Abstract
Background: Polycystic ovarian syndrome (PCOS) is a neuroendocrine metabolic disorder characterized by an irregular menstrual cycle. Treatment for PCOS using synthetic drugs is effective. However, PCOS patients are attracted towards natural remedies due to the effective therapeutic outcomes with natural drugs and the limitations of allopathic medicines. In view of the significance of herbal remedies, herein, we discuss the role of different herbs in PCOS. Methods: By referring to the Scopus, PubMed, Google Scholar, Crossref and Hinari databases, a thorough literature search was conducted and data mining was performed pertaining to the effectiveness of herbal remedies against PCOS. Results: In this review, we discuss the significance of herbal remedies in the treatment of PCOS, and the chemical composition, mechanism of action and therapeutic application of selected herbal drugs against PCOS. Conclusions: The present review will be an excellent resource for researchers working on understanding the role of herbal medicine in PCOS.
Keywords: herbal medicine, infertility, insulin and obesity
1. Introduction
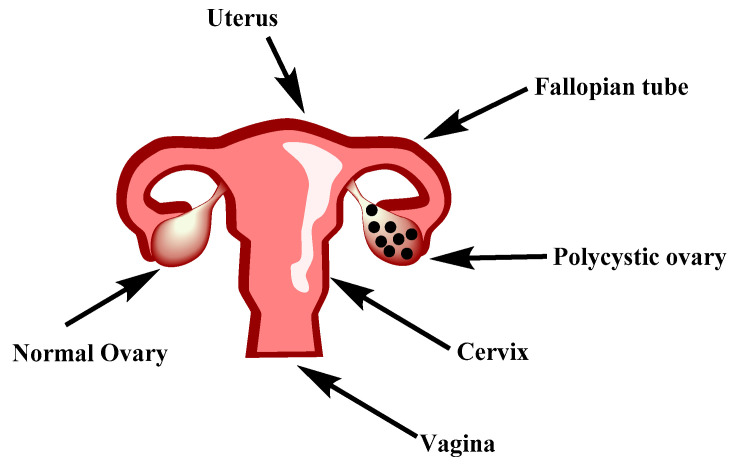
Polycystic ovarian syndrome (PCOS) is a compounded disorder characterized by elevated androgen levels, menstrual irregularities and cysts on either one or both ovaries [1]. The difference between a normal and polycystic ovary is presented in Figure 1. PCOS is believed to be a genetically complex endocrine disorder of undetermined etiology with a complicated pathophysiology [2]. The World Health Organization (WHO) stated that PCOS affected over 116 million women worldwide in 2012. One in five Indian women are affected by PCOS [3]. Globally, 1.55 million incident cases of PCOS in women of reproductive age (15–49 years) were reported, representing an increase in the rate of 4.47% (2.86–6.37%) from 2007 to 2017 [4]. A large-scale survey conducted across India in 2020 showed that around 16% of female respondents between the ages of 20 and 29 years suffered from PCOS [5].
Figure 1.
Differentiation between a normal and polycystic ovary.
The precise etiology and pathophysiology of PCOS are currently being studied. Based on genetics, metabolic factors, and the clinical characteristics of PCOS, several ideas have been proposed, both in utero and postnatally. Obesity exacerbates all aspects of PCOS due to underlying metabolic disturbance. Medical treatment became the preferred treatment over surgical resection of the ovaries when options such as clomiphene and follicle-stimulating hormone (FSH) became available. There was renewed interest in the surgical treatment of PCOS when laparoscopic treatment became popular. Newer technologies such as ultrasound to image ovaries were a breakthrough in the history of PCOS [6]. In around 75% of instances, the cause of infertility is a lack of ovulation. PCOS is the primary cause of infertility in persons who have it.
1.1. Etiology
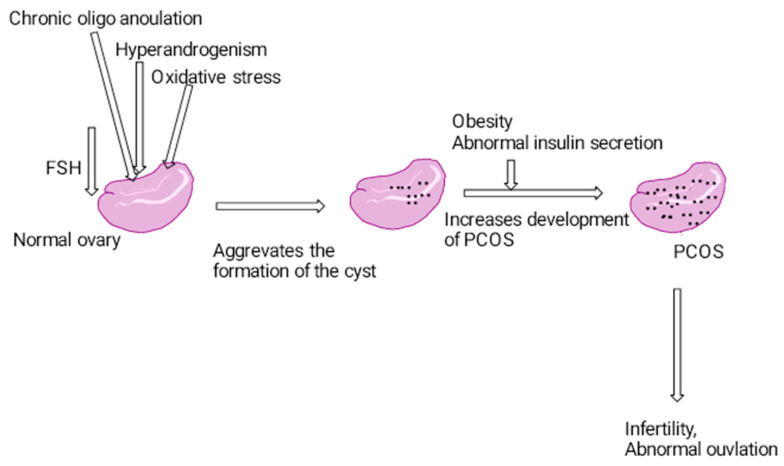
The major etiology behind PCOS is primary disordered gonadotropin secretions, ovarian and adrenal hyperandrogenism and disorder of insulin resistance [7]. The regulation of gonadotropin-releasing hormone (GnRH) is uncontrolled, which may lead to increased luteinizing hormone (LH) and decreased FSH; this may lead to the suppression of the response of ovarian follicles to FSH, elevated anti-Mullerian hormone (AMH), follicular arrest and the increased secretion of testosterone, estradiol and dehydroepiandrosterone [8]. Disrupted ovarian synthesis of steroid hormones in these diseases may result in an increase in circulating androgens, which may be more pronounced in women with polycystic ovarian syndrome [9] (Figure 2). Hyperinsulinism and hypogonadism are considered as the capability of insulin to stimulate gonadal and adrenal androgen production, and this hyperinsulinism is also one of the major risk factors of PCOS [10]. In PCOS, immature follicle development was observed due to increased LH levels and decreasing levels of FSH. Similarly, the increased production of androgens and reduced blood levels of aromatase were observed. Excessive androgens in PCOS is due to elevated abdominal fat, and this may lead to hyperinsulinemia and dyslipidemia. An increase in cell androgen production and hyperinsulinemia reduces sex hormone binding globulin (SHBG) to increase circulating testosterone levels. All these factors may aggravate the disease’s progression [11].
Figure 2.
Pathogenesis of polycystic ovarian syndrome.
1.2. Pathophysiology
The interplay between neuroendocrine levels, metabolic levels and ovarian anomalies may contribute to the development of polycystic ovarian syndrome. However, insulin resistance and obesity are the leading causes of PCOS in the majority of people. Elevated insulin levels cause abnormalities in the hypothalamic–pituitary–ovarian axis [12]. This deviation may lead to the suppression of insulin’s effect on post receptors, elevation of blood levels of free fatty acids, androgens and cytokinins such as TNF-α and IL-6 and the maximum deposition of leptin and resistin in adipocytes in the abdomen [13]. Elevated androgen levels in the blood may cause adipocytes to inhibit adiponectin, resulting in an insulin sensitivity reduction as well as an insulin increase in the blood. However, insulin may also stimulate the synthesis of aldo-keto reductase 1c3 (AKR1C3), which in turn promotes the release of adipose androgens present in females [14]. Oxidative stress also has a role in the etiology of many reproductive problems and anomalies in certain women, including infertility, repeated abortions and preeclampsia. Reductions in blood amino acid levels were found to be significantly induced in PCOS women as compared to healthy controls [15]. In addition to amino acids, a deficiency in vitamins may also induce the metabolic abnormalities that might lead to the development of cysts in the ovary [16]. In a few cases, genome variation in susceptible genes such as cytochrome P1A1, CYP17A1, (CYP1A1), CYP11A, CYP19, 17β-hydroxysteroid dehydrogenase (HSD17B6), androgen receptor (AR), insulin receptor (INSR), sex hormone-binding globulin (SHBG), insulin receptor substrate 1 (IRS1) and peroxisome proliferator-activated receptor gamma (PPAR-γ) [17] is responsible for PCOS. The major signs of PCOS are abnormal blood androgen levels and sugar levels, the presence of cysts in the ovaries and premenstrual syndrome. Obesity, depression, anxiety, eating disorders and chronic metabolic syndrome are also prevalent symptoms in PCOS patients [18]. Furthermore, it will cause an increase in blood pressure and cholesterol levels, which can lead to the development of heart disease and diabetes mellitus. The diagnosis of polycystic ovarian syndrome is performed as per NICHD, AES and RDC [19]. Regular measurement of androgen levels, ultrasonography, the morphology of polycystic ovaries and the wide heterogeneity of polycystic ovarian syndrome are the currently used parameters to predict PCOS [20].
