Abstract
Background
Cutaneous melanoma is amongst the most aggressive of all skin cancers. Neoadjuvant treatment is a form of induction therapy, given to shrink a cancerous tumour prior to the main treatment (usually surgery). The purpose is to improve survival and surgical outcomes. This review systematically appraises the literature investigating the use of neoadjuvant treatment for stage III and IV cutaneous melanoma.
Objectives
To assess the effects of neoadjuvant treatment in adults with stage III or stage IV melanoma according to the seventh edition American Joint Committee on Cancer (AJCC) staging system.
Search methods
We searched the following databases up to 10 August 2021 inclusive: Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, LILACS and four trials registers, together with reference checking and contact with study authors to identify additional studies. We also handsearched proceedings from specific conferences from 2016 to 2020 inclusive.
Selection criteria
Randomised controlled trials (RCTs) of people with stage III and IV melanoma, comparing neoadjuvant treatment strategies (using targeted treatments, immunotherapies, radiotherapy, topical treatments or chemotherapy) with any of these agents or current standard of care (SOC), were eligible for inclusion.
Data collection and analysis
We used standard Cochrane methods. Primary outcomes were overall survival (OS) and adverse effects (AEs). Secondary outcomes included time to recurrence (TTR), quality of life (QOL), and overall response rate (ORR). We used GRADE to evaluate the certainty of the evidence.
Main results
We included eight RCTs involving 402 participants. Studies enrolled adults, mostly with stage III melanoma, investigated immunotherapies, chemotherapy, or targeted treatments, and compared these with surgical excision with or without adjuvant treatment. Duration of follow‐up and therapeutic regimens varied, which, combined with heterogeneity in the population and definitions of the endpoints, precluded meta‐analysis of all identified studies. We performed a meta‐analysis including three studies.
We are very uncertain if neoadjuvant treatment increases OS when compared to no neoadjuvant treatment (hazard ratio (HR) 0.43, 95% confidence interval (CI) 0.15 to 1.21; 2 studies, 171 participants; very low‐certainty evidence). Neoadjuvant treatment may increase the rate of AEs, but the evidence is very uncertain (26% versus 16%, risk ratio (RR) 1.58, 95% CI 0.97 to 2.55; 2 studies, 162 participants; very low‐certainty evidence). We are very uncertain if neoadjuvant treatment increases TTR (HR 0.51, 95% CI 0.22 to 1.17; 2 studies, 171 participants; very low‐certainty evidence). Studies did not report ORR as a comparative outcome or measure QOL data.
We are very uncertain whether neoadjuvant targeted treatment with dabrafenib and trametinib increases OS (HR 0.28, 95% CI 0.03 to 2.25; 1 study, 21 participants; very low‐certainty evidence) or TTR (HR 0.02, 95% CI 0.00 to 0.22; 1 study, 21 participants; very low‐certainty evidence) when compared to surgery. The study did not report comparative rates of AEs and overall response, and did not measure QOL.
We are very uncertain if neoadjuvant immunotherapy with talimogene laherparepvec increases OS when compared to no neoadjuvant treatment (HR 0.49, 95% CI 0.15 to 1.64; 1 study, 150 participants, very low‐certainty evidence). It may have a higher rate of AEs, but the evidence is very uncertain (16.5% versus 5.8%, RR 2.84, 95% CI 0.96 to 8.37; 1 study, 142 participants; very low‐certainty evidence). We are very uncertain if it increases TTR (HR 0.75, 95% CI 0.31 to 1.79; 1 study, 150 participants; very low‐certainty evidence). The study did not report comparative ORRs or measure QOL.
OS was not reported for neoadjuvant immunotherapy (combined ipilimumab and nivolumab) when compared to the combination of ipilimumab and nivolumab as adjuvant treatment. There may be little or no difference in the rate of AEs between these treatments (9%, RR 1.0, 95% CI 0.75 to 1.34; 1 study, 20 participants; low‐certainty evidence). The study did not report comparative ORRs or measure TTR and QOL.
Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) likely results in little to no difference in OS when compared to neoadjuvant nivolumab monotherapy (P = 0.18; 1 study, 23 participants; moderate‐certainty evidence). It may increase the rate of AEs, but the certainty of this evidence is very low (72.8% versus 8.3%, RR 8.73, 95% CI 1.29 to 59; 1 study, 23 participants); this trial was halted early due to observation of disease progression preventing surgical resection in the monotherapy arm and the high rate of treatment‐related AEs in the combination arm. Neoadjuvant combination treatment may lead to higher ORR, but the evidence is very uncertain (72.8% versus 25%, RR 2.91, 95% CI 1.02 to 8.27; 1 study, 23 participants; very low‐certainty evidence). It likely results in little to no difference in TTR (P = 0.19; 1 study, 23 participants; low‐certainty evidence). The study did not measure QOL.
OS was not reported for neoadjuvant immunotherapy (combined ipilimumab and nivolumab) when compared to neoadjuvant sequential immunotherapy (ipilimumab then nivolumab). Only Grade 3 to 4 immune‐related AEs were reported; fewer were reported with combination treatment, and the sequential treatment arm closed early due to a high incidence of severe AEs. The neoadjuvant combination likely results in a higher ORR compared to sequential neoadjuvant treatment (60.1% versus 42.3%, RR 1.42, 95% CI 0.87 to 2.32; 1 study, 86 participants; low‐certainty evidence). The study did not measure TTR and QOL.
No data were reported on OS, AEs, TTR, or QOL for the comparison of neoadjuvant interferon (HDI) plus chemotherapy versus neoadjuvant chemotherapy. Neoadjuvant HDI plus chemotherapy may have little to no effect on ORR, but the evidence is very uncertain (33% versus 22%, RR 1.75, 95% CI 0.62 to 4.95; 1 study, 36 participants; very low‐certainty evidence).
Authors' conclusions
We are uncertain if neoadjuvant treatment increases OS or TTR compared with no neoadjuvant treatment, and it may be associated with a slightly higher rate of AEs. There is insufficient evidence to support the use of neoadjuvant treatment in clinical practice. Priorities for research include the development of a core outcome set for neoadjuvant trials that are adequately powered, with validation of pathological and radiological responses as intermediate endpoints, to investigate the relative benefits of neoadjuvant treatment compared with adjuvant treatment with immunotherapies or targeted therapies.
Keywords: Adult; Humans; Antineoplastic Agents; Antineoplastic Agents/adverse effects; Ipilimumab; Melanoma; Melanoma/drug therapy; Melanoma/pathology; Melanoma, Cutaneous Malignant; Neoplasm Staging; Nivolumab; Randomized Controlled Trials as Topic; Skin Neoplasms; Skin Neoplasms/drug therapy; Skin Neoplasms/pathology
Plain language summary
What are the benefits and risks of neoadjuvant treatment (drug treatment prior to surgery to remove a tumour) for melanoma, a type of skin cancer?
What did we want to find out?
Cutaneous melanoma is a very aggressive form of skin cancer. It is generally fatal if detected at an advanced stage. Earlier treatment may allow for surgical removal of the tumour and an improved chance of long‐term survival. Neoadjuvant treatment is drug treatment administered before surgery, to reduce the tumour size so that it is easier to remove, to reduce complications of surgery, and to reduce the risk of spread of the disease. New drug types, immunotherapies and targeted treatments, have been developed which may be effective for neoadjuvant use.
We wanted to find out if neoadjuvant treatment of stage III or IV melanoma helps people live longer, and to compare adverse (unwanted) effects with neoadjuvant treatment and routine care.
What did we do?
We searched the medical literature for randomised controlled trials that compared certain types of treatments for melanoma skin cancer. The types of treatment included are:
‐ targeted treatments ‐ such as dabrafenib and trametinib;
‐ immunotherapies ‐ such as ipilimumab and nivolumab;
‐ chemotherapy ‐ such as dacarbazine and temozolomide;
‐ topical treatments ‐ such as imiquimod;
‐ radiotherapy.
We considered both single‐drug and combination‐drug treatments. We described and compared the results from these studies, taking into account the differences between the studies.
What did we find?
We identified eight randomised controlled trials that included 402 adults. The majority of people had stage III melanoma and were treated in hospital. Most studies used immunotherapies or targeted treatments, and compared these with surgery, with or without adjuvant treatment (treatment given after surgery to remove the tumour, to reduce the risk of the tumour coming back). No studies considered the impact of treatment on quality of life, and most studies did not compare tumour response rates after different treatments.
We are uncertain whether neoadjuvant treatment helps people live longer when compared with no neoadjuvant treatment. It may lead to more adverse events, and we are uncertain if it increases the time until the tumour comes back.
We are uncertain whether neoadjuvant targeted treatment with dabrafenib and trametinib helps people live longer, compared with no neoadjuvant treatment, or if it can increase the time until the tumour comes back. The study did not compare safety outcomes with each treatment.
We are uncertain if neoadjuvant immunotherapy with talimogene laherparepvec (T‐VEC) helps people live longer when compared with no neoadjuvant treatment. It may lead to more adverse events. We are uncertain if it increases the time until the tumour comes back.
No data were reported on whether neoadjuvant immunotherapy with combined ipilimumab and nivolumab helps people live longer, when compared with adjuvant (treatment given only after surgery) combined ipilimumab and nivolumab. There may be little or no difference in the rate of adverse events. No data were reported on whether neoadjuvant immunotherapy with combined ipilimumab and nivolumab increases the time until the tumour comes back.
Neoadjuvant combination of ipilimumab and nivolumab likely results in little or no difference in how long people live, when compared with neoadjuvant nivolumab. It may increase the rate of adverse events, but our confidence in the evidence is very low. It is worth noting that this trial was stopped early as patients in the neoadjuvant nivolumab arm may not be able to receive surgery due to disease progression and also because of a high rate of treatment‐related adverse events in the combination treatment arm. Combination treatment may lead to higher tumour response rates, but our confidence in the evidence is very low. The time until the tumour comes back may not be different.
No data were available on whether neoadjuvant immunotherapy with combined ipilimumab and nivolumab helps people live longer, when compared with neoadjuvant sequential treatment with ipilimumab and nivolumab. It likely results in fewer adverse events compared to sequential treatment, and may result in higher tumour response rates. The sequential treatment arm of the trial stopped recruiting patients due to a high incidence of severe AEs. Data on the time taken for the tumour to return were not collected.
No data were reported on whether neoadjuvant high‐dose interferon plus chemotherapy, when compared to neoadjuvant chemotherapy, can help people live longer, increase the time taken for the tumour to reoccur, reduce adverse events, or impact quality of life. It may have little to no effect on tumour response rates.
What does this mean?
We are uncertain if neoadjuvant treatment of stage III or IV melanoma will help people to live longer, or to have more time before the disease recurs. We are also uncertain if the benefits of neoadjuvant treatment outweigh the risks of adverse events.
How up to date is this evidence?
The evidence is up to date to August 2021.
Summary of findings
Summary of findings 1. Neoadjuvant treatment compared to no neoadjuvant treatment.
| Neoadjuvant treatment compared to no neoadjuvant treatment | |||||||
| Patient or population: stage III or IV cutaneous melanoma Setting: hospital Intervention: neoadjuvant treatment Comparison: surgery with or without adjuvant treatment | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with surgery +/‐ adjuvant treatment | Risk with neoadjuvant treatment | ||||||
| Overall survival (OS), measured by the number of deaths over time Median follow‐up: 18.6 to 24 months |
Study population | HR 0.43 (0.15 to 1.21) | 171 (2 RCTsa) | ⊕⊝⊝⊝ Very low b,c | |||
| 100 per 1000 | 44 per 1000 (16 to 120) | ||||||
| Adverse events, assessed with: CTCAE criteria v4 Median follow‐up: 2 to 4 years |
Study population | RR 1.58, 95% CI 0.97 to 2.55 | 162 (2 RCTsd) | ⊕⊝⊝⊝ Very low b,c,e | |||
| 165 per 1000 | 260 per 1000 (160 to 420) | ||||||
| Overall response rate (ORR), assessed with: radiological assessment using RECIST v1.1 criteria | This outcome was not reported as a comparative outcome as it was measured only in the neoadjuvant arm. | ‐ | ‐ | ‐ | |||
| Time to recurrence (TTR),measured by the number of disease recurrence events over time. Assessed with radiological assessment using RECIST v1.1 criteria Median follow‐up: 18.6 to 24 months |
Study population | HR 0.51 (0.22 to 1.17) | 171 (2 RCTsa) | ⊕⊝⊝⊝ Very low b,c,e | |||
| 400 per 1000 | 229 per 1000 (106 to 450) | ||||||
| Quality of life ‐ not measured | This outcome was not measured for this comparison. | ‐ | ‐ | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived as follows: Assumed risk in the control population: 1 year OS rate = 90% (Balch 2009). Assumed risk in the control population: 1 year TTR rate = 60% (Eggermont 2021). Assumed risk in the control population: toxicity rate across control arms in the included trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; HR: hazard ratio; RECIST: response evaluation criteria in solid tumours; RCT: randomised controlled trial; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aMeta‐analysis of outcomes from Amaria 2018a and Dummer 2020b. bDowngraded one level for risk of bias for high risk of performance and detection bias in both trials, and one level for risk of publication bias, as results for the largest trial are only published as abstracts to date, and in a clinical trials database; no peer reviewed publication is available. cDowngraded one level for imprecision due to the small number of events and wide confidence intervals. dMeta‐analysis of outcomes from Blank 2018 and Dummer 2020b. eDowngraded one level for inconsistency (between‐study heterogeneity).
Summary of findings 2. Neoadjuvant targeted treatment (BRAF/MEK inhibition) compared to no neoadjuvant treatment.
| Neoadjuvant BRAF/MEK inhibition compared to no neoadjuvant treatment | ||||||
| Patient or population: stage III or IV cutaneous melanoma Setting: hospital Intervention: neoadjuvant BRAF/MEK inhibition Comparison: surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with surgery | Risk with Neoadjuvant BRAF/MEK inhibition | |||||
| Overall survival (OS), measured by number of deaths over time, Median follow‐up: 18.6 months | Study population | HR 0.28 (0.03 to 2.25) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 100 per 1000 | 29 per 1000 (3 to 211) | |||||
| Adverse events, assessed with: CTCAE criteria v4 Follow‐up: duration of treatment, total of 52 weeks of treatment | The overall incidence of Grade 3 and 4 adverse events was not reported. Eight treatment related Grade 3 adverse events occurred in the neoadjuvant dabrafenib and trametinib arm. | ‐ | 14 (1 RCT) |
⊕⊕⊝⊝ LOWc | Number of participants reflects those recruited to the neoadjuvant treatment arm only. | |
| Overall response rate (ORR), assessed with: radiological assessment using RECIST v1.1 criteria Follow‐up: 8 weeks | This outcome was assessed only in the neoadjuvant arm, and there are no comparative results. The rate of overall response (complete response and partial response) in the neoadjuvant arm was 85% (response‐evaluable population). | ‐ | 13 (1 RCT) |
⊕⊕⊝⊝ LOWd,e | Number of participants reflects those recruited to the neoadjuvant treatment arm only. One person was excluded as they withdrew consent prior to commencing treatment. | |
| Time to recurrence (TTR), measured by the number of disease recurrence events over time, assessed with: radiological assessment using RECIST v1.1 criteria Median follow‐up: 18.6 months | Study population | HR 0.02 (0.00 to 0.22) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,e | TTR was described as event free survival in the trial publication. | |
| 400 per 1000 | 10 per 1000 (0 to 106) | |||||
| Quality of Life ‐ not measured | This outcome was not measured for this comparison. | ‐ | ‐ | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived as follows: Assumed risk in the control population: 1 year OS rate = 90% (Balch 2009). Assumed risk in the control population: 1 year TTR rate = 60% (Eggermont 2021). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; HR: hazard ratio, RECIST: Response evaluation criteria in solid tumours; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels for risk of bias, as risk of performance and detection bias were high for this outcome, and risk of other bias was high, due to early cessation of the trial based on an unplanned interim analysis where the prespecified criteria for early discontinuation were not met. Differences in adjuvant treatment options between treatment arms further confound outcomes.
bDowngraded one level for imprecision, as the number of events was small and the confidence intervals wide.
cDowngraded two levels for risk of bias: one level as risk of performance and detection bias were high for this outcome, and a second level for risk of other bias as adverse events in the control arm were not recorded. dDowngraded one level for risk of bias, as risk of detection bias was considered high for this outcome. eDowngraded one level for imprecision, as the number of events was small.
Summary of findings 3. Neoadjuvant immunotherapy (talimogene laherparepvec) compared to no neoadjuvant treatment.
| Neoadjuvant talimogene laherparepvec compared to no neoadjuvant treatment | |||||||
| Patient or population: stage III and IV cutaneous melanoma Setting: hospital Intervention: neoadjuvant talimogene laherparepvec Comparison: surgery | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with surgery | Risk with Neoadjuvant talimogene laherparepvec | ||||||
| Overall survival (OS), measured by the number of deaths over time, Follow‐up: 24 months | Study population | HR 0.49 (0.15 to 1.64) | 150 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | |||
| 100 per 1000 | 50 per 1000 (16 to 159) | ||||||
| Adverse Events, assessed with: CTCAE v4.0 Follow‐up: 24 months | Study population | RR 2.84 (0.96 to 8.37) | 142 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,d,e | |||
| 58 per 1000 | 165 per 1000 (57 to 391) | ||||||
| Overall response rate (ORR), assessed with: radiological response according to RECIST v1.1 Follow‐up: 13 to 18 weeks | This outcome was assessed only in the neoadjuvant treatment arm, and there are no comparative results. The rate of overall response (complete response and partial response) in the neoadjuvant arm was 13.2% (80% CI 8.3 to 19.5). | ‐ | 76 (1 RCT) |
⊕⊝⊝⊝ VERY LOWa,b,d | Number of participants reflects those recruited to the neoadjuvant treatment arm only. | ||
| Time to recurrence (TTR), measured by the number of disease recurrence events over time, assessed with: radiological assessment using RECIST v1.1 Follow‐up: 24 months | Study population | HR 0.75 (0.31 to 1.79) | 150 (1 RCT) |
⊕⊝⊝⊝ VERY LOWa,c,e | |||
| 400 per 1000 | 318 per 1000 (146 to 599) | ||||||
| Quality of Life ‐ not measured | This outcome was not measured for this comparison. | ‐ | ‐ | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived as follows: Assumed risk in the control population: 1 year OS rate = 90% (Balch 2009). Assumed risk in the control population: 1 year TTR rate = 60% (Eggermont 2021). Assumed risk in the control population: toxicity rate across control arms in the included trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common terminology criteria for adverse events; HR: hazard ratio; RECIST: Response evaluation criteria in solid tumours; RCT: randomised controlled trial; RR: Risk ratio; | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
aDowngraded one level for risk of publication bias, as results only published as abstracts to date, and in a clinical trials database; no peer reviewed publication is available. bDowngraded one level for imprecision as the number of events was small. c Downgraded one level for risk of bias due to high risk of performance and detection bias. Differences in adjuvant treatment options between treatment arms further confounds outcomes. d Downgraded one level for risk of bias due to high risk of detection bias. e Downgraded one level for imprecision as confidence intervals are wide.
Summary of findings 4. Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to adjuvant immunotherapy (combined ipilimumab and nivolumab).
| Neoadjuvant combined ipilimumab and nivolumab compared to adjuvant ipilimumab and nivolumab | ||||||
| Patient or population: stage III cutaneous melanoma Setting: hospital Intervention: neoadjuvant ipilimumab plus nivolumab Comparison: adjuvant ipilimumab plus nivolumab | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with adjuvant ipilimumab plus nivolumab | Risk with neoadjuvant ipilimumab plus nivolumab | |||||
| Overall survival (OS) | This outcome was not reported for this comparison. | ‐ | ‐ | ‐ | ||
| Adverse events, assessed with: CTCAE criteria Median follow‐up: 25.6 months | Study population | RR 1.00 (0.75 to 1.34) | 20 (1 RCT) | ⊕⊕⊝⊝ LOW a,b | ||
| 900 per 1000 | 900 per 1000 (675 to 1000) | |||||
| Overall response rate (ORR), assessed with: radiological assessment according to RECIST v1.1 Follow‐up: 6 weeks | This outcome was assessed only in the neoadjuvant treatment arm, and there are no comparative results. The rate of overall response (complete response and partial response) in the neoadjuvant arm was 40%. | ‐ | 10 (1 RCT) | ⊕⊕⊝⊝ LOWc,d | Number of participants reflects those recruited to the neoadjuvant treatment arm only. | |
| Time to recurrence ‐ not measured | This outcome was not measured for this comparison. | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not measured | This outcome was not measured for this comparison. | ‐ | ‐ | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived from the toxicity rate across control arms in the included trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common terminology criteria for adverse events; RECIST: Response evaluation criteria in solid tumours; RCT: randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias, as risk of performance bias and detection bias were considered high for this outcome. bDowngraded one level for imprecision as confidence interval were wide and fail to exclude important benefit or important harm. cDowngraded one level due to risk of bias, as risk of detection bias was considered high for this outcome. dDowngraded one level due to imprecision, as the number of events was small.
Summary of findings 5. Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (nivolumab).
| Neoadjuvant combined ipilimumab and nivolumab compared to neoadjuvant nivolumab | ||||||
| Patient or population: stage III and IV cutaneous melanoma Setting: hospital Intervention: neoadjuvant ipilimumab combined with nivolumab, with adjuvant nivolumab Comparison: neoadjuvant and adjuvant nivolumab | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with neoadjuvant nivolumab | Risk with neoadjuvant ipilimumab plus nivolumab | |||||
| Overall survival (OS), measured by number of deaths over time Median follow‐up: 15 months | No difference was seen in OS between the treatment arms (P = 0.18). No HR or absolute number of events were reported, and it was not possible to extract data reliably from the published Kaplan Meier curves. | ‐ | 23 (1 RCT) |
⊕⊕⊕⊝ MODERATEa | ||
| Adverse events, assessed with: CTCAE criteria Median follow‐up: 15 months | Study population | RR 8.73 (1.29 to 59.00) | 23 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Trial was discontinued early, due to an observation of disease progression preventing surgical resection in the PD‐1 monotherapy arm, and a high rate of grade 3 TRAES in the combination arm | |
| 83 per 1000 | 728 per 1000 (108 to 1000) | |||||
| Overall response rate (ORR), assessed with: radiological response according to RECIST v1.1 Follow‐up: up to 12 weeks | Study population | RR 2.91 (1.02 to 8.27) | 23 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 250 per 1000 | 728 per 1000 (255 to 1000) | |||||
| Time to recurrence (TTR), measured by the number of disease recurrence events over time, assessed with: RECIST v.1 Median follow‐up: 15 months | No difference was seen in TTR between treatment arms (P = 0.19). No HR or absolute numbers of events were reported, and it was not possible to extract data reliably from published Kaplan Meier curves. | ‐ | 23 (1 RCT) |
⊕⊕⊝⊝ LOWa,b | TTR was described as progression‐free survival in the trial publication. | |
| Quality of life ‐ not measured | This outcome was not measured for this comparison. | ‐ | ‐ | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived as follows: Assumed risk in the control population: toxicity rate across control arms in the included trials. Assumed risk in the control population: response rate across control arms in the included trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common terminology criteria for adverse events; PD‐1: programmed death‐1; RCT: randomised controlled trial; RECIST: response evaluation criteria in solid tumours; RR: risk ratio; TRAEs: treatment related adverse events. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for imprecision as the number of events was small. bDowngraded one level for risk of bias, as the risk of performance bias and/or detection bias were considered high for this outcome. cDowngraded one level for imprecision as the confidence intervals were wide.
Summary of findings 6. Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (sequential treatment with ipilimumab then nivolumab).
| Neoadjuvant combined ipilimumab and nivolumab (two different dosing regimens) compared to neoadjuvant sequential ipilimumab then nivolumab | ||||||
| Patient or population: stage III cutaneous melanoma Setting: hospital Intervention: neoadjuvant ipilimumab plus nivolumab (two different dosing regimens) Comparison: neoadjuvant sequential ipilimumab then nivolumab | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sequential ipilimumab then nivolumab | Risk with neoadjuvant ipilimumab plus nivolumab (two different dosing regimens) | |||||
| Overall survival (OS) | This outcome was not reported for this comparison. | ‐ | ‐ | ‐ | ||
| Adverse events, assessed with: CTCAE Median follow‐up: 32 months | The overall incidence of Grade 3 and 4 adverse events was not reported. Immune‐related Grade 3 to 4 adverse events at 12 weeks were reported in 40% of participants treated with nivo1ipi3, in 20% of participants treated with nivo3ipi1 and in 50% of participants in the sequential treatment arm. The sequential treatment arm closed early due to a high incidence of severe adverse events. | ‐ | 86 (1 RCT) | ⊕⊕⊝⊝ LOW a,b | ||
| Overall response rate (ORR), assessed with: radiological assessment according to RECIST v1.1 Follow‐up: 6 weeks | Study population | RR 1.42 (0.87 to 2.32) | 86 (1 RCT) | ⊕⊕⊝⊝ LOW c,d | ||
| 423 per 1000 | 601 per 1000 (368 to 982) | |||||
| Time to response ‐ not measured | This outcome was not measured for this comparison | ‐ | ‐ | ‐ | ||
| Quality of life ‐ not measured | This outcome was not measured for this comparison. | ‐ | ‐ | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived as follows: Assumed risk in the control population: toxicity rate across control arms in the included trials. Assumed risk in the control population: response rate across control arms in the included trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common terminology criteria for adverse events; Nivo1ipi3: Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg; Nivo3ipi1: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg; RECIST: Response evaluation criteria in solid tumours; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias, as risk of performance bias and detection bias was considered high for this outcome. bDowngraded one level for risk of bias as only immune‐related adverse events reported. cDowngraded one level for imprecision as confidence intervals are wide. dDowngraded one level due to risk of bias, as risk of detection bias was considered high for this outcome.
Summary of findings 7. Neoadjuvant immunotherapy (high dose interferon) plus chemotherapy compared to neoadjuvant chemotherapy.
| Neoadjuvant chemotherapy and interferon compared to neoadjuvant chemotherapy | ||||||
| Patient or population: stage III and IV cutaneous melanoma Setting: hospital Intervention: neoadjuvant chemotherapy and interferon Comparison: neoadjuvant chemotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy alone | Risk with neoadjuvant interferon plus chemotherapy | |||||
| Overall survival (OS) | This outcome was not reported for this comparison. | — | — | — | ||
| Adverse events | This outcome was not reported for this comparison. | — | — | — | ||
| Overall response rate (ORR), measured after 8 weeks of treatment and prior to surgery. | Study population | RR 1.75 (0.62 to 4.95) | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a,b,c | The definition of ORR in this trial included complete, partial responses and stable disease, so is not directly comparable with other ORR outcomes in this review. | |
| 222 per 1000 | 333 per 1000 (150 to 586) | |||||
| Time to recurrence — not reported | This outcome was not reported for this comparison. | — | — | — | ||
| Quality of life — not reported | This outcome was not reported for this comparison. | — | — | — | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is derived as follows: Assumed risk in the control population: response rate across control arms in the included trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to serious risk of bias, as the risk of detection bias was rated high for this outcome. bDowngraded one level due to serious risk of bias, as the risk of selective reporting and publication bias were rated high for this outcome. cDowngraded one level for imprecision, as confidence intervals are very wide.
Background
A glossary of the terms used is provided in Appendix 1.
Description of the condition
Cutaneous melanoma is amongst the most aggressive of all skin cancers (Garbe 2016). It is a type of skin cancer originating in the melanin‐producing melanocytes, which are found between the outer layer of the skin (the epidermis) and the layer beneath (the dermis) (Garbe 2016). It typically presents in distinctive subtypes, such as superficial spreading melanoma, nodular melanoma, and acral lentiginous melanoma (present on acral surfaces such as the sole, and occurs more commonly in populations at low‐risk for non‐acral melanomas such as Asian and African populations).
Melanocytes become cancerous as a result of unrepaired DNA damage or other genetic alterations, or both (Curtin 2005; Eggermont 2014). Genetic and environmental factors which increase the risk of melanoma include exposure to sunlight and ultraviolet (UV) radiation; a high number of moles (naevi); fair skin; age; family history; and a history of previous melanoma (Whiteman 2011).
Cutaneous melanoma occurs mainly in European, Oceanic and North American populations, accounting for almost 82% of the global incidence, and almost 64% of the mortality related to the disease (Ferlay 2015). In 2020, there was a global incidence of 324,635 cases (1.7% of total cancer cases) and 57,043 deaths (0.6% of total cancer mortality) (Sung 2021). The incidence of cutaneous melanoma is increasing, and the death rate is declining at a lower rate than for many other neoplasms (Global Burden of Disease 2016). Incidence and mortality are higher in men than women (Sung 2021).
Dermoscopy is commonly used to diagnose melanoma, while histopathology assessments together with clinical and radiological examination are used to stage the disease; sentinel node biopsy is used for staging higher‐risk melanomas (Garbe 2016; Michielin 2019). Melanoma is staged according to the American Joint Committee on Cancer (AJCC) Melanoma Staging criteria. This review uses the seventh edition of these staging criteria (AJCC 2011); the eighth edition was published subsequent to the review protocol (AJCC 2017). In stage 0 melanoma (in situ melanoma), the abnormal melanocytes have not started to spread into deeper layers. In stages I and II melanoma, invasive cancer has formed, but there is no spread to lymph nodes or distant sites. With stage III melanoma, the melanoma has spread to the lymph nodes or lymphatic channels, and it may or may not be ulcerated. In stage IV melanoma, cancer has spread elsewhere in the body, with the brain, lung, liver, distant lymph nodes and other areas of the skin being the most common places of metastasis. The different stages are further subdivided based on prognostic variables into categories A, B, or C, e.g. IIIA, IIIB, IIIC. Prognostic variables in melanoma include Breslow depth, ulceration, mitotic rate, number and site of distant metastases, and serum lactate dehydrogenase (LDH) levels (Balch 2009). Tumour‐infiltrating lymphocytes (white blood cells that migrate into a tumour and help kill tumour cells as part of the host immune response to cancer) have been identified as potential prognostic factors in melanoma (Thomas 2013). Molecular characterisation of the tumour is recommended for stage IIC, III and IV melanomas (Michielin 2019). Ten‐year survival ranges from 93% for stage IA to 39% for stage IIC. At stage III, five‐year survival rates range from 78% to 40%, while for stage IV disease it ranges from 10% to 25%, dependent on LDH levels (Balch 2009). Melanoma‐specific survival is expected to increase across all the more severe disease stages with the widespread adoption of effective immunotherapies; whilst stage‐specific survival data is not yet available for people with stage IIIC and IV melanoma, data from the Surveillance, Epidemiology and End Results Program (SEER) in the USA indicates that the death rate from melanoma has declined since 2014 (SEER 2022).
Melanoma tumours have a high mutational load, due to the combination of driver genetic mutations and continuous exposure to the carcinogen, UV radiation (Curtin 2005). Currently, available treatments target the BRAF mutation, and research is ongoing to identify therapeutic agents which target the other mutations (Posch 2013). The high mutational load and immunogenicity of melanoma tumours contributed to the early investigation and use of checkpoint inhibitors for this disease (Postow 2015).
Description of the intervention
Neoadjuvant treatment is a form of induction therapy, given as a first step to shrink a cancerous tumour prior to the main treatment, which is generally surgery (NCI 2021). The aim is to improve survival outcomes, reduce surgical morbidity and improve patient outcomes (Tahrini 2011). It also allows for the provision of 'real‐time' information on tumour behaviour in response to systemic treatment, useful for translational analyses and potentially more personalised treatment regimens. Neoadjuvant treatment is generally administered for a preplanned, fixed period of time prior to a surgical procedure; optional adjuvant treatment can be used in the postoperative period. Numerous therapeutic approaches can be used, including cytotoxic chemotherapy, radiation therapy, topical agents, immunotherapies, and targeted treatments. These approaches work by diverse pharmacological and physiological mechanisms to reduce tumour volume.
Neoadjuvant treatment regimens are not included in the current European Society of Medical Oncology (ESMO) treatment guidelines for cutaneous melanoma (Michielin 2019). The National Comprehensive Cancer Network (NCCN) guidelines recommend that people with extensive resectable disease at very high risk of recurrence, or where there is uncertainty regarding the resectability of nodal disease, undergo assessment by a multidisciplinary tumour board and be considered for neoadjuvant systemic therapy, preferably in the context of a clinical trial (NCCN 2021). These guidelines note that there is currently insufficient data to recommend any specific agent as neoadjuvant therapy for melanoma. Neoadjuvant treatment is included as a treatment option for distant metastases in the European Consensus Guidelines 2016 issued by the European Organisation for Research and Treatment of Cancer (EORTC) and the European Association of Dermato‐Oncology (EADO) (Garbe 2016), referencing research using neoadjuvant treatment with high‐dose interferon (HDI) (Moschos 2006).
How the intervention might work
Many therapeutic approaches have been investigated for their utility as neoadjuvant treatments for melanoma. The effect of neoadjuvant treatment for melanoma may operate through an immuno‐modulatory effect, rather than a direct anti‐tumour effect (Johnson 2015a; Moschos 2006). Immunotherapies and targeted treatments have demonstrated survival benefits in stage III and IV melanoma; their usefulness is somewhat tempered by a proportion of non‐responders, and the development of tumour resistance over time (Johnson 2015; Zhao 2017). The therapeutic hypothesis for neoadjuvant therapy is that use of these agents for earlier stages of the disease, before changes in the tumour microenviornment facilitating immune evasion occur, may lead to greater treatment benefits in a larger proportion of people (Braeuer 2014; Davar 2013).
Chemotherapy
Cytotoxic chemotherapy was previously the mainstay of systemic treatment for stage IIIC and IV cutaneous melanoma. Many regimens have been investigated, with varying impact on clinical outcomes, but no regimen demonstrated an improvement in overall survival (OS) (Pasquali 2018). Dacarbazine, an alkylating agent (Lexicomp, 20th Ed), works by disrupting the DNA replication mechanisms of the tumour. It has been investigated as monotherapy for stage IIIC and IV disease, as well as in neoadjuvant and adjuvant treatment strategies for earlier‐stage melanoma (Buzaid 1998; Kim 2009). It has now largely been displaced by newer agents, but may be used in palliative chemotherapy, and as treatment in countries where newer treatments are not available or not reimbursed. Temozolomide is an oral analogue of dacarbazine, with demonstrated non‐inferiority to dacarbazine (Middleton 2000; Patel 2011). Combination chemotherapy regimens targeting multiple mechanisms of cell growth and replication have also been investigated, using agents including the vinca alkaloids, such as vindesine and vinblastine (inhibitors of microtubular assembly), taxanes such as paclitaxel (inhibitors of microtubule disassembly), platinum analogues such as cisplatin or carboplatin (alkylating agents), and nitrosoureas such as lomustine, carmustine and fotemustine (alkylating agents) (Bhatia 2009). Compared to monotherapy, combination regimens are associated with an increase in toxicity, a slightly higher response rate, and no significant improvement in OS (Pasquali 2018).
Immunotherapy
Interleukin‐2 (IL‐2) and interferon alpha (IFN‐alpha) were amongst the earliest immunotherapies used in clinical practice for the management of stage III and IV melanoma (Kirkwood 2012); both have fallen out of routine use due to the availability of less toxic and more efficacious agents. IFN‐alpha is authorised for adjuvant treatment of stage II and III melanoma, having demonstrated improvements in recurrence‐free survival (RFS), and potentially an increase in OS (Mocellin 2013, Najjar 2019). The greatest effect is likely seen in those with ulcerated melanomas with palpable nodes (Eggermont 2012; Eggermont 2020; Wheatley 2007). The mechanism of action of IFN‐alpha in melanoma is unknown, and is possibly linked to its immuno‐stimulatory effects on antigen‐presenting cells, leading to an increase in tumour‐infiltrating lymphocytes producing an innate immune response to the tumour (Heise 2016; Moschos 2006). IL‐2 has demonstrated improvements in clinical outcomes in a small proportion of people with advanced melanoma (approximately 10%), but severe toxicity and the absence of a biomarker to predict efficacy limits its use (Amaria 2015). The mechanism of action is unclear; it has a variety of effects at the tumour site, including stimulating the production of cytokines, increasing vascular permeability, and promoting the differentiation and proliferation of T lymphocytes.
Ipilimumab is a cytotoxic T‐lymphocyte associated protein 4 (CTLA‐4) inhibitor, which has shown survival benefits in advanced disease (Hodi 2010; Robert 2011), and also as an adjuvant treatment for stage III disease (Eggermont 2016). It exerts its effects via T cells, stimulating an immune response against the tumour; additional local actions may supplement this effect (Postow 2015). Pembrolizumab and nivolumab target the programmed death‐1 (PD‐1) pathway, disruption of which potentiates the T‐cell response to the tumour, and may influence other immune responses in B cells and natural killer cells (Postow 2015). CTLA‐4 and PD‐1 inhibitors are classified as checkpoint inhibitors, which are now the standard of care treatments for stage IIIC and IV melanoma. These agents are associated with improvements in survival outcomes, an immune‐related side‐effect profile, and durable responses in some people (Postow 2015). RCTs have demonstrated the synergistic effect of combined CTLA‐4/PD‐1 inhibition with ipilimumab and nivolumab in stage IIIC and IV melanoma (Larkin 2015; Wolchok 2017, Wolchok 2021, Hodi 2016). PD‐1 blockade with pembrolizumab or nivolumab is now considered standard of care as adjuvant therapy in people with stage III melanoma, which is at high risk of recurrence following surgical resection (Michielin 2019; NCCN 2021). It is associated with increases in recurrence free survival (RFS) compared with ipilimumab or placebo (Eggermont 2018; Weber 2017), although the impact on OS is unclear (Ascierto 2020). Pembrolizumab has recently been approved in the USA as adjuvant treatment for stage IIB or IIC melanoma following complete resection, based on an increase in RFS compared with placebo (Luke 2022).
