Abstract
FLASH radiotherapy (FLASH-RT) is an emerging ultra-high dose (>40 Gy/s) delivery that promises to improve the therapeutic potential by limiting toxicities compared to conventional RT while maintaining similar tumor eradication efficacy. Image-guidance is an essential component of modern RT that should be harnessed to meet the special emerging needs of FLASH-RT and its associated high risks in planning and delivering of such ultra-high doses in short period of times. Hence, this contribution will elaborate on the imaging requirements and possible solutions in the entire chain of FLASH-RT treatment, from the planning, through the setup and delivery with online in vivo imaging and dosimetry, up to the assessment of biological mechanisms and treatment response. In patient setup and delivery, higher temporal sampling than in conventional RT should ensure that the short treatment is delivered precisely to the targeted region. Additionally, conventional imaging tools such as cone-beam computed tomography (CBCT) will continue to play an important role in improving patient setup prior to delivery, while techniques based on magnetic resonance imaging (MRI) or positron-emission-tomography (PET) may be extremely valuable for either Linac or particle FLASH therapy, to monitor and track anatomical changes during delivery. In either planning or assessing outcomes, quantitative functional imaging could supplement conventional imaging for more accurate utilization of the biological window of the FLASH effect, selecting for or verifying things such as tissue oxygen and existing or transient hypoxia on the relevant time scales of FLASH-RT delivery. Perhaps most importantly at this time, these tools might help improve the understanding of the biological mechanisms of FLASH-RT response in tumor and normal tissues. The high dose deposition of FLASH provides an opportunity to utilize pulse-to-pulse imaging tools such as Cherenkov or radiation acoustic emission imaging. These could provide individual pulse mapping or assessing the 3D dose delivery superficially or at tissue depth, respectively. In summary, the most promising components of modern RT should be used for safer application of FLASH-RT, and new promising developments could be advanced to cope with its novel demands but also exploit new opportunities in connection with the unique nature of pulsed delivery at unprecedented dose rates, opening a new era of biological image guidance and ultra-fast, pulse-based in vivo dosimetry.
Keywords: FLASH-RT, biological image-guidance, online imaging, in vivo dosimetry
1. Introduction
Imaging is an integral part of conventional radiotherapy planning and delivery1. In treatment planning, it is applied for target and organ-at-risk (OAR) definitions and for virtual simulation purposes. In delivery, it is applied for localization and tracking of the tumor. Two notions are typically employed of “image-guidance” and “adaptation”, both to ensure that treatment plans can account for daily changes during (intra-fraction) or between (inter-fraction) treatment deliveries2–5. Historically, radiotherapy 3D planning relied on computed tomography (CT) imaging for contouring and dose calculation. 4D CT is typically utilized for the evaluation of motion extent and the guidance of internal target volume (ITV) delineation. More recently, positron emission tomography (PET) and magnetic resonance imaging (MRI) have been used to improve the gross or clinical target (GTV/CTV) definitions via functional information and/or better soft tissue discrimination6. Corrections for daily setup errors have traditionally utilized onboard imaging with cone beam CT (CBCT) or Mega Voltage (MV) imaging technologies7,8, and newly emerging MR-Linac technologies are being introduced clinically for identifying and tracking the target just before and even during treatment9,10. Though these technologies continue to evolve in conventional radiotherapy with photons primarily, but also in particle therapy11–13, the nature of ultra-fast dose rate or FLASH radiotherapy may demand more stringent requirements for image-guidance.
FLASH radiotherapy (FLASH-RT) is an ultra-high dose rate (>40 Gy/s) regimen when compared to conventional (~0.1 Gy/s) delivery14,15. FLASH-RT has demonstrated tremendous ability to improve the radiotherapy therapeutic index (tumor control to side effects ratio) by at least 20–30% in preclinical studies16–18 and even in early clinical trial cases19. However, these studies were limited to simplistic irradiation scenarios where treatment planning and delivery risks can be monitored effectively. The transition to more real-world cancer treatment cases to reap the benefits of FLASH-RT will undoubtedly require further image-guidance developments to safeguard the accurate and effective delivery of FLASH-RT. In addition to this clinical need, there is still a lot of ambiguity regarding the manifestation of the FLASH effect and its underlying radiobiology from oxygen depletion to immune modulation. The oxygen depletion theory, which traces its roots to the hockey stick phenomenon of the 1950s and 1960s seems to be the more discussed interpretation to date20,21. For instance, Petersson et al investigated models of oxygen kinetics during irradiation and developed a time-dependent model of the oxygen enhancement ratio in mammalian cells with oxygen depletion. The model was evaluated in terms of dose and dose-rate dependency and was fit to experimental in vitro and in vivo datasets21. However, in vivo measurements of oxygen have shown quite modest depletion in the range of a change in pO2 = 0.2 mmHg per Gy of dose22. So, the protection in case of normal tissue due to oxygen depletion is yet to provide a convincing argument for tumor response mechanisms, and the magnitudes of depletion do not obviously match what would be needed for transient radiobiological hypoxia. In any case, better quantitative understanding of the FLASH-RT underlying mechanisms will also aid in developing better planning and achieve better treatment responses.
It is possible that the instantaneous nature of FLASH-RT delivery lends itself to more instantaneous measurements of imaging as well, such that less conventional approaches could be employed. The density of energy per unit time is near 1000× higher in FLASH-RT than in conventional RT, implying that at least 30 dB higher signal-to-noise ratio (SNR), and so this can provide opportunities for monitoring the dose delivery, which are not possible with standard low dose rates. Examples of this come from ultrasound imaging of the radiation-induced acoustic force, or optical imaging of the Cherenkov emission or scintillation23. The potential and value of these technologies are examined here. Additionally, methods to explore the underlying radiobiology mechanisms can be provided by some emerging imaging and sensing tools, and these are examined as well.
2. Imaging for Treatment Planning
At a very top level, the needs for imaging in FLASH-RT treatment planning are similar to those in conventional treatment, but the risks involved with inaccuracies in planning or dose misplacement during delivery are much higher. The possible options in FLASH-RT are either fractionated or single shot treatments, with the major concern that each pulse delivery occurs in a very short period of time, in the order of few microseconds with the total delivery lasting less than a second. For example, the interplay effect between internal motion and radiation delivery would be presumably a much bigger issue as there will be virtually no volumetric dose averaging effect over the motion for FLASH-RT. This concern about the ultrafast timing means that things such as identifying the correct motion state or needs of visualizing oxygen distributions become more important to ensure safe and accurate delivery. However, the very fast delivery may on the one hand mitigate motion management issues but would require performing the imaging for planning in exactly the same motion state in which the ultrafast delivery at the predetermined dose rate threshold will then occur or stop the beam after few pulses if the motion state is not matched, organ deformations have taken place, or the FLASH dose rate is not met. During planning, interplay effects can be evaluated by calculating dose in different phases of a 4D CT simulation, when this can be deemed a good representation of the treatment scenario. In terms of target and OAR delineation, within established imaging methods in RT, it may be important to consider new segmentation tasks and sub-volumes versus full-volume segmentations with multimodality imaging and advanced artificial intelligence (AI) methods24. In fact, it might become more important to identify relevant (sub)areas, which might benefit from the FLASH effect, e.g., in terms of organs-at-risk (OARs) protection for dose escalation to the tumor.
While CT has been the standard imaging tool for treatment planning in RT, as it provides the relative electron densities (and roughly stopping power ratio, SPR) needed for dose calculation in photon (particle) therapy, magnetic resonance imaging (MRI) is also more widely used in a number of centers due to its superior soft tissue contrast for target and OAR identification. In particular, the latest advances of combined MR-Linac technologies9, along with the prospects of similar solutions also in particle therapy13, have motivated several research efforts toward MR-only radiotherapy planning workflows, which are greatly supported by advances in AI to provide synthetic CT data for planning from the available MR acquisitions25. These MR-only workflows could represent a new paradigm providing an (even time-resolved) patient model in treatment position, which would certainly benefit treatment accuracy in both fractionated and single shot FLASH-RT treatments. Moreover, depending on the MR equipment capabilities and affordability (especially in terms of acquisition time) protocols, such new combined solutions could also open new prospects for biological guidance with quantitative assessment of biomarkers, which could turn out to be relevant for the FLASH-RT effects, to be then imaged close in time to the delivery (see next section).
In terms of functional imaging, PET is a well-proven nuclear medicine modality, which is playing an increasing role in radiotherapy, not only for improving target definition and identifying sub-volumes of higher risk, especially FDG-PET, but also for dose escalation studies26. In a conventional radiotherapy treatment of lung cancer, for example, an adaptive escalated dose based on FDG-avid region detected by mid-treatment FDG-PET improved local tumor control at 2-year follow-up27. These and other similar findings have motivated the recent development of a combined PET-Linac for biological image guidance at the treatment site28. This solution is conceptually similar to the MR-Linac, but focuses on the functional imaging capabilities of PET, harnessed toward high-quality time resolved imaging for real-time tumor tracking. In addition to the wide adoption of FDG, other novel PET tracers have been developed and applied targeting various aspects of tumor biology, including cell proliferation (FLT), cell death (Annexin), and tumor hypoxia (FMISO, Faza, ATSM, etc.)29. Specifically, tracers of low oxygen pressure, or hypoxia, may be of valuable interest for FLASH-RT planning. In such case, hypoxia imaging can be used to guide to the potential benefits from FLASH-RT for a particular tumor. In addition, the level of oxygenation pressure (pO2) in surrounding normal tissues may be used to assess the level of normal tissue protection in treatment planning. In addition to PET, other oxygen sensing tracers using other imaging technologies (see also Section 5 on “Imaging for biological mechanisms and treatment response assessment”) may be necessary with varying trade-offs30, or the development of dedicated oxygen and reactive oxygen species (ROS) sensing nanoparticle probes. These will likely be needed in pre-clinical work to optimize FLASH-RT efficacy. It is possible that tumor hypoxia imaging could provide insight into the mechanisms of the FLASH-RT effect as well, if areas of high or low oxygen are seen as being more susceptible to protection. Mechanistic studies in pre-clinical imaging models would be required to understand these processes31, before such information could be reliably used for treatment planning, which are evolving areas. These unknowns associated with FLASH-RT have limited its current applicability to superficial tumors, where such planning conditions are easier to control19.
