Abstract
T cells from Mycobacterium avium-infected C57BL/6 mice reacted to culture filtrate, envelope, and cytosol proteins and to fractions obtained from these proteins. Multiple targets were recognized, such as 29- to 45-kDa and <21-kDa antigens of the culture filtrate, antigens of around 30 kDa in the envelope and cytosol, and 45- to 116-kDa proteins in the envelope.
The identification of the key antigenic targets of the immune response to mycobacteria is of pivotal importance in the design and testing of new vaccine candidates against mycobacterial pathogens, most notably Mycobacterium tuberculosis. Work from several laboratories has identified secreted/exported proteins from M. tuberculosis as the major targets of a protective immune response to experimental tuberculosis infections (2, 3, 5, 12, 13, 17, 18, 20). These antigens are also favored targets during an immune response in human patients infected with the tubercle bacilli (17). However, other proteins believed not to be excreted by the mycobacterial cells have also been identified as important targets, namely in the induction of protective immune responses in experimental animals (14, 23), suggesting that the complete picture of the immune response to mycobacterial infections may be more complex with regard to the antigenic repertoire recognized in vivo. Mycobacterium avium is an opportunistic pathogen that is thought to interfere, in certain areas of the world, with the efficacy of the only tuberculosis vaccine in use today, the attenuated Mycobacterium bovis strain bacille Calmette-Guérin (BCG) (9). The reason for the failure of vaccination trials is not known, but it has been speculated that sensitization of human beings to antigens from environmental mycobacteria might affect BCG efficacy, most likely because all mycobacteria would share common antigens. Sharing of antigens between nontuberculous mycobacteria and BCG was already shown to occur at the immunological level in mice (15). However, except for two defined antigens, the latter study used crude extracts that combined many different antigens. The sharing of common antigens between M. avium, BCG, and M. tuberculosis will be understood in the near future thanks to efforts in the area of genomics. However, we still need studies in the fields of proteomics and immunology to generate functional data and therefore to be able to critically analyze the genomic information, namely studying native proteins rather than recombinant ones, as the latter may lack the immunogenicity of the former (1, 21). Thus, we initiated the characterization of the T-cell response to M. avium protein antigens using a mouse model of infection.
We isolated antigens from M. avium 2447, an AIDS patient isolate obtained from F. Portaels (Institute of Tropical Medicine, Antwerp, Belgium) that forms smooth transparent colonies when cultured on solid media. Mycobacteria from log-phase cultures were inoculated into Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose (Sauton P+G) (8) and with no detergent, at a final concentration of approximately 5 × 106 CFU/ml (according to the absorbance measured at 600 nm), and grown at 37°C without shaking. The number of viable bacteria and the smooth transparent morphotype were confirmed by plating serial dilutions of the cultures on solid Middlebrook 7H10 medium (Difco, Sparks, Md.). At the end of log phase (i.e., at day 15 as evaluated from the previous growth curves), cultures were centrifuged for processing of bacterial antigens. Culture filtrate proteins were obtained from the filter-sterilized supernatant of the culture by ultrafiltration in a stirred cell (Millipore, Bedford, Mass.) with an Amicon YM membrane (molecular weight cut-off [MWCO] of 3,000) (Millipore). The concentrate was precipitated with 80% ammonium sulfate, and the precipitate was washed in a Centriprep (MWCO of 3,000) (Millipore). Cytosolic and envelope proteins were obtained after the pellet was washed twice with phosphate-buffered saline (PBS), resuspended in PBS containing 0.1% Tween 80 (Sigma), 1 mM MgCl2 (Merck, Darmstadt, Germany) and 1 mM benzamidine (Sigma) (10), and disrupted through sonication with pulses of 1 min at maximum power, with the sample kept in ice during the whole procedure. The sonicate was centrifuged to discard intact mycobacteria (30 min at 2,700 × g), and the supernatant was dialyzed against PBS (MWCO of 12,000). The suspension was then ultracentrifuged for 2 h at 150,000 × g. The pellet containing the envelope proteins was resuspended in PBS, and the supernatant enriched in cytosolic proteins was precipitated with 80% ammonium sulfate and dialyzed against PBS.
