Abstract
1. The spatial organization of a population can influence the spread of information, behaviours, and pathogens. Territory size and territory overlap, components of spatial organization, provide key information as these metrics may be indicators of habitat quality, resource dispersion, contact rates, and environmental risk (e.g., indirectly transmitted pathogens). Furthermore, sociality and behaviour can also shape space use, and subsequently, how space use and habitat quality together impact demography.
2. Our study aims to identify factors shaping the spatial organization of wildlife populations and assess the impact of epizootics on space use. We also use network analysis to describe spatial organization and connectivity of social groups.
3. Here, we assessed the seasonal spatial organization of Serengeti lions and Yellowstone wolves at the group level. We examine the factors predicting mean territory size and mean territory overlap for each population using generalized additive models. We further explore the mechanisms by which disease perturbations could cause changes in spatial organization.
4. We demonstrate that lions and wolves were similar in that group-level factors, such as number of groups, shaped spatial organization more than population-level factors, such as population density. Factors shaping territory size were slightly different than factors shaping territory overlap; for example, wolf pack size was an important predictor of territory overlap, but not territory size. Lion spatial networks were more highly connected, while wolf spatial networks varied seasonally. We found that resource dispersion may be more important for driving territory size and overlap for wolves than for lions. Additionally, canine distemper epizootics may alter lion spatial organization, highlighting the importance of including behavioural and movement ecology in studies of pathogen transmission dynamics.
5. We provide insight about when we might expect to observe the impacts of resource dispersion, disease perturbations, and other ecological factors on spatial organization. Our work highlights the importance of monitoring and managing social carnivore populations at the group level. Future research should elucidate the complex relationships between demographics, social and spatial structure, abiotic and biotic conditions, and pathogen infections.
INTRODUCTION
The spatial organization of free-ranging animal populations, such as territory size and overlap (Arden-Clarke, 1986; Belcher & Durrant, 2004), emerges from patterns in resource availability and distribution (Macdonald, 1983), and can drive a population’s demographic rates (Cantor et al., 2012; Pasinelli et al., 2014), consumer-resource dynamics (Murdoch et al., 2003), and disease transmission (Hess et al. 1996; Cross et al. 2005). Similarly, mating systems (Gosden & Svensson, 2008), kin relations (VanderWaal et al., 2009), and human pressures (Lesmerises et al., 2013) can also influence a population’s spatial organization. How predators are distributed on the landscape has repercussions for competing predators and prey (Kittle et al., 2016; Kohl et al., 2019). Thus, recognizing the ecology of observed spatial patterns of a population is important for understanding population dynamics and relationships among species in a community. Here, we ask: which mechanisms drive spatial organization in territorial carnivore populations? We investigate this question by leveraging 60 cumulative years of observations to examine the covariates influencing territory size and territory overlap of African lions (Panthera leo) in Serengeti National Park and gray wolves (Canis lupus) in Yellowstone National Park.
Spatial organization is commonly characterized by territoriality, which is a product of both biotic and abiotic processes in an environment. A territory is an area encompassing vital resources for an individual’s fitness (Macdonald, 1983); this predominantly includes an area to give birth and raise young (e.g., nests, burrows) and food resources. There is a range in territorial behaviour, from acute protection of a transient resource (e.g., speckled wood butterfly: Davies, 1978), to intense defense year-round (e.g., Eurasian otters: Erlinge, 1968; gray wolves: Mech, 1994; African lions: Heinsohn, 1997).
Literature from the last few decades emphasizes how food availability predominantly influences a population’s spatial organization (Lack, 1954; Simon et al., 1975; Ostfeld et al., 1985; Davies & Hartley, 1996; Fuller et al., 2003). However, sociality and behaviour can also shape space use, and space use and habitat quality together can impact demography (Alberts, 2019; Thompson, 2019). Research focusing on the relationship between spatial configuration, sociality, and the abiotic and biotic environment has been a central debate in ecology, and recent work highlights that spatial and social organization cannot be ignored when considering population dynamics or individual fitness (Armansin et al., 2019; He et al., 2019; Paniw et al., 2019). For example, Yellowstone wolves infected with sarcoptic mange have substantially higher survival when they are associated with larger packs and there are higher prey densities (Almberg et al., 2015); however, access to prey and hunting success is influenced by territory location and topography (Kauffman et al., 2007; Nelson et al., 2012). Thus the survival of an infected wolf is necessarily linked to the spatial characteristics of it’s pack. For highly territorial social species, such as lions and wolves, social behaviour like territory defense, infanticide, and scent marking plays an important role in maintaining boundaries and limiting the amount of space that groups share (Packer et al., 1990; Spong & Creel, 2004; Cubaynes et al., 2014; Smith et al., in press). Here we explore variables shaping space use more comprehensively.
Spatial organization may also be affected by biotic and abiotic perturbations, including extreme weather events (Loe et al., 2016; Paniw et al., 2019), harvest (Woodroffe et al., 2006), or infectious disease epizootics (reviewed in Binning et al., 2017). For instance, wolves decrease their daily distance traveled as the severity of their mange infection intensifies, likely because of the increasing energetic demands of thermoregulation as hair loss increases (Cross et al., 2016). Conversely, parasites can manipulate intermediate hosts to travel to habitats where they are more likely to be predated by the definitive hosts – these are areas that hosts often avoid when uninfected (Lafferty & Morris, 1996; Thomas et al., 2002). Thus the consequences of an infection may manifest in changes in space use; for example, male wood mice infected with nematode parasites have larger territories than uninfected males (Brown et al., 1994), and female Tasmanian devils decreased their home range size and overlap following a wide-spread facial tumor disease outbreak (Comte et al., 2020). The relationship between space use and epizootics is a new area of research for territorial, social carnivores.
Network analysis can spatial organization (Croft et al., 2011). When groups in a population (i.e., nodes) interact with each other, these relationships can be quantified as ‘edges’ in a network, and edges can be weighted based on frequency, intensity, duration, or type of interaction. Networks using spatial overlap to form edges connecting nodes have demonstrated utility in elucidating nonrandom relationships (Perkins et al., 2009; Godfrey et al., 2010; VanderWaal et al., 2014), including the identification of parasite transmission pathways via the relationship between home range overlap and the spatiotemporal spread of parasites (i.e., Fenner et al., 2011). Here, we use a network approach to describe population-level connectivity for lion prides in Serengeti and wolf packs in Yellowstone.
These exceptionally well-monitored lion and wolf populations provide a unique opportunity to compare and contrast the spatial organization of group-living carnivores that occur in similar ecosystems and with similar ecology. Serengeti and Yellowstone are both vast expanses of protected land that contain suites of carnivores, mesopredators, and ungulate prey. Both systems experience extreme seasonality, which drives migratory ungulate and predator movement. Lions and wolves are apex predators that live in highly territorial, familial groups (i.e., prides and packs, respectively). Generally, larger groups have higher fitness, higher hunting success, and access to better quality habitat (lions: Packer et al., 1990; Mosser & Packer, 2009; wolves: Tallents et al., 2012; Stahler et al., 2013; MacNulty et al., 2014; but see Kittle et al., 2015). Serengeti lions and Yellowstone wolves have both experienced population-wide exposure to canine distemper virus (CDV) – lions in 1977, 1981, 1994, 1999 and 2008, and wolves in 1999, 2005, and 2008 (Packer et al., 1999; Viana et al., 2015; Cross et al., 2018).