3. Imaging for Setup Correction, Delivery, and Adaptation
The two major reasons for image guidance in standard RT are (1) to ensure initial setup is anatomically matching the CT simulation set up, and (2) to adaptively adjust the plan or delivery if needed to match new geometrical deformations or tissue changes32,33. While conventional use is even more necessary in fractionated treatment, there are much more critical needs in FLASH-RT, where hypofractionation to high doses and ultrafast delivery make each treatment accuracy essentially high, both in terms of successful OAR protection and effective tumor eradication.
In both photon and particle therapy, state-of-the-art image-guided radiation therapy (IGRT) approaches nowadays rely on pre-treatment volumetric confirmation of the patient anatomy at the treatment site using X-ray cone beam CT (CBCT) for daily setup corrections3. Albeit having limited field of view compared to conventional CT, CBCT imaging has proven to be a valuable complimentary tool to other onboard imagers (e.g., electronic portal imaging devices, EPIDs) while providing volumetric imaging at kV-level image contrasts. However, its image quality is compromised by the increased amount of scattered radiation due to the employed irradiation cone impinging on a large area detector, in contrast to the fan beam configuration adopted in diagnostic CT scanners. This typically results in highly unreliable Hounsfield unit (HU) numbers, still deemed sufficient for setup corrections but not for accurate dosimetric recalculation for treatment adaptation in case of large anatomical changes. Over the last years, CBCT image quality has been enhanced via improvements in image reconstruction, from the historical backprojection with the Feldkamp, Davis and Kress (FDK) algorithm to statistical iterative algorithms of better SNR,34 along with different strategies of scatter correction at the projection or image level, including deep learning35. All these improvements have resulted in expanding the role for CBCT to image-guided adaptive radiotherapy36,37, which can also become essential for FLASH-RT setup. In fact, improved on-site imaging accuracy would facilitate the application of FLASH-RT in hypofractionated settings to further enhance the sparing of radiation-induced toxicities when treating larger volumes38. However, CBCT imaging can only capture patient anatomy prior to the delivery, and while it has proven a useful tool to reduce setup errors and enable many life-saving developments39, despite all recent advances, it still suffers from poor soft tissue contrast, often requiring use of surrogates to identify anatomy, and deposits a non-negligible imaging dose. 4D CBCT has recently been introduced on modern Linacs, which provides motion-resolved volumetric imaging prior to the treatment with a typical gantry rotation speed of 1 cycle per minute. For FLASH-RT delivered to motion-sensitive sites such as lung tumor, 4D CBCT is valuable to confirm the motion being similar to what was observed in 4D CT simulation for the ITV delineation. Towards this goal, several methods have been introduced to speed up 4D CBCT acquisition40–42. However, despite this promising application of the intrinsically slow volumetric acquisition of CBCT for time resolved reconstruction of anatomical motion close in time to the treatment delivery, CBCT is not typically used for real-time imaging except in some fluoroscopy settings possibly with dual energy43, still with the risk of increased dose exposure to the patient. Deep learning methods could significantly reduce reconstruction requirements to few views44, however, whether they could reach the quality and efficiency to provide feedback almost simultaneous to the beam delivery, as it would be ideally required for a safe application of FLASH-RT, still remain to be seen.
To overcome the main shortcoming of CBCT-based image-guidance, in the virtual simulation and planning stages, MRI has been routinely utilized to provide superior soft tissue visualization for better delineations of tumor, tumor margin and OARs at no burden of ionizing radiation45. However, in the majority of RT cases, MRI scans were acquired on diagnostic scanners with the patient positioning and immobilization devices different from that at the time of treatments, limiting applicability to patient setup. While dedicated MRI RT simulators are emerging and available in many centers, the imaging setup is still dominated by on-board X-ray imaging technologies on conventional Linacs and particle therapy systems. With the already mentioned latest advances in integrating an MRI scanner with a medical linear accelerator (MR-Linac), on-board MRI imaging is now available in more than 60 centers worldwide, allowing the patient to be finely aligned based on the registration of on-board MR scans to the planning MR scan. Although the MR setup scans provide nice visualization of soft tissue and give no ionizing radiation dose, the lack of 6 degrees of freedom (DoF) couch on current MR-Linac systems is not ideal especially for hypofractionated FLASH-RT. However, these limitations could be overcome in next-generation of MR-Linac devices. Moreover, the initial positive experience within the photon therapy community and the more stringent demand of accurate anatomical confirmation for treatment of moving organs with charged particles have prompted research and development toward next-generation of MR-guidance integrated in particle therapy beamlines and gantries, with first experimental demonstrations already existing and clinical devices anticipated in the next 5 to 10 years13. Besides the anatomical information for setup, and depending on the imaging abilities of the integrated MR scanner (especially in terms of field strength and geometrical arrangement), emerging multiparametric on-board MRI protocols may provide functional information such as pre-/post-treatment oxygenation, inflammation, tumor responses and radiation-induced acute toxicities, which can be explored for the biological modelling and optimization of FLASH-RT.
As an alternative to expensive MR technologies, ultrasound offers a non-invasive, real-time, and radiation-free imaging modality that can provide (even time resolved) 3D-US anatomical visualization for selected anatomical locations of sonic access. This has been historically used for patient setup in prostate and gynecological cancers46. However, another exciting phenomenon is related to radiation-induced acoustics, which is the generation of acoustic waves due to thermoelastic expansion of a substance following the absorption of energy deposited by properly pulsed ionizing radiation. This signal is proportional to radiation dose and can be detected using ultrasound transducers47. This has been shown to be applied to a wide variety of applications such as Linac photon beam dosimetry as mentioned earlier, proton therapy range verification, and radiological imaging47. However, an exciting aspect of this phenomenon, especially in the case of photon Linac, is that the higher dose per pulse of the FLASH-RT irradiation significantly improves the SNR and can provide accurate dose measurement at deeper tissues48, as addressed more in detail in the next section. Hence, the prospects of co-registering these thermoacoustic emissions to an US-based representation of the patient anatomy have revived the interest in US-imaging for patient setup and delivery49, especially in the context of FLASH-RT.
For particle therapy, an alternative to on-site X-ray image guidance, which could reach clinical application earlier than MR-guidance, is planar or volumetric transmission proton/light ion imaging. In fact, even with the most advanced algorithms of synthetic CT generation from CBCT or MR images, only reliable information about the medium relative electron density can be retrieved. However, dose calculations in particle therapy require the knowledge of the tissue stopping power relative (SPR) to water. While dual-energy or even spectral X-ray imaging, already under development for CBCT systems, could offer an alternative solution to this long-standing problem of inaccurate conversion of CT numbers into SPR, transmission imaging with the same radiation quality as for treatment could open the possibility of directly probing the tissue stopping properties for a more accurate determination of the SPR, roughly independent of the different energies used for imaging and treatment. Despite the remaining issues of enhanced scattering (especially for protons), nuclear interactions and requirements of complex detector instrumentation, especially when aiming at single particle tracking, several promising solutions have been developed in the last years, with a first commercial radiographic system close to clinical deployment50. In particular, proton transmission imaging could be used in radiographic mode to enable an optimization of the planning CT calibration to SPR51, potentially also compensating for anatomical changes when using a sufficient number of high quality radiographies52, or provide full tomographic capabilities for a patient model in treatment position to be used for daily replanning. The latter scenario could be also favored by the recent developments of fluence field modulation in combination with pencil-beam delivery, which open up further perspective of dose saving for daily imaging53. But of particular relevance to FLASH-RT is the possibility to exploit information from sparse radiographies, which could ideally be simultaneously acquired to the treatment in the recently proposed scenario of shoot-through FLASH proton therapy54.
For patient setup, an alternative to volumetric imaging that has become standard of care in most radiotherapy centers is surface guidance, based upon optical scanners55. These are especially important in areas of soft tissue anatomy, where restraints are not used and where daily setup requires body manipulation. However, perhaps most relevant to FLASH-RT are the gating capabilities, which can be used to synchronize delivery to parts of the breathing cycle for upper anatomy, such as breast irradiation55. Given the instantaneous delivery of FLASH, the ability to time-gate the delivery to the precise body position will be quite important, and if applied to areas of the upper anatomy56, the use of surface guidance technologies may become critical to avoid small location mistakes in the delivery. These technologies are largely optical surface scanning systems today but have also included radiofrequency and infrared technologies for tracking body surface motion. Future work may also include beam delivery tracking, such as scintillation57 or Cherenkov imaging58 on the patient surface, as is further discussed below. Though these technologies offer tremendous opportunities for FLASH-RT their current image quality is still inferior to existing radiological images based on conventional CT or MRI. Moreover, due to the possible latency times associated with these technologies (i.e., hundreds of ms), they may need to be used in conjunction with prediction algorithms to correctly foresee the motion state at the time of delivery. Given the elevated dose rate of FLASH-RT demands on these prediction algorithms and reduction of latency times will be an important task for application to FLASH-RT.
4. Online Imaging & in vivo Dosimetry
The most advanced implementation of adaptive RT envisions online workflows of combined in vivo imaging and dosimetry toward the final goal of real-time RT adaptation. Several methods have been investigated over the past few years to reconstruct the delivered dose or visualize in vivo the particle beam stopping position within the patient, utilizing the transmitted radiation captured by EPID detectors in photon therapy59 or different secondary emissions, like energetic photons from prompt nuclear de-excitation or positron emission and annihilation in proton and light ion therapy60. But despite continued advances in detector technologies and data processing tools, including computational acceleration by AI61, these techniques are far from being used in routine clinical application. This limitation also applies to the already mentioned possible acquisition of radiographic transmission signals with a detector (e.g., range telescope) distal to the ion beam after the patient, which could lead to similar concepts as EPID-based dosimetry in photon therapy in the possible scenario of shoot-through irradiation for FLASH-RT regimens in particle therapy. Alternative solutions deemed more in reach for clinical deployment rely on the online capturing of the anatomical location, currently limited to fast 2D cine MRI sequences at a few Hz frame rate at modern MR-Linac62, combined with dosimetric calculations based on log-files and synthetic CT generation from the acquired MR data. This may improve with fast reconstruction methods utilizing deep learning running on fast graphical processing units (GPU) in the future63. However, such approaches are especially meant to overcome one of the still outstanding great challenges of modern RT, namely, to properly account for organ motion and deformation during beam delivery. However, the achievable temporal resolution of all above mentioned technologies might still be a challenge for application in time-resolved analysis and online assessment in FLASH-RT unless faster electronics or prediction algorithms could be used to compensate for such possible latency times.