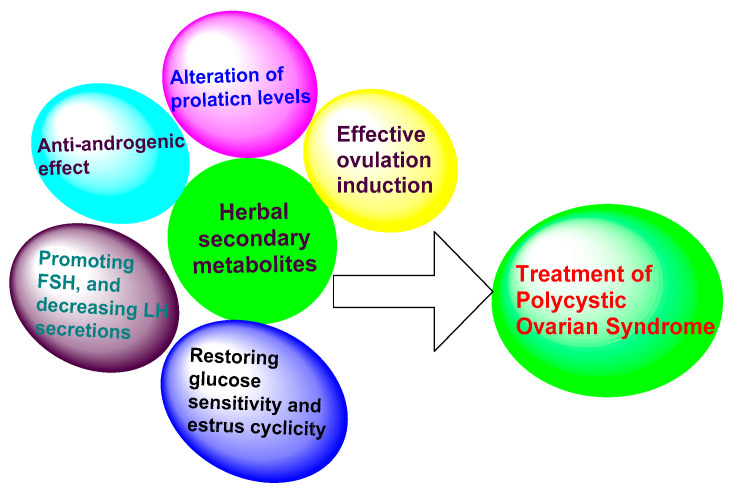
A variety of methods are used in treating PCOS, including laparoscopy and the utilization of allopathic medicines. For instance, in laparoscopy, cysts are removed from the ovaries through a surgical procedure [21,22,23]. Drugs including nafarelin, troglitazone, clomiphene, metformin and spironolactone are currently employed for the treatment of PCOS. However, these drugs can cause major complications with long-term usage, including menstrual abnormalities, nausea, vomiting and gastro-intestinal disturbances, weight gain, increased insulin resistance, less compliance, poor efficacy and more contraindications [7]. Obese people, women of childbearing age, lactating mothers and patients with cardiovascular complications [8] are at a greater risk from the usage of these drugs, due to their side effects. Hence, there is a pressing need to identify and develop drugs of plant origin, which are much more effective than existing allopathic drugs. Recently, the use of herbal medicines by healthcare professionals to treat PCOS has become a major turning point [5]. Herbal medicines are extracts of entire plants or any part of a plant that shows a major therapeutic effect, with fewer side effects when compared to conventional therapy [24]. They have a significant role in prevention, cure and rehabilitation. Herbal drugs are complex interventions with the potential for synergistic and antagonistic interactions between compounds [25]. They are essential for the management of PCOS and have fewer side effects than allopathic medicines [26]. The regular usage of herbs is safe and more efficacious in treating PCOS and suppressing the events that contribute to the development of cysts in PCOS [27]. Currently, herbal remedies are playing a prominent role in treating various chronic disorders, including PCOS. The use of herbal medicines and modifications to the diet may help in treating PCOS more effectively [8]. Different herbs will exert their activity against PCOS through a variety of mechanisms, including the suppression of prolactin levels, anti-androgenic activity, promoting follicle stimulating hormone (FSH), decreasing luteinizing hormone (LH), the induction of ovulation and restoration of glucose sensitivity, estrus cyclicity and enzyme activity (Figure 3).
Figure 3.
Mechanisms through which different herbal secondary metabolites are active in the treatment of PCOS.
Among various herbs, Glycyrrhiza glabra (liquorice), Linum (chaste berry), Vitex negundo (Chinese chaste tree), Foeniculum vulgare (fennel) and Curcuma longa (Turmeric) are potential plant sources that have shown effective action against PCOS [8].
2. Materials and Methods
In view of the significance of herbal remedies in the treatment of PCOS, herein, we discuss the chemical constituents, mechanisms of action and therapeutic application of selected herbal drugs against PCOS by referring to the Scopus, PubMed, Google Scholar, Crossref and Hinari databases. The following is a detailed discussion of the selected herbal drugs that are effective against PCOS via various mechanisms.
3. Results
3.1. Herbs That Increase Ovulatory Cycles
Changes in prolactin levels and hormonal imbalances will have a significant impact on ovulatory cycles. Decreasing prolactin levels or improving the hormonal balance have a positive impact on ovulatory cycles and the treatment of PCOS. These two activities have the potential to reduce cyst formation, dissolve cysts and improve ovulatory cycles. Vitex and turmeric are two herbs that show a beneficial effect in PCOS by increasing the ovulatory cycles.
3.1.1. Vitex agnus castus
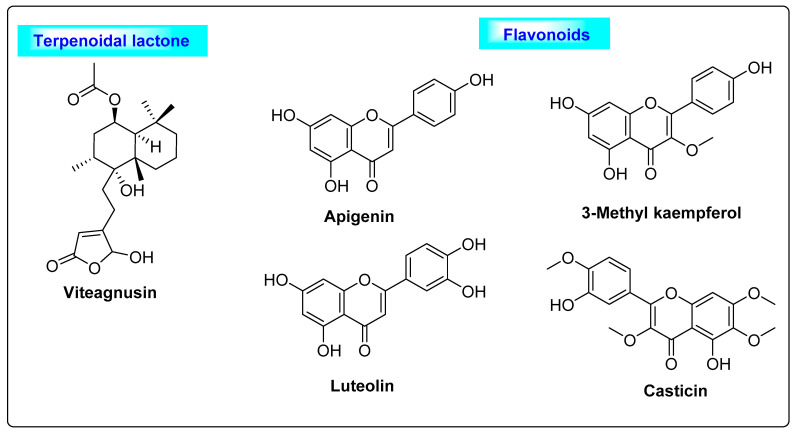
Vitex agnus castus is a Verbenaceae family member that is also known as chaste berry, and it has been used in herbal medicine for the past 2000 years. It is a large shrub native to Europe and is also broadly disseminated in southern regions of the United States. The imbalance of estrogen levels is marked by menstrual cycle abnormalities and premenstrual syndrome, such as insufficiency of the corpus luteum and cyclical mastalgia and post-menopausal hot flashes [28,29,30]. The Vitex fruits mostly contain monoterpenoids, including bornyl acetate, limonene, 1,8–cineol, α-pinene and β-pinene [31,32], and labdane-type diterpenoids, such as viteagnusin, vitexilactone, rotundifuran and vitex lactam A. Moderate amounts of flavanoids such as luteolin, apigenin, 3-methylkaempferol, casticin, chrysoplenetin and chrysosplenol D [33,34] and iridoids such as cynaroside were also reported (Figure 4) [35,36,37]. The diterpenoid viteagnusin and flavonoids including apigenin, 3-methylkaempferol, luteolin and casticin play a major role in suppressing the prolactin levels by inhibiting dopamine-2 (D-2) receptors and increasing the ovulatory cycles [38]. Additionally, the estrogen receptor-β (ERβ)-selective action of the flavonoid apigenin will also help in increasing the ovulatory cycles [39]. All the above mechanisms can further facilitate the suppression of cyst formation in the ovary and may be useful for the treatment of PCOS.
Figure 4.
Terpenoidal lactone and flavonoids of Vitex agnus castus responsible for improving ovulatory cycle.
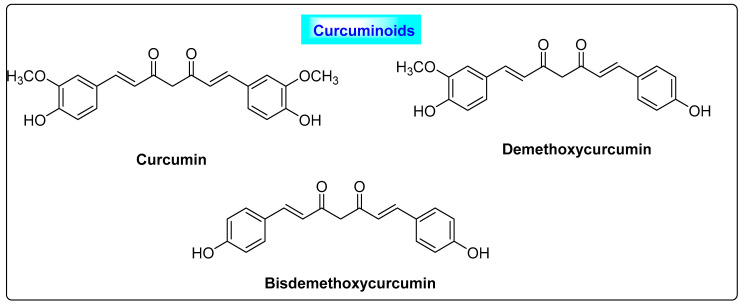
3.1.2. Curcuma longa
Curcuma longa belongs to the family Zingeberaceae and its rhizome is routinely used as a spice in the Asian continent. It is commonly called Curcuma domestica Loir, Curcuma domestica vale, curcuma, yellow ginger, amomum curcuma Jacq, Indian saffron and yellow root. A detailed description of the chemical constituents present in turmeric is provided elsewhere [40]. Turmeric contains a range of primary and secondary metabolites, and more than 250 phytoconstituents have been reported, including carbohydrates (60–70%), proteins (5–10%), terpenes (1.5–5%) and resins. Resins of turmeric, commonly called curcuminoids (2.5–8%), constitute the major secondary metabolites and they are responsible for the color and most of the biological properties. Curcuminoids including curcumin (diferuloylmethane) (71.50–94%), desmethoxycurcumin (6–19.4%) and bisdemethoxycurcumin (0.30–9.10%) are the three most important constituents isolated. It primarily contains phenolic compounds such as curcumins, curcuminoids, ferulic acid, eugenol, ascorbic acid, vanillic acid, caffeic acid, syringic acid, protocatechuic acid, and p-coumaric acid, and terpenoids including turmerone, α-turmerone, camphene, β-sesquiphellandrene, γ-terpinene and carotene (Figure 5) [41]. Curcuminoids have significant effects in the treatment of PCOS. They reduce the follicular sheath and improve the formation of the corpus luteum and the ovulation process. Hence, turmeric improves the histological features of polycystic ovaries. Curcuminoids also suppresses the serum levels of progesterone and elevate the levels of estradiol in women with PCOS [42]. Furthermore, their estrogenic, antihyperlipidemic, antioxidant and hypoglycemic effects are useful in managing PCOS and preventing ovarian cell dysfunction and thereby improving ovulation and fertility.
Figure 5.
Curcuminoids seen in Curcuma longa responsible for improving the PCOS condition.
3.2. Herbs with Anti-Androgen Properties
Elevated blood levels of androgens are also the one of the major etiologies behind PCOS. Hence, drugs with anti-androgen activity are used in the treatment of PCOS. Herbs including Glycyrrhiza glabra, Linum usitatissimum, Mentha spicata, Cocus nucifera and Punica granatum have anti-androgenic action and these herbs could be useful for the management of PCOS.
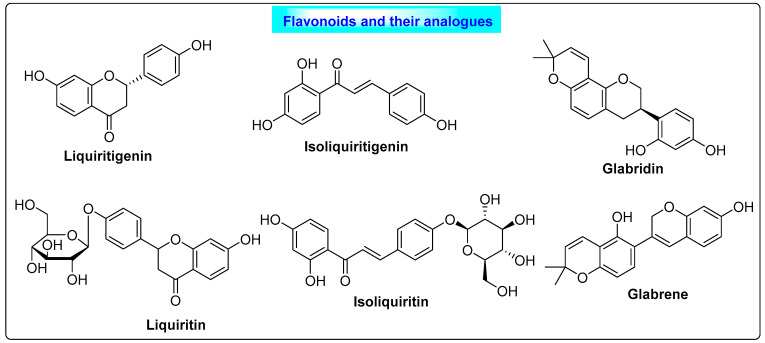
3.2.1. Glycyrrhiza glabra
Glycyrrhiza glabra belongs to the family Fabaceae. It contains mainly 2–9% of glycyrrhizin, glycyrrhizin acid, flavonoids, isoflavonoids, carbohydrates, amino acids and triterpenoid saponins. Liquiritigenin, isoliquiritigenin, liquiritin, isoliquiritin, glabridin and glabrene are the major phytoestrogens found in liquorice [43]. It acts as a potent anti-androgen and helps the body to maintain biosynthesis and the release of estrogen. The flavonoids of Glycyrrhiza (Figure 6) possess estrogenic activity and they interact with estrogenic receptors, and this results in their anti-androgenic effect. Additionally, flavonoids can help in the secretion of insulin, which reduces blood sugar levels and contributes positively to the treatment of PCOS [44]. In addition to its benefits in PCOS, liquorice has other therapeutic roles, such as cough suppression, antibacterial and antiviral activity and treatment for digestive issues, hepatitis and mouth ulcers [3].