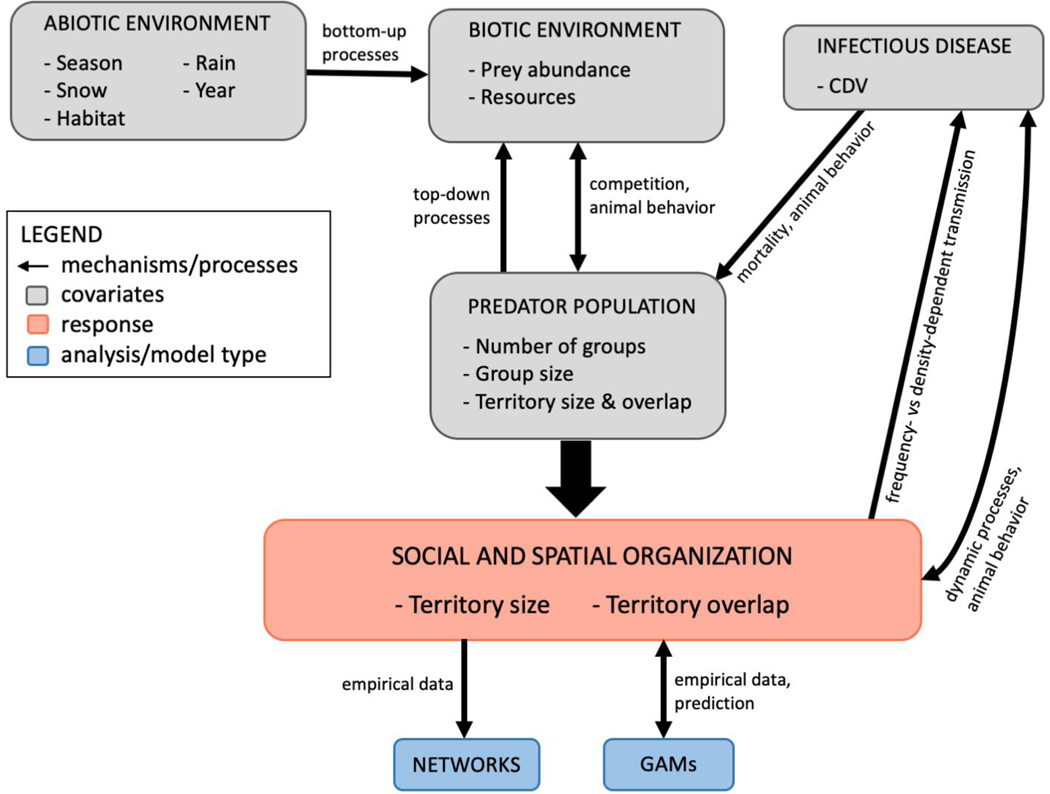
We assess how group-living, resource abundance, epizootics, and environmental factors drive the spatial organization in territorial carnivore populations. First, we describe the lion pride and wolf pack spatial organization using network analysis; second, we examine the group, population, and seasonal covariates influencing these populations’ spatial organization using generalized additive models. Figure 1 displays a workflow connecting the covariates we used in our models and their potential relationships, potential mechanisms and processes underlying those relationships, and our model types.
Figure 1.
Workflow diagram displaying how our dataset (covariates, gray boxes) can relate to spatial organization (response variables, pink box) and the selected analyses (blue boxes). Arrows represent mechanisms or processes that may link the variables and models; direction of the arrow implies the direction of the process.
METHODS
African Lion and Gray Wolf Sociality
Lions and wolves reside in familial social groups. African lion prides are comprised of related females and their offspring typically less than three years old (Pusey & Packer, 1987; Packer et al., 1991). Prides range in size from two to 21 adult females (Pusey & Packer, 1987). Prides are highly territorial and, although rare, interpride interactions are aggressive and can be deadly (Schaller, 1972; Grinnell et al., 1995). Male lions live in smaller groups (1–9 individuals) and fight for access to females; males may have access to more than one female pride territory at a time (Bygott et al., 1979; Pusey & Packer, 1987). Male lions disperse from their natal prides before sexual maturity, and female lions may leave if the pride grows too large and may occupy a neighboring territory, but territories remain exclusive (VanderWaal et al., 2009).
Wolf packs are typically comprised of a breeding pair, their dependent offspring, and a few unrelated individuals. Packs typically range from three to 12 members. Wolves disperse from their packs when the packs is too large to be supported, and to avoid inbreeding packs rely on the emigration of related individuals out of their natal packs and the immigration unrelated individuals into new packs (Mech & Boitani, 2003; VonHoldt et al., 2008). On average, dispersers in the Rocky Mountains relocated about 90–100 km away from their previous pack’s territory (straight-line distance, Jimenez et al., 2017). Wolf pack territories are distinct and interpack conflicts are often aggressive and can result in death, even between related individuals from different packs (Cubaynes et al., 2014; Cassidy et al., 2015).
Study Sites and Location Data
Serengeti National Park (Serengeti) is a vast protected area (14,750-km2) located in northern Tanzania and can be roughly divided into grassland plains (southern portion) and woodland (northern portion) habitats. The Serengeti ecosystem is recognized for the massive annual wildebeest (Connochaetes taurinus) and zebra (Equus burchelli) migrations where ungulates move south in the wet season and north in the dry season. Buffalo (Syncerus caffer) are a common resident ungulate, making them an important dry season food source. Suites of predators also reside in Serengeti, including African lions (Panthera leo).
The lion population increased from the start of monitoring in 1966 to 2014; increases in population size occurred every 5–10 years as numbers jump from a lower equilibrium value to a higher value, with one notable decrease in population size which began after the 1994 canine distemper virus outbreak and persisted for five years (Packer et al., 2005; Fig. S1A). The canine distemper virus (CDV) epizootic in 1994 led to a one-third reduction in the lion population size across all age classes (Roelke-Parker et al., 1996). There were also multiple periods of widespread CDV exposure (1976, 1981, 1993/1994, 1998, 2007), determined via opportunistic serological testing, where population wide declines were not observed (Munson et al., 2008; Viana et al., 2015). We considered the five CDV exposure periods as CDV epizootics.
Lions were monitored weekly (1973–2014) by recording the locations of individually identified lions; the years 1966–1972 were excluded due to a low number of sightings and few monitored prides. These sightings were either opportunistic or aided by the use of very-high-frequency (VHF) telemetry. Individuals were members of prides with known compositions and all lions within observed groups were recorded. In order to retain a focus on the comparison of group-living social carnivores, we did not include solitary lions in this analysis.
Yellowstone National Park (Yellowstone) is a large protected area (8991-km2) characterized by long, harsh winters when elk (Cervus canadensis) and bison (Bison bison) migrations occur in northern Yellowstone. Elk are one of the most abundant ungulates and gray wolves’ (Canis lupus) main prey source (Metz et al., 2012). Central and southern Yellowstone are referred to as the Interior, which is characterized by higher elevations, higher snow levels, and is more heavily forested.
Wolves were reintroduced into Yellowstone in 1995, decades after extirpation. Their population increased rapidly, peaking in 2008 at nearly 200 wolves, before it declined and subsequently stabilized at about 90 wolves between 2010–2016 (Smith et al., 2017a; Fig. S1B). The wolf population experienced three major CDV epizootics between 1997–2016 that were associated with juvenile mortality up to ~80%: 1999, 2005, and 2008 (Almberg et al., 2009). CDV epizootic years were determined using a combination of juvenile seropositivity, observed host symptoms and mortality, and, for a subset of individuals suspected to be infected during outbreaks, the confirmation of CDV infection was achieved via PCR (Almberg et al., 2009).
Wolves were monitored using global positioning system (GPS) collar locations and visual observations aided by VHF collars. Wolves were located approximately biweekly via aerial monitoring throughout the year (1997–2016; Smith & Bangs, 2009; Kohl et al., 2019). Individually identifiable wolves were members of packs with known membership and composition. We excluded any singular wolves or transient groups from our analysis, defined as groups surviving <2 consecutive months. We also removed the years 1995–1996 when the population was being reintroduced.
Calculating Territory Size and Overlap
All individual locations were aggregated by group (Kittle et al., 2015). Kernel density estimation used to to construct seasonal territories with a limited extent and the standard degree of smoothing. Peripheral locations were discarded (5%) to calculate each group’s 95% territory size and removed extreme outlier positions from territories that were probably incorrectly recorded locations; we then averaged the 95% kernel densities for all observed prides/packs over the season to create a covariate called territory size. Seasons were considered to be six months long based on mean temperature and precipitation (rain in Serengeti and snow in Yellowstone). In Serengeti, the wet season was defined as November–April and the dry season was May–October (based on monthly rainfall totals; Weather Atlas, 2020). In Yellowstone, winter was defined as October–March and summer was April–September.
Territories are often inflated with small sample sizes (i.e., small number of individual location coordinates), therefore we assessed whether this was true for the wolf and lion datasets and found that wolf and lion territory size was invariant to sample size. In general, territory size and overlap were fairly stable as the number of locations approached 50, and were fairly stable or slightly declined as the number of locations exceeded 50 (Supporting Information Effects of sample size on network estimation, Fig. S3, S4). There is a trade-off between retaining enough groups to realistically represent the population while ensuring the groups were appropriately sampled (Cross et al., 2012; Gilbertson et al., 2020). We chose to retain most extant groups in the population even if they had few locations. Thus, we discarded any groups with <20 locations per season.
