Abstract
(1) Objective: We performed a systematic review to explore the prevalence of intravenous (IV) rehydration therapy in hospital settings, and we assessed it by patient groups and populations. (2) Methods: A systematic review of major databases and grey literature was undertaken from inception to 28 March 2022. Studies reporting prevalence of IV rehydration therapy in a hospital setting were identified. The data were synthesised in a narrative approach. (3) Results: Overall, 29 papers met the inclusion criteria. The prevalence of IV rehydration therapy in paediatric patients ranged from 4.5% (hospitalised with diarrhoea and dehydration) to 100% (admitted to the emergency department with mild to moderate dehydration caused by viral gastroenteritis), and in adults this ranged from 1.5% (had single substance ingestion of modafinil) to 100% (hospitalised with hypercalcemia). The most common indication for IV rehydration therapy in paediatric patients was dehydration due to fluid loss from the gastrointestinal tract. Other causes included malnutrition, neuromuscular disease, bronchiolitis, and influenza. In adults, indications for IV rehydration therapy were much more diverse: fever, diarrhoea, drug intoxication, hypercalcemia, cancer, and postural tachycardia syndrome; (4) Conclusions: This systematic review showed that IV rehydration therapy in paediatric patients is often used to treat dehydration and diarrhoea, while in adults it has a broader spectrum of use. While IV rehydration therapy is important in correcting fluid problems and electrolyte status, the maintenance fluid prescribing practices vary considerably, and guidelines are scarce.
Keywords: intravenous fluids therapy, IV rehydration, paediatric patients, adult patients
1. Introduction
Intravenous (IV) rehydration therapy is widely used to prevent or correct problems with fluid and electrolyte status when oral administration is not possible, or it is impaired [1]. IV fluids enter the bloodstream directly, bypassing the waiting time associated with oral rehydration. Fluid loss can be caused by surgery, accident, or common conditions such as fever, vomiting, and diarrhoea. Moreover, in some cases requiring hospitalisation such as acute renal failure, hyponatremia, hypercalcemia, acute pancreatitis, and sepsis, which are more common in adults, IV rehydration is the most important part of the treatment and can be lifesaving, even if there is no loss of fluid [2,3]. In addition, IV hydration is used to maintain hydration in paediatric and adult cancer patients or terminal care patients who do not have adequate oral intake [4]. Therefore, IV rehydration therapy has a wide range of uses.
IV rehydration therapy is the procedure by which a specially formulated IV solution is administrated through a tube attached to a needle, which is inserted into a vein. IV solutions contain small amounts of salt (sodium chloride) or sugar (dextrose, glucose, or levulose) that are dissolved in sterile water [1]. One of the most used IV fluids is 0.9% normal saline that contains sterile water and 0.9% sodium chloride. IV rehydration therapy is a simple and effective way of supplying fluids directly into the intravascular fluid compartment. However, an interprofessional team approach is sometimes required to achieve optimum fluid balance for patients [5]. The type, amount, and infusion rate of IV rehydration therapy may vary according to body composition, dehydration level, and cardiac output status of each patient, as well as clinical and hemodynamic parameters such as daily urine output or blood pressure. Therefore, fluid prescription may be difficult, especially in patients with impaired homeostatic mechanisms, such as those with renal or heart failure, or in patients with ongoing excessive losses (e.g., as a result of diarrhoea) [6]. Moreover, incorrect management of fluid assessment and monitoring is associated with adverse outcomes such as hyponatremia (sodium concentration of less than 135 mmol/L (135 mEq/L)), fluid overload, and hyperchloremic acidosis (pH less than 7.35 develops with an increase in ionic chloride) due to inappropriate fluid composition and/or infusion rates/volumes [2]. For example, despite the obvious benefits of IV fluid therapy, excessive fluid administration may lead to various complications [7]. High volumes of IV fluids may be retained in the interstitial space, causing interstitial oedema, impaired organ perfusion, possibly acute pulmonary oedema, and increased mortality [7]. Optimal fluid status not only shortens hospital stay, but may also reduce the incidence of postoperative complications, mortality, and adverse outcomes related to dehydration, such as acute confusion, constipation, urinary tract infections, fatigue, falls, and delayed wound healing, particularly in older adults [8]. Dehydration has also been associated with longer hospital stays, with an annual cost estimate of >1.14 billion USD in 1999 for the diagnosis of primary dehydration [9]. All this clearly shows how important interventions to improve hydration are in older adults.
However, to date, no attempt has been made to collate the literature on the prevalence of IV rehydration therapy in hospital settings, and little is known about this, particularly by patient groups or populations. Information on prevalence of IV rehydration therapy in hospital settings is of upmost importance to aid in hospital decision making (procurement and medical practice), the development of policy, and medical guidelines (i.e., is it over or under used). Therefore, in this systematic review, we provide an overview of the prevalence of IV rehydration therapy in hospital settings in paediatric and adult populations.
2. Materials and Methods
The current systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. Prior to conducting our review, we identified the following questions to guide our search:
The following two questions were established to guide the search:
What is the prevalence of IV rehydration therapy in hospital settings?
Is there a certain age group of people that is more likely to receive IV rehydration therapy in hospital settings?
2.1. Search Strategy
Electronic databases were searched from database inception until 28 March 2022 including PubMed/Medline, Embase, Web of Science, and Scopus. In PubMed, the following search strategy was used: “(“intravenous fluids”[Title/Abstract] OR “parenteral fluids”[Title/Abstract] OR “IV fluids”[Title/Abstract] OR “fluid infusion”[Title/Abstract] OR “fluid administration”[Title/Abstract] OR “fluid therapy”[Title/Abstract] OR “fluid perfusion”[Title/Abstract] OR “intravenous rehydration”[Title/Abstract] OR “parenteral rehydration”[Title/Abstract]) AND (“hospitalised patients”[Title/Abstract] OR “hospitalisation”[Title/Abstract] OR “in hospital”[Title/Abstract])”. The strategy was then adapted for the other databases. Full information on database-specific search strategies can be found in online Supplemental Table S1. Results of the searches were exported to bibliographic database and duplicates removed. Titles and abstracts were screened by two independent reviewers (JG, LS), and then the full paper screening was conducted by the same reviewers before making a final decision on eligibility. Any inconsistencies were discussed and resolved by consensus with a third reviewer (PS).
