD, the precise mechanisms by which PE mediates these effects are still under investigation. Some investigations have pointed out the ability of PE to reduce amyloid deposits, reactive astrocytes, activated microglia, and proinflammatory cytokines and improve mitochondrial function and mitophagy (Bromley-Brits et al., 2011; He et al., 2017; Zhao et al., 2020; Liu et al., 2020; Kodali et al., 2021b). Many other studies have attributed the beneficial effects of PE on brain function to enhanced adult hippocampal neurogenesis and elevated levels of the brain-derived neurotrophic factor (BDNF) due to their involvement in synaptic plasticity and cognitive, memory and mood function (Cotman et al., 2007; Kodali et al., 2016; Choi et al., 2018; Islam et al., 2017). BDNF can also promote neuronal survival, enhance dendritic arborization and facilitate neosynaptogenesis (Greenberg et al., 2009; Islam et al., 2017). A study has also suggested that upregulation of BDNF after PE likely occurs in the hippocampus via increased FNDC5/irisin expression (Wrann et al., 2013). From these perspectives, irisin comes across as an exciting anti-aging or pro-cognitive compound.
Irisin, the cleaved and N-terminal portion of FNDC5 released into the circulation after PE (Islam et al., 2017), is a skeletal muscle-secreted myokine made up of 112 amino acids (Ohtaki, 2016). Irisin was initially discovered in the mouse skeletal muscle as muscles express large amounts of FNDC5 transcripts. However, mRNAs of FNDC5 are also seen in many tissues, including the brain and liver (Huh et al., 2012). Contraction of skeletal muscles leads to increased expression of the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1-α, which induces FNDC5 gene expression, leading to the release of irisin into the bloodstream following its cleavage from FNDC5 (Catalán, 2018). However, the exact mechanism by which FNDC5 is cleaved into irisin remains to be elucidated. Irisin is also one of the adipocytokines released by the adipocytes. Irisin is involved in thermogenesis and the browning response of the white adipose tissue, a process involving the expression of mitochondrial uncoupling protein-1 and other related genes leading to morphological changes and mitochondrial activity observed in brown adipose tissue (Gomarasca, 2020). Irisin is also known for several other functions in different tissues or organs. Examples include inhibition of adipogenic differentiation to facilitate osteogenic differentiation (Zhang et al., 2014) and influencing the function of skeletal muscles, pancreas (Liu et al., 2017), liver (Arias-Loste et al., 2014), brain (Wrann, 2015), and bone (Qiao et al., 2016).
The expression of FNDC5 through the extracellular-signal-regulated kinase 1/2 (ERK1/2) pathway and phosphorylation of cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP response element-binding protein (CREB) has also been shown to play a significant role in neural differentiation and neuroprotection (Hosseini Farahabadi et al., 2015). Furthermore, irisin, the cleaved product of FNDC5, can regulate cell proliferation through the signal transducer and activator of transcription and ERK1/2 mitogen-activated protein kinase (MAPK) pathways (Moon et al., 2013; Liu et al., 2017). Besides, irisin can activate the brain cAMP/PKA/CREB signaling pathways and enhance BDNF, which can rescue synaptic plasticity in a model of AD (Lourenco et al., 2019) and promote neurogenesis and synaptogenesis. B DNF has also been shown to modulate proinflammatory microglia into noninflammatory or antiinflammatory phenotypes. The underlying mechanism involves activating the ERK-CREB pathway through its receptor tropomyosin receptor kinase B. Phosphorylated CREB then reduces the nuclear factor kappa B activation by competing for CREB binding protein-P300, which results in reduced inflammation (Wen et al., 2010; Qin et al., 2016; Dong et al., 2018). Moreover, increased expression of FNDC5 can lead to reduced inflammation via AMP kinase (AMPK) pathway activation (Xiong et al., 2018). However, it was unknown whether such effects were mediated through irisin released from FNDC5 or overexpression of FNDC5 alone (Xiong et al., 2018). Additional studies have also implied that irisin can regulate BDNF expression (Huang et al., 2019). Thus, increased FNDC5 or irisin expression leading to BDNF upregulation in the hippocampus has been proposed as the underlying mechanism promoting cognitive function after skeletal muscle contractions during exercise. However, it remains to be proven whether irisin release by FNDC5 is a critical requirement for enhancing BDNF concentration in the hippocampus. Fig. 1 illustrates the potential mechanisms by which PE-mediated irisin release could improve cognitive function in aging and neurodegenerative disease conditions.
Fig. 1.