Figure 6.
Flavonoids of liquorice that show beneficial effects in PCOS through anti-androgenic activity.
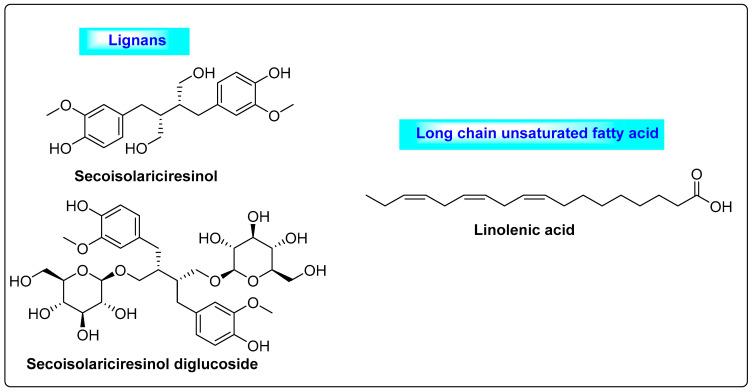
3.2.2. Linum usitatissimum
Linum usitatissimum is commonly called linseed and it belongs to Linaceae. It contains 30–40% of fixed oils, 6–10% mucilage, 25% proteins, saturated and unsaturated fatty acids and lignans (Figure 7). Major lignans include secoisolariciresinol and secoisolariciresinol diglycoside-SDG, and the mucilage of fiber is rich in l-galactose, d-xylose, d-galacturonic acid and l-rhamnose. The secondary metabolites of Linum regulate estrogen production in the body and promote fertility rate as well as the menstrual cycle [45]. Similarly, they decrease androgen levels and considerably reduce elevated levels of testosterone in the blood, which is useful for treating PCOS [46]. Flaxseed also reduces symptoms associated with PCOS, such as hyperandrogenism and hirsutism. Preclinical studies confirmed that the supplementation of flaxseed in the diet decreased the androgen levels in female rats, improved the formation of the corpus luteum and reduced the number of cysts in ovarian follicles [47]. Hence, the regular intake of flaxseed through dietary supplementation could significantly suppress the ovarian volume and follicle size in the ovaries and regulate the frequency of menstrual cycles. It is also used in various disorders, such as neoplasms, diabetes mellitus, arrhythmia, obesity and clotting problems in blood vessels [3].
Figure 7.
Lignans and polyunsaturated fatty acids present in Linum usitatissimum with anti-androgenic activity.
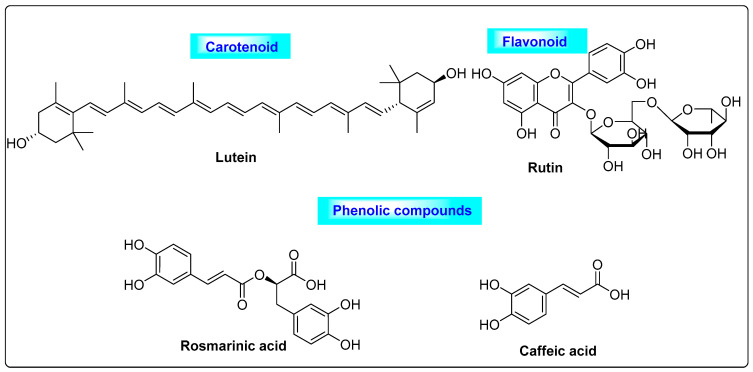
3.2.3. Mentha spicata
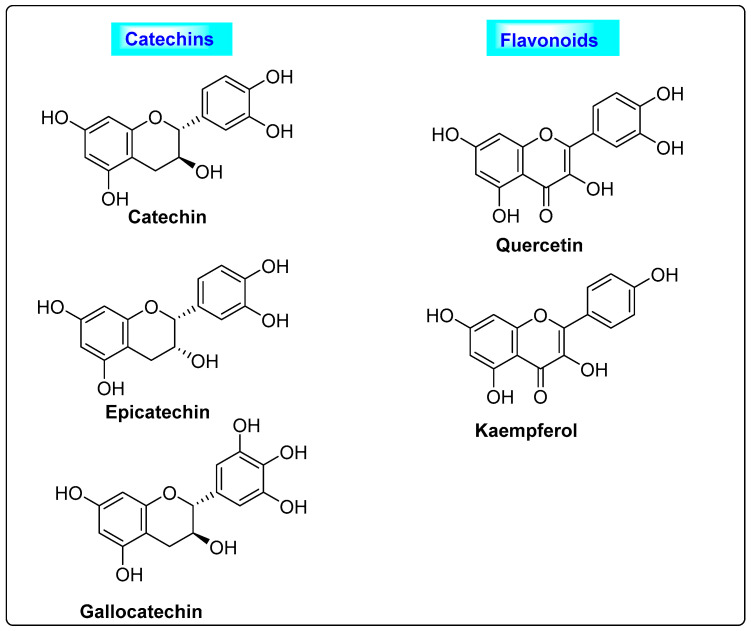
Mentha spicata is a Labiatae member that has been used in culinary applications for many years. It contains carotenoids, such as lutein, and flavonoids and their analogs, including catechin, rutin, xanthomicrol, quercetin-4-glucoside, 5,6-dihydroxy-7,8,3,4-tetramethoxyflavone, sorbifolin, thymosin, hesperidin, gallocatechin-gallate, thymonin, sideritoflavone, ladanein, narirutin, luteolin -7-O-rutinoside, isorhoifolin, eriodictyol-7-O-glucoside and 5-O-demethylnobiletin [48], as well as phenolic compounds such as rosmarinic acid, caffeic acid, salvianolic, dehydro-salvianolic acids and cinnamic acids. The major components that possess good antioxidant effects are lutein, rutin, rosmarinic acid and caffeic acid (Figure 8) [49]. During a 30-day in vivo study of laboratory animals, these compounds reduced the free and total testosterone levels, as well as the degree of hirsutism. Spearmint improves ovarian cysts in PCOS by reducing atretic follicles and enhancing graafian follicles. It has anti-inflammatory, anti-diabetic and anti-cancer properties [50,51]. Mentha regulates the blood ratio of LH and FSH. Based on this regulation of LH/FSH in the blood, it could be useful for the treatment of PCOS. Several preclinical studies have shown that spearmint has anti-androgen properties [52].
Figure 8.
An array of different secondary metabolites of Mentha spicata that possess anti-androgenic activity.
3.2.4. Cocos nucifera
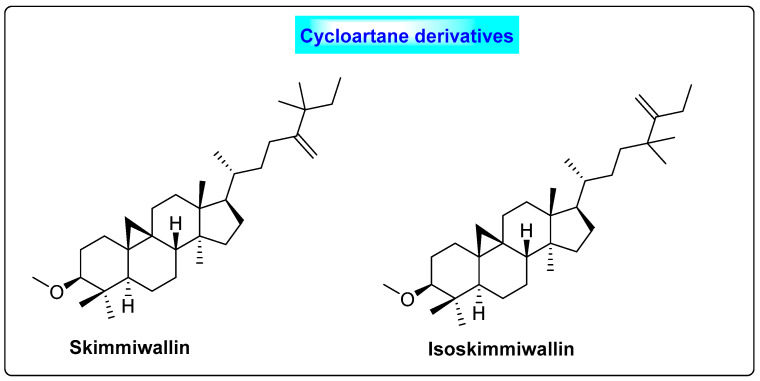
Cocos nucifera belongs to the family Arecaceae. The oil contains primarily alpha-tocopherol and lauric acid, and, in the case of roots, it has rich phenolic compounds, such as flavonoids and saponins [53]. It also contains lupeol methyl ether, skimmiwallin and isoskimmiwallin (Figure 9). C. nucifera contains twenty-five volatile and semi-volatile phytoconstituents [54]. The flavonoid present in Cocos has hypoglycemic effects and reduces blood glucose levels. The lipid methyl (9Z,12Z)-9,12-octadecadienoate present in this plant possesses anti-androgenic properties [55]. C. nucifera regulates the blood levels of sex hormones such as FSH and LH [56]. Preclinical research has shown that Cocos nucifera has a beneficial effect in modifying the histology of the ovaries in PCOS patients, in terms of cyst size and number. Similarly, it also suppresses the weight of the ovary and increases the uterus weight. Based on the regulation of hormones, it could be useful for preventing cyst formation in the ovaries. In India, infusions of a coconut inflorescence taken orally are used to treat menstrual cycle disorders [57]. Similarly, upon oral administration, coconut milk has a contraceptive property [58,59,60].
Figure 9.
Triterpenoids of Cocos nucifera that possess anti-androgenic activity.