2.2. Study Inclusion and Exclusion
Studies were included if they met the following criteria: (1) observational cross-sectional, prospective, or retrospective cohort studies (2) that investigate the frequency of IV rehydration therapy (3) in any population (healthy or with a specific disease condition) (4) in a hospital setting. Published articles that were written in English were included. Review articles, nonhuman studies, conference abstracts, and articles in a language other than English were excluded from the review. Studies investigating IV therapy for resuscitation and studies that reported on IV rehydration solutions with added medications into the IV bolus and goal-directed fluid therapy were excluded.
Studies were excluded at full text for the following criteria: (1) IV fluids for resuscitation, (2) fluid overload related to IV fluids, (3) hyponatraemia related to IV fluids, (4) guided IV therapy or restricted IV fluid therapy, (5) intraoperative/postoperative IV fluid administration, (6) hydration by enteroclysis, (7) outpatient IV rehydration, (8) IV fluids use at home, (9) use of IV fluids not specified, (10) RCT, or (11) combined data for IV fluids and oral rehydration solution.
2.3. Data Extraction
Data were extracted by an independent reviewer (JG) including the following: first author, year, country, type of the study design (cross-sectional, cohort), population, sample size included, participants’ characteristics (e.g., age, sex), number of participants receiving IV rehydration therapy, information of control/comparator group, and the period of observation. A second independent reviewer (LS) validated the data extraction. The data were synthesised in a narrative approach.
2.4. Quality Assessment
Risk of bias in individual studies was assessed by one independent reviewer (JG) and checked by another (LS) using the Critical Appraisal Skills Programme [11]. The Cohort Study checklist was used for the cohort studies [12]. This checklist contains 12 questions to which the reviewer answered ‘yes’, ‘cannot tell’, or ‘no’. The Cohort Study checklist was also used for the cross-sectional studies because there is no separate cross-sectional survey checklist in the CASP series. Questions 6(a) and 6(b), which assessed the follow up of the study, were not applicable to the cross-sectional designs and were therefore marked as not applicable. The quality was evaluated as ‘fair’, ‘good’, and ‘poor’ based on the CASP checklists. Any discrepancies were resolved during a discussion with a third reviewer (PS).
3. Results
3.1. Search Results
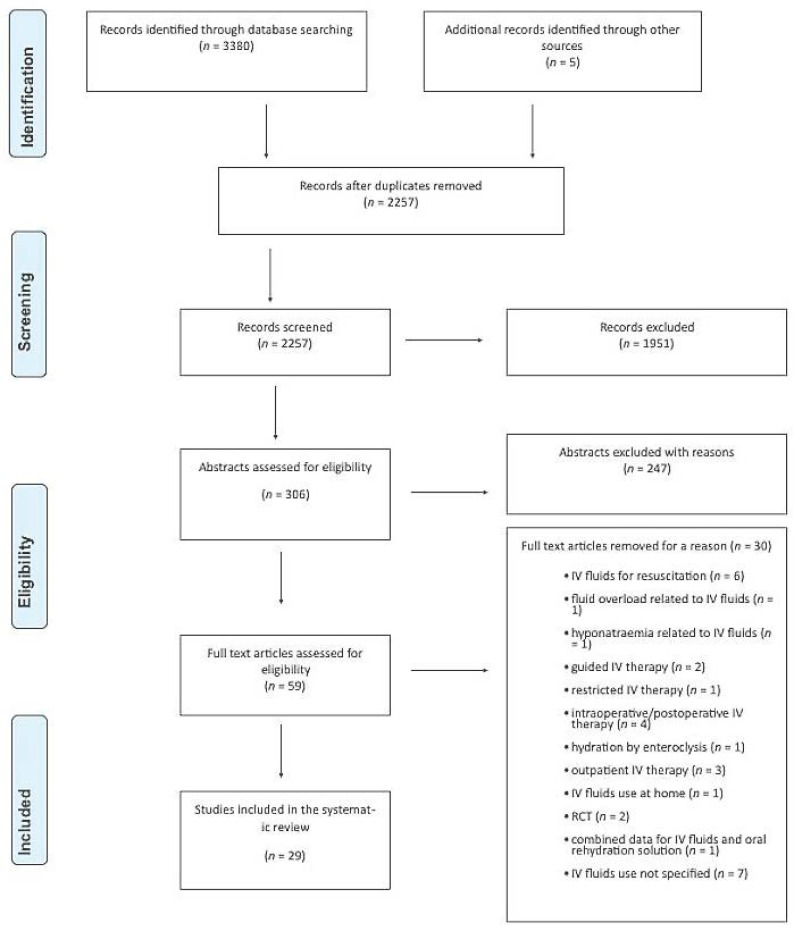
Of 2257 articles screened, we reviewed the full text of 59 studies. After careful review, the authors agreed on the inclusion of 29 studies for the narrative synthesis. Figure 1 shows the PRISMA flow diagram.
Figure 1.
PRISMA flow diagram illustrating article selection.
3.2. Studies’ Characteristics
All included studies were published between 1991 and 2021. The 29 included studies yielded a total of 863,346 patients and the age ranged from 3 days to 87 years old. Study characteristics can be found in Table 1. A total of 51.5% were male.
Table 1.
Demographic characteristics of included studies.