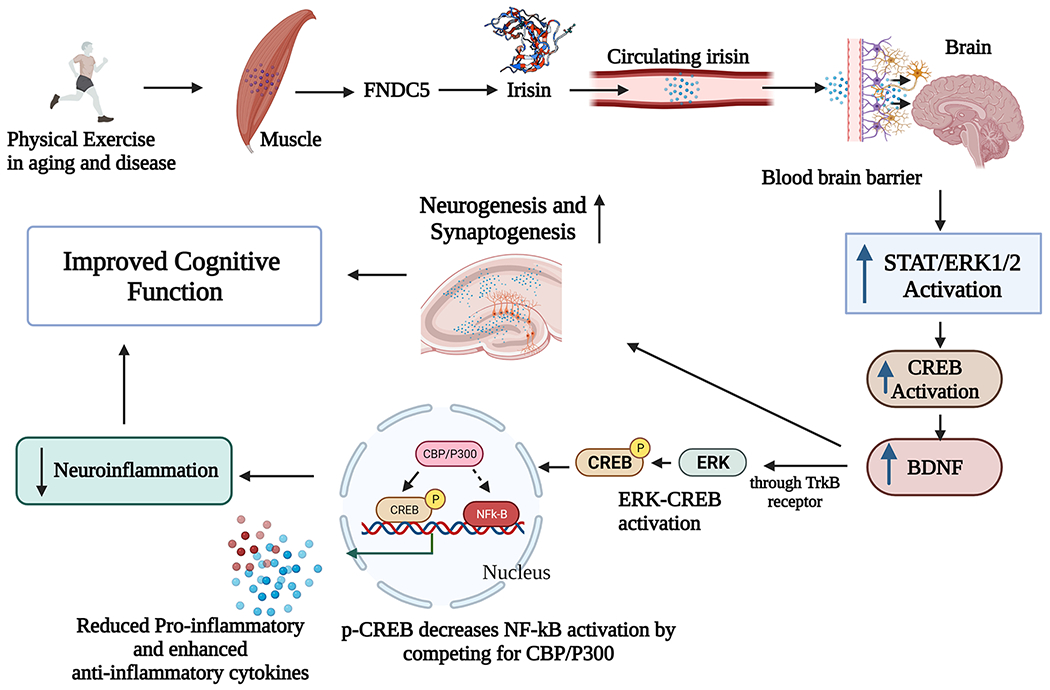
A schematic showing the potential mechanisms by which physical exercise-mediated irisin release could improve cognitive function in aging and neurodegenerative disease conditions. Physical exercise can induce FNDC5 expression in skeletal muscles. FNDC5 protein is then cleaved into irisin, which enters the circulating blood. Irisin can enter the brain by crossing the blood-brain barrier, where it can increase BDNF production through the activation of STAT3/AMPK/ERK signaling, enhancing neurogenesis and synaptogenesis to improve cognitive function. Also, irisin-mediated BDNF increase has the promise to decrease neuroinflammation by reducing the production of NF-kB and pro-inflammatory cytokines IL-6 and IL-1β by activating the ERK-CREB pathway through its receptor trkB. Thus, PE-mediated release of irisin into the bloodstream can potentially also improve cognitive function by reducing neuroinflammation. BDNF: Brain-derived neurotrophic factor; CBP: CREB binding protein; CREB: cyclic adenosine monophosphate (cAMP) response element-binding protein; ERK: extracellular signal-regulated kinases; FNDC5: fibronectin type III domain containing 5; IL-1β: interleukin-1 beta; IL-6: interleukin 6; STAT: signal transducer and activator of transcription; TrkB: Tropomyosin receptor kinase B.
3. Effects of knock-down of FNDC5/irisin on cognitive function in adulthood and aging
To understand the role of irisin in exercise, Islam and colleagues first developed a mouse model with a global knockout of FNDC5 (F5KO mouse) (Islam et al., 2021). Analysis of various tissues from F5KO mice revealed that the FNDC5 gene was knocked out in adipose tissues, muscle, heart, cerebellum, and forebrain, including the hippocampus. However, the irisin levels in the plasma of F5KO mice were comparable to the plasma in wild-type (WT) mice. The reason underlying such discrepancy is unknown, while the authors suggested the possibility of sensitivity or specificity issues (Islam et al., 2021). Next, the authors investigated PE-induced improvements in spatial learning and memory in WT mice and FNDC5 knockout (F5KO) mice using a water maze test. While the WT mice showed better spatial learning and memory retrieval abilities after voluntary PE, F5KO mice did not exhibit such changes, implying that increased irisin release via the cleavage of FNDC5 in the skeletal muscle and/or the brain is critical for PE-mediated improvements in hippocampus-dependent cognitive and memory function. Furthermore, in a novel object recognition test that depends on the integrity of the perirhinal cortex and the hippocampus, aged (21–24 months old) F5KO mice did not prefer the novel object over the familiar object, in contrast to their age-matched WT counterparts preferring the novel object, suggesting that lack of FNDC5 in the forebrain can contribute to age-related recognition memory impairments. Furthermore, compared to WT mice, F5KO mice displayed significant reductions in sustaining long-term potentiation (LTP) in the dentate gyrus (DG) following high-frequency stimulations of the medial perforant pathway, suggesting decreased synaptic plasticity. These results suggested the involvement of FNDC5 in PE-induced enhanced cognitive function, age-related cognitive impairment, and dentate gyrus function. However, since the effect of reinstatement of FNDC5 or exogenous application of irisin was not tested on cognitive function in the aged F5KO mice, it was unclear whether age-related recognition memory impairments and LTP deficits could be alleviated through increased FNDC5 expression or irisin concentration in the forebrain.
To probe the role of irisin in DG function, the authors compared pattern separation function between adult WT and F5KO mice by employing a contextual fear conditioning discrimination learning task (Islam et al., 2021). Pattern separation ability can discriminate similar but not identical experiences in a non-overlapping manner, which depends on the integrity of the DG and the extent of hippocampal neurogenesis (Leutgeb et al., 2007; Christian et al., 2020). F5KO mice took significantly longer times to discriminate between contexts in contextual fear conditioning discrimination learning. Notably, overexpression of irisin through a direct injection of an adeno-associated virus 8 encoding irisin (AAV8-FLAG) into the DG enhanced contextual fear conditioning discrimination learning in both WT and F5KO mice. These results supported the involvement of irisin in pattern separation function. However, it was unclear how increased irisin production in the DG of WT mice improved pattern separation function. In future studies, it remains to be investigated whether such improvements in WT mice reflect enhanced neurogenesis and synaptic plasticity.