3.2.5. Punica granatum
Punica granatum belongs to the family Punicaceae and possesses large amounts of folic acid, vitamins (B2, C and B1), sugars, organic acids and pantothenic acid. Unsaturated and saturated fatty acids are commonly seen in the seeds. The phenolic compounds (catechins and flavonoids) (Figure 10) and phytosterols found in the seed extract have good effects in reducing the complications of PCOS. In women who included Punica granatum in their regular diet, the blood levels of free testosterone, serum estrogen and androstenedione hormone were normalized [61]. Based on the various research conducted on Punica granatum, it is found that the usage of Punica granatum reduces the complications associated with PCOS [3].
Figure 10.
Phenolics present in Punica granatum having anti-androgen properties.
3.3. Herbs That Restore Glucose Sensitivity, Estrus Cyclicity and Enzyme Activity
Decreasing insulin sensitivity and elevated blood glucose levels are also two of the major symptoms observed in women suffering from PCOS. As a result, drugs that increase insulin sensitivity are included in PCOS treatment. Herbs such as Cinnamomum cassia and Aloe vera, which have the same mechanism, can reduce blood glucose as well as regulate the estrus cycle and could be useful.
3.3.1. Cinnamomum cassia
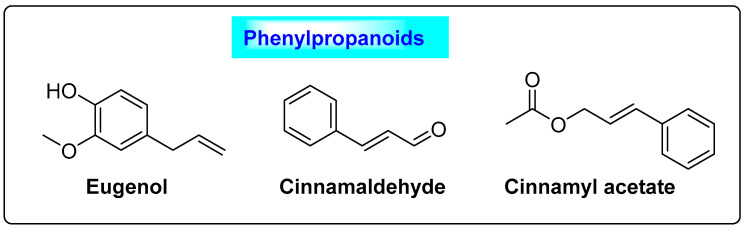
Cinnamomum cassia belongs to the family Lauraceae. An array of polyphenolic compounds has been reported in Cinnamomum cassia, including cinnamyl alcohol, linalool, eugenol, eugenol acetate, methyl eugenol and benzaldehyde, along with procyanidins such as cinnamaldehyde, cinnamyl acetate, caryophyllene, monoterpene, pinene, hydrocarbon, benzyl benzoate, phellandrene, safrole, cymene and cineol (Figure 11) [62]. Cinnamomum contains 80.59% carbohydrates, 59.55–53.1% dietary fiber, 9.5–10.5% moisture, 3.89–4.65% protein, 3.55% ash and vitamins [63]. Phenolic compounds regulate blood insulin levels, as well as promoting glucose uptake and the synthesis of glycogen in the liver [64]. Cinnamon extract improves insulin selectivity in women with PCOS. The procyanidins and polyphenols in cinnamon are responsible for the hypoglycemic effect by stimulating the insulin signaling pathway. Cinnamon is used as an adjunctive in the treatment of PCOS through oral supplementation during the luteal phase, where it could regulate progesterone levels. Similarly, taking cinnamon on a daily basis will help to normalize the menstrual cycle and effectively suppress polycystic ovary syndrome [65]. The ingredients possess good anti-inflammatory and antioxidant properties. They raise the levels of superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) in the blood, while lowering the level of malondialdehyde (MDA) and increasing the likelihood of pregnancy [66]. Moreover, it lowers the fasting blood glucose level and insulin, total cholesterol (TC), low-density lipoprotein (LDL) and triglyceride (TG) concentrations [67]. A series of clinical trials with 183 participants were performed to compare the effects of cinnamon alone and a mixture of herbs to a placebo or a control. Since the I2 of 17% showed that there was no statistical difference between the studies (p = 0.30), the results were combined using a fixed effect model. The overall effect size showed that there was a statistically significant difference in the LDL levels of PCOS patients who consumed cinnamon alone and those who used a mixture of herbs. This means that PCOS patients who ate cinnamon alone or used a mixture of herbs had significantly lower LDL levels.
Figure 11.
Phenylpropanoids of Cinnamomum cassia that exhibit enzymatic activity.
3.3.2. Aloe vera
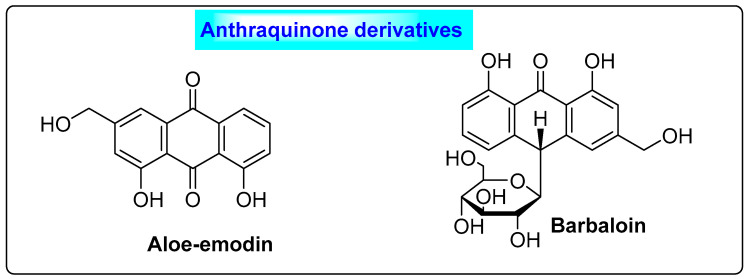
Aloe vera is a Liliaceae plant that contains secondary metabolites such as anthraquinone derivatives, flavonoids, phytosterols, polyphenols and other nutrients. Aloe emodin and barbaloin (Figure 12) are the major constituents of Aloe vera. It was observed that women treated with Aloe vera gel experienced the partial reversion of estrous cyclicity and improved steroidogenic activity. Similarly, in pre-clinical studies on rats, aloe vera not only suppressed 3β-HSD activity and 17β-HSD activity but also reduced the ovary weight, resulting in the suppression of overall androgen secretion. Additionally, it also increased estrogen synthesis by stimulating the flux of the steroidogenesis pathway. It can restore glucose sensitivity, the estrus cycle and the plasma levels of lipoproteins, besides suppressing the biogenesis of cholesterol in the liver [68]. Aloe vera also has a regulating effect on blood lipid and glucose levels, and hence this activity is useful in treating PCOS due to metabolic disturbances [69,70].
Figure 12.
Secondary metabolites of Aloe vera responsible for altering enzymatic activity, which could be beneficial in PCOS.
3.4. Herbs That Promote FSH and Decrease LH Secretions
In PCOS, a common complication is elevated levels of LH and decreased levels of FSH. Hence, drugs that have the ability to elevate the levels of FSH and reduce the concentrations of LH are beneficial in the treatment of PCOS. Herbs including Foeniculum vulgare, Panax ginseng and Cimicifuga racemosa have such actions; hence, they are useful for the treatment of PCOS.
3.4.1. Foeniculum vulgare
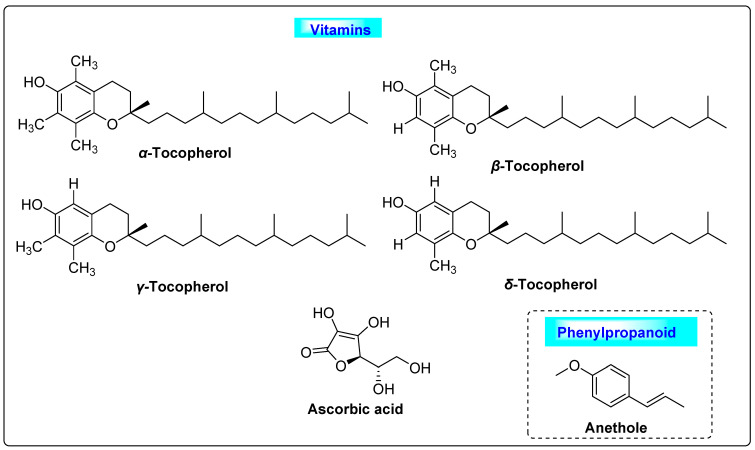
Foeniculum vulgare is usually called fennel and belongs to the family Apiaceae. It has a volatile oil content of 4–5%. Numerous chemical constituents with many therapeutic activities have been seen in fennel. The constituents include trans-anethole, α-pinene estragole, fenchone, 1,8-cineole, β-carotene, myristicin, limonene, β-sitosterol, cinnamic acid, caffeic acid, ferulic acid, fumaric acid, benzoic acid, p-coumaric acid, vanillic acid, kaempferol, quercetin, rutin and vanillin. Fennel contain 50–60% of anethole, phenolic esters, 18–22% of fenchone, fixed oils and proteins [71], as well as vitamins such as α-tocopherol, ascorbic acid, β-tocopherol, γ-tocopherol and δ-tocopherol (Figure 13) [72]. Fennel vitamins have high antioxidant activity and protect the cells from oxidative damage. Anethole promotes menstruation, facilitates birth and also induces estrogenic properties in the ovarian follicle. All these may contribute to the treatment of PCOS. Other pharmacological properties of fennel are useful for the treatment of helminthic infections, neurological disorders and hirsutism. Further, it has tumor suppression, anti-diabetic and hepatoprotective properties [73,74,75].
Figure 13.
Vitamins and phenylpropanoids of Foeniculum vulgare that possess usefulness in PCOS.
3.4.2. Panax ginseng
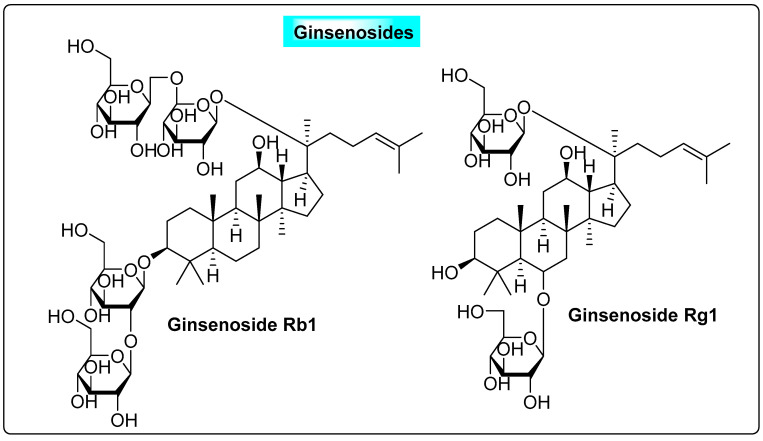
Panax ginseng is known as “the king of herbs” and has been used for more than 2000 years. The saponins found in ginseng are the active molecules responsible for its useful therapeutic activity, and they include major ginsenosides, namely ginsenoside Rb1, Rb2, Rc, Rd, Re, Ro and Ra, and minor ginsenosides (Figure 14). P. ginseng can stimulate the growth of estrogen receptor (ER)-positive (þ) cells in vitro. Ginsenoside Rb1 and Rg1 can stimulate ERs with estrogen-like activity [43]. Hence, this can significantly elevate serum estradiol while suppressing follicle-stimulating hormone (FSH) and luteinizing hormone (LH). A significant decrease in plasma LH levels may be beneficial and effective for improving the fertility rate in PCOS anovulation patients [43]. Ginseng can also help with postmenopausal symptoms such as insomnia, anxiety and depression. It is often used as a natural estrogen replacement therapy, as well as because it can modify the estrus cycle and shows significant estrogenic effects, as suggested by the reversal of atrophy of the vagina and uterus with the upregulated expression of ERα and ERβ in the reproductive tissue. All the above-discussed features of ginseng are positive factors that could contribute to the treatment of PCOS [76].