| Author, Year | Country or Region | Study Duration | Study Design | Sample Size | Age Range | Age Mean (SD) |
Age Median (IQR) |
Sex % Male |
Population | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdul-Mumin, Ervin and Halvorson, 2019 [18] | Ghana | January 2013–December 2014 | Retrospective chart review | 473 | NR | NR | 12 (9–24) months | 56 | Paediatric patients hospitalised with acute gastroenteritis | Good |
| Akech et al., 2018 [13] | Kenya | October 2013–December 2016 | Prospective chart review | 8025 | NR | NR | 12 (8–18) months | 0 | Paediatric patients hospitalised with diarrhoea and dehydration | Good |
| Ben- Shalom, Toker and Schwartz, 2016 [29] | Israel | 2001–2010 | Retrospective chart review | 58 | 0–24 months | 6.8 (5.27) months | NR | 59.7 | Paediatric patients hospitalised with hypernatremic dehydration | Good |
| Blacklock et al., 2015 [38] | Sierra Leone | 26 July 2012–22 September 2012 | Retrospective chart review | 798 | <5–≥60 years | NR | NR | 45 | Paediatric and adult patients hospitalised with cholera during the epidemic | Fair |
| Chow et al., 2009 [14] | China | 1 April 2021 to 31 March 2003 | Retrospective chart review | 7391 | NR | NR | 13 (6–26) months | 59 | Paediatric patients admitted to the hospital with diagnosis of diarrhoea. | Fair |
| Dbaibo et al., 2013 [19] | Lebanon | April 2007–August 2008 | Hospital-based surveillance design | 491 | NR | NR | 12 (0–59) months | NR | Paediatric patients hospitalised with a diagnosis of acute gastroenteritis | Fair |
| Fikrie, Alemayehu and Gebremedhin, 2019 [32] | Ethiopia | July 2015–June 2017 | Retrospective cohort | 381 | 6–59 months | 22.4 (15.8) | NR | 49.6 | Paediatric patients hospitalised with Severe Acute Malnutrition | Good |
| Freedman et al., 2014 [20] | USA | 1 January 2002–31 December 2011 | Retrospective cohort study | 804,000 | NR | 3.1 (3.9) years | NR | 53.1 | Paediatric patients who were diagnosed as having gastroenteritis in an emergency department | Good |
| Heyman et al., 1990 [15] | Malawi | July 1981–July 1986 | Retrospective chart review | 3495 | ≤12–≥24 months | NR | NR | 77 | Paediatric patients hospitalised with diarrhoea or gastroenteritis | Fair |
| Janet el al., 2015 [30] | Spain | 15 July 2012–15 December 2012 | Prospective cohort study | 83 | NR | NR | 4 (1.7–7) years | 56.6 | Paediatric patients with mild-to moderate isonatremic dehydration | Fair |
| Kao et al., 2019 [31] | Taiwan | January 2005–January 2015 | Retrospective chart review | 44 | NR | 9.9 (5.6) years | 11.1 (10.6) | 68.2 | Paediatric patients with chronic neuromuscular disorder who visited the emergency room | Good |
| Lopez-Medina et al., 2012 [33] | USA | 27 April 2009–23 March 2010 | Retrospective cohort study | 73 | 3–179 days | NR | 48 days | 48 | Paediatric patients hospitalised with laboratory-confirmed influenza | Fair |
| Machado et al., 2015 [41] | USA | 1 October 2010–30 September 2013 | Retrospective chart review | 72 | 54–87 years | 70.4 (NR) years | NR | 40 | Patients hospitalised with hypercalcemia | Good |
| Marra et al., 2011 [36] | Brazil | 8 April 2008–9 May 2008 | Retrospective chart review | 3393 | NR | NR | NR | NR | Paediatric and adult patients treated in the hydration tent during dengue fever epidemic | Fair |
| Moineau and Newman, 1990 [21] | Canada | December 1988–April 1989 | Prospective pilot study | 17 | NR | 2.6 (1.7) years | NR | 47 | Paediatric patients admitted to the emergency department with mild to moderate dehydration caused by viral gastroenteritis | Fair |
| Myat et al., 2021 [22] | Myanmar | May 2018–January 2020 | Hospital-based surveillance design | 3226 | 5 days to 59 months | NR | NR | 59.5 | Paediatric patients hospitalised for acute gastroenteritis | Fair |
| Nazurdinov et al., 2018 [23] | Tajikistan | January 2013–December 2014 | Hospital-based surveillance design | 2863 | 0–59 months | NR | NR | 61 | Paediatric patients hospitalised with acute gastroenteritis and rotavirus | Fair |
| Oakley et al., 2016 [34] | Australia | 1 April to 31 October 2011 to 2013 | Retrospective cohort study | 491 | NR | 5.1 (1.9) weeks | NR | 56 | Paediatric patients hospitalised with bronchiolitis | Fair |
| Patwari et al., 1991 [16] | India | January 1989–December 1989 | Retrospective chart review | 5996 | 0–5 years | NR | NR | 64.9 | Paediatric patients who attended hospital with diarrhoea | Fair |
| Perl et al., 2011 [24] | Israel | 1 April 2004–31 March 2006 | Retrospective chart review | 533 | NR | 21.7 (31) months | 13 months | 56.5 | Paediatric patients hospitalised with acute gastroenteritis, rotavirus gastroenteritis, and diarrhoea and vomiting | Good |
| Redondo-Gonzalez et al., 2016 [25] | Spain | 1 January 2003–31 December 2009 | Retrospective cohort study | 17,415 | 7 months–≥14 years | NR | NR | 53.4 | Paediatric patients hospitalised with acute gastroenteritis | Fair |
| Spiller et al., 2009 [39] | USA | 2000–2007 | Retrospective chart review | 137 | 1–82 years | 22 (NR) years | NR | 38 | Adult patients who had single substance ingestion of modafanil | Fair |
| Hasan et al., 2021 [40] | Bangladesh | 2 April 2018–12 May 2018 | Retrospective chart review | 1531 | 0–≥ 30 years | NR | NR | 58.4 | Paediatric and adult patients hospitalised with diarrhoea during epidemic | Good |
| Tewari et al., 2018 [37] | New Delhi | May 2013–September 2013 | Prospective cohort study | 500 | 6 months to 77 years | NR | NR | 53.9 | Paediatric and adult patients hospitalised with fever and other signs of dengue | Fair |
| Thronaes et al., 2021 [35] | Norway | 15 January 2019–15 January 2020 | Prospective longitudinal study | 451 | NR | 68.9 (13.1) years | NR | 60.3 | Adult patients with incurable cancer | Fair |
| Tseng et al., 2018 [42] | USA | January 2010–January 2017 | Retrospective cohort study | 332 | NR | 29.3 (9.5) years | NR | 10 | Adult patients’ postural tachycardia syndrome | Fair |
| Waisbourd-Zinman et al., 2008 [26] | Israel | 1 January 2003–31 December 2006 | Prospective cohort study | 356 | NR | 14.6 (24.7) months | 9 months | 54.5 | Paediatric patients hospitalised with nosocomial rotavirus gastroenteritis | Fair |
| Wathen, MacKenzie and Bothner, 2004 [28] | USA | January–October, 2004 | Prospective cohort study | 182 | 2.7 months to 8.5 years | 1.4 years | 51 | Paediatric patients presenting at the hospital with gastroenteritis and dehydration | Fair | |
| Wildi-Runge et al., 2009 [27] | Switzerland | July 2002–March 2006 | Retrospective chart review | 539 | NR | 1.4 (NR) years | NR | 55.6 | Paediatric patients hospitalised with rotavirus gastroenteritis | Fair |
SD, standard deviation; IQR, interquartile range; NR, not reported.
