Abstract
BACKGROUND
MicroRNA (miRNA) circulating in plasma have been proposed as biomarkers for a variety of diseases and stress measures, including depression, stress, and trauma. However, few studies have examined the relationship between stress and miRNA during pregnancy.
METHODS
In this study, we examined associations between measures of stress and depression during pregnancy with miRNA in early and late pregnancy from the MADRES cohort of primarily low-income Hispanic women based in Los Angeles, California. Extracellular-vesicle-(EV-) associated miRNA were isolated from maternal plasma and quantified using the Nanostring nCounter platform. Correlations for stress-associated miRNA were also calculated for 89 matching cord blood samples.
RESULTS
Fifty miRNA were nominally associated with depression, perceived stress, and prenatal distress, (raw p<0.05) with 17 miRNA shared between two or more stress measures. Two miRNA (miR-150-5p and miR-148b-3p) remained marginally significant after FDR adjustment (p<0.10). Fifteen PANTHER pathways were enriched for predicted gene targets of the 50 miRNA associated with stress. Clusters of maternal and neonate miRNA expression suggest a link between maternal and child profiles.
LIMITATIONS
The study evaluated 142 miRNA and was not an exhaustive analysis of all discovered miRNA. Evaluations for stress, depression and trauma were based on self-reported instruments, rather than diagnostic tools.
CONCLUSIONS
Depression and stress during pregnancy are associated with some circulating EV miRNA. Given that EV miRNA play important roles in maternal-fetal communication, this may have downstream consequences for maternal and child health, and underscore the importance of addressing mental health during pregnancy, especially in health disparities populations.
Keywords: extracellular vesicles, microRNA, pregnancy, depression, perceived stress
Introduction
Pregnancy is a challenging time involving many physical and emotional changes. Stress, anxiety, and prenatal depression affect an estimated 10–20% of pregnant individuals worldwide (Fawcett et al. 2019; Woody et al. 2017), and these conditions may have downstream consequences for both the mother and child. For example, prenatal depression is one central risk factor for postpartum depression (Edwards, Le, and Garnier-Villarreal 2021), and those with moderate prenatal depression may be more likely to experience adverse pregnancy outcomes, including preeclampsia (Acheampong et al. 2021). Depression during pregnancy is also associated with adverse outcomes for the child, including a higher risk of preterm birth and low birth weight (Grote et al. 2010). Children whose parents experienced prenatal depression also have altered biochemical and physiological profiles (Fatima, Srivastav, and Mondal 2017; Field, Diego, and Hernandez-Reif 2006). Given these consequences, understanding the roles of stress and depression during pregnancy is critical for improving clinical care for expectant mothers and their offspring, especially among individuals who are at greater risk of depression. Epigenetic modifications, such as DNA methylation and microRNA (miRNA) modulated gene expression, may play a role in the effects of adversity, stress, and depression during pregnancy on the offspring. Emerging research has reported associations between circulating miRNA levels and neurological and psychiatric disorders, such as bipolar disorder and depression as well as childhood trauma (Maffioletti et al. 2016; Yuan et al. 2018; Gheysarzadeh et al. 2018; Van der Auwera et al. 2019). EV-associated miRNA are hypothesized to contribute to maternal-fetal communication, and we have previously reported that EV-associated miRNA profiles change over time in pregnancy, identifying 64 miRNA that increase with GA and 26 that decrease, possibly supporting a dynamic role for miRNA during pregnancy (Foley et al. 2021). A large fraction of circulating EV miRNA during pregnancy originate in the placenta, and a growing body of evidence has found that placental miRNA profiles may reflect environmental exposures during the prenatal period (Avissar-Whiting et al. 2010; Kappil et al. 2016; Li et al. 2015; Marsit 2015). Additionally, a recent study reported that lifetime stress and negative life events were associated with changes in EV-associated miRNA profiles in breastmilk (Bozack et al. 2021). Because of their responsiveness to many conditions, circulating EV-associated miRNA have been suggested as a biomarker for pregnancy-related conditions (Tsochandaridis et al. 2015; Yang et al. 2020; Balaraman et al. 2016), as well as hypothesized to serve as a diagnostic tool for sub-clinical conditions, allowing early intervention for complications such as gestational diabetes (Zhu et al. 2015). However, impacts of maternal stress and depression on maternal circulating EV-associated miRNA during pregnancy remain largely unknown.
MiRNA associated with major depression in brain tissue have also been identified in cerebrospinal fluid and circulating in serum (Wan et al. 2015). In particular, circulating miR135a and miR-1202 have been proposed as possible biomarkers of depression: much lower levels have been reported among individuals with depression compared with healthy controls (Gheysarzadeh et al. 2018). Very few studies have evaluated associations between stress, depression, and miRNA expression patterns during pregnancy. This has been particularly understudied in health disparities populations, which are at greater risk of stress and depression, due to multiple socio-cultural factors, including acculturation stress (D’Anna-Hernandez, Aleman, and Flores 2015). For example, prior studies on stress and depressive symptoms among Puerto Rican pregnant women living in western Massachusetts reported that higher levels of education, income and living with a partner were associated with lower levels of depressive symptoms (Fortner et al. 2011).
In this study, we examined relationships between several measures of stress and circulating extracellular vesicle (EV) associated miRNA levels among pregnant participants from the MADRES study, an ongoing pregnancy cohort based in Los Angeles, California, USA. Participants for this study were mainly recruited from clinics located in areas with substantial health disparities and included predominantly lower income Hispanic individuals (Bastain et al. 2019). Previous work in this cohort has found that perceived stress and depressive symptoms during pregnancy were higher for participants with less education, identifying as Black, living with a partner rather than married, and among foreign-born Hispanic women who have lived in the US more than 20 years (Toledo-Corral et al. 2021). Investigating how biomarkers are associated with depression and perceived stress during pregnancy is critical to understanding downstream health effects in underserved populations.
Study aims and rationale.
This study aimed to identify relationships between stress measures and levels of 142 EV-associated miRNA in circulation, measured in both early and late pregnancy from 455 women from a low-income, predominantly Hispanic cohort. Using a discovery approach with a large miRNA panel, rather than selecting limited candidates allowed greater detection of miRNA associated with depressive symptomology and stress measures, given a lack of validated miRNA biomarkers for these stress measures. We additionally examined whether miRNA associated with stress measures were correlated with expression levels in cord blood to assess whether maternal stress could be directly affecting their infant’s miRNA profile. Results from this study suggest several miRNA associated with stress and depression that could be used for deeper analyses with children’s health outcomes or used in a biomarker capacity, although research in more diverse populations and situations are needed.
Materials and Methods
Participants.
Participants were recruited from The Maternal and Developmental Risks from Environmental and Social Stressors (MADRES), an ongoing cohort of more than 900 pregnant women and their children who received their prenatal care in Los Angeles, CA, USA (Bastain et al. 2019). Pregnant participants were eligible to participate if they were less than 30 weeks gestation, over 18 years of age, and able to speak English or Spanish. Exclusion criteria included: 1. HIV-positive status, 2. physical, mental, or cognitive disability preventing participation, 3. current incarceration, and 4. multiple pregnancy.
During an early-pregnancy visit (mean=13.6 weeks, sd= 4.2) participants who enrolled in MADRES before 20 weeks gestation completed administered questionnaires, provided biospecimens including a blood sample, and were measured for height and weight. Similar measures and data were also collected at a late pregnancy visit (mean=31.7 weeks, sd=2.0), and a blood sample was also collected at this time point. Among the 455 participants who were included in the current study, N=68 participants completed the early pregnancy visit only, N=192 participants completed the late pregnancy visit only, and N=195 participants completed both visits. Stress measures surveyed in the participants at both visits included depressive symptoms (20 item CES-D, Center for Epidemiological Studies- Depression)(Knight et al. 1997), perceived stress (PSS, Perceived Stress Scale)(Cohen, Kamarck, and Mermelstein 1983), prenatal distress (PDQ, Prenatal Distress Questionnaire)(Yali and Lobel 1999) and traumatic life events (ACE, Adverse Childhood Events)(Felitti et al. 1998). Additional linear models were also run with dichotomized CES-D scores (scores >16 indicating probable depression) and ACE (scores >4 indicating increased odds for major health conditions) (Knight et al. 1997; Felitti et al. 1998).
miRNA Extraction.