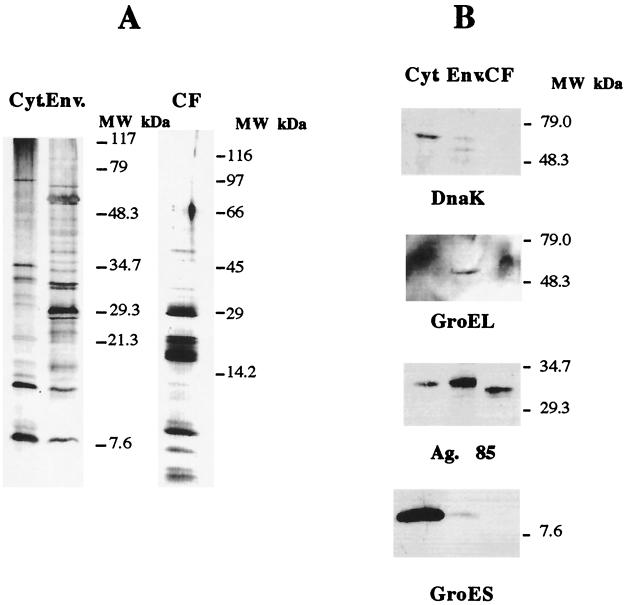
Following the preparative procedures described above, we obtained crude extracts which were analyzed to assess if they were distinct sources of antigens. Cytosolic, envelope, and culture filtrate proteins (20 μg) were separated in a 10 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and analyzed either by silver staining or by Western blotting after transfer to a PROTON nitrocellulose (Schleicher and Schuell, Dassel, Germany) membrane in a semidry electrophoretic transfer cell (Bio-Rad, Richmond, Calif.). During the latter procedure, the membrane was blocked in PBS containing 0.5% Tween 20 (Sigma), incubated for 2 h at room temperature with the antibodies diluted (1:50) in PBS–0.05% Tween–0.37 M NaCl, and washed in the dilution buffer. The primary antibodies used were specific for DnaK (clone HÅT 3), GroEL (clone HÅT 5), GroES (clone HYB 76-1), and Ag85 (clone HYT 27), and they were kindly provided by Peter Andersen (Statens Seruminstitut, Copenhagen, Denmark). The secondary antibody, a horseradish peroxidase-coupled sheep anti-mouse antibody (Amersham-Pharmacia Biotech, Little Chalfont, United Kingdom) was incubated at a 1:500 dilution for 2 h at room temperature. The specific protein complexes were detected using the ECL reagents (Amersham-Pharmacia Biotech).
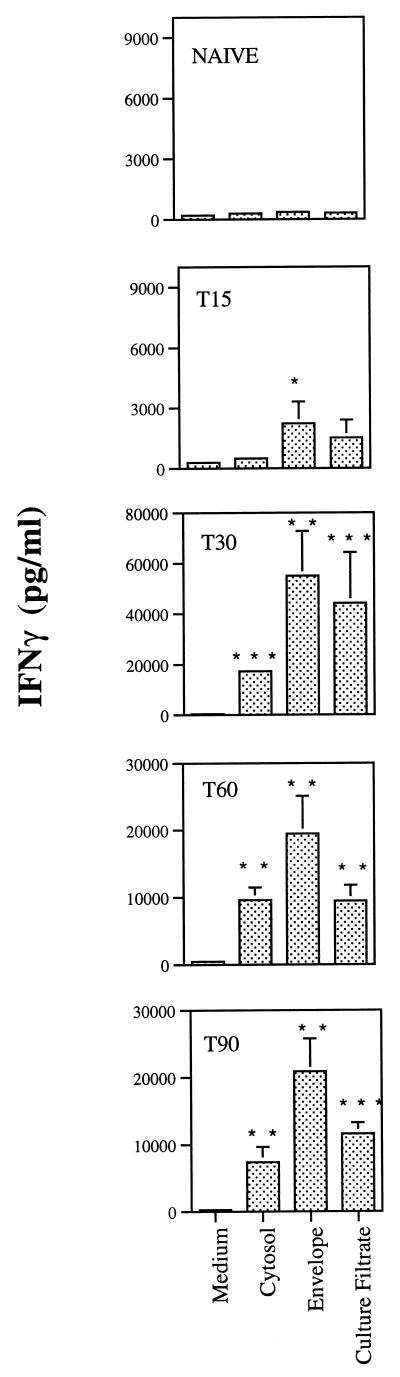
The protein profiles observed in the silver-stained gels for the three fractions were different, with distinct, different major bands obtained with the three preparations (Fig. 1A). The immunochemical analysis by the Western blotting with the panel of monoclonal antibodies against well-defined mycobacterial proteins (DnaK, GroEL, Ag85 complex, and GroES) showed that the three protein preparations corresponded to distinct cellular compartments. Thus, the DnaK and GroES proteins were mostly found in the cytosolic preparations, whereas the GroEL protein was found exclusively in the envelope fraction. Although we could find Ag85 in all three compartments, the molecular weight (MW) pattern varied, with higher-MW forms being found in the cytosol and envelope and a lower-MW form being found in the culture filtrate. We thus conclude that the culture filtrate and envelope preparations have negligible contamination with cytosol proteins. These preparations were then studied in terms of their ability to stimulate T cells isolated from infected mice. Six-week-old C57BL/6J female mice (Harlan Iberia, Madrid, Spain) were infected intravenously with 106 CFU of