To ensure we compared territories with approximately uniform amounts of data, we randomly selected up to 400 locations annually from each group without replacement, aiming for 200 locations per group per season. Estimating a territory using random sampling reduces autocorrelation and provides reasonably small bias and exceptional precision when the smoothing parameter is appropriate (Fieberg, 2007). Lion locations were occasionally identical due to sampling methodology, prohibiting territory estimation. Therefore, if a pride contained <11 unique locations in a given season, we expanded the territory to 1-km2 around those locations. This expansion allowed for territory and overlap estimation and retention of extant prides. We do not expect territories to differ according to collar type or time of day (Demma & Mech, 2011; lions sleep for ~22 hours per day).
Territory overlap was estimated using the volume of intersection between each group. The volume of intersection index is a common overlap metric that ranges from 0 (complete exclusion) to 1 (complete overlap) while it accounts for the three-dimensional shape of the territory kernel (Millspaugh et al., 2004). Volume of intersection can be interpreted as the proportion of space shared between two groups within a season. We summed all overlap measurements per group and averaged them to calculate territory overlap, which describes how much space is shared between that group and neighboring groups in the population.
All group-level attributes were averaged by season before analyses. In summary, the data were calculated for each group, per season, per year, and then averaged. Our two response variables (mean territory size and mean territory overlap) were calculated in the R package adehabitatHR (Calenge, 2006) with the functions kernelUD (‘kern=bivnorm’), getverticeshr, and kerneloverlap. Although not a main goal of this manuscript, we also assessed the validity of using territory overlap as a surrogate for direct contact in the lion and wolf populations (See SI Spatial overlap as a surrogate for direct contact).
Network Analysis
We used network analysis to more broadly describe how well the populations were spatially connected (Fig. 2, S2). Weighted edges were constructed using territory overlap; networks were weighted and unfiltered, which is generally preferred to removing edges below an arbitrary weight threshold, or binary edges (Farine & Whitehead, 2015).
Figure 2.
Wolf seasonal territories (85% isopleths of kernel density estimates for visualization purposes) for packs in summer (top left) and winter (bottom left) for the year 2011, where the thin black outline is the boundary of Yellowstone National Park. The right column shows the corresponding seasonal networks. Each pack was assigned a color, edge width represents total overlap between territories, and network configurations reflect approximate spatial organization (R package igraph, Csardi & Nepusz, 2006). Figure S2 is the equivalent figure for lions.
We explored the population attributes: network size (total number of groups), mean degree (mean number of groups each group spatially overlaps with), proportion disconnected (the proportion of groups that did not share any space with other groups), network density (the proportion of possible edges that are realized), betweenness centrality (how important a group is in spatially connecting the population based on the shortest paths between groups), and closeness centrality (how important a group is in spatially connecting the population based on its network position in relation to other groups). Centrality measures were weighted by territory overlap (volume of intersection ≥0.001), and density and degree were unweighted; attributes were calculated in the R package igraph (Csardi & Nepusz, 2006). Again, group-level attributes were averaged across each season before network analyses (i.e., centrality, degree).
Statistical Analysis
Covariates considered to be potentially important in shaping territory size and territory overlap among groups included: population density (population count within study areas), group size (group size observed), number of groups (total number of distinct groups observed within study areas), season (wolf: summer/winter, lion: dry/wet), rainfall (lions) or snow level (wolf), habitat (lion: woodlands/plains, wolf: northern range/interior), CDV epizootic (number of years since the last period of exposure), and prey population densities (annual counts within study areas). It is likely that territory size and territory overlap are not independent, and so we used these variables as covariates in respective models: territory overlap was used as a predictor of territory size, and territory size was used as a predictor of territory overlap. Prey population sizes from the Serengeti were only available at the ecosystem level for buffalo, Thomson’s gazelle, wildebeest, and zebra from aerial surveys conducted every 2–6 years. These parameters were obtained from previously published studies; see Supporting Information Table S1 for details on each covariate.
We used Generalized Additive Models (GAMs) to understand how our covariates shaped territory size and overlap, while accounting for temporal autocorrelation. Prior to analysis, we screened for variable collinearity, and, in the lion dataset, excluded population density as it was strongly collinear with the number of prides on the landscape (Pearson’s correlation coefficient = 0.79). To screen for variable interactions to include in our GAM models, we employed two machine learning algorithms (using the default parameters): support vector machines (Hastie et al., 2004) and boosted regression trees (Elith et al., 2008). We used the support vector machines for the wolf dataset because they are better suited for dealing with smaller datasets, and boosted regression trees for the lion dataset because they are more powerful and require a larger dataset. These approaches account for missing data, which was common with some lion variables (i.e., prey species abundance). Any covariate or interaction of no predictive value in either machine learning model was excluded from subsequent GAMs, including all prey variables from the lion models. See Fountain-Jones et al., (2019) for more detail about this analytical pipeline.
We anticipated temporal trends would account for a notable portion of the variation in territory size and overlap. We were also particularly interested in the effect of CDV epizootics on spatial organization. Therefore, we constructed and compared three GAMs to determine what covariates best captured variability in territory size and territory overlap. Our models were (in order of increasing complexity):
temporal trend only (temporal trend model, ‘null’ model),
temporal trend with CDV epizootic as a covariate (temporal trend + CDV model), and
the complete model with all covariates considered to be of predictive value in the machine learning models (complete model).
The seasonal component (wet/dry or winter/summer) was modeled as a fixed effect. Tensor products were used as the smoothing functions for each covariate because the covariates were all on different scales. For the temporal trend model (model i) we allowed the year to be a smoothed Gaussian process basis function (tensor product) that is recommended for variables with temporal correlation (Wood & Augustin, 2002). Then, CDV epizootic was added as a numeric covariate (model ii). Finally, we constructed the complete model (model iii) adding the covariates listed in Supporting Information Table S1 (excluding population density in the lion model). We used the Gaussian process basis function for population density, number of groups, group size, and elk abundance as these were also temporally autocorrelated. The default basis functions were used for the remaining covariates.
Each model was fitted using restricted maximum likelihood and models were evaluated using the ‘gam.check’ function. P values were calculated using the anova function since season was a covariate in all of our models. We used the package mgcv (Wood & Augustin, 2002) to construct each GAM. Model selection was performed using the integrated penalized spline approach (Wood & Augustin, 2002) and the temporal trend model and temporal trend + CDV model were compared to the complete model based on AIC values. See Supporting Information and Data Accessibility statement for further details.
All analyses were performed in Program R version 3.6.1 (2017).
RESULTS
Network Analysis
The lion dataset comprised 42 years (1973–2014). The number of prides per season ranged from 2–26, with the lowest counts in the earlier years. Prides had, on average, 6.3 members (range: 2–21, standard deviation = 3.59). Most prides shared space to some extent with other prides – on average each pride overlapped with 7–9 neighboring prides (Fig. 3A), and centrality (Fig. 3B,C) and overlap estimates were moderate (Table S10). Closeness and betweenness centrality, and proportion disconnected (Fig. 3E) did not differ substantially between the wet and dry seasons. Mean degree (Fig. 3A) and network density (Fig. 3D), however, were slightly higher in the wet season.
Figure 3.
Density distributions of five network attributes: (A) mean degree, (B) betweenness centrality, (C) closeness centrality, (D) network density, (E) proportion disconnected, by season (wet/winter = gray, dry/summer = black) for lions in Serengeti National Park (1973–2014, left column) and wolves in Yellowstone National Park (1997–2016, right column).
The wolf dataset comprised 20 years (1997–2016). The number of packs per season ranged from 4–16, with the lowest counts in earlier and later years. Packs had, on average, 10.0 members (range: 2–37; standard deviation = 5.52). Most packs spatially overlapped with others; spatial overlap was greater in winter, which corresponds to a higher network density (Fig. 3D). Each pack’s territory overlapped with 3–5 neighboring packs on average (Fig. 3A). Closeness centrality was low (Fig. 3C), indicating that there is a large distance between packs. There were stark differences in population attributes in the summer versus winter (Fig. 2), with a higher mean degree (Fig. 3A), density (Fig. 3D), and fewer disconnected packs (Fig. 3E) in winter (Table S10), but betweenness centrality was more consistent throughout the year (Fig. 3B).
Lions and wolves both experienced seasonal decreases in the proportion of groups spatially connected – wet season in Serengeti and winter in Yellowstone (Fig. 3E). Lion prides are more disconnected in the wet season likely because prey and water are spread out across the landscape, whereas in the dry season, lions congregate at watering holes or at good hunting locations with reliable prey sources (Hopcraft et al., 2005; Kittle et al., 2016). Wolves are more disconnected in summer likely because they purposefully den away from other packs, and prey are more spread out on the landscape, thus reducing the need to hunt in the same areas (Kauffman et al., 2007).