Early research with granulocyte‐macrophage colony stimulating factor (GM‐CSF) showed some impact on disease response in advanced melanoma (Hoeller 2001; Ridolfi 2002; Si 1996), thought to be mediated by stimulation of dendritic cells to trigger a host immune response. This led to the development of talimogene laherparepvec, an oncolytic viral immunotherapy derived from herpes simplex virus‐1 (HSV‐1), which is designed to produce GM‐CSF intra‐lesionally (Andtbacka 2015). It has shown benefit compared to GM‐CSF in the treatment of regionally or distantly metastatic melanoma (stage IIIB, IIIC and IV) in the absence of visceral metastases and normal LDH levels (Kaufman 2014). Concomitant administration of sargramostim, a GM‐CSF‐secreting vaccine adjuvant, with ipilimumab also demonstrated improved treatment outcomes in a phase III RCT (Hodi 2014).
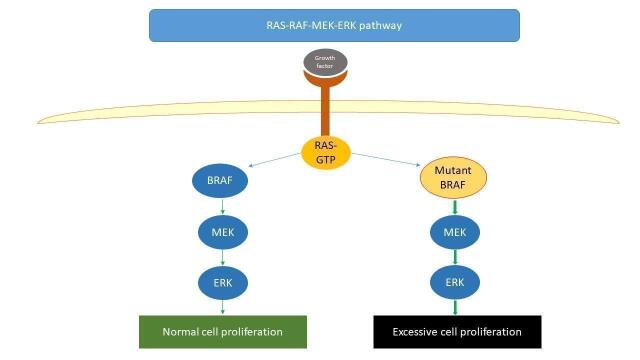
Targeted treatments
In approximately 50% of cutaneous melanomas there is a mutation in the BRAF gene, which causes cell proliferation and tumour growth (Eggermont 2014); inhibition of this effect can have a damaging effect on tumour growth (Figure 1, Davies 2002). BRAF inhibitors were first licensed in Europe in 2011 based on improvements in survival outcomes compared with dacarbazine (Hauschild 2012; McArthur 2014). Subsequently, MEK inhibitors were licensed for concomitant use with BRAF inhibitors, exhibiting a synergistic effect in prolonging progression‐free survival (PFS) and OS compared to BRAF inhibitor monotherapy, and overcoming the challenge of treatment resistance, with durable responses in some people with favourable survival characteristics (Larkin 2014; Long 2017a; Robert 2015; Robert 2019). Adjuvant treatment with dabrafenib and trametinib for stage III melanoma following surgical resection has demonstrated an increase in RFS compared with placebo; the impact on OS is less certain (Dummer 2020a; Long 2017b).
1.
Simplified diagram of the RAS‐RAF‐MEK‐ERK pathway
Bevacizumab, an anti‐vascular epithelial growth factor (VEGF) monoclonal antibody, is an anti‐angiogenic agent that exerts its effects by reducing the growth of blood vessels required by growing tumours. Studies have shown activity in melanoma (Kim 2012; Kruijff 2012; Varker 2007), and a phase III RCT in the adjuvant setting has shown an increase in disease‐free survival but no demonstrated effect on OS (Corrie 2017). Axitinib is an oral anti‐VEGF agent, which exerts its effects similarly to bevacizumab, and is primarily used in renal cell carcinoma. It has produced both complete and partial responses in people with previously treated metastatic melanoma (Algazi 2015; Fruehauf 2011).
Topical agents
Imiquimod is a toll‐like receptor (TLR) 7 agonist which acts as an immune response modifier, although its precise mechanism of action is far from clear (Lexicomp, 20th Ed). It is currently used for the topical treatment of superficial basal cell carcinoma and a number of other indications, including genital warts, actinic keratosis (EMA 2021), and as adjuvant treatment or as monotherapy for lentigo maligna (Lallas 2021). There are documented case series of its use for the treatment of melanoma, in particular for people with multiple cutaneous in‐transit metastases (Florin 2012).
Radiation Therapy
Radiation therapy uses high‐energy radiation to shrink tumours and kill cancer cells by damaging their DNA so that they can no longer replicate. Radiation therapy has traditionally had a peripheral role in the management of melanoma, used primarily in the management of brain metastases (stereotactic ablative radiation therapy) and for symptom control. Radiation therapy can be considered after resection of bulky nodal disease, to reduce the risk of disease recurrence in the radiation field, but has no impact on disease‐free survival (DFS) and OS (Dummer 2015). Preclinical models have shown a potential synergistic effect of radiation therapy with immunotherapy, with some clinical evidence for the abscopal effect, and many reported case studies and case series (Barker 2014; Chandra 2015), although the underlying molecular mechanisms of this effect are poorly understood (Reynders 2015). Clinical trials are underway which are investigating the concomitant use of various dosing schedules of radiation therapy with immunotherapy for systemic treatment of advanced disease (Kang 2016).
Why it is important to do this review
Neoadjuvant treatment strategies are standard of care in a number of solid tumours, including breast, oesophageal and ovarian cancers (Korde 2021; Wright 2016). While not universally implemented as a treatment strategy in the current treatment paradigm for stage III and IV melanoma, there has historically been interest in this area. Neoadjuvant treatment is a suggested option in the 2016 European consensus guidelines for the management of distant metastases of melanoma (Garbe 2016). With no treatment regimens authorised in the neoadjuvant setting, it is important to identify and appraise the underlying evidence base for neoadjuvant treatment recommendations.
With the latest clinical advances in the treatment of stage IIIC and IV melanoma, there is ongoing research interest in utilising these new agents in earlier stages of the disease. To evaluate the benefit of newer agents, it is necessary to systematically analyse the evidence for the use of neoadjuvant treatments for stage III and IV melanoma. There is no published high‐quality systematic review of the trials investigating neoadjuvant treatment strategies for stage III and IV melanoma. This review provides physicians, researchers and patients with a systematic appraisal of the existing literature investigating the use of neoadjuvant treatment for cutaneous melanoma. It provides comparative evidence for the relative efficacy of neoadjuvant treatment and a new generation of drug treatments.
Objectives
To assess the effects of neoadjuvant treatment in adults with stage III or stage IV melanoma according to the seventh edition American Joint Committee on Cancer (AJCC) staging system.
Methods
Criteria for considering studies for this review
Types of studies
We conducted this review in accordance with the methods outlined in the peer‐reviewed and published review protocol (Gorry 2018). We only included prospectively randomised controlled trials (RCTs) investigating neoadjuvant treatment approaches for cutaneous melanoma, in people with AJCC seventh edition stage III or IV cutaneous melanoma. Cluster‐randomised trials were also eligible for inclusion. We excluded non‐randomised studies and cross‐over studies. We only searched for and included health economics studies that were conducted alongside clinical effectiveness studies, i.e. we did not conduct an additional search for health economic studies.
Types of participants
Eligible participants were adults aged 18 years and over with AJCC (seventh edition) stage III and IV cutaneous melanoma enrolled in trials of neoadjuvant treatment. We excluded people with stage I and II disease due to their better prognosis, in line with the review protocol.
Types of interventions
Neoadjuvant treatment is administered prior to surgery, as part of a regimen which includes surgery, and may or may not include further adjuvant treatment following surgery, with the same or different treatment(s). We considered all types of systemic therapies, radiotherapy or topical drug therapy for the neoadjuvant treatment of stage III and IV melanoma, including:
targeted treatments;
immunotherapy;
chemotherapy;
topical agents;
radiation therapy.
As well as monotherapy, we included combinations of the named interventions and treated them as separate treatment strategies. We considered any treatment schedule (i.e. sequence, doses, combinations etc.), as long as it met the defined criteria for neoadjuvant treatment. A neoadjuvant treatment strategy had to be clearly specified and meet the following criteria:
confirmed disease stage in accordance with the AJCC seventh edition criteria;
predefined systemic or local treatment prior to planned surgical procedure;
planned surgical procedure;
may or may not include continued treatment after the surgical procedure.
Controls or comparators of interest included standard of care (SOC) or placebo. We considered SOC to be surgical removal of the tumour, with or without subsequent adjuvant treatment involving any of the above treatments, with or without specified observation periods.
Types of outcome measures
There were no defined outcome sets for neoadjuvant trials in melanoma prior to the development of this protocol (COMET Initiative 2017). We selected two primary outcomes and eight secondary outcomes for this review, as outlined below. Details of the definitions applied for each outcome are provided in Appendix 1. Some of these outcomes (pathological complete response, overall response rate) are measured immediately after neoadjuvant treatment at the point of surgery, whereas longer‐term time to event outcomes such as time to recurrence and overall survival are measured continuously throughout the administration of trial treatments and in the follow‐up phase.
Primary outcomes
Overall survival (OS), expressed as a hazard ratio (HR).
Adverse events (AEs), expressed as the proportion of participants with Grade three or four AEs on the Common Terminology Criteria for Adverse Events (CTCAE) scale (CTCAE 2010).
Secondary outcomes
Overall Response Rate (ORR), expressed as the percentage of participants showing each of complete response (CR) and partial response (PR).
Time to recurrence (TTR), expressed as an HR.
Quality of life (QOL), as defined by the validated quality‐of‐life measures or instruments used in each trial.
Progression‐free survival (PFS), expressed as an HR.
Disease‐free survival (DFS), expressed as an HR.
Economic evaluation will be described, expressed as the cost per Quality Adjusted Life Year (QALY) and cost per Life Year Gained (LYG).
Pathological complete response (pCR) rate, expressed as the rate of participants showing an absence of residual invasive and in situ cancer on hematoxylin and eosin evaluation of the complete resected specimen and all sampled regional lymph nodes following completion of neoadjuvant systemic therapy.
Surgical outcomes (qualitative description as there is not an established measure of surgical outcomes available).
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press or in progress). We reported the search outcomes according to the methodological requirements of Cochrane and as per the PRISMA standards (Rethlefsen 2021).
Electronic searches
The Cochrane Skin Information Specialist (Liz Doney) searched the following databases up to 10 August 2021 using strategies based on the draft strategy for MEDLINE in our published protocol (Gorry 2018):
the Cochrane Skin Specialised Register 2021 using the search strategy in Appendix 2;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2021, Issue 8, in the Cochrane Library using the search strategy in Appendix 3;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 4;
Embase via Ovid (from 1974) using the strategy in Appendix 5; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 6.
Trial Registers
We searched the following trial registries up to 10 September 2021 using the search terms ‘melanoma’ and restricting to randomised trials only:
ClinicalTrials.gov (www.clinicaltrials.gov);
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/); and
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
We did not search the ISRCTN trials registry as these trials are all registered in the ICTRP database (see Differences between protocol and review).
Searching other resources
Searching reference lists
We checked the bibliographies of included studies for further references to relevant trials.
Searching within other reviews
We checked any systematic reviews identified as part of our database search that were related to this review title, to identify any missing trials, and scanned their reference lists to identify additional relevant trials. We also checked the reference lists of published international guidelines (INMC 2019; Michielin 2019; NCCN 2021).
Searching by contacting relevant individuals or organisations
We contacted experts in the field to obtain additional information on relevant trials (Table 8).
1. Contact with authors.
| Date contacted | Information requested | Date replied | Information received |
| 20 July 2018 | We contacted the Istituto Tumori to obtain information on the current status of EUDRACT2014‐000334‐30. | N/A | N/A |
| 20 July 2018 | We contacted the regulatory department of Philogen to obtain information on the current status of EUDRACT 2015‐002549‐72/NCT02938299. | N/A | N/A |
| 21 August 2018 28 November 2018 |
We contacted the named Principal Investigator, and the specified results point of contact, to obtain more in‐depth information on the trial design and results (NCT00525031). | N/A | N/A |
| 21 August 2018 | We contacted Dr Rozeman to obtain a copy of a poster published at ASCO and identified through handsearching of abstracts. (NCT02437279) | 23 August 2018 | Directed to publication due shortly in Nature Medicine (published 8 October 2018 and used as the primary reference source for the review). |
| 7 November 2018 | We contacted Dr C Blank to obtain additional information on the NCT02437279 trial (Blank 2018), specifically:
(NCT02437279) |
10 November 2018 15 November 2018 |
No patients with lentigo maligna/mucosal/uveal melanoma were enrolled in the study. INMC scoring criteria were used to define pathological complete response in the trial (Tetzlaff 2018). The definition of relapse‐free survival is as per published paper. Radiographic ORR data provided. HRs and 95% confidence intervals cannot be provided as the trial was not powered for this comparison. |
| 30 October 2018 | We contacted the lead authors of the Combi‐Neo trial, Drs Amaria, Wargo and Burton, seeking some additional information as follows.
(NCT02231775) |
2 November 2018 | Patients with lentigo maligna, mucosal or uveal melanoma were eligible for the study, but no patients were enrolled. No quality of life assessments were conducted as part of the study. There was no formal assessment of perioperative complications as part of the study. Randomisation was based on stage (IIIB/IIIC/M1a vs M1B/M1C). There were no additional prespecified outcomes in the trial protocol. |
| 27 May 2021 | We contacted the lead author, Dr Tarhini, to obtain the trial protocol and OS and PFS outcomes disaggregated by treatment arm. (NCT01608594) |
N/A | N/A |
| 28 May 2021 | We contacted the lead author, Dr Albertini, to obtain the trial protocol and OS and RFS outcomes disaggregated by treatment arm. (NCT00590824) | N/A | N/A |
| 21 August 2018 | We contacted the lead author, Dr Andtbacka, to obtain more in‐depth information on the trial NCT02211131, including a copy of a poster presented at ASCO 2018. | N/A | N/A |
| 14 January 2019 | We contacted Dr de Gruijl to obtain information regarding eligibility for inclusion in the review, specifically:
|
14 November 2019 | Recruited patients were clinical stage I or II, based on initial diagnosis by Breslow thickness. A publication was provided with additional information (Koster 2017). |
| 10 May 2022 | We contacted the named principle investigator of NCT04139902 to determine if Kelly 2019 was in fact referring to this RCT. | 10 May 2022 | Confirmed that Kelly 2019 is referring to NCT04139902. |
ASCO: American Society for Clinical Oncology; N/A: Not applicable; OS: overall survival; PFS: progression free survival; RCT: randomised controlled trial
Hand searching of conference proceedings
We handsearched conference proceedings from the European Society of Medical Oncology from 2016 to 2020, and the Society for Melanoma Research from 2016 to 2019. We did not handsearch the ASCO abstracts as specified in the review protocol as they were indexed in MEDLINE (see Differences between protocol and review).
Unpublished literature
We contacted original authors/investigators for clarification and further data where trial reports were unclear (Table 8).
Secondary endpoints
We did not perform separate searches for information relating to secondary endpoints including AEs and quality of life data. We considered data on these outcomes contained in included studies only.
Data collection and analysis
We authored the review using Review Manager software (Review Manager 2020), as per Cochrane requirements.
Selection of studies
We used Covidence to assess the references identified through the search. Three authors (CG, HOD and SB) assessed the relevance of all the identified titles and abstracts identified in the search. CG obtained the full text of potentially relevant studies, which three authors (one of CG, HOD, SB) then reviewed for eligibility. Another review author (LMcC) resolved any discordant decisions through discussion to reach consensus. When necessary, we contacted study authors to obtain additional information to ascertain eligibility status (see Table 8).
Data extraction and management
Three authors (CG, HOD, LMcC) conducted data extraction independently and in duplicate, using a data extraction form piloted on two studies. The review authors were not blinded to any of the study information. They extracted the following data:
descriptive information on the population, including participant characteristics and disease stage;
trial methods, including study start date, duration of follow‐up, and funding source;
intervention and comparator details, including treatment name, dose, method of administration, duration of treatment and follow‐up;
primary and secondary outcomes as specified above (Types of outcome measures);
trial outcome data.
A third author (SB or HOD) reviewed the extracted data for accuracy; authors resolved any disagreements by consensus. Where there were multiple reports of the same study, we extracted data from each report separately and used the most complete publication as the primary reference.
Assessment of risk of bias in included studies
Two authors (two of CG, HOD, LMcC) assessed risk of bias independently using the Cochrane risk of bias tool, according to the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They evaluated risk of bias for the specified domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data and selective outcome reporting. Authors categorised risk of bias as ‘low risk’, high risk’ or ‘unclear risk’ for each domain, according to the criteria in the Cochrane Handbook. A third party (LMcC, HOD) resolved any disagreements in the assessments.
Measures of treatment effect
We used hazard ratios (HRs) and their corresponding 95% confidence intervals (95% CIs) for time‐to‐event outcomes (OS, time to recurrence, DFS, PFS). We extracted HRs directly from the original studies when reported. We used the generic inverse variance function in RevMan to input HRs and CIs, with the variance and standard error estimated using the reported HRs and CIs (Higgins 2011). We followed the methods proposed by Parmar and colleagues where summary survival statistics were not available (Parmar 1998).
We calculated risk ratios (RR) and their corresponding 95% CIs for dichotomous outcomes, including tumour response rate, where appropriate.
Unit of analysis issues
All included studies had a parallel‐group design, so the unit of analysis is at the individual participant level. We had planned to account for within‐study correlation for multiple‐arm trials, by calculating an average of the relevant pair‐wise comparisons from the study and calculating a variance for the study, accounting for the correlation between the comparisons (Higgins 2011). We included one multi‐arm trial in the review. This trial was not designed for any formal hypothesis testing of differences between treatment arms, therefore we pooled the results of the two similar arms without accounting for within‐trial correlations. More details are provided in the section Effects of interventions. As prespecified at the protocol stage, If we identify cluster‐randomised trials in future updates we plan to use the published effect estimates taking clustering into account (Gorry 2018).
Dealing with missing data
We conducted analyses using the intention‐to‐treat (ITT) population. We contacted the authors to obtain any missing data. If the ITT analyses were not available, we performed the analysis using the ‘as treated’ population, i.e. those participants who received the planned trial treatment with data reported.
We assessed the risk of attrition bias by examining dropout rates, withdrawals and loss to follow‐up, as part of the risk of bias assessment. Where relevant, we described any methods employed in the publication to address incomplete data, including any sensitivity analyses.
Assessment of heterogeneity
An assessment of clinical and methodological heterogeneity, performed as part of assessment for suitability for meta‐analysis, considered trial design, treatments administered, disease stage of the participants, and duration of follow‐up. We assessed statistical heterogeneity using the I2 statistic, with interpretation of the I2 statistic based on the ranges provided in the Cochrane Handbook for Systematic Revies of Itnerventions (Deeks 2022), as follows:
0 to 40%: might not be important;
30 to 60%: may represent moderate heterogeneity;
50 to 90%: may represent substantial heterogeneity; and
75 to 100%: considerable heterogeneity.
Assessment of reporting biases
We have included a narrative description of the risk of reporting bias for the primary outcomes for each study.
Data synthesis
The review protocol stated that a meta‐analysis of outcomes would only be undertaken if participants, interventions, comparisons and outcomes were considered sufficiently similar across the identified trials to produce a clinically meaningful result. We undertook assessment of heterogeneity as described above. We had planned to use a random‐effects model for meta‐analysis if sufficient studies were identified; however, as we only included three studies in the meta‐analysis, we implemented a fixed‐effect model as outlined in the review protocol, due to the difficulty of estimating between trial heterogeneity. We conducted analyses using RevMan Web.
Subgroup analysis and investigation of heterogeneity
The review protocol stated that, where possible, we would conduct subgroup analysis examining the effect of the intervention according to disease stage. The relevant data were not available for any of the identified studies, so no subgroup analyses are presented.
Sensitivity analysis
We had planned to undertake sensitivity analyses excluding studies at high risk of bias, and by using unblinded assessments of disease progression. These were not possible as data meeting these criteria were not available from the identified studies.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to rate the certainty of evidence for selected outcomes included in the summary of findings tables (GRADE Handbook 2013). This process involved assessing the certainty of the evidence according to the risk of bias, inconsistency, indirectness, imprecision, and publication bias, as outlined in the Cochrane Handbook (Schünemann 2022). Two authors conducted the assessment independently, with a third author resolving any disputes. Three authors conducted the GRADE assessment (CG, HOD, LMcC), and graded evidence as high, moderate, low or very low.
Summary of findings tables are presented for the most clinically relevant comparisons identified in the review:
neoadjuvant treatment compared to no neoadjuvant treatment;
neoadjuvant targeted treatment (BRAF/MEK combination) compared to no neoadjuvant treatment;
neoadjuvant immunotherapy (talimogene laherparepvec) compared to no neoadjuvant treatment;
neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to adjuvant immunotherapy (combined ipilimumab and nivolumab);
neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (nivolumab);
neoadjuvant immunotherapy (combined ipilimumab and nivolumab, two different dosing regimens) compared to neoadjuvant immunotherapy (sequential treatment with ipilimumab then nivolumab);
neoadjuvant immunotherapy (high dose interferon) plus chemotherapy compared to neoadjuvant chemotherapy.
The review protocol prespecified two primary outcomes (OS and AEs) and three secondary outcomes (ORR, PFS and QOL) for inclusion in summary of findings tables. Subsequent to the publication of the protocol, the International Neoadjuvant Melanoma Consortium (INMC) published recommendations on trial design for neoadjuvant treatments for melanoma, including recommendations on selection of relevant trial endpoints (INMC 2019). Based on these recommendations, we decided to include time to recurrent disease (TTR) in the summary of findings tables, in lieu of progression‐free survival (see Differences between protocol and review).
We constructed the summary of finding tables using GRADEpro software (GRADEpro GDT).
Results
Description of studies
This section describes the outcomes of our review. Preliminary review findings were previously published (Gorry 2020).
Results of the search
In accordance with the review protocol (Gorry 2018), we searched databases listed under Electronic searches (on 10 August 2021) and retrieved 8564 records. Handsearching of the grey literature, including clinical trials registers, identified a further 18 records. After removal of duplicates, we screened a total of 8532 records and excluded 8176 records based on titles and abstracts. We obtained the full text of the remaining 356 records, of which we excluded 293 studies (299 references) following full‐text review; the majority (231 studies) did not closely match our inclusion criteria and are not presented in the Characteristics of excluded studies section. We presented the reasons for excluding 36 studies (reported in 42 references) in Characteristics of excluded studies and categorised 11 studies reported in 23 references as ongoing (see Characteristics of ongoing studies). We also identified three studies that are awaiting classification (Characteristics of studies awaiting classification).
We included eight studies reported in 57 references (Characteristics of included studies). We included three studies in our quantitative meta‐analysis. For a further description of our screening process, see the study flow diagram (Figure 2).
2.
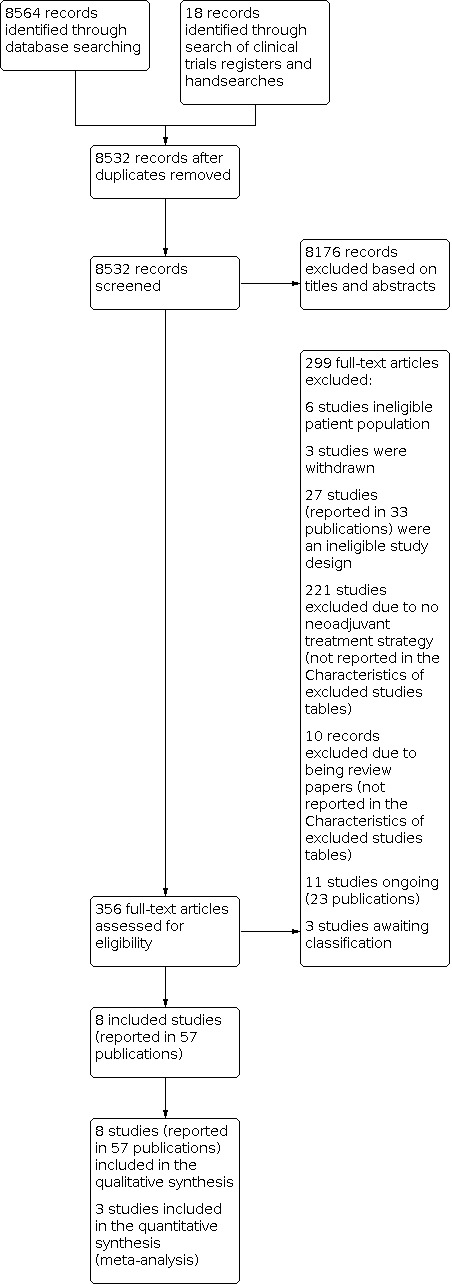
PRISMA Study flow diagram.
Dealing with missing data
Where we did not have sufficient data in the identified studies to determine eligibility, we contacted the authors via email where possible. The details of contacts with authors, questions posed, and answers received are provided in Table 8. We used the additional information provided to exclude one study due to an ineligible population (van den Hout 2013). After determining eligibility, we contacted the study authors if there were missing data for key outcomes for the review (Table 8); additional data were provided by the authors for Amaria 2018a, Amaria 2018b, and Blank 2018.
Included studies
We identified eight trials that were eligible for the review (Albertini 2018; Amaria 2018a; Amaria 2018b; Blank 2018; Dummer 2020b; Hwu 2017; Rozeman 2019; Tarhini 2018).
Design
All eight included trials were Phase I or Phase II RCTs, designed as parallel‐group trials. We did not identify any cluster‐randomised trials. All trials were open‐label in design. The trials were all funded by pharmaceutical companies, except for Albertini 2018, where the source of funding was multiple grants, and a trial investigator was the CEO of the company which owned the intervention.
Sample sizes
Sample sizes were small in all trials; the largest trial enrolled 150 participants (Dummer 2020b), whilst the smallest trials enrolled 20 participants (Albertini 2018; Blank 2018).
Setting
Trials were conducted in the USA (Albertini 2018; Amaria 2018a; Amaria 2018b; Dummer 2020b; Hwu 2017; Tarhini 2018), Europe (Blank 2018; Dummer 2020b; Rozeman 2019) and Australia (Dummer 2020b; Rozeman 2019). Two were multicentre trials (Dummer 2020b; Rozeman 2019).
Participants
The eight trials randomised 402 participants. The population comprised men (62%) and women (38%) aged 18 years and over (range 18 to 82 years). Most participants had favourable performance status (79% had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0, and 97% had normal LDH levels). All randomised participants had AJCC seventh edition stage III or IV melanoma; the majority had stage IIIB (38%) or IIIC (47%) melanoma. Three trials did not report the proportion of participants with each disease stage (Albertini 2018; Hwu 2017; Rozeman 2019).
Interventions
Detailed descriptions of the drugs and dosing schedules used are provided in the Characteristics of included studies tables. There were six trials administering various combinations of neoadjuvant immunotherapy agents (ipilimumab with nivolumab, nivolumab, TVEC, ipilimumab with HDI, interleukin monoclonal antibody), one trial administering neoadjuvant immunotherapy in combination with chemotherapy (HDI with temozolomide), and one trial of neoadjuvant targeted therapy (dabrafenib and trametinib). Three studies compared neoadjuvant treatment to SOC (surgery with or without adjuvant treatment), three compared to neoadjuvant immunotherapy (nivolumab, sequential ipilimumab then nivolumab, ipilimumab plus HDI), one compared to neoadjuvant chemotherapy, and one to adjuvant treatment with an experimental interleukin monoclonal antibody. Duration of the neoadjuvant treatment phase varied from four to 12 weeks.
We investigated the following treatment comparisons.
Neoadjuvant targeted treatment (BRAF/MEK combination) versus no neoadjuvant treatment: one study (Amaria 2018a).
Neoadjuvant immunotherapy (oncolytic viral immunotherapy with talimogene laherparepvec (T‐VEC)) versus no neoadjuvant treatment: one study (Dummer 2020b).
Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) versus adjuvant immunotherapy (nivolumab): one study (Blank 2018);
Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) versus neoadjuvant immunotherapy (nivolumab): one study (Amaria 2018b);
Neoadjuvant combined immunotherapy (combined ipilimumab and nivolumab, at two different dosing regimens schedules, versus sequential neoadjuvant immunotherapy (sequential with ipilimumab then nivolumab): one study (Rozeman 2019);
Neoadjuvant immunotherapy (HDI) plus chemotherapy versus neoadjuvant chemotherapy: one study (Hwu 2017);
Neoadjuvant immunotherapy (experimental interleukin monoclonal antibody hu14.18‐IL2) versus adjuvant immunotherapy (experimental interleukin monoclonal antibody hu14.18‐IL2): one study (Albertini 2018);
Neoadjuvant immunotherapy (ipilimumab 10 mg/kg plus HDI) versus neoadjuvant immunotherapy (ipilimumab 3 mg/kg plus HDI): one study (Tarhini 2018).
Outcomes
Length of follow‐up and maturity of the time‐to‐event outcome data varied considerably across the studies. Duration of follow‐up ranged from 15 months to over four years. Studies reported data that met the requirements for inclusion in the analysis as per the review protocol for the following outcomes.
Overall survival (OS) (three studies)
Adverse events (AEs) (six studies)
Overall response rate (ORR) (radiological) (seven studies)
Pathological complete response (pCR) (six studies)
Time to recurrence (TTR) (three studies)
Disease‐free survival (DFS) (three studies)
Surgical outcomes (two studies)
No studies reported on the prespecified outcomes of QOL, progression‐free survival, and economic evaluation.
Feasibility of meta‐analysis
There were important differences between the trials, for example, the different dosing schedules of immunotherapies and consequently different treatment effects, and the underlying differences in the mechanism of action between immunotherapies, targeted treatments, and chemotherapy. There were some differences in the proportions of participants with stage IIIB, IIIC, and stage IV disease randomised to the trials, which impact the baseline risk of disease recurrence (Balch 2009). Furthermore, time‐to‐event outcomes (OS, TTR, DFS) are likely to be impacted by differences in follow‐up duration between the treatment arms, and in differences in the adjuvant treatment strategies employed in the trials, which would be expected to impact these outcomes.
Differences in the definitions of measured recurrence endpoints also impact feasibility of meta‐analysis; some trials measured recurrence endpoints from point of randomisation, whereas some measured from the point of surgery, and there were also differences in the censoring rules for a recurrence event, meaning that the endpoints were not comparable. Based on these important differences between trials, we concluded that a meta‐analysis of all identified trials was not feasible and would not provide useful data for clinical decision‐making.
We deemed three studies to be sufficiently similar in terms of interventions and methodological design to include in a meta‐analysis (Amaria 2018a; Blank 2018; Dummer 2020b). These three studies all compared neoadjuvant treatment to surgery, with or without subsequent adjuvant treatment. There are limitations to this analysis, not least differences in the proportions of participants with each disease state, the proportions receiving adjuvant treatment and the types of adjuvant treatment, and the different underlying mechanisms of action of immunotherapy agents compared with targeted treatments. The outcomes of the meta‐analysis are presented in Table 1 and Effects of interventions.
Excluded studies
We presented the reasons for excluding 36 studies (42 references) in the Characteristics of excluded studies table. Reasons for the exclusion were as follows: ineligible study design (27 studies in 33 publications), ineligible population (six studies), and study withdrawn (three studies). We excluded a further 231 studies because they were narrative review articles (n = 10) or did not deliver a neoadjuvant treatment regimen (n = 221).
Ongoing studies
We identified 11 ongoing studies in 23 publications, which we describe in the Characteristics of ongoing studies table. These studies are, in the main, investigating combinations of the interventions of interest.
Studies awaiting classification
Please see Characteristics of studies awaiting classification. Three studies are awaiting classification. We identified one study through the search of the EUDRA‐CT database (EudraCT 2014‐000334‐30). No results were posted for this study, and we received no response from the contact persons (Table 8). We could not access two studies identified through the electronic database search (de Braud 1994; Kleeberg 1986).
Risk of bias in included studies
The risk of bias assessments for each study are detailed in the Characteristics of included studies table, and summarised in Figure 3 and Figure 4. We did not consider any of the trials to be at low risk of bias for all domains. Unclear risk of bias was common; all included trials were at unclear risk of bias for at least one domain. We did not formally assess evidence for publication bias, but considered trials to be at high risk of bias where no peer‐reviewed publications were available. Further details and justifications for the assessments are provided in the Characteristics of included studies table and the risk of bias tables for the individual studies. We considered the external validity of the included trials to be poor for efficacy endpoints, as they were not designed or powered to detect differences in efficacy between treatment arms.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
With the exception of two trials (Amaria 2018a; Dummer 2020b), we considered the risk of selection bias to be unclear with regard to issues related to random sequence generation. In most cases, the methods were not reported, and there was inadequate information to assess the risk.
Allocation concealment
We considered the risk of selection bias with regard to allocation concealment to be unclear for all trials except Dummer 2020b, due to the absence of the relevant information in the published study reports.
Blinding
Performance bias
All eligible trials were open‐label trials, and as such all those involved in the trials were aware of treatment allocation. Therefore, we considered there to be a high risk of performance bias for TTR, DFS and other outcomes, such as certain AEs, where the outcomes are somewhat dependent on the treatment choices taken during the trial. This can, in turn, be influenced by physician knowledge of the treatment allocation. We rated the risk of bias for OS to be unclear, as OS outcomes are likely confounded by subsequent off‐trial treatment, in addition to choices made during the trial.
Detection bias
We rated detection bias to be high in five of the included trials, and unclear in three as there was insufficient data to assess the risk of detection bias (Hwu 2017; Rozeman 2019; Tarhini 2018). We applied this rating to subjective outcomes such as adverse events, pCR, ORR, and TTR. We deemed detection bias for OS to be low. Detection bias for pCR outcomes was low for Blank 2018, as the endpoint was assessed by a blinded pathologist.
Incomplete outcome data
We considered the risk of attrition bias to be low for studies which published CONSORT diagrams and provided analyses in the ITT population. Attrition bias was low for five trials, and unclear for three trials (Hwu 2017; Rozeman 2019; Tarhini 2018). Hwu 2017 only reported data for 50 of the 52 participants recruited, with no information provided on the missing participants. Similarly, for the efficacy analyses, Tarhini 2018 only reported outcomes for 28 of the 30 participants enrolled.
Selective reporting
We deemed reporting bias to be low in four studies with either a published trial protocol (Amaria 2018b; Blank 2018, Dummer 2020b), or where the authors stated in personal correspondence that no other outcomes were included and that all outcomes had been reported (Amaria 2018a). Reporting bias was unclear in four trials where the protocol was unavailable.
Other potential sources of bias
We considered other potential sources of bias to be high in four included trials (Albertini 2018; Amaria 2018a; Dummer 2020b; Hwu 2017). No peer‐reviewed results were available for Dummer 2020b and Hwu 2017, so we considered both to be at high risk of publication bias. Two studies allowed various adjuvant treatment options to be administered to participants in line with local SOC, which could confound time‐to‐event outcomes (Amaria 2018a; Dummer 2020b), and as such we considered these to be at high risk of bias. Additionally, one trial was halted early and did not meet the prespecified criteria for early cessation of trial treatment for efficacy (Amaria 2018a); outcomes could be biased as a result (Bassler 2010).
Amaria 2018b used a procedure for confirming if people were eligible for the trial, involving a consensus panel of medical and surgical oncologists coupled with the stated eligibility criteria, which has the potential to produce an enriched trial population. We rated this as unclear risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Eight trials were eligible for the review. One of these trials presented all results as a pooled analysis of both treatment arms (Albertini 2018). Efforts to contact the authors to obtain trial outcomes disaggregated by treatment arm failed. Thus, this trial is described narratively, but no comparative results are presented, and it is excluded from the summary of findings tables. Similarly, one trial provided only pooled results for time‐to‐event outcomes, but disaggregated results for ORR and pCR (Tarhini 2018); this trial is described narratively, and comparative results are presented for ORR and pCR outcomes; it is excluded from the summary of findings tables. Studies are described individually in 'Characteristics of included studies'.
Neoadjuvant treatment versus no neoadjuvant treatment (three studies)
Please see Table 1.
We conducted a meta‐analysis of three trials (Amaria 2018a; Blank 2018; Dummer 2020b), deemed methodologically similar, to determine if there was a treatment benefit with neoadjuvant treatment compared to no neoadjuvant treatment (surgery with or without adjuvant treatment). We pooled results for outcomes where trial publications reported data, and where the definitions of the endpoints were sufficiently similar to justify pooling. Details of the included studies can be found in Characteristics of included studies.
Primary outcomes
Overall survival (2 RCTs, 171 participants)
We are uncertain if neoadjuvant treatment increases OS (HR 0.43, 95% CI 0.15 to 1.21; heterogeneity: Chi2 = 0.21, df = 1, P = 0.65, I2 = 0%; very low‐certainty evidence; Analysis 1.1). We downgraded certainty by one level for imprecision, due to the small number of events and wide confidence intervals, one level for risk of bias due to high risk of performance and detection bias, and one level for publication bias as the findings from the largest trial are not published in a peer‐reviewed journal. There were also concerns regarding indirectness, as adjuvant treatment provided in the largest trial does not reflect current clinical practice.
1.1. Analysis.