Figure 14.
Useful anti-PCOS ginsenosides present in Panax ginseng.
3.4.3. Cimicifuga racemosa
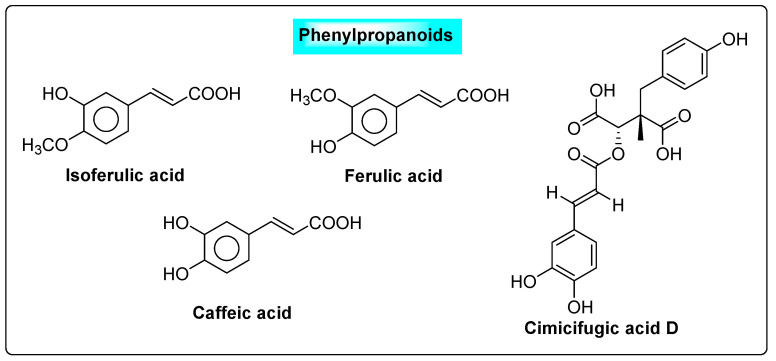
Cimicifuga racemosa is also called black cohosh and belongs to the family Ranunculaceae. Over the last several decades, a large number of researchers have focused nearly entirely on two groups of compounds (triterpene glycosides and phenolic acids). The major phenolic components of black cohosh include hydroxycinnamic acids, ferulic acid, isoferulic acid and caffeic acid, as well as their condensation products with glycolyl phenylpropanoids, commonly known as cimicifugic acids (Figure 15) [77]. These are responsible for the suppression of cysts in the ovary. These compounds exhibit their effects by specifically interacting with the hypothalamus and pituitary estrogen receptors (ERα). The binding of compounds to α-estrogen receptors in the pituitary gland will reduce LH production. The flavonoids decreased the blood levels of LH and also improved pregnancy rates in patients who had ever used clomiphene during a menstrual cycle [78].
Figure 15.
Phenylpropanoids present in Cimicifuga racemosa.
3.4.4. Pimpinella anisum L.
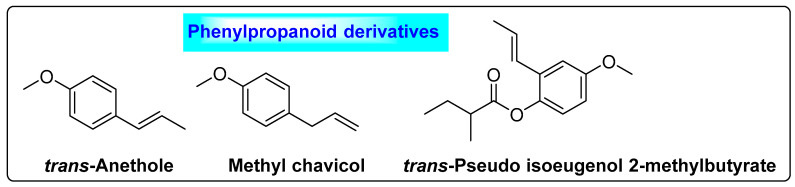
Pimpinella anisum L. is commonly called anisum or sweet cumin and belongs to the family Apiaceae. It contains 9% moisture, 35% sugars, 18% protein, 16% lipids, 7% ash, 5% starch, 12–25% crude fiber, as well as 2–7% essential oil [79]. Additionally, it contains oleoresin, which is a yellowish-green to orange-brown liquid. The major constituents of aniseed oil are trans-anethole (90%), anisketone, anisaldehyde and methyl chavicol. Other minor constituents present in anisum include γ-himachalene (2–4%), trans-pseudo isoeugenol 2-methylbutyrate (1.3%), p-anisaldehyde (1%) and methyl chavicol (0.9–1.5%) (Figure 16) [80,81]. Anethole helps to relieve oligomenorrhea and improve quality of life in women who are undergoing treatment for PCOS. The phenolic ingredients possess phytoestrogenic features, which may play a greater role in the regulation and improvement of menstrual cycles and LH/FSH secretion in women with PCOS and play an important role in relieving PCOS complications [74].
Figure 16.
Phenylpropanoids present in Pimpinella anisum L. that possess anti-PCOS activity.
3.4.5. Trigonella foenum-graecum
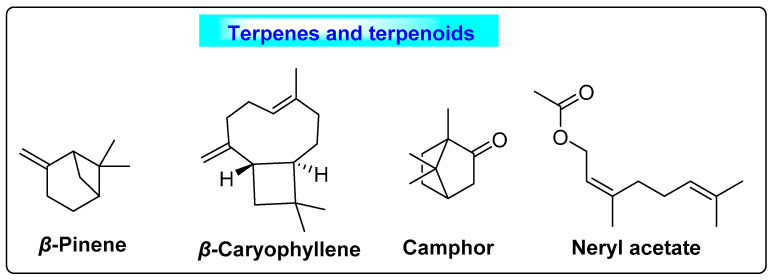
Trigonella foenum-graecum is commonly called “fenugreek” and belongs to the family Fabaceae. The fenugreek fruit contains 8–10% moisture, 45% carbohydrates, 15–28% protein, 6–12% lipid, 4–8% ash, 8–16% fiber and 0.2–0.3% essential oil [82]. The primary components of fenugreek seed oil responsible for the suppression of cysts are β-pinene, β-caryophyllene, camphor and neryl acetate (Figure 17). In the case of volatile oil, the fenugreek seed is rich in sesquiterpenes, n-alkanes and some oxygenated components [83]. It also contains phytosterols, terpenoids, flavonoids (naringenin, saponaretin, lilyn, kaempferol, isovitexin, orientin, vitexin, isoorientin, luteolin and quercetin) alkaloids (choline, trigonelline and carpaine) and saponins (fenugrin, foenugracin, trigonoesides, glycoside, yamogenin, smilagenin, yuccagenin, sarsasapogenin, hederagin, tigonenin, diosgenin) [84]. Clinical trials demonstrated that it was effective in alleviating PCOS symptoms when women took two capsules daily through the diet [85,86]. Anisum reduced the cyst size, as well as ovary volume, in women who received it daily as a supplement to their diet over a period of 90 days [87]. Similarly, it also decreases the LH/FSH ratio; significant maintenance of the menstrual cycle was seen following oral supplementation. Based on these actions, this herb could have a useful and significant effect on PCOS [74].
Figure 17.
Terpenes and terpenoids of Trigonella foenum-graecum that suppress cyst formation.
3.5. Effective Ovulation Induction Agents
The most common complication of PCOS is infertility or frequent pregnancy termination due to the patient’s lack of carrying capacity. As a result, drugs used to stimulate ovulation are included in PCOS treatment. Herbs such as Zingiber officinalis and Tribulus terrestris, which have the same action and promote ovulation, might be useful for the treatment of PCOS.
3.5.1. Zingiber officinalis
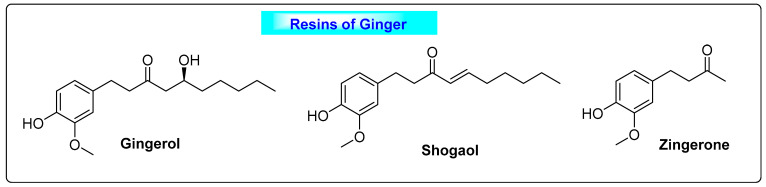
Zingiber officinalis is commonly called ginger and belongs to the family Zingeberaceae. The essential oil of ginger contains around 60-65 compounds. The major active phytochemicals are geraniol, gingerol, curcumin, α-curcumene, geranial, neral, borneol, linalool, β-sesquiphellandrene, afarnesene, sabinene, camphene, gamma-terpinene and terpinen-4-ol. The resin component of ginger comprises the constituents paradol, zingerol, zingiberene, zingiberon, shogaol, ascorbic acid, β-carotene, p-coumaric acid and caffeic acid [88,89]. Additionally, ginger also contains flavonoids and phenolics that are beneficial in PCOS. Gingerol and shogaol are potent antioxidant compounds, along with zingerone and a mild amount of oily resin ginger; all of these have demonstrated an anti-prostaglandin effect by inhibiting prostaglandin production and suppressing arachidonic acid production (Figure 18) [90]. Ginger will increase the fertility index, the testosterone level in serum and the testes and seminal vesicle weight, and increase the motility of sperm as well as the sperm count in males. The flavonoids and phenolic compounds in ginger could maintain the balance of estrogen and progesterone, with their specific pharmacological and physiological effects. Similarly, they could regulate the sex hormones in the blood [91]. Ginger’s phytoestrogen component can balance the estrogen to progesterone ratio, and thus it could be used to treat PCOS [92].
Figure 18.
Resinous substances of Zingiber officinalis.