M. avium 2447 (smooth transparent morphotype) through a lateral tail vein at different time points and sacrificed on the same day at different times of infection to perform the in vitro stimulation of splenic cells. At days 15, 30, 60, and 90 after infection, spleens were removed and single-cell suspensions were prepared. Cells were washed with RPMI–2% fetal calf serum (FCS), and the erythrocytes were lysed with a hemolytic solution (155 mM NH4Cl–10 mM KHCO3, pH 7.2). The cells were cultured in Dulbecco's modified Eagle medium (Life Technologies, Paisley, United Kingdom) supplemented with HEPES buffer, pyruvate, and 10% FCS. The cells were cultured in 96-well, round-bottom, microtiter tissue culture plates (Nunc, Roskilde, Denmark), each well containing 2 × 105 cells in a volume of 200 μl with no stimulus or incubation in the presence of antigen at a final concentration of 4 μg of crude extract/ml. Culture supernatants from triplicate wells were harvested 72 h later for the detection of gamma interferon (IFN-γ) as a readout of the response of those T cells to the different antigens using an enzyme-linked immunosorbent assay (ELISA) as previously described (22). As shown in Fig. 2, the envelope proteins were strong stimulators of IFN-γ production from day 15 of infection. The three preparations had antigens that stimulated T cells from mice infected for 30, 60, or 90 days, with the highest amount of IFN-γ being produced at day 30 of infection.
FIG. 1.
Analysis of crude extracts of M. avium 2447 proteins. Cytosol (Cyt.), envelope (Env.), and culture filtrate (CF) preparations were analyzed by gradient (10 to 20%) SDS-PAGE followed by silver staining (A) or transferred to nitrocellulose and incubated with antibodies against DnaK, GroEL, Ag85 complex, or GroES followed by ECL-dependent detection of antigen-positive bands (B).
FIG. 2.
Antigenicity of the crude extracts of M. avium 2447 proteins. Single-cell suspensions were prepared from spleens of mice infected for 15 (T15), 30 (T30), 60 (T60), or 90 (T90) days with M. avium 2447 or noninfected (naive) animals and stimulated in vitro with 4 μg of cytosol, envelope, or culture filtrate proteins per ml. IFN-γ release was quantified by ELISA in the 72-h culture supernatants. Results are expressed as means of values of triplicate samples ±1 standard deviation performed on cells pooled from four mice. Statistically significant differences compared to values for nonstimulated cells are labeled: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P = 0.00.