Regarding populations, 21 studies were conducted on paediatric patients. Four studies reported the prevalence of IV rehydration in children admitted to the hospital with diagnosis of diarrhoea [13,14,15,16]; ten studies considered children admitted to the hospital with diagnosis of acute gastroenteritis [17,18,19,20,21,22,23,24,25,26]; three studies considered children hospitalised with dehydration [27,28,29]; one study considered children admitted to the hospital with chronic neuromuscular disorder [30]; one study considered children admitted to the hospital with severe acute malnutrition [31]; one study considered children hospitalised with laboratory-confirmed influenza [32]; and one study considered children hospitalised with bronchiolitis [33].
Eight studies were conducted on adult patients. One study investigated the prevalence of IV rehydration in patients with incurable cancer [34]; two studies investigated patients with fever and other signs of dengue [35,36]; one study investigated patients admitted to the cholera isolation ward [37]; one study investigated patients who had single substance ingestion of modafinil [38]; one study investigated patients with diarrhoea [39]; one study investigated patients with hypercalcemia [40]; and one study investigated patients with postural tachycardia syndrome [41].
3.3. Frequency of Intravenous Rehydration in Paediatric Patients
Of the 29 included studies, 21 reported the prevalence of IV rehydration in paediatric patients. This review found that the use of IV rehydration therapy varied considerably amongst paediatric patients. The prevalence ranged from 4.5% to 100% (Table 2).
Table 2.
Frequency of IV rehydration in paediatric and adult patients.
| Author, Year | Sample Size | Exposure Group n (%) |
Comparator Group n (%) | Overall n (%) |
Effect Size | Population |
|---|---|---|---|---|---|---|
| Chow et al., 2009 [14] | 7391 | 3548 (48%) | - | - | - | Paediatric patients admitted to the hospital with diagnosis of diarrhoea |
| Patwari et al., 1991 [16] | 5996 | 366 (6.1%) | - | - | - | Paediatric patients who came to the hospital with diarrhoea |
| Akech et al., 2018 [13] | 8025 | 3569 (45%) | - | - | - | Paediatric patients hospitalised with diarrhoea and dehydration |
| Heyman et al., 1990 [15] | 3495 | 1310 (37.5%) | - | - | - | Paediatric patients hospitalised with diarrhoea or gastroenteritis |
| Abdul-Mumin, Ervin and Halvorson, 2019 [17] | 473 | 365 (77%) | - | - | - | Paediatric patients hospitalised with acute gastroenteritis |
| Freedman et al., 2014 [19] | 804,000 | 148,780 (18.5%) | - | - | - | Paediatric patients who were diagnosed as having gastroenteritis in an emergency department |
| Moineau and Newman, 1990 [20] | 17 | 17 (100%) | - | - | - | Paediatric patients admitted to the emergency department with mild to moderate dehydration caused by viral gastroenteritis |
| Wathen, MacKenzie and Bothner, 2004 [27] | 182 | 182 (100%) | - | - | - | Paediatric patients presenting at the hospital with gastroenteritis and dehydration |
| Waisbourd-Zinman et al., 2008 [25] | 356 | 239 (67%) | - | - | - | Paediatric patients hospitalised with nosocomial rotavirus gastroenteritis |
| Perl et al., 2011 [23] | 533 | Rotavirus positive (n = 202) 187 (92%) |
Rotavirus negative (n = 331) 249 (75%) |
436 (82%) | 4.06 (2.28–7.21) | Paediatric patients hospitalised with acute gastroenteritis, rotavirus gastroenteritis, and diarrhoea, and vomiting |
| Dbaibo et al., 2013 [18] | 491 | Rotavirus positive (n = 136) 136 (100%) |
Rotavirus negative (n = 351) 351 (96.3%) |
491 (97.4%) | p = 0.0234 | Paediatric patients hospitalised with a diagnosis of acute gastroenteritis |
| Nazurdinov et al., 2018 [22] | 2863 | Rotavirus positive (n = 1207) 1097 (91%) |
Rotavirus negative (n = 1656) 1433 (87%) |
2530 (88.5%) | NR | Paediatric patients hospitalised with acute gastroenteritis and rotavirus. |
| Myat et al., 2021 [21] | 2977 | Rotavirus positive (n = 1320) 770 (58.3%) |
Rotavirus negative (n = 1657) 880 (53.1%) |
1650 (55.5%) | <0.01 | Paediatric patients hospitalised for acute gastroenteritis |
| Redondo-Gonzalez et al., 2016 [24] | 17,415 | Rotavirus positive (n = 1657) 75 (4.5%) |
Rotavirus negative (n = 15,758) 230 (1.6%) |
NR | 3.2 (2.46–4.18) | Paediatric patients hospitalised with acute gastroenteritis |
| Wildi-Runge, 2009 [26] | 539 | 378 (70.1%) | - | - | - | Paediatric patients hospitalised with rotavirus gastroenteritis |
| Ben- Shalom et al., 2016 [28] | 58 | 58 (100%) | - | - | - | Paediatric patients hospitalised with hypernatremic dehydration |
| Janet et al., 2015 [29] | 83 | 83 (100%) | - | - | - | Paediatric patients with mild-to moderate isonatremic dehydration |
| Lopez-Medina et al., 2012 [32] | 73 | 39 (53%) | - | - | - | Paediatric patients hospitalised with laboratory-confirmed influenza |
| Kao et al., 2019 [30] | 44 | 69 (34%) | - | - | - | Paediatric patients with chronic neuromuscular disorder who visited the emergency room |
| Fikrie, Alemayehu and Gebremedhin, 2019 [31] | 381 | 87 (22.8%) | - | - | - | Paediatric patients hospitalised with Severe Acute Malnutrition |
| Oakley et al., 2016 [33] | 491 | 65 (31%) | - | - | - | Paediatric patients hospitalised with bronchiolitis |
| Hasan et al., 2021 [39] | 1531 | Patients during 2018 epidemic (n = 562) 333 (59.3%) |
Patients during the seasonally matched periods (n = 969) 450 (46.4%) |
783 (51%) | OR 95%CI 1.7 (1.4–2.1) |
Paediatric and adult patients hospitalised with diarrhoea during epidemic |
| Marra et al., 2011 [35] | 3393 | 824 (24.3%) | - | - | - | Paediatric and adult patients treated in the hydration tent during dengue fever epidemic |
| Tewari et al., 2018 [36] | 500 | 45 (9.2%) | - | - | - | Paediatric and adult patients hospitalised with fever and other signs of dengue |
| Thronaes et al., 2021 [34] | 451 | 203 (45%) | - | - | - | Adult patients with incurable cancer |
| Spiller et al., 2009 [38] | 137 | 2 (1.5%) | - | - | - | Adult patients who had single substance ingestion of modafanil |
| Blacklock et al., 2015 [37] | 798 | 767 (96.1%) | - | - | - | Paediatric and adult patients hospitalised with cholera during the epidemic |
| Machado et al., 2015 [40] | 72 | Calcium supplement syndrome positive (n = 15) 15 (100%) |
Calcium supplement syndrome negative (n = 57) 0 (0%) |
15 (22%) | NR | Patients hospitalised with hypercalcemia |
| Tseng et al., 2018 [41] | 332 | 21 (6.3%) | - | - | - | Adult patients with postural tachycardia syndrome |
n, participant; NR, not reported.