3.5.2. Tribulus terrestris
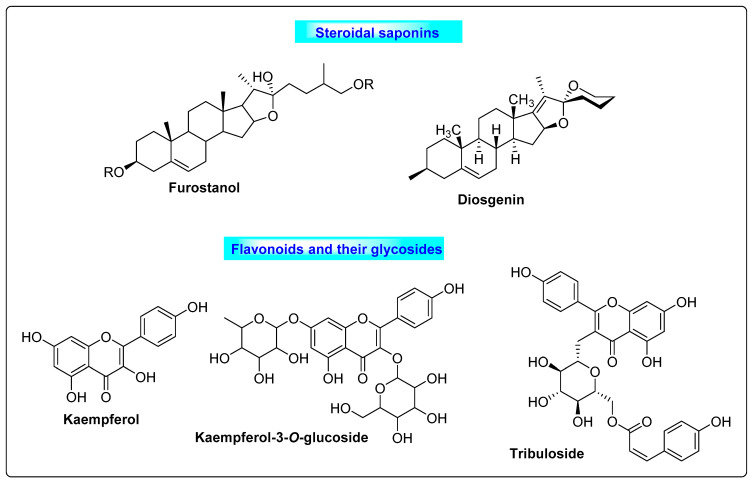
Tribulus terrestris is commonly known as Gokharu, or puncture vine, and belongs to the family Zygophyllaceae. The major chemical constituents are furostanol and spirostanol, and saponins such as tigogenin, diosgenin, gitogenin, hecogenin, neogitogenin, neohecogenin, chlorogenin, ruscogenin, protodioscin and protogracillin. Kaempferol, kaempferol-3-glucoside, kaempferol-3-rutinoside and tribuloside are also potent therapeutic compounds derived from Tribulus (Figure 19). Saponins obtained from TT possess hypoglycemic properties [93]. TT significantly reduced the serum glucose level, serum triglyceride level and serum cholesterol in a study. It normalized estrous cyclicity, steroidal hormonal levels and ovarian follicular growth. Many compounds from Tribulus are effective ovarian stimulants and act as fertility tonics for women, making it a good choice for women with polycystic ovaries [94].
Figure 19.
Saponins and flavonoids present in Tribulus terrestris that have positive effects on PCOS by inducing ovulation.
4. Discussion
Previously, a meta-analysis of several herbs was conducted to compare active ingredients that are effective against PCOS [95,96]. After studying the chemical contents of the herbs that are useful for treating PCOS, we believe that phenylpropanoids, flavonoids and their glycosides play a significant role in treating PCOS through several mechanisms. Other plant compounds with anti-PCOS efficacy include steroids, such as steroidal saponins and cycloartane derivatives, terpenoids, phenolics, catechins, resins, lignans and curcuminoids. As a result, we believe that a polyherbal formulation including a combination of flavonoids, phenylpropanoids and other ingredients with various mechanisms of action may be useful in controlling PCOS. A number of researchers have conducted human randomized controlled clinical studies, involving a total of 361 individuals, including 184 in the experimental group and 177 in the control group. On this basis, all patients in the group received luteal support combined with traditional Chinese medicine, whereas the control group received luteal support alone. For the meta-analysis, the researchers used random effects models. The combined SMD value of the three studies was −2.38, with a 95% confidence interval of [−2.82, −1.93], and it was statistically significant (Z = 10.50, p < 0.00001), indicating that the integrated traditional Chinese and Western medical treatment was superior to a purely Western medical treatment in lowering serum plasminogen activator inhibitor type-1 (PAI-I) levels in PCOS patients at risk of abortion. Similarly, polyherbal formulations containing three or more herbs in a tablet, capsule or pill have a favorable impact on PCOS. After 3 months of therapy, extremely significant benefits were discovered in terms of lowering discomfort during menstruation, reducing menstrual irregularity, normalizing follicular growth and ovulation and considerably reducing obesity in PCOS patients [97]. Hence, we conclude that a polyherbal formulation will have a greater impact in terms of decreasing the symptoms as well as treating PCOS in a more efficient manner.
5. Conclusions
PCOS is the most common hormonal illness in women from adolescence to pre-menopause, with a variety of complications, including infertility, metabolic and cardiovascular issues and long-term health issues that can last a lifetime. Synthetic medications have shown excellent management for the treatment of PCOS, but substantial adverse drug reactions make their value for long-term cure questionable. To enhance recovery rates and acceptance, patients are increasingly relying on herbal therapy as an alternative to synthetic medications for the control and treatment of PCOS. The current review provides a thorough review of herbs that are beneficial for PCOS and related complications. We have reviewed various key medicinal herbs, their primary chemical constituents and their specialized significance in PCOS management. We are certain that our evaluation will be of significant use to researchers working on herbal therapies to treat PCOS.
Acknowledgments
The authors acknowledge the Management and Principal of Vignan Pharmacy College, for supporting this review work. Akram Ashames would like to thank the Deanship of Graduate Studies and Research, Ajman University, for providing partial funding of the article processing charges. Akram Ashames, Richie R. Bhandare and Afzal B. Shaik would like to thank the Dean’s Office at the College of Pharmacy and Health Sciences, Ajman University, UAE and St. Mary’s Group of Institutions, Andhra Pradesh, India for their support.
Author Contributions
Conceptualization, J.N.L. and A.B.S.; methodology, J.N.L.; software, A.A.; validation, A.N.B., S.S.M.K. and L.P.N. formal analysis, A.B.S.; investigation, A.A.; resources, R.R.B.; data curation, N.H.; writing—original draft preparation, J.N.L.; writing—review and editing, A.B.S.; visualization, A.A.; project administration, N.H.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable. No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
The current review provides a thorough review of herbs that are beneficial for PCOS and related complications. We have reviewed various key medicinal herbs, their primary chemical constituents and their specialized significance in PCOS management. This evaluation will be of significant use to researchers working on herbal therapies to treat PCOS.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ndefo U.A., Eaton A., Green M.R. Polycystic ovary syndrome: A review of treatment options with a focus on pharmacological approaches. Pharm. Ther. 2013;38:336–355. [PMC free article] [PubMed] [Google Scholar]
- 2.Bharathi R.V., Swetha S., Neerajaa J., Madhavica J.V., Janani D.M., Rekha S.N., Ramya S., Usha B. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertil. Soc. J. 2017;22:313–316. doi: 10.1016/j.mefs.2017.05.007. [DOI] [Google Scholar]
- 3.Yadav K., Ghadge P., Langeh A., Kalbhare S., Phadtare P., Bhoite R. A Review on Herbal Medicinal Plant for Treatment of Polycystic Ovarian Syndrome (PCOS) Asian J. Pharm. Res. Dev. 2020;8:83–87. [Google Scholar]
- 4.Liu J., Wu Q., Hao Y., Jiao M., Wang X., Jiang S., Han L. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum. Reprod. 2021;36:1108–1119. doi: 10.1093/humrep/deaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abasian Z., Rostamzadeh A., Mohammadi M., Hosseini M., Rafieian-Kopaei M. A review on role of medicinal plants in polycystic ovarian syndrome: Pathophysiology, neuroendocrine signaling, therapeutic status and future prospects. Middle East Fertil. Soc. J. 2018;23:255–262. doi: 10.1016/j.mefs.2018.04.005. [DOI] [Google Scholar]
- 6.Ganie M.A., Vasudevan V., Wani I.A., Baba M.S., Arif T., Rashid A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J. Med. Res. 2019;150:333–344. doi: 10.4103/ijmr.IJMR_1937_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon C.Y., Cho I.H., Park K.S. Therapeutic Effects and Mechanisms of Herbal Medicines for Treating Polycystic Ovary Syndrome: A Review. Front. Pharmacol. 2020;11:1192. doi: 10.3389/fphar.2020.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goswami P.K., Khale A. Natural Remedies for Polycystic Ovarian Syndrome (PCOS): A Review. Int. J. Pharm. Phytopharm. Res. 2012;1:396–402. [Google Scholar]
- 9.Chan Y.K., Boram L., Kyoung S.P. Oriental herbal medicine and moxibustion for polycystic ovary syndrome A meta-analysis. Medicine. 2018;43:97. doi: 10.1097/MD.0000000000012942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoberg E., Orjala J., Meier B., Sticher O. Diterpenoids from the fruits of Vitex agnus-castus. Phytochemistry. 1999;52:1555–1558. doi: 10.1016/S0031-9422(99)00181-8. [DOI] [Google Scholar]
- 11.Balen A. The pathophysiology of polycystic ovary syndrome: Trying to understand PCOS and its endocrinology. Best Pract. Res. Clin. Obstet. Gynaecol. 2004;18:685–706. doi: 10.1016/j.bpobgyn.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Begum G.S., Shariff A., Ayman G., Mohammad B., Housam R., Khaled N. Assessment of Risk Factors for development of polycystic ovarian syndrome. Int. J. Contemp. Med. Res. 2017;4:2454–7379. [Google Scholar]
- 13.Pelluri R., Srikanth K., Paritala H., Ravi V., Kamma S.P., Piduguralla K.D., Venkateswarlu U. The role of high serum uric acid levels in androgenic and non-androgenic polycystic ovarian syndrome patients. Clin. Epidemiol. Glob. Health. 2021;12:100910. doi: 10.1016/j.cegh.2021.100910. [DOI] [Google Scholar]
- 14.O’Reilly M., Gathercole L., Capper F., Arlt W., Tomlinson J. Effect of insulin on AKR1C3 expression in female adipose tissue: In-vivo and in-vitro study of adipose androgen generation in polycystic ovary syndrome. Lancet. 2015;385:S16. doi: 10.1016/S0140-6736(15)60331-2. [DOI] [PubMed] [Google Scholar]
- 15.Amini L., Tehranian N., Movahedin M., Tehrani F.R., Ziaee S. Antioxidants and management of polycystic ovary syndrome in Iran: A systematic review of clinical trials. Iran. J. Reprod. Med. 2015;13:1–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Susan A., Jason A.A., Caroline A.S., Alan B. Herbal medicine for the management of polycysticovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogeni sm; a review of the laboratory evidence for effects with corroborative clinical findings. Complement. Altern. Med. 2014;14:511. doi: 10.1186/1472-6882-14-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman R.J., Dewailly D., Legro R.S., Hickey T.E. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 18.Khanage S.G., Subhash T.Y., Bhaiyyasaheb I.R. Herbal drugs for the treatment of Polycystic ovary syndrome (pcos) and its Complications. Pharm. Res. 2019;2:5–13. [Google Scholar]
- 19.Dunne N., Slater W. The Natural Diet Solution for PCOS and Infertility: How to Manage Polycystic Ovary Syndrome Naturally. Natural Solutions for PCOS, Health Solutions Plus; Seattle, WA, USA: 2006. [Google Scholar]
- 20.Elsheikh M., Caroline M. Polycystic Ovary Syndrome. Oxford University Press; Oxford, UK: 2008. [Google Scholar]
- 21.Legro R.S. Clomiphene, Metformin, or Both for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 22.Decio A. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007;131:61–67. doi: 10.1016/j.ejogrb.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Felenbam A. Laparoscopic treatment of polycystic ovaries with insulated needle cautery: A reappraisal. Fertil. Steril. 2000;73:266–269. doi: 10.1016/s0015-0282(99)00534-8. [DOI] [PubMed] [Google Scholar]
- 24.Miller L.G., Murray W.J. Herbal Medicinals: A Clinician’s Guide. Routledge; London, UK: 1998. p. 326. [Google Scholar]
- 25.Tilburt J.C., Kaptchuk T.J. Bulletin of the World Health Organization. 86th ed. World Health Organization; Geneva, Switzerland: 2008. pp. 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anonymous. Zanzibar Traditional and Alternative Medicine Policy, Ministry of Health and Social Welfare in Collaboration with: World Health Organization. 2008. [(accessed on 15 September 2021)]. Available online: https://www.afro.who.int/publications/zanzibar-traditional-and-alternative-medicine-policy-2008.