F5KO mice also exhibited abnormal function of adult-born neurons in the hippocampus. Studies with the birth-dating marker 5′-bromodeoxyuridine and c-fos, a marker of neuronal activity, revealed that PE induced abnormal activation patterns in adult-born neurons in F5KO mice compared to WT mice. Altered activation of adult born neurons in FSKO mice can impair sparse coding critically required for normal pattern separation function (McAvoy et al., 2016). Sparse coding involves the activity of fewer neurons within a relevant time window, which is a pattern of neuronal activity considered necessary for pattern separation function involving discrimination of similar but not identical experiences in a non-overlapping manner. However, neuronal population sparseness and expansion recoding are independent properties, as neural populations can be expanded into either a sparse or dense code by changing the intrinsic excitability of the neurons (Cayco-Gajic and Silver, 2019). In the DG, the sparse coding has been suggested to involve adult-born neurons because they promote feedback inhibition through mossy cells and hilar gamma-amino butyric acid-positive interneurons, which, in turn, facilitates the recruitment of non-overlapping populations of dentate granule cells to encode similar but not identical inputs (McAvoy et al., 2016). Interestingly, the number of newborn neurons in the hippocampus did not differ between WT and F5KO mice in baseline conditions, and PE similarly improved the number of newborn neurons in both genotypes of mice. However, the newborn neurons in F5KO mice did not display the enhanced total dendritic length and dendritic arbors typically seen in WT mice after PE (Islam et al., 2021). Quantification of dendritic spines of newborn neurons in the molecular layer of the dorsal hippocampus also revealed reduced spine density in sedentary F5KO mice, which did not improve even with PE. Furthermore, single nuclei RNA-sequencing of adult-born neurons revealed an abnormal transcriptional profile in newborn neurons from F5KO mice, with or without PE. Overall, the features of newborn neurons implied that FNDC5 expression could influence newborn neuron development and maturation, and their deficiency can alter newborn neurons at the transcriptional level. The involvement of FNDC5 in the differentiation of newly born neurons could also be gleaned from several previous studies. These include the involvement of FNDC5 in neuronal differentiation through the ERK1/2 pathway (Hashemi et al., 2013; Zhang and Zhang, 2016). Also, reduced and increased neuronal differentiation of neuronal precursor cells has been observed with knockdown or overexpression of the Fndc5 gene, respectively (Huh, 2018; Hashemi et al., 2013). However, in the absence of neurogenesis studies following the reinstatement of FNDC5 expression or exogenous irisin administration in F5KO mice, it was unclear whether neurogenesis alterations were linked solely to the loss of FNDC5 expression or loss of both FNDC5 and irisin.
4. Promise of irisin to improve cognitive function in AD models
The next set of experiments by Islam and colleagues examined the role of FNDC5/irisin in AD models (Islam et al., 2021). They first chose six-month-old amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mice, which exhibited reduced Fndc5 gene expression in the hippocampus (Islam et al., 2021). A previous study has also shown reduced irisin concentration in the hippocampus of these mice (Lourenco et al., 2019). This mouse model also starts to display amyloid plaques, gliosis, and cognitive deficits around 6 months of age (Jankowsky et al., 2004, 2021). A reduction in Fndc5 gene expression has also been seen in the parahippocampal gyrus of AD patients compared to controls (Wan et al., 2020). The authors generated the APP/PS1-F5KO progeny by crossing F5KO mice with the APP/PS1 mice and tested the contextual fear conditioning discrimination learning, which demonstrated an exaggerated cognitive decline in APP/PS1-F5KO mice (Islam et al., 2021). Such changes were also associated with higher levels of soluble Aβ-40 in the cortex but not in the hippocampus. Next, the authors probed the therapeutic potential of peripherally delivered irisin in 8 months old APP/PS1 mice. Tail-vein injection of AAV8-irisin-FLAG in these mice resulted in overexpression of irisin in the liver, which led to increased irisin concentration in the circulating blood. While AAV8-irisin-FLAG administration did not increase irisin mRNA expression in the brain, it did increase the overall irisin concentration in the brain, implying that irisin from the circulating blood entered the brain through BBB. However, the hippocampal irisin concentration did not alter significantly. In Barnes and Morris water maze tasks performed two months later, AAV8-irisin-FLAG-treated AD mice displayed improved hippocampal-dependent spatial learning and memory ability. Such results suggest that an overall increase in irisin concentration in the brain, without a hippocampus-specific increase, can improve hippocampus-dependent cognitive function.
Similar cognitive improvements were also seen with tail-vein injections of AAV8-irisin-FLAG into the 5X familial AD (5XFAD) mice, another AD model showing the aggressive progression of cognitive impairments, and pathological markers (Oakley et al., 2006; Ohno et al., 2007). Furthermore, in APP/PS1 mice, irisin treatment improved context discrimination/pattern separation tasks. Improved cognitive function in AD mice observed following peripheral administration of irisin by Islam and colleagues is congruous with a previous study showing that enhancing brain FNDC5/irisin through an intra-cerebroventricular administration of adenoviral vector expressing full-length FNDC5 (AdFNDC5) can rescue object memory and contextual memory impairments in Aβ oligomer-infused mice (Lourenco et al., 2019). In the latter study, AdFNDC5 enhanced FNDC5/irisin mRNA expression and protein levels in the cerebral cortex. The study also showed that intravenous administration of AdFNDC5 can enhance irisin in the hippocampus (Lourenco et al., 2019). Brain tissue analysis revealed reduced reactive astrocytes and activated microglia in APP/PS1 mice receiving irisin (Islam et al., 2021). Specifically, irisin treatment downregulated astrocyte- and microglia-specific genes in AD mice but did not influence hippocampal neurogenesis, synaptic plasticity genes, or hippocampal/cortical amyloid plaque load. While it is unclear how irisin regulated reactive astrocytes and activated microglia in the AD brain, investigation in cultured astrocytes suggested that irisin possibly modulated reactive astrocytes by acting on αvβ5 integrin receptor complexes expressed on them. Additional investigations in the study also suggested that circulating irisin can cross the blood-brain barrier (BBB). While the study did not measure changes in proinflammatory cytokines or microglia with activated inflammasomes in the AD brain, a previous cell culture study has suggested that irisin can reduce the release of proinflammatory cytokines Interleukin-6 (IL-6) and IL-1β and the expression of cyclooxygenase-2 (COX-2), a proinflammatory mediator in astrocytes (Wang et al., 2018). Furthermore, a study testing the effects of irisin in macrophages stimulated with lipopolysaccharide has suggested that irisin can significantly alleviate inflammatory signaling by decreasing the levels of multiple inflammatory mediators such as toll-like receptor 4, myeloid differentiation primary response 88, and the phosphorylated nuclear factor kappa B (Mazui--Bialy et al., 2017). Fig. 2 illustrates the effects of deletion of irisin in AD mice and the potential mechanisms by which peripheral irisin treatment can improve cognitive function in AD mice.