Four studies reported the prevalence of IV rehydration in children admitted to the hospital with diagnosis of diarrhoea [13,14,15,16]. The prevalence ranged from 6.1% to 48%. Five studies reported the prevalence of IV rehydration in children admitted to the hospital with diagnosis of acute gastroenteritis [17,19,20,25,27]. The prevalence ranged from 18.5% to 100%. Six studies reported the prevalence of IV rehydration in children with diagnosis of acute gastroenteritis who also tested positive for rotavirus [18,21,22,23,24,26]. The prevalence in children with rotavirus ranged from 4.5% to 100%. Two studies reported the prevalence of IV rehydration in children hospitalised with dehydration [28,29]. The prevalence was 100%. One study reported the prevalence of IV rehydration in children admitted to the hospital with chronic neuromuscular disorder [30]. The prevalence was 34%. One study reported the prevalence of IV rehydration in children admitted to the hospital with severe acute malnutrition [31]. The prevalence was 22.8%. One study reported the prevalence of IV rehydration in children hospitalised with laboratory-confirmed influenza [32]. The prevalence was 53%. One study reported the prevalence of IV rehydration in children hospitalised with bronchiolitis [33]. The prevalence was 31%.
These results show that the highest prevalence of IV rehydration therapy was observed amongst those hospitalised with dehydration [28,29] and rotavirus-positive gastroenteritis [18], while the lowest prevalence was also observed amongst those hospitalized with acute gastroenteritis but only amongst rotavirus-negative patients [24]. This suggests that type and severity of illness may play a role in the prevalence of IV rehydration therapy. Furthermore, patients with rotavirus-positive gastroenteritis were administrated IV rehydration therapy more often compared to rotavirus-negative patients.
This review showed that the most common indication for IV rehydration therapy in paediatric patients was dehydration due to fluid loss from the gastrointestinal tract. The second most common indication for IV rehydration was influenza, followed by neuromuscular disease, bronchiolitis, and malnutrition.
3.4. Frequency of Intravenous Rehydration in Adult Patients
Of the 29 included studies, 8 reported the prevalence of IV rehydration in adult patients. Similar to the paediatric patients, the use of IV rehydration therapy varied considerably amongst adult patients. The prevalence ranged from 1.5% to 100% (Table 2).
Two studies [35,36] reported the prevalence of IV rehydration in patients with fever and other signs of dengue. The prevalence ranged from 9.2% to 24.3%. One study [39] reported the prevalence of IV rehydration in patients admitted to the hospital with diarrhoea. The prevalence was 51%. One study [34] reported the prevalence of IV rehydration in patients with incurable cancer. The prevalence was 45%. One study [38] reported the prevalence of IV rehydration in patients who had single substance ingestion of modafinil. The prevalence was 1.5%. One study [37] reported the prevalence of IV rehydration in patients admitted to the cholera isolation ward. The prevalence was 96.1%. One study [40] reported the prevalence of IV rehydration in patients hospitalised with calcium supplement syndrome. The prevalence was 22%. One study [41] reported the prevalence of IV rehydration in patients hospitalised with postural tachycardia syndrome. The prevalence was 6.3%.
The results show that the highest prevalence of IV rehydration therapy was observed amongst patients hospitalised with cholera [37], whilst the lowest prevalence of IV rehydration therapy was seen amongst patients who had single substance ingestion of modafanil [38]. We also found a relatively high prevalence of IV rehydration amongst patients with diarrhoea [39]. Although, this review showed that IV rehydration was required in all paediatric patients suffering from dehydration [20,27], IV therapy amongst dehydrated adult patients was not so frequent [35].
This review indicated that in adult patients, the most common indication for IV rehydration therapy was dehydration due to fluid loss caused by cholera, and the second most common indication for IV rehydration was dehydration caused by diarrhoea, followed by cancer, fever, hypercalcemia, postural tachycardia syndrome, and drug intoxication.
4. Discussion
This systematic review of 29 studies demonstrated that there are substantial differences in the prevalence of IV rehydration therapy in both paediatric and adult populations. The prevalence of IV rehydration therapy in paediatric patients ranged from 4.5% to 100% and in adult patients ranged from 1.5% to 100%. In paediatric patients, IV rehydration therapy was required more frequently (80%) due to dehydration owing to fluid loss from the gastrointestinal tract, while other causes included malnutrition, neuromuscular disease, bronchiolitis, and influenza. In adults, indications for IV rehydration therapy were much more diverse: fever, diarrhoea, drug intoxication, hypercalcemia, cancer, and postural tachycardia syndrome.