- 27.Benzie I.F., Galor S.W. Herbal Medicine: Biomolecular and Clinical Aspects. Volume 7. CRC Press; Boca Raton, FL, USA: 2011. [PubMed] [Google Scholar]
- 28.Merz P.G., Schrödter A., Rietbrock S., Gorkow C., Löw D. Prolactin secretion and tolerance during treatment with an Agnus castus extract—Effect on prolactin secretion. Phytopharm. Forsch. Klin. Anwend. 1996;104:447–453. doi: 10.1055/s-0029-1211483. [DOI] [PubMed] [Google Scholar]
- 29.Webster D.E., Lu J., Chen S.N., Farnsworth N.R., Wang Z.J. Activation of the mu-opiate receptor by Vitex agnus-castus methanol extracts: Implication for its use in PMS. J. Ethnopharmacol. 2006;106:216–221. doi: 10.1016/j.jep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Burdette J.E., Xu H., Gu C., Van Breemen R.B., Bhat K.P., Booth N., Constantinou A.I., Pezzuto J.M., Fong H.H., et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen J.M., Katsiotis S.T. Variation in essential oil yield and composition of Cretan Vitex agnuscastus L. fruits. J. Essent. Oil Res. 1999;11:599–605. doi: 10.1080/10412905.1999.9701221. [DOI] [Google Scholar]
- 32.Senatore F., Napolitano F., Ozcan M. Chemical composition and antibacterial activity of essential oil from fruits of Vitex agnus-castus L. (Verbenaceae) growing in Turkey. J. Essent. Oil-Bear. Plants. 2003;6:185–190. doi: 10.1080/0972-060X.2003.10643349. [DOI] [Google Scholar]
- 33.Wollenweber E., Mann K. Flavonols from fruits of Vitex agnus-castus. Planta Med. 1983;48:126–127. doi: 10.1055/s-2007-969905. [DOI] [PubMed] [Google Scholar]
- 34.Hirobe C., Qiao Z.S., Takeya K., Itokawa H. Cytotoxic flavonoids from Vitex agnus-castus. Phytochemistry. 1997;46:521–554. doi: 10.1016/S0031-9422(97)00127-1. [DOI] [PubMed] [Google Scholar]
- 35.Görler K., Öhlke D., Soicke H. Iridoid derivatives from Vitex agnus-castus. Planta Med. 1985;50:530–531. doi: 10.1055/s-2007-969589. [DOI] [PubMed] [Google Scholar]
- 36.Ono M., Yamasaki T., Konoshita M., Ikeda T., Okawa M., Kinjo J., Yoshimitsu H., Nohara T. Five new diterpenoids, viteagnusins A-E, from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2008;56:1621–1624. doi: 10.1248/cpb.56.1621. [DOI] [PubMed] [Google Scholar]
- 37.Ono M., Nagasawa Y., Ikeda T., Tsuchihashi R., Okawa M., Kinjo J., Yoshimitsu H., Nohara T. Three new diterpenoids from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2009;57:1132–1135. doi: 10.1248/cpb.57.1132. [DOI] [PubMed] [Google Scholar]
- 38.Shao N.C., Brent Friesenb J., Donna W., Dejan N., Richard B., Breemena V. Phytoconstituents from Vitex agnus-castus fruits. Fitoterapia. 2011;82:528–533. doi: 10.1016/j.fitote.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shishodia S., Singh T., Chaturvedi M.M. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007;595:127–148. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 40.Wojcik M., Krawczyk M., Wojcik P., Cypryk K., Wozniak L.A. Molecular mechanisms underlying curcumin-mediated therapeutic effects in type 2 diabetes and cancer. Oxidative Med. Cell. Longev. 2018;2018:9698258. doi: 10.1155/2018/9698258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanvir E.M., Hossen M.S., Hossain M.F., Afroz R., Gan S.H., Khalil M.I., Karim N. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual. 2017:8471785. doi: 10.1155/2017/8471785. [DOI] [Google Scholar]
- 42.Jimenez A.S., Monroy A., Alavez S. Curcumin and insulin resistance-molecular targets and clinical evidences. Biofactors. 2016;42:561–580. doi: 10.1002/biof.1302. [DOI] [PubMed] [Google Scholar]
- 43.Yang H., Kim H.J., Pyun B.-J., Lee H.W. Licorice ethanol extract improves symptoms of polycytic ovary syndrome in Letrozole induced female rats. Integr. Med. Res. 2018;7:264. doi: 10.1016/j.imr.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baby B.T., Rani S., Rasheed S.P., Bency B. Polycystic ovarian syndrome: Therapeutic potential of herbal remedies—A review. Int. J. Herb. Med. IJHM. 2016;91:91–96. [Google Scholar]
- 45.Jelodar G., Masoomi S., Rahmanifar F. Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iran. J. Basic Med. Sci. 2018;21:645–650. doi: 10.22038/IJBMS.2018.25778.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aziz E., Ameer K., Elham A., Solmaz M.D., Amir H., Magali C. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021;14:103106. [Google Scholar]
- 47.Kamel H.H. Role of phyto-oestrogens in ovulation induction in women with polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;168:60–63. doi: 10.1016/j.ejogrb.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Aires A., Marrinhas E., Carvalho R., Dias C., Saavedra M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res. Int. 2016;2016:5201879. doi: 10.1155/2016/5201879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soković M.D., Vukojević J., Marin P.D., Brkić D.D., Vajs V., Van Griensven L.J. Chemical composition of essential oils of thymus and mentha species and their antifungal activities. Molecules. 2009;14:238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rooney S., Pendry B. Phytotherapy for polycystic ovarian syndrome: A review of the literature and evaluation of practitioners’ experiences. J. Herbal Med. 2014;4:159–171. doi: 10.1016/j.hermed.2014.05.001. [DOI] [Google Scholar]
- 51.Wang J., Ji J., Song Z., Zhang W., He X., Li F., Zhang C., Guo C., Wang C., Yuan C. Hypocholesterolemic effect of emodin by simultaneous determination of in vitro and in vivo bile salts binding. Fitoterapia. 2016;110:116–122. doi: 10.1016/j.fitote.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Xueli C., Chong T., Jingyi W., Baochang H., Jinbang X. Meta-analysis of therapeutic efficacy and effects of integrated traditional Chinese and Western medicine on coagulation and fibrinolysis system in patients with threatened abortion and polycystic ovary syndrome. Am. J. Transl. Res. 2022;14:2768–2778. [PMC free article] [PubMed] [Google Scholar]
- 53.Costa C.T., Bevilaqua C.M., Morais S.M., Camurca V.A.L., Maciel M.V., Braga R.R. Anthelmintic activity of Cocos nucifera L. on intestinal nematodes of mice. Res. Vet. Sci. 2010;88:101–103. doi: 10.1016/j.rvsc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Yong J.W., Ge L., Ng Y.F., Tan S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules. 2009;14:5144–5164. doi: 10.3390/molecules14125144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Escalante Erosa F., Gamboa-León M.R., Lecher J.G., Arroyo-Serralta G.A., Zizumbo-Villareal D., Oropeza-Salín C., Peña-Rodríguez L.M. Major components from the epicuticular wax of Cocos nucifera. Rev. Soc. Quím. Mex. 2002;46:247–250. [Google Scholar]
- 56.Lima E.B.C., Sousa C.N.S., Meneses L.N., Ximenes N.C., Júnior S., Vasconcelos G.S., Lima N.B.C., Patrocínio M.C.A., Macedo D., Vasconcelos S.M.M. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz. J. Med. Biol. Res. 2015;48:953–964. doi: 10.1590/1414-431x20154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhandary M.J., Chandrashekar K.R., Kaveriappa K.M. Medical ethnobotany of the Siddis of Uttara Kannada district, Karnataka, India. J. Ethnopharmacol. 1995;47:149–158. doi: 10.1016/0378-8741(95)01274-H. [DOI] [PubMed] [Google Scholar]
- 58.Brondegaard V.J. Contraceptive plant drugs. Planta Med. 1973;23:167–172. doi: 10.1055/s-0028-1099428. [DOI] [PubMed] [Google Scholar]
- 59.Sachs M., von Eichel J., Asskali F. Wound management with coconut oil in Indonesian folk medicine. Chirurg. 2002;73:387–392. doi: 10.1007/s00104-001-0382-4. [DOI] [PubMed] [Google Scholar]
- 60.Hirschhorn H.H. Botanical remedies of the former Dutch East Indies (Indonesia). Part I: Eumycetes, Pteridophyta, Gymnospermae, Angiospermae (Monocotyledones only) J. Ethnopharmacol. 1983;7:123–156. doi: 10.1016/0378-8741(83)90016-8. [DOI] [PubMed] [Google Scholar]
- 61.Hossein K.J., Leila K., Ebrahim T.K., Nazanin S.J., Farzad P., Elham R. The effect of pomegranate juice extract on hormonal changes of female Wistar rats caused by polycystic ovarian syndrome. Biomed. Pharmacol. J. 2015;8:971–977. doi: 10.13005/bpj/849. [DOI] [Google Scholar]