Comparison 1: Neoadjuvant treatment compared to no neoadjuvant treatment, Outcome 1: Overall survival
Adverse events (2 RCTs, 162 participants)
Neoadjuvant treatment may be associated with a higher rate of adverse events, but the outcome is very uncertain (RR 1.58, 95% CI 0.97 to 2.55; heterogeneity: Chi2 = 10.44, df = 1, P = 0.001, I2 = 90%; very low‐certainty evidence; Analysis 1.2). We downgraded certainty one level for high risk of performance and detection bias, and one level for publication bias. We also downgraded one level for imprecision due to the width of the confidence intervals, and one level for inconsistency due to between‐trial heterogeneity.
1.2. Analysis.

Comparison 1: Neoadjuvant treatment compared to no neoadjuvant treatment, Outcome 2: Adverse events
Secondary outcomes
Overall response rate
We could not report this outcome as a comparative outcome as it was measured only in the neoadjuvant arm, and could not be included in the meta‐analysis.
Time to recurrence (2 RCTs, 171 participants)
We are uncertain if neoadjuvant treatment increases TTR (HR 0.51, 95% CI 0.22 to 1.17; heterogeneity: Chi2 = 7.41, df = 1, P = 0.006, I2 = 87%, very low‐certainty evidence, Analysis 1.3). We downgraded certainty by one level for risk of bias (high risk of performance and detection bias in both trials), one level for publication bias, one level for imprecision due to the width of the confidence intervals, and one level for inconsistency (between‐trial heterogeneity). There were also concerns regarding indirectness, as adjuvant treatment provided in the largest trial does not reflect current clinical practice.
1.3. Analysis.

Comparison 1: Neoadjuvant treatment compared to no neoadjuvant treatment, Outcome 3: Time to recurrence
Pathological complete response
We could not report this outcome as a comparative outcome as it was measured only in the neoadjuvant arm, and could not be included in the meta‐analysis.
Other secondary endpoints
Studies did not collect data on QOL, surgical outcomes or economic evaluation. DFS and PFS were not prespecified trial endpoints and were therefore not reported. These secondary endpoints could not be included in the meta‐analysis.
Individual trial outcomes
For descriptive reporting of the individual trial outcomes, we grouped the trials into the treatment categories as identified in the review protocol. This section is laid out under the following subheadings:
targeted treatments;
immunotherapies;
chemotherapy;
topical agents;
radiotherapy.
Comparisons from the identified trials are listed under each treatment category. The trial PICO is presented, followed by the primary and secondary outcomes. Each study is presented only once, in the category where it is considered most relevant.
1.1. Neoadjuvant BRAF/MEK treatment versus no neoadjuvant treatment (one study)
Please see Table 2.
PICO: Amaria 2018a studied neoadjuvant treatment consisting of an eight‐week treatment course of dabrafenib and trametinib prior to surgery, followed by up to 44 weeks of adjuvant treatment, compared with no neoadjuvant treatment, surgery, and optional SOC adjuvant treatment. Eligible people had stage IIIB, IIIC or IV melanoma, with a BRAF V600 mutation. The trial randomised 21 participants: 14 to neoadjuvant treatment and seven to the control arm. The median follow‐up was 18.6 months.
Primary outcomes
Overall survival
We are uncertain whether neoadjuvant BRAF/MEK inhibition increases OS; HR 0.28, 95% CI 0.03 to 2.25; Analysis 2.1. Median OS was not reached in either arm. We rated the certainty of the evidence as very low, downgraded one level for risk of performance and detection bias, and one level for high risk of other bias, due to early cessation of the trial based on an unplanned interim analysis where the prespecified criteria for early discontinuation were not met. We downgraded one level for imprecision as the confidence intervals were wide and due to the small number of events; additionally, the trial was not powered to detect differences between treatment arms. Differences in adjuvant treatment options between treatment arms, and between current clinical practice, further confound outcomes and raise concerns regarding indirectness, although we did not formally downgrade the evidence for this.
2.1. Analysis.

Comparison 2: Neoadjuvant targeted treatment (BRAF/MEK inhibition) compared to no neoadjuvant treatment, Outcome 1: Overall survival
Adverse events
Safety was not reported as a comparative outcome. The overall incidence of Grade 3 and 4 AEs was not reported. Eight treatment‐related AEs (TRAEs) of Grade 3 occurred in the neoadjuvant arm. We rated the certainty of the evidence as low, downgraded one level for risk of performance and detection bias, and one level for other bias, as the AEs in the control arm were not recorded.
Secondary outcomes
Overall response rate
ORR was not a comparative outcome. The rate of ORR (complete responses (CR) plus partial responses (PR)) in the neoadjuvant treatment arm was 85% (11/13 participants). An additional two participants achieved stable disease (15%). The analysis was limited to 13 participants as one person withdrew consent prior to initiating therapy. We rated the certainty of the evidence as low and downgraded one level for risk of detection bias and one level for imprecision as the number of events was small.
Time to recurrence (corresponding to trial definition of event‐free survival)
The trial primary endpoint was event‐free survival (EFS), defined as “time from randomisation to recurrence event (local or distant disease development, or death)”, which correlates with the review definition of TTR. A difference in TTR (EFS) was observed between treatment arms, HR 0.02 (95% CI 0.00 to 0.22) (Analysis 2.2). Median TTR (EFS) was 19.7 months (95% CI 16.2 to not estimable) in the intervention arm and 2.9 months (95% CI 1.7 to not estimable) in the control arm. We rated the certainty of the evidence as very low, downgraded by two levels for high risk of bias, and one level for imprecision as the number of events was small.
2.2. Analysis.

Comparison 2: Neoadjuvant targeted treatment (BRAF/MEK inhibition) compared to no neoadjuvant treatment, Outcome 2: Time to recurrence
Pathological complete response
pCR was not a comparative outcome. The rate of pCR in the neoadjuvant treatment arm was 58% in participants who underwent surgery (7 of 12 participants). The analysis was limited to 12 participants, as one participant withdrew consent prior to initiating therapy, and one withdrew consent prior to undergoing surgery. Two additional participants (17%) achieved a pathological partial response.
Other secondary endpoints
The study did not collect data on QOL, surgical outcomes or economic evaluation. DFS and PFS were not prespecified trial endpoints.
2.1. Neoadjuvant talimogene laherparepvec (T‐VEC) versus no neoadjuvant treatment (one study)
Please see Table 3.
PICO: Dummer 2020b investigated the use of neoadjuvant T‐VEC compared with surgery alone in people with Stage IIIB, C and Stage IV M1a melanoma. A total of 150 participants were randomised, 76 to the T‐VEC arm and 74 to the surgery arm. This trial reported outcomes with 80% confidence intervals. We calculated 95% confidence intervals for outcomes reporting 80% confidence intervals using a normal approximation prior to data input for analysis.
Primary outcomes
Overall survival
There was a numerical advantage in OS for participants treated with T‐VEC, but the increase was not statistically significant (HR for OS 0.49, 95% CI 0.15 to 1.64; Analysis 3.1). The two‐year OS rates were 88.9% (80% CI 83 to 92.8) with T‐VEC, and 77.4% (80% CI 70.2 to 83) with surgery. Three‐year OS rates were published without confidence intervals and so are not presented here. We rated the certainty of the evidence as very low, downgraded by one level for high risk of performance and detection bias, one level for publication bias as no peer‐reviewed data is available, and one level for imprecision as the number of events was small and the confidence intervals wide. Differences in adjuvant treatment options between treatment arms, and also from current clinical practice, further confounds outcomes.
3.1. Analysis.

Comparison 3: Neoadjuvant immunotherapy (talimogene laherparepvec) compared to no neoadjuvant treatment, Outcome 1: Overall survival
Adverse events
The study did not report the overall incidence of Grade 3 and 4 AEs. Treatment‐related adverse events were higher with T‐VEC compared with surgery (RR 2.84, 95% CI 0.96 to 8.37; Analysis 3.2), but not statistically different. Grade ≥ 3 treatment‐emergent adverse events were more common with T‐VEC (16.4% vs 5.8%), as were serious treatment‐related adverse events (17.8% vs 2.9%). Surgery occurred as planned in 75% of participants in the T‐VEC arm, and 93% in the control arm. We rated the certainty of the evidence as very low, downgraded by one level for publication bias, one level for high risk of detection bias, and one level for imprecision as the confidence intervals were wide.
3.2. Analysis.

Comparison 3: Neoadjuvant immunotherapy (talimogene laherparepvec) compared to no neoadjuvant treatment, Outcome 2: Adverse events
Secondary outcomes
Overall response rate
ORR was not a comparative outcome. The rate of ORR (CR+PR) in the neoadjuvant treatment arm was 13.2%; the absolute number of events was not specified. We rated the certainty of the evidence as very low, downgraded by two levels for risk of detection bias and publication bias, and one level for imprecision due to the small number of events.
Time to recurrence (corresponding to trial definition of relapse‐free survival)
The trial endpoint of relapse‐free survival, defined as the time from randomisation to the date of the first of local, regional or distant recurrence of melanoma or death due to any cause, corresponds with the review outcome of TTR. The trial did not provide the absolute number of recurrence events in each arm; the difference between arms was not statistically significant (HR 0.75, 95% CI 0.31 to 1.79; Analysis 3.3). The study reported outcomes with 80% confidence intervals, which we accounted for when estimating the variance for data input for analysis. Relapse‐free survival rates at one year were 33.7% with T‐VEC (80% CI 26.8 to 40.8), vs 21.9% (80% CI 15.9 to 28.7) with surgery alone, and at two years were 29.5% with T‐VEC (80% CI 22.9 to 36.4) versus 16.5% (80% CI 11 to 22.9) with surgery alone. Of note, at least 11% of participants in the T‐VEC arm and 29% in the surgery arm received subsequent adjuvant therapy; this difference may confound TTR outcomes. It also raises concerns regarding indirectness, as the adjuvant treatments received are unlikely to reflect current clinical practice, although we did not formally downgrade the evidence for this. We rated the certainty of the evidence as very low, downgraded by one level for high risk of detection bias, one level for imprecision as the confidence intervals were very wide, and one level for publication bias as no peer‐reviewed data are available.
3.3. Analysis.

Comparison 3: Neoadjuvant immunotherapy (talimogene laherparepvec) compared to no neoadjuvant treatment, Outcome 3: Time to recurrence
Pathological complete response
pCR was reported as a comparative outcome, with a rate of 2.7% in the surgery arm compared with 17.1% (80% CI 11.6 to 24) in the TVEC arm. It is unclear how pCR was observed in the surgery arm, and as such we excluded this result from the analysis.
Surgical outcomes
The trial reported the rate of R0 surgical resection (microscopically margin‐negative resection), with no difference between treatment arms (P = 0.594).
Other secondary outcomes
The study did not report data on QOL, economic evaluation, DFS, or PFS.
2.2. Neoadjuvant combined ipilimumab and nivolumab versus adjuvant combined ipilimumab and nivolumab (one study)
Please see Table 4.
PICO: Blank 2018 investigated the effects of combined ipilimumab and nivolumab as neoadjuvant therapy, compared to adjuvant therapy. Eligible people had palpable stage III melanoma. The study randomised 10 participants to each arm.
Primary outcomes
Overall survival
This outcome was not reported for this comparison. The trial did not report the HR or confidence intervals, and we could not obtain the data accurately from the published Kaplan Meier curves. Data are immature, with median OS not reached in either arm. We requested the data from the authors, who declined to provide it on the basis that the trial was not powered to detect differences in this outcome.
Data with a median follow‐up of 48 months indicate a four‐year OS rate of 90% in the neoadjuvant arm and 70% in the adjuvant treatment arm.
Adverse events
The trial did not report the overall incidence of Grade 3 and 4 AEs. No difference in the incidence of Grade 3 to 4 TRAEs was observed (RR 1.00, 95% CI 0.75 to 1.34; P = 1.0), 20 participants (Analysis 4.1). With additional follow‐up (median 30 months), of the 90% participants who had developed one or more Grade 3 to 4 AEs across both arms, all had recovered to Grade ≤ 1, except for Grade 2 endocrine toxicities requiring hormonal supplementation that were ongoing in eight (50%) of the 16 participants remaining alive. We rated the certainty of the evidence as low, downgraded by one level for high risk of performance and detection bias, and one level for imprecision as the effect size was null and the confidence interval was wide.
4.1. Analysis.

Comparison 4: Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to adjuvant immunotherapy (combined ipilimumab and nivolumab), Outcome 1: Adverse events
Secondary outcomes
Overall response rate
ORR was not a comparative outcome. The rate of ORR (CR+PR) in the neoadjuvant treatment arm was 40%. We rated the certainty of the evidence as low and downgraded by one level for a risk of detection bias and one level for imprecision due to the small number of events
Pathological complete response
pCR was not a comparative outcome. In the neoadjuvant arm, three out of nine participants evaluable for pCR achieved a pCR (33%); three participants achieved a near complete pCR (≤ 10% viable tumour cells) and one achieved a pathological partial response.
Disease‐free survival (corresponding to trial definition of relapse‐free survival)
The trial endpoint of relapse‐free survival, defined as time from surgery until the date of first relapse (local, regional, or distant metastases), corresponds closely with the review protocol definition of DFS. Two participants in the neoadjuvant arm experienced a DFS (RFS) event, versus four in the adjuvant arm. No HR or absolute number of events were reported, and we could not extract the data accurately from the published Kaplan Meier curves. We requested the data from the authors, who declined to provide it on the basis that the trial was not powered to detect differences in this outcome. Data with longer follow‐up (median 48 months) indicate a four‐year relapse rate of 60% in both treatment arms.
Other secondary outcomes
Surgery‐related AEs were reported. One Grade 3 to 4 surgery‐related AE was reported in the neoadjuvant arm (wound infection) and two were reported in the adjuvant arm (wound infection).
The trial did not collect data on TTR, QOL, surgical outcomes or economic evaluation. Participants in long‐term follow‐up (post‐trial) were invited to complete the EORTC‐QLQ‐C30 questionnaire (median time from randomisation: 30 months). When compared with a registry cohort matched for age, gender, education and marital status, participants reported statistically significant and clinically meaningful lower scores (worse QOL) in functional domains, and higher scores (worse QOL) in the symptom burden of fatigue, compared to the controls. Physical functioning and global QOL scores did not differ between trial participants and controls.
2.3. Neoadjuvant combined ipilimumab and nivolumab versus neoadjuvant nivolumab (one study)
Please see Table 5.
PICO: Amaria 2018b investigated the effects of either neoadjuvant combined ipilimumab and nivolumab or nivolumab monotherapy for nine weeks, followed by surgery, then up to 13 cycles of adjuvant nivolumab. Eligible people had stage IIIB and IIIC melanoma. The trial randomised 23 individuals, 12 to nivolumab and 11 to combined ipilimumab and nivolumab.
Primary outcomes
Overall survival
No difference in OS was observed (P = 0.18). The trial did not report HRs or the absolute number of events, and we could not extract the data accurately from the published Kaplan Meier curves. Median OS was not reached in either arm. The certainty of the evidence was graded as moderate, downgraded by one level for imprecision as the number of events was small.
Adverse events
The overall incidence of Grade 3 to 4 AEs was not reported. There were differences between the treatment arms in terms of Grade 3 to 4 TRAEs (RR 8.73, 95% CI 1.29 to 59.0; 23 participants; Analysis 5.1). The risk ratio showed a higher risk for combination treatment (P = 0.0263). We rated the certainty of the evidence as very low, downgraded by one level as we considered the risk of performance and detection bias to be high for this outcome, and two levels for imprecision due to the width of the confidence intervals and the small number of events.
5.1. Analysis.

Comparison 5: Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (nivolumab), Outcome 1: Adverse events
The trial was halted early by the Data Safety Monitoring Board, on the basis of an early observation of disease progression, preventing surgical resection during neoadjuvant nivolumab treatment (17%, 2 out of 12 participants), and high rates of Grade 3 TRAEs during neoadjuvant combination treatment (73%, 8 out of 11 participants).
Secondary outcomes
Overall response rate
Combination ipilimumab and nivolumab was associated with a higher radiological response rate than nivolumab (RR 2.91, 95% CI 1.02 to 8.27; P = 0.045; 23 participants; Analysis 5.2). We rated the certainty of the evidence as very low, downgraded by two levels for imprecision as the number of events was small and the confidence intervals wide, and one level for high risk of detection bias.
5.2. Analysis.

Comparison 5: Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (nivolumab), Outcome 2: Overall response rate
Time to recurrence (corresponding to trial definition of progression‐free survival)
The trial endpoint of progression‐free survival, defined as “time from randomisation to development of radiographic progression before surgery, disease recurrence after surgical resection or death from any cause”, correlates with the review definition of TTR. No difference in TTR (PFS) was observed (P = 0.19). The trial did not report the HR or absolute number of events, and we could not obtain the data accurately from the published Kaplan Meier curves. We rated the certainty of the evidence as low, downgraded by one level for high risk of detection bias and one level for imprecision as the number of events was small.
Disease‐free survival (corresponding to trial definition of recurrence‐free survival)
The trial endpoint of recurrence‐free survival, defined as “time from surgical resection to date of documented disease recurrence” correlates with the review definition of DFS. No difference in DFS (RFS) was observed (P = 0.58). The trial did not report the HR or absolute number of events, and we could not obtain the data accurately from the published Kaplan Meier curves.
Pathological complete response
There was a numerically higher rate of pCR with combined ipilimumab and nivolumab compared to nivolumab alone (RR 1.82, 95% CI 0.56 to 5.88; P = 0.3184; 23 participants; Analysis 5.3), but the difference was not statistically significant.
5.3. Analysis.

Comparison 5: Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (nivolumab), Outcome 3: Pathological Complete Response
Other secondary outcomes
This trial did not collect data on QOL, surgical outcomes or economic evaluation.
2.4. Neoadjuvant combined ipilimumab and nivolumab (different dosing regimens) compared to neoadjuvant sequential ipilimumab then nivolumab (one study)
Please see Table 6.
PICO: The three‐arm trial by Rozeman 2019 studied two regimens of neoadjuvant combined ipilimumab and nivolumab, using different dosing regimens, compared with sequential treatment with ipilimumab followed by nivolumab. Only people with stage III melanoma were eligible for this trial. The study enrolled 30 participants into each combination treatment arm and 26 to the sequential treatment arm. We pooled the combination arms in the analysis below, therefore no adjustment for between‐arm correlation is required (Higgins 2011).
Primary outcomes
Overall survival
The study did not report data for this outcome. No HR or confidence intervals were reported, and we could not obtain the data accurately from the published Kaplan Meier curves. To date, two participants have died in one of the combination arms (ipilimumab 3 mg/kg plus nivolumab 1 mg/kg).
Adverse events
The study did not report the overall incidence of Grade 3 and 4 AEs. Fewer immune‐related Grade 3 to 4 AEs were reported in the combination treatment arm compared to the sequential treatment arm (RR 0.6, 95% CI 0.35 to 1.03; P = 0.0663; 86 participants; Analysis 6.1). The sequential treatment arm closed early due to a high incidence of severe AEs. We rated the certainty of the evidence as low, downgraded by two levels for risk of performance and detection bias, and risk of other bias as only immune‐related AEs were reported.
6.1. Analysis.

Comparison 6: Neoadjuvant immunotherapy (combined ipilimumab and nivolumab (2 different dosing regimens) compared to neoadjuvant immunotherapy (sequential ipilimumab then nivolumab), Outcome 1: Adverse events
Secondary outcomes
Overall response rate
Neoadjuvant combination ipilimumab and nivolumab was associated with a numerically higher rate of ORR (CR+PR) compared to neoadjuvant sequential therapy (RR 1.42, 95% CI 0.87 to 2.32; P = 0.1658; 86 participants; Analysis 6.2). We rated the certainty of the evidence as low, downgraded by one level for a risk of detection bias, and one level for imprecision as the confidence intervals were wide and the study was not powered to detect differences in this outcome.
6.2. Analysis.

Comparison 6: Neoadjuvant immunotherapy (combined ipilimumab and nivolumab (2 different dosing regimens) compared to neoadjuvant immunotherapy (sequential ipilimumab then nivolumab), Outcome 2: Overall response rate
Pathological complete response
There was a statistically significant higher rate of pCR with neoadjuvant combination ipilimumab and nivolumab compared to neoadjuvant sequential therapy (RR 2.24, 95% CI 1.06 to 4.71; P = 0.0335; 86 participants; Analysis 6.3).
6.3. Analysis.

Comparison 6: Neoadjuvant immunotherapy (combined ipilimumab and nivolumab (2 different dosing regimens) compared to neoadjuvant immunotherapy (sequential ipilimumab then nivolumab), Outcome 3: Pathological complete response
Disease‐free survival (corresponding to trial definition of relapse‐free survival)
The trial endpoint of relapse‐free survival, defined as time from surgery until the date of first relapse (local, regional, or distant metastases), corresponds closely with the review protocol definition of DFS. Median DFS (RFS) was not reached with 8.3 months, 17.7 months or 24 months follow‐up. DFS (RFS) at 24 months was estimated at 84% across all three arms (95% CI 76 to 92).
Time to recurrence
A HR for TTR was not reported. We could not obtain the data accurately from the published Kaplan Meier curves.
Other secondary outcomes
Only surgery‐related AEs were reported. Similar incidences of surgery‐related all‐grade AEs, and Grade 3 and 4 AEs, were reported across the treatment arms. Four Grade 3 to 4 surgery‐related AEs were reported in each treatment arm, with wound infection being the most common.
The trial did not report data on QOL, surgical outcomes or economic evaluation.
2.5. High‐dose interferon plus chemotherapy versus chemotherapy (one study)
Please see Table 7.
PICO: Hwu 2017 studied the use of neoadjuvant temozolomide in combination with HDI compared with neoadjuvant temozoloamide alone, in people with stage IIIB, IIIC, and IV melanoma. The trial included 52 participants, 27 in the temozolomide monotherapy arm, and 25 in the combination therapy arm.
Primary outcomes
Overall survival
This outcome was not reported for this comparison. Efforts to contact the authors failed.
Adverse events
The trial did not report AE data according to the CTCAE criteria, and therefore did not report this outcome in line with the predefined outcomes of this review. More serious AEs (SAEs) were reported in the temozolomide plus HDI arm: 8 (32%) compared with none in the temozolomide arm. The reported SAEs with combination therapy were increased leukocytes (4%), increased neutrophils (4%), lymphopenia (12%) and decreased platelets (12%).
Secondary outcomes
Overall response rate
ORR was greater with combination therapy (RR 1.75, 95% CI 0.62 to 4.95; P = 0.29; 36 participants; Analysis 7.1). Of note, the reported ORR includes participants who achieved a best response of stable disease, as well as CR and PR, and so is not directly comparable with other ORR outcomes in this review. We rated the certainty of the evidence as very low, downgraded by two levels for risk of detection bias and high risk of selective reporting and publication bias as no data are available from a peer‐reviewed source. We downgraded by one level for imprecision as the confidence intervals were wide and the number of events small.
7.1. Analysis.

Comparison 7: Neoadjuvant immunotherapy (high dose interferon) and chemotherapy compared to neoadjuvant chemotherapy, Outcome 1: Overall response rate
Other secondary outcomes
The trial did not report data on TTR, pCR, DFS, PFS, QOL, surgical outcomes or economic evaluation.
2.6. Neoadjuvant hu14.18‐IL2 versus adjuvant hu14.18‐IL2 (one study)
PICO: Albertini 2018 investigated the efficacy of neoadjuvant and adjuvant treatment with hu14.18‐IL2, an experimental cytokine immunotherapy, versus adjuvant hu14.18‐IL2, in people with stage III or IV melanoma. Hu‐IC is a humanized monoclonal antibody (mAb) covalently linked to two molecules of IL‐2 at the Fc region. The hu14.18 mAb recognises GD2, a disialoganglioside found in tumours of neuroectodermal origin. The trial randomised 20 individuals, 11 to the neoadjuvant arm and nine to the adjuvant arm. The trial was not powered for formal hypothesis testing. Median follow‐up of surviving participants is longer than four years (range 31.8 to 70.4 months).
Reported outcomes included recurrence‐free survival (analogous to disease‐free survival as per review protocol definition), adverse events (limited to Grade 3 to 4 AEs, and AEs requiring dose modification, and excluding previously documented AEs associated with hu14.18‐IL2), and OS. The trial did not present outcome data disaggregated by treatment arm, so we can not report them here. Efforts to contact the authors to obtain this information failed.
2.7. Neoadjuvant ipilimumab 10mg/kg plus high‐dose interferon versus neoadjuvant ipilimumab 3mg/kg plus high‐dose interferon
PICO: Tarhini 2018 investigated the efficacy of neoadjuvant treatment with either 10 mg/kg or 3 mg/kg ipilimumab, in combination with HDI in both treatment arms, in people with stage III melanoma. The trial randomised 28 people, 14 to each arm. The trial was not powered for formal hypothesis testing. The study hypothesis was that neoadjuvant ipilimumab in combination with HDI is safe and associated with durable pCR. The median follow‐up in responding participants was 32 months.
The primary endpoint was safety, with secondary endpoints including pCR, radiological response rate (no definition provided), PFS (no definition provided) and OS. The trial did not present OS, PFS and safety outcomes disaggregated by treatment arm, so we can not report them here. Efforts to contact the authors to obtain this information failed.
There were no differences in the rate of pCR or radiological response rate between treatment arms. A pCR was observed in five of 14 participants treated with ipilimumab 3 mg/kg, and in four of 14 participants in the ipilimumab 10 mg/kg arm (RR 1.25, 95% CI 0.42 to 3.70; Analysis 8.1). Radiological preoperative response rate was four of 14 participants with ipilimumab 3 mg/kg and six of 14 participants with ipilimumab 10 mg/kg (RR 0.67, 95% CI 0.24 to 1.86; Analysis 8.2), although the trial did not clearly define what it considered a radiological response. The trial did not report surgical outcomes directly, but stated that there was no delay to planned surgery secondary to ongoing toxicity resulting from the neoadjuvant induction phase.
8.1. Analysis.

Comparison 8: Neoadjuvant immunotherapy (ipilimumab 10mg/kg plus high‐dose interferon) compared to neoadjuvant immunotherapy (ipilimumab 3mg/kg plus high‐dose interferon), Outcome 1: Pathological complete response
8.2. Analysis.