Fig. 2.
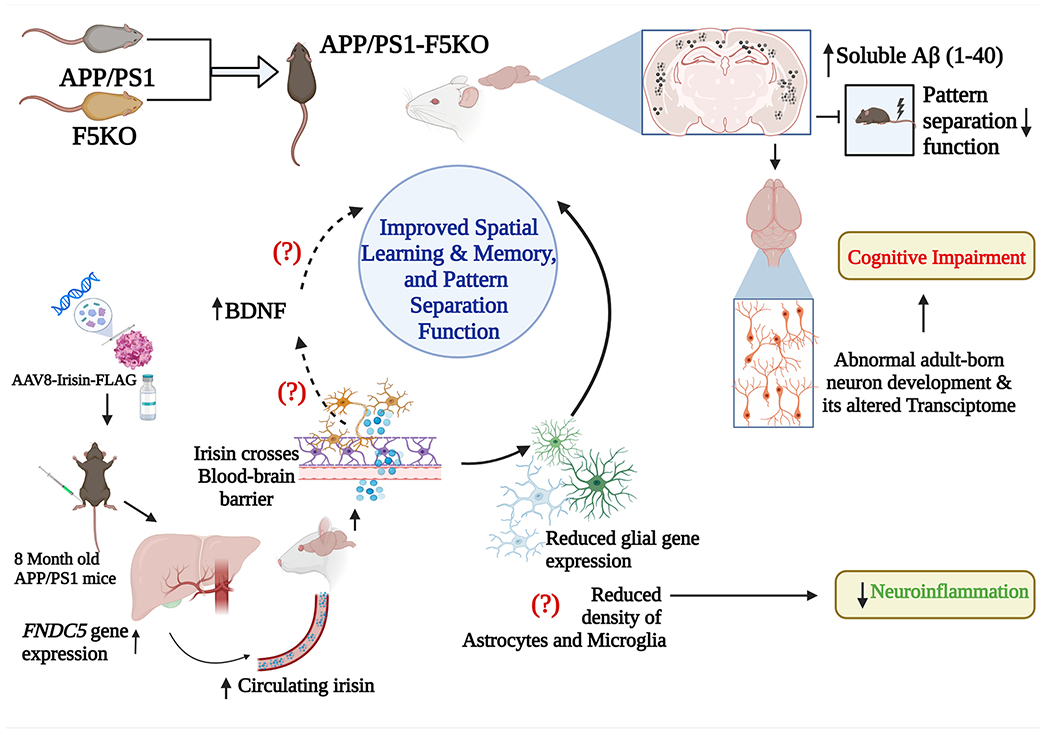
A schematic showing the effects of global KO of FNDC5 in APP/PS1 mice (a model of AD) and the potential mechanisms by which peripheral irisin treatment could improve cognitive function in AD mice. Breeding of AD mouse (APP/PS1) with FNDC5 gene knock-out (F5KO) mouse produced an AD mouse model with F5KO (APP/PS1-F5KO). Such mice displayed impaired pattern separation function and increased soluble amyloid-beta (Aβ 1–40) concentration, and abnormal development of adult born neurons with altered transcriptome. On the other hand, intravenous administration of AAV8-irisin-FLAG in APP/PS1 mice increased FNDC5 overexpression in the liver, which increased irisin release into the circulating blood. Peripheral irisin enters the brain by crossing the blood-brain barrier, modulating glial cell gene expression, and possibly reducing the numbers of reactive astrocytes and activated microglia, the perpetrators of chronic neuroinflammation. Such antiinflammatory effects likely improved cognitive function in AAV8-irisin-FLAG treated APP/PS1 mice. Irisin also potentially increased BDNF concentration in the brain, which likely also contributed to improved cognitive function in APP/PS1 mice. AAV: Adeno-associated virus; Aβ: amyloid-beta; BDNF: brain-derived neurotrophic factor; F5KO: fibronectin type III domain containing 5 gene knock-out.