In both populations, there was a wide spread of seasonal betweenness centrality values, and about half of the values were zero, indicating that peripheral groups were highly connected in some seasons but not others (Fig. 3B). The distribution of network density was fairly predictable within a season, but differed between seasons (Fig. 3D); a greater proportion of edges were realized in the wet (lions) and winter (wolves) seasons. In general, the lion population had a higher centrality and mean degree than the wolf population (Table S10), and some prides served as central hubs in the network while other prides were weakly connected. The wolf population in Yellowstone is characterized by many stable but weak connections among packs, with a few important packs serving as central hubs. In both populations, the most connected groups were either the most centrally located, or were groups with a small territory that was predominantly shared with neighboring groups.
Statistical Modeling Results
Lion territories were larger when there were more prides on the landscape and as overlap among prides increased. Mean territory size also grew the longer it had been since a CDV epizootic, especially when five or more years had passed (Fig. 4A, S8a). Territories were slightly larger in the wet season, and territories generally became larger through the time series. The complete model explained significantly more deviance (82.5%) in the data compared to the temporal trend model (25.4%) or temporal trend + CDV model (49.7%); thus the complete model was our top model (Table S2a–S5a).
Figure 4.
The percentage of deviance explained by each term used in the (A,B) territory size and (C,D) territory overlap complete models (model iii) for (A,C) lions in Serengeti and (B,D) wolves in Yellowstone. Color indicates the scale or type of term: red = group-level, gray = population-level, blue = temporal trends. Deviance explained was calculated as the difference between the complete model and the model with the covariate of interest removed; the smoothing terms from the complete model were used for continuity.
The amount of territory overlap between a lion pride and neighboring prides increased when the pride had more members, a larger territory size, and when there were more prides on the landscape. Mean territory overlap was also higher the longer it had been since a CDV epizootic, especially when four or more years had passed (Fig. 4C, S8b). The complete model was the top model (Table S5b) and explained a large amount of deviance in pride-level connectivity (89.5%). The temporal trend model explained 39.6% of the deviance, and the temporal trend + CDV model explained 63.8% of the deviance (Table S3b, S4b).
The relationship between lion territory size and territory overlap was positive, and both territory size and overlap were important predictors of the other. Adding CDV to the temporal models nearly doubled the deviance explained for both territory size and overlap. Territory overlap tended to increase as rainfall increased, and rain explained quite a bit of the deviance; however, rain was not statistically significant. Taken together, we can conclude that lions expand their territories during the wet season and are more likely to overlap with neighboring prides. Interestingly, as the number of prides increased, so did territory size and overlap, suggesting that lions do not contract their territories readily (except following CDV exposure).
We were particularly interested in the effect of CDV on lion spatial organization, so we explored how spatial and network metrics (i.e., territory size, territory overlap, degree, or betweenness centrality) changed the year following each CDV exposure period (Fig. 5, Fig. S5; Supporting Information: Post-hoc mechanism exploration). We found that changes in territory size, overlap, and degree were the most extreme following the 1994 epizootic (Fig. 5, S5); for instance, about 85% prides decreased territory overlap with other prides by at least 10%, and about 62% of prides decreased their degree and territory size by at least 10%. After the 1998 epizootic, lions similarly decreased their territory size, overlap, and degree.
Figure 5.
(Top row) Serengeti lion networks during the 1994 canine distemper virus (CDV) epizootic and the year post-epizootic (1995); colored nodes represent different prides, node size represents territory size, and edge width corresponds to total overlap between territories. (Bottom row) Boxplots comparing four spatial or network attributes – territory size, territory overlap, degree, and betweenness centrality – between the epizootic year (E, gray) and the post-epizootic year (P, white). Network layouts are force-directed (igraph; Csardi & Nepusz, 2006).
Temporal trends (season and year) were the only significant covariates predicting wolf territory size (Fig. 4B, Tables S6a–S8a), and they explained 79.4% of the deviance in territory sizes (Table S6a). The complete model had the lowest AIC value but all models were within ~3 △AIC because temporal trends dominated (Table S9a).
Wolf pack territory overlap increased when there were more packs on the landscape, population density increased, and pack size was larger (Fig. 4D, Table S6b). The relationship between territory overlap and population density was nonlinear such that overlap increased as population density increased until about 90 wolves/1000-km2, then stabilized. Mean territory overlap was higher in the winter, which corresponded with larger territory size and snow, both of which were significant predictors. The complete model was the top model (Table S9b) and it explained 95.0% of the deviance, which was a large improvement from the temporal trend model (52.4%, Table S7b) and the temporal trend + CDV model (53.8%, Table S8b).
There was a slight increasing trend in territory overlap throughout the timeseries, whereas territory size declined in the early years as wolves established territories following reintroduction, and then territory sizes slowly increased and have potentially stabilized in recent years (Fig. S10, S11). This pattern tracks trends in population size: rapid increase followed by a decline and stabilization (Fig. S1). Wolf spatial organization was driven seasonally such that territory size and overlap were both greater in the winter, yet as snow levels increased and limited wolf movement, overlap declined. The relationship between territory size and overlap was positive, although territory size was more important for predicting territory overlap than overlap was for predicting size. These results suggest that territory size is highly predictable by season, whereas territory overlap generally increases as territory size increases but is modulated by the number of packs in the population, snow pack, and population size.
Generally, group-level covariates shaped territory attributes more than population-level for both lions and wolves (Fig. 4, Table S2, S6). We found that the number of groups in the population was significant in three of the four top models; this was the most notable similarity between lion and wolf modeling results. The population-level covariates with the strongest influence on territory size and overlap included CDV epizootics (lions only) and precipitation as rain (lions only) or snow (wolves only). Generally, covariates shaping territory size were similar to covariates shaping territory overlap, although the magnitude of the covariates’ importance differed.
DISCUSSION
We assessed how environmental factors, biotic interactions, and infectious disease epizootics alter network topology and drive the seasonal territory patterns of two social carnivores: lions in Serengeti National Park and wolves in Yellowstone National Park. Lion populations were highly connected with some prides serving as hubs connecting peripheral prides; wolf packs were weakly connected, but these connections were quite stable through time. Covariates shaping territory size were similar to covariates shaping territory overlap, although the magnitude of the covariates’ importance differed in each model. The results of this study provide insights into how: 1) the number of groups in a population, 2) territory size and overlap, and 3) disease perturbations might impact space use.
First, we found that the number of groups in a study area or population was unequivocally important in shaping spatial organization. This is important because, for social species like lions and wolves, groups act as the functional units comprising the population. Groups raise offspring and compete for territories and resources, and the fitness of solitary individuals is often drastically reduced (Packer & Ruttan, 1988; Packer et al., 1990; Almberg et al., 2015). More recent research describing the spatial and social processes of group-living territorial species has benefitted from explicit consideration of groups (e.g., spinner dolphins: Karczmarski et al., 2005; orcas: Baird & Whitehead, 2000, primates: Kasper & Voelkl, 2009, spotted hyena: Ilany et al., 2015; meerkats: Bateman et al., 2015, and some birds: Ke et al., 2017). Our results therefore support the growing focus of conservation or management for social species on maintaining viable groups, rather than focusing on a specific population size.
Second, lion and wolf territory size and overlap had a complex relationship that suggested that as a territory grows, so does territory overlap, and vice versa. If groups had more neighboring groups, then overlap was more likely to increase. This implies that groups were not as good at maintaining territory boundaries when they had to travel more widely across larger territories and when there were more potential intruders in close proximity. Importantly, this relationship was influenced by season, which likely relates to resource availability and resource dispersion since prey are migratory in both systems. In particular, prey are aggregated on the landscape within specific habitats with respect to season. For example, when elk are scarce, wolf packs in Yellowstone utilize overlapping hunting areas due to favorable topography and elk occurrence (Kauffman et al., 2007), and this a likely mechanism for increasing territory overlap. Yet elk density was not significant in our wolf territory size and overlap models, indicating that elk density does not necessarily capture the heterogeneous distribution of elk.