Blood samples from pregnant participants were collected in EDTA vacutainers. Plasma was separated and frozen at −80°C in 500ul aliquots for early and late visits during pregnancy. EV-associated miRNA were extracted from plasma using the Qiagen ExoRNeasy kit, as described previously (Foley et al. 2021) with an EV enrichment step followed by miRNA isolation. Extractions followed the manufacturer’s instructions, with a second addition of 100uL chloroform and an additional round of phase separation. The purified miRNA was frozen at −80°C until quantification.
Quantification.
Total miRNA quantity was measured by BioAnalyzer using a small RNA kit (Agilent Technologies, Inc. USA). If a sufficient quantity of miRNA was measured in the sample (>100pg/ul), 3ul of the purified miRNA was prepared on the NanoString NCounter with Human v3 miRNA expression assay (Nanostring Technologies, Inc.) using a modified protocol: after addition of the hybridized probes, samples were diluted 1:2, rather than 1:10 as prescribed. Samples were then processed and assessed following the rest of the recommended NanoString protocol.
Statistical analyses.
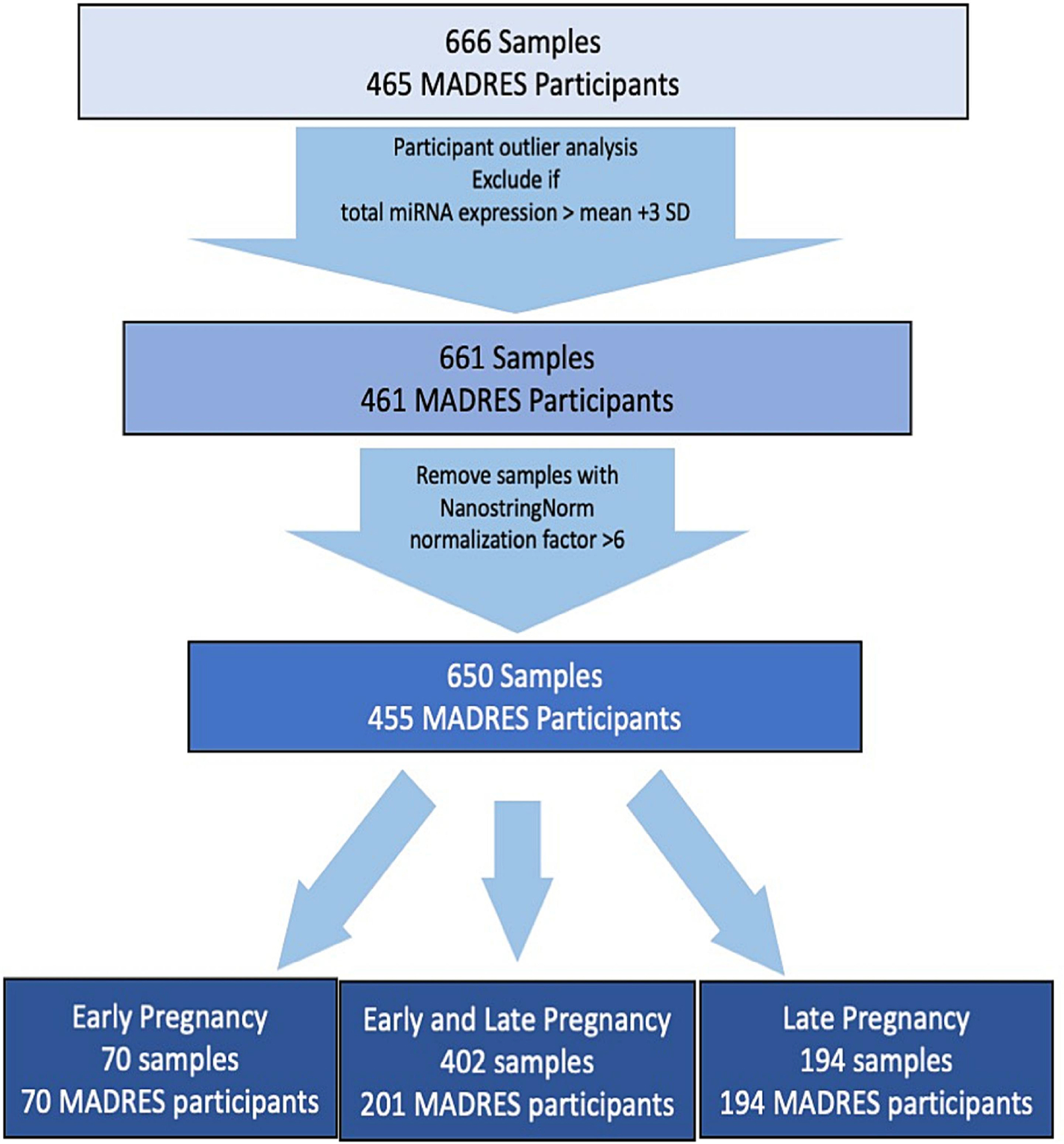
Quantified miRNA RCC files for each sample were converted to text files using NSolver (v. 4.0.70, Nanostring Technologies) for downstream processing in R (v.4.1.1). Early and late pregnancy miRNA counts were log2 transformed and normalized using the NanoString Norm (v. 1.2.1) R Package. Sample specific backgrounds were calculated using the geometric mean of the negative controls included on the nCounter platform, similar to previous analyses of Nanostring miRNA data (Howe et al. 2021; Cook et al. 2019; Huen et al. 2019). Machine-read values were used for counts falling below the sample-specific limits of detection. Participants with outlier total miRNA counts (higher or lower than the mean total log-transformed count +/− 3 standard deviations) were excluded (N=4) to limit influence from extreme high-expression samples. Samples were excluded if normalization factors flagged by NanoStringNorm were >6, suggesting unusually low miRNA content (Waggott et al. 2012). Participants were excluded if they did not have a recorded maternal age during pregnancy or gestational age (GA) at birth, because standardized maternal age and GA at sample collection could not be determined. A total of 650 samples from 455 unique participants were retained (Fig. 1). One hundred forty-two miRNA with values above background in a least 50% of samples in either the first or third trimester were selected for further analysis.
Figure 1. Sample Selection flowchart for miRNA samples.
Separate mixed effect linear regression models were used to investigate the associations between counts for each of the 142 miRNA and each stress measure using the NLME (v. 3.1–143) package in R. In sum, each miRNA was used in a linear mixed model for each stress metric (continuous and dichotomized CES-D, continuous PSS, continuous PDQ and continuous and dichotomized ACE) and two sensitivity analyses, for a total of eight models per miRNA, then false-discovery rate (FDR) adjusted for multiple testing within each stress or analysis. Suggestive values after False Discovery Rate (FDR) adjustment were reported for FDR p<0.10. Sensitivity analyses using data stratified for each timepoint (early and late pregnancy) used the full set of covariates and a random effect for chip group as before. An additional sensitivity analysis was performed for participants with two timepoints using the difference of their stress measures.
Potential confounders and precision variables were selected based on a priori criteria, which have been previously described for studies in this cohort that used the same miRNA data (Foley et al. 2021). Briefly, covariates known or suspected to affect circulating miRNA profiles (GA, BMI, maternal age, fetal sex, gravidity), as well as SES and acculturation proxy variables (preferred language, race and ethnicity, education) and study variables (recruitment site, study entry time) were considered. Final covariates included: GA at sample collection, maternal age standardized at 12 weeks, recruitment site, study entry time (before or after 20 weeks gestation), race and ethnicity (US-born White Hispanic, Foreign-born white Hispanic, other Hispanic, Black non-Hispanic, other non-Hispanic), gravidity (primigravida or multigravida), fetal sex, preferred language (English or Spanish), maternal education (Did not finish high school, graduated high school, some college or completed college), and pre-pregnancy BMI (continuous). Models also included random effects for participant ID and chip group, to account for participants who provided samples at both time points, and for variation in sample quantification over time, respectively. Maternal age was standardized at 12 weeks using maternal date of birth and the best available GA at birth. Best available GA at birth was determined using a hierarchy of available data: (1) ultrasound measurement of crown-rump length in first trimester (<14 weeks GA); (2) trimester ultrasound measurement of fetal biparietal diameter in the second trimester (<28 weeks GA); (3) obstetric clinical estimate abstracted from the participant’s medical records; (4) date of the participant’s self-reported last menstrual period.