Thus, it is clear that PE induces increased expression of FNDC5 in skeletal muscles (Wrann et al., 2013), followed by the release of its cleaved form irisin into the circulating blood (Lu et al., 2016), eventually resulting in enhanced concentration of irisin in the brain. PE also enhances FNDC5 expression and BDNF concentration in the brain, including the hippocampus (Cotman et al., 2007; Choi et al., 2018; Kim and Song, 2018; Tu et al., 2020). BDNF has a role in synaptic plasticity, neosynaptogenesis, neuroprotection, cognitive and mood function, and hippocampal neurogenesis (Greenberg et al., 2009; Kuipers and Bramham, 2006; Kim and Song, 2018; Wang and Holsinger, 2018; Harley et al., 2021). Therefore, the question is whether irisin is an upstream mediator of BDNF synthesis and release in the brain following PE. A previous study by Wrann and associates has suggested that hippocampal BDNF is induced through FNDC5 (Wrann et al., 2013). In a cell culture study, a forced expression of FNDC5 through adenovirus in primary cortical neurons considerably increased the expression of the Bdnf gene and several other genes involved in hippocampal function. Also, RNAi-mediated knockdown of FNDC5 reduced Bdnf gene expression. Bdnf gene expression was also controlled by PGC-1α, a transcriptional regulator of Fndc5 gene expression. This link was evident from reduced Bdnf gene expression in PGC-1α KO mice. Additional assays showed that neurons stimulated with recombinant BDNF displayed reduced Fndc5 gene expression, implying a homeostatic FNDC5/BDNF feedback loop (Wrann et al., 2013). Furthermore, peripheral delivery of FNDC5 to the liver through adenoviral vectors induced elevated irisin levels in the circulating blood, which increased Bdnf expression in the hippocampus and other neuroprotective genes in the forebrain, including the hippocampus (Wrann et al., 2013). Thus, following PE, BDNF upregulation in the hippocampus could occur through increased FNDC5 expression in the brain, including the hippocampus, or increased irisin concentration in the brain without a significant upregulation in the hippocampus. However, whether a direct exogenous administration of irisin into the hippocampus alone can enhance BDNF concentration and improve cognitive function is unclear. Additional studies are needed in the future to address the above issues, as the investigation in AD models did not determine whether improved cognitive function following systemic administration of irisin involved increased expression of Fndc5 and Bdnf genes and/or elevated levels of BDNF in the hippocampus (Islam et al., 2021).
5. Summary and future perspectives
In summary, the study by Islam and colleagues suggested that FNDC5 and irisin, the secreted part of the exercise hormone FNDC5, can influence cognitive function. This conclusion was supported by findings that genetic deletion of Fndc5 leads to loss of PE-induced improved cognitive function in adult mice, cognitive dysfunction in aged mice, and exaggerated cognitive impairments in AD models. Additionally, the authors showed that systemic administration of AAV8-irisin-FLAG leads to overexpression of irisin in the liver and the circulating blood, from where it enters the brain by crossing the BBB and improves cognitive function in AD models. However, several issues require further investigation. It is unclear how irisin entering the brain from the circulating blood acts on neural cells to improve cognitive function. While increased circulating irisin levels result in enhanced irisin concentration in the brain (Islam et al., 2021), it is unknown whether such irisin increase in the brain can enhance BDNF protein concentration in the hippocampus or downstream upregulation of BDNF in the hippocampus following irisin entry into the brain is required for the beneficial effects of irisin on cognitive function. Studies on the effects of systemic administration of AAV8-irisin-FLAG in mouse models with altered BDNF signaling or mice with specific deletion of BDNF in the hippocampus (Adachi et al., 2008; Taliaz et al., 2010; Lindholm and Castrén, 2014; Harb et al., 2021) may address this issue.
Furthermore, specific receptors that bind to irisin in neural cells are mostly unknown. The study by Islam and coworkers suggested that irisin can bind to αVβ5 integrin receptor complexes on astrocytes, consistent with previous studies (Greenhill, 2019; Kim et al., 2018). Nevertheless, it is unknown whether irisin can act directly on neurons and microglia through specific receptors. It is an important issue because newborn neurons in F5KO mice did not exhibit the enhanced dendritic arbors typically seen after PE and systemic administration of AAV8-irisin-FLAG modulated activated microglia (Islam et al., 2021). Moreover, the effects of systemically administered AAV8-irisin-FLAG on suppressing neuroinflammation in AD models need further investigation. Notably, it is unknown whether irisin can significantly inhibit neuroinflammatory cascades, such as the activation of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasomes (Heneka et al., 2013; Saresella et al., 2016; Feng et al., 2020) and senescent cells (Reddy et al., 2017) in the AD brain. Both inflammasomes and senescent cells are known to perpetuate the release of adverse proinflammatory cytokines such as IL-1, IL18, and IL-6 in the AD brain (Feng et al., 2020; Saez-Atienzar and Masliah, 2020). Finally, it remains to be addressed whether systemic administration of FLAG-tagged recombinant irisin would be as efficacious as AAV8-irisin-FLAG. If irisin levels can be enhanced pharmacologically, the effects of irisin treatment could be tested in clinical trials to prevent age-related cognitive dysfunction, restrain the evolution of age-related mild cognitive impairment into AD, or slow down the progression of cognitive decline in individuals diagnosed with the early stage of AD.
Acknowledgments
The authors are supported by grants from the National Institute of Neurological Disorders and Stroke (1R01NS106907 to A.K.S.) and the Department of Defense (W81XWH-19–1-0548 and W81XWH-20–1-0568 to A.K.S.). The figure was created with BioRender.com.
Footnotes
Competing Interests
The authors declare that there are no competing interests.