Despite the generic notion that FLASH-RT delivery is instantaneous and thereby “freezes motion” and so may not require any motion management, the reality for current Linac-based technologies (as well as particle beam deliveries), is that the desired dose is delivered in a sequence of pulses and not as “a single shot” for practical and safety considerations. In a study by Wilson et al.64, they conjecture that to achieve the FLASH effect, the irradiation beam should be pulsed at a frequency on the order of 100 Hz (i.e., time from one pulse to the next (ΔToff)≈10 ms) and the dose-per-pulse (dose rate) should be greater than 1 Gy (≥ 106), thus a fraction delivery in this case could best last few tenths of a seconds (ΔTfraction ≈100 ms). Though this time is still relatively short, and the exact numbers are still being worked out, it highlights the need for a completely new era of online dosimetry utilizing technologies such as radiation optics (Cherenkov emission [CE])23 and ionizing radiation acoustics imaging (iRAI)48 that are capable of pulse-based detection, as discussed in the following. In Figure 1, redrawn from Wilson et al, it can be seen that with CE or iRAI single pulse dosimetry is permissible, so for a 10 Gy fraction dose, there will be 10 pulses required where each pulse is typically of 1–6μs duration and the time between pulses (ΔToff) is about 10 ms (pulse repetition rate = 100 Hz) and dose rate per pulse of ≥ 106 Gy/s), providing multiple opportunities (though short) for intervention if any misalignment issues (or errors) may arise between these pulses that would warrant halting the delivery. However, unlike conventional radiotherapy where the beam off process could add few more negligible pulses, this is not the case in FLASH where a significant amount of dose could be delivered during such a period. For instance, 2 unaccounted pulses could result in an additional 2 Gy to the patient. Hence, there are far more stringent requirements for better electronic and feedback circuits to ensure safe and accurate pulse-by-pulse delivery within this ΔToff window and at the end of delivered fraction (ΔTfraction). It worth noting that even though there could be currently technical challenges to use the per-pulse information directly for a corrective action during delivery, it could still provide very important time-resolved information to monitor retrospectively if the FLASH irradiation conditions and proper dose targeting were maintained during the entire irradiation, to correlate with treatment outcomes.
Figure 1.
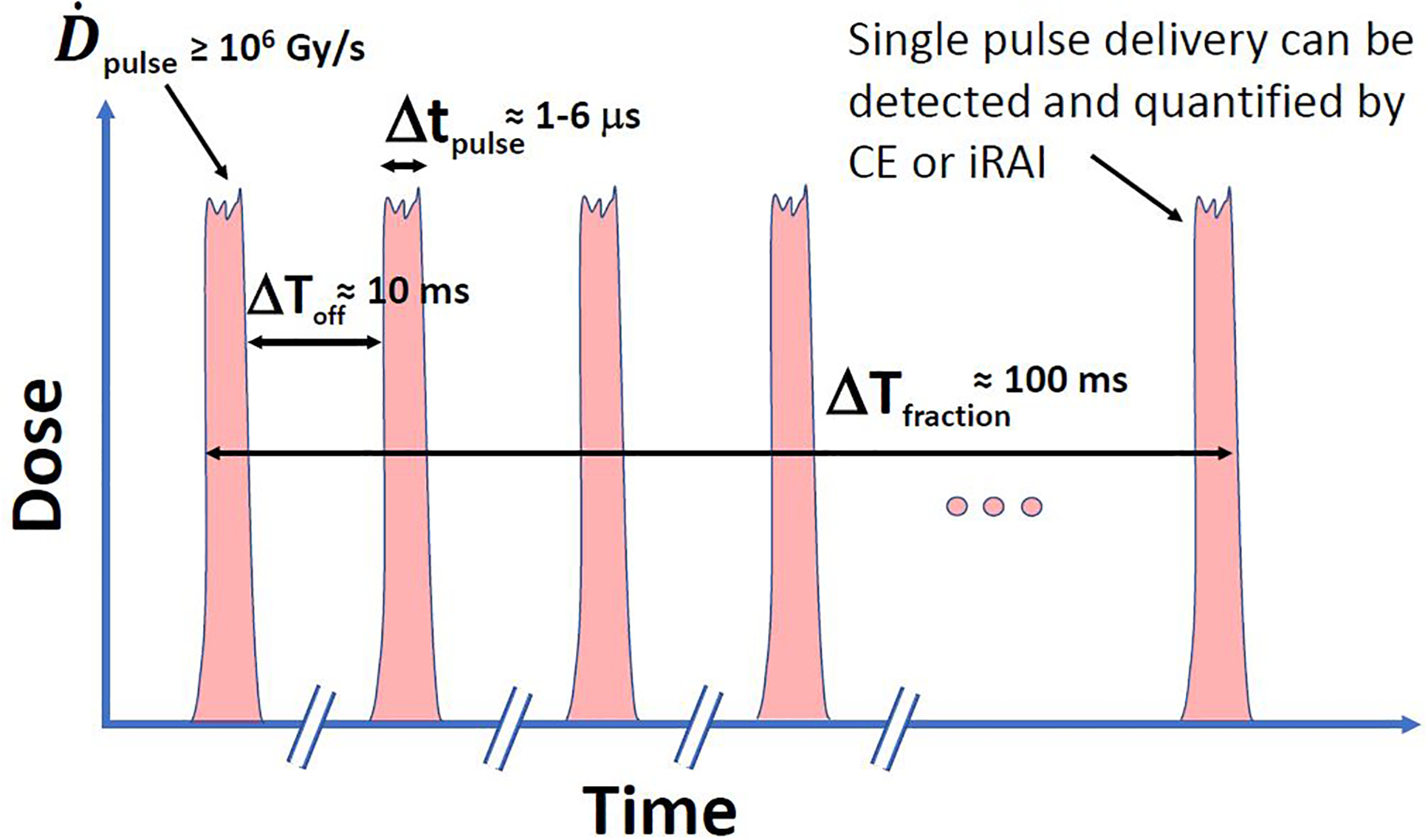
An idealized illustration of a beam FLASH-RT pulse structure, with highinstantaneous dose rate per pulse () and short pulse lengths (Δtpulse). With new pulse-based dosimetry, opportunities for detecting individual pulses using ultra-fast CE or iRAI detection can be potentially realized to ensure safe and accurate dosimetry for every pulse.
Existing technologies for surface guidance might also be used, although their feedback times would need to be improved for the ultra-short delivery of FLASH-RT. This is definitely a new era of not only ultra-fast dose rate but also ultra-fast dosimetry to ensure safe and accurate delivery.
4.A. Optical Imaging for in vivo Dosimetry
Cherenkov and scintillation imaging have been established in a number of pilot Linac-based centers to track photon and electron beam shape during irradiation along with dose delivery using non-contact imaging cameras65,66 or with point probe sensors67. With time-gated intensified cameras, they can capture light just during the short radiation bursts of a Linac or pulsed source and reject most of the ambient room light. When scintillators are used as well, the bright signal can be a direct indicator of dose at that location, when calibrated to absolute units68,69. In FLASH-RT, imaging these can be invaluable tools to assess beam delivery and dose because they can be imaged at the speed of individual pulses (1–6μs) and can be used to track each pulse and the total delivery. The imaging is often time-gated to the Linac pulses (Figure 2a), allowing light capture just during the Linac pulses, thereby minimizing or removing background light. Systematic design can be used to image radiation dose in water tanks (Figure 2b) or in the surface of human patients (Figure 2c). The light signals from a FLASH-RT beam would be 1000× brighter than in conventional RT, and so the ability to image beams in water tanks and patients is expected to be very good, and likely achievable with low-cost camera technology, although the need for fast imaging of each pulse would require time-gating to the frequency of the Linac. Lateral and depth profile images are possible to fully quantify the three-dimensional dose distributions in water tanks23,70, while limited to surface images in applications to patients. Applicability to protons and light ions, for which Cherenkov and luminescence emissions have recently been reported, interestingly below the expected theoretical threshold limits71, could further benefit from similar trends of enhancing the instantaneous current of the beam pulses in FLASH-RT, however on different time scales from ns to μs, depending on the underlying accelerator technology.
Figure 2.
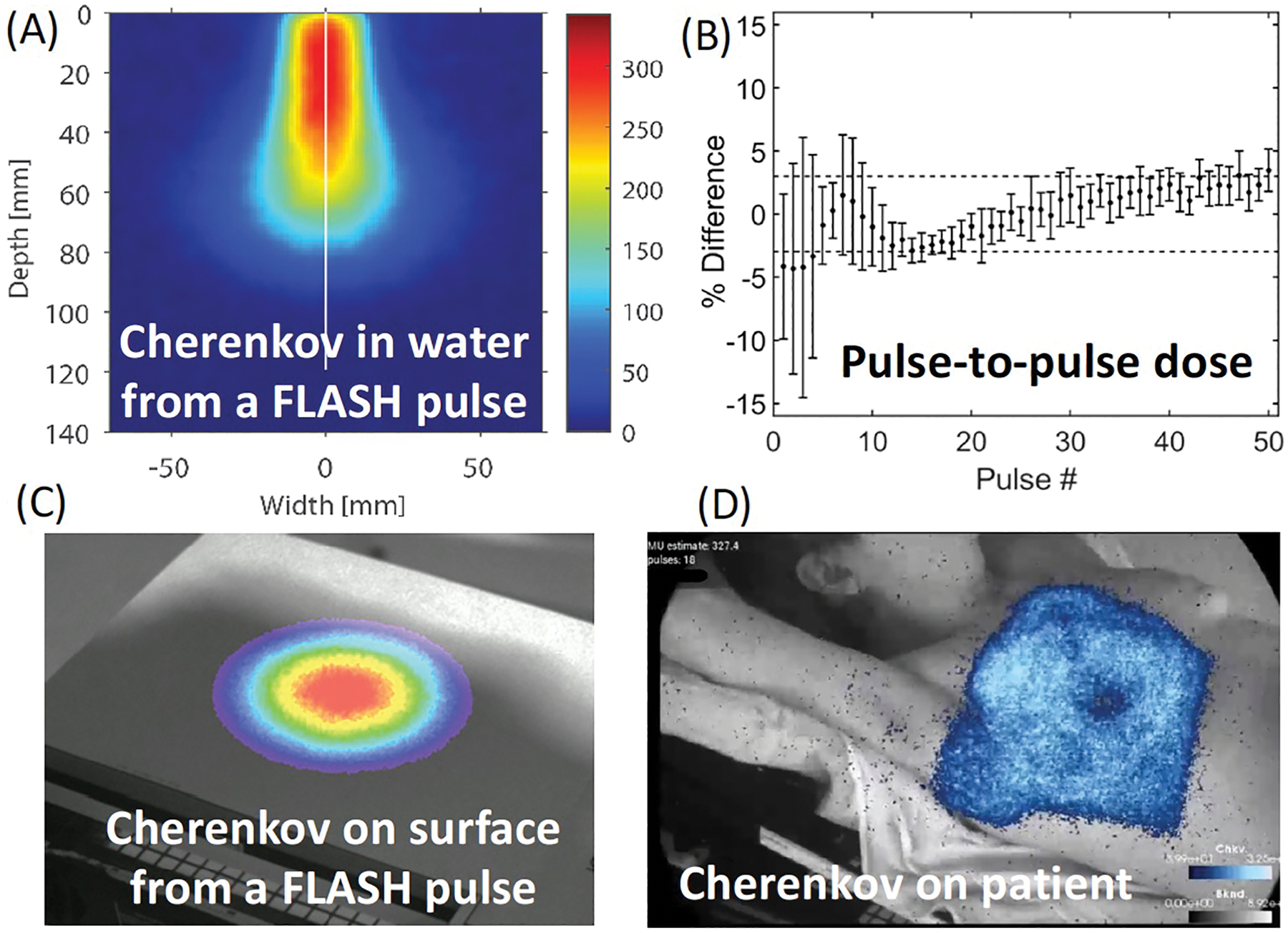
Images from a time-gated camera for acquisition of each Linac pulse (a), from emission in a water phantom for depth dose72, and on the surface of a board for lateral profile73. The ability to image this light signal during each pulse (c) shows the value for pulse-to-pulse verification of delivery. This capability could be extended to on-patient imaging in future work, with the example shown in (d) of Cherenkov light from a standard whole breast radiotherapy patient74.