Cord blood miRNA was measured for 89 infants of participants and paired with maternal late pregnancy samples. miRNA was quantified from 500uL of plasma and assessed according to the same procedures as the maternal samples, via Nanostring. Cord blood counts were normalized independently with NanoStringNorm, under the same parameters as maternal samples. To assess the similarity between cord blood miRNA profiles and late pregnancy miRNA profiles, Spearman correlations between late pregnancy maternal and cord blood EV miRNA counts were examined for the 50 miRNA which were found to be associated with CES-D, PSS, or PDQ scores before multiple testing correction. Correlation dendrograms were created using the package “pheatmap” (v.1.0.12) in R. Pairwise Spearman correlation test statistics and p-values were calculated using the cor.test function from “stats” (v. 4.1.2) package in R.
Pathway Analysis.
Predicted gene targets for miRNA that were suggestively associated with each of the stress metrics were identified using mirDIP v. 5.0.2.3 (Tokar et al. 2018). MirDIP indexes 24 databases of predicted and/or validated miRNA targets and assigns an integrated score for each interaction. Unique predicted target genes were restricted to integrated scores in the highest quartile (>0.80) and were used to identify potential pathways modulated by stress-associated miRNA. PANTHER v. 16.0 (Mi et al. 2021) was used to perform statistical overrepresentation tests for PANTHER pathways.
Results
Population description.
Participants recruited to MADRES and selected for miRNA analysis were generally healthy (Table 1), with nearly 90% of babies born at term or late term (37–42 weeks gestation). Less than 10% of babies were born preterm. Thirty-one percent of participants were expecting their first child, while 64% were expecting a second or later child, with some participants pregnant with their 5th or 6th child. MADRES participants were largely Hispanic (81%) and nearly 11% identified as Black. Obesity is prevalent in this population, with 33.2% and 36.5% of participants being overweight and obese, respectively, according to CDC levels of pre-pregnancy BMI categories. In this cohort, the prevalence of probable prenatal depression was 28% in early pregnancy and 18% in late pregnancy (Toledo-Corral et al. 2021).
Table 1:
Demographics of the Study Population.
| Study Population | |||
| n | Mean (SD) | ||
| Maternal Age | Standardized at 12 weeks | 455 | 28.5 (5.9) years |
| Pregnancy timing | Early pregnancy samples | 263 | 13.2 (4.2) weeks |
| Late pregnancy samples | 387 | 31.6 (2.0) weeks | |
| n | Percent | ||
| Gestational Age at Birth | Preterm (<37 weeks) | 43 | 9.4% |
| Term (37–40 weeks) | 287 | 63.1% | |
| Late term (> 40weeks) | 121 | 26.6% | |
| Information not available | 4 | 0.9% | |
| Fetal Sex | Female | 221 | 48.6% |
| Male | 230 | 50.5% | |
| Information not available | 4 | 0.9% | |
| Parity | Nulliparous | 141 | 31.0% |
| Primiparous or higher | 292 | 64.2% | |
| Information not available | 22 | 4.8% | |
| Language | English | 293 | 64.4% |
| Spanish | 158 | 34.7% | |
| Declined to answer | 4 | 0.9% | |
| Race/Ethnicity | US-Born White Hispanic | 160 | 35.1% |
| Foreign-Born White Hispanic | 183 | 40.2% | |
| Other Hispanic | 26 | 5.7% | |
| Black Non-Hispanic | 49 | 10.8% | |
| Non-Hispanic Other | 28 | 6.2% | |
| Unknown or declined to answer | 9 | 2.0% | |
| Education | Less than 12th grade (Did not finish high school) | 124 | 27.2% |
| Completed 12th grade (Graduated high school) | 132 | 29.0% | |
| Some college or completed college | 190 | 41.8% | |
| Declined to answer | 9 | 2.0% | |
| Pre-pregnancy BMI | Underweight or Normal Weight (< 25 kg/m2) | 134 | 29.4% |
| Overweight (25 kg/m2 – 29.9 kg/m2) | 151 | 33.2% | |
| Class 1–3 Obese (> 30 kg/m2) | 166 | 36.5% | |
| Declined to answer or information unavailable | 4 | 0.9% | |
Stress metrics in MADRES.
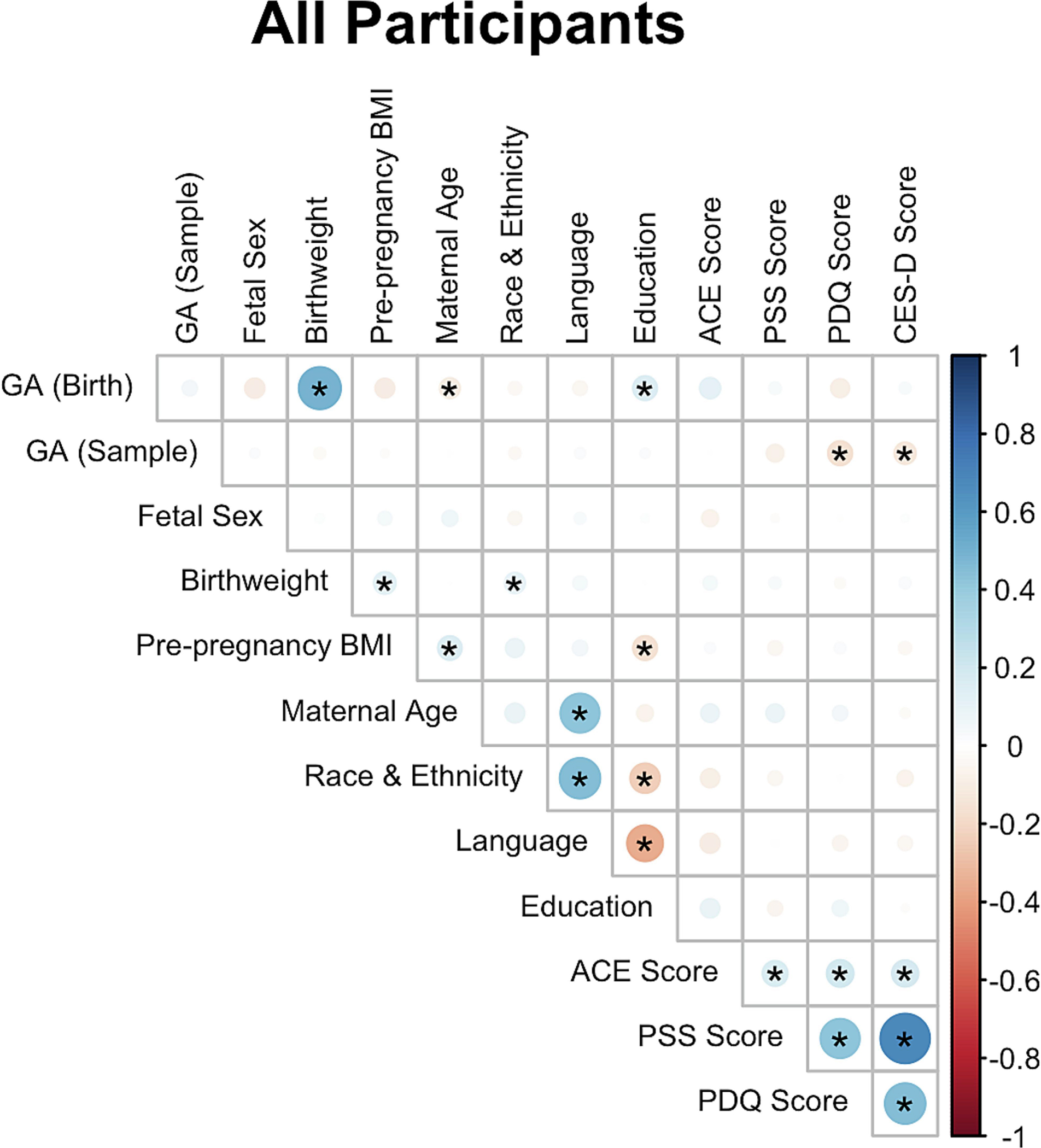
The stress metrics (PSS, PDQ, CES-D, and ACE) were all positively and significantly correlated with each other (Fig. 2). CES-D scores ranged from 0 to 46, with 123 of 450 responding participants (27.3%) scoring above 16 at either timepoint, suggesting probable depression. PSS scores ranged from 0 to 32, with a mean score of 12.8 across both timepoints. PDQ scores ranged from 0 to 16, with an average score of 4.4 across both timepoints. ACE scores ranged from 0 to 10, with 66 of 338 responding participants (19.5%) scoring greater than or equal to 4, associated with increased risk of psychological and medical consequences (Knight et al. 1997; Felitti et al. 1998).
Figure 2. Correlation plots for attributes of the MADRES participants profiled for miRNA.