Acute gastroenteritis is a disease with high morbidity and mortality affecting the paediatric population. Dehydration is the most common complication of acute gastroenteritis and therefore a frequent reason for consultation in paediatric emergency departments [29]. The most appropriate treatment method is still in debate. One of the main discussions is regarding the volume and the rate of administration of fluid used for IV rehydration, leading to great variability in the practice in paediatric emergency care [2]. We found that the highest prevalence of IV rehydration therapy was observed amongst paediatric patients who were hospitalised with dehydration [28,29] and rotavirus gastroenteritis [18]. Another finding is that in general, it appears that IV rehydration is much more frequently needed in rotavirus-positive than rotavirus-negative patients. Compared to patients with rotavirus-negative gastroenteritis, patients with rotavirus-positive gastroenteritis had a higher incidence of vomiting, lethargy, and dehydration. However, interestingly, the lowest prevalence of IV rehydration therapy was also seen amongst those hospitalised with rotavirus gastroenteritis [24]. This may be because a wide age range of children (7 months–14 years) were included in this study. On the other hand, dehydration may develop not only in enteritis, but also in the course of other infections in paediatric patients and IV rehydration may be needed. For example, in infants infected with influenza, both nutrition and fluid intake decrease due to fever and respiratory abnormalities, and nausea and diarrhoea increase fluid loss. Moreover, bacterial and viral co-infections can exacerbate these conditions. Therefore, half of hospitalised infants undergo IV rehydration [32]. Although nasogastric hydration has been found to be safe and effective in infants hospitalised for bronchiolitis, it is known that 31% of them are administered IV fluid hydration, and these are sicker infants who are followed up in the intensive care unit and receive IV antibiotic therapy [33]. One of the most important problems in children with neuromuscular diseases or in children in underdeveloped countries (based on malnutrition) is weakness/fatigue, infections, and metabolic disturbances, and all of these can deteriorate the general health status of patients and cause dehydration [30,31]. Therefore, dehydration requiring IV rehydration treatment is present in 23% of patients with severe acute malnutrition and 34% of children with neuromuscular disease admitted to the emergency department [30,31]. Considering the aforementioned data, it becomes clear that there are many factors, such as severity of illness and concomitant infections, affecting IV rehydration therapy in paediatric practice, and treatment should be individualised.
Regarding the adult population, the highest prevalence of IV rehydration therapy was observed amongst patients hospitalised with cholera, and almost all patients admitted received IV fluids, because there was a history of vomiting, which may have influenced the decision to treat with IV fluids in these patients; thus, they could not consume oral rehydration solution [37]. Whilst the lowest prevalence (1.5%) of IV rehydration therapy was seen amongst patients who had single substance ingestion of modafanil [38]. Additionally, patients with postural tachycardia syndrome often have gastrointestinal symptoms, and sometimes, these symptoms can be so severe that non-oral nutritional/hydration support, including IV fluids, may be required. Tseng et al. found this ratio as 6.3% [41]. In the Machado et al.’s study, IV rehydration was administered in all patients positive for calcium supplement syndrome (22% of hypercalcemia cases); supplements were discontinued, and calcium level at discharge was found as normal in 80% of patients [40]. IV rehydration therapies are also frequently used for electrolyte disturbances. However, this review showed that IV rehydration was required mostly for dehydration-related hypernatremia in children, while it was also necessary for hypercalcemia in adults. Although IV rehydration therapies have many clear indications, there are still some dilemmas and ethical issues regarding its role in end of life or palliative care [42]. Withholding and withdrawing hydration from terminally ill patients poses many ethical challenges. For example, from the perspective of Islam, rules governing the care of terminally ill patients are derived from the principle that injury and harm should be prevented or avoided. The hastening of death by the withdrawal of food and drink is forbidden, but Islamic law permits the withdrawal of futile, death-delaying treatment, including life support [43]. In the present review, Thronaes et al. et al., found that IV rehydration was applied in half of incurable cancer patients [34]. Therefore, withholding and withdrawing artificial nutrition and hydration must be evaluated in specific situations (terminally ill patients, palliative care, dementia, aged patients) and always case by case in an individual manner. It is important to treat patients appropriately to their cultural and spiritual needs.
Despite the fact that standard rehydration guidelines for a range of conditions and different settings exist, IV fluids tend to be overutilised. In fact, IV fluids are so ubiquitous in hospitals that one would forget considering the indications. For instance, the World Health Organisation advises the use of oral rehydration to treat mild or moderate dehydration secondary to diarrhoea, and IV rehydration to only treat severe dehydration [44]; many emergency departments and primary care physicians prefer to use IV fluids over oral rehydration solutions for children who are dehydrated [45,46]. Such findings indicate a large gap between clinical guidelines and current clinical practice. Furthermore, Abdul-Mumin [17] reported that 70% of paediatric patients who had no dehydration status at the time of admission received IV fluids. This could reflect both incorrect use of IV solutions and poor evaluation/documentation of hydration status. Therefore, the initial assessment of the patient should also include the decision on whether the patient actually requires IV rehydration, and if that is the case, tailor it to the specific needs of that patient.
The findings of our study should be interpreted within its limitations. First, we had to include highly heterogeneous populations. Moreover, owing to many original studies included in the review not reporting IV rehydration prevalence by definite age-range groups, it was not possible to provide accurate data for prevalence by age group. Future studies reporting prevalence of IV rehydration should attempt to report data by definite age-range groups. Second, evaluation was made independent of the type, content, and infusion rate of IV rehydration therapy. Third, the prevalence and indications of IV rehydration therapy were assessed, but outcomes could not be evaluated. Nonetheless, our systematic review is the first and included a large number of studies regarding important issue on IV rehydration therapy. Finally, while studies reported the condition for which the populations were hospitalised, the exact reasons for hospitalisation (e.g., complications or exacerbations) were not reported.
5. Conclusions
In conclusion, IV rehydration therapy can be applied both as a part of the primary treatment in cases caused by dehydration and as a supportive treatment in some conditions, such as drug intoxication, cancer, and postural tachycardia syndrome. While IV rehydration therapy is often implemented owing to dehydration and diarrhoea in children, it is used in a broader spectrum in adults. While IV rehydration therapy is critical, especially for hospitalised patients, maintenance fluid prescribing practices vary considerably, and guidelines are scarce. Therefore, in some cases, an interprofessional team approach is required to achieve optimum fluid balance for these patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/epidemiologia4010002/s1, Table S1: Database search strategy; Table S2: Risk of bias in cohort studies; Table S3: Risk of bias in cross sectional studies.