4.B. Acoustic Imaging for in vivo Dosimetry
Ionizing radiation acoustic imaging (iRAI), which is the result of pressure waves generated following tissue irradiation by a pulsed beam, has the ability to map the 3D dose deposition of individual Linac pulses at deep seated tumors47. This is further improved by the intrinsic higher dose per pulse of FLASH-RT, leading to improved SNR and better dose visualization without excessive need of preamplification as in conventional radiotherapy48. This has been demonstrated recently at the University of Michigan using a 6 MeV electron beam from a modified Varian CLinac that delivered 320 Gy/s at 100 cm source-to-axis distance (SAD). To demonstrate the linearity of iRAI as a dosimetry tool under FLASH-RT dose rates, iRAI measurements were conducted using a single element ultrasound transducer in a homogenous gelatin phantom, with the dose per pulse varied by changing the SAD of the transducer and phantom. The results showed a highly linear relationship between the iRAI signal amplitude and the Linac dose per pulse (R2 = 0.9998), with a repeatability of 1% and a dose resolution error less than 2.5% when compared to radiochromic film dose measurements, as shown in Figure 3.
Figure 3.
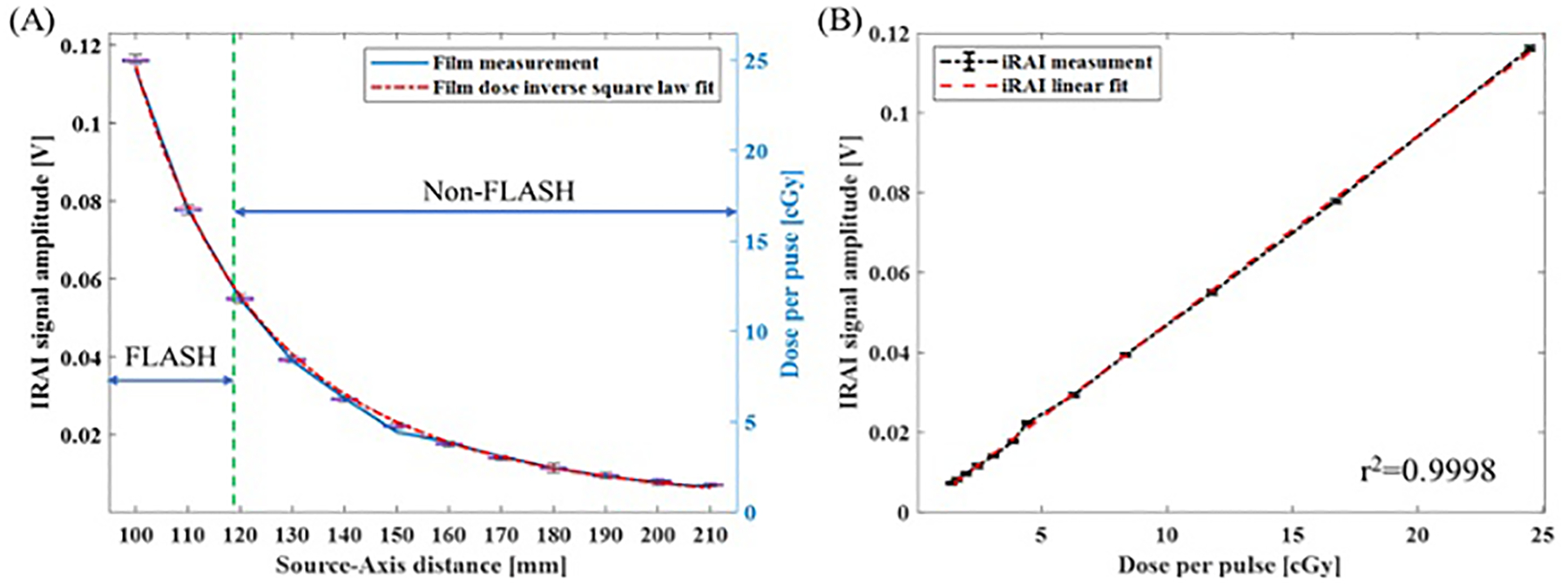
Linearity of iRAI dose measurement and comparison with film measurement. (a) The iRAI dose measurement compared with film measurement along with different SAD; (b) The linearity of iRAI dosimetric measurement48.
To measure how the deposited dose changes with tissue depth (defined as a percentage depth dose [PDD] curve) during FLASH-RT, a special phantom consisting of porcine gelatin and water fixed in a water tank was used, as shown in Figures 4a, b. Confirmation with film measurements was setup using a custom 3D printed radiochromic film holder with a distance of 2 mm between each piece of film. The curve depicted in Figure 4c shows the iRAI measured dose vs. depth (blue curve), with similar trends to the film measurement. To quantify iRAI measurements, a correction factor was introduced to compensate for the electron beam divergence and its energy changes as a function of depth using Monte Carlo simulated fluence changes. The iRAI measurements of the depth-dependent dose after applying this correction factor, as shown by the red curve, have an excellent agreement with the measurements from the film, with a root-mean square error (RMSE) of 0.0243 (2.43%). Building upon these results, the feasibility of a dual-modality ultrasound and iRAI imaging system that co-registers the iRAI measured dose image with soft tissue anatomy was demonstrated using an ex vivo model of rabbit liver, proving feasibility for live imaging application48. Figure 5a shows the iRAI/US co-registered image, with the false color (red) outline being the iRAI image of the radiation field boundaries with. The ex vivo rabbit liver was translated with respect to the fixed beam with Figure 5b showing the field position relative to the liver at different time points in translation. It is worth noting that these images are formed from single Linac pulses, demonstrating the potential to use this technique for pulse-to-pulse dosimetry and beam localization (since US images can be taken in between Linac pulses to give real time anatomical feedback), which will be vital for FLASH-RT. However, the application of this system in clinical FLASH-RT may benefit from further optimization of the acquisition system as suggested by recent Monte Carlo simulations75.
Figure 4.