Comparison 8: Neoadjuvant immunotherapy (ipilimumab 10mg/kg plus high‐dose interferon) compared to neoadjuvant immunotherapy (ipilimumab 3mg/kg plus high‐dose interferon), Outcome 2: Objective response rate
3. Chemotherapy
One study comprised chemotherapy (temozolomide) as a comparator (Hwu 2017), and is described under heading 2.5 (Table 7).
4. Topical agents
We did not find any relevant studies with topical agents as an intervention or comparator.
5. Radiotherapy
We did not find any relevant studies with radiotherapy as an intervention or comparator.
Discussion
Summary of main results
This Cochrane Review identified the available evidence for the use of neoadjuvant therapies in stage III and IV cutaneous melanoma. We identified eight RCTs with results that are included in this review, in addition to 11 ongoing RCTs. Most trials investigated combinations of treatments already authorised for advanced melanoma, and primarily investigated the effects of immunotherapy agents. Due to the diversity of regimens investigated, differences in trial methodology and definition of endpoints, and significant differences in the use of adjuvant treatment, we did not consider a meta‐analysis of all identified studies to be appropriate. We conducted a meta‐analysis that included outcomes from three trials, following qualitative assessment of similarities between the trials. The meta‐analysis did not increase the certainty in the results, primarily as the underlying methodological challenges that led to high levels of uncertainty in the individual assessments were replicated across the three included trials. A summary of the review outcomes for the most clinically relevant comparisons is provided below.
Neoadjuvant treatment compared to no neoadjuvant treatment
Following meta‐analysis of three trials (Amaria 2018a; Blank 2018; Dummer 2020b), we are very uncertain if neoadjuvant treatment increases OS when compared to no neoadjuvant treatment (Table 1). Neoadjuvant treatment may increase the rate of AEs compared to no neoadjuvant treatment, but the evidence is very uncertain. We are also very uncertain if neoadjuvant treatment increases TTR. ORR was not reported as a comparative outcome in the included trials, and QOL was not reported for this comparison.
Neoadjuvant targeted treatment (BRAF/MEK inhibition) compared to no neoadjuvant treatment
One trial compared neoadjuvant targeted therapy with dabrafenib and trametinib, with no neoadjuvant treatment (Amaria 2018a). We are very uncertain whether neoadjuvant targeted treatment with dabrafenib and trametinib increases OS (Table 2) or TTR when compared to no neoadjuvant treatment. AEs and ORR were not reported as comparative outcomes, and QOL data were not collected for this comparison. ORR was highest with targeted therapies (Table 2).
Neoadjuvant immunotherapy (talimogene laherparepvec) compared to no neoadjuvant treatment
One trial compared neoadjuvant immunotherapy with talimogene laherparepvec (T‐VEC) to no neoadjuvant treatment (Dummer 2020b). We are very uncertain if neoadjuvant immunotherapy with T‐VEC increases OS (Table 3) when compared to no neoadjuvant treatment. It may be associated with a higher rate of AEs, but the evidence is very uncertain. We are also very uncertain if it increases TTR. ORR was not reported as a comparative outcome and QOL data were not collected.
Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to adjuvant immunotherapy (combined ipilimumab and nivolumab)
One trial compared neoadjuvant immunotherapy with combined ipilimumab and nivolumab, to adjuvant immunotherapy with combined ipilimumab and nivolumab (Blank 2018). OS was not reported (Table 4). There may be little or no difference in the rate of AEs between these treatment options. ORR was not reported as a comparative outcome; TTR and QOL data were not collected. No difference in DFS was seen after four years of follow‐up.
Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (nivolumab)
One trial compared neoadjuvant immunotherapy with combined ipilimumab and nivolumab to neoadjuvant nivolumab (Amaria 2018b). Neoadjuvant combination therapy likely results in little to no difference in OS when compared to neoadjuvant nivolumab monotherapy (Table 5). Combination therapy may result in a higher rate of AEs, but the certainty of evidence is very low. It is worth noting that Amaria 2018b was halted early on the basis of an early observation of disease progression preventing surgical resection in the nivolumab arm and the high rate of treatment‐related AEs in the combination treatment arm. Neoadjuvant combination treatment may result in higher ORR when compared to neoadjuvant nivolumab monotherapy, although the evidence is very uncertain, and likely results in little to no difference in TTR or DFS. QOL data were not collected.
Neoadjuvant immunotherapy (combined ipilimumab and nivolumab (two different dosing regimens) compared to neoadjuvant immunotherapy (sequential ipilimumab then nivolumab)
One trial compared neoadjuvant immunotherapy with combined ipilimumab and nivolumab to neoadjuvant sequential immunotherapy (ipilimumab followed by nivolumab) (Rozeman 2019). OS was not reported (Table 6). Fewer immune‐related Grade 3 to 4 AEs were reported in the combination treatment arm compared to the sequential treatment arm; the sequential treatment arm closed early due to a high incidence of severe AEs. ORR was higher with the combination treatment also. Data on TTR and QOL were not collected. Similar rates of DFS (85%) were seen across all three treatment arms.
Neoadjuvant immunotherapy (high dose interferon) and chemotherapy compared to neoadjuvant chemotherapy
One trial compared the neoadjuvant combination of immunotherapy with HDI and chemotherapy with temozolomide, to neoadjuvant chemotherapy with temozolomide alone (Hwu 2017). No data were reported on OS, AEs, TTR, or QOL for this comparison (Table 7). Neoadjuvant HDI plus chemotherapy may have little to no effect on ORR, but the evidence is very uncertain.
Overall completeness and applicability of evidence
We considered the external validity of the included trials to be low, due to small sample size and lack of representativeness of the participants, limited duration of follow‐up, and inconsistent use of adjuvant therapy in the control arms. A number of the included trials compared neoadjuvant treatment strategies rather than comparing neoadjuvant treatment with SOC, which is surgery, adjuvant treatment and observation. The challenges posed by inconsistencies in trial design, for example in the heterogenous dosing regimens and treatment combinations of immunotherapies, are reflected in the limited feasibility of meta‐analysis of the included studies.
A total of 402 people participated in the included clinical trials. Most trials were single‐centre trials, and recruited participants primarily from the USA, the Netherlands, and Australia. The participants recruited to the included trials are unlikely to be representative of the broader population of people with melanoma, given that such a large proportion had an ECOG PS of 0 (79%) and LDH levels less than the upper limit of normal (97%). This may be at least partially explained by the fact that the included trials are early stage, phase I and II trials. This may be rectified to some extent in the larger ongoing RCTs, which will also recruit in more centres and in more countries, which will improve the representativeness of the population. It has been previously noted that people recruited to melanoma RCTs may not be fully representative of the broader population, and those unrepresented people may have poorer outcomes (Van Zeijl 2020). There were some differences in the proportions of participants with stage IIIB, IIIC, and stage IV disease randomised to the trials (Characteristics of included studies), which impacts baseline risk of disease recurrence (Balch 2009), and therefore creates heterogeneity across the identified trials.
Some trials did not appropriately control for adjuvant treatment in the control arm, and we downgraded the certainty of the evidence due to high risk of bias and imprecision. These trials permitted a range of optional adjuvant treatments, many of which do not reflect current SOC, administered at the discretion of the investigator. Additionally, a number of trials compared two neoadjvuant treatment arms, rather than comparing neoadjuvant treatment to SOC. Feasibility of meta‐analysis was reduced by the inconsistent use of adjuvant therapy across both the intervention and comparator arms in the included trials. Many of the identified trials would have been designed and initiated prior to adjuvant immunotherapy/targeted therapy becoming SOC; however, the absence of a consistent approach to adjuvant therapy, aligned with current treatment guidelines, limits the generalisability of the outcomes of these studies to current practice, particularly for time to event outcomes such as OS and TTR. Most of the identified ongoing trials include planned adjuvant treatment in both the intervention and control arm, which will address this deficiency in the evidence base.
There were important differences between the trials, for example, the different dosing schedules of immunotherapies and consequently different treatment effects, and the underlying differences in mechanism of action between immunotherapies, targeted treatments and chemotherapy. The trials included in this review considered mainly immunotherapy type agents, a reflection of the mode of action of these agents and their ability to impact the tumour environment as well as the tumour itself. In terms of the ongoing trials identified, all included an immunotherapy agent in the neoadjuvant arm, and most included a combination of agents, as combinations of immunotherapies, or immunotherapies with targeted treatments. New drug treatments continue to come on stream for melanoma (e.g. relatlimab in, a lymphocyte activation gene‐3 (LAG‐3) antibody administered in combination with nivolumab for unresectable or metastatic melanoma) and SOC for the disease will continue to evolve. Future iterations of this review will need to incorporate a broader range of immunotherapy interventions in particular, to maintain relevance to clinical practice. Only one of the included trials considered chemotherapy; chemotherapy does not feature as an intervention or comparator in any of the ongoing trials. This reflects the broader shift away from traditional chemotherapy drugs in favour of targeted treatments and immuno‐stimulating agents (Robert 2016). Similarly, no trials of topical agents or radiation therapy were identified in the review.
Differences in the definitions of measured recurrence endpoints also impact feasibility of meta‐analysis; some trials measured recurrence endpoints from point of randomisation, whereas some measured from the point of surgery, and there were also differences in the censoring rules for a recurrence event, meaning that the endpoints were not comparable.
The importance of ORR as a surrogate endpoint for efficacy of neoadjuvant treatment is subject to debate. While ORR with immunotherapy regimens targeting the PD‐L1/CTLA‐4 pathways is modest, clinical outcomes such as recurrence events appear infrequent. In this review, ORR was higher with combination treatment than single‐agent regimens (Table 5; Table 6; Table 7), and was highest with targeted therapies (Table 2), and lowest with T‐VEC (Table 3). It is unclear if ORR is a reasonable surrogate for recurrence and survival events in neoadjuvant treatment of melanoma.
Similarly, the importance of pCR as a surrogate endpoint for efficacy of neoadjuvant treatment is an ongoing point of discussion. In this review, rates of pCR reported across the included trials varied from 17.1% (T‐VEC, Dummer 2020b) to 58% (BRAF/MEK combination, Amaria 2018a). Rates may be higher with combination immunotherapy compared with nivolumab monotherapy or sequential ipilimumab and nivolumab (Amaria 2018b; Rozeman 2019). The correlation between pCR and relapse and survival outcomes was investigated in a number of studies. Establishing and routinely applying a single definition of pCR is a key starting point for determining the relationship between pCR outcomes and survival outcomes. A pooled analysis of data from a selection of single armed‐trials examined the correlation between pathological responses and survival outcomes (Menzies 2021), and early indicators are that particularly for immunotherapies, pathological responses may be appropriate indicators of long‐term response. Selection of appropriate endpoints for neoadjuvant trials is an ongoing regulatory challenge and there is little evidence in the literature of a standardised endpoint emerging. Pathological response rates were accepted by the Food and Drug Administration (FDA) in the regulatory authorisation of neoadjuvant pertuzumab for breast cancer (FDA 2020; Prowell 2012).
We did not find any studies that examined QOL or PFS with neoadjuvant treatment. Similarly, no studies considered the economic impact of neoadjuvant treatment. Measurement of surgical outcomes was rare, and thus we are uncertain if neoadjuvant treatment impacts surgical outcomes. The approach to the definition and recording of AEs varied across the studies, which limits the comparability of the safety profiles of the neoadjuvant regimens.
There remain a number of important gaps in the evidence base for neoadjuvant treatment of advanced melanoma. There are challenges in establishing surrogate markers which will satisfy both regulators and funders of new technologies. Demonstrating differences in OS is a difficult challenge for trial design and follow‐up, due to the longer OS now experienced by people with stage III and IV melanoma with effective treatments in the adjuvant and metastatic settings. Measuring OS in neoadjuvant trials will require many years of follow‐up, and the outcome will be heavily confounded by adjuvant treatments, and treatments received in the metastatic setting. It must be considered if further extending OS should remain as a primary aim of neoadjuvant treatments of melanoma, or whether patient‐important outcomes, such as time to disease recurrence or time without symptoms, are more relevant objectives for future clinical research in this field.
The current trials provide little information about the consequences of neoadjuvant treatment for the current treatment paradigm in the metastatic setting, for example, the potential for retreatment with immunotherapy, potential for resistance emerging to targeted therapy, and determining if there is a risk of a poorer response in later stage disease. Research into these issues will be important in providing information for shared decision‐making around whether to opt for neoadjuvant treatment, and also potentially to identify people who are most likely to benefit from neoadjuvant treatment.
Overall, the included trials were consistent in indicating early signals of potential efficacy of neoadjuvant treatment compared to surgery alone. Further trials will be needed to identify the benefits of neoadjuvant treatment compared with adjuvant treatment; these phase III trials will likely recruit a more diverse patient population which will improve the external validity of the evidence base.
Quality of the evidence
The relatively small number of trials identified, and the small number of participants enrolled on these studies, limits the robustness of the conclusions which can be drawn from this review.
The level of certainty in the evidence for time to event outcomes such as OS and TTR was very low, downgraded consistently for imprecision and high risk of bias. Publication bias was also a concern with some of the trials, with no peer‐reviewed data available. Identified trials were almost all early‐stage clinical trials, not designed nor adequately powered to detect differences in time to event outcomes. Differences in the definitions of measured recurrence endpoints also impacted the feasibility of meta‐analysis; some trials measured recurrence endpoints from point of randomisation, whereas some measured from the point of surgery, and there were also differences in the censoring rules for a recurrence event, meaning that the endpoints were not comparable. Additionally, some of the included trials did not adequately provide for the use of SOC adjuvant treatment in the control arm. Time‐to‐event outcomes (OS, TTR, DFS) are more likely to be impacted by differences in follow‐up duration between the treatment arms, and in differences in the adjuvant treatment strategies employed in the trials, than other outcomes such as ORR and pCR. Duration of follow‐up ranged from 15 months to over four years in the included studies. Based on these important differences between trials, we concluded that a meta‐analysis of all identified trials was not feasible and would not provide useful data for clinical decision‐making.
The meta‐analysis did not increase the certainty in the results of the OS and TTR outcomes, primarily as the underlying methodological challenges that led to high levels of uncertainty in the individual assessments were replicated across the included trials.
ORR outcomes were commonly not described comparatively, as they are only measured in the neoadjuvant treatment arm. This, coupled with the high risk of detection bias in this outcome, led to us reporting the certainty in the ORR outcomes as low to very low.
Potential biases in the review process
The literature search was extensive and is expected to have identified all eligible RCTs. There remains a possibility that an eligible trial may have been omitted. We reviewed other published review articles, none of which identified any other eligible RCTs (Agreements and disagreements with other studies or reviews).
We have clearly identified where our methods have deviated from those predefined in the review protocol (see Differences between protocol and review). We did not adjust for between‐arm correlation in the only trial included which had more than two treatment arms (Rozeman 2019). We opted to pool the results from two treatments arms, and compare with the sequential treatment arm without any adjustments, as the trial itself was not powered for any formal hypothesis testing, and all results reported are simply descriptive.
We could not access three of the identified publications, and have categorised these studies as awaiting classification (Characteristics of studies awaiting classification). Since de Braud 1994 and Kleeberg 1986 are older publications, it is unlikely they would have been eligible for inclusion as the AJCC seventh edition staging criteria would not have been used in enrolling participants, and therefore we do not consider there is a serious risk of bias to our review as a result of their exclusion.
We did attempt to contact authors where all the required information to determine inclusion/exclusion was not available, or to obtain additional results (Table 8). We have highlighted in the Characteristics of included studies where we used data obtained directly from the authors. There is a chance that some ongoing studies may have completed and results may be available since we contacted the authors.
Agreements and disagreements with other studies or reviews
A published systematic review of neoadjuvant treatment in melanoma (Yu 2016) identified two trials for inclusion, Hwu 2017 and a study by Aryian and colleagues (Ariyan 1982). We excluded the latter from this review (Excluded studies) as participants could select which treatment arm they wished to be allocated to, an outdated disease staging classification was used, and it was not possible to determine if the enrolled participants were consistent with the eligible population for this review. The review concluded that there was no strong evidence to support the routine use of neoadjuvant treatment for surgically resectable stage III or oligometastatic melanoma.
A separate systematic review, which included single‐arm trials and excluded phase I trials, identified four RCTs, all of which are included in this review (Boulva 2021). The authors concluded that a meta‐analysis was not possible due to the variability of the treatment arms and reported outcomes, and the paucity of homogenous randomised data. They did note the encouraging preliminary results, which suggest that neoadjuvant treatment may be safe and feasible, and may be associated with a high pathological response rate. More mature data, phase III trials, and an understanding of the correlation between pCR and long‐term outcomes, are all required.
Khunger 2019 provided an overview of recent developments in neoadjuvant therapeutics for melanoma, and identified a number of single‐arm trials that we excluded from this review as they were non‐randomised studies (Excluded studies). A number of studies identified in our review were not mentioned in Khunger 2019 (Albertini 2018; Hwu 2017). Khunger 2019 highlighted the benefit of neoadjuvant treatment in providing biospecimens before and after therapy, which may provide "mechanistic insights and biomarker findings", offering insight into the biologic and immunologic response to novel therapeutics.
Similarly, Versluis 2020 and Pelster 2020 provided a summary of neoadjuvant trials of checkpoint inhibitors, neither of which identified any additional RCTs other than those included in our review. Versluis 2020 noted the large number of ongoing trials of neoadjuvant treatment using immunotherapies for a large range of tumour types, and highlighted the potential for 'reverse translation' using preclinical testing to lead to personalised neoadjuvant treatment regimens for patients in the future. They note the need to validate the relationship between pathologic response and longer‐term RFS, and for phase III RCTs to demonstrate an RFS benefit with neoadjuvant treatment compared with adjuvant treatment. Pelster 2020 arrived at similar conclusions, highlighting the potential benefit of combination regimens with appropriate dose adjustments to moderate toxicity, and the importance of harmonising neoadjuvant trial design.
Authors' conclusions
Implications for practice.
We are uncertain if neoadjuvant treatment can improve overall survival (OS), compared with the standard of care (SOC) in people with stage III or IV melanoma. We are uncertain whether neoadjuvant treatment improves time to recurrence (TTR). Overall response rate (ORR) is highest with targeted treatment, and with combination regimens compared with single‐treatment regimens. There remains the possibility that neoadjuvant immunotherapy may reduce the likelihood of successful surgical resection due to rapid disease progression (Amaria 2018b), or that treatment‐related adverse events (AEs) may delay surgery. Neoadjuvant treatment may be associated with a slightly higher rate of AEs compared with SOC, and we are uncertain if the benefits outweigh the risks of harm. The identified trials were early‐phase clinical trials with poor external validity, inadequately powered to detect differences in outcomes between treatment arms, and confidence intervals often overlapped no effect. The majority of trials were using immunotherapy agents as neoadjuvant treatment, but in most trials the control arm did not reflect the current SOC, which is adjuvant immunotherapy or targeted treatment. Immunotherapies and targeted therapies do not currently have regulatory authorisation in the USA or Europe for neoadjuvant treatment of melanoma. Use of immunotherapy in the neoadjuvant setting could have significant cost issues for health services, due to the potential for cost‐offsets of immunotherapy treatment in the recurrent disease setting. This may be offset by the additional healthcare resource use in administering treatment in the neoadjuvant setting, where potentially many people would be considered eligible for treatment.
Implications for research.
Phase III randomised controlled trials (RCTs) of sufficient size, duration of follow‐up and with adequate power to detect differences in time‐to event outcomes such as relapse‐free survival (RFS), are required to reduce the imprecision consistently highlighted as a quality issue in this review. The use of control arms which provide current standard of care adjuvant treatment with immunotherapy or targeted treatments, will be essential to recruit participants to these trials, and to elicit the benefits of neoadjuvant treatment versus adjuvant treatment. Blinding would be appropriate to address some of the concerns regarding performance and detection bias. The ongoing studies identified in this review are larger trials, including some phase III trials with appropriate adjuvant treatment as part of the control arm, will go some way to addressing the deficiencies in the certainty of evidence as highlighted in this review.
The use of optional or non‐standard of care adjuvant therapy was highlighted as a potential source of bias in this review. Since the inception of this review protocol, adjuvant treatment with immunotherapy or targeted therapy has become the accepted standard of care for stage III melanoma following surgical resection. Thus, future trials evaluating neoadjuvant treatment strategies should include adjuvant treatment with immunotherapies or targeted therapies in the control arm, to identify if there is meaningful clinical benefit to patients beyond that offered by adjuvant treatment. Future neoadjuvant trials identified in the review include an adjuvant treatment component in the investigational arm also, and will improve the quality of the available evidence. There are exceptions to this, for example where the objective of the trial is to risk‐stratify participants to avoid adjuvant treatment.
A challenge highlighted in the identified trials is the heterogeneity in trial design, including the use of varied definitions of trial endpoints. A defined core outcome set for neoadjuvant treatment trials is necessary. INMC 2019 goes some way towards this, suggesting that recurrence‐free survival, event‐free survival and distant metastatic‐free survival are the important survival outcomes to include in these trials, but unfortunately does not provide clear definitions of these endpoints. INMC 2019 similarly highlights the importance of consistent application of pathological scoring measures in order to validate its use as a surrogate outcome, and to enable cross‐trial comparisons; Tetzlaff 2018 is a welcome start. Further investigation of the relationship between radiographic response, pathological response and survival outcomes is required, as has been initiated by Menzies 2021, and if it potentially differs depending on treatment type, e.g. immunotherapies versus targeted treatments.
The included studies did not collect or report data on quality of life (QOL) outcomes, and rarely on surgical outcomes. The absence of data on QOL outcomes and on surgical outcomes makes a holistic assessment of the benefits and risks of neoadjuvant treatment challenging. These endpoints should be considered for inclusion in future neoadjuvant trial designs as a priority. The potential impact of neoadjuvant treatment on surgical outcomes, such as delays or cancellation of surgery due to AEs or disease progression, or differences in the R0 resection rate, or time to subsequent healing postsurgery, are relevant outcomes to patients and health systems alike, and should be considered in subsequent trials of neoadjuvant treatment. Reaching consensus on the most important surgical outcomes to record will be important. Similarly, information on QOL with neoadjuvant treatment will be important for informed decision‐making by patients, if electing to undergo neoadjuvant treatment or indeed, if considering entering a clinical trial of neoadjuvant treatment. Information on QOL is also important for economic evaluation of treatments, and prospective collection of data within RCTs can provide the information necessary to provide evidence of additional benefit to Health Technology Assessment agencies as well as regulatory authorities, thus facilitating patient access to treatment. The potential for neoadjuvant treatment to reduce drug costs should also be examined when designing future trials of neoadjuvant treatment, particularly if short courses of neoadjuvant treatment could reduce the risk of recurrence and therefore the high costs incurred in the metastatic setting. This is especially important given that the high costs associated with these drugs prohibits access in the metastatic setting in many countries (Kandolf Sekulovic 2018).
Treatments are associated with high costs in every jurisdiction, and consideration of the opportunity cost of funding these drugs as neoadjuvant treatment will be required. Data relevant to Health Technology Assessment agencies, as well as regulatory bodies, should be collected in trials, to ensure neoadjuvant treatment regimens are ultimately available to those who may benefit from them. Such endpoints include preference‐based measures of QOL, quantifying resource use, and cost offsets in the recurrent disease setting. It will also be essential to establish the nature of the relationship between pathological complete response (pCR), TTR and OS.
Histological or cytological staging was required prior to enrolment in the identified studies included in this review. In clinical practice, patient selection will be an important component of the provision of neoadjuvant treatment, requiring careful assessment of the likelihood of successful resection of the tumour, and balancing the potential risks of delaying surgery with the potential benefits of providing upfront systemic treatment. A recent publication from the INMC provides suggestions for harmonising the approach to issues of surgical relevance in neoadjuvant trials for melanoma, such as the definition of resectability, and the extent and scope of routine surgery (Van Akkooi 2022). The adoption of the eighth edition of the American Joint Committee on Cancer (AJCC) melanoma staging criteria, and the use of these criteria for staging melanoma in participants enrolled in future clinical trials, will impact on the comparability of future trials and trial outcomes with the trials included in this review, where all included trials used the seventh edition criteria.
Finally, we currently have little information about the consequences of neoadjuvant treatment for the available treatment options in the recurrent or metastatic disease setting, for example retreatment with immunotherapy, the potential for resistance emerging to targeted therapy etc. Neoadjuvant trials provide an opportunity to engage in translational research, to obtain extensive personalised information on immune response to treatment, genomic markers, and long‐term clinical outcomes, and to potentially inform personalised treatment choices for patients in future. Research in this area will be important to adequately inform choices on whether to opt for neoadjuvant treatment, and to identify those patients who are most likely to benefit.
History
Protocol first published: Issue 3, 2018
Acknowledgements
Acknowledgements from the authors
We wish to acknowledge the support of the Health Research Board in Ireland for providing funding for the conduct of this review.
We thank the Cochrane Skin Group, in particular Emma Mead and Elizabeth Doney for advice, assistance in protocol design, and designing search strategies, and Helen Scott, Robert Boyle and Laura Prescott for their assistance and advice. We thank Corynne Marchal, Managing Editor of the Cochrane Lung Cancer Group for invaluable assistance in accessing some full‐text articles for the review.
We thank Gilliosa Spurrier from Melanoma France for her review and commentary on the protocol from a consumer perspective. We thank Dr Karren Komitas (University of Texas School of Dentistry at Houston, TX, USA) for providing translation of Russian language abstracts. We thank Dr Aisling O' Leary for proofreading and helpful contributions.
We wish to thank all the investigators who responded to our requests for additional information regarding trial eligibility and trial outcomes.
Editorial and peer‐reviewer contributions
Cochrane Skin supported the authors in the development of this review. The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Toby Lasserson, Cochrane Evidence Production & Methods Directorate
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): c/o Cochrane Production Service (individual chose not to be publicly acknowledged)
Peer‐reviewers (provided comments and recommended an editorial decision): Mary S Brady, Gastric and Mixed Tumor Service, Memorial Sloan Kettering Cancer Cente (clinical/content review); Nobuyuki Horita, Chemotherapy Center, Yokohama City University Hospital (clinical/content review); Enrico Zelin, Dermatology Clinic of Trieste, Maggiore Hospital, University of Trieste, Trieste, Italy (clinical/content review); Yana G. Najjar, UPMC Hillman Cancer Center (clinical/content review); Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review), Anne Littlewood, Cochrane Oral Health, University of Manchester (search review).
Appendices
Appendix 1. Glossary
| Term | Explanation |
| Abscopal effect | The abscopal effect is a phenomenon in which localised radiotherapy of a tumour is associated with the regression of other tumours at a distance from the irradiated site, likely mediated by activation of the immune system. The underlying molecular mechanism of the abscopal effect is poorly understood. |
| Adjuvant treatment | Treatment given to patients after the primary therapy, which is usually surgical removal of the tumour, when there is a high risk of future recurrence based on tumour stage and histology. |
| Adverse event | An unfavourable outcome that occurs during or after the use of a drug or other intervention, but is not necessarily caused by it. |
| Alkylating agent | A type of drug that is used in the treatment of cancer. It interferes with the cell's DNA and inhibits cancer cell growth (NCI 2021). |
| AJCC TNM staging | A system for disease staging that assigns the pathological disease stage according to the T (primary tumour), N (regional lymph nodes) and M (distant metastasis) status of the melanoma. Stage IV melanoma includes all patients with metastases to distant sites. |
| Angiogenesis | The formation of new blood vessels. |
| Anti‐angiogenic | Anti‐angiogenic agents act to prevent new blood vessels, which tumours require to grow, from forming. |
| Anti‐vascular epithelial growth factor (VEGF) monoclonal antibody | A substance that binds to receptors disabling a signalling protein called vascular endothelial growth factor (VEGF). VEGF may be found on some types of cancer cells and stimulates the formation of blood vessels. There are different types of anti‐VEGF monoclonal antibodies being studied in the treatment of cancer. These substances are a type of angiogenesis inhibitor (i.e. may prevent the growth of new blood vessels that tumours need to grow) and a type of monoclonal antibody (NCI 2021). |
| Bias | Bias is defined as a systematic error, or deviation from the truth, in results. Biases can lead to under‐estimation or over‐estimation of the true intervention effect and can vary in magnitude: some are small (and trivial compared with the observed effect) and some are substantial (so that an apparent finding may be due entirely to bias) (Boutron 2021). |
| BRAF mutation | Serine/threonine‐protein kinase B‐Raf, an oncogene occurring in ~50% cutaneous melanoma. |
| BRAF, RAS, NF1 and Triple Wild Type | Classification of melanomas based on four genomic categories, proposed by the Cancer Genome Atlas. |
| Checkpoint inhibitors | Drugs that "block 'checkpoint' proteins that stop the immune system from attacking the cancer cells" (NCI 2021); these agents override the signalling/activation of immune system checkpoints, encouraging the immune system to recognise cancer and generate an immune response directed towards the cancer. Checkpoint inhibitors used in clinical practice for the treatment of melanoma include PD‐1 and CTLA‐4 targeted agents. |
| CTCAE | Common Terminology Criteria for Adverse Events, is a descriptive terminology which can be utilised for AE reporting. A grading or severity term is provided for each AE term. |
| Cytokine | A protein made by certain immune and non‐immune cells which acts on the immune system. Cytokines can stimulate or inhibit the immune system. Exogenous cytokines are used to help the body fight cancer, infections, and other diseases. Examples of cytokines are interleukins, interferons, and colony‐stimulating factors (filgrastim, sargramostim). |
| Cytotoxic | A substance that kills cells, including cancer cells. These agents may stop cancer cells from dividing and growing and may cause tumours to shrink in size (NCI 2021). |