Resource dispersion is the prevailing hypothesis for the evolution of territoriality (Ostfeld et al., 1985; Maher & Lott, 2000; Johnson et al., 2002). Yet there are other plausible drivers of spatial organization, such as kinship (Frase & Armitage, 1984; Rogers, 1987; Pravosudova et al., 2001), land tenure (Benson et al., 2004; Elbroch et al., 2016), and protection of young or infanticide (Wolff & Peterson, 1998; Smith et al., 2015). These drivers are not mutually exclusive and their relationship can be complex. For example, cougar (Puma concolor) territories are well described by resource dispersion, and individuals often utilize territory vacated by a cougar of the same sex (Elbroch et al., 2016). The strong influence of season in shaping wolf spatial organization implies that resource dispersion is an important driver of space use. African lions are tolerant of dispersing daughters, which frequently bud off from existing prides and occupy neighboring territories where they show considerable overlap with their mothers’ range for the first 2–5 years but decline by 10 years post-dispersal (VanderWaal et al., 2009); this indicates kin relationships among lions may be an important driver of spatial organization.
Third, we identified changes in lion spatial organization following the CDV epizootic that were not observed for wolves (Fig. 5). For the 1994 epizootic, we postulate that differences in space use arose in part from the fact that the major die-off in the lion population killed individuals of all age classes due to a coinfection (Roelke-Parker et al., 1996; Munson et al., 2008), whereas CDV epizootics in the wolf population primarily affected mostly pups and yearlings (Almberg et al., 2009). The loss of adult lions that maintain territory boundaries may have been a contributing covariate to prides’ contraction in space (McComb, et al. 1994; Heinsohn et al., 1996; Mosser & Packer 2009). As social species, which individuals die within a group can have disproportionate impacts on the behaviour of group members (i.e., ‘social disruption’; e.g., Borg et al., 2015). Social species such as lions (Heinsohn & Packer 1995; Davidson et al., 2011), wolves (Borg et al., 2015), whales (Wade et al., 2012), and badgers (Carter et al., 2007) exhibit behavioural changes (e.g., territory reconfiguration, dispersal, group dissolution) following the death (e.g.,due to harvest, cull, disease) of group members. However, social disruption mechanisms would not explain the effect we observed of CDV epizootics with limited mortality in lions, such as in 1998.
Infectious diseases such as CDV can alter the behaviour of infected individuals. CDV infection may impede movement through symptoms such as muscle tremors and lethargy, which could subsequently lead to the reductions in territory size and overlap we observed. Sick or weakened animals may behaviourally avoid healthy animals in order to reduce their risk of injury during an aggressive intergroup encounter. Alternatively, infectious diseases may also alter the behaviour of healthy individuals, as observed in guppies (Poecilia reticulata; Houde & Torio, 1992). Similar to our observations for lions following the 1994 epizootic, female Tasmanian devils decreased their home range size and home range overlap following a large-scale outbreak of facial tumor disease (Comte et al., 2020). Such disease-induced behavioural changes can have significant impacts on subsequent transmission dynamics. For instance, when raccoons are infected with rabies, individual movement may decline due to imparied mobility, which can alter epizootic size and spread (Reynolds et al., 2015). While we lack the data to distinguish between mechanisms underlying changes in spatial organization, our results support the importance of incorporating spatial and behavioural ecology into studies of pathogen transmission dynamics.
Changes in space use may also result from competition (Apps et al., 2006). Top predators have been shown to alter sympatric carnivores species’ space use (reviewed in Davis et al., 2018; Wang et al., 2020). Both lions and wolves have a larger effect on the space use patterns of other carnivores (e.g., hyenas, cheetahs, coyotes, cougars) rather than the other way around; this has been described for lions the Serengeti system (Vanak et al., 2013; Kittle et al., 2016; Swanson et al., 2016) and wolves in the northern Rocky Mountains (Berger & Gese, 2007; Kortello et al., 2007; Bartnick et al., 2013). Therefore, we do not believe that the occurrence of other species would have a substantial impact on lion and wolf spatial organization.
Data Limitations and Future Directions
Our analyses might have been strengthened by larger sample sizes (i.e., more locations per group each season) such that more locations per group per season could increase the precision of our territory estimates (Seaman et al., 1999). Additionally, more detailed data on prey distribution in both Serengeti and Yellowstone may have improved our models. Other studies demonstrated a shift in Serengeti lion territories as they follow prey migration, resulting in annual territory overlap that is not necessarily simultaneous, or territories with little overlap where prey abundance is high (reviewed in Hanby et al., 1995). Wolves in Yellowstone may shift their space use patterns to improve access to elk (Kauffman et al., 2007). We were unable to assess this because our prey data were at the scale of the study area, and annual prey counts may not explain the spatial and seasonal distribution of prey on the landscape and hence access to prey for these territorial species. Gathering more detailed data at the group-level is an area of future research.
Another area for future research is the impact of human activities and tourism on lion and wolf space use. Human activity has been shown to affect carnivore movement and space use (Boydston et al., 2003; Foster et al., 2010; Ordiz et al., 2011; Smith et al., 2017b; Zeller et al., 2019), however, this has not been comprehensively assessed in either Serengeti lions or Yellowstone wolves and we were unable to include it in our analysis.
CONCLUSION
In summary, we identified ecological drivers shaping the seasonal space use of two apex territorial species. While there is abundant literature about the effects of spatial or social organization on population dynamics in free-ranging animals (e.g., pathogen transmission, reviewed in White et al., 2017), there have been few attempts to elucidate drivers of mammalian spatial organization as we have done here. Our work highlights the importance of monitoring and managing social carnivore populations at the group level. We found that resource dispersion may be more important for driving territory size and overlap for wolves than for lions. Finally, canine distemper exposure, even with limited mortality, may alter lion spatial organization, demonstrating the importance of including behavioural and movement ecology in studies of infectious disease transmission dynamics. We hope that future research builds off of this work to elucidate the complex relationships between a population’s demographics, social and spatial structure, abiotic and biotic conditions, and pathogen infections.
DATA ACCESSIBILITY
Data available from the Dryad Digital Repository https://doi.org/10.5061/dryad.vq83bk3qd (Brandell et al., 2020).
Supplementary Material
REFERENCES
- Alberts SC 2019. Social influences on survival and reproduction: Insights from a long-term study of wild baboons. Journal of Animal Ecology 88:47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almberg ES, Cross PC, Dobson AP, Smith DW, Metz MC, Stahler DR, and Hudson PJ 2015. Social living mitigates the costs of a chronic illness in a cooperative carnivore. Ecology Letters 18:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almberg ES, Mech LD, Smith DW, Sheldon JW, and Crabtree RL 2009. A serological survey of infectious disease in Yellowstone National Park’s canid community. PLoS ONE 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps CD, McLellan BN, and Woods JG 2006. Landscape partitioning and spatial inferences of competition between black and grizzly bears. Ecography 29:561–572. [Google Scholar]