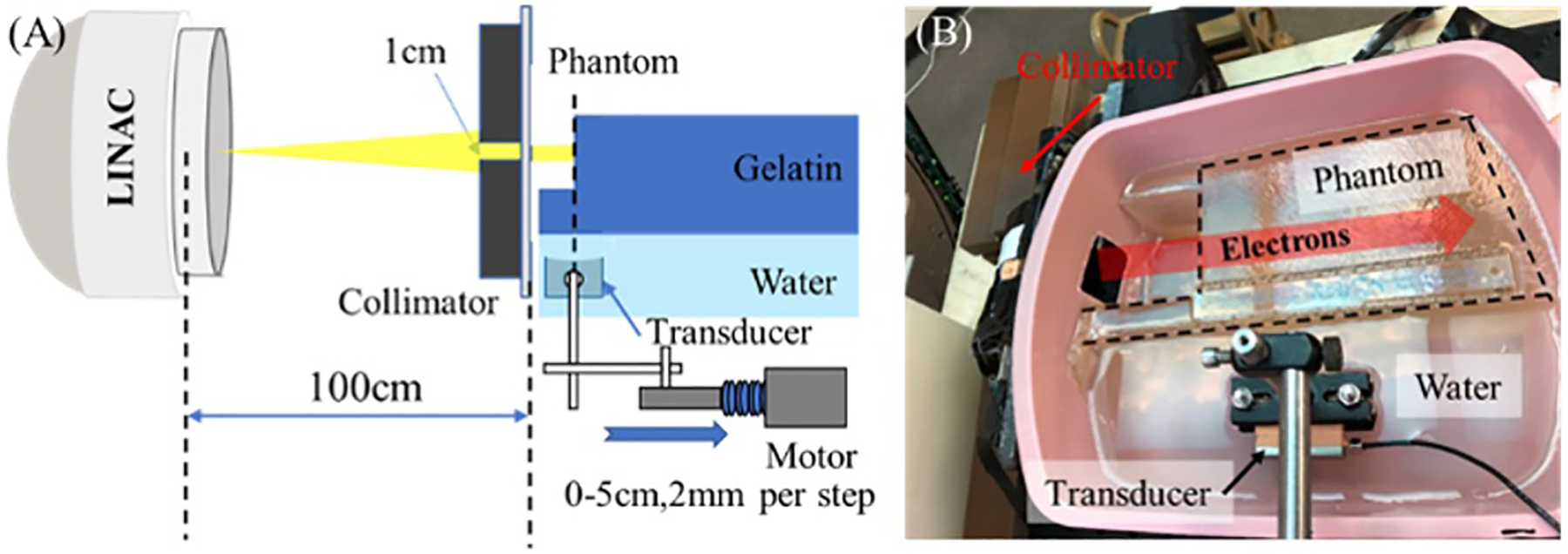
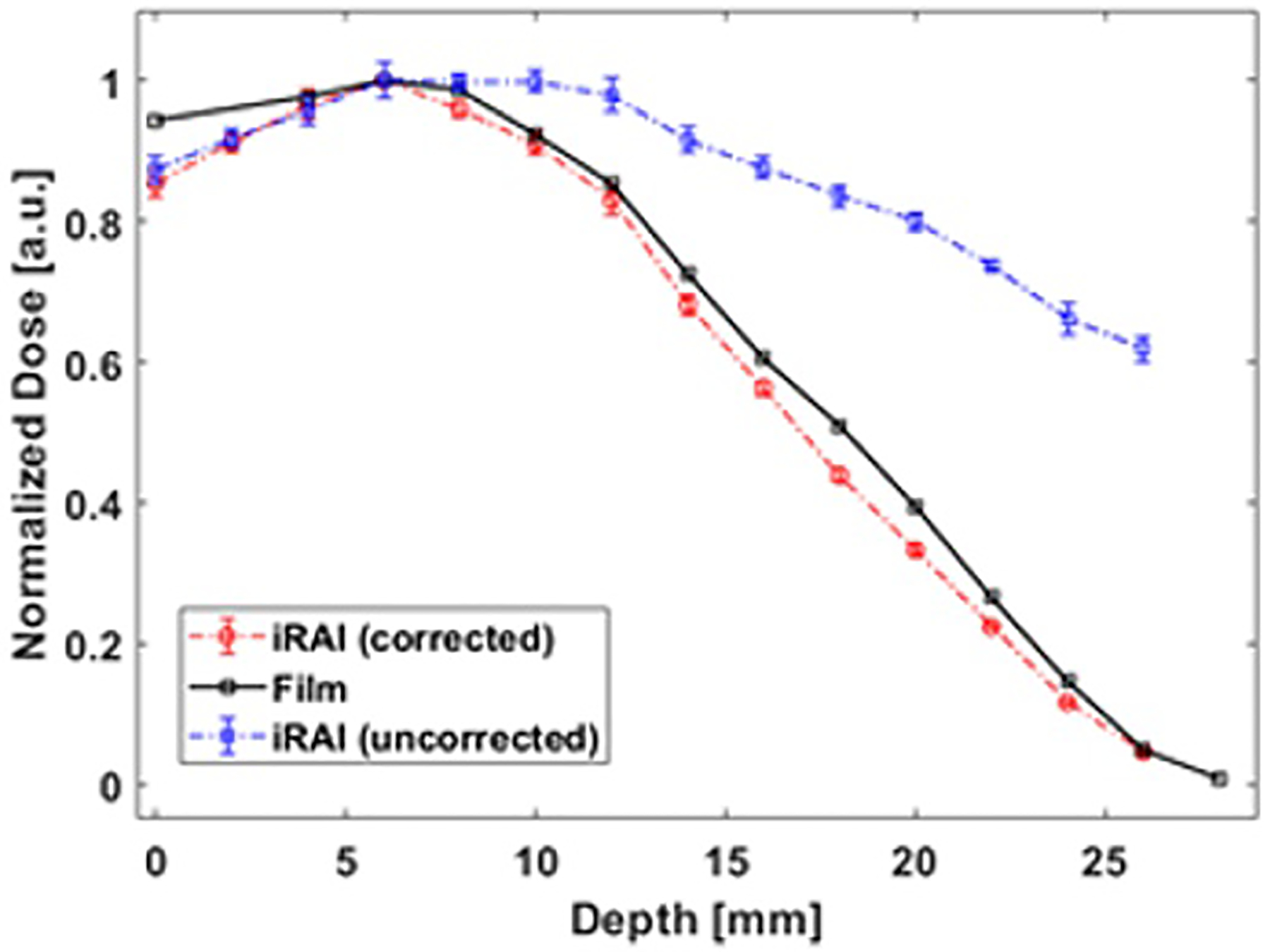
The experimental setup for comparing iRAI and film dose measurements as a function of depth. (a) Schematic of the setup. (b) Photograph of the setup. (c) Uncorrected (blue curve) and corrected (red curve) iRAI measurements of depth dependent dose in the phantom in comparison with the normalized film measurement (black curve)48.
Figure 5.
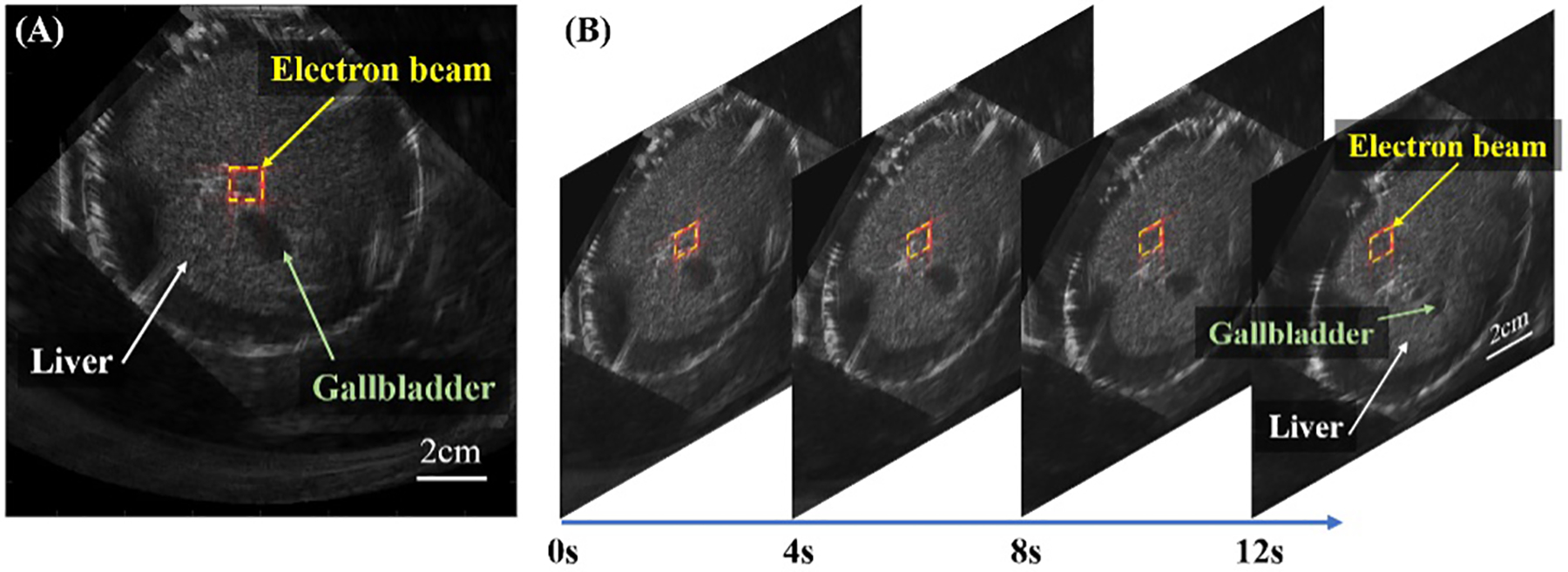
iRAI/US dual-modality imaging of a rabbit liver phantom ex vivo. (a) A registered and combined IRAI and US image, with the radiation field boundaries in red (b) The multi-frame combined images from real-time IRAI and US dual-modality imaging at different time points when the phantom was translated.48
Depending on the pulsing time (micro)structure and peak current, iRAI promises to be also an attractive method to monitor the Bragg peak position (if stopped within the patient) and reconstruct the delivered dose in FLASH particle therapy47,76. In fact, despite the still ongoing developments in terms of optimal detector technology (especially regarding sensitivity and bandwidth) and the current limitations to proof-of-concept studies primarily at standard dose rates from commercially available synchrocyclotrons or artificially pulsed cyclotrons and synchrotrons with controller circuits77, the prospects of ultra-high dose rate delivery for FLASH-RT are expected to favor detectability of iRAI signals with protons and light ions at deeper tissues, potentially also on a single pulse or microstructure basis in high frequency accelerators. The clinical implementation is similar to routine ultrasound imaging where an ultrasound transducer is placed onto the patient surface (e.g., abdomen) and acoustically coupled with ultrasound gel with the exception that this transducer would be optimized for iRAI and it would be held mechanically in a single position for the duration of the treatment session.
Despite the excitement associated with these technologies, there are several limitations that need to be addressed to enable robust clinical implementation. The SNR luckily is not an issue due to the high dose rate of FLASH, however, achieving form factors that can be easily integrated into the clinical workflow is still a work in progress. For example, having a transducer that is in a form factor (e.g., compactness) that allows it to be placed on a location that is optimal to collect iRAI signals while not interfering with the treatment beam (which will be less stringent for FLASH RT since the iRAI signals would have sufficiently high enough SNR to give more flexibility on transducer placement). Additionally, anatomical locations must be considered due to the nature of acoustic propagation in tissue with certain regions more conducive to iRAI (e.g., liver or prostate). Moreover, devising feedback systems that can translate the findings of these in vivo dosimetry into an active process is yet to be worked out.
5. Imaging for Biological Mechanisms and Treatment Response Assessment
Full clinical exploitation of FLASH-RT will remain strictly dependent on our understanding of the underlying biological mechanisms and our ability to visualize the corresponding relevant quantities at unprecedented time scales. For example, one of the current hypotheses around FLASH-RT effects postulates a close connection with oxygen levels in tissue78. However, oxygen imaging within tissue has remained a long-standing challenge of conventional RT, given its transient and micro-heterogeneous distributions. This becomes especially problematic in FLASH-RT where there are purported to be transient local depletions of oxygen due to hydrolysis-mediated radicals scavenging the available oxygen. To date there have not been any PET-FMISO studies of oxygen in FLASH-RT, but because of the transient nature of oxygen, the timescales of this would likely not work, because of the longtime of PET imaging and the very short transient depletions expected from the irradiation. Faster methods such as electrodes, near-infrared spectroscopy of hemoglobin, or phosphorescence quenching are likely the only tools available that would be sufficiently fast to measure oxygen on the relevant timescale of seconds or less, as discussed in the following.