Author Contributions
Writing—original draft, J.G. and P.S.; Writing—review and editing, A.K., G.F.L.S., N.V., P.C.I., A.C., and L.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
Guillermo F. López Sánchez is funded by the European Union—Next Generation EU.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hoorn E.J. Intravenous fluids: Balancing solutions. J. Nephrol. 2016;30:485–492. doi: 10.1007/s40620-016-0363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brossier D.W., Tume L.N., Briant A.R., Chaparro C.J., Moullet C., Rooze S., Verbruggen S.C.A.T., Marino L.V., Alsohime F., Beldjilali S., et al. ESPNIC clinical practice guidelines: Intravenous maintenance fluid therapy in acute and critically ill children—A systematic review and meta-analysis. Intensiv. Care Med. 2022;48:1691–1708. doi: 10.1007/s00134-022-06882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudnick M.R., Fay K., Wahba I.M. Fluid administration strategies for the prevention of contrast-associated acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2022;31:414–424. doi: 10.1097/MNH.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 4.Hwa Y.L., Kull M.R. The why and how of maintaining hydration during cancer therapy. Curr. Opin. Support. Palliat. Care. 2020;14:324–332. doi: 10.1097/SPC.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 5.Wood C. Fluid management: An update for perioperative practitioners. J. Perioper. Pr. 2021;31:71–79. doi: 10.1177/1750458920964174. [DOI] [PubMed] [Google Scholar]
- 6.Frost P. Intravenous fluid therapy in adult inpatients. BMJ. 2014;350:g7620. doi: 10.1136/bmj.g7620. [DOI] [PubMed] [Google Scholar]
- 7.Crosignani A., Spina S., Marrazzo F., Cimbanassi S., Malbrain M.L.N.G., Van Regenemortel N., Fumagalli R., Langer T. Intravenous fluid therapy in patients with severe acute pancreatitis admitted to the intensive care unit: A narrative review. Ann. Intensiv. Care. 2022;12:1–15. doi: 10.1186/s13613-022-01072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno C., Collier A., Holyday M., Lambert K. Interventions to Improve Hydration in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2021;13:3640. doi: 10.3390/nu13103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao H., Barber J., Campbell E.S. Economic burden of dehydration among hospitalized elderly patients. Am. J. Health Pharm. 2004;61:2534–2540. doi: 10.1093/ajhp/61.23.2534. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ Br. Med. J. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Critical Appraisal Skills Programme CASP Checklists. 2022. [(accessed on 15 September 2022)]. Available online: https://casp-uk.net/
- 12.Critical Appraisal Skills Programme CASP Cohort Study Checklist. 2022. [(accessed on 15 September 2022)]. Available online: https://casp-uk.net/images/checklist/documents/CASP-Cohort-Study-Checklist/CASP-Cohort-Study-Checklist_2018.pdf.
- 13.Akech S., Ayieko P., Gathara D., Agweyu A., Irimu G., Stepniewska K., English M., Ngarngar S., Aduro N., Mutai L., et al. Risk factors for mortality and effect of correct fluid prescription in children with diarrhoea and dehydration without severe acute malnutrition admitted to Kenyan hospitals: An observational, association study. Lancet Child Adolesc. Health. 2018;2:516–524. doi: 10.1016/S2352-4642(18)30130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow C., Choi K., Nelson E.A.S., Chan P., Mast T.C., Distefano D., Tam J.S., Bresee J.S. Use of intravenous fluids in Hong Kong children hospitalised for diarrhoea and relationship to severity and aetiology. Vaccine. 2009;27:F55–F60. doi: 10.1016/j.vaccine.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 15.Heymann D.L., Mbvundula M., Macheso A., McFarland D.A., Hawkins R.V. Oral rehydration therapy in Ma-lawi: Impact on the severity of disease and on hospital admissions, treatment practices, and recurrent costs. Bull. World Health Organ. 1990;68:193–197. [PMC free article] [PubMed] [Google Scholar]
- 16.Patwari A.K., Kumar H., Anand V.K., Aneja S., Sharma D. Diarrhea training and treatment unit: Experience from a teaching hospital. Indian J. Pediatr. 1991;58:775–781. doi: 10.1007/BF02825434. [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Mumin A., Ervin S., Halvorson E.E. Clinical characteristics associated with increased resource utilization of hospitalized children under 5 years with acute gastroenteritis at a tertiary hospital in the northern region of Ghana: A retrospective study. Pan Afr. Med. J. 2019;33:186. doi: 10.11604/pamj.2019.33.186.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dbaibo G., Rajab M., Inati A., Mikhael R., Choueiry E., Al-Tannir M., Salam O., Ramakrishnan G., DeAntonio R. Hospital-based surveillance study of rotavirus gastroenteritis in children under 5 years of age in Lebanon. Trials Vaccinol. 2013;2:25–30. doi: 10.1016/j.trivac.2013.08.002. [DOI] [Google Scholar]
- 19.Freedman S.B., Hall M., Shah S.S., Kharbanda A.B., Aronson P.L., Florin T.A., Mistry R.D., Macias C.G., Neuman M.I. Impact of Increasing Ondansetron Use on Clinical Outcomes in Children With Gastroenteritis. JAMA Pediatr. 2014;168:321–329. doi: 10.1001/jamapediatrics.2013.4906. [DOI] [PubMed] [Google Scholar]
- 20.Moineau G., Newman J. Rapid intravenous rehydration in the pediatric emergency department. Pediatr. Emerg. Care. 1990;6:186–188. doi: 10.1097/00006565-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Myat T.W., Thu H.M., Tate J.E., Burnett E., Cates J.E., Parashar U.D., Kyaw Y.M., Khaing T.E.E., Moh K.M., Win N.N., et al. Rotavirus infection among children under five years of age hospitalized with acute gastroenteritis in Myanmar during 2018–2020—Multicentre surveillance before rotavirus vaccine introduction. Vaccine. 2021;39:6907–6912. doi: 10.1016/j.vaccine.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nazurdinov A.B., Azizov Z.A., Tishkova F., Turkov S.M., Daniels D.S., Leshem E. Rotavirus hospitalizations among children <5 years of age-Tajikistan, 2013–2014. Vaccine. 2018;36:7794–7797. doi: 10.1016/j.vaccine.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Perl S., Goldman M., Berkovitch M., Kozer E. Characteristics of rotavirus gastroenteritis in hospitalized children in Israel. Isr. Med. Assoc. J. IMAJ. 2011;13:274–277. [PubMed] [Google Scholar]