- Arden-Clarke CHG 1986. Population density, home range size and spatial organization of the Cape clawless otter, Aonyx capensis, in a marine habitat. Journal of Zoology 209:201–211. [Google Scholar]
- Armansin NC, Stow AJ, Cantor M, Leu ST, Klarevas-Irby JA, Chariton AA, and Farine DR 2019. Social Barriers in Ecological Landscapes: The Social Resistance Hypothesis. Trends in Ecology and Evolution. [DOI] [PubMed] [Google Scholar]
- Baird RW, and Whitehead H. 2000. Social organization of mammal-eating killer whales: group stability and dispersal patterns. Canadian Journal of Zoology 78:2096–2105. [Google Scholar]
- Bartnick TD, Van Deelen TR, Quigley HB, and Craighead D. 2013. Variation in cougar (Puma concolor) predation habits during wolf (Canis lupus) recovery in the southern Greater Yellowstone Ecosystem. Canadian Journal of Zoology 91:82–93. [Google Scholar]
- Bateman AW, Lewis MA, Gall G, Manser MB, and Clutton-Brock TH 2015. Territoriality and home-range dynamics in meerkats, Suricata suricatta: A mechanistic modelling approach. Journal of Animal Ecology 84:260–271. [DOI] [PubMed] [Google Scholar]
- Belcher CA, and Darrant JP 2004. Home range and spatial organization of the marsupial carnivore, Dasyurus maculatus maculatus (Marsupialia: Dasyuridae) in south-eastern Australia. Journal of Zoology 262:271–280. [Google Scholar]
- Benson JF, Chamberlain MJ, and Leopold BD 2004. Land tenure and occupation of vacant home ranges by bobcats (Lynx rufus). Journal of Mammalogy 85:983–988. [Google Scholar]
- Berger KM, and Gese EM 2007. Does interference competition with wolves limit the distribution and abundance of coyotes? Journal of Animal Ecology 76:1075–1085. [DOI] [PubMed] [Google Scholar]
- Binning SA, Shaw AK, and Roche DG 2017. Parasites and host performance: Incorporating infection into our understanding of animal movement. Integrative and Comparative Biology 57:267–280. [DOI] [PubMed] [Google Scholar]
- Borg BL, Brainerd SM, Meier TJ, and Prugh LR 2015. Impacts of breeder loss on social structure, reproduction and population growth in a social canid. Journal of Animal Ecology 84:177–187. [DOI] [PubMed] [Google Scholar]
- Boydston EE, Kapheim KM, Szykman M, and Holekamp KE 2003. Individual Variation in Space Use By Female Spotted Hyenas. Journal of Mammalogy 84:1006–1018. [Google Scholar]
- Brandell EE, Fountain-Jones NM, Gilbertson MLJ, Cross PC, Hudson PJ, Smith DW, … Craft ME (2020). Data from: Group density, disease, and season shape territory size and overlap of social carnivores. Dryad Digital Repository, 10.5061/dryad.vq83bk3qd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ED, Macdonald DW, Tew TE, and Todd IA 1994. Apodemus sylvaticus infected with Heligmosomoides polygyrus (Nematoda) in an arable ecosystem: epidemiology and effects of infection on the movements of male mice. Journal of Zoology 234:623–640. [Google Scholar]
- Calenge C. 2006. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecological Modelling 7:516–519. [Google Scholar]
- Cantor M, Wedekin LL, Guimaraes PR, Daura-Jorge FG, Rossi-Santos MR, and Simoes-Lopes PC 2012. Disentangling social networks from spatiotemporal dynamics: the temporal structure of a dolphin society. Animal Behaviour 84:641–651. [Google Scholar]
- Carter SP, Delahay RJ, Smith GC, Macdonald DW, Riordan P, Etherington TR, Pimley ER, Walker NJ, and Cheeseman CL 2007. Culling-induced social perturbation in Eurasian badgers Meles meles and the management of TB in cattle: An analysis of a critical problem in applied ecology. Proceedings of the Royal Society B: Biological Sciences 274:2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy KA, MacNulty DR, Stahler DR, Smith DW, and Mech LD 2015. Group composition effects on aggressive interpack interactions of gray wolves in Yellowstone National Park. Behavioral Ecology 26:1352–1360. [Google Scholar]
- Comte S, Carver S, Hamede R, and Jones M. 2020. Changes in spatial organization following an acute epizootic: Tasmanian devils and their transmissible cancer. Global Ecology and Biogeography:e00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, Madden JR, Franks DW, and James R. 2011. Hypothesis testing in animal social networks. Trends in Ecology and Evolution 26:502–507. [DOI] [PubMed] [Google Scholar]
- Cross PC, Almberg ES, Haase CG, Hudson PJ, Maloney SK, Metz MC, Munn AJ, Nugent P, Putzeys O, Stahler DR, Stewart AC, and Smith DW 2016. Energetic costs of mange in wolves estimated from infrared thermography. Ecology 97:1938–1948. [DOI] [PubMed] [Google Scholar]
- Cross PC, Creech TG, Ebinger MR, Heisey DM, Irvine KM, and Creel S. 2012. Wildlife contact analysis: Emerging methods, questions, and challenges. Behavioral Ecology and Sociobiology 66:1437–1447. [Google Scholar]
- Cross PC, Lloyd-Smith JO, Johnson PLF, and Getz WM 2005. Duelling timescales of host movement and disease recovery determine invasion of disease in structured populations. Ecology Letters 8:587–595. [Google Scholar]
- Cross PC, van Manen FT, Viana M, Almberg ES, Bachen D, Brandell EE, Haroldson MA, Hudson PJ, Stahler DR, and Smith DW 2018. Estimating distemper virus dynamics among wolves and grizzly bears using serology and Bayesian state-space models. Ecology and Evolution 8:8726–8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csardi G, and Nepusz T. 2006. The igraph software package for complex network research. InterJournal, Complex Systems 1695:1–9. [Google Scholar]
- Cubaynes S, Macnulty DR, Stahler DR, Quimby KA, Smith DW, and Coulson T. 2014. Density-dependent intraspecific aggression regulates survival in northern Yellowstone wolves (Canis lupus). Journal of Animal Ecology 83:1344–1356. [DOI] [PubMed] [Google Scholar]
- Davidson Z, Valeix M, Loveridge AJ, Madzikanda H, and Macdonald DW 2011. Socio-spatial behaviour of an African lion population following perturbation by sport hunting. Biological Conservation 144:114–121. [Google Scholar]
- Davies NB 1978. Territorial defence in the speckled wood butterfly (Pararge aegeria): The resident always wins. Animal Behaviour 26:138–147. [Google Scholar]
- Davies NB, and Hartley IR 1996. Food Patchiness, Territory Overlap and Social Systems: An Experiment with Dunnocks Prunella modularis. The Journal of Animal Ecology 65:837. [Google Scholar]
- Davis CL, Rich LN, Farris ZJ, Kelly MJ, Di Bitetti MS, Di Blanco Y, Albanesi S, Farhadinia MS, Gholikhani N, Hamel S, Harmsen BJ, Wultsch C, Kane MD, Martins Q, Murphy AJ, Steenweg R, Sunarto S, Taktehrani A, Thapa K, Tucker JM, Whittington J, Widodo FA, Yoccoz NG, and Miller DAW 2018. Ecological correlates of the spatial co-occurrence of sympatric mammalian carnivores worldwide. Ecology Letters 21:1401–1412. [DOI] [PubMed] [Google Scholar]
- Demma DJ, and Mech LD 2011. Accuracy of Estimating Wolf Summer Territories by Daytime Locations. The American Midland Naturalist 165:436–445. [Google Scholar]
- Elbroch LM, Lendrum PE, Quigley H, and Caragiulo A. 2016. Spatial overlap in a solitary carnivore: Support for the land tenure, kinship or resource dispersion hypotheses? Journal of Animal Ecology 85:487–496. [DOI] [PubMed] [Google Scholar]
- Elith J, Leathwick JR, and Hastie T. 2008. A working guide to boosted regression trees. Journal of Animal Ecology 77:802–813. [DOI] [PubMed] [Google Scholar]
- Erlinge S. 1968. Territoriality of the Otter Lutra lutra L. Oikos January:81–98. [Google Scholar]
- Farine DR, and Whitehead H. 2015. Constructing, conducting and interpreting animal social network analysis. Journal of Animal Ecology 84:1144–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner AL, Godfrey SS, and Bull MC 2011. Using social networks to deduce whether residents dispersers spread parasites in a lizard population. Journal of Animal Ecology 80:835–843. [DOI] [PubMed] [Google Scholar]
- Fieberg J. 2007. Kernel density estimators of home range: smoothing and the autocorrelation red herring. Ecology 88:1059–1066. [DOI] [PubMed] [Google Scholar]
- Foster RJ, Harmsen BJ, and Doncaster CP 2010. Habitat Use by Sympatric Jaguars and Pumas Across a Gradient of Human Disturbance in Belize. Biotropica 42:724–731. [Google Scholar]
- Fountain-Jones N, Machado G, Carver S, Packer C, Mendoza M, and Craft ME 2019. How to make more from exposure data? An integrated machine learning pipeline to predict pathogen exposure. Journal of Animal Ecology. [DOI] [PubMed] [Google Scholar]