References
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM, 2008. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol. Psychiatry 63, 642–649. 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANON 2019. Alzheimer’s disease facts and figures (Statistical resource for U.S. data related to Alzheimer’s disease No. Alzheimers Dement 2019;15(3), 2019. [Google Scholar]
- Arias-Loste MT, Ranchal I, Romero-Gómez M, Crespo J, 2014. Irisin, a link among fatty liver disease, physical inactivity and insulin resistance. Int J. Mol. Sci 15, 23163–23178. 10.3390/ijms151223163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF, 2015. Neuroinflammation in the normal aging hippocampus. Neuroscience 309, 84–99. 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettio LEB, Rajendran L, Gil-Mohapel J, 2017. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav Rev 79, 66–86. 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- Bromley-Brits K, Deng Y, Song W, 2011. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp 2920. 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán V, Frühbeck G, Gómez-Ambrosi J, 2018. Chapter 8 - Inflammatory and Oxidative Stress Markers in Skeletal Muscle of Obese Subjects. In: del Moral AM, Aguilera García CM (Eds.), Obesity. Academic Press, pp. 163–169. 10.1016/B978-0-12-812504-5.00008-8. [DOI] [Google Scholar]
- Cayco-Gajic NA, Silver RA, 2019. Re-evaluating circuit mechanisms underlying pattern separation. Neuron 101, 584–602. 10.1016/j.neuron.2019.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, van Praag H, Spiegelman BM, Gage FH, Tanzi RE, 2018. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361. 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Ming G-L, Song H, 2020. Adult neurogenesis and the dentate gyrus: predicting function from form. Behav. Brain Res 379, 112346. 10.1016/j.bbr.2019.112346. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A, 2007. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472. 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dong Y, Pu K, Duan W, Chen H, Chen L, Wang Y, 2018. Involvement of Akt/CREB signaling pathways in the protective effect of EPA against interleukin-1β-induced cytotoxicity and BDNF down-regulation in cultured rat hippocampal neurons. BMC Neurosci. 19, 52. 10.1186/s12868-018-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Clavaguera F, Potier M-C, 2019. The prion-like propagation hypothesis in Alzheimer’s and Parkinson’s disease. Curr. Opin. Neurol 32, 266–271. 10.1097/WCO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- Fan Z, Brooks DJ, Okello A, Edison P, 2017. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 140, 792–803. 10.1093/brain/aww349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktäschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA, 2019. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci 22, 401–412. 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y-S, Tan Z-X, Wu L-Y, Dong F, Zhang F, 2020. The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Res Rev. 64, 101192 10.1016/j.arr.2020.101192. [DOI] [PubMed] [Google Scholar]
- Glatigny M, Moriceau S, Rivagorda M, Ramos-Brossier M, Nascimbeni AC, Lante F, Shanley MR, Boudarene N, Rousseaud A, Friedman AK, Settembre C, Kuperwasser N, Friedlander G, Buisson A, Morel E, Codogno P, Oury F, 2019. Autophagy is required for memory formation and reverses age-related memory decline. Curr. Biol 29 (435–448), e8 10.1016/j.cub.2018.12.021. [DOI] [PubMed] [Google Scholar]
- Gomarasca M, Banff G, Lombardi G, 2020. Chapter Four - Myokines: The endocrine coupling of skeletal muscle and bone. In: Makowski GS (Ed.), Advances in Clinical Chemistry. Elsevier, pp. 155–218. 10.1016/bs.acc.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL, 2009. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci 29, 12764–12767. 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill C, 2019. Irisin receptor in osteocytes identified. Nat. Rev. Endocrinol 15, 63. 10.1038/s41574-018-0151-9. [DOI] [PubMed] [Google Scholar]
- Gurău F, Baldoni S, Prattichizzo F, Espinosa E, Amenta F, Procopio AD, Albertini MC, Bonafè M, Olivieri F, 2018. Anti-senescence compounds: a potential nutraceutical approach to healthy aging. Ageing Res. Rev 46, 14–31. 10.1016/j.arr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Harb M, Jagusch J, Durairaja A, Endres T, Leßmann V, Fendt M, 2021. BDNF haploinsufficiency induces behavioral endophenotypes of schizophrenia in male mice that are rescued by enriched environment. Transl. Psychiatry 11, 233. 10.1038/s41398-021-01365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley SBR, Willis EF, Shaikh SN, Blackmore DG, Sah P, Ruitenberg MJ, Bartlett PF, Vukovic J, 2021. Selective ablation of BDNF from microglia reveals novel roles in self-renewal and hippocampal neurogenesis. J. Neurosci 41 (19), 4172–4186. 10.1523/JNEUROSCI.2539-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi M-S, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, Nasr-Esfahani MH, Baharvand H, 2013. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 231, 296–304. 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK, 2008. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging 29, 129–147. 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X-F, Liu D-X, Zhang Q, Liang F-Y, Dai G-Y, Zeng J-S, Pei Z, Xu G-Q, Lan Y, 2017. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front. Mol. Neurosci 10, 144. 10.3389/fnmol.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kurnmer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng T-C, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT, 2013. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, Braendgaard H, Gottrup H, Lolk A, Wermuth L, Jacobsen S, Laugesen LP, Gergelyffy RG, Høgh P, Bjerregaard E, Andersen BB, Siersma V, Johannsen P, Cotman CW, Waldemar G, Hasselbalch SG, 2016. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J. Alzheimers Dis 50, 443–453. 10.3233/JAD-150817. [DOI] [PubMed] [Google Scholar]
- Hosseini Farahabadi SS, Ghaedi K, Ghazvini Zadegan F, Karbalaie K, Rabiee F, Nematollahi M, Baharvand H, Nasr-Esfahani M-H, 2015. ERK1/2 is a key regulator of Fndc5 and PGC1α expression during neural differentiation of mESCs. Neuroscience 297, 252–261. 10.1016/j.neuroscience.2015.03.069. [DOI] [PubMed] [Google Scholar]