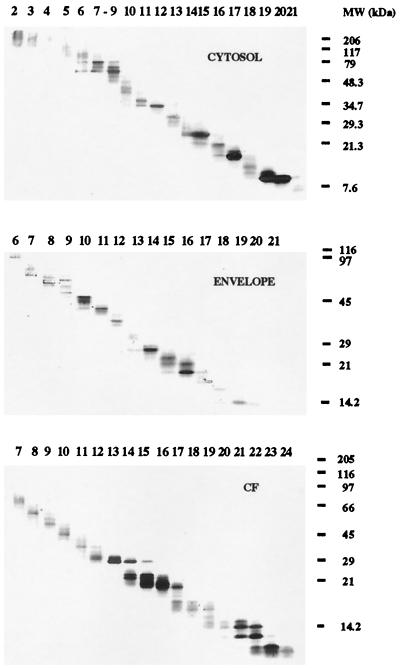
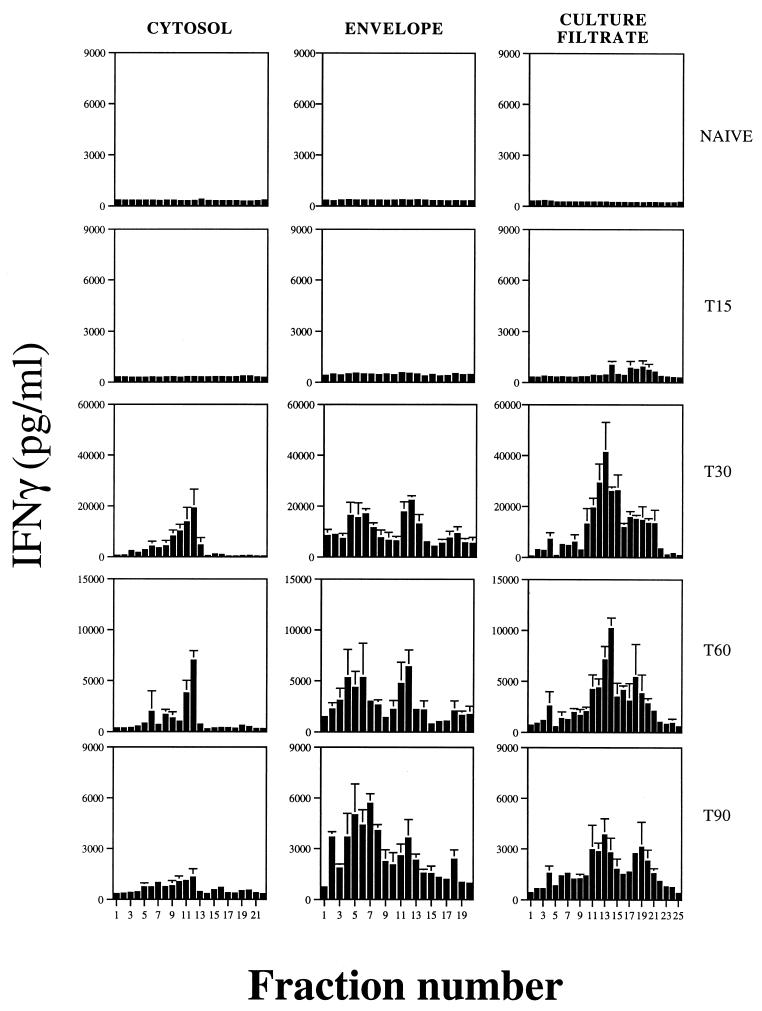
To obtain panels of MW fractions from the culture filtrate, envelope, and cytosol preparations, the multielution technique (4) was used. Briefly, 7 mg of culture filtrate or cytosolic proteins or 9 mg of envelope proteins was separated by SDS-PAGE (with a gradient gel of 10 to 20% acrylamide), and the gel was prepared for electroelution as described before (4). The proteins were electroeluted (40 V) for 20 min into a 2-mM phosphate buffer in a whole-gel eluter (Bio-Rad) in a cold room. The fractions were collected and analyzed (40 μl of each fraction) in a gradient SDS-PAGE (10 to 20% acrylamide) after fixation and silver staining (16). Protein concentration was quantified by the Micro BCA method (Pierce, Rockford, Ill.). The fractions were stabilized with 0.5% FCS in PBS. The SDS-PAGE analysis of the fractions obtained showed that most of them contained proteins in a very narrow range of MWs (Fig. 3), thereby greatly reducing the complexity of the crude extracts. The fractions obtained were then used to study the antigenic specificity of the T cells from infected mice, as detailed above (Fig. 4). The in vitro stimulation of spleen cells with the MW fractions obtained from the three different compartments at a final concentration of 2 μg/ml showed that the response to the culture filtrate fractions emerged at day 15, earlier than the response to the cytosol or envelope fractions. At day 30 of infection, the peak of the response, the strongest stimulators of T cells were found among fractions of culture filtrate in the 29,000-to-45,000 MW range. Fraction 13 from the culture filtrate, which was enriched in proteins in the 30-kDa region (where proteins such as Ag85 complex, a group of proteins that are highly immunogenic among mycobacteria (11, 24), are expected to locate), induced the strongest IFN-γ production. Fractions 12 of the cytosol and envelope, which contain proteins in the same region of MW, were also very immunogenic. Fractions from the envelope between 45 and 116 kDa contained highly stimulatory proteins that were absent from the corresponding MW fractions of the culture filtrate. On the other hand, there was a group of fractions in the culture filtrate below 21 kDa (fractions 17 to 21), which did not include the lowest-MW fractions, that were also inducers of IFN-γ production.