Phosphorescent quenching oxygen measurements have been completed in recent studies to track oxygen transients during and after electron beam FLASH-RT79. These were carried out at Dartmouth, with an injectable phosphorescent agent, OxyPhor (Oxygen Enterprises, Philadelphia PA)80, that is biocompatible and stays either intravenous or interstitial (i.e., extracellular) for most of its biological lifetime, and allows time-gated lifetime sensing which is quenched by molecular oxygen79. Sensing of this species lifetime provides a direct estimate of the local tissue partial pressure (pO2) from the Stern-Volmer equation fitting. In vivo measurements of pO2 showed depletion at the level of 1–2mmHg per 10 Gy of dose delivered. This depletion rate was substantially less than would be required to deplete oxygen in normal tissues, however it could deplete regions of tissue that were already near hypoxia, such as niche areas away from capillaries or near low flow blood vessels. Studies of how this can be used in vivo are still ongoing, however one of the more useful discoveries has been that the Cherenkov light from each pulse of the radiation can be sufficient to excite the Oxyphor itself, thereby allowing for direct luminescence imaging of pO2 from the tissue, without the need for any additional light source81.
In particle therapy, possibilities to trace oxygen or other elements concentrations at an intriguingly fast timescale, mainly constrained by the irradiation time (micro)structure and the subsequent (sub)nanosecond nuclear de-excitation processes, have been reported in the context of prompt gamma spectroscopy82. In this proof-of-concept study (Figure 6), elemental concentration changes of 1% for calcium and 2% for oxygen in adipose, brain, breast, liver, muscle, and bone-related tissue surrogates were clearly identified for proton, helium and carbon ion irradiation at clinical beam intensities and acquisition times. Although clinical utility will in the end depend on the sensitivity of the method in realistic in vivo applications, which will likely be constrained by the limited amount of irradiation induced nuclear emissions and the detection efficiency of multi-MeV prompt gamma rays, the almost instantaneous nature of this process might favor possible future applications also in the context of FLASH-RT.
Figure 6.
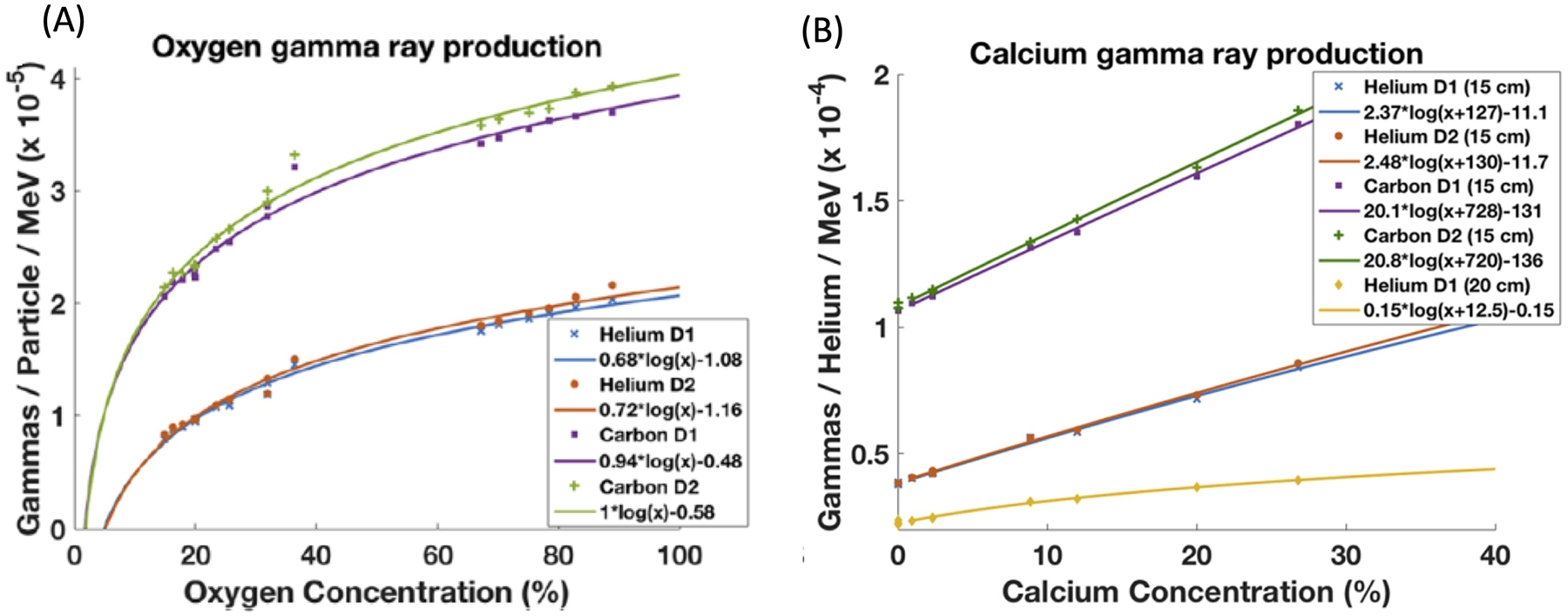
Experimental data points showing prompt gamma yields detected at different distances of 15 cm and 20 cm from the beam axis for substances of different oxygen (a) and calcium (b) concentrations, irradiated with helium and carbon ion beams. The solid lines are fits to the data points82.
Despite the more recent insights and rapidly increasing research efforts, the application of FLASH radiotherapy does not remain without controversy. For instance, a study from MD Anderson found that FLASH-RT did not spare normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome83. It is unclear from the literature whether the used dose rate of 35 Gy/sec was inadequate or other confounding factors. In their interpretation of this variability, they suggested physical conditions (beam characteristics) as well as cell types that may impact FLASH-RT response. Using Casarett’s classification of radiation sensitivity, they mention that gastrointestinal epithelial cells belong to groups I and II from being vegetative intermitotic, i.e., continually dividing without differentiating to differentiating intermitotic, i.e., differentiating into mature non-dividing cells after a finite number of divisions. While lymphocytes, immune cells, are known to be sensitive to RT. Also, they note that heart and splenic irradiation do not deplete hematopoietic stem cells unless specifically targeted. This is in contrast with lung alveolar cells and brain neurons that belong to groups III and IV, respectively, and tend to be less radiosensitive than groups I and II.
Another interpretation is immune modulation84. Now whether FLASH-RT can trigger enhanced immune cell similar to SBRT85, for instance, is still unclear. The STING pathway and type 1 interferon (IFN) signaling have been suggested as a mechanistic link between radiation dose and fractionation with antitumor immunity. However, the relationship with dose rate is yet to be determined. Similarly, more studies are still needed to clarify if the immune response or other biological responses such as DNA damage is different following FLASH-RT compared to conventional RT64. Among the other popular hypotheses for the radioprotective effect of FLASH-RT is the relative sparing of stem cells residing in hypoxic niches in normal tissues86. This hypoxic niche has been reported in the bone marrow, mesenchymal stem cells, and neural stem cells among others87. However, this does not help explain the FLASH-RT effect in hypoxic tumors. Hence, many more molecular signatures beyond oxygen concentrations may need to be targeted and ideally visualized in vivo and at the irradiation site, on time scales comparable to the single pulses of the FLASH-RT delivery.
With several pre-clinical and even clinical studies on the horizon, it will be possible to gain additional insights into the mechanisms underlying the FLASH effect both for tumor and normal tissue from the systematic analysis of treatment dose response. In fact, response assessment is essential when dealing with high-risk high reward modality such as FLASH-RT. On the one hand, this could follow the common practice in conventional radiotherapy using anatomical images (CT or MRI) or functional imaging (Nuclear PET or SPECT, or MRI)26,88,89 at different time points before, during (in case of fractionated regimens) and after RT. In case of X-ray CT imaging, recent progress enabled by micro-CT with their high spatial resolutions as well as photon counting detector technologies and spectral imaging that should be evaluated in terms of improved diagnostic quality for the targeted application. Ideally, all these imaging examinations should be correlated with the relevant biological imaging to better elucidate the mechanisms leading to the phenomenological observations of treatment response. However, the application of imaging biomarkers for FLASH-RT is still in its infancy as this treatment modality continues to evolve.
Depending on the type of tumor eradication and normal tissue sparing aimed with the FLASH-RT, even emerging imaging technologies like phase contrast and dark field imaging could be considered to highlight pathophysiological changes at better image contrast than with conventional imaging modalities90,91. This could for example apply to pulmonary structures or brain injuries, which could be imaged with greater details than conventional imaging approaches, as shown by recent clinical92 and pre-clinical93,94 diagnostic investigations with prototype setups. Although availability of these technologies remains limited and clinical applicability in tomographic setups is still questionable both in terms of technical feasibility and imaging dose, they could play an important role in in vivo preclinical research to provide new insights, which could bring us a step further in our understanding of tumor and normal tissue responses to FLASH-RT95, to pave the way for a more efficient clinical translation.
6. Conclusion and Outlook
Image-guidance for FLASH-RT represents a new clinical need that would go beyond current conventional radiotherapy practices, given its therapeutic potential as well as its associated high risks in planning and delivering of such ultra-high doses in short periods of time.
In planning the treatment and assessing its outcome, quantitative functional imaging would be expected to play a major role to supplement conventional imaging of the patient anatomy based on X-rays and MRI, to foster a more accurate utilization of the biological window of the FLASH effect. If the oxygen depletion phenomenon does dominate FLASH radiobiology effects, then assessment of the oxygen pressure distributions may be needed in addition to traditional anatomical delineations. This can be partly achieved by existing PET imaging hypoxia tracers but will require the development of more sensitive measures, especially of surrounding normal tissue levels to benefit from oxygen depletion currently available in pre-clinical settings, and at the relevant time scales of FLASH-RT delivery. Additional emerging imaging technologies could play an important role in shedding new light onto our understanding of the underlying biological mechanisms and the related manifestations of treatment response both at the tumor and normal tissue levels.
For delivery, conventional onboard imaging such as CBCT will continue to play an important role in improving pre-treatment patient setup, while emerging techniques based on MRI-Linac or PET-Linac, along with their counterparts meanwhile also envisioned for particle therapy, may be invaluable to monitor anatomical and functional changes. On the other hand, new techniques based on Cherenkov emission and radiation acoustics may find much needed clinical utility for mapping and assessing the 3D dose delivery superficially or at tissue depth, respectively. The high dose rate provides an opportunity to image radiation deposition with tools that are not previously viable at lower dose rates, such as the ability to image the volumetric delivery of dose with acoustic tomography methods. The development of these novel tools can help with a new era of ultra-fast (pulse-based) FLASH dosimetry for FLASH-RT, as the improved SNR offers new ways to image the dose deposition. However, there remain several challenges for offline and online detection and monitoring of FLASH-RT in terms of sensitivity, spatial and temporal resolutions that need to be addressed for photon and particle beam modalities. Methods based on AI and faster electronics and advanced beam control systems may help resolve many of the impending reconstruction problems and optimize the associated clinical workflow61.
