Abstract
Purpose
Ultrasound-guided interscalene nerve block (UISB) is commonly used to alleviate postoperative pain during shoulder arthroscopy. This retrospective observational study aimed to evaluate the intraoperative advantages and analgesic effects of preoperative UISB.
Patients and Methods
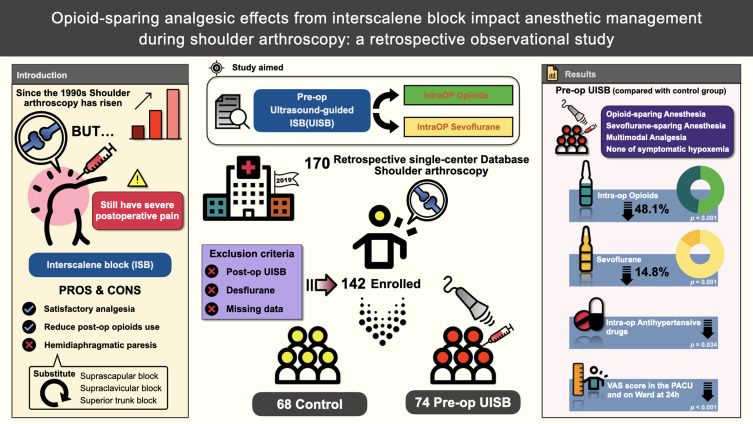
In this retrospective observational study, a total of 170 patients underwent shoulder arthroscopy at a tertiary medical center in southern Taiwan throughout 2019. After applying the exclusion criteria, 142 of these cases were included, with 74 and 68 in the UISB group and control groups, respectively. The primary outcome was the evaluation of intraoperative morphine milligram equivalent (MME) consumption. Secondary outcomes were sevoflurane consumption, the use of intraoperative antihypertensive drugs, and postoperative visual analog scale (VAS) scores in the post-anesthesia care unit (PACU) and in the ward at 24 h after surgery.
Results
Preoperative UISB effectively reduced opioids and volatile gases during surgery, supported by a 48.1% and 14.8% reduction in the median intraoperative MME and sevoflurane concentrations, respectively, and showed less need for antihypertensive drugs. The preoperative UISB group also showed significantly better performance on the VAS in both the PACU and ward.
Conclusion
Taken together, the preoperative UISB reduced not only intraoperative MME and sevoflurane consumption but also had satisfactory VAS scores in both the PACU and ward without any symptomatic respiratory complications. In summary, preoperative UISB is a reliable adjuvant analgesic technique and a key factor in achieving opioid-sparing and sevoflurane-sparing anesthesia and multimodal analgesia during shoulder arthroscopy.
Keywords: interscalene block, multimodal analgesia, opioid-sparing anesthesia, shoulder arthroscopy, volatile-sparing anesthesia
Graphical Abstract
Introduction
Shoulder surgery is a growing field in geriatric orthopedics and sports medicine. Since the late 1990s, an increasing number of shoulder surgeries that require arthrotomy have been widely performed by arthroscopy. Minimally invasive arthroscopic techniques have shown excellent benefits in terms of functional outcomes and shortened length of hospitalization.1 However, the incidence of severe pain after shoulder arthroscopy remains high, preventing recovery, and opioid use is usually required for several days.2
Interscalene nerve block (ISB) has been commonly used to alleviate postoperative pain and provide optimal analgesia during shoulder arthroscopy.3,4 Ultrasound-guided interscalene nerve block (UISB) can reduce opioid-related adverse effects such as pruritus, nausea, vomiting, consciousness disturbance, and constipation.5,6 On average, UISB can also reduce postoperative visual analog scale (VAS) scores for at least 8 h and decrease opioid use between 8 and 12 h.7 In addition, regional anesthesia, such as peripheral nerve block or neuraxial anesthesia, which is an important component of multimodal analgesia, has been proven to reduce postoperative opioid consumption.8 In recent years, the efficacy of postoperative pain control with supraclavicular, suprascapular, and other relevant blocks during shoulder arthroscopy has also been widely compared.9–12 However, these trials focused mainly on the postoperative opioid-sparing effects of nerve blocks and did not show an impact on the intraoperative consumption of volatile anesthetics and opioids.
Therefore, we conducted a retrospective study to test the intraoperative volatile- and opioid-sparing effects of UISB performed preoperatively. This study aimed to evaluate intraoperative morphine milligram equivalent (MME) consumption and investigate intraoperative sevoflurane consumption, intraoperative antihypertensive drugs, and the assessment of postoperative pain in different periods.