- Huang L, Yan S, Luo L, Yang L, 2019. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Mol. Med. Rep 19, 1074–1082. 10.3892/mmr.2018.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, 2018. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res 41 (1), 14–29 10.1007/s12272-017-0994-y. [DOI] [PubMed] [Google Scholar]
- Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS, 2012. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61, 1725–1738. 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Young MF, Wrann CD, 2017. The Role of FNDC5/Irisin in the Nervous System and as a Mediator for Beneficial Effects of Exercise on the Brain. In: Spiegelman B (Ed.), Hormones, Metabolism and the Benefits of Exercise, Research and Perspectives in Endocrine Interactions. Springer International Publishing, Cham, pp. 93–102. 10.1007/978-3-319-72790-5_8. [DOI] [Google Scholar]
- Islam MR, Valaris S, Young MF, Haley EB, Luo R, Bond SF, Mazuera S, Kitchen RR, Caldarone BJ, Bettio LEB, Christie BR, Schmider AB, Soberman RJ, Besnard A, Jedrychowski MP, Kim H, Tu H, Kim E, Choi SH, Tanzi RE, Spiegelman BM, Wrann CD, 2021. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab 3, 1058–1070. 10.1038/s42255-021-00438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR, 2004. Mutant presenilins specifically elevate the levels of the 42-residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet 13, 159–170. 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jia R-X, Liang J-H, Xu Y, Wang Y-Q, 2019. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 19, 181. 10.1186/s12877-019-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe E, Ringman JM, 2019. Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ 367, l6217. 10.1136/bmj.l6217. [DOI] [PubMed] [Google Scholar]
- Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman V-JA, Novick SJ, Strutzenberg TS, Pascal BD, Le PT, Brooks DJ, Roche AM, Gerber KK, Mattheis L, Chen W, Tu H, Bouxsein ML, Griffin PR, Baron R, Rosen CJ, Bonewald LF, Spiegelman BM, 2018. Irisin mediates effects on bone and fat via αV integrin receptors. Cell 175, 1756–1768. 10.1016/j.cell.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OY, Song J, 2018. The role of irisin in Alzheimer’s disease. J. Clin. Med 7, 407. 10.3390/jcm7110407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK, 2015. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature and reduced glial activation. Sci. Rep 5, 8075. 10.1038/srep08075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Megahed T, Mishra V, Shuai B, Hattiangady B, Shetty AK, 2016. Voluntary running exercise-mediated enhanced neurogenesis does not obliterate retrograde spatial memory. J. Neurosci 36, 8112–8122. 10.1523/JNEUROSCI.0766-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Attaluri S, Madhu LN, Shuai B, Upadhya R, Gonzalez JJ, Rao X, Shetty AK, 2021a. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell 20, e13277. 10.1111/acel.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Mishra V, Hattiangady B, Attaluri S, Gonzalez JJ, Shuai B, Shetty AK, 2021b. Moderate, intermittent voluntary exercise in a model of Gulf War Illness improves cognitive and mood function with alleviation of activated microglia and astrocytes, and enhanced neurogenesis in the hippocampus. Brain Behav. Immun 97, 135–149. 10.1016/j.bbi.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers SD, Bramham CR, 2006. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr. Opin. Drug Discov. Devel 9, 580–586. [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M-B, Moser EI, 2007. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966. 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Li W, Cheng H, Li G, Zhang L, 2020. Mitochondrial damage and the road to exhaustion. Cell Metab. 32, 905–907. 10.1016/j.cmet.2020.11.004. [DOI] [PubMed] [Google Scholar]
- Lindholm JSO, Castrén E, 2014. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav. Neurosci 8, 143. 10.3389/fnbeh.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Du F, Li X, Wang M, Duan R, Zhang J, Wu Y, Zhang Q, 2017. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS One 12, e0175498. 10.1371/journal.pone.0175498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chu JMT, Yan T, Zhang Y, Chen Y, Chang RCC, Wong GTC, 2020. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer’s disease mice. J. Neuroinflamm 17, 4. 10.1186/s12974-019-1653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Holtzman DM, 2019. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179, 312–339. 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, Goncalves RA, Clarke JR, Beckman D, Staniszewski A, Berman H, Guerra LA, Forny-Germano L, Meier S, Wilcock DM, de Souza JM, Alves-Leon S, Prado VF, Prado MAM, Abisambra JF, Tovar-Moll F, Mattos P, Arancio O, Ferreira ST, De Felice FG, 2019. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med 25, 165–175. 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Li H, Shen S-W, Shen Z-H, Xu M, Yang C-J, Li F, Feng Y-B, Yun J-T, Wang L, Qi H-J, 2016. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis. 15, 93. 10.1186/s12944-016-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, Stevens M, Thornton D, 2017. The business of anti-aging science. Trends Biotechnol. 35, 1062–1073. 10.1016/j.tibtech.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Mazur-Bialy AI, Pocheć E, Zarawski M, 2017. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int. J. Mol. Sci 18 (4), 701 10.3390/ijms18040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy KM, Scobie KN, Berger S, Russo C, Guo N, Decharatanachart P, Vega-Ramirez H, Miake-Lye S, Whalen M, Nelson M, Bergami M, Bartsch D, Hen R, Berninger B, Sahay A, 2016. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron 91, 1356–1373. 10.1016/j.neuron.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H-S, Dincer F, Mantzoros CS, 2013. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 62, 1131–1136. 10.1016/j.metabol.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, Wilkins HM, Brooks WM, Billinger SA, Swerdlow RH, Burns JM, 2017. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS One 12, e0170547. 10.1371/journal.pone.0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R, 2006. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci 26, 10129–10140. 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R, 2007. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol. Dis 26, 134–145. 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, 2016. Chapter 37 - Irisin. In: Takei Y, Ando H, Tsutsui K (Eds.), Handbook of Hormones, 329. Academic Press, San Diego, pp. e37–e43. 10.1016/B978-0-12-801028-0.00037-4. [DOI] [Google Scholar]