FIG. 3.
Fractionation of cytosol, envelope, and culture filtrate (CF) proteins. Proteins were separated by SDS-PAGE and electroeluted as described in the text. The different MW fractions were analyzed on a silver-stained SDS-PAGE gel.
FIG. 4.
Antigenicity of the fractions obtained from cytosol, envelope, and culture filtrate proteins (the fraction numbers on this figure correspond to the numbers in Fig. 3). Spleen cells of noninfected mice or mice infected with M. avium 2447 were stimulated in vitro with 2 μg of each fraction per ml. IFN-γ release was quantified by ELISA with culture supernatants.
The antigen repertoire recognized by IFN-γ-secreting T cells during an M. avium infection in mice revealed a highly diverse set of protein antigens. The antigen targets were not confined to the secreted/exported proteins but rather were promiscuous among all compartments of the mycobacterial cell when grossly dissected into secreted/exported (culture filtrate), envelope, and cytoplasmic proteins. Several groups have favored secreted/exported proteins as the major targets of the protective immune response to M. tuberculosis (2, 3, 5, 12, 13, 17, 18, 20). In addition, cell wall-associated proteins have also been shown to evoke an immune response during infection (17), suggesting their shedding from the cell wall during growth of mycobacteria inside the vacuoles of the infected macrophage. Finally, the response to cytoplasmic antigens would, according to some, appear later in infection as a result of the killing of the mycobacteria, and this response would be associated not with a protective IFN-γ-mediated immune response but most likely with a type 2 immune response, which is putatively associated with removal of the dead mycobacteria (19). Our data confirm the above paradigm by showing important reactivity towards secreted/exported as well as envelope proteins. However, IFN-γ-secreting T cells were also found to respond to the cytosolic proteins, namely to fractions of around 30 to 32 kDa, characteristic of the Ag85 complex. Although it is believed that this is a typical secretion antigen (24, 25), we detected its presence in both the somatic (cytosol and envelope) and secreted fractions. Curiously, the former forms had a higher MW than the secreted form. The fact that Ag85 is found in the envelope is not surprising due to its function in the synthesis of the cell wall (7). Its presence in the cytosol may be due to contamination during preparation of the antigens, e.g., by its release from the cell wall during sonication of the bacilli, and it could justify reactivity to the cytosol. Otherwise, we are rather confident that cross-contamination represents negligible components of each fraction, since we found no traces of DnaK, GroEL, or GroES in the culture filtrates, whereas their presence in the cytoplasm or envelope was clearly detectable by Western blotting. This finding contrasts with the data reported for M. tuberculosis, where the heat shock proteins DnaK, GroEL, and GroES were found in the short-term culture filtrate (3). Although GroES has been described as a major T-cell antigen recognized by cells from tuberculosis patients and infected animals (6, 18), we failed to observe any reactivity to the fractions of the cytosol expected to contain this antigen. This might be explained by the fact that only the molecule isolated from culture filtrates is able to stimulate T cells, as elegantly shown by Rosenkrands and colleagues (21).