Materials and Methods
This retrospective observational study was approved by the Institutional Review Board (IRB) of Kaohsiung Chang Gung Memorial Hospital (IRB approval no.:202101342B0). This manuscript complies with the applicable guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.13
Data Collection
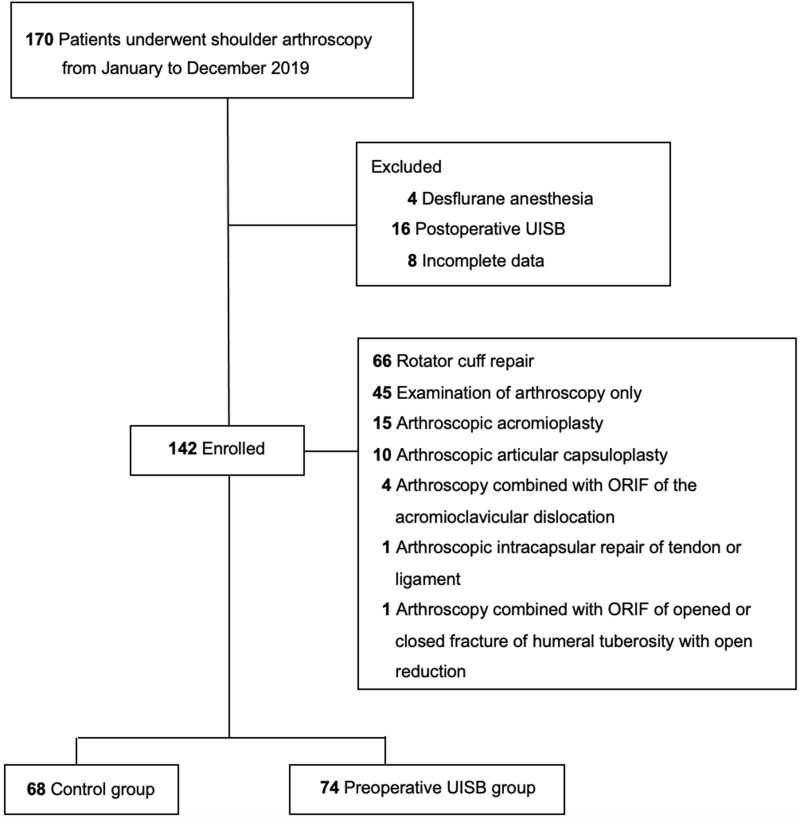
This study was conducted through a comprehensive review of the anesthesia records of patients who underwent arthroscopic shoulder surgery between January and December 2019. A total of 170 general anesthesia records were retrieved from our single-center electronic database. The exclusion criteria were cases of desflurane anesthesia (n = 4), postoperative UISB (n = 16), and those with incomplete data (n = 8). Finally, 142 patients were analyzed and allocated into two groups: 74 and 68 patients in the preoperative and control groups, respectively (Figure 1). Among the 142 patients, 66 patients received rotator cuff repair, 15 patients received arthroscopic acromioplasty, 10 patients received arthroscopic articular capsuloplasty, four patients received arthroscopy combined with open reduction and internal fixation (ORIF) of an acromioclavicular dislocation, one patient received arthroscopic intracapsular repair of a tendon or ligament, and one patient received arthroscopy combined with ORIF of an opened or closed fracture of the humeral tuberosity. The remaining 45 patients underwent arthroscopic examination.
Figure 1.
Flow diagram of the control group (n = 68) and the preoperative UISB group (n = 74).
Ultrasound-Guided Interscalene Nerve Block
Patients who underwent preoperative UISB did not receive any premedication in the induction area where the UISB was performed before arriving to the operating room. After setting the standard American Society of Anesthesiologists (ASA) monitoring and supplemental oxygen, the C5 to C8 nerve roots were identified using real-time sonography with a total volume of 20 mL of a mixture of 15 mL 0.5% levobupivacaine and 5 mL 2% lidocaine. After UISB completion, the sensory block was assessed every 5 min for at least 30 min by loss of temperature and pinprick sensation on the dermatomes of C5 to C8 (if at least C5-C7 were blocked, the whole shoulder joint was completely anesthetized). The success of the preoperative UISB was defined as loss of temperature and pinprick sensation within 30 min. In Taiwan, the cost of UISB is not included in the National Health Insurance. Therefore, to ensure quality and efficacy, all UISB procedures and testing for successful blockade at our institution were performed by experienced anesthesiologists in charge of orthopedic anesthesia.
Assessment of Block Onset and Possible Pulmonary Complications
Unexpected phrenic nerve blockade is common following UISB and may impair diaphragmatic and respiratory functions, especially in patients with pre-existing pulmonary pathology. All patients in the preoperative UISB group were frequently monitored using a pulse oximeter with 3 L/min oxygen administration via nasal cannula. After confirming the success of the UISB, the patient was immediately sent to the operating room for anesthesia induction. Only one of the 74 patients in the preoperative UISB group complained of discomfort in breathing, but hypoxemia was not observed under pulse oximeter monitoring (>97%).