Cross-sectional analysis of miRNA associated with stress and depression measures.
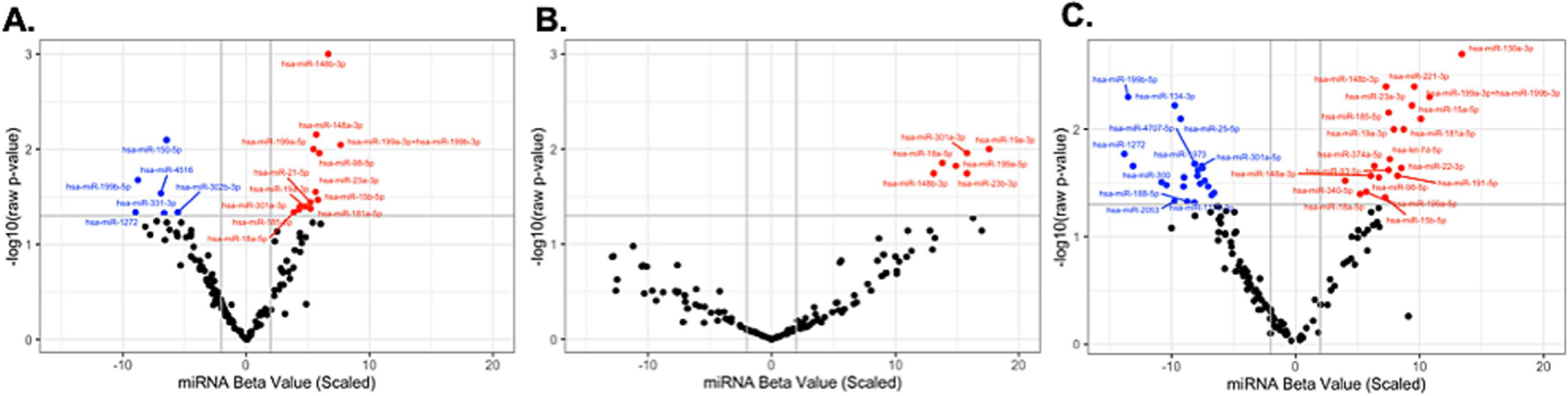
In linear mixed-effects models, 50 unique miRNA were associated with at least one of the three main stress metrics (CES-D, PSS, PDQ) before false discovery rate (FDR) adjustment, suggesting that a wide range of miRNA may be responsive to stress and depressive symptoms. Nineteen of these miRNA were associated with CES-D, 43 with PSS, and 9 with PDQ. 17 of these miRNA were shared between two or more stress measures, suggesting some overlap between downstream effects of stressors (Fig. 3). Sensitivity analyses with early or late pregnancy data sets partially supported these findings, considering loss of power due to fewer observations (SI tables 5–7).
Figure 3. miRNA associated with each of the three stress measures during pregnancy.
A.) miRNA associated with CESD. B.) miRNA associated with PSS. C.) miRNA associated with PDQ.
Longitudinal analysis of miRNA associated with changes in stress and depression.
Longitudinal analyses of miRNA count changes associated with changes in stress measure scores were also evaluated for 195 participants with two timepoints (SI Fig. 1 and SI Tables 8–10). Nine miRNA from the main analysis were also suggestively associated with changes in CES-D scores (FDR p<0.1). Likewise, 3 miRNA were suggestively associated with changes in PDQ scores (FDR p<0.1). However, zero miRNA were associated with changes in PSS scores (FDR p>0.1).
After multiple testing correction, lower levels of miR-150-5p were marginally associated with higher PDQ scores (FDR p=0.09). This miRNA was also associated with higher CES-D scores prior to multiple testing correction (raw p=0.008, FDR p=0.27). Lower levels of a second miRNA, miR-148b-3p, was also suggestively associated with higher CES-D scores after multiple testing correction (raw p=0.001, FDR p<0.1), and with higher PSS scores before multiple testing correction (raw p=0.004, FDR p=0.12) (SI Table 1).
Gene target analysis.
Predicted target genes of miRNA associated with the three stress measures were enriched in several PANTHER pathways, including insulin signaling, p53 pathways and several growth factor pathways (FGF, IGF, TGF-beta, and PDGF) (Fig. 4). Three pathways were shared between the three stress measures: TGF-beta signaling pathway, gonadotropin-releasing hormone receptor pathway, and insulin/IGF activated protein kinase cascade. Other pathways were specific to stress factors, such as integrin signaling pathway (PSS only) and FGF signaling (CES-D only).
Figure 4. PANTHER Pathways shared between miRNA associated with two or more stress measures.
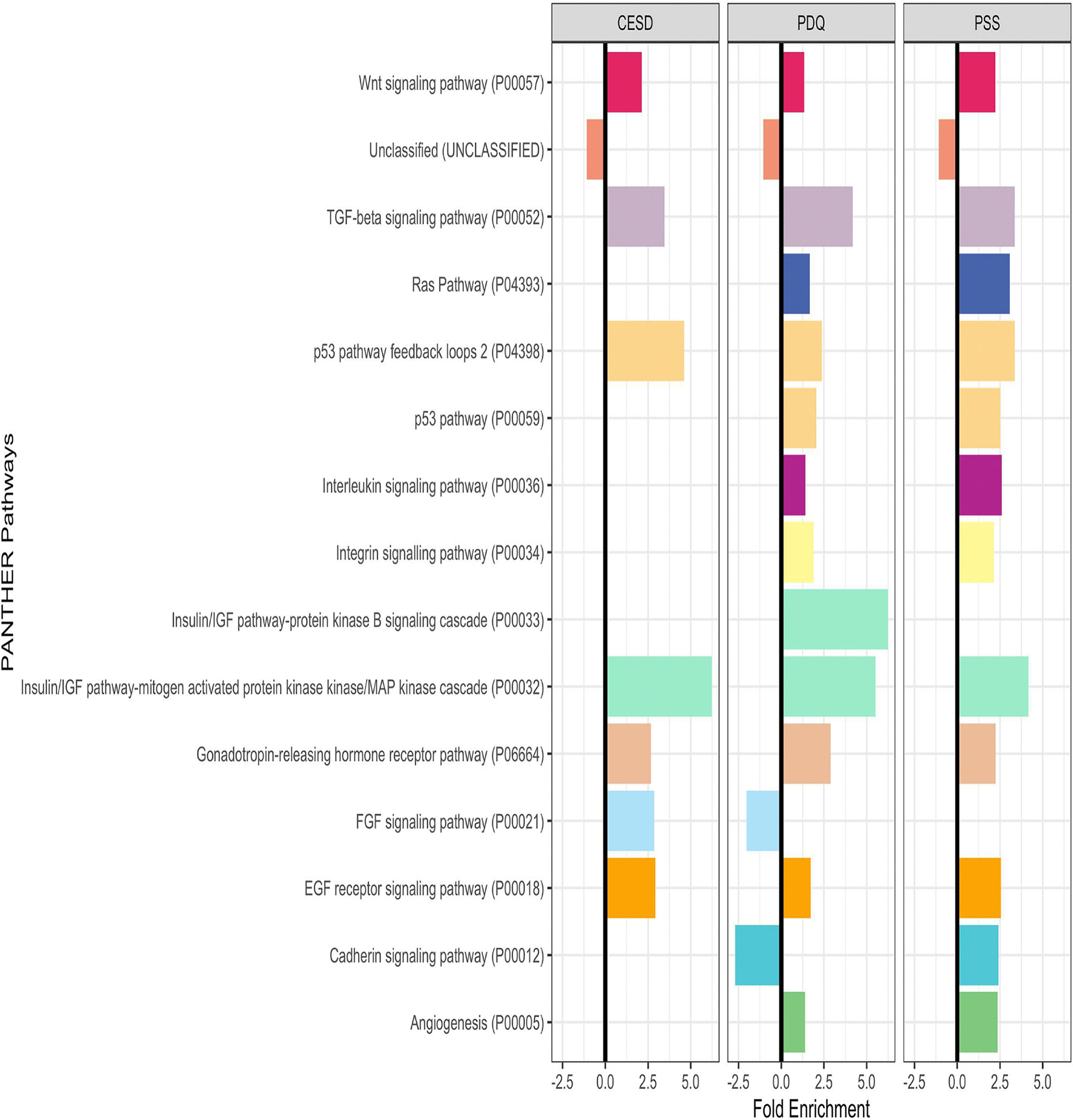
PANTHER pathways sharing critical features (i.e. p53 and Insulin/IGF) share colors. All bars have FDR p-value <0.05, missing bars have FDR p-value >0.05. For tables of these values, see SI Table 3.