| Cytotoxic T lymphocyte associated protein 4 (CTLA‐4) | A "checkpoint" protein found on T cells (a type of immune cell) that helps keep the body’s immune responses in check. When CTLA‐4 is bound to another protein called B7, it helps keep T cells from killing other cells, including cancer cells. Some anticancer drugs, called immune checkpoint inhibitors, are used to block CTLA‐4. When this protein is blocked, the “brakes” on the immune system are released and the ability of T cells to kill cancer cells is increased (NCI 2021). |
| Dentritic cells | Dentritic cells (DCs) are a type of cell within the immune system, and act as a messenger cell (antigen presenting cell) activating T cell and B cell immune responses to antigenic (foreign) material. |
| Dermoscopy | Examination of the skin using skin surface microscopy, to evaluate pigmented skin lesions and diagnose melanoma (DermNet 2004). |
| Disease‐free survival | Defined as the length after primary cancer treatment ends that the patient survives without any signs or symptoms of that cancer. Sometimes described as recurrence free survival. |
| Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) | A cytokine that stimulates stem cells ("cells with the potential to develop into many different types of cells in the body" (NLM 2016)) to create granulocytes and monocytes (types of white blood cells), thereby, boosting the immune response. |
| Histopathology | The study of diseased cells and tissues using a microscope (NCI 2021). |
| Immuno‐modulatory | A substance that stimulates or suppresses the immune system and may help the body fight cancer, infection, or other diseases. Specific immunomodulating agents, such as monoclonal antibodies, cytokines, and vaccines, affect specific parts of the immune system. Nonspecific immunomodulating agents, such as BCG and levamisole, affect the immune system in a general way (NCI 2021). |
| Immunotherapy | A type of therapy that uses substances to stimulate or suppress the immune system to help the body fight cancer, infection, and other diseases. Some types of immunotherapy only target certain cells of the immune system. Others affect the immune system in a general way. Types of immunotherapy include cytokines, vaccines, bacillus Calmette‐Guerin (BCG), and some monoclonal antibodies such as PD‐1 inhibitors and CTLA‐4 inhibitors (NCI 2021). |
| Imprecision | In the context of GRADE, imprecision refers to random error in the trial, an aspect of the quality of the evidence. It is generally considered in terms of the confidence intervals, specifically the width of the confidence intervals, and whether they cross the boundary of no treatment effect, or not (Guyatt 2011a) |
| Inconsistency | In the context of GRADE, inconsistency refers to differences in relative risk reductions across trials for a given outcome (Guyatt 2011b). |
| Indirectness | In the context of GRADE, indirectness refers to differences between the study population, interventions, or outcomes, and those defined by the review question. It can also refer to a lack of head‐to‐head trials between the interventions of interest (Guyatt 2011c). |
| Isolated limb perfusion | A procedure that may be used to deliver anticancer drugs directly to an arm or leg. The flow of blood to and from the limb is temporarily stopped with a tourniquet (a tight band around the limb), and anticancer drugs are put directly into the blood of the limb. This allows the person to receive a high dose of drugs in the area where the cancer occurred (NCI 2021). |
| Loco‐regional | Restricted to a localised region of the body. |
| MEK inhibitor | Mitogen‐activated protein kinase (MEK) is part of the RAS‐RAF‐MEK‐ERK signalling pathway. A MEK inhibitor is a type of protein kinase inhibitor which targets this pathway, to reduce tumour growth and proliferation. |
| Melanocytes | A cell in the skin and eyes that produces and contains the pigment called melanin (NCI 2021). |
| Meninges | The three membranes (the dura mater, arachnoid, and pia mater) that line the skull and vertebral canal and enclose the brain and spinal cord. |
| Metastases | The spread of cancer cells from the place where they first formed to another part of the body. In metastasis, cancer cells break away from the original (primary) tumour, travel through the blood or lymph system, and form a new tumour in other organs or tissues of the body. The new, metastatic tumour is the same type of cancer as the primary tumour. For example, if breast cancer spreads to the lung, the cancer cells in the lung are breast cancer cells, not lung cancer cells (NCI 2021). |
| Metastectomy | Surgery to remove one or more metastases (tumours formed from cells that have spread from the primary tumour). When all metastases are removed, it is called a complete metastasectomy (NCI 2021). |
| Mitotic rate | A measure of how fast cancer cells are dividing and growing. To find the mitotic rate, the number of cells dividing in a certain amount of cancer tissue is counted. Mitotic rate is used to help find the stage of melanoma (a type of skin cancer) and other types of cancer. Higher mitotic rates are linked with lower survival rates (NCI 2021). |
| Monoclonal antibody (MAB) | "A type of targeted drug therapy. Some monoclonal antibodies are a type of immunotherapy. Monoclonal means all one type. So each MAB is a lot of copies of one type of antibody. They are made in a laboratory. Monoclonal antibodies work by recognising and finding specific proteins on cancer cells. Each monoclonal antibody recognises one particular protein. So different monoclonal antibodies have to be made to target different types of cancer. They work in different ways depending on the protein they are targeting" (CRUK 2017). |
| Mucosal melanoma | Mucosal melanoma is a rare form of melanoma (around 1% cases of melanomas) that's occurs on mucosal surfaces. Mucous membranes are those surfaces that line cavities within the body, such as the respiratory tract and genitourinary tract. Mucosal melanoma is caused by melanocytes in these mucosal surfaces becoming cancerous. |
| Neoadjuvant treatment | Treatment that is given before the (usually) surgical treatment of a primary tumour with the aim of improving the results of surgery or (chemo)radiotherapy and preventing the development of metastases. |
| Oncolytic | An oncolytic agent is characterised by causing oncolysis or destruction of tumour cells. |
| Overall response rate | The percentage of people in a study or treatment group who have a partial or complete response to the treatment within a certain period of time. A partial response is a decrease in the size of a tumor or in the amount of cancer in the body, and a complete response is the disappearance of all signs of cancer in the body. In a clinical trial, measuring the overall response rate is one way to see how well a new treatment works (NCI 2021). |
| Overall survival | Defined as the time from start of treatment (or randomisation) to death from any cause. |
| Pathological Complete Response | Defined as an absence of residual invasive and in situ cancer on hematoxylin and eosin evaluation of the complete resected specimen and sampled regional lymph nodes, following completion of neoadjuvant systemic therapy. Pathological complete response has been included as an endpoint in neoadjuvant clinical trials, and has been considered a clinically relevant endpoint by regulators examining the efficacy of neoadjuvant treatment for breast cancer. Research into its clinical significance in melanoma is ongoing. |
| Primary melanoma | The description of the original site of the tumour (the primary location) as opposed to a disease stage. |
| Progammed Death‐1 (PD‐1) regulatory pathway | Programmed Death‐1 (PD‐1) is a protein found on T cells (a type of immune cell) that helps keep the body's immune responses in check. When PD‐1 is bound to another protein called PD‐L1, it helps keep T cells from killing other cells, including cancer cells. When the PD‐1 protein is blocked so that PD‐L1 cannot attach, the 'brakes' on the immune system are released and the ability of T cells to kill cancer cells is increased. |
| Programmed Death‐Ligand 1 (PD‐L1) | Programmed Death‐Ligand 1 (PD‐L1) is a protein which binds to the PD‐1 protein and prevents T cell activation against cancer cells. |
| Progression Free survival | Defined as the time from start of treatment (or randomisation) to disease progression or death from any cause. |
| R0 surgical resection | R0 resection indicates a microscopically margin‐negative resection, in which no gross or microscopic tumour remains in the primary tumour bed. |
| RAS‐RAF‐MEK‐ERK pathway | Also known as the MAPK/ERK pathway, this is a signalling pathway that regulates cell production; it comprises a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. |
| Sentinel lymph node biopsy | Removal and examination of the sentinel node(s), the first lymph node(s) to which cancer cells are likely to spread from a primary tumor (NCI 2021). |
| Sterotactic radiotherapy | A type of external radiation therapy that uses special equipment to position the patient and precisely deliver radiation to a tumour. The total dose of radiation is divided into several smaller doses given over several days. Stereotactic radiation therapy is used to treat brain tumours and other brain disorders. It is also being studied in the treatment of other types of cancer, such as lung cancer. Also called stereotactic external‐beam radiation therapy and stereotaxic radiation therapy (NCI 2021). |
| Talimogene laherparepvec | A drug used to treat melanoma that has recurred (come back) after surgery. Talimogene laherparepvec is made with a form of the herpes virus that has been changed in the laboratory to infect and break down cancer cells without harming normal cells. Talimogene laherparepvec is injected directly into tumours in the skin and lymph nodes. It is a type of oncolytic virus therapy. Also called Imlygic and T‐VEC (NCI 2021). |
| Time to Recurrence (TTR) | Time from date of randomisation to local relapse/recurrence or regional relapse/recurrence or distant metastases or death from any cause. Sometimes described as event free survival. |
| Toll‐like receptor (TLR) 7 agonist | Toll‐like receptors (TLRs) are proteins which play a key role in the immune system, recognising infectious agents and mediating cytokine production as part of the immune response. TLR agonists activate the TLR‐mediated reponse. TLR 7 is encoded by the TLR7 gene in humans. |
| Treatment related adverse events (TRAEs) | Treatment emergent adverse events (TEAE) are undesirable events not present prior to medical treatment, or an already present event that worsens either in intensity or frequency following the treatment. |
| Tumour infiltrating lymphocytes | Tumour infiltrating lymphocytes (TILs) are white blood cells that migrate into a tumour, and are implicated in killing tumour cells as part of a host immune response to cancer. The presence of TILs is often associated with better clinical outcomes. TILs contain a mixture of different cell types, such as B cells, macrophages etc.; T cells are usually the most abundant type of cell. |
| Vascularity | In medical terms, this is the state of blood vessel development and functioning in an organ or tissue. |
| Vascular permeability | Describes the capacity of a blood vessel wall to allow for the flow of small molecules (e.g. drugs) or cells (e.g. lymphocytes) in and out of the blood vessel. |
| An abbreviated version of this glossary was previously published (Gorry 2018; Gorry 2020) | |
Appendix 2. Cochrane Skin Specialised Register via the Cochrane Register of Studies (CRS‐Web)
((Melanoma* or “skin Neoplas*” or “skin cancer*”) and (neoadjuvant* or immunotherap* or “Interleukin‐2” or “Interferon‐alpha*” or ipilimumab or nivolumab or pembrolizumab or “cytotoxic t lymphocyte antigen 4 inhibitor*” or “CTLA‐4 inhibitor*” or “programmed death‐ligand 1” or “PD‐L1 inhibitor*” or “checkpoint inhibitor*” or “interleukin‐2” or “interferon alpha*” or “targeted treatment*” or ((braf or mek or mapk) and inhibitor*) or dacarbazine or temozolomide or vindesine or vinblastine or paclitaxel or cisplatin or carboplatin or lomustine or carmustine or fotemustine or bendamustine or tamoxifen or vemurafenib or dabrafenib or trametinib or cobimetinib or “mitogen activated protein kinase inhibitor*” or “antineoplastic agent*” or chemotherap* or “cancer vaccine*” or “talimogene laherparepvec” or “intra‐lesional treatment*” or imiquimod or bevacizumab or axitinib or (topical and neoadjuvant)))
Appendix 3. CENTRAL (Cochrane Library) search strategy
#1 [mh melanoma [mj]] #2 melanoma*:ti,ab #3 [mh "skin neoplasms" [mj]] #4 (skin next neoplas*):ti,ab #5 (skin next cancer*):ti,ab #6 {or #1‐#5} #7 [mh "neoadjuvant therapy" [mj]] #8 neoadjuvant*:ti,ab #9 #7 or #8 #10 [mh immunotherapy [mj]] #11 [mh Interleukin‐2 [mj]] #12 [mh interferon‐alpha [mj]] #13 (ipilimumab or nivolumab or pembrolizumab):ti,ab #14 cytotoxic t lymphocyte antigen 4 inhibitor*:ti,ab #15 CTLA‐4 inhibitor*:ti,ab #16 programmed death‐ligand 1:ti,ab #17 PD‐L1 inhibitor*:ti,ab #18 (checkpoint next inhibitor*):ti,ab #19 (interleukin‐2):ti,ab #20 (interferon next alpha*):ti,ab #21 (targeted next treatment*):ti,ab #22 ((braf or mek or mapk) next inhibitor*):ti,ab #23 (vemurafenib or dabrafenib or trametinib or cobimetinib):ti,ab #24 mitogen activated protein kinase inhibitor*:ti,ab #25 [mh "antineoplastic agents" [mj]] #26 chemotherap*:ti,ab #27 MeSH descriptor: [Tamoxifen] explode all trees #28 MeSH descriptor: [Dacarbazine] explode all trees #29 MeSH descriptor: [Vinblastine] explode all trees #30 MeSH descriptor: [Vindesine] explode all trees #31 MeSH descriptor: [Paclitaxel] explode all trees #32 MeSH descriptor: [Cisplatin] explode all trees #33 MeSH descriptor: [Carboplatin] explode all trees #34 MeSH descriptor: [Lomustine] explode all trees #35 MeSH descriptor: [Carmustine] explode all trees #36 (dacarbazine or temozolomide or vindesine or vinblastine or paclitaxel or cisplatin or carboplatin or lomustine or carmustine or fotemustine or bendamustine or tamoxifen):ti,ab #37 MeSH descriptor: [Cancer Vaccines] explode all trees #38 talimogene laherparepvec:ti,ab #39 intra‐lesional treatment*:ti,ab #40 imiquimod:ti,ab #41 MeSH descriptor: [Bevacizumab] explode all trees #42 Bevacizumab:ti,ab #43 axitinib:ti,ab #44 (topical and neoadjuvant):ti,ab #45 {or #9‐#44} #46 #6 and #45
Appendix 4. MEDLINE (Ovid) search strategy
1. exp *Melanoma/ 2. melanoma$.ti,ab. 3. exp *Skin Neoplasms/ 4. skin neoplas$.ti,ab. 5. skin cancer$.ti,ab. 6. or/1‐5 7. exp Neoadjuvant Therapy/ 8. neoadjuvant$.ti,ab. 9. 7 or 8 10. exp *Immunotherapy/ 11. exp Interleukin‐2/ 12. exp Interferon‐alpha/ 13. (ipilimumab or nivolumab or pembrolizumab).ti,ab. 14. cytotoxic t lymphocyte antigen 4 inhibitor$.ti,ab. 15. CTLA‐4 inhibitor$.ti,ab. 16. programmed death‐ligand 1.ti,ab. 17. PD‐L1 inhibitor$.ti,ab. 18. checkpoint inhibitor$.ti,ab. 19. (interleukin‐2 or interferon alpha$).ti,ab. 20. targeted treatment$.ti,ab. 21. ((braf or mek or mapk) and inhibitor$).ti,ab. 22. (vemurafenib or dabrafenib or trametinib or cobimetinib).ti,ab. 23. mitogen activated protein kinase inhibitor$.ti,ab. 24. exp *Antineoplastic Agents/ 25. chemotherap$.ti,ab. 26. exp Tamoxifen/ 27. exp Dacarbazine/ 28. exp Vinblastine/ 29. exp Vindesine/ 30. exp Paclitaxel/ 31. exp Cisplatin/ 32. exp Carboplatin/ 33. exp Lomustine/ 34. exp Carmustine/ 35. (dacarbazine or temozolomide or vindesine or vinblastine or paclitaxel or cisplatin or carboplatin or lomustine or carmustine or fotemustine or bendamustine or tamoxifen).ti,ab. 36. exp Cancer Vaccines/ 37. talimogene laherparepvec.ti,ab. 38. intra‐lesional treatment$.ti,ab. 39. imiquimod.ti,ab. 40. exp Bevacizumab/ 41. Bevacizumab.ti,ab. 42. axitinib.ti,ab. 43. (topical and neoadjuvant).ti,ab. 44. or/9‐43 45. randomized controlled trial.pt. 46. controlled clinical trial.pt. 47. randomized.ab. 48. placebo.ab. 49. clinical trials as topic.sh. 50. randomly.ab. 51. trial.ti. 52. 45 or 46 or 47 or 48 or 49 or 50 or 51 53. exp animals/ not humans.sh. 54. 52 not 53 55. 6 and 44 and 54
[Lines 45‐54: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 5. Embase (Ovid) search strategy
1. exp *Melanoma/ 2. melanoma$.ti,ab. 3. exp metastatic melanoma/ 4. exp *skin tumor/ 5. skin neoplas$.ti,ab. 6. skin cancer$.ti,ab. 7. or/1‐6 8. exp neoadjuvant therapy/ 9. neoadjuvant$.ti,ab. 10. exp *immunotherapy/ 11. exp *interleukin 2/ 12. exp *alpha interferon/ 13. exp ipilimumab/ 14. exp nivolumab/ 15. exp pembrolizumab/ 16. cytotoxic t lymphocyte antigen 4 inhibitor$.ti,ab. 17. CTLA‐4 inhibitor$.ti,ab. 18. programmed death‐ligand 1.ti,ab. 19. PD‐L1 inhibitor$.ti,ab. 20. checkpoint inhibitor$.ti,ab. 21. (interleukin‐2 or interferon alpha$).ti,ab. 22. (ipilimumab or nivolumab or pembrolizumab).ti,ab. 23. targeted treatment$.ti,ab. 24. ((braf or mek or mapk) and inhibitor$).ti,ab. 25. exp vemurafenib/ 26. exp dabrafenib/ 27. exp trametinib/ 28. exp cobimetinib/ 29. (vemurafenib or dabrafenib or trametinib or cobimetinib).ti,ab. 30. mitogen activated protein kinase inhibitor$.ti,ab. 31. exp *antineoplastic agent/ 32. chemotherap$.ti,ab. 33. exp *chemotherapy/ 34. exp *tamoxifen/ 35. exp dacarbazine/ 36. exp vinblastine/ 37. exp vindesine/ 38. exp *paclitaxel/ 39. exp *cisplatin/ 40. exp *carboplatin/ 41. exp lomustine/ 42. exp carmustine/ 43. (dacarbazine or temozolomide or vindesine or vinblastine or paclitaxel or cisplatin or carboplatin or lomustine or carmustine or fotemustine or bendamustine or tamoxifen).ti,ab. 44. exp cancer vaccine/ 45. exp talimogene laherparepvec/ 46. talimogene laherparepvec.ti,ab. 47. intra‐lesional treatment$.ti,ab. 48. imiquimod.ti,ab. 49. exp imiquimod/ 50. exp bevacizumab/ 51. Bevacizumab.ti,ab. 52. exp axitinib/ 53. axitinib.ti,ab. 54. (topical and neoadjuvant).ti,ab. 55. or/8‐54 56. crossover procedure.sh. 57. double‐blind procedure.sh. 58. single‐blind procedure.sh. 59. (crossover$ or cross over$).tw. 60. placebo$.tw. 61. (doubl$ adj blind$).tw. 62. allocat$.tw. 63. trial.ti. 64. randomized controlled trial.sh. 65. random$.tw. 66. or/56‐65 67. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 68. human/ or normal human/ 69. 67 and 68 70. 67 not 69 71. 66 not 70 72. 7 and 55 and 71 [Lines 56‐71: Based on terms suggested for identifying RCTs in Embase (section 3.6.2) in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 6. LILACS search strategy
neoadjuvant and (melanoma$ or neoplas$ or cancer$) and skin
In LILACS we searched using the above terms and the Controlled clinical trials topic‐specific query filter.
Appendix 7. Abbreviations and acronyms
| Abbreviation | Definition |
| AE | Adverse event |
| AJCC | American Joint Committee on Cancer |
| ASCO | American Society of Clinical Oncology |
| BRAF | v‐raf murine sarcoma viral oncogene homolog B1 |
| CR | Complete response |
| CTLA‐4 | Cytotoxic T‐lymphocyte associated protein 4 |
| DFS | Disease‐free survival |
| DNA | Deoxyribonucleic acid |
| DOR | Duration of response |
| EADO | European Association of Dermato‐Oncology |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| EMA | European Medicines Agency |
| EORTC | European Organisation for Research and Treatment of Cancer |
| ESMO | European Society of Medical Oncology |
| FDA | Food and Drug Administration |
| GM‐CSF | Granulocyte‐macrophage colony‐stimulating factor |
| HDI | High‐dose interferon |
| HR | Hazard ratio |
| HSV‐1 | Herpes simplex virus‐1 |
| IFN‐alpha | Interferon alpha |
| IL‐2 | Interleukin‐2 |
| ILP | Isolated limb perfusion |
| INMC | International Neoadjuvant Melanoma Consortium |
| ITT | Intention‐to‐treat |
| IV | Intravenous |
| LDH | Lactate dehydrogenase |
| LYG | Life years gained |
| MEK | Mitogen‐activated protein kinase |
| NCCN | National Comprehensive Cancer Network |
| ORR | Overall response rate |
| OS | Overall survival |
| pCR | Pathological complete response |
| PD | Progressive disease |
| PD‐1 | Programmed death‐1 |
| PD‐L1 | Programmed death ligand‐1 |
| PFS | Progression‐free survival |
| PR | Partial response |
| QALY | Quality adjusted life year |
| RCT | Randomised controlled trial |
| RFS | Relapse free survival |
| RT | Radiation therapy |
| SD | Stable disease |
| SOC | Standard of care |
| TLR | Toll‐like receptor |
| TRAE | Treatment related adverse event |
| TTR | Time to recurrence |
| T‐VEC | Talimogene laherparepvec |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
Data and analyses
Comparison 1. Neoadjuvant treatment compared to no neoadjuvant treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 2 | 171 | Hazard Ratio (IV, Fixed, 95% CI) | 0.43 [0.15, 1.21] |
| 1.2 Adverse events | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.97, 2.55] |
| 1.3 Time to recurrence | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 0.51 [0.22, 1.17] |
Comparison 2. Neoadjuvant targeted treatment (BRAF/MEK inhibition) compared to no neoadjuvant treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Time to recurrence | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Neoadjuvant immunotherapy (talimogene laherparepvec) compared to no neoadjuvant treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Overall survival | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Time to recurrence | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to adjuvant immunotherapy (combined ipilimumab and nivolumab).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Neoadjuvant immunotherapy (combined ipilimumab and nivolumab) compared to neoadjuvant immunotherapy (nivolumab).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Overall response rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.3 Pathological Complete Response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Neoadjuvant immunotherapy (combined ipilimumab and nivolumab (2 different dosing regimens) compared to neoadjuvant immunotherapy (sequential ipilimumab then nivolumab).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 Overall response rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.3 Pathological complete response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Neoadjuvant immunotherapy (high dose interferon) and chemotherapy compared to neoadjuvant chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Overall response rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Neoadjuvant immunotherapy (ipilimumab 10mg/kg plus high‐dose interferon) compared to neoadjuvant immunotherapy (ipilimumab 3mg/kg plus high‐dose interferon).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Pathological complete response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.2 Objective response rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albertini 2018.
| Study characteristics | ||
| Methods | Open‐label, randomised, single‐centre translational study. There were initially four groups, but two groups where participants were treated with cilengitide were discontinued due to safety concerns. Enrollment commenced 28 March 2008, trial end date 4 May 2012 |
|
| Participants | Inclusion criteria: Recurrent stage III or IV melanoma (AJCC seventh ed.), no more than 3 sites of disease, ECOG 0 to 1, cutaneous, ocular, mucosal or unknown primary melanoma Exclusion criteria: Brain metastases (active or inactive) Country: USA Number of recruited patients: 20 Recruited participant characteristics: Stage III: 65% Stage IV: 35% BRAF positive: not specified Male: 65% ECOG PS 0: 80% LDH < ULN: not specified |
|
| Interventions | A treatment course of hu14.18‐IL2 comprised 4‐hour continuous intravenous infusion on days 1, 2 and 3, at a daily dose of 6 mg/m2/day (28‐day treatment cycle) Group 1, neoadjuvant treatment
Group 2, adjuvant treatment
|
|
| Outcomes |
Not measured: pCR, ORR, TTR, PFS, DFS, economic evaluation, quality of life Median follow‐up of surviving patients is > 4 years (range 31.8 to 70.4 months) |
|
| Funding source | Support was provided by NIH R01 CA032685, R01 CA087025, R35 CA166105, P30 CA014520 from the National Cancer Institute, by resources at the William S. Middleton Memorial Veterans Hospital, Madison, WI, and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. Additional support was provided by grants from the Midwest Athletes for Childhood Cancer Fund, the Crawdaddy Foundation, the Stand Up to Cancer Foundation, the St. Baldrick’s Foundation, the Hyundai Hope on Wheels Program, Ann’s Hope Foundation, the Tim Eagle Memorial, and the Jay Van Sloan Memorial from the Steve Leuthold Family. | |
| Declarations of interest | The authors have the following financial or other conflicts of interests to disclose related to this publication: Dr. Hans Loibner is CEO for Apeiron Biologics AG, and Apeiron Biologics AG has ownership of the hu18.18‐IL2 immunocytokine used in this study. The remaining authors reported no financial or other conflicts of interest to disclose related to this publication. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "“patients were randomised….using permutated blocks of size 4…" Comment: insufficient information provided to assess ROB in random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "“patients were randomised….using permutated blocks of size 4…" Comment: Insufficient information provided to assess ROB in allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open‐label trial" Comment: Knowledge of treatment assignment could impact disease management and therefore both RFS and OS. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Open‐label trial" Comment: Assuming RFS assessed by unblinded assessor, ROB high. Considered low for OS. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used for OS. Two participants excluded from RFS analysis, considered unlikely to impact outcome Comment: Considered low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available, all outcomes specified in published paper are reported on Comment: Unclear risk |
| Other bias | High risk | Comment: Trial not powered for formal hypothesis testing and any conclusions regarding comparative efficacy drawn from the results should be considered to have a high ROB. |
Amaria 2018a.
| Study characteristics | ||
| Methods | Single‐centre, open‐label, randomised Phase II trial. Recruitment commenced 23 October 2014. The trial was halted following an interim safety analysis in April 2016; recruitment to the intervention arm continues as a single‐armed study. |
|
| Participants | Inclusion criteria: AJCC seventh edition Stage III and IV cutaneous melanoma aged 18 years of over, of any gender and ethnicity, with surgically resectable disease and BRAF V600E/K mutation Exclusion criteria: people with previous exposure to BRAF/MEK inhibitors, or ongoing use of cancer therapy Country: USA Participants randomised: 21 Recruited participant characteristics: Stage IIIB: 24% Stage IIIC: 62% Stage IV: 14% BRAF+: 100% Male: 62% ECOG PS 0: 90% LDH < ULN: 90% |
|
| Interventions | Arm 1: dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily) for 8 weeks prior to surgery, and for 44 weeks following surgery (52 weeks total treatment) Arm 2: definitive surgery in line with current local practice guidelines, and the choice of receiving adjuvant therapy following surgery (including IFN‐alpha, IL‐2, cisplatin, vinblastine, dacarbazine, ipilimumab or observation). |
|
| Outcomes | Outcomes included in this review
Outcomes measured but not included in this review:
Median duration of follow‐up: 18.6 months |
|
| Funding source | Novartis Pharmaceuticals Corporation | |
| Declarations of interest | RNA reports grants from Merck, Bristol‐Myers Squibb, and Array Biopharma, all outside the submitted work. MTT reports personal fees from Myriad Genetics, Seattle Genetics, and Galderma, all outside the submitted work. MCA reports grants from Pfizer Australia, and non‐financial support from Merck and Bristol‐Myers Squibb Australia, outside the submitted work. W‐JH reports research grants from Merck, Bristol‐Myers Squibb, MedImmune, GlaxoSmithKline, and has served on an advisory board for Merck, all outside the submitted work. HAT reports personal fees from Novartis, grants from Merck and Celgene, and grants and personal fees from BMS and Genentech, all outside the submitted work. JEG reports advisory board participation with Merck and Castle Biosciences. CNS and VG report patents for gut microbiome pending. RB reports grants from NIH. AL reports personal fees from BMS, Novartis, Merck, and Genentech/Roche; personal fees and non‐financial support from ArcherDX and Beta‐Cat; grants and non‐financial support from Medimmune/Astra Zeneca and Sanofi; and grants, personal fees, and non‐financial support from Janssen, all outside the submitted work. MKW reports personal fees from Merck and EMD Serono, outside the submitted work. PS reports consultant or advisor fees from Bristol‐Myers Squibb, GlaxoSmithKline, AstraZeneca, Amgen, Jounce, Kite Pharma, Neon, Evelo, EMD Serono, and Astellas, during the conduct of the study; stock from Jounce, Kite Pharma, Evelo, Constellation, and Neon outside the submitted work; and has a patent licensed to Jounce for a novel immunotherapy outside the submitted work. MAD reports personal fees from Novartis, BMS, and Vaccinex; grants from Astra Zeneca and Merck; and grants and personal fees from Roche/Genentech and Sanofi Aventis, all outside the submitted work. JAW has received compensation for a speaker’s bureau and honoraria from Dava Oncology, Bristol‐Myers Squibb, and Illumina, and has served on advisory committees for GlaxoSmithKline, Roche/Genentech, Novartis, and Astra Zeneca. All other authors declare no competing interests. |
|
| Notes | People with lentigo maligna, uveal and mucosal melanomas were not specifically excluded from enrolment, however no participants with these conditions were enrolled into the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Participants were assigned a unique number at the time of enrolment. Randomisation was implemented by the Clinical Trial Conduct web site maintained by the Department of Biostatistics at the University of Texas MD Anderson Cancer Center". Additional information was provided by the authors, randomisation was based on stage IIIB/C/M1a versus M1b/M1c. Comment: probably conducted appropriately given the involvement of biostatistics department |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Enrolment and randomisation were done by the trial's designated research nurse" Comment: insufficient detail provided for judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "no masking of assignments was attempted and patients, investigators, and data analysts and assessors were aware of treatment assignment" Comment: Knowledge of treatment allocation could influence choices regarding patient care while on trial, which could influence subjective outcomes including safety. Risk of bias for objective outcome of OS is unclear, as knowledge of trial treatment assignment could affect subsequent care. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "patients, investigators, and data analysts and assessors were aware of treatment assignment" Comment: the objective OS outcome is considered to be at low risk of bias.The assessment of unclear risk of bias applies to subjective outcomes‐ safety, RFS, pCR and ORR outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients were assessable for the primary endpoint (event‐free survival at 12 months)" Comment: CONSORT diagram provided, detailed information on the missing patients provided, and missing data handled appropriately in the time‐to‐event analysis (ITT population) |
| Selective reporting (reporting bias) | Low risk | Quote: N/A Comment: the trial protocol is not publicly available to inform this judgement. All outcomes described in the publication were reported on. The authors stated that there were no additional pre‐specified outcomes in the trial protocol. |
| Other bias | High risk | Quote: "At the time of this interim safety analysis, seven EFS events had occurred: five in patients assigned to standard of care and two in those assigned to dabrafenib and trametinib. The reported p value (p<0.0001) did not cross the O'Brien‐Fleming stopping boundary for seven events...." Comment: Trial did not meet the pre‐specified criteria for early cessation of trial treatment for efficacy, and outcomes could be biased as a result (Bassler 2010). Quote: "Patients assigned to the standard of care group....were offered standard of care adjuvant therapy including interferon‐alpha2b, pegylated interferon‐alpha2b, ipilimumab, biochemotherapy..., infusional interleukin‐2, cisplatin, vinblastine and dacarbazine or observation". "Patients in the neoadjuvant plus adjuvant dabrafenib and trametinib group....received up to 44 weeks adjuvant dabrafenib and trametinib (52 weeks total treatment)." Comment: Protocol driven imbalance in trial follow‐up treatments, relevant now that the efficacy of adjuvant dabrafenib and trametinib in reducing relapse rate in Stage III melanoma is known (Long 2017b). |
Amaria 2018b.
| Study characteristics | ||
| Methods | Single‐centre, randomised, open‐label, non‐comparative Phase II trial. The trial was halted following an assessment by the Data Safety Monitoring Board due to early observation of disease progression preventing surgical resection in the nivolumab arm, and high rates of Grade 3 TRAEs with combination therapy. |
|
| Participants | Inclusion criteria: ECOG PS 0 to 1, Stage IIIB, C and IV resectable melanoma. People with lentigo maligna, uveal and mucosal melanomas were not specifically excluded from enrolment; one person with letigo maligna was recruited but no people with uveal or mucosal melanoma. Exclusion criteria: active or known autoimmune disease, prior treatment with PD‐1, PD‐L1 or CTLA‐4 targeted treatment, bone, brain or leptomeningeal metastases. Country: USA Participants randomised: 23 Recruited participant characteristics: Stage IIIB: 39% Stage IIIC: 43% Stage IV: 14% BRAF+: 48% Male: 83% ECOG PS 0: 100% LDH < ULN: 91% |
|
| Interventions | Arm 1: nivolumab 3 mg/kg via IV route every two weeks for up to 4 doses, followed by planned surgical resection and adjuvant nivolumab for up to 13 doses over 6 months. Arm 2: Nivolumab 1 mg/kg via IV route and ipilimumab 3 mg/kg via IV route every three weeks for up to 3 doses, followed by planned surgical resection and adjuvant nivolumab for up to 13 doses over 6 months. |
|
| Outcomes | =Outcomes included in this review
Outcomes measured but not included in this review:
Median duration of follow‐up: approximately 15 months |
|
| Funding source | Bristol‐Myers Squibb | |
| Declarations of interest | RNA received grants from Merck, Bristol‐Myers Squibb and Array Biopharma, all outside the submitted work. S.M.R. received support from National Institutes of Health
T32 Training Grant T32 CA 009666, outside the submitted work. HAT received personal fees from Novartis, grants from Merck and Celgene, and grants and personal fees from BMS and Genentech, all outside of the submitted work. MAD received personal fees from Novartis, BMS and Vaccinex, grants from AstraZeneca and Merck, and grants and personal fees from Roche/Genentech and Sanofi‐Aventis, all outside the submitted work. W‐JH received research grants from Merck, Bristol‐Myers Squibb, MedImmune, and GlaxoSmithKline and has served on an advisory board for Merck, all outside the submitted work. MKW received personal fees from Merck and EMD Serono, outside the submitted work. JG has participated in the advisory board of Merck and Castle Biosciences. AJL received personal fees from BMS, Novartis, Merck and Genentech/Roche, personal fees and nonfinancial support from ArcherDX and Beta‐Cat, grants and nonfinancial support from Medimmune/AstraZeneca and Sanofi and grants, personal fees and nonfinancial support from Janssen, all outside the submitted work. VG reports a US patent (PCT/US17/53,717), consultant fees from Microbiome DX, and honoraria from CAP18, outside of the submitted work. A.R. reports a US patent (PCT/US17/53,717) and is supported by the Kimberley Clark Foundation Award for Scientific Achievement provided by MD Anderson’s Odyssey Fellowship Program. MCA is supported by the National Health and Medical Research Council of Australia CJ Martin Early Career Fellowship (1148680), and reports advisory board participation, travel support and honoraria from Merck Sharpe and Dohme. CNS reports a US patent (PCT/US17/53,717), outside of the submitted work. PS received consultant or advisor fees from Bristol‐Myers Squibb, GlaxoSmithKline, AstraZeneca, Amgen, Jounce, Kite Pharma, Neon, Evelo, EMD Serono and Astellas, during the conduct of the study; has stocks from Jounce, Kite Pharma, Evelo, Constellation and Neon, outside the submitted work; and has a patent licensed to Jounce, outside the submitted work. MTT reports personal fees from Myriad Genetics, Seattle Genetics and Novartis, all outside the submitted work. JAW reports a US patent (PCT/US17/53,717), has received compensation for speaker’s bureau and honoraria from Dava Oncology, Bristol‐Myers Squibb and Illumina and has served on advisory committees for GlaxoSmithKline, Roche/Genentech, Novartis and AstraZeneca. All other authors declare no competing interests. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “we conducted a randomised…” “Subject randomisation will be conducted by the Clinical Trials Conduct Website maintained by the Department of Biostatistics at the University of Texas M.D. Anderson Cancer Centre” Comment: insufficient information on the random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote:“Subjects will be approved for treatment after ….Subjects will then be assigned a subject number and will be eligible for randomisation.” Comment: insufficient detail provided for judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: N/A. The study is recorded as open‐label in the supplementary appendix. Comment: knowledge of treatment allocation could influence choices regarding patient care while on trial, which could influence subjective outcomes including safety. Risk of bias for objective outcome of OS is unclear, as knowledge of trial treatment assignment could affect subsequent care. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: N/A Comment: there is no mention of blinding outcome assessors in publication or appendix, so probably not blinded. The assessment of high risk of bias applies to subjective outcomes: safety, RFS, pCR and ORR outcomes. We consider the objective OS outcome to be at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: N/A Comment: CONSORT diagram provided in Supplmenetary Appendix. ITT is used for the analysis of time‐to‐event data. |