Intraoperative Anesthesia Management and Postoperative Pain Management
After preoperative preparation, all 142 patients underwent surgery under general anesthesia, and mechanical ventilation was performed by placing an endotracheal tube. The patients regularly received intravenous fentanyl 2 mcg/kg and propofol 2 mg/kg mixed with lidocaine 20 mg for induction. Rocuronium 0.6 mg/kg or cis-atracurium 0.2 mg/kg was used as the neuromuscular blocking agent to facilitate endotracheal intubation. Cis-atracurium and neostigmine have been used for neuromuscular blocking agent and reversal during our routine anesthetic management. Sugammadex was administered together with rocuronium only if the patient agreed to pay an additional cost of approximately US$200.
In addition, prophylaxis for postoperative nausea and vomiting (PONV), such as dexamethasone and/or ondansetron, was routinely administered at our institution based on patient risk factors, following guideline recommendations.14 Sevoflurane, a modern inhalation anesthetic, was used to maintain anesthesia using an anesthesia machine, and a fresh gas flow of 30–50% oxygen with air, depending on the pulse oximetry value, was maintained at 2 L/min. The depth of anesthesia was monitored using the bispectral index (BIS) value, which was maintained between 40 and 60 for anesthesia induction and intraoperative maintenance to ensure adequate anesthetic depth. Furthermore, antihypertensive agents, including intravenous labetalol and nicardipine, were administered according to the anesthesiologist’s preference if the patient’s blood pressure increased by 30% from the baseline. In both the UISB and control groups, intravenous opioids were still used for analgesia if the VAS score was > 3 in the PACU.
Primary and Secondary Outcomes
The primary outcome was the evaluation of intraoperative MME consumption. Opioids are commonly used to supplement general anesthetics and are the most widely used analgesic agents for intraoperative surgical stimuli. In our practice, some types of opioids, including fentanyl, alfentanil, and morphine, are administered intravenously during shoulder arthroscopy. For consistent comparison, we converted all types and routes of administration of opioid doses into MME.15
The consumption of intraoperative sevoflurane, use of intraoperative antihypertensive drugs, assessment of postoperative pain in different periods, including the post-anesthesia care unit (PACU) and the ward at 24 h, MME consumption in the PACU and ward, satisfaction with anesthesia, length of hospitalization, and incidence of PONV were all established as secondary outcomes. Data on sevoflurane consumption was collected from the electronic data recorded in our anesthesia machine, including Carestation 620 (GE Datex-Ohmeda, Madison, WI, USA), Avance (GE Datex-Ohmeda, Madison, WI, USA), S/5 ADU (GE Datex-Ohmeda, Madison, WI, USA), and Primus (Drägerwerk AG, Lübeck, Germany). The pain assessment in the ward 24 h after surgery was divided into “at rest” and “during movement”. The VAS score (10-cm scale from 1 to 10; 0, no pain; 10, worst possible pain) was used to assess postoperative pain response in the study. VAS scores and opioid consumption in the ward were evaluated and recorded by well-trained nurse anesthetists at 24 h after surgery. Furthermore, 24 h after the operation, we routinely obtained a satisfaction questionnaire based on the patient’s experience with anesthesia, with a score of 1–5 (1 = poor, 2 = fair, 3 = average, 4 = good, and 5 = excellent).
Statistical Analysis
Categorical variables, such as sex, ASA physical status, and antiemetic use, are presented as raw numbers or percentages. Chi-square or Fisher’s exact test was used to compare the groups. Continuous numeric data were tested using the Student’s t-test (normality) or Mann−Whitney U-test (non-normality). All numerical data are presented as median (25–75%). SPSS® (version 22.0; IBM® Corp., Armonk, NY, USA) was used for all statistical analyses. Statistical significance was set at P < 0.05. The sample size was determined based on Kreutziger et al,16 who had demonstrated that ISB reduced the total MME of shoulder reduction by 85%. Assuming alpha = 0.05 and power = 0.90, 60 people in each group are determined, resulting in a total sample size of 120.
Results
In total, 74 and 68 of the 142 enrolled patients were assigned to the preoperative UISB and control groups, respectively (Figure 1). Table 1 summarizes the demographic characteristics and comorbidities of the study groups. Sex, body weight, ASA physical status, duration of anesthesia, and comorbidities, including hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), congestive heart failure (CHF), cerebrovascular disease, cerebrovascular accident (CVA), end-stage renal disease (ESRD), and cancer, were not significantly different between the groups (Table 1). However, the age distribution in both groups was statistically different (p = 0.033), and the median was 60.5 and 56.5 in the preoperative UISB and control groups, respectively.
Table 1.