Concluding, FLASH-RT is an exciting treatment modality that promises to change not only the practice of radiotherapy but also its adjuvant role in the field of oncology. However, the full-fledged application in radiotherapy will require further developments beyond the state-of-the-art in image-guidance techniques for better planning, delivery, and treatment response assessment, as presented in this contribution.
References
- 1.Bourland J, ed Image-Guided Radiation Therapy. 1st ed. Boca Raton: CRC Press; 2012. doi: 10.1201/b11627. [DOI] [Google Scholar]
- 2.Bortfeld T, Schmidt-Ullrich R, De Neve W, Wazer D, eds. Image-guided IMRT. Berlin: Springer-Verlag; 2006. [Google Scholar]
- 3.De Los Santos J, Popple R, Agazaryan N, et al. Image guided radiation therapy (IGRT) technologies for radiation therapy localization and delivery [published online ahead of print 2013/05/15]. Int J Radiat Oncol Biol Phys. 2013;87(1):33–45. [DOI] [PubMed] [Google Scholar]
- 4.Yan D Adaptive radiotherapy: merging principle into clinical practice [published online ahead of print 2010/03/12]. Semin Radiat Oncol. 2010;20(2):79–83. [DOI] [PubMed] [Google Scholar]
- 5.Dawson LA, Jaffray DA. Advances in Image-Guided Radiation Therapy. Journal of Clinical Oncology. 2007;25(8):938–946. [DOI] [PubMed] [Google Scholar]
- 6.Goyal S, Kataria T. Image Guidance in Radiation Therapy: Techniques and Applications. Radiology Research and Practice. 2014;2014:705604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alaei P, Spezi E. Imaging dose from cone beam computed tomography in radiation therapy. Physica Medica. 2015;31(7):647–658. [DOI] [PubMed] [Google Scholar]
- 8.Santanam L, Esthappan J, Mutic S, et al. Estimation of setup uncertainty using planar and MVCT imaging for gynecologic malignancies [published online ahead of print 2008/06/10]. Int J Radiat Oncol Biol Phys. 2008;71(5):1511–1517. [DOI] [PubMed] [Google Scholar]
- 9.Corradini S, Alongi F, Andratschke N, et al. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol. 2019;14(1):92–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noel CE, Parikh PJ, Spencer CR, et al. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncologica. 2015;54(9):1474–1482. [DOI] [PubMed] [Google Scholar]
- 11.Landry G, Hua C-h. Current state and future applications of radiological image guidance for particle therapy. Medical Physics. 2018;45(11):e1086–e1095. [DOI] [PubMed] [Google Scholar]
- 12.MacKay RI. Image Guidance for Proton Therapy [published online ahead of print 2018/03/20]. Clin Oncol (R Coll Radiol). 2018;30(5):293–298. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann A, Oborn B, Moteabbed M, et al. MR-guided proton therapy: a review and a preview [published online ahead of print 2020/05/31]. Radiat Oncol. 2020;15(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245). [DOI] [PubMed] [Google Scholar]
- 15.Favaudon V, Fouillade C, Vozenin MC. Ultrahigh dose-rate, “flash” irradiation minimizes the side-effects of radiotherapy. Cancer Radiother. 2015;19(6–7):526–531. [DOI] [PubMed] [Google Scholar]
- 16.Montay-Gruel P, Acharya MM, Petersson K, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. P Natl Acad Sci USA. 2019;116(22):10943–10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol. 2017;124(3):365–369. [DOI] [PubMed] [Google Scholar]
- 18.Vozenin MC, De Fornel P, Petersson K, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res. 2019;25(1):35–42. [DOI] [PubMed] [Google Scholar]
- 19.Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22. [DOI] [PubMed] [Google Scholar]
- 20.Boscolo D, Scifoni E, Durante M, Krämer M, Fuss MC. May oxygen depletion explain the FLASH effect? A chemical track structure analysis. Radiother Oncol. 2021;162:68–75. [DOI] [PubMed] [Google Scholar]
- 21.Petersson K, Adrian G, Butterworth K, McMahon SJ. A Quantitative Analysis of the Role of Oxygen Tension in FLASH Radiation Therapy. International Journal of Radiation Oncology*Biology*Physics. 2020;107(3):539–547. [DOI] [PubMed] [Google Scholar]
- 22.Cao X, Zhang R, Esipova TV, et al. Quantification of Oxygen Depletion During FLASH Irradiation In Vitro and In Vivo [published online ahead of print 2021/04/13]. Int J Radiat Oncol Biol Phys. 2021;111(1):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashraf MR, Rahman M, Zhang R, et al. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Frontiers in Physics. 2020;8(328). [Google Scholar]
- 24.Seo H, Badiei Khuzani M, Vasudevan V, et al. Machine learning techniques for biomedical image segmentation: An overview of technical aspects and introduction to state-of-art applications. Medical Physics. 2020;47(5):e148–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmér E, Karlsson A, Nordström F, et al. Synthetic computed tomography data allows for accurate absorbed dose calculations in a magnetic resonance imaging only workflow for head and neck radiotherapy. Physics and Imaging in Radiation Oncology. 2021;17:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaidi H, Alavi A, Naqa IE. Novel Quantitative PET Techniques for Clinical Decision Support in Oncology [published online ahead of print 2018/10/17]. Semin Nucl Med. 2018;48(6):548–564. [DOI] [PubMed] [Google Scholar]
- 27.Kong FM, Ten Haken RK, Schipper M, et al. Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non-Small-Cell Lung Cancer: A Phase 2 Clinical Trial [published online ahead of print 2017/06/02]. JAMA Oncol. 2017;3(10):1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oderinde OM, Shirvani SM, Olcott PD, Kuduvalli G, Mazin S, Larkin D. The technical design and concept of a PET/CT linac for biology-guided radiotherapy [published online ahead of print 2021/07/15]. Clin Transl Radiat Oncol. 2021;29:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nature Reviews Clinical Oncology. 2012;9(12):674–687. [DOI] [PubMed] [Google Scholar]
- 30.Jansen J, Knoll J, Beyreuther E, et al. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Medical Physics. 2021;48(7):3982–3990. [DOI] [PubMed] [Google Scholar]
- 31.Favaudon V, Labarbe R, Limoli CL. Model studies of the role of oxygen in the FLASH effect. Medical Physics. 2021;n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glide-Hurst CK, Lee P, Yock AD, et al. Adaptive Radiation Therapy (ART) Strategies and Technical Considerations: A State of the ART Review From NRG Oncology [published online ahead of print 2021/01/21]. Int J Radiat Oncol Biol Phys. 2021;109(4):1054–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brock KK. Adaptive Radiotherapy: Moving Into the Future. Seminars in radiation oncology. 2019;29(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Lu H, Liang Z, et al. An experimental study on the noise properties of x-ray CT sinogram data in Radon space. Physics in Medicine and Biology. 2008;53(12):3327–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Z, Chen Y, Zhang Y, Ge Y, Yin F-F, Ren L. Augmentation of CBCT Reconstructed From Under-Sampled Projections Using Deep Learning [published online ahead of print 2019/04/23]. IEEE Trans Med Imaging. 2019;38(11):2705–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen BS, Hawkins PG, Polan DF, et al. Early Changes in Serial CBCT-Measured Parotid Gland Biomarkers Predict Chronic Xerostomia After Head and Neck Radiation Therapy [published online ahead of print 2018/07/14]. Int J Radiat Oncol Biol Phys. 2018;102(4):1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posiewnik M, Piotrowski T. A review of cone-beam CT applications for adaptive radiotherapy of prostate cancer. Physica Medica. 2019;59:13–21. [DOI] [PubMed] [Google Scholar]
- 38.Montay-Gruel P, Acharya MM, Gonçalves Jorge P, et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin Cancer Res. 2021;27(3):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy [published online ahead of print 2002/07/20]. Int J Radiat Oncol Biol Phys. 2002;53(5):1337–1349. [DOI] [PubMed] [Google Scholar]
- 40.Yoganathan SA, Maria Das KJ, Mohamed Ali S, Agarwal A, Mishra SP, Kumar S. Evaluating the four-dimensional cone beam computed tomography with varying gantry rotation speed [published online ahead of print 2016/02/27]. Br J Radiol. 2016;89(1060):20150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santoso AP, Song KH, Qin Y, et al. Evaluation of gantry speed on image quality and imaging dose for 4D cone-beam CT acquisition. Radiation Oncology. 2016;11(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maurer J, Pan T, Yin FF. Slow gantry rotation acquisition technique for on-board four-dimensional digital tomosynthesis [published online ahead of print 2010/03/17]. Med Phys. 2010;37(2):921–933. [DOI] [PubMed] [Google Scholar]
- 43.Sajja S, Lee Y, Eriksson M, et al. Technical Principles of Dual-Energy Cone Beam Computed Tomography and Clinical Applications for Radiation Therapy. Advances in Radiation Oncology. 2020;5(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen L, Zhao W, Xing L. Patient-specific reconstruction of volumetric computed tomography images from a single projection view via deep learning. Nature biomedical engineering. 2019;3(11):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chin S, Eccles CL, McWilliam A, et al. Magnetic resonance-guided radiation therapy: A review [published online ahead of print 2019/10/28]. J Med Imaging Radiat Oncol. 2020;64(1):163–177. [DOI] [PubMed] [Google Scholar]
- 46.Camps SM, Fontanarosa D, de With PHN, Verhaegen F, Vanneste BGL. The Use of Ultrasound Imaging in the External Beam Radiotherapy Workflow of Prostate Cancer Patients [published online ahead of print 2018/04/06]. Biomed Res Int. 2018;2018:7569590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hickling S, Xiang L, Jones KC, et al. Ionizing radiation-induced acoustics for radiotherapy and diagnostic radiology applications. Medical Physics. 2018;45(7):e707–e721. [DOI] [PubMed] [Google Scholar]
- 48.Oraiqat I, Zhang W, Litzenberg D, et al. An ionizing radiation acoustic imaging (iRAI) technique for real-time dosimetric measurements for FLASH radiotherapy. Medical Physics. 2020;47(10):5090–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Oraiqat I, Lei H, Carson PL, Ei Naqa I, Wang X. Dual-Modality X-Ray-Induced Radiation Acoustic and Ultrasound Imaging for Real-Time Monitoring of Radiotherapy. BME Frontiers. 2020;2020:9853609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeJongh EA, DeJongh DF, Polnyi I, et al. Technical Note: A fast and monolithic prototype clinical proton radiography system optimized for pencil beam scanning [published online ahead of print 2021/01/01]. Med Phys. 2021;48(3):1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins-Fekete CA, Brousmiche S, Hansen DC, Beaulieu L, Seco J. Pre-treatment patient-specific stopping power by combining list-mode proton radiography and x-ray CT [published online ahead of print 2017/06/29]. Phys Med Biol. 2017;62(17):6836–6852. [DOI] [PubMed] [Google Scholar]