| Selective reporting (reporting bias) | Low risk | Quote: N/A Comment: trial protocol available. No evidence of selective reporting bias. |
| Other bias | Unclear risk | Quote: "The trial was stopped early by the Data Safety Monitoring Board..." Comment: truncated RCTs are known to overestimate treatment effects (Bassler 2010). However since the trial was not designed to detect relative efficacy, we rated the risk of bias as unclear rather than high. Quote: "Subjects will be approved for treatment after a consensus panel of medical and surgical oncologists has determined that the disease is amenable to surgical resection and after the subject has passed screening evaluations….Subjects will then be assigned a subject number" Comment: subjective screening in addition to objective criteria gives rise to the risk of an enriched trial population relative to the stated objective criteria. |
Blank 2018.
| Study characteristics | ||
| Methods | Single centre, open‐label, randomised, non‐comparative Phase Ib trial | |
| Participants | Inclusion criteria: aged 18 years or older, with histologically confirmed resectable stage III melanoma with palpable lymph node metastases and no history of in‐transit metastases within the last 6 months, WHO performance status 0 to 1 and normal LDH levels Exclusion criteria: prior immunotherapy targeting CTLA‐4, PD‐1 or PD‐L1, radiotherapy before or after surgery within the trial Country: Netherlands Participants randomised: 21 Recruited participant characteristics: Stage IIIB: 70% Stage IIIC: 30% BRAF +: 70% Male: 65% ECOG PS 0: not specified LDH < ULN: 100% |
|
| Interventions | Arm 1 Neo‐adjuvant arm: ipilimumab 3 mg/kg IV plus nivolumab 1 mg/kg IV every 3 weeks, for 2 doses prior to complete lymph node dissection (CLND) at week 6, then 2 doses further doses starting at week 6 post‐CLND. Arm 2 Adjuvant arm: ipilimumab 3 mg/kg IV plus nivolumab 1 mg/kg IV every 3 weeks for 4 doses, commencing at week 6 post‐CLND. |
|
| Outcomes | Coprimary endpoints
Secondary endpoints
Data were reported in the pivotal publication for distant metastases free survival and OS. Median duration of follow‐up: 3 years. |
|
| Funding source | Bristol Myers Squibb | |
| Declarations of interest | CUB reports personal fees for advisory roles for MSD, BMS, Roche, GSK, Novartis, Pfizer, GenMab, and Lilly, and grants from BMS, NanoString, and Novartis, outside the submitted work. EAR reports travel support from NanoString Technologies and MSD, outside the submitted work. LFF, KS, BvdW, PK, OK, MvdB, DP, A Broeks, HAM, SA, StM, LMP, LGG‐O, A Bruining, and HvT have nothing to disclose. JVvT reports travel support from Roche, outside the submitted work. RMG has a financial interest in Adaptive Biotechnologies. SW is an employee of and is a stockholder in NanoString Technologies, has an advisory role with Roche, and is a former employee of the Oncofactor Corporation, outside the submitted work. DSP reports research support from BMS. JBAGH reports that NKI received fees for his advisory roles from BMS, MSD, Roche, Neon Therapeutics, Immunocore, Novartis, AstraZeneca/ MedImmune, Pfizer, and Ipsen; NKI received grants from BMS, Merck, Novartis, and Neon Therapeutics, outside the submitted work. ACJvA reports personal fees for an advisory role with Amgen, Bristol‐Myers Squibb, Novartis, MSD‐Merck, and Merck‐Pfizer, and grants from Amgen and Novartis, all outside the submitted work. TNS is consultant for Adaptive Biotechnologies, AIMM Therapeutics, Amgen, Neon Therapeutics, Scenic Biotech, and reports grant/research support from Merck, BristolMyers Squibb, and Merck KGaA; he is a stockholder in AIMM Therapeutics and Neon Therapeutics |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation will be done by the trial office of the AVL…”. “Patients... were included into the OpACIN trial and randomised to receive…” Comment: insufficient information provided on sequence generation to assess risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patient randomisation will only be accepted from authorised investigators or through their authorised data manager or authorised staff member” Comment: insufficient information provided on allocation concealment to assess risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: N/A. The study is recorded as open‐label in the supplementary appendix. Comment: knowledge of treatment allocation could influence choices regarding patient care while on trial, which could influence subjective outcomes including safety. Risk of bias for objective outcome of OS is unclear, as knowledge of trial treatment assignment could affect subsequent care. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Overall survival Quote: N/A Comment: the objective OS outcome is considered to be at low risk of bias. Pathological Complete Reponse Quote: "reviewed by one blinded pathologist, who scored the percentage vial tumour cells in the surgery material" Comment: pCR is considered low risk Other Subjective outcomes Quote: N/A. Comment: the assessment of high risk of bias applies only to RFS, ORR and safety outcomes Overall rated as high risk as the majority of outcomes relevant to this review are subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: N/A Comment: CONSORT diagram provided. ITT population is used for the analysis of time‐to‐event data. |
| Selective reporting (reporting bias) | Low risk | Quote: N/A Comment: trial protocol available online, all prespecified outcomes are reported in trial publication. |
| Other bias | Low risk | No other potential biases identified |
Dummer 2020b.
| Study characteristics | ||
| Methods | Phase II randomised, open‐label multicentre trial. Accrural commenced February 2015. | |
| Participants | Inclusion criteria: aged ≥18 years, melanoma Stage IIIB, IIIC, or IVM1a with at least 1 injectable lesion ≥ 10mm in longest diameter (cutaneous, subcutaneous or nodal), ECOG PS 0 to 1, adequate haematological, hepatic and renal function, LDH < ULN. Exclusion criteria: primary ocular and mucosal melanoma, history of autoimmune disease, immunodeficiency, immunosuppression, active herpetic lesions or prior complications of HSV‐1 infection. Number of participants: 150 Countries: USA, Australia, Brazil, France, Greece, Poland, Russia, Spain, Switzerland Recruited participant characteristics: Stage IIIB: 41% Stage IIIC: 41% Stage IV: 18% Male: 64% BRAF+: not specified ECOG PS 0: not specified LDH < ULN: assumed 100% in accordance with inclusion criteria |
|
| Interventions | Arm 1: 6 doses of T‐VEC over 12 weeks, prior to surgery. T‐VEC is administered until planned surgery (12 weeks), no injectable tumours or intolerance. The initial dose (day 1 week 1) is at a concentration of 106 (1 million) plaque forming units (PFU)/ml, with a maximum of 4ml administered (reference 2). Subsequent doses (day 1 week 4, 6, 8, 10, 12) are given at a higher dose 108 (100 million) PFUs/ml, again with a maximum of 4ml administered each cycle (reference 2). Drug is administered by intralesional injection into cutaneous, subcutaneous and/or nodal lesions. Arm 2: Immediate surgical resection, with optional adjuvant treatment. |
|
| Outcomes | Outcomes included in this review.
Outcomes measured but excluded from this review.
Median duration of follow‐up: 24 months |
|
| Funding source | Not stated, sponsored by Amgen. | |
| Declarations of interest | None stated | |
| Notes | Not available as a peer‐reviewed publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Trial protocol section 5.1 "Upon confirmation of eligibility, the site staff will use the Interactive Voice Response (IVR) system to randomize a subject… The IVR system will assign a randomization number..." Comment: use of a centralised electronic randomisation system in a multicentre trial is likely to lead to a low ROB |
| Allocation concealment (selection bias) | Low risk | Quote: Trial protocol section 5.1 "Upon confirmation of eligibility, the site staff will use the Interactive Voice Response (IVR) system to randomize a subject…. The IVR system will assign a randomization number. Judgement: use of a centralised electronic randomisation system in a multicentre trial is likely to lead to a low ROB |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: "Open label trial". Judgement: some potential for bias in choice of subsequent management for participants, which could impact OS, so ROB for this outcome is considered high. Choice of adjuvant treatment declared prior to enrolment, but it seems some participants received it post‐enrolment anyway, so this may impact TTR. ROB considered low for ORR and pCR outcomes. Overall, rated high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not explicitly stated but seems that radiographic tumour assessments and pCR assessment conducted locally and unblinded, and so are associated with a high risk of bias, as is TTR. For OS, low ROB. Overall rated high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Time to event outcomes (RFS, OS) analysed in ITT population so at low ROB. Similairly pCR and ORR analysed in ITT population so considered low ROB, despite high number of patients lost to follow‐up. No information available on participants who did not receive protocol‐specified surgery in either arm. Unclear if this may impact outcomes. Overall rated low risk of bias |
| Selective reporting (reporting bias) | Low risk | Reference: Section 10.1 trial protocol, all outcomes specified here are reported, excluding 3 and 5 year RFS and OS, which is due to the immaturity of the data. |
| Other bias | High risk | No published peer reviewed paper available, only abstracts and data filed in clinical trials database. Funding sources unclear No information available about subsequent adjuvant treatment received by participants, which is likely to have a significant impact on TTR and OS outcomes. |
Hwu 2017.
| Study characteristics | ||
| Methods | Single‐centre, randomised Phase II trial | |
| Participants | Inclusion criteria: stage IIIB, IIIC or IVM1a melanoma with potentially resectable disease, aged 18 years or older, and ECOG PS 0 to 1. Exclusion criteria: autoimmune disease, active infection or immunosuppressive disease, concurrent corticosteroids, current significant psychiatric illness, significant cardiac or pulmonary dysfunction. Country: USA Recruited participant characteristics: Stage III: not specified Stage IV: not specified BRAF+: not specified Male: not specified ECOG PS 0: not specified LDH < ULN: not specified |
|
| Interventions | Arm 1: Temozolomide 150 mg/m2/day via the oral route, for 7 days followed by a 7 day break. A cycle is 8 weeks in duration. Arm 2: Temozolomide 150 mg/m2/day via the oral route, for 7 days followed by a 7 day break, plus pegylated interferon‐alpha 2b 0.5 mcg/kg via subcutaneous injection once weekly. A cycle is 8 weeks in duration. In each arm, participants received one 8‐week cycle prior to surgery. For participants with a response, 3 additional cycles of the assigned treatment could be administered as adjuvant therapy. |
|
| Outcomes | Primary outcome was pooled overall response, defined as complete response plus partial response plus stable disease. Reponse rate was also reported in each treatment arm. Median duration of follow‐up: unknown |
|
| Funding source | Sponsor: MD Anderson Cancer Centre. Study was funded by Schering Plough and Merck. | |
| Declarations of interest | No declarations of interest are provided. | |
| Notes | Not available as a peer‐reviewed publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: N/A Comment: no information is provided about sequence generation to assess risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: N/A Comment: no information is provided about allocation concealment to assess risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: N/A Comment: probably open‐label, as there is no placebo control and obvious difference between treatment arms. Knowledge of treatment allocation could influence choices regarding patient care while on trial, which could influence subjective outcomes including safety. Risk of bias for objective outcome of OS is unclear, as knowledge of trial treatment assignment could affect subsequent care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: N/A Comment: insufficient information to assess risk of bias. No information provided on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: N/A Comment: it is reported that 52 participants were recruited to the study, but outcomes are reported only for 50 participants. No information is provided regarding the reasons for participant dropout. However as this is a non‐comparative study the implication of this missing data is difficult to assess. |
| Selective reporting (reporting bias) | Unclear risk | Quote: N/A Comment: the trial protocol is not publicly available to inform this judgement. |
| Other bias | High risk | No other potential biases identified. Study is not published in a peer‐reviewed journal; therefore, we consider the risk of publication bias to be high. |
Rozeman 2019.
| Study characteristics | ||
| Methods | Phase II open‐label multi centre randomised trial | |
| Participants | Inclusion Criteria: 18 years or over, cytologically or histologically confirmed resectable macroscopic (Stage III) melanoma, WHO PS 0 or 1, measurable lymph node metastases, normal LDH Exclusion Criteria: prior exposure to immune‐checkpoint inhibitors, distantly metastasised melanoma, active autoimmune disease, history of in‐transit metastases within the last 6 months. Countries: Australia, Netherlands, Austria, Sweden Participants: 86 Recruited participant characteristics: Stage III: 100% BRAF+: not specified Males: 57% ECOG PS 0: not specified LDH<ULN: 99% |
|
| Interventions | Arm 1: ipilimumab 3 mg/kg plus nivolumab 1 mg/kg once every three weeks for 2 doses Arm 2: ipilimumab 1 mg/kg plus nivolumab 3 mg/kg once every three weeks for 2 doses Arm 3: Ipilimumab 3 mg/kg once every three weeks for 2 doses followed (> 2 hours and < 24 hours) by nivolumab 3 mg/kg once every 2 weeks for 2 doses. Surgery is scheduled at week 6 in all treatment arms. No adjuvant treatment was scheduled. |
|
| Outcomes | Primary endpoints
Secondary endpoints
Outcomes measured but not included in the review: Distant metastatic free survival Mediation duration of follow‐up: at least 32 months |
|
| Funding source | Bristol Myers Squibb and the Netherlands Cancer Institute | |
| Declarations of interest | CB: Advisory roles (BMS, MSD, Roche, Novartis, GSK, Pfizer, Lilly, GenMab, Pierre Fabre), Research grants (BMS, Novartis, Nano String). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: N/A Comment: no information is provided about sequence generation to assess risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: N/A Comment: no information is provided about allocation concealment to assess risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: N/A Comment: knowledge of treatment allocation could influence choices regarding patient care while on trial, which could influence subjective outcomes including safety. Risk of bias for objective outcome of OS is unclear, as knowledge of trial treatment assignment could affect subsequent care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: N/A Comment: insufficient information to assess risk of bias. No information provided on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: N/A Comment: EFS data reported for ITT population. Currently no CONSORT diagram available. |
| Selective reporting (reporting bias) | Unclear risk | Quote: N/A Comment: the trial protocol is not publicly available to inform this judgement. |
| Other bias | Low risk | No other potential biases identified |
Tarhini 2018.
| Study characteristics | ||
| Methods | Phase II open‐label single‐centre RCT | |
| Participants | Inclusion criteria: 18 years or greater, ECOG PS 0 to 1, surgically resectable Stage III melanoma, adequate organ function Exclusion criteria: distant metastatic disease, known risk factors for bowel perforation, history of some autoimmune disorders, underlying heart conditions, prior CTLA‐4 treatment or CD137 agonist. Country: USA Number of participants recruited: unknown, planned to recruit 30 participants Recruited participant characteristics: Stage IIIB: 10% Stage IIIC: 83% BRAF+: not specified Males: 60% ECOG PS 0: 53% LDH < ULN: not specified |
|
| Interventions | Arm 1: ipilimumab 10 mg/kg via IV infusion once every 3 weeks for 2 doses followed by definitive surgery. Concurrent interferon alpha‐2b at 20 million units (MU)/m2/day IV for 5 consecutive days each week for 4 weeks, then 10 MU/m2/day on alternate days for 3 days each week, via the subcutaneous route for 2 weeks, followed by definitive surgery. Following recovery from surgery, ipilimumab 10 mg/kg once every 3 weeks for 2 doses, then once every 12 weeks for 4 additional doses. Following surgery, IFN is resumed at the 10 MU dose for 46 additional weeks. Arm 2: ipilimumab 3 mg/kg via IV infusion once every three weeks for 2 doses followed by definitive surgery. Concurrent interferon alpha‐2b at 20 MU/m2/day IV for 5 consecutive days each week for 4 weeks, then 10 MU/m2/day on alternate days for 3 days each week, via the subcutaneous route for 2 weeks, followed by definitive surgery. Following recovery from surgery, ipilimumab 3 mg/kg once every 3 weeks for 2 doses, then once every 12 weeks for 4 additional doses. Following surgery, IFN is resumed at the 10 MU dose for 46 additional weeks. |
|
| Outcomes | Primary outcome
Secondary outcomes
Median duration of follow‐up for responding patients is 32 months. |
|
| Funding source | Research grant support from Merck, BMS and Adaptive biotechnologies. Sponsored by University of Pittsburgh | |
| Declarations of interest | Tarhini: Research grant support from Merck, BMS and Adaptive Biotechnologies, and consultant for Merck, BMS, HUYA, Pfizer, Sanofi, Novartis, Genetech‐Roche, Array Biopharma, Newlink genetics Kirkwood: consulting/advisory role with BMS, Merck, Novartis, Roche, Genentec, EMD Serrono, Array biopharma, Prometheus Yusko, Rytlewski employees of Adaptive Biotechnologies |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised" Judgement: no information is provided about sequence generation to assess risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: N/A Judgement: no information is provided about allocation concealment to assess risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: 'open‐label trial' Judgement: knowledge of treatment allocation could influence treatment choices regarding patient care while on trial, which could influence subjective outcomes including safety. Risk of bias for OS unclear, as knowledge of trial treatment could affect subsequent care. Overall rated as high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | OS: Quote: N/A Judgement: the objective outcome OS is considered to be low risk of bias. Other subjective outcomes: TTR, ORR, pCR, safety ‐ insufficient information to assess risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: N/A Judgement: 30 participants enrolled, only 28 included in the efficacy analysis, no reasons for dropout reported |
| Selective reporting (reporting bias) | Unclear risk | Quote: N/A Judgement: Insufficient information to assess risk of bias, as trial protocol not available |
| Other bias | Low risk | No additional sources of bias were identified. |
AEs: adverse events; AJCC: American Joint Committee on Cancer; BRAF+: tested positive for the BRAF mutation; CLND: complete lymph node dissection; CTLA‐4: cytotoxic T‐lymphocyte‐associated antigen‐4; DFS: disease‐free survival; ECOG PS: European Cooperative Oncology Group Performance Status; EFS: event free survival; HSV: herpes simplex virus; IFN: interferon; IL‐2: interleukin‐2; ITT: intention‐to‐treat; IV: intravenous; LDH: lactate dehydrogenase; LDH < ULN: lactate dehydrogenase less than the upper limit of normal; N/A: not applicable; ORR: overall response rate; OS: overall survival; pCR: pathological complete response; PD: programmed death; PD‐L1: programmed death ligand‐1; PFS: progression‐free survival; ROB: risk of bias; RECIST: Response Evaluation Criteria in Solid Tumours; TRAEs: treatment‐related adverse events; TTR: time to recurrence; T‐VEC: talimogene laherparepvec; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| 2006‐005350‐79 | This study is not an RCT. |
| Ahmann 1976 | This study is not an RCT. |
| Ariyan 1982 | This study is not an RCT. |
| Buzaid 1998 | This study is not an RCT. |
| DeCOG 2010 | This study is not an RCT. |
| Dillman 2015 | This study is not an RCT. |
| Koyanagi 2005 | This study is not an RCT. |
| Long 2019 | This study is not an RCT. |
| Madu 2016 | This study is not an RCT. |
| Moschos 2006 | This study is not an RCT. |
| Mudigonda 2016 | This study is not an RCT. |
| Najjar 2017 | This study is not an RCT. |
| Najjar 2018 | This study is not an RCT. |
| NCT01720407 | Ineligible population: lentigo maligna |
| NCT02036086 | This study is not an RCT. |
| NCT02303951 | This study is not an RCT. |
| NCT02736123 | Study withdrawn as Principal Investigator leaving clinical trial centre. |
| NCT03313206 | Ineligible population: mucosal melanoma |
| NCT03618641 | This study is not an RCT. |
| NCT04007588 | Study withdrawn (slow accrual) |
| NCT04013854 | Ineligible study design |
| NCT04495010 | Study withdrawn by Sponsor as business objectives have changed. |
| NCT04741997 | Ineligible study design |
| Notohardjo 2020 | Ineligible population |
| O'Connor 1978 | Ineligible population |
| Passalacqua 1996 | This study is not an RCT. |
| Prieto 2016 | This study is not an RCT. |
| Reijers 2019 | This study is not an RCT. |
| Samoylenko 2019 | This study is not an RCT. |
| Schermers 2019 | This study is not an RCT. |
| Shah 2010 | This study is not an RCT. |
| Stiles 2017 | This study is not an RCT. |
| Urosevic 1996 | This study is not an RCT. |
| Van den Hout 2010 | Ineligible population |
| Van Den Hout 2013 | Ineligible population |
| Wargo 2015 | This study is not an RCT. |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
de Braud 1994.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | Study could not be sourced. All efforts to locate the study failed. |
EudraCT 2014‐000334‐30.
| Methods | Phase II open‐label RCT |
| Participants | Adults aged 18 year and over, with Stage III melanoma, ECOG PS 0 to 1, BRAF wildtype with no prior adjuvant treatment |
| Interventions | Esomeprazole for oral use No additional information provided |
| Outcomes | Primary endpoint: changes in the immune profile induced by treatment in the tumour‐draining lymph nodes Secondary endpoint: disease‐free survival, gene expression profiling |
| Notes | Fondazione IRCCS ''Istituto Nazionale dei Tumori'' |
Kleeberg 1986.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | Study cannot be located |
ECOG PS: European Cooperative Oncology Group Performance Status; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02858921.
| Study name | A Phase II, Randomsied, Open‐label Study of Neoadjuvant Dabrafenib, Trametinib and/or Pembrolizumab in BRAF Mutant Resectable Stage IIIB/C Melanoma (NeoTrio) |
| Methods | Phase II randomised open‐label trial. |
| Participants | Inclusion criteria: 18 years or over, resectable stage IIIB, IIIC or IV cutaneous melanoma, BRAFV600 mutation positive, ECOG PS 0 to 1, adequate organ function, and expected life expectancy greater than 12 months. Exclusion criteria: uveal or mucosal melanoma, prior anti‐cancer treatment for melanoma (excluding surgery, adjuvant radiation therapy or adjuvant IFN or ipilimumab), active autoimmune disease, active infection, history or evidence of cardiovascular disease. |
| Interventions | Participants are randomised to 1 of 3 arms: Arm 1: sequential dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily) for 2 weeks, followed by pembrolizumab 200 mg IV at weeks 2, 4, 6, and 9, surgery at week 12, then adjuvant pembrolizumab every 3 weeks from week 12 for 50 weeks. Arm 2: concurrent dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily) and pembrolizumab 200 mg IV every 3 weeks for 52 weeks, with surgery at week 12. Arm 3: pembrolizumab 200 mg IV every 3 weeks for 52 weeks, with surgery at week 12. |
| Outcomes | Primary outcome
Secondary outcomes (relevant to this review)
|
| Starting date | November 2017 |
| Contact information | monica.osorio@melanoma.org.au |
| Notes | Sponsor: Melanoma Institue Australia, in collaboration with Merck Sharpe & Dohme and Novartis. |
NCT02938299.
| Study name | A Phase III, open‐label, randomised, controlled multicentre study of the efficacy of L19IL2/L19TNF neoadjuvant intratumoral treatment followed by surgery, versus surgery alone, in clinical stage IIIB and IIIC melanoma patients. |
| Methods | Phase III, open‐label, multicentre RCT |
| Participants | Inclusion criteria: 18 years or over, with stage IIIB or IIIC melanoma, eligible for complete surgical resection, ECOG PS 0 to 1, and expected life expectancy greater than 24 months. Exclusion criteria: uveal or mucosal melanoma, active infection, recent history of or current active cardiovascular disease or active autoimmune disease. |
| Interventions | Arm 1: intratumoural administration of L19IL2 and L19TNF into all injectable cutaneous, subcutaneous and nodal tumours once weekly for up to 4 weeks, followed by surgical resection within 4 weeks of completing treatment. Arm 2: surgical resection of melanoma tumour lesions within 4 weeks of randomisation |
| Outcomes | Primary outcome: recurrence‐free survival rate at 1 year following randomisation Secondary outcomes
|
| Starting date | July 2016 |
| Contact information | regulatory@philogen.com |
| Notes |
NCT03567889.
| Study name | An Open‐Label, Randomized, Controlled Multi‐Center Study of The Efficacy of Daromun (L19IL2 + L19TNF) Neoadjuvant Intratumoral Treatment Followed by Surgery and Adjuvant Therapy Versus Surgery and Adjuvant Therapy in Clinical Stage IIIB/C Melanoma Patients |
| Methods | Phase III, open‐label, multicentre RCT |
| Participants | Inclusion criteria: ≥18 years, ECOG PS 0 to 1, stage IIIB and IIIC (AJCC v7) metastatic melanoma, life expectancy ≥ 24 months, eligible for complete surgical resection of all metastases, and be a candidate for intralesional therapy with at least one injectable cutaneous, subcutaneous, or nodal melanoma lesion (≥ 10 mm in longest diameter) or with multiple injectable lesions that in aggregate have a longest diameter of ≥ 10 mm. Exclusion criteria
|
| Interventions | Arm 1: daromun plus surgery and adjuvant therapy 4‐week period of neoadjuvant treatment with daromun (L19IL2/L19TNF) via intratumoural injection, followed by surgery, then at investigators discretion commencing adjuvant therapy within four weeks of surgery. Arm 2: surgery and adjuvant therapy Surgery within 4 weeks of randomisation followed by adjuvant therapy at investigators discretion. |
| Outcomes | Primary
Secondary
|
| Starting date | 20 September 2018 |
| Contact information | Contact: regulatory@philogen.com |
| Notes |
NCT03698019.
| Study name | NCT03698019 |
| Methods | Phase II open‐label, multicentre RCT |
| Participants | Inclusion criteria: stage III or IV resectable melanoma, including those with mucosal or acral origin, medically fit and adequate organ function. Prior non‐immunotherapy adjuvant treatment is permitted. Exclusion criteria: uveal melanoma, previous neoadjuvant treatment for melanoma, previous immunotherapy, active autoimmune disease. |
| Interventions | Arm A: adjuvant pembrolizumab, commencing within 84 days after surgical resection, at a dose of 200 mg IV every 3 weeks for up to 18 cycles in the absence of disease progression or unacceptable toxicity. Arm B: neoadjuvant pembrolizumab, 200 mg IV every 3 weeks for 3 cycles, then surgery within 3 weeks. Within 84 days, participants commence adjuvant pembrolizumab at a dose of 200 mg IV every 3 weeks or up to 15 cycles in the absence of disease progression or unacceptable toxicity. |
| Outcomes | Primary outcome measures
Secondary outcome measures relevant to this review
|
| Starting date | 6 December 2018 |
| Contact information | Not specified |
| Notes | People with mucosal and acral melanoma are eligible for this study, but excluded from the systematic review protocol |
NCT04133948.
| Study name | NCT04133948 |
| Methods | Phase Ib, multicentre, open‐label RCT |
| Participants | Inclusion criteria: ≥18 years, with resectable stage III cutaneous melanoma, normal LDH and WHO performance status 0 to 1. Exclusion criteria: uveal or mucosal melanoma, distantly metastasised melanoma, history of in‐transit metastases within the last 6 months, active autoimmune disease, prior checkpoint inhibitor therapy or targeted BRAF/MEK treatment, prior radiation therapy, significant cardiovascular disease. |
| Interventions | Arm A: for IFN‐gamma high patients, participants will receive presurgically 2 courses nivolumab 240 mg IV every 3 weeks Arm B: for IFN‐gamma high patients, participants will receive presurgically 2 courses nivolumab 240 mg IV every 3 weeks plus domatinostat 200 mg twice daily on days 1 to 14 every 3 weeks Arm C: for IFN‐gamma low patients, participants will receive presurgically 2 courses nivolumab 240 mg every 3 weeks + domatinostat 200 mg twice daily, on days 1 to 14 every 3 weeks Arm D: for IFN‐gamma low patients, participants will receive presurgically 2 courses nivolumab 240 mg every 3 weeks + ipilimumab 80 mg every 3 weeks + domatinostat. Participants in arm D will start with once‐daily dosing scheme of domatinostat 200 mg, on days 1 to 14 every 3 weeks. Based on safety data of the first 5 participants in this arm, the next participants will be treated with either a higher dosing scheme (200 mg twice daily, days 1 to 14, every 3 weeks), a lower dosing scheme (100 mg once daily, days 1 to 14, every 3 weeks), or the same dosing scheme (200 mg once daily, days 1 to 14, every 3 weeks). |
| Outcomes | Primary
Secondary
|
| Starting date | 27 December 2019 |
| Contact information | Contact: Christian Blank, Prof. +31205129111 c.blank@nki.nl Contact: Irene Reijers, MD +31205129111 i.reijers@nki.nl |
| Notes |
NCT04139902.
| Study name | NCT04139902 |
| Methods | Phase II open‐label RCT |
| Participants | Adults aged over 18 years with stage III B/C/D or oligometastatic stage IV A melanoma with lymph node (LN) and/or in‐transit and/or oligometastatic disease who have yet to undergo definitive surgery are eligible to enrol. Inclusion Criteria: adults aged ≥ 18 years with stage IIIB, IIIC, IIID or IVA cutaneous melanoma (as per AJCC v 8 staging criteria), who have yet to undergo definitive surgery, have ECOG PS 0 to 1 and adequate organ function. Exclusion criteria: uveal or mucosal melanoma, active autoimmune disease, prior treatment with a checkpoint inhibitor, IDO inhibitor, active central nervous system metastases. |
| Interventions | Arm 1: dostarlimab (TSR‐042) Preoperative phase: dostarlimab (TSR‐042) 500 mg IV, on cycle 1 day 1, and then again on cycle 2 day 1. Postoperative phase: dostarlimab (TSR‐042) 500 mg IV for 4 doses every 3 weeks (cycles 3 to 4) and then 1000 mg IV every 6 weeks for 6 doses (cycles 5 to 10) for approximately 48 weeks. Arm 2: dostarlimab (TSR‐042) and TSR‐022 (combination) Preoperative phase: dostarlimab (TSR‐042) 500 mg and TSR‐022 300 mg will be administered through an IV over 30 minutes, on cycle 1 day 1 and then again on cycle 2 day 1. Postoperative phase: dostarlimab (TSR‐042) will be administered through an IV over 30 minutes for 4 doses every 3 weeks (cycles 3 to 4), and then 1000 mg will be administered through an IV over 30 minutes every 6 weeks for 6 doses (cycles 5 to 10) for approximately 48 weeks. TSR‐022 will not be administered in the postoperative phase . |
| Outcomes | Primary
Secondary outcomes relevant to this review
|
| Starting date | Not specified |
| Contact information | Diwakar Davar, MD davard@upmc.edu Amy J Rose, BSN kennaj@upmc.edu |
| Notes |
NCT04303169.
| Study name | MSD2020 |
| Methods | Phase I/II open‐label, multicentre, rolling‐arm umbrella platform design |
| Participants | Inclusion criteria: confirmed stage IIIB, IIIC or IIID melanoma amenable to surgery, adequate organ function. Exclusion criteria: uveal or mucosal melanoma, active autoimmune disease of current immunosuppressive therapy, not naive to talimogene laherparepvec and other oncolytic viruses. |
| Interventions | Arm 1: neoadjuvant pembrolizumab (dose not specified), tumour resection surgery, then adjuvant pembrolizumab for a total duration of treatment of approximately 1 year. Arm 2: neoadjuvant pembrolizumab (dose not specified) in combination with V937 (other names Coxsackievirus CVA21, dose not specified), tumour resection surgery, then adjuvant pembrolizumab for a total duration of treatment of approximately one year. Arm 3: neoadjuvant pembrolizumab (dose not specified) in combination with vibostolimab (dose not specified), tumour resection surgery, then adjuvant pembrolizumab for a total duration of treatment of approximately one year. |
| Outcomes | Primary outcomes
Secondary outcomes relevant to this review
|
| Starting date | Not specified |
| Contact information | Not specified |
| Notes | Study sponsored by Merck Sharpe and Dohme |
NCT04401995.
| Study name | NCT04401995 |
| Methods | Phase II, randomised, open‐label trial |
| Participants | Inclusion criteria: aged 18 years and older, with stage IIIB‐IIID cutaneous (or unknown primary) melanoma with palpable nodal disease and/or in‐transit disease who have yet to undergo definitive surgery are eligible to enrol, with measurable disease and ECOG PS 0 to 1. Exclusion criteria include uveal or mucosal melanoma, active CNS metastases, prior treatment with anti‐PD1/anti‐PD‐L1/anti‐PD‐L2/anti‐CD137/ BRAF/MEK inhibitors, ongoing immunosuppressive therapy, history of allergic or hypersensitivity reaction of IFN‐alpha or ipilimumab. |
| Interventions | Both arms: following the prime phase and restaging systemic scans, participants will undergo surgical resection. Arm 1: nivolumab and CMP‐001 combination Prime phase ‐ nivolumab 240 mg IV, every 2 weeks starting with cycle 2 (cycles 2, 4, 6) for 6 weeks in combination with CMP‐001 5 mg subcutaneous 1st dose, and the remaining once weekly injections, 10 mg intra‐tumorally will be administered weeks 2 to 7. Boost phase ‐ nivolumab 480 mg IV, every 4 weeks and CMP‐001 5 mg subcutaneous every 4 weeks up to 48 weeks. Arm 2: nivolumab Prime phase ‐ nivolumab 240 mg IV, every 2 weeks starting with cycle 2 (cycles 2, 4, 6) for 6 weeks. Boost phase ‐ nivolumab 480 mg IV, every 4 weeks starting from the time of surgery recovery for up to 48 weeks. |
| Outcomes | Primary outcomes
Secondary outcomes relevant to this review
|
| Starting date | Not specified |
| Contact information | Contact: Amy Rose, RN kennaj@upmc.edu Contact: Tiffany Devine, RN devinetl3@upmc.edu |
| Notes |
NCT04708418.