Demographic and Clinical Characteristics
| Variables (Unit) | N (%) /Median (IQR) | Control Group (n = 68) | Preoperative UISB Group (n = 74) | p-value |
|---|---|---|---|---|
| Sex (female/male) | 70/72 | 38/30 | 32/42 | 0.132 |
| Age (years) | 58.0 (50.0–65.0) | 56.5 (47.5–61.0) | 60.5 (50.8–67.3) | 0.033 |
| Body weight (kg) | 67.0 (59.8–75.3) | 64.5 (58.3–73.8) | 69.0 (60.8–78.0) | 0.164 |
| ASA | 0.327 | |||
| I | 9 (6.3%) | 6 (8.8%) | 3 (4.1%) | |
| II | 114 (80.3%) | 55 (80.9%) | 59 (79.7%) | |
| III | 19 (13.4%) | 7 (10.3%) | 12 (16.2%) | |
| Duration of anesthesia (h) | 2.17 (1.75–2.67) | 2.14 (1.78–2.65) | 2.25 (1.88–2.76) | 0.337 |
| Comorbidities | ||||
| Hypertension | 44 (31.0%) | 21 (30.9%) | 23 (31.1%) | 0.980 |
| DM | 18 (12.7%) | 7 (10.3%) | 11 (14.9%) | 0.413 |
| COPD | 1 (0.7%) | 0 (0.0%) | 1 (1.4%) | 1.000 |
| CAD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| CHF | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Cerebrovascular disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| CVA | 2 (1.4%) | 0 (0.0%) | 2 (2.7%) | 0.497 |
| ESRD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Cancer | 2 (1.4%) | 1 (1.5%) | 1 (1.4%) | 1.000 |
Note: Numeric values are expressed as medians (interquartile ranges) or numbers (%).
Abbreviations: IQR, interquartile range; UISB, ultrasound-guided interscalene nerve block; ASA, American Society of Anesthesiologists classification; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; ESRD, end-stage renal disease.
Intraoperative variables, such as intraoperative sevoflurane consumption, MME consumption, and the use of antihypertensive agents, were analyzed in both groups (Table 2). Intraoperative MME consumption was 48.1% lower in the preoperative UISB group than that in the control group (0.056 vs 0.108 mg/kg/h, p < 0.001). Similarly, the preoperative UISB group showed a statistically significant 14.8% reduction in intraoperative sevoflurane consumption compared to the control group (0.201 vs 0.236 mL/kg/h, p = 0.001). We also found that antihypertensive agents, including labetalol and nicardipine, were used significantly less in the preoperative UISB group than in the control group during surgery (p < 0.034).
Table 2.
Intraoperative Medication Administration in Both Groups
| Variables (Unit) | N(%)/Median (IQR) | Control Group (n = 68) | Preoperative UISB Group (n = 74) | p-value |
|---|---|---|---|---|
| Intraoperative MME* consumption (mg/kg/h) | 0.074(0.051–0.115) | 0.108(0.078–0.134) | 0.056 (0.036–0.074) | <0.001 |
| Sevoflurane consumption (mL/kg/h) | 0.221(0.177–0.274) | 0.236(0.202–0.294) | 0.201 (0.161–0.250) | 0.001 |
| Antihypertensive drug use and dosage | 0.034 | |||
| None | 110 (77.5%) | 49 (72.1%) | 61 (82.4%) | |
| 1 | 28 (19.7%) | 15 (22.0%) | 13 (17.6%) | |
| 2 | 4 (2.8%) | 4 (5.9%) | 0(0.0%) | |
| Labetalol (mg) | 11 (0–0) | 2.5 (0–2.5) | 0 (0–2.5) | 0.087 |
| Nicardipine (mg) | 25 (0–0) | 1.0 (0–1.0) | 0 (0–1.0) | 0.183 |
Notes: Numeric values are expressed as medians (interquartile ranges) or numbers (%). *Opioid consumption was converted to MME.
Abbreviations: IQR, interquartile range; UISB, ultrasound-guided interscalene nerve block; MME, morphine milligram equivalent.
As the patients were sent to the PACU, the preoperative UISB group demonstrated lower MME consumption (0.007 [0.001–0.012] mg/kg vs 0.015 [0.007–0.023] mg/kg, p = 0.055) than the control group. However, the difference was not statistically significant (Table 3). MME consumption in the ward also did not show significant differences between the two groups (p = 0.761). The preoperative UISB group showed satisfactory postoperative analgesia compared to the control group (VAS score 0.5 [0–1.0] vs 3.0 [3.0–4.0], p < 0.001) in the PACU. After returning to the ward, the preoperative UISB group also showed statistically satisfactory VAS scores during movement (2.0 [1.0–2.0] vs 3.0 [3.0–4.0], p < 0.001) and at rest (1.0 [0–1.0] vs 1.0 [1.0–2.0], p < 0.001) at 24 h after surgery. We also analyzed the incidence of PONV, which was classified as postoperative dizziness, postoperative nausea (PON), postoperative vomiting (POV), and the drug class of antiemetics used in the PACU. However, there were no statistically significant differences in postoperative dizziness (P = 0.848), PON (P = 0.607), POV (P = 0.311), or antiemetic use (P = 0.696) between the two groups in the PACU. Other variables, such as length of hospitalization (p = 0.432) and satisfaction with anesthesia (p = 0.472), did not show significant differences.