- 52.Palaniappan P, Meyer S, Kamp F, et al. Deformable image registration of the treatment planning CT with proton radiographies in perspective of adaptive proton therapy [published online ahead of print 2020/05/05]. Phys Med Biol. 2021;66(4):045008. [DOI] [PubMed] [Google Scholar]
- 53.Dickmann J, Sarosiek C, Rykalin V, et al. Experimental realization of dynamic fluence field optimization for proton computed tomography [published online ahead of print 2020/06/24]. Phys Med Biol. 2020;65(19):195001. [DOI] [PubMed] [Google Scholar]
- 54.Verhaegen F, Wanders RG, Wolfs C, Eekers D. Considerations for shoot-through FLASH proton therapy [published online ahead of print 2021/02/12]. Phys Med Biol. 2021;66(6):06nt01. [DOI] [PubMed] [Google Scholar]
- 55.Al-Hallaq H, Batista V, Kugele M, Ford E, Viscariello N, Meyer J. The role of surface-guided radiation therapy for improving patient safety [published online ahead of print 2021/08/29]. Radiother Oncol. 2021;163:229–236. [DOI] [PubMed] [Google Scholar]
- 56.Freislederer P, Kugele M, Ollers M, et al. Recent advanced in Surface Guided Radiation Therapy [published online ahead of print 2020/08/02]. Radiat Oncol. 2020;15(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jenkins CH, Naczynski DJ, Yu SJ, Xing L. Monitoring external beam radiotherapy using real-time beam visualization [published online ahead of print 2015/01/08]. Med Phys. 2015;42(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarvis LA, Hachadorian RL, Jermyn M, et al. Initial Clinical Experience of Cherenkov Imaging in External Beam Radiation Therapy Identifies Opportunities to Improve Treatment Delivery [published online ahead of print 2020/11/24]. Int J Radiat Oncol Biol Phys. 2020. doi: 10.1016/j.ijrobp.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olaciregui-Ruiz I, Beddar S, Greer P, et al. In vivo dosimetry in external beam photon radiotherapy: Requirements and future directions for research, development, and clinical practice. Physics and imaging in radiation oncology. 2020;15:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parodi K, Polf JC. In vivo range verification in particle therapy [published online ahead of print 2018/11/14]. Med Phys. 2018;45(11):e1036–e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui S, Tseng HH, Pakela J, Ten Haken RK, El Naqa I. Introduction to machine and deep learning for medical physicists [published online ahead of print 2020/05/18]. Med Phys. 2020;47(5):e127–e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurz C, Buizza G, Landry G, et al. Medical physics challenges in clinical MR-guided radiotherapy [published online ahead of print 2020/05/07]. Radiat Oncol. 2020;15(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng G, Guo Y, Zhan J, et al. A review on deep learning MRI reconstruction without fully sampled k-space. BMC Medical Imaging. 2021;21(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? [published online ahead of print 2020/02/06]. Front Oncol. 2019;9:1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pogue BW, Zhang R, Glaser A, et al. Cherenkov imaging in the potential roles of radiotherapy QA and delivery. Journal of Physics: Conference Series. 2017;847:012046. [Google Scholar]
- 66.Zhang R, Glaser AK, Gladstone DJ, Fox CJ, Pogue BW. Superficial dosimetry imaging based on Čerenkov emission for external beam radiotherapy with megavoltage x-ray beam. Medical Physics. 2013;40(10):101914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oraiqat I, DeBruin S, Pearce R, et al. Silicon Photomultipliers for Deep Tissue Cerenkov Emission Detection During External Beam Radiotherapy. IEEE Photonics Journal. 2019;11(4):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zlateva Y, Muir BR, El Naqa I, Seuntjens JP. Cherenkov emission-based external radiotherapy dosimetry: I. Formalism and feasibility. Medical Physics. 2019;46(5):2370–2382. [DOI] [PubMed] [Google Scholar]
- 69.Zlateva Y, Muir BR, Seuntjens JP, El Naqa I. Cherenkov emission-based external radiotherapy dosimetry: II. Electron beam quality specification and uncertainties. Medical Physics. 2019;46(5):2383–2393. [DOI] [PubMed] [Google Scholar]
- 70.Rahman M, Ashraf MR, Zhang R, et al. Electron FLASH Delivery at Treatment Room Isocenter for Efficient Reversible Conversion of a Clinical LINAC. International Journal of Radiation Oncology*Biology*Physics. 2021;110(3):872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano Y, Yamamoto S. Estimation of the fractions of luminescence of water at higher energy than Cerenkov-light threshold for various types of radiation. Journal of Biomedical Optics. 2019;24(6):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashraf MR, Rahman M, Zhang R, et al. Technical Note: Single-pulse beam characterization for FLASH-RT using optical imaging in a water tank [published online ahead of print 2021/03/18]. Med Phys. 2021;48(5):2673–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahman M, Ashraf MR, Zhang R, et al. Spatial and temporal dosimetry of individual electron FLASH beam pulses using radioluminescence imaging [published online ahead of print 2021/05/21]. Phys Med Biol. 2021. doi: 10.1088/1361-6560/ac0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hachadorian R, Bruza P, Jermyn M, et al. Correcting Cherenkov light attenuation in tissue using spatial frequency domain imaging for quantitative surface dosimetry during whole breast radiation therapy. J Biomed Opt. 2018;24(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ba Sunbul NH, Zhang W, Oraiqat I, et al. A simulation study of ionizing radiation acoustic imaging (iRAI) as a real-time dosimetric technique for ultra-high dose rate radiotherapy (UHDR-RT) [published online ahead of print 2021/08/26]. Med Phys. 2021. doi: 10.1002/mp.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jolly S, Owen H, Schippers M, Welsch C. Technical challenges for FLASH proton therapy. Physica Medica. 2020;78:71–82. [DOI] [PubMed] [Google Scholar]
- 77.Diffenderfer ES, Verginadis II, Kim MM, et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System [published online ahead of print 2020/01/14]. Int J Radiat Oncol Biol Phys. 2020;106(2):440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Favaudon V, Labarbe R, Limoli CL. Model studies of the role of oxygen in the FLASH effect [published online ahead of print 2021/08/19]. Med Phys. 2021. doi: 10.1002/mp.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao X, Rao Allu S, Jiang S, et al. Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nature Communications. 2020;11(1):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esipova TV, Barrett MJP, Erlebach E, Masunov AE, Weber B, Vinogradov SA. Oxyphor 2P: A High-Performance Probe for Deep-Tissue Longitudinal Oxygen Imaging. Cell Metabolism. 2019;29(3):736–744.e737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pogue BW, Feng J, LaRochelle EP, et al. Maps of in vivo oxygen pressure with submillimetre resolution and nanomolar sensitivity enabled by Cherenkov-excited luminescence scanned imaging. Nature Biomedical Engineering. 2018;2(4):254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magalhaes Martins P, Dal Bello R, Ackermann B, et al. PIBS: Proton and ion beam spectroscopy for in vivo measurements of oxygen, carbon, and calcium concentrations in the human body. Scientific Reports. 2020;10(1):7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venkatesulu BP, Sharma A, Pollard-Larkin JM, et al. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Scientific Reports. 2019;9(1):17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durante M, Bräuer-Krisch E, Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy [published online ahead of print 2017/11/28]. Br J Radiol. 2018;91(1082):20170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marciscano AE, Haimovitz-Friedman A, Lee P, et al. Immunomodulatory Effects of Stereotactic Body Radiation Therapy: Preclinical Insights and Clinical Opportunities [published online ahead of print 2019/03/06]. Int J Radiat Oncol Biol Phys. 2021;110(1):35–52. [DOI] [PubMed] [Google Scholar]
- 86.Pratx G, Kapp DS. Ultra-High-Dose-Rate FLASH Irradiation May Spare Hypoxic Stem Cell Niches in Normal Tissues [published online ahead of print 2019/05/31]. Int J Radiat Oncol Biol Phys. 2019;105(1):190–192. [DOI] [PubMed] [Google Scholar]
- 87.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche [published online ahead of print 2010/08/05]. Cell Stem Cell. 2010;7(2):150–161. [DOI] [PubMed] [Google Scholar]
- 88.Jeraj R, Bradshaw T, Simončič U. Molecular Imaging to Plan Radiotherapy and Evaluate Its Efficacy. Journal of Nuclear Medicine. 2015;56(11):1752. [DOI] [PubMed] [Google Scholar]
- 89.Gurney-Champion OJ, Mahmood F, van Schie M, et al. Quantitative imaging for radiotherapy purposes. Radiother Oncol. 2020;146:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfeiffer F, Bech M, Bunk O, et al. Hard-X-ray dark-field imaging using a grating interferometer [published online ahead of print 2008/01/22]. Nat Mater. 2008;7(2):134–137. [DOI] [PubMed] [Google Scholar]
- 91.Willer K, Fingerle AA, Gromann LB, et al. X-ray dark-field imaging of the human lung—A feasibility study on a deceased body. PLOS ONE. 2018;13(9):e0204565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gassert FT, Urban T, Frank M, et al. X-ray Dark-Field Chest Imaging: Qualitative and Quantitative Results in Healthy Humans [published online ahead of print 2021/08/25]. Radiology. 2021. doi: 10.1148/radiol.2021210963:210963. [DOI] [PubMed] [Google Scholar]
- 93.Burkhardt R, Gora T, Fingerle AA, et al. Early detection of radiation-induced lung damage with X-ray dark-field radiography in mice [published online ahead of print 2020/11/20]. Eur Radiol. 2021;31(6):4175–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romano M, Bravin A, Mittone A, et al. A Multi-Scale and Multi-Technique Approach for the Characterization of the Effects of Spatially Fractionated X-ray Radiation Therapies in a Preclinical Model. Cancers. 2021;13(19):4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Viermetz M, Gustschin N, Schmid C, et al. Dark-field computed tomography reaches the human scale [published online ahead of print 2022/02/09]. Proc Natl Acad Sci U S A. 2022;119(8). [DOI] [PMC free article] [PubMed] [Google Scholar]