- Qiao X, Yong Qiao X, Nie Y, Ma Y, Xian Ma Y, Chen Y, Cheng R, Yin W, Yao Yinrg W, Hu Y, Xu W, Ming Xu W, Xu L, Zhi Xu L, 2016. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep 6, 18732. 10.1038/srep18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Bouchard R, Pugazhenthi S, 2016. Regulation of cyclic AMP response element-binding protein during neuroglial interactions. J. Neurochem 136, 918–930. 10.1111/jnc.13497. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK, 2006. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 5, 545–558. 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK, 2008. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus 18, 931–944. 10.1002/hipo.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Williams J, Smith F, Bhatti JS, Kumar S, Vijayan M, Kandimalla R, Kuruva CS, Wang R, Manczak M, Yin X, Reddy AP, 2017. MicroRNAs, Aging, Cellular Senescence, and Alzheimer’s Disease. Prog. Mol. Biol. Transl. Sci 146, 127–171. 10.1016/bs.pmbts.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Saez-Atienzar S, Masliah E, 2020. Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nat. Rev. Neurosci 21, 433–444. 10.1038/S41583-020-0325-z. [DOI] [PubMed] [Google Scholar]
- Saresella M, La Rosa F, Piancone F, Zoppis M, Marventano I, Calabrese E, Rainone V, Nemni R, Mancuso R, Clerici M, 2016. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener 11, 23. 10.1186/s13024-016-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Kodali M, Upadhya R, Madhu LN, 2018. Emerging anti-aging strategies-scientific basis and efficacy. Aging Dis. 9, 1165–1184. 10.14336/AD.2018.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A, 2010. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry 15, 80–92. 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu T, Peng J, Jiang Y, 2020. FNDC5/Irisin: a new protagonist in acute brain injury. Stem Cells Dev. 29, 533–543. 10.1089/scd.2019.0232. [DOI] [PubMed] [Google Scholar]
- van der Kant R, Goldstein LSB, Ossenkoppele R, 2020. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci 21, 21–35. 10.1038/s41583-019-0240-3. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH, 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci 25, 8680–8685. 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y-W, Al-Ouran R, Mangleburg CG, Perumal TM, Lee TV, Allison K, Swarup V, Funk CC, Gaiteri C, Allen M, Wang M, Neuner SM, Kaczorowski CC, Philip VM, Howell GR, Martini-Stoica H, Zheng H, Mei H, Zhong X, Kim JW, Dawson VL, Dawson TM, Pao P-C, Tsai L-H, Haure-Mirande J-V, Ehrlich ME, Chakrabarty P, Levites Y, Wang X, Darnmer EB, Srivastava G, Mukherjee S, Sieberts SK, Omberg L, Dang KD, Eddy JA, Snyder H, Chae Y, Amberkar S, Wei W, Hide W, Preuss C, Ergun A, Ebert PJ, Airey DC, Mostafavi S, Yu L, Klein H-U, Accelerating Medicines Partnership-Alzheimer’s Disease Consortium, Carter, Collier GW, Golde DA, Levey TE, Bennett AI, Estrada DA, Townsend K, Zhang TM, Schadt B, De Jager E, Price PL, Ertekin-Taner ND, Liu N, Shulman Z, Mangravite JM, Logsdon LM, B. A, 2020. Meta-analysis of the Alzheimer’s disease human brain transcriptome and functional dissection in mouse models. Cell Rep. 32, 107908 10.1016/j.celrep.2020.107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li H, Wang H, Wang J-H, Song F, Sun Y, 2018. Irisin exerts neuroprotective effects on cultured neurons by regulating astrocytes. Mediat. Inflamm 2018, 9070341. 10.1155/2018/9070341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Holsinger RMD, 2018. Exercise-induced brain-derived neurotrophic factor expression: therapeutic implications for Alzheimer’s dementia. Ageing Res Rev. 48, 109–121. 10.1016/j.arr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Wang S, Colonna M, 2019. Microglia in Alzheimer’s disease: a target for immunotherapy. J. Leukoc. Biol 106, 219–227. 10.1002/JLB.MR0818-319R. [DOI] [PubMed] [Google Scholar]
- Wen AY, Sakamoto KM, Miller LS, 2010. The role of the transcription factor CREB in immune function. J. Immunol 1 185 (11), 6413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, 2015. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 1, 55–61. 10.3233/BPL-150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM, 2013. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659. 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Jiang Q, McDermott J, Han J-DJ, 2018. Aging and Alzheimer’s disease: comparison and associations from molecular to system level. Aging Cell 17, e12802. 10.1111/acel.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X-Q, Geng Z, Zhou B, Zhang F, Han Y, Zhou Y-B, Wang J-J, Gao X-Y, Chen Q, Li Y-H, Kang Y-M, Zhu G-Q, 2018. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism 83, 31–41. 10.1016/j.metabol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Xu L, Long J, Su Z, Xu B, Lin M, Chen Y, Long D, 2019. Restored presynaptic synaptophysin and cholinergic inputs contribute to the protective effects of physical running on spatial memory in aged mice. Neurobiol. Dis 132, 104586 10.1016/j.nbd.2019.104586. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang W, 2016. Can irisin be a linker between physical activity and brain function. Biomol. Concepts 7, 253–258. 10.1515/bmc-2016-0012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang L-J, Tang D, 2014. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 63, 514–525. 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- Zhao N, Yan Q-W, Xia J, Zhang X-L, Li B-X, Yin L-Y, Xu B, 2020. Treadmill exercise attenuates Aβ-induced mitochondrial dysfunction and enhances mitophagy activity in APP/PS1 transgenic mice. Neurochem. Res 45, 1202–1214. 10.1007/s11064-020-03003-4. [DOI] [PubMed] [Google Scholar]