Table 3.
Postoperative Clinical Presentation in Both Groups
| Variables (Unit) | N (%) /Median (IQR) | Control Group (n = 68) | Preoperative UISB Group (n = 74) | p-value |
|---|---|---|---|---|
| PACU | ||||
| MME in the PACU (mg/kg) | 0.011 (0.006–0.015) | 0.015 (0.007–0.023) | 0.007 (0.001–0.012) | 0.055 |
| VAS score in the PACU | 1.0 (0.0–3.0) | 3.0 (3.0–4.0) | 0.5 (0.0–1.0) | <0.001 |
| Postoperative | ||||
| MME in the ward (mg/kg) | 0.050 (0.037–0.063) | 0.049 (0.030–0.068) | 0.050 (0.032–0.068) | 0.761 |
| VASM in the ward | 2.0 (1.0–3.0) | 3.0 (3.0–4.0) | 2.0 (1.0–2.0) | <0.001 |
| VASR in the ward | 1.0 (0.0–1.0) | 1.0 (1.0–2.0) | 1.0 (0.0–1.0) | <0.001 |
| Satisfaction of anesthesia | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 0.472 |
| Length of hospitalization (day) | 2.5 (1.5–3.0) | 2.5 (2.0–3.5) | 2.5 (1.5–3.0) | 0.432 |
| PONV | ||||
| Postoperative dizziness | 18 (12.7%) | 9 (13.2%) | 9 (12.2%) | 0.848 |
| PON | 3 (2.1%) | 2 (2.9%) | 1 (1.4%) | 0.607 |
| POV | 9 (6.3%) | 6 (8.8%) | 3 (4.1%) | 0.311 |
| Antiemetics | 0.696 | |||
| None | 62 (43.7%) | 31 (45.6%) | 31 (41.9%) | |
| 1 | 77 (54.2%) | 35 (51.5%) | 42 (56.8%) | |
| 2 | 3 (2.1%) | 2 (2.9%) | 1 (1.4%) |
Note: M, during movement; R, at rest.
Abbreviations: IQR, interquartile range; UISB, ultrasound-guided interscalene nerve block; PACU, post-anesthesia care unit; MME, morphine milligram equivalent; VAS, visual analog scale; PONV, postoperative nausea and vomiting; PON, postoperative nausea; POV, postoperative vomiting.
Discussion
The effectiveness of preoperative UISB in lowering opioids and anesthetics during surgery was further supported by statistically significant reductions of 48.1% and 14.8% in reducing the median intraoperative MME consumption (p < 0.001) and sevoflurane concentration (p = 0.001) compared with the control group. Through literature review, this study is the first to present and precisely calculate the intraoperative sevoflurane concentration in milliliters per kilogram and per hour; the preoperative UISB group also showed less fluctuation and better hemodynamic stability as evidenced by the use of fewer classes of antihypertensive drugs (p = 0.034). Our study also found reduced postoperative MME in the PACU (p = 0.055), although the difference was not statistically significant. In contrast, for the assessment of postoperative analgesia, the preoperative UISB group had significantly satisfactory VAS scores in the PACU and ward at 24 h after surgery.
Opioids have long been considered not only complementary to general anesthesia but are also widely used, even in the perioperative period. However, recent clinical studies have suggested that opioid-free or opioid-sparing anesthesia might provide equal and adequate analgesia,17–19 and reduce opioid-related adverse effects such as ileus, delirium, sleep disturbance,20 respiratory depression, PONV,18 hyperalgesia21 and even the promotion of malignancies.22 Zinboonyahgoon et al23 conducted a prospective observational study showing that truncal regional anesthesia, a peripheral nerve block, reduced postoperative opioid consumption for up to two weeks after breast surgery, especially among patients with higher baseline catastrophizing. Another systematic review indicated that high-dose intraoperative opioids, especially remifentanil, led to increased postoperative VAS scores and analgesic consumption, termed “secondary to opioid-induced hyperalgesia”.24
Multimodal analgesia is defined as the simultaneous use of different analgesics or techniques to target different receptors within nociceptive or neuropathic pathways, thereby reducing surgical stress responses, acute postoperative pain,25 and chronic postoperative pain.26,27 Different studies have demonstrated that surgical stimuli are managed with other hypnotics or analgesics, including nerve block, intravenous acetaminophen, ketamine, and alpha-2 agonists, such as dexmedetomidine or clonidine, magnesium, and lidocaine alone or in combination, as an opioid alternative to achieve opioid-free or opioid-sparing anesthesia.28,29 To prevent the side effects of opioids and to avoid the disadvantages of single-agent analgesia, opioid-sparing or opioid-free anesthesia is provided by applying multimodal techniques, especially a peripheral nerve block.