- 62.Jayaprakasha G.K., Rao L.J., Sakariah K.K. Chemical composition of volatile oil from Cinnamomum zeylanicum buds. Z. Nat. Sect. C J. Biosci. 2002;57:990–993. doi: 10.1515/znc-2002-11-1206. [DOI] [PubMed] [Google Scholar]
- 63.Muchuweti M., Kativu E., Mupure H., Chidewe C., Ndhlala R.A., Benhura N.A.M. Phenolic composition and antioxidant properties of some spices. Am. J. Food Technol. 2007;2:414–420. doi: 10.3923/ajft.2007.414.420. [DOI] [Google Scholar]
- 64.Evans P., Halliwell B. Micronutrients: Oxidant/antioxidant status. Br. J. Nutr. 2001;85((Suppl. 2)):67–74. doi: 10.1079/BJN2000296. [DOI] [PubMed] [Google Scholar]
- 65.Wang J.G., Anderson R.A., Graham G.M., Chu M.C., Sauer M.V., Guarnaccia M.M. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: A pilot study. Fertil. Steril. 2007;88:240–243. doi: 10.1016/j.fertnstert.2006.11.082. [DOI] [PubMed] [Google Scholar]
- 66.Khodaeifar F., Fazljou S.M.B., Khaki A., Torbati M., Ouladsahebmadarek E., Khaki A.A. Investigating the Role of Hydroalcoholic Extract of Apium graveolens and Cinnamon zeylanicum on Metabolically Change and Ovarian Oxidative Injury in a Rat Model of Polycystic Ovary Syndrome. Int. J. Women’s Health Reprod. Sci. 2019;7:92–98. doi: 10.15296/ijwhr.2019.15. [DOI] [Google Scholar]
- 67.Ainehchi N., Farshbaf-Khalili A., Ghasemzadeh A., Hamdi K., Khaki A., Ouladsahebmadarek E., Delazar A., Bakhtyari F., Mazandarani M. The Effect of Herbal Medicine Supplementation on Clinical and Para-clinical Outcomes in Women with PCOS: A Systematic Review and Meta-analysis. Int. J. Women’s Health Reprod. Sci. 2019;7:423–433. doi: 10.15296/ijwhr.2019.72. [DOI] [Google Scholar]
- 68.Desai B.N., Maharjan R.H., Nampoothiri L.P. Aloe barbadensis Mill. formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacogn. Res. 2012;4:109–115. doi: 10.4103/0974-8490.94736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farideh Z.Z., Bagher M., Ashraf A., Akram A., Kazem M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertil. 2010;11:169–174. [PMC free article] [PubMed] [Google Scholar]
- 70.Badgujar S.B., Patel V.V., Bandivdekar A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014;2014:842674. doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conforti F., Statti G., Uzunov D., Menichini F. Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp. piperitum (Ucria) coutinho seeds. Biol. Pharm. Bull. 2006;29:2056–2064. doi: 10.1248/bpb.29.2056. [DOI] [PubMed] [Google Scholar]
- 72.Križman M., Baričevič D., Prošek M. Determination of phenolic compounds in fennel by HPLC and HPLC-MS using a monolithic reversed-phase column. J. Pharm. Biomed. Anal. 2007;43:481–485. doi: 10.1016/j.jpba.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 73.Shahidi F., Hossain A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018;3:8–75. doi: 10.31665/JFB.2018.3149. [DOI] [Google Scholar]
- 74.Sadrefozalayi S., Farokhi F. Effect of the aqueous extract of Foeniculum vulgare (fennel) on the kidney in experimental PCOS female rats. Avicenna J. Phytomed. 2014;4:110. [PMC free article] [PubMed] [Google Scholar]
- 75.Pak S.C., Lim S.C., Nah S.Y., Lee J., Hill J.A., Bae C.S. Role of Korean red ginseng total saponins in rat infertility induced by polycystic ovaries. Fertil. Steril. 2005;84:1139–1143. doi: 10.1016/j.fertnstert.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 76.Wuttke W., Seidlova W.D., Gorkow C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: Effects on menopause symptoms and bone markers. Maturitas. 2003;44((Suppl. 1)):67–77. doi: 10.1016/S0378-5122(02)00350-X. [DOI] [PubMed] [Google Scholar]
- 77.Frei K.S., Schaffner W., Rahlfs V.W., Bodmer C., Birkhäuser M. Cimicifuga racemosa dried ethanolic extract in menopausal disorders: A double-blind placebo-controlled clinical trial. Maturitas. 2005;51:397–404. doi: 10.1016/j.maturitas.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Schultz J.M., Herrmann K. Occurrence of hydroxybenzoic acid and hydroxycinnamic acid in spices. IV. Phenolics of spices. Z. Lebensm.-Unters.-Forsch. 1980;171:193–199. [Google Scholar]
- 79.Moghazi E.L., Ali A.M., Ross K., Mottaleb B.U.T. Flavonoids of Pimpinella anisum L. growing in Egypt. Fitoterapia. 1979;50:267–268. [Google Scholar]
- 80.Kumaravel S., Alagusundaram K. Antimicrobial activity and phytochemical analysis of selected Indian spices. J. Pure Appl. Microbiol. 2014;8:4131–4136. [Google Scholar]
- 81.Parthasarathy V.A., Chempakam B., Zachariah T.J. Chemistry of Spices. CABI; Oxfordshire, UK: 2008. pp. 405–407. [Google Scholar]
- 82.Girardon P., Bessiere J.M., Baccou J.C., Sauvaire Y. Volatile constituents of fenugreek seeds. Planta Med. 1985;6:533–534. doi: 10.1055/s-2007-969591. [DOI] [PubMed] [Google Scholar]
- 83.Ahmad A., Alghamdi S.S., Mahmood K., Afzal M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 2016;23:300–310. doi: 10.1016/j.sjbs.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khanna A., John F., Das S., Thomas J.R., Maliakel B. Efficacy of a novel extract of fenugreek seeds in alleviating vasomotor symptoms and depression in perimenopausal women: A randomized, double-blinded, placebo-controlled study. J. Food Biochem. 2020;44:1350–1357. doi: 10.1111/jfbc.13507. [DOI] [PubMed] [Google Scholar]
- 85.Dayani S.A., Karunathilaka L.P., Kodituwakku N.D., Karunarathne Y.A. Clinical efficacy of Ayurveda treatment regimen on Subfertility with Poly Cystic Ovarian Syndrome (PCOS) Ayu. 2010;31:24–27. doi: 10.4103/0974-8520.68203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhingardive K.B., Sarvade D.D., Bhatted S. Clinical efficacy of Vamana Karma with Ikshwaaku Beeja Yoga followed by Shatapushpadi Ghanavati in the management of Artava Kshayawsr to polycystic ovarian syndrome. Ayu. 2017;38:127–132. doi: 10.4103/ayu.AYU_192_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anand S., Amrita J., Sushil K.G., Manashi B., Pawan K., Harry G., Debasis B. Efficacy of a Novel Fenugreek Seed Extract (Trigonella foenum-graecum, Furocyst TM) in Polycystic Ovary Syndrome (PCOS) Int. J. Med. Sci. 2015;12:825–831. doi: 10.7150/ijms.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charles D.J. Antioxidant properties of spices, herbs and other sources. Front. Nat. Prod. 2013;1:4310–4614. [Google Scholar]
- 89.Cheng X.L., Liu Q., Peng Y.B., Qi L.W., Li P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011;129:1785–1792. doi: 10.1016/j.foodchem.2011.06.026. [DOI] [Google Scholar]
- 90.Atashpour S., Kargar J.H., Kargar J.Z., Maleknasab M. Comparison of the effects of ginger extract with clomiphene citrate on sex hormones in rats with polycystic ovarian syndrome. Int. J. Reprod. Biomed. 2017;15:561–568. doi: 10.29252/ijrm.15.9.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ali B.H., Blunden G., Tanira M.O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 92.Poojari P., Paramanya A., Singh D., Ali A. Herbal Medicines: A Boon for Healthy Human Life. Academic Press; Cambridge, MA, USA: 2022. Polycystic ovarian syndrome: Causes and therapies by herbal medicine; pp. 435–451. [Google Scholar]
- 93.Saiyed A., Jahan N., Makbul S.A.A., Ansari M., Bano H., Habib S.H. Effect of combination of Withania somnifera Dunal and Tribulus terrestris Linn on letrozole induced polycystic ovarian syndrome in rats. Integr. Med. Res. 2016;5:293–300. doi: 10.1016/j.imr.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chhatre S., Nesari T., Somani G., Kanchan D., Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014;8:45–54. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 96.Moini Jazani A., Nasimi Doost Azgomi H., Nasimi Doost Azgomi A., Nasimi Doost Azgomi R. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS) DARU J. Pharm. Sci. 2019;27:863–877. doi: 10.1007/s40199-019-00312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhiman K. Ayurvedic intervention in the management of uterine fibroids: A Case series. Ayu. 2014;35:303. doi: 10.4103/0974-8520.153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable. No new data were created or analyzed in this study.