- Frase BA, and Armitage KB 1984. Foraging Patterns of Yellow-Bellied Marmots: Role of Kinship and Individual Variability. Behavioral Ecology and Sociobiology 16:1–10. [Google Scholar]
- Fuller TK, Mech LD, and Cochrane JF 2003. Wolf Population Dynamics. Pages 161–191 in Mech LD and Boitani L, editors. Wolves: Behavior, Ecology, and Conservation. [Google Scholar]
- Gilbertson MLJ, White LA, and Craft ME 2020. Trade-offs with telemetry-derived contact networks for infectious disease studies in wildlife. Methods in Ecology and Evolution:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey SS, Moore JA, Nelson NJ, and Bull CM 2010. Social network structure and parasite infection patterns in a territorial reptile, the tuatara (Sphenodon punctatus). International Journal for Parasitology 40:1575–1585. [DOI] [PubMed] [Google Scholar]
- Gosden TP, and Svensson EI 2008. Spatial and temporal dynamics in a sexual selection mosaic. Evolution: International Journal of Organic Evolution 62:845–856. [DOI] [PubMed] [Google Scholar]
- Grinnell J, Packer C, and Pusey AE 1995. Cooperation in male lions: kinship, reciprocity or mutualism? Animal Behavior 49:95–105. [Google Scholar]
- Hanby JP, Bygott JD, and Packer C. 1995. Ecology, Demography, and Behavior of Lions in Two Contrasting Habitats: Ngorongoro Crater and the Serengeti Plains. Pages 315–331 in Sinclaire ARE and Arcese P, editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. The University of Chicago Press. [Google Scholar]
- Hastie T, Rosset S, Tibshirani R, and Zhu J. 2004. The Entire Regularization Path for the Support Vector Domain Description. Journal of Machine Learning Research 5:1391–1415. [Google Scholar]
- He P, Maldonado-Chaparro AA, and Farine DR 2019. The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behavioral Ecology and Sociobiology 73. [Google Scholar]
- Heinsohn R. 1997. Group territoriality in two populations of African lions. Animal Behaviour 53:1143–1147. [DOI] [PubMed] [Google Scholar]
- Heinsohn R, and Packer C. 1995. Complex Cooperative Strategies in Group-Territorial African Lions. Science 269:1260–1262. [DOI] [PubMed] [Google Scholar]
- Heinsohn R, Packer C, and Pusey AE 1996. Development of cooperative territoriality in juvenile lions. Proceedings of the Royal Society of London Series B: Biological Sciences 263:475–479. [DOI] [PubMed] [Google Scholar]
- Hess G. 1996. Disease in metapopulation models: implications for conservation. Ecology 77:1617–1632. [Google Scholar]
- Houde AE, and Torio AJ 1992. Effect of parasitic infection on male color pattern and female choice in guppies. Behavioral Ecology 3:346–351. [Google Scholar]
- Ilany A, Booms AS, and Holekamp KE 2015. Topological effects of network structure on long-term social network dynamics in a wild mammal. Ecology Letters 18:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez MD, Bangs EE, Boyd DK, Smith DW, Becker SA, Ausband DE, Woodruff SP, Bradley EH, Holyan J, and Laudon K. 2017. Wolf dispersal in the Rocky Mountains, Western United States: 1993–2008. Journal of Wildlife Management 81:581–592. [Google Scholar]
- Johnson DDP, Kays R, Blackwell PG, and Macdonald DW 2002. Does the resource dispersion hypothesis explain group living? Trends in Ecology and Evolution 17:563–570. [Google Scholar]
- Karczmarski L, Würsig B, Gailey G, Larson KW, and Vanderlip C. 2005. Spinner dolphins in a remote Hawaiian atoll: Social grouping and population structure. Behavioral Ecology 16:675–685. [Google Scholar]
- Kasper C, and Voelkl B. 2009. A social network analysis of primate groups. Primates 50:343–356. [DOI] [PubMed] [Google Scholar]
- Kauffman MJ, Varley N, Smith DW, Stahler DR, MacNulty DR, and Boyce MS 2007. Landscape heterogeneity shapes predation in a newly restored predator-prey system. Ecology Letters 10:690–700. [DOI] [PubMed] [Google Scholar]
- Ke D-H, Deng Y-H, Guo W-B, and Huang Z-H 2017. A quadratic correlation between long-term mean group size and group density in a cooperatively breeding passerine. Ecology and Evolution 7:8719–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittle AM, Anderson M, Avgar T, Baker JA, Brown GS, Hagens J, Iwachewski E, Moffatt S, Mosser A, Patterson BR, Reid DEB, Rodgers AR, Shuter J, Street GM, Thompson ID, Vander Vennen LM, and Fryxell JM 2015. Wolves adapt territory size, not pack size to local habitat quality. Journal of Animal Ecology 84:1177–1186. [DOI] [PubMed] [Google Scholar]
- Kittle AM, Bukombe JK, Sinclair ARE, Mduma SAR, and Fryxell JM 2016. Landscape-level movement patterns by lions in western Serengeti: Comparing the influence of inter-specific competitors, habitat attributes and prey availability. Movement Ecology 4:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl MT, Ruth TK, Metz MC, Stahler DR, Smith DW, White PJ, and MacNulty DR 2019. Do prey select for vacant hunting domains to minimize a multi-predator threat? Ecology Letters 22:1724–1733. [DOI] [PubMed] [Google Scholar]
- Kortello AD, Hurd TE, and Murray DL 2007. Interactions between cougars (Puma concolor) and gray wolves (Canis lupus) in Banff National Park, Alberta. Ecoscience 14:214–222. [Google Scholar]
- Lack D. 1954. The natural regulation of animal numbers. Page The Natural Regulation of Animal Numbers. [Google Scholar]
- Lafferty KD, and Morris AK 1996. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77:1390–1397. [Google Scholar]
- Lesmerises F, Dussault C, and St-Laurent MH 2013. Major roadwork impacts the space use behaviour of gray wolf. Landscape and Urban Planning 112:18–25. [Google Scholar]
- Loe LE, Hansen BB, Stien A, Albon SD, Bischof R, Carlsson A, Irvine RJ, Meland M, Rivrud IM, Ropstad E, Veiberg V, and Mysterud A. 2016. Behavioral buffering of extreme weather events in a high-Arctic herbivore. Ecosphere 7:1–13. [Google Scholar]
- Macdonald DW 1983. The ecology of social behaviour. Nature 301:379–384. [Google Scholar]
- MacNulty DR, Tallian A, Stahler DR, and Smith DW 2014. Influence of Group Size on the Success of Wolves Hunting Bison. PLoS ONE 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher CR, and Lott DF 2000. A Review of Ecological Determinants of Territoriality within Vertebrate Species. The American Midland Naturalist 143:1–29. [Google Scholar]
- McComb K, Packer C, and Pusey A. 1994. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Animal Behaviour 47:379–387. [Google Scholar]
- Mech LD 1994. Buffer Zones of Territories of Gray Wolves as Regions of Intraspecific Strife. Journal of Mammalogy 75:199–202. [Google Scholar]
- Mech LD, and Boitani L. 2003. Wolves: behavior, ecology, and conservation. University of Chicago Press. [Google Scholar]
- Metz MC, Smith DW, Vucetich JA, Stahler DR, and Peterson RO 2012. Seasonal patterns of predation for gray wolves in the multi-prey system of Yellowstone National Park. Journal of Animal Ecology 81:553–563. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Gitzen RA, Kernohan BJ, Larson MA, and Clay CL 2004. Comparability of three analytical techniques to assess joint space use. Wildlife Society Bulletin 32:148–157. [Google Scholar]
- Mosser A, and Packer C. 2009. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Animal Behaviour 78:359–370. [Google Scholar]
- Munson L, Terio KA, Kock R, Mlengeya T, Roelke ME, Dubovi E, Summers B, Sinclair ARE, and Packer C. 2008. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS ONE 3:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch WW, Briggs CJ, and Nisbet RM 2003. Consumer-resource dynamics. Princeton University Press. [Google Scholar]
- Nelson AA, Kauffman MJ, Middleton AD, Jimenez MD, Mcwhirter DE, Barber J, and Gerow K. 2012. Elk migration patterns and human activity influence wolf habitat use in the Greater Yellowstone Ecosystem. Ecological Applications 22:2293–2307. [DOI] [PubMed] [Google Scholar]
- Ordiz A, Støen O-G, Delibes M, and Swenson JE 2011. Predators or prey? Spatio-temporal discrimination of human-derived risk by brown bears. Oecologia 166:59–67. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS 1985. Limiting Resources and Territoriality in Microtine Rodents. The American Naturalist 126:1–15. [Google Scholar]
- Packer C, Altizer S, Appel M, Brown E, Martenson J, O’Brien SJ, Roelke-Parker M, Hofmann-Lehmann R, and Lutz H. 1999. Viruses of the Serengeti: Patterns of infection and mortality in African lions. Journal of Animal Ecology 68:1161–1178. [Google Scholar]