From the literature review of several trials, the UISB group showed better performance on the postoperative VAS score, and the effect lasted for at least 12 h.30–34 In our study, the preoperative UISB group showed better performance in VAS scores both in the PACU (0.5 [0–1.0] vs 3.0 [3.0–4.0], p < 0.001) and in the ward at 24 h after surgery (1.0 [0–1.0] vs 1.0 [1.0–2.0], p < 0.001, at rest; 2.0 [1.0–2.0] vs 3.0 [3.0–4.0], p < 0.001, during movement). UISB is a peripheral nerve block that is mostly performed after shoulder arthroscopy. Ultrasound-guided peripheral nerve block techniques have been developed in recent decades with improvements in sonographic resolution. Through the assistance of ultrasound, practitioners can perform real-time local injections, which reduces damage to vessels, nerves, or soft tissues35 and improves the success rate.36 However, some may have questioned whether ISB can lead to nerve injury, which is difficult to identify after shoulder arthroscopy. In 2012, Sviggum et al37 investigated 1655 participants who underwent elective total shoulder arthroplasty during a 15-year study period and concluded that the use of ISB does not increase the risk of peripheral nerve injury during total shoulder arthroplasty. In addition to Horner syndrome,38 a serious complication of ISB is associated with diaphragmatic paresis from a phrenic nerve block.39 Many attempts have been made to mitigate pulmonary dysfunction associated with regional anesthesia of the brachial plexus.9–12 Their results did not mention whether the patients had any symptomatic respiratory complications between the patient groups. However, there was no significant difference in symptomatic dyspnea within 24 h between the two groups. In our trial, only one patient who received preoperative UISB had respiratory discomfort but did not suffer from symptomatic hypoxemia.
A previous study showed that volatile anesthetics are strongly associated with PONV.40 Frauenknecht et al18 published a systematic review and meta-analysis of trials, including 23 randomized controlled trials in 2019, and showed that opioid-inclusive anesthesia was more strongly associated with PONV than opioid-free anesthesia. Consequently, reducing sevoflurane and intraoperative opioid consumption can reduce PONV. In our study, PONV occurred less frequently in patients who underwent preoperative UISB (PON, 1.4% vs 2.9%; POV, 4.1% vs 8.8%). However, the differences in postoperative dizziness (p = 0.848), PON (p = 0.607), and POV (p = 0.311) between the two groups were not significant. We speculate that these results may be due to routine PONV prophylaxis followed by recommendations based on patient risk factors.41
Our patients underwent UISB before general anesthesia without perioperative desaturation. No neurological or respiratory complications were found in the PACU or ward within 24 h after surgery. The preoperative UISB guarantees opioid-sparing and sevoflurane-sparing anesthesia for shoulder arthroscopy. However, this study has several limitations. Our study may have potential biases inherent to its retrospective design and could not predict whether the results would be certified in a prospective evaluation. The sample size was enough to perform further analyses and was limited to single-center observations. Some novel monitoring methods for nociception, such as nociceptive level (NOL®, Medasense Biometrics Ltd., Ramat Gan, Israel) monitors, are not yet available in Taiwan.42 Further prospective randomized controlled studies using NOL and BIS monitoring in each patient are still needed and may provide more precise guidance and evaluation of the consumption of intraoperative opioids and volatile anesthetics. This study did not consider the differences in surgical techniques and seniority among orthopedic surgeons. Finally, our results may only be applicable to arthroscopic shoulder surgery.
Conclusion
In this retrospective observational study, we concluded that preoperative UISB effectively reduced surgical stress without perioperative respiratory complications. Compared to the control group, the analgesic effect was also reflected in the preservation of intraoperative MME and sevoflurane consumption, with reductions of 48.1% and 14.8%, respectively. It also provides satisfactory intraoperative hemodynamic stability by significantly reducing the use of antihypertensives. For postoperative analgesia, the preoperative UISB group also showed better VAS scores in the PACU and ward 24 h after surgery. In summary, the preoperative UISB is a reliable adjuvant analgesic technique and a key factor in achieving opioid-sparing and sevoflurane-sparing anesthesia and multimodal analgesia during shoulder arthroscopy.
Acknowledgments
We appreciate the assistance of the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital.
Data Sharing Statement
The data presented in this study are available from the corresponding authors upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital (IRB number: 202101342B0), and will be conducted according to the Declaration of Helsinki.