ACE scores associated with miRNA profiles.
Because ACE scores reflect childhood trauma, which does not change over pregnancy, its associations with miRNA were evaluated separately for each timepoint. For circulating miRNA sampled in early pregnancy, 10 miRNA were associated with higher ACE scores before, but not after FDR adjustment, while 1 miRNA was associated with higher ACE scores in late pregnancy before, but not after FDR adjustment (SI Table 2.) Of these, two miRNA were shared with any of the three stress measures previously considered (CES-D, PSS, or PDQ): miR-150-5p and miR-98-5p, again suggesting overlap in downstream effects of stressors.
Correlations between maternal and cord blood samples.
Spearman correlations between maternal miRNA counts in late pregnancy and cord blood ranged from 0.4 to −0.3, with 158 pairwise correlations significant before adjustment (raw p-value<0.05), and 0 suggestive after FDR- adjustment (FDR p-value<0.1). (Fig. 5) Clusters of expression profiles between cord blood and late pregnancy plasma using miRNA expression data showed that miRNA in the maternal samples did not always cluster with the same miRNA in the cord blood samples, suggesting that deeper research into miRNA networks between parent and child could be a rich source of biomarker data. Associations between pregnancy and cord blood samples for miRNA ranged between 0.4 and −0.3, with no miRNA significant after FDR correction (p>0.05).
Figure 5. Heatmap of miRNA in cord blood and late pregnancy plasma samples.
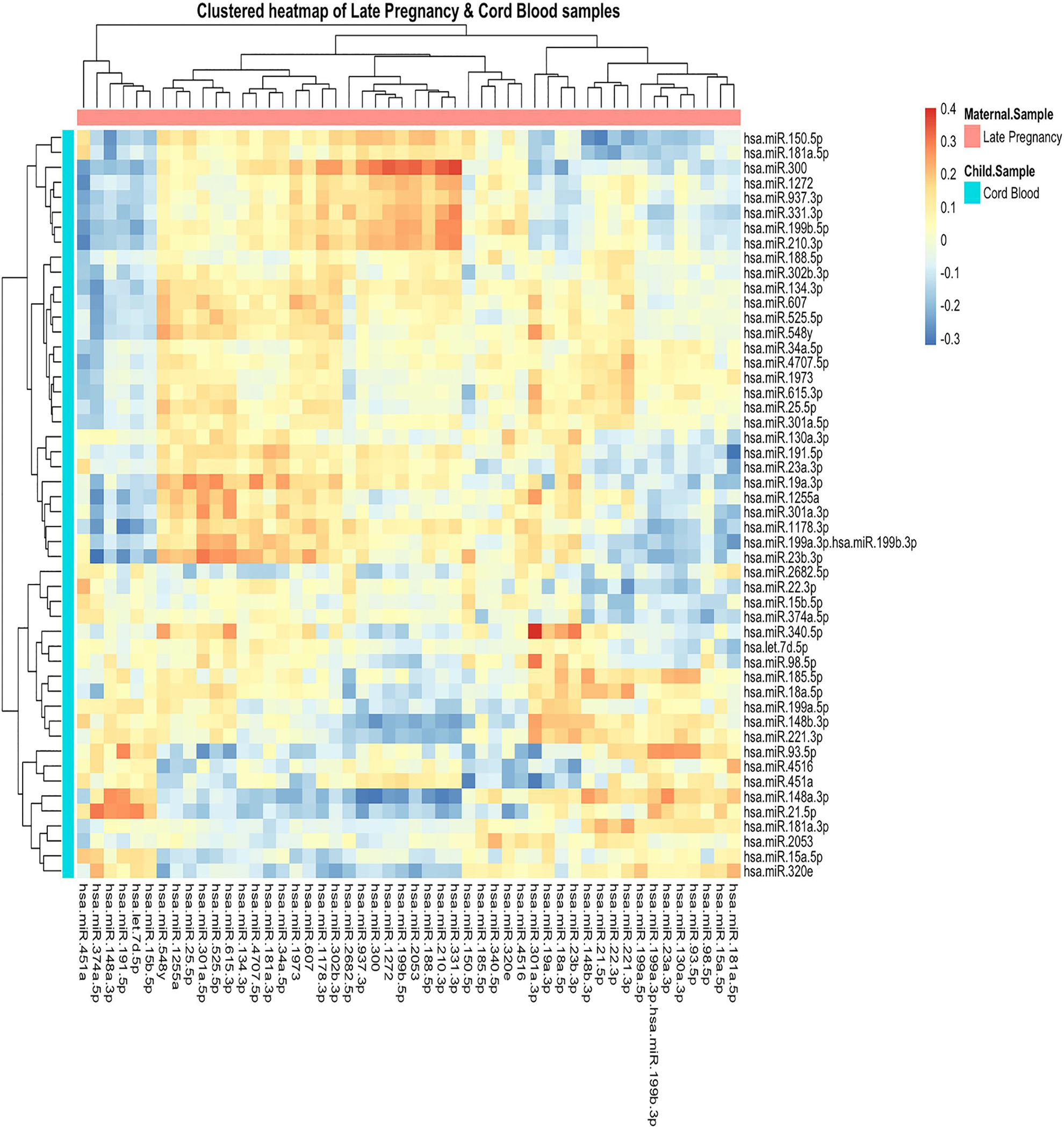
Spearman correlations ranged from 0.4 to −0.3. Dendrograms show clustering across sample types for positive and negative correlations.
Discussion
In a cohort of predominantly Hispanic pregnant individuals from urban Los Angeles, California, USA, we identified 50 miRNA that were nominally associated with at least one stress measure during pregnancy, 17 of which were shared between two or more of the stress measures examined. For two of these miRNA (miR-150-5p and miR-148b-3p), associations remained marginally statistically significant after multiple testing correction. Ten miRNA in early pregnancy and 1 miRNA in late pregnancy were significantly associated with ACE scores (raw p-value <0.05). However, none of these associations remained statistically significant after FDR-adjustment (SI Table 2).
We found that higher levels of prenatal distress were associated with lower levels of miR-150-5p. Consistent with this finding, studies in rats have reported that knocking out miR-150-5p increases anxiety-like behavior (Zhang et al. 2019). Predicted targets of miR-150-5p include several brain-related genes, such as SV2 glycoproteins and BASP1 (SI Table 4, mirdb.org), which have been implicated in depression-like behaviors in mice and associated with post-natal brain development, respectively (Sun et al. 2021; Manganas et al. 2021). We also found that CES-D scores were positively associated with lower expression levels of miR-148b-3p. Reduced levels of mir-148b-3p have been associated with impaired glucose metabolism in mouse models, suggesting that increases in this miRNA in pregnancy will modulate glucose sensitivity and thus energy availability (Pracht et al. 2021). Previous evidence suggests blood glucose irregularities may be associated with early pregnancy depression in Proyecto Bueno Salud, a predominantly Hispanic cohort (Ertel et al. 2014).
Four of the miRNA we identified (let-7d-3p, miR-34a-5p, miR221-3p and miR-451a) have been previously described as biomarkers with high predictive power for depression (Kuang et al. 2018). Target genes of these miRNA are involved in signaling pathways and brain functions associated with depression and respond to treatment with anti-depressants. However, inconsistency among miRNA profiles from patients with depression suggests that miRNA responses to depression and stress are multi-faceted and may differ between people or situations although they target the same or similar pathways involved in brain function and synaptic plasticity.
Fifteen PANTHER pathways were significantly enriched for predicted gene targets of the 50 unique miRNA that were associated with at least one of the three main stress measures (PSS, CES-D, and PDQ). Pathways were overrepresented in insulin/IGF/MAP kinase pathways, TGF-beta signaling, Wnt signaling, GnRH signaling, EGF receptor signaling and p53 pathways for all three stress measures. The prevalence of growth factor and kinase pathways among these suggests that these miRNAs may have wider downstream effects, as has been suggested for miR-486-5p and miR-486-3p, which have substantial pleiotropic activity in cancer and other disorders (ElKhouly, Youness, and Gad 2020). Several of the pathways are also involved in cell cycle and apoptosis regulation and have been previously associated with depression or stress (e.g., p53 pathways and FGF signaling) (Ruan et al. 2015; Garbett et al. 2015). Mice deficient in elements of the p53 pathway show increased anxiety- and depression-like behaviors (Ruan et al. 2015). The IGF pathway and Ras pathway have functions in synaptic plasticity and signal transduction and may also be disrupted in depression (Marsden 2013). Enrichment in these pathways suggests that miRNA could be responsible for dampening these pathways and producing stress-related and depressive symptomology in pregnant women, or that these symptoms modulate miRNA expression.