The fractionation techniques utilized in this work are not precise enough to identify single antigens but are suitable for an initial screening of the responses against the whole proteome. They are adequate for the kind of kinetic study presented here, which would be extremely laborious with other methods, such as those relying on two-dimensional separation procedures. They also have the advantage of using native antigens, which may differ from the recombinant products. The data generated here may, on the other hand, guide us to select groups of antigens separated through more potent techniques. Our data also raise interesting speculations regarding the field of vaccine development by suggesting that antigens from different compartments may be adequate candidates for the generation of protection. Also, it will be important to understand whether an improvement on the protective efficacy of subunit vaccines based on culture filtrate protein can be obtained by adding proteins from other sources of the mycobacterial cell. The fact that the responses to the antigens showed distinct kinetics raises the possibility that antigens from different compartments may be involved in protection at distinct stages of the disease. In this context it should be mentioned that vaccination with HSP60-expressing DNA vaccines had a major impact on experimental tuberculosis when performed during infection as a therapeutical vaccine, whereas a similar construct expressing a secreted antigen had no effect (14); on the other hand, both antigens prevented infection when given prior to bacillary challenge (3, 23). It would be interesting to follow up the present observations and reanalyze the antigenic repertoire in experimental tuberculosis. Finally, it should be stressed that the reactivities against M. avium proteins observed here were determined by the proteins expressed during culture in a particular medium. Other proteins may be expressed in other media and, more importantly, other proteins may be expressed in vivo and not in vitro. It is therefore still necessary to broaden this type of analysis to fully understand the nature of the immunogenic proteome of M. avium in the context of infection.
Acknowledgments
We are indebted to P. Andersen for his support in the setting up of the analytical methods used, for the gift of reagents, and for fruitful discussions.
The work was supported by contracts ERBIC18CT970254 from the INCO/DC Programme (European Commission) and BIA247/94 from the PRAXIS Programme (Lisbon). T.F.P. and J.F.C. received fellowships from PRAXIS.
REFERENCES
- 1.Abou-Zeid C, Gares M-P, Inwald J, Janssen R, Zhang Y, Young D B, Hetzel C, Lamb J R, Baldwin S L, Orme I M, Yeremeev V, Nikonenko B V, Apt A S. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856–1862. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Heron I. Simultaneous electroelution of whole SDS-polyacrylamide gels for the direct analysis of complex protein mixtures. J Immunol Methods. 1993;161:29–39. doi: 10.1016/0022-1759(93)90195-d. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes P F, Mehra V, Rivoire B, Fong S-J, Brennan P J, Voegtline M S, Minden P, Houghten R A, Bloom B R, Modlin R L. Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J Immunol. 1992;148:1835–1840. [PubMed] [Google Scholar]
- 7.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 8.Collins F M, Lamb J R, Young D B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988;56:1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine P E M. BCG vaccination against tuberculosis and leprosy. Br Med Bull. 1988;44:691–703. doi: 10.1093/oxfordjournals.bmb.a072277. [DOI] [PubMed] [Google Scholar]
- 10.Hirschfield G R, McNeil M, Brennan P J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990;172:1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huygen K, Palfliet K, Jurion F, Hilgers J, ten Berg R, Van Vooren J-P, De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1988;56:3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leal I S, Smedegård B, Andersen P, Appelberg R. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect Immun. 1999;67:5747–5754. doi: 10.1128/iai.67.11.5747-5754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindblad E B, Elhay M J, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowrie D B, Tascon R E, Bonato V L D, Lima V M F, Faccioli L H, Stavropoulos E, Colston M J, Hewinson R G, Silva C L. Therapy of tuberculosis in mice by DNA vaccination. Nature (London) 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 15.Lozes E, Denis O, Drowart A, Jurion F, Palfliet K, Vanonckelen A, de Bruyn J, de Cock M, van Vooren J-P, Huygen K. Cross-reactive immune responses against Mycobacterium bovis BCG in mice infected with non-tuberculous mycobacteria belonging to the MAIS-group. Scand J Immunol. 1997;46:16–26. doi: 10.1046/j.1365-3083.1997.d01-99.x. [DOI] [PubMed] [Google Scholar]
- 16.Morrissey J. Silver stain for proteins in polyacrylamide gels, a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 17.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 18.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 19.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 20.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenkrands I, Weldingh K, Ravn P, Brandt L, Højrup P, Rasmussen P B, Coates A R, Singh M, Mascagni P, Andersen P. Differential T-cell recognition of native and recombinant Mycobacterium tuberculosis GroES. Infect Immun. 1999;67:5552–5558. doi: 10.1128/iai.67.11.5552-5558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva R A, Pais T F, Appelberg R. Evaluation of interleukin 12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–5585. [PubMed] [Google Scholar]
- 23.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 24.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiker H G, Harboe M, Nagai S. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J Gen Microbiol. 1991;137:875–884. doi: 10.1099/00221287-137-4-875. [DOI] [PubMed] [Google Scholar]