Patient Consent for Publication
The Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital waived the need to obtain informed consent due to the retrospective nature of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99–104. doi: 10.1177/036354659502300117 [DOI] [PubMed] [Google Scholar]
- 2.Hurley ET, Maye AB, Thompson K, et al. Pain control after shoulder arthroscopy: a systematic review of randomized controlled trials with a network meta-analysis. Am J Sports Med. 2021;49(8):2262–2271. doi: 10.1177/0363546520971757 [DOI] [PubMed] [Google Scholar]
- 3.Hadi H, Tadros BJ, Kochhar T, Dhinsa BS. The role of interscalene brachial plexus block anaesthesia in arthroscopic shoulder surgery; a prospective study. J Clin Orthop Trauma. 2021;16:154–156. doi: 10.1016/j.jcot.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warrender WJ, Syed UAM, Hammoud S, et al. Pain management after outpatient shoulder arthroscopy: a systematic review of randomized controlled trials. Am J Sports Med. 2017;45(7):1676–1686. doi: 10.1177/0363546516667906 [DOI] [PubMed] [Google Scholar]
- 5.Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65(6):608–624. doi: 10.1111/j.1365-2044.2009.06231.x [DOI] [PubMed] [Google Scholar]
- 6.Hughes MS, Matava MJ, Wright RW, Brophy RH, Smith MV. Interscalene brachial plexus block for arthroscopic shoulder surgery: a systematic review. J Bone Joint Surg Am. 2013;95(14):1318–1324. doi: 10.2106/JBJS.L.01116 [DOI] [PubMed] [Google Scholar]
- 7.Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth Analg. 2015;120(5):1114–1129. doi: 10.1213/ANE.0000000000000688 [DOI] [PubMed] [Google Scholar]
- 8.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691–697. doi: 10.1001/jamasurg.2017.0898 [DOI] [PubMed] [Google Scholar]
- 9.Abdallah FW, Wijeysundera DN, Laupacis A, et al. Subomohyoid anterior suprascapular block versus interscalene block for arthroscopic shoulder surgery: a multicenter randomized trial. Anesthesiology. 2020;132(4):839–853. doi: 10.1097/ALN.0000000000003132 [DOI] [PubMed] [Google Scholar]
- 10.Auyong DB, Hanson NA, Joseph RS, Schmidt BE, Slee AE, Yuan SC. Comparison of anterior suprascapular, supraclavicular, and interscalene nerve block approaches for major outpatient arthroscopic shoulder surgery: a randomized, double-blind, noninferiority trial. Anesthesiology. 2018;129(1):47–57. doi: 10.1097/aln.0000000000002208 [DOI] [PubMed] [Google Scholar]
- 11.Hussain N, Goldar G, Ragina N, Banfield L, Laffey JG, Abdallah FW. Suprascapular and interscalene nerve block for shoulder surgery: a systematic review and meta-analysis. Anesthesiology. 2017;127(6):998–1013. doi: 10.1097/aln.0000000000001894 [DOI] [PubMed] [Google Scholar]
- 12.Kang R, Jeong JS, Chin KJ, et al. Superior trunk block provides noninferior analgesia compared with interscalene brachial plexus block in arthroscopic shoulder surgery. Anesthesiology. 2019;131(6):1316–1326. doi: 10.1097/ALN.0000000000002919 [DOI] [PubMed] [Google Scholar]
- 13.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi: 10.1213/ANE.0000000000000002 [DOI] [PubMed] [Google Scholar]
- 15.BPM Books. Back INPMH. 3rd ed. Cardiff, UK: BPM Books; 2001. [Google Scholar]
- 16.Kreutziger J, Hirschi D, Fischer S, Herzog RF, Zbinden S, Honigmann P. Comparison of interscalene block, general anesthesia, and intravenous analgesia for out-patient shoulder reduction. J Anesth. 2019;33(2):279–286. doi: 10.1007/s00540-019-02624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiore JF, Olleik G, El-Kefraoui C, et al. Preventing opioid prescription after major surgery: a scoping review of opioid-free analgesia. Br J Anaesth. 2019;123(5):627–636. doi: 10.1016/j.bja.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 18.Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. 2019;74(5):651–662. doi: 10.1111/anae.14582 [DOI] [PubMed] [Google Scholar]
- 19.Olausson A, Svensson CJ, Andrell P, Jildenstal P, Thorn SE, Wolf A. Total opioid-free general anaesthesia can improve postoperative outcomes after surgery, without evidence of adverse effects on patient safety and pain management: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2022;66(2):170–185. doi: 10.1111/aas.13994 [DOI] [PubMed] [Google Scholar]
- 20.O’Gara BP, Gao L, Marcantonio ER, Sleep SB. Pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. 2021;135(6):1132–1152. doi: 10.1097/ALN.0000000000004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558–1568. doi: 10.1016/S0140-6736(19)30430-1 [DOI] [PubMed] [Google Scholar]
- 22.Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110(6):1636–1643. doi: 10.1213/ANE.0b013e3181de0ab6 [DOI] [PubMed] [Google Scholar]