Among the miRNA shared between stress measures, two miRNAs (miR-150-5p and miR-98-5p) were also associated with ACE measures before FDR adjustment, suggesting that some miRNA may be altered due to early life trauma in addition to ongoing stress. Furthermore, two of the miRNA nominally associated with stress measures in MADRES, miR-98-5p and miR-15b-5p have been recognized as critical nodes that may contribute to the molecular mechanisms underpinning important brain functions disrupted in Alzheimer’s disease. The third miRNA, miR-486, has been associated with several types of cancer as well as regulation of fetal hemoglobin synthesis and obesity in children (ElKhouly, Youness, and Gad 2020; Marzano et al. 2018). While speculative, this evidence suggests that alterations in this miRNA during pregnancy could have downstream consequences for the offspring, given the intimate ties between childhood depression and obesity (Sutaria et al. 2019). Evidence presented in this study on miRNAs shared between these stress measures suggests miRNA have wide-ranging roles in the effects of stress and depression, whether we consider stressors, the perception of stress or psychological impact of stressors. Additionally, the evidence for some of these miRNA in non-pregnant depressed or stressed individuals suggests that these miRNA are not specific to pregnancy and depression, but rather associated with depressive symptomology, regardless of pregnancy status. Future analysis of these miRNA and their downstream targets may point to mechanisms ultimately associated with cognitive function and stress load, which may affect maternal and child health outcomes.
Limitations.
In this study, prenatal depression was measured using the CES-D, which collects self-reported depressive symptoms and is therefore not a diagnostic tool. The current study did not examine diagnosed depression or previous depressive episodes prior to pregnancy, and did not have additional pre-pregnancy samples, especially from those with depression as controls for direct intra-subject comparison. However, the modeling strategy applied here included non-depressed pregnant women from the MADRES cohort (CES-D scores <16), and does more accurately reflect miRNA associated with stress and depression than using only depressed or stressed participants. Depression and other mental health issues are likely underdiagnosed in the MADRES cohort, as well as in many underserved and low-income populations (Lorant 2003), and are likely to be highly correlated, so that miRNA associated with one or more of these stressors should be considered across stressors due to strong pleiotropic effects. Additionally, we acknowledge that miRNA profiles from circulating EVs do not necessarily reflect profiles in brain tissue, and the 142 miRNA treated in this study are a limited set of the thousands of discovered miRNA in humans. However, these circulating EV-associated miRNA likely play specific roles in communication between tissues, including maternal-fetal communication (Bidarimath et al. 2017; Lee, Saadeldin, and Oh 2015). Stress-associated dysregulation of this communication pathway may have potential consequences for the health of both the mother and child. Although the 50 miRNA associated with the stress measures were not significantly correlated between mother and child, these miRNA were still expressed, suggesting that maternal miRNA profiles may influence neonatal expression levels to some extent.
Our results suggest that depressive symptomology and stress during pregnancy affects circulating EV- associated miRNA profiles in mothers. These results are consistent with prior studies which have reported that circulating miRNA profiles differ in non-pregnant people with depression, bipolar disorder, and that miRNA profiles change in response to anti-depressant treatment (Maffioletti et al. 2016; Camkurt et al. 2020; Ceylan et al. 2020; Lopez et al. 2014). MiRNA have been proposed as potential biomarkers for depression, although none are yet well-established (Yuan et al. 2018; Gheysarzadeh et al. 2018). MiRNA with altered expression due to trauma, stress, and depression may affect critical pathways involved in children’s brain function as well as potentially affecting other developing systems in utero. In a clinical context, early assessment of stress, trauma and depression could be used to provide expectant parents with additional resources to limit negative effects on their child’s development and reduce the risks of developing other affective disorders.
While the consequences of stress-induced miRNA changes in pregnancy remain largely unknown, miRNA are estimated to regulate 60% of protein-coding genes, so that even small changes in EV-associated miRNA could have widespread impacts on gene expression in other tissues, affecting multiple downstream target pathways. Although miRNA profiles are not yet characterized or used for diagnostic assessment, future research may tie maternal expression of miRNA to children’s vulnerabilities via these pathways, especially those associated with brain growth and development. These consequences underscore the clinical relevance of evaluating depressive symptoms and stress in pregnancy as well as supporting parents and children, especially from underserved groups in which stress and depression are more prevalent and yet under-diagnosed.
Supplementary Material
Highlights.
Fifty miRNA were nominally associated with stress, depressive symptoms, and prenatal distress (raw p-value<0.05).
Two miRNA (miR-150-5p and miR-148b-3p) were suggestively associated with depressive symptoms after FDR correction (FDR p-value<0.10)
Predicted gene targets for these stress- and depression-associated miRNA were significantly enriched in IGF/Insulin, p53, Ras, EGF, and MAP kinase pathways.
miRNA expression profiles clustered between late pregnancy and cord blood, suggesting a link between maternal miRNA expression during pregnancy and child miRNA expression.
Acknowledgements
The authors would like to thank the MADRES participants, study staff and community clinic partners for their contributions to this work, as well as the SCEHSC lab and USC Genomics Core for their work on miRNA samples.
Funding
This work was supported by National Institute on Minority Health and Health Disparities (NIHMD, www.nimhd.nih.gov) grant 5R01MD011698 and P50MD015705. Laboratory efforts were also supported by the Southern California Environmental Health Sciences Center core facility under grant P30ES007048. CGH is supported by an NIEHS Pathway to Independence Award (R00 ES030400). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations.
- miRNA
microRNA
- GA
gestational age
- MADRES
Maternal and Developmental Risks from Environmental and Social Stressors
- EV
extracellular vesicle
- FDR
false discovery rate
- CES-D
Center for Epidemiologic Studies – Depression
- PSS
Perceived Stress Scale
- PDQ
Prenatal Distress Questionnaire
- ACE
Adverse Childhood Events
Footnotes
Declarations of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheampong Kwabena, Pan Xiongfeng, Kaminga Atipatsa Chiwanda, Wen Shi Wu, and Liu Aizhong. 2021. “Risk of Adverse Maternal Outcomes Associated with Prenatal Exposure to Moderate-Severe Depression Compared with Mild Depression: A Fellow-up Study.” Journal of Psychiatric Research 136 (April): 32–38. 10.1016/j.jpsychires.2021.01.036. [DOI] [PubMed] [Google Scholar]