- Packer C, Gilbert DA, Pusey AE, and O’Brieni SJ 1991. A molecular genetic analysis of kinship and cooperation in African lions. Nature 351:562–565. [Google Scholar]
- Packer C, Hilborn R, Mosser A, Kissui B, Borner M, Hopcraft G, Wilmshurst J, Mduma S, and Sinclair ARE 2005. Ecological change, group territoriality, and population dynamics in Serengeti lions. Science 307:390–393. [DOI] [PubMed] [Google Scholar]
- Packer C, and Ruttan L. 1988. The Evolution of Cooperative Hunting. The American Naturalist 132:159–198. [Google Scholar]
- Packer C, Scheel D, and Pusey AE 1990. Why lions form groups: food is not enough. The American Naturalist 136:1–19. [Google Scholar]
- Paniw M, Maag N, Cozzi G, Clutton-Brock T, and Ozgul A. 2019. Life history responses of meerkats to seasonal changes in extreme environments. Science 363:631–635. [DOI] [PubMed] [Google Scholar]
- Pasinelli G, Runge JP, and Schiegg K. 2014. Source-sink status of small and large wetland fragments and growth rate of a population network. Pages 216–238 Sources, sinks and sustainability. [Google Scholar]
- Perkins SE, Cagnacci F, Stradiotto A, Arnoldi D, and Hudson PJ 2009. Comparison of social networks derived from ecological data : implications for inferring infectious disease dynamics. Journal of Animal Ecology 78:1015–1022. [DOI] [PubMed] [Google Scholar]
- Pravosudova EV, Grubb TC Jr, and Parker PG 2001. The Influence of Kinship on Nutritional Condition and Aggression Levels in Winter Social Groups of Tufted Titmice. The Condor 103:821–828. [Google Scholar]
- Pusey AE, and Packer C. 1987. The Evolution of Sex-Biased Dispersal in Lions. Behavior 101:275–310. [Google Scholar]
- Reynolds JJH, Hirsch BT, Gehrt SD, and Craft ME 2015. Raccoon contact networks predict seasonal susceptibility to rabies outbreaks and limitations of vaccination. Journal of Animal Ecology 84:1720–1731. [DOI] [PubMed] [Google Scholar]
- Roelke-Parker ME, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, O’Brien SJ, Pospischil A, Hofmann-Lehmann R, Lutz H, Mwamengele GLM, Mgasa MN, Machange GA, Summers BA, and Appel MJG 1996. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LL 1987. Effects of Food Supply and Kinship on Social Behavior, Movements, and Population Growth of Black Bears in Northeastern Minnesota. Wildlife Monographs 97:3–72. [Google Scholar]
- Schaller GB 1972. The Serengeti lion: a study of predator-prey relations. University of Chicago Press, Chicago, IL. [Google Scholar]
- Seaman DE, Millspaugh JJ, Kernohan BJ, Brundige GC, Raedeke KJ, and Gitzen RA 1999. Effects of Sample Size on Kernel Home Range Estimates. The Journal of Wildlife Management 63:739–747. [Google Scholar]
- Simon CA 1975. The influence of food abundance on territory size in the iguanid lizard Sceloporus jarrovi. Ecology 56:993–998. [Google Scholar]
- Smith D, Stahler D, Stahler E, Metz M, Cassidy K, Cassidy B, Koitzsch L, Harrison Q, Cato E, and McIntyre R. 2017a. Yellowstone Wolf Project Annual Report. Page Yellowstone National Park Wolf Project Annual Report 2016. Yellowstone National Park, WY, USA. [Google Scholar]
- Smith DW, and Bangs EE 2009. Reintroduction of wolves to Yellowstone National Park: history, values, and ecosystem restoration. Pages 92–125 Reintroduction of top-order predators. Oxford: Wiley-Blackwell. [Google Scholar]
- Smith DW, Cassidy KA, Stahler DR, MacNulty DR, Harrison Q, Balmford B, Stahler EE, Brandell EE, and Coulson T. (n.d.). Population Dynamics and Demography. Page Yellowstone Wolves: Science and Discovery in the World’s First National Park. University of Chicago Press. [Google Scholar]
- Smith DW, Metz MC, Cassidy KA, Stahler EE, McIntyre RT, Almberg ES, and Stahler DR 2015. Infanticide in wolves: seasonality of mortalities and attacks at dens support evolution of territoriality. Journal of Mammalogy 96:1174–1183. [Google Scholar]
- Smith JA, Suraci JP, Clinchy M, Crawford A, Roberts D, Zanette LY, and Wilmers CC 2017b. Fear of the human ‘super predator’ reduces feeding time in large carnivores. Proceedings of the Royal Society B: Biological Sciences 284:20170433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong G, and Creel S. 2004. Effects of kinship on territorial conflicts among groups of lions, Panthera leo. Behavioral Ecology and Sociobiology 55:325–331. [Google Scholar]
- Stahler DR, Macnulty DR, Wayne RK, and Smith DW 2013. The adaptive value of morphological, behavioural and life-history traits in reproductive female wolves. Journal of Animal Ecology 82:222–234. [DOI] [PubMed] [Google Scholar]
- Swanson A, Arnold T, Kosmala M, Forester J, and Packer C. 2016. In the absence of a “landscape of fear”: How lions, hyenas, and cheetahs coexist. Ecology and Evolution 6:8534–8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallents LA, Randall DA, Williams SD, and MacDonald DW 2012. Territory quality determines social group composition in Ethiopian wolves Canis simensis. Journal of Animal Ecology 81:24–35. [DOI] [PubMed] [Google Scholar]
- Team, R. C. 2017. R: A language and environment for statistical computing. [Google Scholar]
- Thomas F, Schmidt-Rhaesa A, Guilhaume M, Manu C, Durand P, and Renaud F. 2002. Do hairworms (Nematomorpha) manipulate the water seeking behaviour of their terrestrial hosts? Journal of Evolutionary Biology 15:356–361. [Google Scholar]
- Thompson NA 2019. Understanding the links between social ties and fitness over the life cycle in primates. Behaviour 156:859–908. [Google Scholar]
- Vanak AT, Fortin D, Thaker M, Ogden M, Owen C, Greatwood S, and Slotow R. 2013. Moving to stay in place: Behavioral mechanisms for coexistence of African large carnivores. Ecology 94:2619–2631. [DOI] [PubMed] [Google Scholar]
- VanderWaal KL, Atwill ER, Isbell LA, and McCowan B. 2014. Linking social and pathogen transmission networks using microbial genetics in giraffe (Giraffa camelopardalis). Journal of Animal Ecology 83:406–414. [DOI] [PubMed] [Google Scholar]
- VanderWaal KL, Mosser A, and Packer C. 2009. Optimal group size, dispersal decisions and postdispersal relationships in female African lions. Animal Behaviour 77:949–954. [Google Scholar]
- Viana M, Cleaveland S, Matthiopoulos J, Halliday J, Packer C, Craft ME, Hampson K, Czupryna A, Dobson AP, Dubovi EJ, Ernest E, Fyumagwa R, Hoare R, Hopcraft JGC, Horton DL, Kaare MT, Kanellos T, Lankester F, Mentzel C, Mlengeya T, Mzimbiri I, Takahashi E, Willett B, Haydon DT, and Lembo T. 2015. Dynamics of a morbillivirus at the domestic–wildlife interface: Canine distemper virus in domestic dogs and lions. Proceedings of the National Academy of Sciences 112:1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonholdt BM, Stahler DR, Smith DW, Earl DA, Pollinger JP, and Wayne RK 2008. The genealogy and genetic viability of reintroduced Yellowstone grey wolves. Molecular Ecology 17:252–274. [DOI] [PubMed] [Google Scholar]
- Wade PR, Reeves RR, and Mesnick SL 2012. Social and Behavioural Factors in Cetacean Responses to Overexploitation: Are Odontocetes Less “Resilient” Than Mysticetes? Journal of Marine Biology 2012:1–15. [Google Scholar]
- Wang Y, Allen ML, and Wilmers CC 2020. Mesopredators display behaviourally plastic responses to dominant competitors when scavenging and communicating. bioRxiv preprint. [Google Scholar]
- White LA, Forester JD, and Craft ME 2017. Using contact networks to explore mechanisms of parasite transmission in wildlife. Biological Reviews 92:389–409. [DOI] [PubMed] [Google Scholar]
- Wolff JO, and Peterson JA 1998. An offspring-defense hypothesis for territoriality in female mammals. Ethology Ecology & Evolution 10:227–239. [Google Scholar]
- Wood SN, and Augustin NH 2002. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecological Modelling 157:157–177. [Google Scholar]
- Woodroffe R, Donnelly CA, Cox DR, Bourne FJ, Cheeseman CL, Delahay RJ, Gettinby G, McInerney JP, and Morrison WI 2006. Effects of culling on badger Meles meles spatial organization: Implications for the control of bovine tuberculosis. Journal of Applied Ecology 43:1–10. [Google Scholar]
- Zeller KA, Wattles DW, Conlee L, and Destefano S. 2019. Black bears alter movements in response to anthropogenic features with time of day and season. Movement Ecology 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository https://doi.org/10.5061/dryad.vq83bk3qd (Brandell et al., 2020).