| Study name | Phase II Randomized Study of Neoadjuvant Pembrolizumab Alone or in Combination With CMP‐001 in Patients With Operable Melanoma: Efficacy and Biomarker Study |
| Methods | Phase II randomised, single‐blind trial Estimated enrolment: 54 participants |
| Participants | Adults aged ≥18 years, ECOG PS 0 to 1, resectable T0, Tx or T1‐4; and N2b, N2c, N3b or N3c melanoma with injectable lesions. People with mucosal or uveal melanoma are excluded. |
| Interventions | Experimental: arm A (pembrolizumab) Neoadjuvant phase: participants receive pembrolizumab IV over 30 minutes on day 1. Treatment repeats every 21 days for up to 3 cycles in the absence of disease progression or unacceptable toxicity. Surgery: participants undergo surgery 1 to 2 weeks after completion of neoadjuvant phase. Adjuvant phase: after recovery from surgery, participants receive pembrolizumab IV over 30 minutes on day 1 of every other cycle. Treatment repeats every 21 days for up to 16 cycles in the absence of disease progression or unacceptable toxicity. Experimental: arm B (CMP‐001, pembrolizumab) Neoadjuvant phase: participants receive CMP‐001 SC on day 1 of cycle 1 and then intratumorally on days 8 and 15 of cycle 1, days 1, 8, and 15 of cycle 2, and day 1 of cycle 3. Participants also receive pembrolizumab IV over 30 minutes on day 8 of each cycle. Treatment repeats every 21 days for up to 3 cycles in the absence of disease progression or unacceptable toxicity. Surgery: participants undergo surgery 1 to 2 weeks after completion of neoadjuvant phase. Adjuvant phase: after recovery from surgery, participants receive pembrolizumab IV over 30 minutes on day 1 of every other cycle. Treatment repeats every 21 days for up to 16 cycles in the absence of disease progression or unacceptable toxicity. |
| Outcomes | Primary:
Secondary:
|
| Starting date | 19 March 2021 Estimated primary completion date: November 2021 |
| Contact information | Principal Investigator: Ahmad Tarhini ECOG‐ACRIN Cancer Research Group Sponsored by the National Cancer Institute |
| Notes |
NCT04722575.
| Study name | Neoadjuvant plus adjuvant therapy with combination or sequence of vemurafenib, cobImetinib, and atezolizumab in patients with high‐risk, surgically resectable BRAF mutated and wild‐type melanoma |
| Methods | Phase II, open‐label RCT |
| Participants | People aged 18 years and over, with stage IIIB, IIIC, IIID or IV resectable melanoma, known BRAF mutation status, ECOG PS 0 to 1 |
| Interventions | Arm A BRAF‐mutated patients. Over a period of 6 weeks (1) + (2):
After surgery and a second screening period (up to 6 weeks): atezolizumab 1200 mg IV for 52 weeks Arm B BRAF‐mutated patients. Over a period of 6 weeks (1) + (2) + (3):
After surgery and a second screening period (up to six weeks): atezolizumab 1200 mg IV for 52 weeks Arm C BRAF‐WT patients. Over a period of six weeks (1) + (2):
After surgery and a second screening period (up to six weeks): atezolizumab 1200 mg IV for 52 weeks |
| Outcomes | Primary:
Secondary:
|
| Starting date | October 2020, primary completion date March 2022 |
| Contact information | Marcello Curvietto curvietto.ma@gmail.com Paola Schiavo neotim@cr‐technology.com |
| Notes |
NCT04949113.
| Study name | NADINA |
| Methods | Randomised open‐label phase III trial |
| Participants | Projected enrolment 420 participants Inclusion criteria
Exclusion criteria:
|
| Interventions | Experimental arm: 2 cycles of neoadjuvant ipilimumab (80 mg) + nivolumab (240 mg) every 3 weeks followed by a total lymph node dissection (TLND) and if applicable, resection of in‐transit metastases. Participants not achieving a pathologic response in arm A will also receive adjuvant nivolumab 480 mg every 4 weeks, 11 cycles. In case of BRAF V600E/K mutation‐positivity, participants will be treated with adjuvant dabrafenib plus trametinib for 46 weeks instead. Control arm: standard upfront total lymph node dissection and if applicable, resection of in‐transit metastases followed by 12 cycles adjuvant nivolumab 480 mg every 4 weeks. |
| Outcomes | Primary
Secondary outcomes of relevance to this review
|
| Starting date | Expected 8 July 2021 |
| Contact information | Prof Christian Blank, c.blank@nki.nl |
| Notes |
AEs: adverse events; AJCC: American Joint Committee on Cancer; BRAF: v‐raf murine sarcoma viral oncogene homolog B1; CLND: complete lymph node dissection; CNS: central nervous system; CTCAE: common terminology criteria for adverse events; CTLA‐4: cytotoxic T‐lymphocyte‐associated antigen‐4; DFS: disease‐free survival; ECOG PS: European Cooperative Oncology Group Performance Status; EFS: event free survival; HSV: herpes simplex virus; IDO: indoleamine 2,3‐dioxygenase; IFN: interferon; IL‐2: interleukin‐2; ITT: intention‐to‐treat; IV: intravenous; LDH: lactate dehydrogenase; LDH < ULN: lactate dehydrogenase less than the upper limit of normal; MEK: Mitogen‐activated protein kinase; N/A: not applicable; ORR: overall response rate; OS: overall survival; pCR: pathological complete response; PD: programmed death; PD‐L1: programmed death ligand‐1; PET: positron emission tomography; PFS: progression‐free survival; pPR: pathological partial reponse; RECIST: Response Evaluation Criteria in Solid Tumours; SC: subcutaneously; Tim‐3: T cell immunoglobulin and mucin‐domain containing‐3; TRAEs: treatment‐related adverse events; TTR: time to recurrence; T‐VEC: talimogene laherparepvec; WHO: World Health Organization; WT: wild‐type
Differences between protocol and review
We did not search the ISRCTN database as all trials are listed in the WHO ICTRP database. We did not handsearch ASCO abstracts as they are indexed in MEDLINE. We conducted additional handsearching of ESMO and SMR abstracts from 2017 to 2020 to ensure the most up‐to‐date publications could be identified and included.
We had planned to include progression‐free survival as an outcome in the summary of findings tables. Recently published work has highlighted recurrence‐free survival, event‐free survival and distant metastatic‐free survival as clinically‐relevant survival outcomes for people undergoing neoadjuvant treatment of melanoma (INMC 2019), and it was considered more relevant to users of the review to include one or more of these endpoints in the summary of findings tables. The time‐to‐recurrent disease outcome defined in the review protocol (broadly analogous to event‐free survival in the INMC publication) was instead included in the summary of findings tables, where reported by the included trials. Progression‐free survival outcomes, where reported, were reported in the results section of the review.
Following feedback from clinical reviewers, we changed the title of the review from 'Neoadjuvant treatment for malignant and metastatic melanoma' to 'Neoadjuvant treatment for stage III and IV cutaneous melanoma'. This was a more precise and accurate description of the population included in our review.
Contributions of authors
CG was the contact person with the editorial base. CG co‐ordinated contributions from the co‐authors and wrote the final draft of the review. CG, HOD, SB, LMcC screened papers against eligibility criteria. CG obtained data on ongoing and unpublished studies. CG, HOD, LMcC appraised the quality of papers. CG, HOD, LMcC extracted data for the review and sought additional information about papers. CG entered data into RevMan. CG, SS analysed and interpreted data. CG, SS, IC worked on the methods sections. CG, RB, EB, MB drafted the clinical sections of the background and responded to the clinical comments of the referees. CG, SS, IC responded to the methodology and statistics comments of the referees. KC was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Sources of support
Internal sources
-
National Centre for Pharmacoeconomics, Ireland
CG received funding support for a PhD examining the cost‐effectiveness of treatment strategies for skin cancer in the Irish healthcare setting.
External sources
-
Health Research Board, Ireland
Financial grant to cover costs of completion of Cochrane Review
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Claire Gorry: none known. Laura McCullagh: none known. Helen O'Donnell: none known. Sarah Barrett: none known. Susanne Schmitz: no relevant interests; statistical editor for Cochrane Anaesthesia and Cochrane Emergency and Critical Care. Michael Barry: none known. Kay Curtin: consultant for Cancer Trials Ireland (Patient Consultant to Melanoma Committee). Eamon Beausang: no relevant interest; Plastic Surgeon, St James's Hospital, Dublin. Rupert Barry: Founder of DermView Ltd, a dermatology clinic chain; Dermatologist, St James Hospital, Dublin. Imelda Coyne: none known.
New
References
References to studies included in this review
Albertini 2018 {published data only (unpublished sought but not used)}
- Albertini MR, Yang RK, Ranheim EA, Hank JA, Zuleger CL, Weber S, et al. Pilot trial of the hu14.18-IL2 immunocytokine in patients with completely resectable recurrent stage III or stage IV melanoma. Cancer Immunology Immunotherapy 2018;67(10):1647-58. [DOI: 10.1007/s00262-018-2223-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00590824. Pilot hu14.18-IL2 in resectable recurrent stage III or stage IV melanoma. clinicaltrials.gov/ct2/show/NCT00590824 (first received 11 January 2008). [Accessed 25 May 2021 https://clinicaltrials.gov/ct2/show/NCT00590824]
- Yang R, Albertini M, Hank J, Loibner H, Gillies S, Sondel P, et al. Tumor infiltrating lymphocyte (TIL) percentage as a prognostic biomarker for overall and relapse free survival in Hu14.18-IL2 treated resectable recurrent stage 3 or 4 melanoma patients. Journal for ImmunoTherapy of Cancer 2017;5(Suppl 2):86. [DOI: 10.1186/s40425-017-0289-3] [DOI] [Google Scholar]
- Yang RK, Kuznetsov IB, Ranheim EA, Wei JS, Sindiri S, Gryder BE, et al. Outcome-related signatures identified by whole transcriptome sequencing of resectable stage III/IV melanoma evaluated after starting Hu14.18-IL2. Clinical Cancer Research 2020;26(13):3296-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amaria 2018a {published and unpublished data}
- Amaria RN, Prieto P, Tetzlaff M, Diab A, Patel S, Woodman SE, et al. Treatment with neoadjuvant + adjuvant dabrafenib and trametinib (D+T) is associated with improved relapse free survival (RFS) versus standard of care (SOC) therapy in patients with high-risk resectable BRAF mutant melanoma. Pigment Cell and Melanoma Research 2017;30(1):78. [Google Scholar]
- Amaria RN, Prieto PA, Tetzlaff MT, Reuben A, Andrews MC, Wargo JA, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncology 2018;19(2):181-93. [DOI] [PubMed] [Google Scholar]
- NCT02231775. Dabrafenib and trametinib before and after surgery in treating patients with stage IIIB-C melanoma with BRAF V600 mutation [Neoadjuvant and adjuvant dabrafenib and trametinib in patients with clinical stage III melanoma (Combi-Neo)]. clinicaltrials.gov/ct2/show/NCT02231775 (first received 4 September 2014).
- Prieto PA, Amaria R, Reuben A, Gopalakrishnan V, Spencer C, Jiang H, et al. Novel neoadjuvant targeted therapy trial with dabrafenib + trametinib yields high response rates and improved relapse-free survival over standard of care (SOC) therapy in patients with high-risk resectable BRAF-mutant melanoma. Annals of Surgical Oncology 2017;24(Suppl 1):S7. [Google Scholar]
- Wargo JA, Amaria RN, Prieto PA, Andrews MC, Tetzlaff MT, Futreal PA, et al. Relapse-free survival and target identification to enhance response with neoadjuvant and adjuvant dabrafenib + trametinib (D+T) treatment compared to standard-of-care (SOC) surgery in patients (pts) with high-risk resectable BRAF-mutant metastatic melanoma. Journal of Clinical Oncology 2017;35(15_suppl):9587-9587. [Google Scholar]
- Wargo JA, Amaria RN, Ross MI, Saw RP, Gershenwald JE, Hwu P, et al. Neoadjuvant BRAF (dabrafenib) and MEK (trametinib) inhibition for high-risk resectable stage III and IV melanoma. Journal of Clinical Oncology 2015;33:TPS9091. [Google Scholar]
- Woodman SE, Prieto P, Andrews MC, Amaria RN, Tetzlaff M, Diab A, et al. Novel neoadjuvant targeted therapy trial yields insight into molecular mechanisms of response. Cancer Research: American Association for Cancer Research Annual Meeting 2017;77(13 Suppl):Abstract CT156. [Google Scholar]
Amaria 2018b {published and unpublished data}
- Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nature Medicine 2018;24(11):1649-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaria RN, Reddy SM, Tawbi HA-H, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant (neo) immune checkpoint blockade (ICB) in patients (Pts) with high-risk resectable metastatic melanoma (MM). Journal of Clinical Oncology 2018;36(15_suppl):9510-9510. [Google Scholar]
- NCT02519322. Neoadjuvant and adjuvant checkpoint blockade [Neoadjuvant and adjuvant checkpoint blockade in patients with clinical stage III or oligometastatic stage IV melanoma]. clinicaltrials.gov/ct2/show/nct02519322 (first received 10 August 2015).
- Reddy SM, Amaria RN, Spencer CN, Tetzlaff M, Reuben A, Andrews M, et al. Neoadjuvant nivolumab versus combination ipilimumab and nivolumab followed by adjuvant nivolumab in patients with resectable stage III and oligometastatic stage IV melanoma: Preliminary findings. Journal for ImmunoTherapy of Cancer 2017;5(Suppl 2):86. [Google Scholar]
Blank 2018 {published and unpublished data}
- Blank C, Van Akkooi A, Rozeman EA, Kvistborg P, Van Thienen HV, Stegenga B, et al. (Neo-)adjuvant ipilimumab + nivolumab (IPI + NIVO) in palpable stage 3 melanoma-initial data from the OpACIN trial. Annals of Oncology 2016;27(Suppl 6):Abstract 4587. [Google Scholar]
- Blank CU, Rozeman EA, Fanchi LF, Sikirska K, de Wiel B, Schumacher TN, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nature Medicine 2018;24:1655-61. [NCT02437279] [DOI] [PubMed] [Google Scholar]
- Blank CU, Van Akkooi A, Rozeman L, Kvistborg P, Van Thienen JV, Stegenga B, et al. (Neo-)adjuvant ipilimumab + nivolumab (IPI + NIVO) in palpable stage 3 melanoma-the OpACIN trial. Pigment Cell and Melanoma Research 2017;30(1):85. [Google Scholar]
- Blank CU, Versluis JM, Reijers IL, Sikorska K, Houdt WJ, Thienen JV, et al. 3-year relapse-free survival (RFS), overall survival (OS) and long-term toxicity of (neo)adjuvant ipilimumab (IPI) + nivolumab (NIVO) in macroscopic stage III melanoma (OpACIN trial). Annals of Oncology 2019;30(Suppl 5):v535. [Google Scholar]
- Boekhout AH, Jozwiak K, Rozeman EA, Janssen SH, Van de Poll-Franse LV, Blank CU. Patient-reported outcomes of patients treated with (neo)adjuvant immune checkpoint combination therapy in high-risk stage III macroscopic melanoma: A matched cohort study. Journal of Clinical Oncology 2019;37(15 Suppl):9588. [Google Scholar]
- Rozeman EA, Blank CU, Van Akkooi AC, Kvistborg P, Fanchi L, Van Thienen JV, et al. Neoadjuvant ipilimumab + nivolumab (IPI+NIVO) in palpable stage III melanoma: Updated data from the OpACIN trial and first immunological analyses. Journal of Clinical Oncology 2017;35(15 Suppl):9586. [Google Scholar]
- Rozeman EA, Fanchi L, Van Akkooi AC, Kvistborg P, Thienen JV, Stegenga B, et al. (Neo-)adjuvant ipilimumab 1 nivolumab (IPI1NIVO) in palpable stage 3 melanoma-updated relapse free survival (RFS) data from the OpACIN trial and first biomarker analyses. Annals of Oncology 2017;28(Suppl 5):v432. [Google Scholar]
- Rozeman EA, Franchi L, Kuilman T, Krijgsman O, Akkooi AC, Kvistborg P, et al. Biomarker analysis from the OpACIN trial (Neo-/adjuvant ipilimumab + nivolumab (IPI+NIVO) in palpable stage 3 melanoma). Journal for the ImmunoTherapy of Cancer 2017;5(Suppl 2):86. [Google Scholar]
- Rozeman EA, Sikorska K, Van De Wiel BA, Franchi LF, Krigsman O, Thienen HV, et al. 30 months relapse-free survival, overall survival, and long-term toxicity update of (neo)adjuvant ipilimumab (ipi) 1 nivolumab (nivo) in macroscopic stage III melanoma (OPACIN trial). Annals of Oncology 2018;29(Suppl 10):X42. [Google Scholar]
- Versluis JM, Reijers IL, Rozeman EA, Sikorska K, Houdt WJ, Thienen JV, et al. 1097P 4-year relapse-free survival (RFS), overall survival (OS) and long-term toxicity of (neo)adjuvant ipilimumab (IPI) + nivolumab (NIVO) in macroscopic stage III melanoma: opACIN trial. Annals of Oncology 2020;31:S742-3. [Google Scholar]
Dummer 2020b {published data only (unpublished sought but not used)}
- Andtbacka RH, Chastain M, Li A, Shilkrut M, Ross MI. Phase 2, multicenter, randomized, open-label trial assessing efficacy and safety of talimogene laherparepvec (T-VEC) neoadjuvant treatment (tx) plus surgery vs surgery for resectable stage IIIB/C and IVM1a melanoma (MEL). Journal of Clinical Oncology 2015;33(15_suppl):TPS9094. [Google Scholar]
- Andtbacka RH, Shabooti M, Li A, Chastain M, Shilkrut M. A phase 2, randomized, open-label trial assessing efficacy and safety of talimogene laherparepvec (T-VEC) neoadjuvant treatment (TX) plus surgery vs. surgery for resectable stage IIIB-IVM1a melanoma. Cancer Research: American Association for Cancer Research 2015;75(14 Suppl):Abstract B13. [Google Scholar]
- Dummer R, Gyorki D, Hyngstrom J, Berger A, Conry R, Demidov L, et al. 3-year results of the phase 2 randomized trial for talimogene laherparepvec (T-VEC) neoadjuvant treatment plus surgery vs surgery in patients with resectable stage IIIB-IVM1a melanoma. Journal for Immunotherapy of Cancer 2020;8(Suppl 3):A263. [Google Scholar]
- Dummer R, Gyorki D, Hyngstrom J, Berger A, Conry R, Dermidov L, et al. A randomized phase 2 study of neoadjuvant talimogene laherparepvec (T-VEC) plus surgery vs surgery for resectable stage IIIB-IVM1a melanoma: 2-year primary analysis of recurrence-free survival (RFS). Journal for Immunotherapy of Cancer 2019;7(Suppl 1):P455. [Google Scholar]
- Dummer R, Gyorki DE, Hyngstrom J, Berger A, Conry R, Demidov L, et al. Improved recurrence-free survival (RFS) from a randomized phase 2 study of neoadjuvant (neo) talimogene laherparepvec (T-VEC) plus surgery (surg) vs surg for resectable stage IIIB-IVM1a melanoma (MEL). Pigment Cell and Melanoma Research 2020;33(1):188. [Google Scholar]
- Dummer R, Gyorki DE, Hyngstrom J, Berger A, Conry R, Demidov L, et al. Primary 2-year (yr) results of a phase II, multicenter, randomized, open-label trial of efficacy and safety for talimogene laherparepvec (T-VEC) neoadjuvant (neo) treatment (tx) plus surgery (surg) vs surg in patients (pts) with resectable stage IIIB-IVM1. Annals of Oncology 2019;30(Suppl 5):v903. [Google Scholar]
- Dummer R, Gyorki DE, Hyngstrom JR, Berger AC, Conry RM, Demidov LV, et al. One-year (yr) recurrence-free survival (RFS) from a randomized, open label phase II study of neoadjuvant (neo) talimogene laherparepvec (TVEC) plus surgery (surgx) versus surgx for resectable stage IIIB-IVM1a melanoma (MEL). Journal of Clinical Oncology 2019;37(15 Suppl):9520. [Google Scholar]
- NCT02211131. Efficacy and safety of talimogene laherparepvec neoadjuvant treatment plus surgery versus surgery alone for melanoma. clinicaltrials.gov/ct2/show/nct02211131 (first received 7 August 2014).
- NCT02211131. Efficacy and safety of talimogene laherparepvec neoadjuvant treatment plus surgery versus surgery alone for melanoma [A phase 2, multicenter, randomized, open-label trial assessing the efficacy and safety of talimogene laherparepvec neoadjuvant treatment plus surgery versus surgery alone for resectable, stage IIIB to IVM1a melanoma]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID-NCT02211131 (first received 5 August 2015).
- Ross MI, Dummer R, Gyorki DE, Hyngstrom J, Berger A, Conry R, et al. Primary 2-year results of phase 2, multicenter, randomized, open-label trial of efficacy and safety for talimogene laherparepvec (T-VEC) neoadjuvant treatment plus surgery versus surgery in patients with resectable stage IIIB-IVM1a melanoma. Annals of Surgical Oncology 2020;20(Suppl 1):S7. [Google Scholar]
Hwu 2017 {published data only (unpublished sought but not used)}
- Chakravarti N, Ivan D, Ross MI, Ilagan JL, Warneke CL, Kim K, et al. Biomarker study in patients with resectable AJCC stage IIIc or Stage IV (M1a) melanoma treated in a randomised phase II neoadjuvant trial. Journal of Clinical Oncology 2013;31(Suppl):Abstract 9047. [Google Scholar]
- NCT00525031. Temozolomide alone or with pegylated interferon-alpha 2b (PGI) in melanoma patients [Randomized phase II neoadjuvant study of temozolomide alone or with pegylated interferon-alpha 2b in patients with resectable American Joint Committee on Cancer (AJCC) stage IIIB/IIIC or stage IV (M1a) metastatic melanoma]. clinicaltrials.gov/ct2/show/nct00525031 (first received 5 September 2007).
Rozeman 2019 {published and unpublished data}
- 2016-001984-35. Multicenter phase 2 study to identify of the optimal neo-adjuvant combination scheme of ipilimumab and nivolumab (OpACIN-neo). clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2016-001984-35 (first received April 2017).
- Blank CU, Rozeman EA, Menzies AM, Van de Wiel BA, Adhkari C, Sikorska K, et al. OpACIN-neo: A multicenter phase II study to identify the optimal neo-adjuvant combination scheme of ipilimumab (IPI) and nivolumab (NIVO). Annals of Oncology 2018;29(Suppl 8):viii734. [Google Scholar]
- Blank CU, Versluis JM, Rozeman EA, Menzies AM, Reijers IL, Krigsman O, et al. 36-months and 18-months relapse-free survival after (neo)adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma patients -update of the OpACIN and OpACIN-neo trials. Cancer Research 2020;80(16):Abstract 3412. [Google Scholar]
- NCT02977052. Optimal neo-adjuvant combination scheme of ipilimumab and nivolumab [Multicenter phase 2 study to identify of the optimal neo-adjuvant combination scheme of ipilimumab and nivolumab (OpACIN-neo)]. clinicaltrials.gov/ct2/show/NCT02977052 (first received 30 November 2016).
- NCT02977052. Optimal neo-adjuvant combination scheme of ipilimumab and nivolumab OpACIN-neo. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=NCT02977052 (first received 2 November 2016).
- Reijers IL, Rozeman EA, Menzies AM, Versluis JM, Van de Wiel BA, Sikorska K, et al. Different pathologic response rates between Australia and Europe in macroscopic stage III melanoma patients upon neoadjuvant ipilimumab plus nivolumab in the phase II OpACIN-neo trial. Cancer Research 2020;80(16 Suppl):Abstract 3271. [Google Scholar]
- Reijers ILM, Rozeman EA, Menzies AM, Versluis JM, Van de Wiei BA, Sikorska K, et al. Continental differences in pathologic response with neoadjuvant ipilimumab (IPI) plus nivolumab (NIVO) in patients with macroscopic stage III melanoma in the phase II OpACIN-neo trial. Annals of Oncology 2019;30(Suppl 5):v541. [Google Scholar]
- Rozeman EA, Hoefsmit EP, Reijers IL, Saw RP, Versluis JM, Krijgsman O, et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nature Medicine 2021;27:256-63. [DOI] [PubMed] [Google Scholar]
- Rozeman EA, Menzies AM, Krijgsman O, Hoefsmit EP, de Wiel BA, Sikorska K, et al. 18-months relapse-free survival (RFS) and biomarker analyses of OpACIN-neo: A study to identify the optimal dosing schedule of neoadjuvant (neoadj) ipilimumab (IPI) 1 nivolumab (NIVO) in stage III melanoma. Annals of Oncology 2019;30(Suppl 5):v910. [Google Scholar]
- Rozeman EA, Menzies AM, Akkooi ACJ, Adhikari C, Bierman C, de Wiel BA, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncology 2019;20(7):948-60. [DOI] [PubMed] [Google Scholar]
- Rozeman EA, Menzies AM, de Wiel BA, Adhikari C, Blank CU, et al. LBA42 - OpACIN-neo – A multicenter phase 2 study to identify the optimal neo-adjuvant combination scheme of ipilimumab (IPI) and nivolumab (NIVO). In: ESMO Congress, Munich 2018. 2018.
- Rozeman EA, Menzies AM, Versluis JM, Reijers IL, Krigsman O, Hoefsmit EP, et al. 36 months and 18 months relapse-free survival (RFS) after (neo) adjuvant ipilimumab (IPI) + nivolumab (NIVO) in macroscopic stage III melanoma (OpACIN and OpACIN-neo trial). Journal of Translational Medicine 2020;18(Suppl 1):No pagination. [Google Scholar]
- Rozeman EA, Van Akkooi AC, Menzies AM, Smith M, Scoyler RA, Pronk LM, et al. Multicenter phase 2 study to identify the optimal neo-adjuvant combination scheme of ipilimumab (IPI) and nivolumab (NIVO) (OpACIN-neo). Journal of Clinical Oncology 2018;36(15 Suppl):TPS9606. [Google Scholar]
- Rozeman EA, Van Akkooi AC, Routier E, Menzies AM, Eriksson H, Smith M, et al. Multicenter phase 2 study to identify the optimal neo-adjuvant combination scheme of ipilimumab (IPI) and nivolumab (NIVO) (OpACIN-neo). Journal of Clinical Oncology 2017;35(15 Suppl):TPS9600. [Google Scholar]
- Versluis JM, Rozeman EA, Menzies AM, Reijers IL, Krigsman O, Hoefsmit EP, et al. Update of the OpACIN and OpACIN-neo trials: 36-months and 24-months relapse-free survival after (neo)adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma patients. Journal for Immunotherapy of Cancer 2020;8(Suppl 2):A2. [Google Scholar]
Tarhini 2018 {published data only (unpublished sought but not used)}
- Khunger A, Sarikonda G, Tsau J, Alfonso Z, Gao J, Pahuja A, et al. Predictive value of circulating B cells and T cell subsets in melanoma patients treated with neoadjuvant ipilimumab and interferon. Journal of Clinical Oncology 2020;38(15 Suppl):e15036. [Google Scholar]
- NCT01608594. Neoadjuvant combination therapy with ipilimumab and highdose IFN-α2b for melanoma. clinicaltrials.gov/ct2/show/NCT01608594 (first received 31 May 2012).
- Tarhini A, Lin Y, Lin H, Rahman A, Vallabhaneni P, Mendiratta P, et al. Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-alpha2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire. Journal for Immunotherapy of Cancer 2018;6(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhini A, Rahman Z, Lin Y, Vallabhaneni P, Tawbi H, Gnan A, et al. Neoadjuvant combination immunotherapy with ipilimumab (3mg/kg or 10mg/kg) and high dose IFN-a2b in locally/regionally advanced melanoma. Journal of Clinical Oncology 2016;34(15 Suppl):9585. [Google Scholar]
- Vallabhaneni P, Achkar T, Vignali M, Benzeno S, Rytleski J, Tarhini AA. Clonality of T cell repertoire in the tumor (TME) and peripheral blood of regionally advanced melanoma patients (pts) treated with neoadjuvant ipilimumab (ipi) and high dose interferon-alpha (HDI). Journal of Clinical Oncology 2017;35(15 Suppl):9585. [Google Scholar]
References to studies excluded from this review
2006‐005350‐79 {published data only}
- 2006-005350-79. A single arm, single-centre, open-label, exploratory trial of recombinant Interleukin-21 administered subcutaneously for 4 weeks as neo-adjuvant treatment prior to sentinel lymph node/complete lymph node dissection followed by 8 weeks of adjuvant treatment in subject with stage III malignant melanoma. www.clinicaltrialsregister.eu/ctr-search/search?query=2006-005350-79 (first received 22 July 2008).
Ahmann 1976 {published data only}
- Ahmann DL, Bisel HF, Edmonson JH, Hahn RG, O'Connell MJ, Frytak S. Phase II study of VP-16-213 versus dianhydrogalactitol in patients with metastatic malignant melanoma. Cancer Treatment Reports 1976;60(11):1681-2. [PubMed] [Google Scholar]
Ariyan 1982 {published data only}
- Ariyan S, Kirkwood J, Mitchell M, Nordlund J, Lerner A, Papac R. Intralymphatic and regional surgical adjuvant immunotherapy in high-risk melanoma of the extremities. Surgery 1982;92(3):459-63. [PubMed] [Google Scholar]
Buzaid 1998 {published data only}
- Buzaid AC, Colome M, Bedikian A, Eton O, Legha SS, Papadopoulos N, et al. Phase II study of neoadjuvant concurrent biochemotherapy in melanomao patients with local-regional metastases. Melanoma Research 1998;8(6):549-56. [DOI] [PubMed] [Google Scholar]
DeCOG 2010 {published data only}
- 2010-022103-21/DE. Neoadjuvant treatment of locoregional metastases in malignant melanoma (AJCC stage IIIB/C) with Multiferon: A phase IIa DeCOG trial. www.clinicaltrialsregister.eu/ctr-search/trial/2010-022103-21/DE (first received 19 November 2010).
Dillman 2015 {published data only}
- Dillman RO, Bayer ME, Langford JA, Hseih CY, Wyatt TJ, Liang G, et al. Phase III multicenter trial of eltrapuldencel-T: Autologous dendritic cells loaded with irradiated autologous tumour cells (DC-TC) in granulocyte-macrophage colony stimulating factor (GM-CSF) in patients with metastatic melanoma (INTUS trial). Journal of Clinical Oncology 2015;33(15 Suppl 1):TPS9082. [Google Scholar]
Koyanagi 2005 {published data only}
- Koyanagi K, O'Day SJ, Gonzalez R, Lewis K, Robinson WA, Amatruda TT, et al. Serial monitoring of circulating melanoma cells during neoadjuvant biochemotherapy for stage III melanoma: Outcome prediction in a multicenter trial. Journal of Clinical Oncology 2005;23(31):8057-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2019 {published data only}
- Long GV, Saw RP, Lo S, Nieweg OE, Shannon KF, Gonzalez M, et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB-C, BRAF V600 mutation-positive melanoma (NeoCombi): a single-arm, open-label, single-centre, phase 2 trial. Lancet Oncology 2019;20(7):961-71. [DOI] [PubMed] [Google Scholar]
Madu 2016 {published data only}
- Blankenstein SA, Rohaan MW, Klop WM, Van Der Hiel B, Van De Wiel BA, Haanen JB, et al. Neoadjuvant cytoreductive treatment with BRAF/MEK inhibition of prior unresectable regionally advanced melanoma to allow complete surgical resection: REDUCTOR trial. Journal of Clinical Oncology 2019;37(15 Suppl):9587-87. [DOI] [PubMed] [Google Scholar]
- Madu MF, Rozeman EA, Hage JA, Wouters M, Klop W, Thienen JV, et al. Neoadjuvant cytoreductive treatment of regionally advanced melanoma with braf/mek inhibition: study protocol of the REDUCTOR (cytoreductive treatment of dabrafenib combined with trametinib to allow complete surgical resection in patients with braf mutated, prior unresectable stage iii or iv melanoma) trial. Clinical Skin Cancer 2016;1(1):48-52. [Google Scholar]
- NTR4654. Trial with melanoma patients who are treated with dabrafenib and trametinib to make the tumor smaller, so it can be surgically removed [Cytoreductive treatment of dabrafenib combined with trametinib to allow complete surgical resection in patients with BRAF mutated, prior unresectable stage III or IV melanoma]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=NTR4654 (first received 20 June 2014).
Moschos 2006 {published data only}
- Moschos SJ, Edington HD, Land SR, Rao UN, Jukic D, Shipe-Spotloe J, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumour regression in association with modulation of tumour infiltrating host cellular immune responses. Journal of Clinical Oncology 2006;24(19):3164-71. [DOI] [PubMed] [Google Scholar]
Mudigonda 2016 {published data only}
- Mudigonda TV, Wyman K, Spigel DR, Dahlman KB, Greco FA, Puzanov I, et al. A phase II trial of erlotinib and bevacizumab for patients with metastatic melanoma. Pigment Cell and Melanoma Research 2016;29(1):101-3. [DOI] [PubMed] [Google Scholar]
Najjar 2017 {published data only}
- Najjar YG, Ding F, Lin Y, VanderWeele R, Butterfield L, Tarhini A. Melanoma antigen-specific effector T cell cytokine secretion patterns in patients treated with ipilimumab. Journal of Translational Medicine 2017;15(39):no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
Najjar 2018 {published data only}
- Najjar Y, Lin H, Lin Y, McCurry D, Davar D, Drabick J, et al. A phase 1 study of neoadjuvant combination immunotherapy with pembrolizumab (P) and high dose IFN-α2b(HDI) in locally/regionally advanced melanoma. Pigment Cell and Melanoma Research 2019;32(1):92-172. [Google Scholar]
NCT01720407 {published data only}
- NCT01720407. Relevance of imiquimod as neo-adjuvant treatment to reduce excision size and the risk of intralesional excision in lentigo malignant of the face (ImiReduc). clinicaltrials.gov/ct2/show/NCT01720407 (first received 2 November 2012).
NCT02036086 {published data only}
- NCT02036086. Study of neo-adjuvant use of vemurafenib plus cobimetinib for BRAF mutant melanoma with palpable lymph node metastases. clinicaltrials.gov/ct2/show/NCT02036086 (first received 14 January 2014).
NCT02303951 {published data only}
- NCT02303951. Neoadjuvant vemurafenib + cobimetinib in melanoma: NEO-VC. clinicaltrials.gov/ct2/show/NCT02303951 (first received 1 December 2014).
NCT02736123 {published data only}
- NCT02736123. Neoadjuvant combination biotherapy with ipilimumab and nivolumab or nivolumab alone [Neoadjuvant combination biotherapy with ipilimumab and nivolumab or nivolumab alone in patients with locally/regionally advanced/recurrent melanoma: a randomized efficacy, safety and biomarker study]. clinicaltrials.gov/ct2/show/NCT02736123 (first received 13 April 2016).
NCT03313206 {published data only}
- NCT03313206. Neoadjuvant treatment associated with maintenance therapy by anti-pd1 immunotherapy in patients with resectable head and neck mucosal melanoma (IMMUQ). clinicaltrials.gov/ct2/show/NCT03313206 (first received 18 October 2017).
NCT03618641 {published data only}
- Davar, D, Karunamurthy, A, Hartman, D, DeBlasio, R, Chauvin, J M, Ding, Q et al. Phase ii trial of neoadjuvant nivolumab (NIVO) and intra-tumoral (IT) CMP-001 in high-risk resectable melanoma (neo-c-nivo): Final results. Journal for Immunotherapy of Cancer 2020;8(Suppl 3):A185-186. [Google Scholar]
- Davar D, Karunamurthy A, Hartman D, Ka M, Menna C, Burkette J, et al. O34 Phase II trial of neoadjuvant nivolumab (Nivo) and intra-tumoral (IT) CMP-001 in high risk resectable melanoma (MEL): Preliminary results. Journal for ImmunoTherapy of Cancer 2019;7(Suppl 1):283. [Google Scholar]
- NCT03618641. CMP-001 in combo with nivolumab in stage IIIB/C/D melanoma patients with clinically apparent lymph node disease [Neoadjuvant Phase II study of TLR9 agonist CMP-001 in combination with nivolumab in stage IIIB/C/D melanoma patients with clinically apparent lymph node disease]. clinicaltrials.gov/ct2/show/NCT03618641 (first received 7 August 2021).
NCT04007588 {published data only}
- NCT04007588. A phase II trial of neoadjuvant treatment with PD-1 inhibition (Nivolumab) with or without IDO inhibition (BMS-986205) and with or without CTLA-4 inhibition (Ipilimumab) in resectable stage III or IV melanoma. clinicaltrials.gov/ct2/show/NCT04007588 (first received 5 July 2019).
NCT04013854 {published data only}
- NCT04013854. Adjuvant treatment determined by pathological response to neoadjvuant nivolumab [Adjuvant nivolumab or ipilimumab + nivolumab determined by pathological response to a single dose of neoadjvuant nivolumab]. clinicaltrials.gov/ct2/show/NCT04013854?term=NCT04013854&draw=2&rank=1 (first received 10 July 2019).
NCT04495010 {published data only}
- NCT04495010. Neoadjuvant nivolumab+ipilimumab followed by adjuvant nivolumab or neoadjuvant nivolumab+ipilimumab followed by adjuvant observation compared with adjuvant nivolumab in treatment-naive high-risk melanoma participants (CheckMate 7UA) [A phase 2, randomized study of neoadjuvant nivolumab plus ipilimumab followed by adjuvant nivolumab or neoadjuvant nivolumab plus ipilimumab followed by either adjuvant nivolumab or postsurgical observation depending on pathologic response compared with adjuvant nivolumab in treatment-naive patients with resectable clinically detectable stage iii melanoma]. clinicaltrials.gov/ct2/show/NCT04495010 (first received 31 July 2020).
NCT04741997 {published data only}
- NCT04741997. Adjuvant therapy based on pathologic response after neoadjuvant encorafenib binimetinib in melanoma [A randomized pilot trial of adjuvant therapy based on pathologic response after neoadjuvant encorafenib and binimetinib in advanced melanoma]. clinicaltrials.gov/ct2/show/NCT04741997 (first received 5 February 2021).
Notohardjo 2020 {published data only}
- Notohardjo, JCL, Bekkenk, MW, Van Den Tol, MP, Van Den Eertwegh, AJM, De Gruijl, TD. Local immunotherapy in early stage melanoma - Protocol synopsis for a randomized phase 2 trial. [Dutch] [Lokale immuuntherapie bij melanoom in een vroeg stadium – protocol synopsis voor een gerandomiseerde fase 2-studie]. Nederlands Tijdschrift voor Dermatologie en Venereologie 2020;30(6):16-8. [Google Scholar]
O'Connor 1978 {published data only}
- O'Connor TP, Labandter HP, Hiles RW, Bodenham DC. A clinical trial of BCG immunotherapy as an adjunct to surgery in the treatment of primary malignant melanoma. British Journal of Plastic Surgery 1978;31(4):317-22. [PubMed] [Google Scholar]
Passalacqua 1996 {published data only}
- Passalacqua R, Cocconi G, Dodero A, Bella M, Sarra S, Tonato M, et al. Long-term results of neoadjuvant chemotherapy in patients with local-regional recurrence of melanoma. Melanoma Research 1997;7 Suppl 1:S119. [Google Scholar]
- Passalacqua R, Cocconi G, Dodero A, Bella M, Sarra S, Tonato M, et al. Neoadjuvant chemotherapy in patients with local-regional recurrence of melanoma. Tumori 1996;82(Suppl):108. [Google Scholar]
Prieto 2016 {published data only}