- Avissar-Whiting Michele, Veiga Keila R., Uhl Kristen M., Maccani Matthew A., Gagne Luc A., Moen Erika L., and Marsit Carmen J.. 2010. “Bisphenol A Exposure Leads to Specific MicroRNA Alterations in Placental Cells.” Reproductive Toxicology 29 (4): 401–6. 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman Sridevi, Schafer Jordan J., Tseng Alexander M., Wertelecki Wladimir, Yevtushok Lyubov, Zymak-Zakutnya Natalya, Chambers Christina D., and Miranda Rajesh C.. 2016. “Plasma MiRNA Profiles in Pregnant Women Predict Infant Outcomes Following Prenatal Alcohol Exposure.” Edited by Ryabinin Andrey E. PLOS ONE 11 (11): e0165081. 10.1371/journal.pone.0165081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastain Theresa M., Chavez Thomas, Habre Rima, Girguis Mariam S., Grubbs Brendan, Toledo-Corral Claudia, Amadeus Milena, et al. 2019. “Study Design, Protocol and Profile of the Maternal And Developmental Risks from Environmental and Social Stressors (MADRES) Pregnancy Cohort: A Prospective Cohort Study in Predominantly Low-Income Hispanic Women in Urban Los Angeles.” BMC Pregnancy and Childbirth 19 (1): 189. 10.1186/s12884-019-2330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidarimath Mallikarjun, Khalaj Kasra, Kridli Rami T., Kan Frederick W. K., Koti Madhuri, and Tayade Chandrakant. 2017. “Extracellular Vesicle Mediated Intercellular Communication at the Porcine Maternal-Fetal Interface: A New Paradigm for Conceptus-Endometrial Cross-Talk.” Scientific Reports 7 (1): 40476. 10.1038/srep40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozack Anne K., Colicino Elena, Rodosthenous Rodosthenis, Bloomquist Tessa R., Baccarelli Andrea A., Wright Robert O., Wright Rosalind J., and Lee Alison G.. 2021. “Associations between Maternal Lifetime Stressors and Negative Events in Pregnancy and Breast Milk-Derived Extracellular Vesicle MicroRNAs in the Programming of Intergenerational Stress Mechanisms (PRISM) Pregnancy Cohort.” Epigenetics 16 (4): 389–404. 10.1080/15592294.2020.1805677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camkurt Mehmet Akif, Karababa İbrahim Fatih, Erdal Mehmet Emin, Kandemir Sultan Basmacı, Fries Gabriel R., Bayazıt Hüseyin, Ay Mustafa Ertan, et al. 2020. “MicroRNA Dysregulation in Manic and Euthymic Patients with Bipolar Disorder.” Journal of Affective Disorders 261 (January): 84–90. 10.1016/j.jad.2019.09.060. [DOI] [PubMed] [Google Scholar]
- Ceylan Deniz, Tufekci Kemal Ugur, Keskinoglu Pembe, Genc Sermin, and Özerdem Ayşegül. 2020. “Circulating Exosomal MicroRNAs in Bipolar Disorder.” Journal of Affective Disorders 262 (February): 99–107. 10.1016/j.jad.2019.10.038. [DOI] [PubMed] [Google Scholar]
- Cohen Sheldon, Kamarck Tom, and Mermelstein Robin. 1983. “A Global Measure of Perceived Stress.” Journal of Health and Social Behavior 24 (4): 385. 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cook Joanna, Bennett Phillip R., Kim Sung Hye, Teoh Tiong Ghee, Sykes Lynne, Kindinger Lindsay M., Garrett Alice, Binkhamis Reem, MacIntyre David A., and Terzidou Vasso. 2019. “First Trimester Circulating MicroRNA Biomarkers Predictive of Subsequent Preterm Delivery and Cervical Shortening.” Scientific Reports 9 (1): 5861. 10.1038/s41598-019-42166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez Kimberly L., Aleman Brenda, and Flores Ana-Mercedes. 2015. “Acculturative Stress Negatively Impacts Maternal Depressive Symptoms in Mexican-American Women during Pregnancy.” Journal of Affective Disorders 176 (May): 35–42. 10.1016/j.jad.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Lisa M., Le Huynh-Nhu, and Garnier-Villarreal Mauricio. 2021. “A Systematic Review and Meta-Analysis of Risk Factors for Postpartum Depression Among Latinas.” Maternal and Child Health Journal 25 (4): 554–64. 10.1007/s10995-020-03104-0. [DOI] [PubMed] [Google Scholar]
- ElKhouly Aisha M., Youness RA, and Gad MZ. 2020. “MicroRNA-486–5p and MicroRNA-486–3p: Multifaceted Pleiotropic Mediators in Oncological and Non-Oncological Conditions.” Non-Coding RNA Research 5 (1): 11–21. 10.1016/j.ncrna.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel Karen A., Silveira Marushka, Pekow Penelope, Braun Barry, Manson JoAnn E., Solomon Caren G., Markenson Glenn, and Chasan-Taber Lisa. 2014. “Prenatal Depressive Symptoms and Abnormalities of Glucose Tolerance during Pregnancy among Hispanic Women.” Archives of Women’s Mental Health 17 (1): 65–72. 10.1007/s00737-013-0379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima Mahino, Srivastav Saurabh, and Mondal Amal Chandra. 2017. “Prenatal Stress and Depression Associated Neuronal Development in Neonates.” International Journal of Developmental Neuroscience 60 (1): 1–7. 10.1016/j.ijdevneu.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Fawcett Emily J., Fairbrother Nichole, Cox Megan L., White Ian R., and Fawcett Jonathan M.. 2019. “The Prevalence of Anxiety Disorders During Pregnancy and the Postpartum Period: A Multivariate Bayesian Meta-Analysis.” The Journal of Clinical Psychiatry 80 (4). 10.4088/JCP.18r12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti Vincent J, Anda Robert F, Nordenberg Dale, Williamson David F, Spitz Alison M, Edwards Valerie, Koss Mary P, and Marks James S. 1998. “Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults.” American Journal of Preventive Medicine 14 (4): 245–58. 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Field Tiffany, Diego Martinez, and Hernandez-Reif Maria. 2006. “Prenatal Depression Effects on the Fetus and Newborn: A Review.” Infant Behavior and Development 29: 445–55. 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Foley Helen Bermudez, Howe Caitlin G., Eckel Sandrah P., Chavez Thomas, Gevorkian Lili, Reyes Eileen Granada, Kapanke Bethany, et al. 2021. “Extracellular Vesicle-Enriched MiRNA Profiles across Pregnancy in the MADRES Cohort.” Edited by Spradley Frank T.. PLOS ONE 16 (5): e0251259. 10.1371/journal.pone.0251259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortner Renée Turzanski, Pekow Penelope, Dole Nancy, Markenson Glenn, and Chasan-Taber Lisa. 2011. “Risk Factors for Prenatal Depressive Symptoms among Hispanic Women.” Maternal and Child Health Journal 15 (8): 1287–95. 10.1007/s10995-010-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett Krassimira A., Vereczkei Andrea, Kálmán Sára, Brown Jacquelyn A., Taylor Warren D., Faludi Gábor, Korade Željka, Shelton Richard C., and Mirnics Károly. 2015. “Coordinated Messenger RNA/MicroRNA Changes in Fibroblasts of Patients with Major Depression.” Biological Psychiatry 77 (3): 256–65. 10.1016/j.biopsych.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysarzadeh Ali, Sadeghifard Nourkhoda, Afraidooni Loghman, Pooyan Farahnaz, Mofid MohammadReza, Valadbeigi Hassan, Bakhtiari Hadi, and Keikhavani Sattar. 2018. “Serum-Based MicroRNA Biomarkers for Major Depression: MiR-16, MiR-135a, and MiR-1202.” Journal of Research in Medical Sciences 23 (1): 69. 10.4103/jrms.JRMS_879_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote Nancy K., Bridge Jeffrey A., Gavin Amelia R., Melville Jennifer L., Iyengar Satish, and Katon Wayne J.. 2010. “A Meta-Analysis of Depression During Pregnancy and the Risk of Preterm Birth, Low Birth Weight, and Intrauterine Growth Restriction.” Archives of General Psychiatry 67 (10): 1012. 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe Caitlin G, Foley Helen B, Kennedy Elizabeth M, Eckel Sandrah P, Chavez Thomas a, Faham Dema, Grubbs Brendan H, et al. 2021. “Extracellular Vesicle MicroRNA in Early versus Late Pregnancy with Birth Outcomes in the MADRES Study.” Epigenetics, March, 1–17. 10.1080/15592294.2021.1899887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen Karen, Lizarraga Daneida, Kogut Katherine, Eskenazi Brenda, and Holland Nina. 2019. “Age-Related Differences in MiRNA Expression in Mexican-American Newborns and Children.” International Journal of Environmental Research and Public Health 16 (4): 524. 10.3390/ijerph16040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappil Maya A., Li Qian, Li An, Dassanayake Priyanthi S., Xia Yulin, Nanes Jessica A., Landrigan Philip J., et al. 2016. “In Utero Exposures to Environmental Organic Pollutants Disrupt Epigenetic Marks Linked to Fetoplacental Development.” Environmental Epigenetics 2 (1): dvv013. 10.1093/eep/dvv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Robert G., Williams Sheila, McGee Rob, and Olaman Susan. 1997. “Psychometric Properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a Sample of Women in Middle Life.” Behaviour Research and Therapy 35 (4): 373–80. 10.1016/S0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- Kuang Wei-Hong, Dong Zai-Quan, Tian Lian-Tian, and Li Jin. 2018. “MicroRNA-451a, MicroRNA-34a-5p, and MicroRNA-221–3p as Predictors of Response to Antidepressant Treatment.” Brazilian Journal of Medical and Biological Research 51 (7): e7212. 