- 24.Redondo-González O., Tenías-Burillo J.M. A multifactorial regression analysis of the features of community-acquired rotavirus requiring hospitalization in Spain as represented in the Minimum Basic Data Set. Epidemiol. Infect. 2016;144:2509–2516. doi: 10.1017/S0950268816000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waisbourd-Zinman O., Ben-Ziony S., Solter E., Scherf E., Samra Z., Ashkenazi S. Hospitalizations for nosocomial rotavirus gastroenteritis in a tertiary pediatric center: A 4-year prospective study. Am. J. Infect. Control. 2009;37:465–469. doi: 10.1016/j.ajic.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Wildi-Runge S., Allemann S., Schaad U.B., Heininger U. A 4-year study on clinical characteristics of children hospitalized with rotavirus gastroenteritis. Eur. J. Pediatr. 2009;168:1343–1348. doi: 10.1007/s00431-009-0934-z. [DOI] [PubMed] [Google Scholar]
- 27.Wathen J.E., MacKenzie T., Bothner J.P. Usefulness of the Serum Electrolyte Panel in the Management of Pediatric Dehydration Treated With Intravenously Administered Fluids. Pediatrics. 2004;114:1227–1234. doi: 10.1542/peds.2004-0457. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Shalom E., Toker O. and Schwartz, S. Hypernatremic Dehydration in Young Children: Is There a Solution? Isr. Med. Assoc. J. IMAJ. 2016;18:95–99. [PubMed] [Google Scholar]
- 29.Janet S., Molina J.C., Marañon R., García-Ros M. Effects of Rapid Intravenous Rehydration in Children With Mild-to-Moderate Dehydration. Pediatr. Emerg. Care. 2015;31:564–567. doi: 10.1097/PEC.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 30.Kao W.-T., Tseng Y.-H., Jong Y.-J., Chen T.-H. Emergency room visits and admission rates of children with neuromuscular disorders: A 10-year experience in a medical center in Taiwan. Pediatr. Neonatol. 2019;60:405–410. doi: 10.1016/j.pedneo.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Fikrie A., Alemayehu A., Gebremedhin S. Treatment outcomes and factors affecting time-to-recovery from severe acute malnutrition in 6–59 months old children admitted to a stabilization center in Southern Ethiopia: A retrospective cohort study. Ital. J. Pediatr. 2019;45:46. doi: 10.1186/s13052-019-0642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Medina E., Ardura M.I., Siegel J.D., Brock E., Sánchez P.J. 2009 Influenza A in Infants Hospitalized at Younger than 6 Months. J. Pediatr. 2012;160:626–631.e1. doi: 10.1016/j.jpeds.2011.09.060. [DOI] [PubMed] [Google Scholar]
- 33.Oakley E., Bata S., Rengasamy S., Krieser D., Cheek J., Jachno K., Babl F.E. Nasogastric Hydration in Infants with Bronchiolitis Less Than 2 Months of Age. J. Pediatr. 2016;178:241–245.e1. doi: 10.1016/j.jpeds.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Thronæs M., Løhre E.T., Kvikstad A., Brenne E., Norvaag R., Aalberg K.O., Moen M.K., Jakobsen G., Klepstad P., Solberg A., et al. Interventions and symptom relief in hospital palliative cancer care: Results from a prospective longitudinal study. Support. Care Cancer. 2021;29:6595–6603. doi: 10.1007/s00520-021-06248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marra A.R., De Matos G.F.J., Janeri R.D., Machado P.S., Schvartsman C., Dos Santos O.F.P. Managing patients with dengue fever during an epidemic: The importance of a hydration tent and of a multidisciplinary approach. BMC Res. Notes. 2011;4:335. doi: 10.1186/1756-0500-4-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tewari V.V., Tewari K., Mehta R. Clinical and hematological profile of patients with dengue fever at a tertiary care hospital—An observational study. Mediterr. J. Hematol. Infect. Dis. 2017;10:e2018021. doi: 10.4084/mjhid.2018.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blacklock A., Sesay A., Kamara A., Kamara M., Blacklock C. Characteristics and clinical management of patients admitted to cholera wards in a regional referral hospital during the 2012 epidemic in Sierra Leone. Glob. Health Action. 2015;8:25266. doi: 10.3402/gha.v8.25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiller H.A., Borys D., Griffith J.R., Klein-Schwartz W., Aleguas A., Sollee D., Anderson D.A., Sawyer T.S. Toxicity from modafinil ingestion. Clin. Toxicol. 2009;47:153–156. doi: 10.1080/15563650802175595. [DOI] [PubMed] [Google Scholar]
- 39.Hasan S.M.T., Das S., Faruque A.S.G., Khan A.I., Clemens J.D., Ahmed T. Taking care of a diarrhea epidemic in an urban hospital in Bangladesh: Appraisal of putative causes, presentation, management, and deaths averted. PLOS Neglected Trop. Dis. 2021;15:e0009953. doi: 10.1371/journal.pntd.0009953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machado M.C., Bruce-Mensah A., Whitmire M., Rizvi A.A. Hypercalcemia Associated with Calcium Supplement Use: Prevalence and Characteristics in Hospitalized Patients. J. Clin. Med. 2015;4:414–424. doi: 10.3390/jcm4030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng A.S., Traub N.A., Harris L.A., Crowell M.D., Dnp C.R.H., Goodman B.P., DiBaise J.K. Factors Associated With Use of Nonoral Nutrition and Hydration Support in Adult Patients With Postural Tachycardia Syndrome. J. Parenter. Enter. Nutr. 2018;43:734–741. doi: 10.1002/jpen.1493. [DOI] [PubMed] [Google Scholar]
- 42.Cardenas D. Ethical issues and dilemmas in artificial nutrition and hydration. Clin. Nutr. ESPEN. 2020;41:23–29. doi: 10.1016/j.clnesp.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Alsolamy S. Islamic Views on Artificial Nutrition and Hydration in Terminally Ill Patients. Bioethics. 2012;28:96–99. doi: 10.1111/j.1467-8519.2012.01996.x. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers, 4th Revision. 2005. [(accessed on 15 September 2022)]. Available online: https://www.who.int/publications/i/item/9241593180.
- 45.Ozuah P.O., Avner J.R., Stein R.E.K. Oral Rehydration, Emergency Physicians, and Practice Parameters: A National Survey. Pediatrics. 2002;109:259–261. doi: 10.1542/peds.109.2.259. [DOI] [PubMed] [Google Scholar]
- 46.Conners G.P., Barker W.H., Mushlin A.I., Goepp J.G.K. Oral versus intravenous: Rehydration preferences of pediatric emergency medicine fellowship directors. Pediatr. Emerg. Care. 2000;16:335–338. doi: 10.1097/00006565-200010000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.