- Prieto PA, Cooper Z, Gopalakrishnan V, Tetzlaff MT, Bassett T, Amaria RN, et al. Treatment with neoadjuvant targeted therapy yields high response rates and pathologic complete responses in patients with resectable metastatic melanoma. Annals of Surgical Oncology 2016;20(Suppl 1):S130-1. [Google Scholar]
Reijers 2019 {published data only}
- Reijers I, Rozeman EA, Menzies AM, Van De Wiel BA, Eriksson H, Suijkerbuijk K, et al. Personalized response-driven adjuvant therapy after combination ipilimumab and nivolumab in high-risk resectable stage III melanoma: PRADO trial. Journal of Clinical Oncology 2019;37(15 Suppl):TPS9605. [Google Scholar]
- Van Den Heuvel NM, Reijers IL, Versluis JM, Rozeman EA, Jozwiak K, Blommers KH, et al. Health-related quality of life in stage III melanoma patients treated with neoadjuvant ipilimumab and nivolumab followed by index lymph node excision only versus therapeutic lymph node dissection: 24-week results of the PRADO trial. Annals of Oncology 2020;31(Suppl 4):S737. [Google Scholar]
Samoylenko 2019 {published data only}
- Samoylenko I, Korotkova OV, Shakhray E, Zabotina T, Berdnikov S, Tabakov D, et al. Recurrence-free survival (RFS) and objective response rate (ORR) phase 1/2 study of intralesional (IL) neoadjuvant (neo) anti-PD1 agents (aPD1) for stage IIIB-IV melanoma (MEL). Journal of Clinical Oncology 2019;37(15 Suppl):e14171. [Google Scholar]
Schermers 2019 {published data only}
- Schermers B, Franke V, Rozeman EA, de Wiel BA, Bruining A, Wouters MW et al. Surgical removal of the index node marked using magnetic seed localization to assess response to neoadjuvant immunotherapy in patients with stage III melanoma. British Journal of Surgery 2019;106(5):519-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2010 {published data only}
- Shah GD, Socci ND, Gold JS, Wolchok JD, Carvajal RD, Panageas KS, et al. Phase II trial of neoadjuvant temozolomide in resectable melanoma patients. Annals of Oncology 2010;21(8):1718-22. [DOI] [PubMed] [Google Scholar]
Stiles 2017 {published data only}
- Stiles ZE, Fleming MD, Luce EA, Deneve JL. Neoadjuvant talimogene laherparepvec for the treatment of metastatic melanoma. American Surgeon 2017;83(7):E266-8. [PubMed] [Google Scholar]
Urosevic 1996 {published data only}
- Urosevic V, Chollet P, Adenis A, Chauvergne J, Fargeot P, Roche H, et al. Results of a phase II trial with cystemustine in advanced malignant melanoma. A trial of the EORTC Clinical Screening Group. European Journal of Cancer 1996;32A(1):181-2. [DOI] [PubMed] [Google Scholar]
Van den Hout 2010 {published data only}
- Van Den Hout MF, Sluijter BJ, Santegoets SJ, Van Den Eertwegh AJ, Meijer S, Van Den Tol P, et al. Arming the melanoma sentinel lymph node against tumor spread: The effects of local administration of combined low-dose CpG-B PF-3512676 and GM-CSF. Cancer Research 2010;70(8 Suppl):5618. [Google Scholar]
Van Den Hout 2013 {published data only (unpublished sought but not used)}
- Van Den Hout MF, Koster BD, Scheper RJ, Van De Ven R, Sluijter BJ, Molenkamp BG, et al. Pre-operative intradermal administration of CpG-B +/- GM-CSF in stage I-III melanoma patients arms the sentinel lymph node: Evidence for reduced tumor spread. Journal for ImmunoTherapy of Cancer 2013;1(Suppl 1):P92. [Google Scholar]
Wargo 2015 {published data only}
- Wargo J, Gershenwald J, Cormier JN, Lee J, Griffin KC, Amaria R, et al. Is it time for a shift in the treatment of advanced resectable stage III and stage IV oligometastatic melanoma? Results of a pilot trial and plans for a randomized trial of neoadjuvant therapy versus upfront surgery. Annals of Surgical Oncology 2015;22(Suppl 1):S127. [Google Scholar]
References to studies awaiting assessment
de Braud 1994 {published data only}
- Braud F, Bajetta E, Comella G, Queirolo P, Sertoli M, Calabresi F, et al. A pilot study with dacarbazine (DTIC) and tamoxifen (TAM) and interferon alpha-2A (rIFN-a 2A) and IL-2 or CVD (CDDP and VDS and DTIC) and TAM and rIFN-a 2A +/- IL-2 in metastatic melanoma for the B.R.E.M.I.M (BRM in Melanoma) Italian Cooperative Group. Annals of Oncology 1994;5(Suppl 8):171. [Google Scholar]
EudraCT 2014‐000334‐30 {published data only (unpublished sought but not used)}
- EudraCT 2014-000334-30. Immunomodulatory effect of esomeprazole antitumoral and high-dose under neoadjuvant and adjuvant in patients with melanoma in stage III. Randomized pilot study treatment vs control. www.clinicaltrialsregister.eu/ctr-search/trial/2014-000334-30/results (first received 27 March 2014).
Kleeberg 1986 {published data only}
- Kleeberg UR, Brocker EB, Carrel S, Hoffman J, Kerekjarto M, Leujeune F. Clinical trial in patients with malignant melanoma: comparison of adjuvant Interferon-alpha-2 with Interferon-gamma with Iscador M and a control group without treatment after resection of high-risk primaries (+ 3mm) of stage I and/or lymph-node metastases [Adjuvante Therapiestudie bei Patienten mit malignem Melanom: vergleich von Interferon r Alpha‐2 mit r Gamma mit Iscador M und einer unbehandelten Kontrollgruppe nach Resektion prognostisch ungünstiger Primärtumoren (+ 3mm) im Stadium I bzw. nach kurativer Resektion von Lymphknotennetastasen (Stadium IIb) MMCG 18871/DK 80/1]. Cochrane Central Register of Controlled Trials (CENTRAL) 1986:2-46.
References to ongoing studies
NCT02858921 {published data only}
- Gonzalez M, Menzies AM, Saw R, Spillane AJ, Nieweg OE, Shannon KF, et al. Determining optimal sequencing of anti-PD-1 and BRAF-targeted therapy: a phase II randomised study of neoadjuvant pembrolizumab with/without dabrafenib and trametinib (D+T) in BRAF V600 mutant resectable stage IIIb/c/d melanoma (NeoTrio trial). Journal of Clinical Oncology 2018;36(15_suppl):TPS9604.
- Gonzalez M, Menzies AM, Saw R, Thompson JF, Spillane AJ, Howle J, et al. A phase II, randomised, open label study of neoadjuvant pembrolizumab with/without dabrafenib and trametinib (D/T) in BRAF V600 mutant resectable stage IIIB/C/D melanoma (NeoTrio Trial). Annals of Oncology 2017;28(Suppl 5):v445.
- NCT02858921. Neoadjuvant dabrafenib, trametinib and/or pembrolizumab in BRAF mutant resectable stage III melanoma (NeoTrio). clinicaltrials.gov/ct2/show/NCT02858921 (first received 8 August 2016).
NCT02938299 {published data only (unpublished sought but not used)}
- 2015-002549-72. A Phase III, open-label, randomized, controlled multi-center study of the efficacy of L19IL2/L19TNF neoadjuvant intratumoral treatment followed by surgery versus surgery alone in clinical stage IIIB and IIIC melanoma patients. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2015-002549-72 (first received July 2015).
- NCT02938299. Neoadjuvant L19IL2/L19TNF- Pivotal Study (Pivotal) [A Phase III, open-label, randomized, controlled multi-centre study of the efficacy of L19IL2/L19TNF neoadjuvant intratumoral treatment followed by surgery versus surgery alone in clinical stage IIIB and IIIC melanoma patients]. clinicaltrials.gov/ct2/show/NCT02938299 (first received 19 October 2016).
NCT03567889 {published data only}
- EUDRA CT 2021-003064-27. An open-label, randomized, controlled multi-center study of the efficacy of daromun (L19IL2 + L19TNF) neoadjuvant intratumoral treatment followed by surgery and adjuvant therapy versus surgery and adjuvant therapy in clinical stage IIIB/C melanoma patients [Estudio multicéntrico controlado, aleatorizado y abierto sobre la eficacia del tratamiento intratumoral neoadyuvante con Daromun (L19IL2 + L19TNF) seguido de cirugía y una terapia adyuvante frente a cirugía y una terapia adyuvante en pacientes con melanoma en estadio IIIB/C]. www.clinicaltrialsregister.eu/ctr-search/trial/2021-003064-27/ES (first received 19 November 2021).
- Miura JT, Zager JS. Neo-DREAM study investigating Daromun for the treatment of clinical stage IIIB/C melanoma. Future Oncology 2019;15(32):3665-74. [DOI: 10.2217/fon-2019-0433] [DOI] [PubMed] [Google Scholar]
- NCT03567889. Efficacy of daromun neoadjuvant intratumoral treatment in clinical stage IIIB/C melanoma patients (Neo-DREAM) [An open-label, randomized, controlled multi-center study of the efficacy of daromun (L19IL2 + L19TNF) neoadjuvant intratumoral treatment followed by surgery and adjuvant therapy versus surgery and adjuvant therapy in clinical stage IIIB/C melanoma patients]. clinicaltrials.gov/ct2/show/NCT03567889 (first received 26 June 2018).
NCT03698019 {published data only}
- NCT03698019. Pembrolizumab in treating patients with stage III/IV high-risk melanoma before and after surgery [A phase II randomized study of adjuvant versus neoadjuvant pembrolizumab (MK-3475) for clinically detectable stage III/IV high-risk melanoma]. clinicaltrials.gov/ct2/show/NCT03698019 (first received 5 October 2018).
- Patel SP, Othus M, Moon J, Tetzlaff M, Buchbinder EI, Sondak VK, et al. S1801: A randomized trial of adjuvant versus neoadjuvant pembrolizumab for melanoma. Journal of Clinical Oncology 2021;39(15_suppl):TPS9585. [Google Scholar]
- Patel SP, Othus M, Moon J, Tetzlaff MT, Buchbinder EI, Sondak VK, et al. S1801: A randomized phase II trial of adjuvant versus neoadjuvant pembrolizumab (PEM) for melanoma. Journal of Clinical Oncology 2020;38(15_suppl):TPS10090. [Google Scholar]
NCT04133948 {published data only}
- NCT04133948. Multicenter phase 1b trial testing the neoadjuvant combination of domatinostat, nivolumab and ipilimumab in ifn-gamma signature-low and ifn-gamma signature-high recist 1.1-measurable stage iii cutaneous or unknown primary melanoma. clinicaltrials.gov/ct2/show/NCT04133948 (first received 21 October 2019).
- Reijers IL, Rozeman EA, Dimitriadis P, Krijgsman O, Bosch LJ, Cornelissen S, et al. P01.15 Personalized combination of neoadjuvant domatinostat, nivolumab (NIVO) and ipilimumab (IPI) in macroscopic stage III melanoma patients stratified according to interferon-gamma (IFN-gamma) signature – the DONIMI study. Journal for Immunotherpy of Cancer 2020;8. [DOI: 10.1136/jitc-2020-ITOC7.28] [DOI] [Google Scholar]
NCT04139902 {published data only}
- Kelly Z, Najjar Y, Zarour H, et al. Randomized phase II neoadjuvant study: PD-1 inhibitor TSR-042 vs. combination PD-1 inhibitor TSR-042 and Tim-3 inhibitor TSR-022 in borderline resectable stage III or oligometastatic stage IV melanoma. Journal for Immunotherapy of Cancer 2019;7(Suppl 1):P457. [Google Scholar]
- NCT04139902. PD-1 inhibitor dostarlimab (TSR-042) vs. combination of Tim-3 inhibitor TSR-022 and PD-1 inhibitor dostarlimab (TSR-042) [Randomized phase II neoadjuvant study of PD-1 inhibitor dostarlimab (TSR-042) vs. combination of Tim-3 inhibitor TSR-022 and PD-1 inhibitor dostarlimab (TSR-042) in resectable stage III or oligometastatic stage IV melanoma (Neo-MEL-T)]. clinicaltrials.gov/ct2/show/NCT04139902 (first received 25 October 2019).
NCT04303169 {published data only}
- EUDRA-CT 2019-003978-22. A Phase 1/2 Open-Label Rolling-Arm Umbrella Platform Design of Investigational Agents With or Without Pembrolizumab or Pembrolizumab Alone in Participants with Melanoma (KEYNOTE-U02): Substudy 02C [Ph 1/2 Substudy of Neoadjuvant Oncological Treatment(s) in MEL]. www.clinicaltrialsregister.eu/ctr-search/trial/2019-003978-22/IT (first received 21 January 2021).
- NCT04303169. Substudy 02C: Safety and efficacy of pembrolizumab in combination with investigational agents or pembrolizumab alone in participants with stage III melanoma who are candidates for neoadjuvant therapy (MK-3475-02C) [A phase 1/2 open-label rolling-arm umbrella platform design of investigational agents with or without pembrolizumab or pembrolizumab alone in participants with melanoma (KEYMAKER-U02): Substudy 02C]. clinicaltrials.gov/ct2/show/NCT04303169 (first received 10 March 2020).
NCT04401995 {published data only}
- NCT04401995. Study of TLR9 agonist CMP-001 in combination with nivolumab vs. nivolumab [Randomized neoadjuvant pilot phase II study of TLR9 agonist CMP-001 in combination with nivolumab vs. nivolumab in stage IIIB/C/D melanoma patients with an integrated imaging biomarker]. clinicaltrials.gov/ct2/show/NCT04401995 (first received 26 May 2020).
NCT04708418 {published data only}
- NCT04708418. A study evaluating whether pembrolizumab alone or in combination with CMP-001 improves efficacy in patients with operable melanoma [Phase II randomized study of neoadjuvant pembrolizumab alone or in combination with CMP-001 in patients with operable melanoma: efficacy and biomarker study]. clinicaltrials.gov/show/NCT04708418 (first posted 14 January 2021).
NCT04722575 {published data only}
- EUCTR2018-004841-17-IT. A phase II randomized non-comparative study, with neoadjuvant plus adjuvant therapy with combination or sequence of vemurafenib, cobImetinib, and atezolizuMab in patients with high-risk, surgically resectable BRAF mutated and wild-type Melanoma. trialsearch.who.int/?TrialID=EUCTR2018-004841-17-IT (first received October 2020).
- NCT04722575. Combination or sequence of vemurafenib, cobimetinib, and atezolizumab in high-risk, resectable melanoma (NEO-TIM) [NEOadjuvant Plus adjuvant therapy with combination or sequence of vemurafenib, cobimetinib, and atezolizumab in patients with high-risk, surgically resectable BRAF mutated and wild-type melanoma]. clinicaltrials.gov/ct2/show/NCT04722575 (first posted 25 January 2021).
NCT04949113 {published data only}
- EUCTR2021-001492-16-NL. Multicenter phase 3 trial comparing NeoADjuvant Ipilimumab + Nivolumab versus standard Adjuvant nivolumab in macroscopic stage III melanoma – NADINA - NADINA [Onderzoek bij melanoom patiënten met uitzaaiingen in de lymfeklieren naar behandeling met ipilimumab en nivolumab voorafgaand aan de operatie in vergelijking met standaard nivolumab behandeling na de operatie.]. trialsearch.who.int/?TrialID=EUCTR2021-001492-16-NL (first received 1 June 2021).
- NCT04949113. Neoadjuvant ipilimumab plus nivolumab versus standard adjuvant nivolumab in macroscopic stage III melanoma (NADINA) [Multicenter phase 3 trial comparing neoadjuvant ipilimumab plus nivolumab versus standard adjuvant nivolumab in macroscopic stage III melanoma - NADINA]. clinicaltrials.gov/ct2/show/NCT04949113 (first received 2 July 2021).
Additional references
AJCC 2011
- Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. AJCC Cancer Staging Manual 7th Edition. 7th edition. Springer, 2011. [Google Scholar]
AJCC 2017
- Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th edition. Springer Cham, 2017. [Google Scholar]
Algazi 2015
- Algazi AP, Cha E, McCalmont T, Bastian BC, Hwang J, Pampaloni MH, et al. The combination of axitinib followed by paclitaxel/carboplatin yields extended survival in advanced BRAF wild-type melanoma: results of a clinical/correlative prospective phase II clinical trial. British Journal of Cancer 2015;112(8):1326-31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amaria 2015
- Amaria RN, Reuben A, Cooper ZA, Wargo JA. Update on use of aldesleukin for treatment of high-risk metastatic melanoma. ImmunoTargets and Therapy 2015;4:79-89. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andtbacka 2015
- Andtbacka RH, Chastain M, Li A, Shilkrut M, Ross MI. Phase 2, multicenter, randomised, open-label trial assessing efficacy and safety of talimogene laherparepvec (T-VEC) neoadjuvant treatment (tx) plus surgery vs surgery for resectable stage IIIB/C and IVM1a melanoma (MEL). Journal of Clinical Oncology 2015;33(15 Suppl):[Epub ahead of print]. [DOI: 10.1200/jco.2015.33.15_suppl.tps9094] [DOI]
Ascierto 2020
- Ascierto PA, Del Vecchio M, Mandala M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncology 2020;21(11):1465-77. [DOI] [PubMed] [Google Scholar]
Balch 2009
- Balch CM, Gershenwald JE, Soong S, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology 2009;27(36):6199-206. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2014
- Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. International Journal of Radiation Oncology, Biology, Physics 2014;88(5):986-97. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. Journal of the Americal Medical Association 2010;303(12):1180-7. [DOI: 10.1001/jama.2010.310] [DOI] [PubMed] [Google Scholar]
Bhatia 2009
- Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology 2009;23(6):488-96. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Boulva 2021
- Boulva K, Apte S, Yu A, Tran A, Shorr R, Song X, et al. Contempory neoadjuvant therapies for high-risk melanoma: a systematic review. Cancers 2021;13(8):1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2021
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Braeuer 2014
- Braeuer RR, Watson IR, Wu CJ, Mobley AK, Kamiya T, Shoshan E, et al. Why is melanoma so metastatic? Pigment Cell and Melanoma Research 2014;27(1):19-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chandra 2015
- Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015;4(11):e1046028. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Skin Specialised Register 2021
- Cochrane Skin. More about the Cochrane Skin Specialised Register. skin.cochrane.org/search-methods (accessed 2 December 2021).
COMET Initiative 2017
- COMET Initiative. Developing a Core Outcome Set (COS) for melanoma trials. www.comet-initiatve.org/studies/details/783 (accessed 28th April 2017).
Corrie 2017
- Corrie P, Marshall A, Lorigan P, Gore ME, Tahir S, Faust G, et al. Adjuvant bevacizumab as treatment for melanoma patients at high risk of recurrence: Final results for the AVAST-M trial. Journal of Clinical Oncology 2017;35(15 Suppl):9501. [DOI: 10.1200/JCO.2017.35.15_suppl.9501] [DOI] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 2017. Available at covidence.org.
CRUK 2017
- Cancer Research UK. General Cancer Information. www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/targeted-cancer-drugs/types/monoclonal-antibodies (accessed 29 January 2018).
CTCAE 2010
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; Published: 28 May 2009 (v4.03: 14 June 2010). Available at evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
Curtin 2005
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. New England Journal of Medicine 2005;353(20):2135-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
Davar 2013
- Davar D, Tarhini AA, Kirkwood JM. Adjuvant immunotherapy of melanoma and development of new approaches using the neoadjuvant approach. Clinics in Dermatology 2013;31(3):237-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2002
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
DermNet 2004
- DermNET NewZealand. Dermoscopy. By: Dr Amanda Oakley MBChB FRACP, Dept of Dermatology Waikato Hospital, Hamilton, New Zealand, 2004. www.dermnetnz.org/topics/dermoscopy (accessed 27 December 2017).
Dummer 2015
- Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U, ESMO Guidelines Committee. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2015;26(Suppl 5):v126-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dummer 2020a
- Dummer R, Hauschild A, Santinami M, Atkinson V, Mandala M, Kirkwood JM, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. New England Journal of Medicine 2020;383:1139-48. [DOI] [PubMed] [Google Scholar]
Eggermont 2012
- Eggermont AM, Suciu S, Testori A, Kruit WH, Marsden J, Punt C, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: Results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. European Journal of Cancer 2012;48(2):218-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eggermont 2014
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet 2014;383(9919):816-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eggermont 2016
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. New England Journal of Medicine 2016;375(19):1845-55. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eggermont 2018
- Eggermont AM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. New England Journal of Medicine 2018;378:1789-1801. [DOI] [PubMed] [Google Scholar]
Eggermont 2020
- Eggermont AM, Rutkowski P, Dutriaux C, Hofman-Wellenhof R, Dziewulski P, Marples M, et al. Adjuvant therapy with pegylated interferon-alfa2b vs observation in stage II B/C patients with ulcerated primary: Results of the European Organisation for Research and Treatment of Cancer 18081 randomised trial. European Journal of Cancer 2020;133:94-103. [DOI] [PubMed] [Google Scholar]
Eggermont 2021
- Eggermont AM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncology 2021;22(5):643-54. [DOI] [PubMed] [Google Scholar]
EMA 2021
- European Medicines Agency. Aldara - EMEA/H/C/000179 -II/0067. Annex I - Summary of product characteristics. Last updated 13 December 2021. Available at www.ema.europa.eu/en/medicines/human/EPAR/aldara.
FDA 2020
- Food and Drug Administration. Pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval; July 2020. Available at www.fda.gov/regulatory-information/search-fda-guidance-documents/pathological-complete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use.
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Florin 2012
- Florin V, Desmedt E, Vercambre-Darras S, Mortier L. Topical treatment of cutaneous metastases of malignant melanoma using combined imiquimod and 5-fluorouracil. Investigational New Drugs 2012;30(4):1641-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fruehauf 2011
- Fruehauf J, Lutzky J, McDermott D, Brown CK, Meric JB, Rosbrook B, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clinical Cancer Research 2011;17(23):7462-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garbe 2016
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Bastholt L, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guidelines-Update 2016. European Journal of Cancer 2016;63:201-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Global Burden of Disease 2016
- Global Burden of Disease Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1151-210. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE Handbook 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available at gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 6 October 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-1302. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. [DOI] [PubMed] [Google Scholar]
Hauschild 2012
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380(9839):358-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heise 2016
- Heise R, Amann PM, Ensslen S, Marquardt Y, Czaja K, Beer D, et al. Interferon alpha signalling and its relevance for the upregulatory effect of transporter proteins associated with antigen processing (TAP) in patients with malignant melanoma. PlosOne 2016;11(1):e0146325. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hodi 2010
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 2010;363(8):711-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodi 2014
- Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomised clinical trial. JAMA 2014;312(17):1744-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodi 2016
- Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncology 2016;17(11):1558-68. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoeller 2001
- Hoeller C, Jansen B, Heere-Ress E, Pustelnik T, Mossbacher U, Schlagbauer-Wadl H, et al. Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. Journal of Investigative Dermatology 2001;117(2):371-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
INMC 2019
- Amaria RN, Menzies AM, Burton EM, Scolyer RA, Tetzlaff MA, Antdbacka R, et al. Neoadjuvant systemic therapy in melanoma: recommendations of the International Melanoma Consortium. Lancet Oncology 2019;20(7):E378-89. [DOI: 10.1016/S1470-2045(19)30332-8] [DOI] [PubMed] [Google Scholar]
Johnson 2015
- Johnson DB, Menzies AM, Zimmer L, Eroglu Z, Ye F, Zhao S, et al. Acquired BRAF inhibitor resistance: A multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. European Journal of Cancer 2015;51(18):2792-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2015a
- Johnson AS, Crandall H, Dahlman K, Kelley MC. Preliminary results from a prospective trial of preoperative combined BRAF and MEK-targeted therapy in advanced BRAF mutation-positive melanoma. Journal of the American College of Surgeons 2015;220(4):581-93.e1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kandolf Sekulovic 2018
- Kandolf Sekulovic L, Guo J, Agarwala S, Hauschild A, McArthur G, Cinat G, et al. Access to innovative medicines for metastatic melanoma worldwide: Melanoma World Society and European Association of Dermato-oncology survey in 34 countries. European Journal of Cancer 2018;104:201-9. [DOI] [PubMed] [Google Scholar]
Kang 2016
- Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. Journal for ImmunoTherapy of Cancer 2016;4(51):1-20. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 2014
- Kaufman HL, Andtbacka RHI, Collichio FA, Amatruda T, Senzer NN, Chesney J, et al. Primary overall survival (OS) from OPTiM, a randomised phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. Journal of Clinical Oncology 2014;32(15 Suppl):9008a. [DOI: 10.1200/jco.2014.32.15_suppl.9008a] [DOI] [Google Scholar]
Kelly 2019
- Kelly Z, Najjar Y, Zarour H, Kirkwood J, Rapisuwon S, Wang H, et al. Randomized phase II neoadjuvant study: PD-1 inhibitor TSR-042 vs. combination PD-1 inhibitor TSR-042 and Tim-3 inhibitor TSR-022 in borderline resectable stage III or oligometastatic stage IV melanoma. Journal for ImmunoTherapy of Cancer 2019;7(Suppl 1):P457. [Google Scholar]
Khunger 2019
- Khunger A, Buchwald ZS, Lowe M, Khan MK, Delman KA, Tarhini AA. Neoadjuvant therapy of locally/regionally advanced melanoma. Therapeutic Advances in Medical Oncology 2019;11:1758835919866959. [DOI: 10.1177/1758835919866959] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2009
- Kim KB, Legha SS, Gonzalez R, Anderson CM, Johnson MM, Liu P, et al. A randomized phase III trial of biochemotherapy versus interferon-alpha-2b for adjuvant therapy in patients at high risk for melanoma recurrence. Melanoma Research 2009;19(1):42-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2012
- Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, et al. BEAM: a randomised phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. Journal of Clinical Oncology 2012;30(1):34-41. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkwood 2012
- Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA: A Cancer Journal for Clinicians 2012;62(5):309-35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korde 2021
- Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO Guideline. Journal of Clinical Oncology 2021;39(13):1485-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koster 2017
- Koster BD, den Hout M, Sluijter B, Molenkamp BG, Vuylsteke R, Baars A, et al. Local adjuvant therapy with low-dose CpG-B offers durable protection against disease recurrence in clinical stage I-II melanoma: data from two randomised phase II trials. Clinical Cancer Research 2017;23(19):5679-86. [DOI] [PubMed] [Google Scholar]
Kruijff 2012
- Kruijff S, Bastiaannet E, Brouwers AH, Nagengast WB, Speijers MJ, Suurmeijer AJH, et al. Use of S-100B to evaluate therapy effects during bevacizumab induction treatment in AJCC stage III melanoma. Annals of Surgical Oncology 2012;19(2):620-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lallas 2021
- Lassas A, Moscarella E, Kittler H, Longo C, Thomas L, Zalaudek I, et al. Real-world experience of off-label use of imiquimod 5% as an adjuvant therapy after surgery or as a monotherapy for lentigo maligna. British Journal of Dermatology 2021;185(3):675-7. [DOI] [PubMed] [Google Scholar]
Larkin 2014
- Larkin J, Ascierto P, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. New England Journal of Medicine 2014;371(20):1867-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Larkin 2015
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New England Journal of Medicine 2015;373(1):23-34. [PMID: ] [DOI] [PMC free article] [PubMed]
Lexicomp, 20th Ed
- Lacey CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 20th Edition. Ohio: American Pharmacists Association, 2011. [Google Scholar]
Long 2017a
- Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Annals of Oncology 2017;28(7):1631-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2017b
- Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. New England Journal of Medicine 2017;377(19):1813-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Luke 2022
- Luke JL, Rutkowski P, Queirolo P, Del Vecchio M, Mackiewicz J, Chiarion-Sileni V, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet 2022;399(10336):1718-29. [DOI] [PubMed] [Google Scholar]
McArthur 2014
- McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncology 2014;15(3):323-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzies 2021
- Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nature Medicine 2021;27:301-9. [DOI] [PubMed] [Google Scholar]
Michielin 2019
- Michielin O, Akkooi AC, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2019;30:1884-1901. [DOI] [PubMed] [Google Scholar]
Middleton 2000
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. Journal of Clinical Oncology 2000;18(1):158-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mocellin 2013
- Mocellin S, Lens MB, Pasquali S, Pilati P, Chiarion Sileni V. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD008955. [DOI: 10.1002/14651858.CD008955.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Najjar 2019
- Najjar YG, Puligandla M, Lee SJ, Kirkwood JM. An updated analysis of 4 randomized ECOG trials of high-dose interferon in the adjuvant treatment of melanoma. Cancer 2019;125(17):3013-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCN 2021
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Melanoma: Cutaneous. v2.2021. www.nccn.org/guidelines/category_1 (accessed 1 November 2021).
NCI 2021
- National Institutes of Health National Cancer Institute. National Cancer Institute Dictionary of Cancer Terms. www.cancer.gov/publications/dictionaries/cancer-terms (accessed 7 July 2021).
NLM 2016
- National Library of Medicine. Stem cells. Last updated 29 March 2016. Available at medlineplus.gov/stemcells.html.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine 1998;17(24):2815-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pasquali 2018
- Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD011123. [DOI: 10.1002/14651858.CD011123.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2011
- Patel PM, Suciu S, Mortier L, Kruit WH, Robert C, Schadendorf D, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). European Journal of Cancer 2011;47(10):1476-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pelster 2020
- Pelster MS, Amaria RN. Neoadjuvant immunotherapy for locally advanced melanoma. Current Treatment Options in Oncology 2020;21(10):1-13. [DOI: 10.1007/s11864-020-0700-z] [DOI] [PubMed] [Google Scholar]
Posch 2013
- Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 2013;110(10):4015-20. [DOI: 10.1073/pnas.1216013110] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Postow 2015
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. Journal of Clinical Oncology 2015;33(17):1974-82. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prowell 2012
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. New England Journal of Medicine 2012;366(26):2438-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rethlefsen 2021
- Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic reviews. Systematic Reviews 2021;10(1):39. [DOI: 10.1186/s13643-020-01542-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Reynders 2015
- Reynders K, Illidge T, Siva S, Change JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to made a rare event clinically relevant. Cancer Treatment Reviews 2015;41(6):503-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ridolfi 2002
- Ridolfi L, Ridolfi R. Preliminary experiences of intralesional immunotherapy in cutaneous metastatic melanoma. Hepato-gastroenterology 2002;49(44):335-9. [PMID: ] [PubMed] [Google Scholar]
Robert 2011
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England Journal of Medicine 2011;364(26):2517-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Robert 2015
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. New England Journal of Medicine 2015;372(1):30-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Robert 2016
- Robert L, Ribas A, Hu-Lieskovan S. Combining targeted therapy with immunotherapy. Can 1+1 equal more than 2? Seminars in Immunology 2016;28(1):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robert 2019
- Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-Year outcomes with dabrafenib plus trametinib in metastatic melanoma. New England Journal of Medicine 2019;381:626-36. [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook..
SEER 2022
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Melanoma of the Skin. seer.cancer.gov/statfacts/html/melan.html (accessed 6 May 2022).
Si 1996
- Si Z, Hersey P, Coates AS. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Research 1996;6(3):247-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sung 2021
- Sung H, Ferlay J, Siegal R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer Journal for Clinicians 2021;71:209-49. [DOI] [PubMed] [Google Scholar]
Tahrini 2011
- Tarhini AA, Pahuja S, Kirkwood JM. Neoadjuvant therapy for high-risk bulky regional melanoma. Journal of Surgical Oncology 2011;104(4):386-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tetzlaff 2018
- Tetzlaff MT, Messina JL, Stein JE, Xu X, Amaria RN, Blank CU, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Annals of Oncology 2018;29(8):1861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2013
- Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. Journal of Clinical Oncology 2013;31(33):4252-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Akkooi 2022
- Akkooi AC, Hieken TJ, Burton EM, Ariyan C, Ascierto PA, Asero SV, et al. Neoadjuvant Systemic Therapy (NAST) in patients with melanoma: surgical considerations by the International Neoadjuvant Melanoma Consortium (INMC).. Annals of Surgical Oncology 2022;29:3694-708. [DOI] [PubMed] [Google Scholar]
van den Hout 2013
- den Hout MF, Koster BD, Scheper RJ, de Ven R, Sluijter BJ, Molenkamp BG, et al. Pre-operative intradermal administration of CpG-B ± GM-CSF in stage I-III melanoma patients arms the sentinel lymph node: evidence for reduced tumor spread. Journal for Immunotherapy of Cancer 2013;1(Suppl 1):P92. [Google Scholar]
Van Zeijl 2020
- Van Zeijl MC, Ismail RK, Wreede LC, den Eertwegh AJ, Boer A, Dartel M, et al. Real-world outcomes of advanced melanoma patients not represented in phase III trials. International Journal of Cancer 2020;147(12):3461-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varker 2007
- Varker KA, Biber JE, Kefauver C, Jensen R, Lehman A, Young D, et al. A randomised phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Annals of Surgical Oncology 2007;14(8):2367-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Versluis 2020
- Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nature Medicine 2020;26:475-84. [DOI] [PubMed] [Google Scholar]
Weber 2017
- Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Lance Cowey C, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. New England Journal of Medicine 2017;377:1824-35. [DOI] [PubMed] [Google Scholar]
Wheatley 2007
- Wheatley K, Ives N, Eggermont AMM, Kirkwood J, Cascinelli N, Markovic SN, et al. Interferon-alpha as adjuvant therapy for melanoma: an individual patient data meta-analysis of randomised trials. Journal of Clinical Oncology 2007;25(18 Suppl):8526. [DOI: 10.1200/jco.2007.25.18_suppl.8526] [DOI] [Google Scholar]
Whiteman 2011
- Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell and Melanoma Research 2011;24(5):879-97. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolchok 2017
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New England Journal Of Medicine 2017;377(14):1345-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolchok 2021
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. Journal of Clinical Oncology 2021;39(15 Suppl):9506. [Google Scholar]
Wright 2016
- Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology 2016;34(28):3460-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2016
- Yu AM, Tran A, Song X, Nessim C, Ong M. Neoadjuvant therapy: the next breakthrough in melanoma treatment? A systematic review. Canadian Journal of Surgery 2016;59(4 Suppl 1):S141. [Google Scholar]
Zhao 2017
- Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Research 2017;77(4):817-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gorry 2018
- Gorry C, McCullagh L, O'Donnell H, Barrett S, Schmitz S, Barry M, et al. Neoadjuvant treatment for malignant and metastatic cutaneous melanoma. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD012974. [DOI: 10.1002/14651858.CD012974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorry 2020
- Gorry, CM. Cost Effectiveness of Treatment Strategies for Skin Cancer in the Irish Healthcare Setting; 2020. Available at www.tara.tcd.ie/handle/2262/93750.