10.1590/1414-431x20187212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Byeong, Saadeldin Islam, and Oh Hyun Ju. 2015. “Embryonic–Maternal Cross-Talk via Exosomes: Potential Implications.” Stem Cells and Cloning: Advances and Applications, July, 103. 10.2147/SCCAA.S84991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Qian, Kappil Maya A, Li An, Dassanayake Priyanthi S, Darrah Thomas H, Friedman Alan E, Friedman Michelle, et al. 2015. “Exploring the Associations between MicroRNA Expression Profiles and Environmental Pollutants in Human Placenta from the National Children’s Study (NCS).” Epigenetics 10 (9): 793–802. 10.1080/15592294.2015.1066960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Juan Pablo, Lim Raymond, Cruceanu Cristiana, Crapper Liam, Fasano Caroline, Labonte Benoit, Maussion Gilles, et al. 2014. “MiR-1202 Is a Primate-Specific and Brain-Enriched MicroRNA Involved in Major Depression and Antidepressant Treatment.” Nature Medicine 20 (7): 764–68. 10.1038/nm.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant V 2003. “Socioeconomic Inequalities in Depression: A Meta-Analysis.” American Journal of Epidemiology 157 (2): 98–112. 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- Maffioletti Elisabetta, Cattaneo Annamaria, Rosso Gianluca, Maina Giuseppe, Maj Carlo, Gennarelli Massimo, Tardito Daniela, and Bocchio-Chiavetto Luisella. 2016. “Peripheral Whole Blood MicroRNA Alterations in Major Depression and Bipolar Disorder.” Journal of Affective Disorders 200 (August): 250–58. 10.1016/j.jad.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Manganas Louis N., Durá Irene, Osenberg Sivan, Semerci Fatih, Tosun Mehmet, Mishra Rachana, Parkitny Luke, Encinas Juan M., and Maletic-Savatic Mirjana. 2021. “BASP1 Labels Neural Stem Cells in the Neurogenic Niches of Mammalian Brain.” Scientific Reports 11 (1): 5546. 10.1038/s41598-021-85129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden WN 2013. “Synaptic Plasticity in Depression: Molecular, Cellular and Functional Correlates.” Progress in Neuro-Psychopharmacology and Biological Psychiatry 43 (June): 168–84. 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Marsit Carmen. 2015. “Placental Epigenetics in Children’s Environmental Health.” Seminars in Reproductive Medicine 34 (01): 036–041. 10.1055/s-0035-1570028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano F, Faienza MF, Caratozzolo MF, Brunetti G, Chiara M, Horner DS, Annese A, et al. 2018. “Pilot Study on Circulating MiRNA Signature in Children with Obesity Born Small for Gestational Age and Appropriate for Gestational Age.” Pediatric Obesity 13 (12): 803–11. 10.1111/ijpo.12439. [DOI] [PubMed] [Google Scholar]
- Mi Huaiyu, Ebert Dustin, Muruganujan Anushya, Mills Caitlin, Albou Laurent-Philippe, Mushayamaha Tremayne, and Thomas Paul D. 2021. “PANTHER Version 16: A Revised Family Classification, Tree-Based Classification Tool, Enhancer Regions and Extensive API.” Nucleic Acids Research 49 (D1): D394–403. 10.1093/nar/gkaa1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracht Katharina, Meinzinger Julia, Schulz Sebastian R., Daum Patrick, Côrte-Real Joana, Hauke Manuela, Roth Edith, et al. 2021. “MiR-148a Controls Metabolic Programming and Survival of Mature CD19-Negative Plasma Cells in Mice.” European Journal of Immunology 51 (5): 1089–1109. 10.1002/eji.202048993. [DOI] [PubMed] [Google Scholar]
- Ruan CS, Zhou FH, He ZY, Wang SF, Yang CR, Shen YJ, Guo Y, et al. 2015. “Mice Deficient for Wild-Type P53-Induced Phosphatase 1 Display Elevated Anxiety- and Depression-like Behaviors | Elsevier Enhanced Reader.” Neuroscience 293: 12–22. 10.1016/j.neuroscience.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Sun Lidong, Bai Donghao, Lin Maoguang, Eerdenidalai Li Zhang, Wang Fengzhen, and Jin Shangwu. 2021. “MiR-96 Inhibits SV2C to Promote Depression-Like Behavior and Memory Disorders in Mice.” Frontiers in Behavioral Neuroscience 14 (March): 575345. 10.3389/fnbeh.2020.575345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutaria Shailen, Devakumar Delan, Yasuda Sílvia Shikanai, Das Shikta, and Saxena Sonia. 2019. “Is Obesity Associated with Depression in Children? Systematic Review and Meta-Analysis.” Archives of Disease in Childhood 104 (1): 64–74. 10.1136/archdischild-2017-314608. [DOI] [PubMed] [Google Scholar]
- Tokar Tomas, Pastrello Chiara, Rossos Andrea E M, Abovsky Mark, Hauschild Anne-Christin, Tsay Mike, Lu Richard, and Jurisica Igor. 2018. “MirDIP 4.1—Integrative Database of Human MicroRNA Target Predictions.” Nucleic Acids Research 46 (D1): D360–70. 10.1093/nar/gkx1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Corral Claudia M., Gao Lu, Chavez Thomas, Grubbs Brendan, Habre Rima, Dunton Genevieve F., Bastain Theresa, and Breton Carrie V.. 2021. “Role of Race, Ethnicity, and Immigration in Perceived Stress and Depressive Symptomatology Trends During Pregnancy.” Journal of Immigrant and Minority Health, July. 10.1007/s10903-021-01235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsochandaridis Marthe, Nasca Laurent, Toga Caroline, and Levy-Mozziconacci Annie. 2015. “Circulating MicroRNAs as Clinical Biomarkers in the Predictions of Pregnancy Complications.” BioMed Research International 2015: 1–8. 10.1155/2015/294954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera Sandra, Ameling Sabine, Wittfeld Katharina, Rowold Enrique d’Harcourt, Nauck Matthias, Völzke Henry, Suhre Karsten, et al. 2019. “Association of Childhood Traumatization and Neuropsychiatric Outcomes with Altered Plasma Micro RNA-Levels.” Neuropsychopharmacology 44 (12): 2030–37. 10.1038/s41386-019-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggott Daryl, Chu Kenneth, Yin Shaoming, Wouters Bradly G., Liu Fei-Fei, and Boutros Paul C.. 2012. “NanoStringNorm: An Extensible R Package for the Pre-Processing of NanoString MRNA and MiRNA Data.” Bioinformatics (Oxford, England) 28 (11): 1546–48. 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Yunqiang, Liu Yuanhui, Wang Xiaobin, Wu Jiali, Liu Kezhi, Zhou Jun, Liu Li, and Zhang Chunxiang. 2015. “Identification of Differential MicroRNAs in Cerebrospinal Fluid and Serum of Patients with Major Depressive Disorder.” Edited by Jeyaseelan Kandiah. PLOS ONE 10 (3): e0121975. 10.1371/journal.pone.0121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, and Harris MG. 2017. “A Systematic Review and Meta-Regression of the Prevalence and Incidence of Perinatal Depression.” Journal of Affective Disorders 219 (September): 86–92. 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Yali AM, and Lobel M. 1999. “Coping and Distress in Pregnancy: An Investigation of Medically High Risk Women.” Journal of Psychosomatic Obstetrics & Gynecology 20 (1): 39–52. 10.3109/01674829909075575. [DOI] [PubMed] [Google Scholar]
- Yang Haiou, Ma Qianqian, Wang Yu, and Tang Zhenhua. 2020. “Clinical Application of Exosomes and Circulating MicroRNAs in the Diagnosis of Pregnancy Complications and Foetal Abnormalities.” Journal of Translational Medicine 18 (1): 32. 10.1186/s12967-020-02227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Heidi, Mischoulon David, Fava Maurizio, and Otto Michael W.. 2018. “Circulating MicroRNAs as Biomarkers for Depression: Many Candidates, Few Finalists.” Journal of Affective Disorders 233 (June): 68–78. 10.1016/j.jad.2017.06.058. [DOI] [PubMed] [Google Scholar]
- Zhang Wen-Juan, Cao Wen-Yu, Huang Yan-Qing, Cui Yan-Hui, Tu Bo-Xuan, Wang Lai-Fa, Zou Guang-Jing, et al. 2019. “The Role of MiR-150 in Stress-Induced Anxiety-Like Behavior in Mice.” Neurotoxicity Research 35 (1): 160–72. 10.1007/s12640-018-9943-x. [DOI] [PubMed] [Google Scholar]
- Zhu Yanan, Tian Fei, Li Hailing, Zhou Youxia, Lu Jiafeng, and Ge Qinyu. 2015. “Profiling Maternal Plasma MicroRNA Expression in Early Pregnancy to Predict Gestational Diabetes Mellitus.” International Journal of Gynecology & Obstetrics 130 (1): 49–53. 10.1016/j.ijgo.2015.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.