- 23.Zinboonyahgoon N, Vlassakov K, Lirk P, et al. Benefit of regional anaesthesia on postoperative pain following mastectomy: the influence of catastrophising. Br J Anaesth. 2019;123(2):e293–e302. doi: 10.1016/j.bja.2019.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Hoogd S, Ahlers SJ, van Dongen EP, et al. Is intraoperative remifentanil associated with acute or chronic postoperative pain after prolonged surgery? An update of the literature. Clin J Pain. 2016;32(8):726–735. doi: 10.1097/AJP.0000000000000317 [DOI] [PubMed] [Google Scholar]
- 25.Chen YK, Boden KA, Schreiber KL. The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: a narrative review. Anaesthesia. 2021;76(Suppl 1):8–17. doi: 10.1111/anae.15256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermanns H, Hollmann MW, Stevens MF, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123(3):335–349. doi: 10.1016/j.bja.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 27.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X [DOI] [PubMed] [Google Scholar]
- 28.Beloeil H. Opioid-free anesthesia. Best Pract Res Clin Anaesthesiol. 2019;33(3):353–360. doi: 10.1016/j.bpa.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 29.Gabriel RA, Swisher MW, Sztain JF, Furnish TJ, Ilfeld BM, Said ET. State of the art opioid-sparing strategies for post-operative pain in adult surgical patients. Expert Opin Pharmacother. 2019;20(8):949–961. doi: 10.1080/14656566.2019.1583743 [DOI] [PubMed] [Google Scholar]
- 30.Nisar A, Morris MW, Freeman JV, Cort JM, Rayner PR, Shahane SA. Subacromial bursa block is an effective alternative to interscalene block for postoperative pain control after arthroscopic subacromial decompression: a randomized trial. J Shoulder Elbow Surg. 2008;17(1):78–84. doi: 10.1016/j.jse.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 31.Cho SS, Lee DH, Yu EY, Yoon MS, Yoon MS. The effect of preoperative interscalene block using low-dose mepivacaine on the postoperative pain after shoulder arthroscopic surgery. Korean J Pain. 2009;22(3):224–228. doi: 10.3344/kjp.2009.22.3.224 [DOI] [Google Scholar]
- 32.Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103(3):428–433. doi: 10.1093/bja/aep173 [DOI] [PubMed] [Google Scholar]
- 33.Lee HY, Kim SH, So KY, Kim DJ. Effects of interscalene brachial plexus block to intra-operative hemodynamics and postoperative pain for arthroscopic shoulder surgery. Korean J Anesthesiol. 2012;62(1):30–34. doi: 10.4097/kjae.2012.62.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singelyn FJ, Lhotel L, Fabre B. Pain relief after arthroscopic shoulder surgery: a comparison of intraarticular analgesia, suprascapular nerve block, and interscalene brachial plexus block. Anesth Analg. 2004;99(2):589–92, table of contents. doi: 10.1213/01.ANE.0000125112.83117.49 [DOI] [PubMed] [Google Scholar]
- 35.Suresh S, Ecoffey C, Bosenberg A, et al. The European Society of Regional Anaesthesia and Pain Therapy/American Society of Regional Anesthesia and Pain Medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg Anesth Pain Med. 2018;43(2):211–216. doi: 10.1097/AAP.0000000000000702 [DOI] [PubMed] [Google Scholar]
- 36.Kapral S, Greher M, Huber G, et al. Ultrasonographic guidance improves the success rate of interscalene brachial plexus blockade. Reg Anesth Pain Med. 2008;33(3):253–258. doi: 10.1016/j.rapm.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 37.Sviggum HP, Jacob AK, Mantilla CB, Schroeder DR, Sperling JW, Hebl JR. Perioperative nerve injury after total shoulder arthroplasty: assessment of risk after regional anesthesia. Reg Anesth Pain Med. 2012;37(5):490–494. doi: 10.1097/AAP.0b013e31825c258b [DOI] [PubMed] [Google Scholar]
- 38.Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040–2046. doi: 10.2106/JBJS.K.01418 [DOI] [PubMed] [Google Scholar]
- 39.Choromanski DW, Patel PS, Frederick JM, Lemos SE, Chidiac EJ. The effect of continuous interscalene brachial plexus block with 0.125% bupivacaine vs 0.2% ropivacaine on pain relief, diaphragmatic motility, and ventilatory function. J Clin Anesth. 2015;27(8):619–626. doi: 10.1016/j.jclinane.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 40.Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–753. doi: 10.1093/bja/aes276 [DOI] [PubMed] [Google Scholar]
- 41.Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–448. doi: 10.1213/ANE.0000000000004833 [DOI] [PubMed] [Google Scholar]
- 42.Meijer F, Honing M, Roor T, et al. Reduced postoperative pain using Nociception level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. Br J Anaesth. 2020;125(6):1070–1078. doi: 10.1016/j.bja.2020.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]